- Department of Neurology, Faculty of Medicine, Shimane University, Izumo, Japan
This study is to test the hypothesis that apathy in healthy participants is closely related to the prefrontal-basal-ganglia circuit and associated structural changes. We selected 36 healthy aged participants with (n = 18) or without apathy (n = 18) from our database. Participants underwent structural MRI scanning, providing data for voxel-based morphometric analysis to explore gray matter changes associated with apathy. Compared to the non-apathy group, the apathy group showed reduced gray matter volume of the right putamen, whereas volumes of the bilateral inferior frontal gyri and left inferior occipital gyrus showed increase. When depression scores were included in a regression model as a covariate, apathetic participants showed decreased gray matter volume in the right precentral gyrus compared to the non-apathetic participants. These findings suggest that apathy is associated with the gray matter volume in the prefrontal-basal-ganglia network, and may have a neuroanatomical basis distinct from depression in healthy elderly.
Introduction
Apathy is defined as a lack of motivation that is not caused by a disturbance in consciousness, cognitive impairment, or emotional distress (Marin et al., 1991), and this symptom can cause dysfunctions in elaboration, execution and management of goal-directed behaviors (Brown and Pluck, 2000). Nowadays, apathy is recognized as a frequent neuropsychiatric symptom not only in neurodegenerative diseases, such as Parkinson’s disease (Dujardin et al., 2007), Huntington’s disease (Di Maio et al., 1993), and progressive supranuclear palsy (Litvan et al., 1996), but also in stroke (Starkstein et al., 1993), dementia (Kuzis et al., 1999), and brain injury (Diaz et al., 2012). Studies have shown that apathy does influence patients’ quality of life (Yeager and Hyer, 2008) and recovery from illness (Politis et al., 2004). Although the exact mechanism is still not clear, the prefrontal-basal-ganglia system is thought to play a key role in apathy (Levy and Dubois, 2006). Most lesion studies have identified the prefrontal cortex and basal ganglia as target regions responsible for apathy symptoms. Apathy symptoms are observed after direct lesions of prefrontal cortex (Eslinger and Damasio, 1985; Stuss et al., 2000), and they can also appear indirectly in many diseases that are accompanied by lesions in the basal-ganglia (Laplane et al., 1989; Engelborghs et al., 2000). For instance, auto-activation deficit was observed in PSP, in which the basal-ganglia dysfunction caused the prefrontal hypometabolism (D’Antona et al., 1985; Baron, 1994). These findings suggest that apathy is associated with disruption of the network involving the frontal lobe and basal ganglia (Middleton and Strick, 2000; Kimura et al., 2003).
Apathy can occur not only in patient populations but also in healthy individuals free of obvious pathology. However, only one study has assessed the structural changes associated with apathy in healthy participants free of any related brain diseases (Grool et al., 2014). In that study, gray matter volume reductions in the frontal and temporal lobes as well as the thalamus were related to apathy symptoms, but no significant changes in the basal ganglia were noted. One reason for this might be that they assessed apathy symptoms using only three items from a geriatric depression scale. Apathy symptoms should be assessed by more elaborate instruments that have been specifically developed for apathy assessment. In this study, we assessed apathy using the apathy scale originally developed by Starkstein et al. (1992) and subsequently modified for use with the Japanese population (Okada et al., 1997). Here, we studied whether apathetic healthy subjects showed any structural changes in the brain including within the prefrontal-basal-ganglia system.
Materials and Methods
Participants
The participants were selected from among the individuals who participated in health screening at the Shimane Institute of Health Science from 2007 to 2013. The database consisted of 445 subjects. All participants provided informed consent, and the medical ethics committee of Shimane University approved the study protocol. The health check included physical examination, detailed medical history (disease, life habits, medication, and treatment), laboratory blood tests, neuropsychological assessment, and head MRI. The subjects included in this study were required to have no history of neurologic or psychiatric conditions such as cerebrovascular disease, dementia, depression, or other psychiatric illnesses. The age was from 60 to 70 years old. The MRI did not show any brain lesions including silent brain lesions, silent brain infarctions, pathological white matter lesions, and microbleeds.
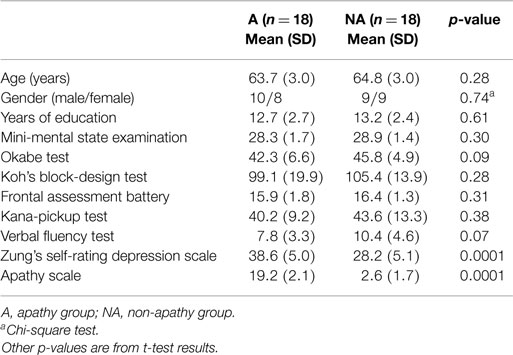
Neuropsychological assessments included the following tests: the mini-mental state examination (MMSE) of general cognitive ability (Folstein et al., 1975), subtests of the Okabe intelligence test, which is a modified and simplified version of the Wechsler adult intelligence scale, including tests assessing information, mental control, digit span, and paired associate and non-associate learning (Kobayashi et al., 1987), and Kohs’ block-design test (Dureman and Sälde, 1959) for visuospatial ability. For assessing frontal lobe executive function we used the frontal assessment battery (FAB) (Dubois et al., 2000), Kana-pickup test (Kaneko, 1990), and verbal fluency test (Ruff et al., 1996). Depression was also assessed using the Japanese version of Zung’s self-rating depression scale (SDS) (Zung, 1965; Fukuda and Kobayashi, 1973). For all included participants, cognitive function test scores were within the normal range, i.e., MMSE score >26, FAB score >12, and Okabe score >35. Apathy was assessed using the apathy scale, which was originally developed by Starkstein et al. (1992) and modified for use with Japanese individuals (Okada et al., 1997). The division score on the apathy scale was 16 points. This division score was chosen as appropriate using our previous study on Japanese stroke patients (Okada et al., 1997). Ultimately, we selected 36 subjects (19 males and 17 females). Eighteen of these had a score on the apathy scale between 16 and 23 and made up the apathy group. Another eighteen had a score between 0 and 5 and comprised the non-apathy group. Statistics about each group’s general information and their neuropsychological examination results are given in Table 1.
MRI Image Acquisition
Scans were performed using a 1.5-T MRI (Symphony, Siemens) at the Shimane Institute of Health Science. Whole brain 3D T1-weighted images (T1WI) were obtained with the following parameters: repetition time (TR) = 1960 ms, echo time (TE) = 3.68 ms, matrix = 256 × 256, field of view (FOV) = 220 mm × 220 mm, voxel size = 1.5 mm × 1.5 mm × 1.5 mm, flip angle = 15°, slice thickness = 1.05 mm, and 120 slices without gap.
Voxel-Based Morphometry
Data analysis was conducted using Statistical Parametric Mapping (SPM) software, Version 8 for Windows (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) and Data Processing Assistant for Resting-State fMRI (Yan and Zang, 2010). Both programs work under MATLAB (MathWorks, Natick, MA, USA). The statistical image analysis was performed using SPM8, and DPARSF software was used for VBM (voxel-based morphometry) analysis. The following five steps were employed: (1) manual reorientation of the images to the center point of the anterior commissure (Friston et al., 1989). (2) The MR images were segmented into three parts: gray matter, while matter, and cerebrospinal fluid using the standard unified segmentation model in SPM8 (Ashburner and Friston, 2005). (3) The gray matter templates were then created using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL), an improved VBM analysis method, which can achieve more accurate inter-subject registration, realignment of small deformations, and better spatial normalization (Ashburner, 2007). (4) After an initial affine registration of the gray matter DARTEL templates to the tissue probability maps in the Montreal Neurological Institute space was implemented, the non-liner warping of gray matter images was performed to the template with 1.5-mm cubic resolution. (5) Images were then modulated to ensure the relative volumes of gray matter were preserved following the spatial normalization procedure. (6) The modulated and normalized gray matter images were smoothed using a 8-mm full-width-at-half-maximum Gaussian kernel.
Statistical Analysis
Differences in gray matter volume over the whole brain between the two groups were tested in SPM8 using one-way analysis of covariance (ANCOVA), using age and gender as covariates of no interest. A comparison of the two groups using SDS scores as a covariate of no interest was also performed. The significance threshold was set to p < 0.001 with more than 100 voxels in every cluster. Further statistical analysis was carried out using IBM SPSS statistics 21 (IBM SPSS Inc., Chicago, IL, USA), including Chi-square and independent samples t-tests on participant characteristics, where the significance threshold was set to p < 0.05.
Results
There were no significant differences between the apathy and non-apathy groups in terms of age, gender, years of education, and neuropsychological test scores. The apathy group had higher depression scores compared to the non-apathy group (Table 1). Apathy scores were significantly correlated with depression scores (r = 0.58, p < 0.001).
Gray matter volumes were compared between the two groups via a whole brain analysis (Table 2). Significant group differences in the gray matter volume were observed at two clusters in the frontal lobe, one cluster in the occipital lobe, and one cluster in the basal ganglia. Gray matter volume in the right putamen was significantly smaller in the apathy group (Figure 1). On the other hand, gray matter volumes in the inferior frontal gyrus of both hemispheres as well as the left inferior occipital gyrus were actually larger in the apathy group compared to the non-apathy group. Figure 2 shows direct comparisons for gray matter volumes of each region between the two groups (Figure 2).
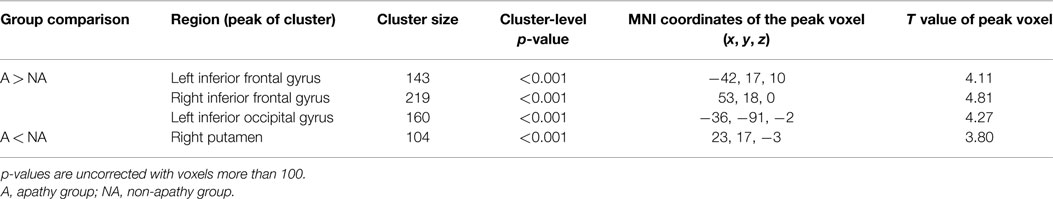
Table 2. Whole brain analysis comparisons of gray matter volume between apathy and non-apathy groups.
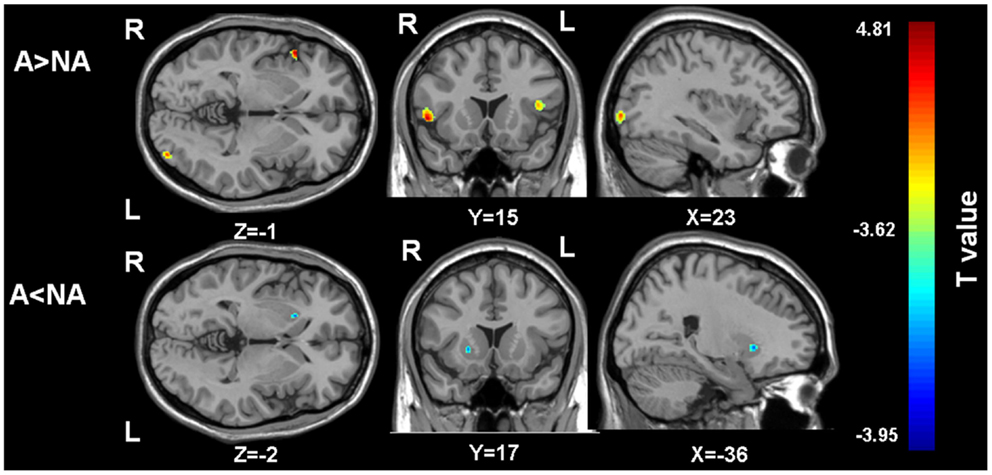
Figure 1. The regions showing gray matter volume differences between the two groups. A, apathy group; NA, non-apathy group. (Threshold at p uncorrected <0.001 and voxels more than 100). A > NA: the regions showing larger gray matter volumes in the apathy group compared to the non-apathy group. A < NA: the regions showing larger gray matter volumes in the apathy group compared to the non-apathy group.
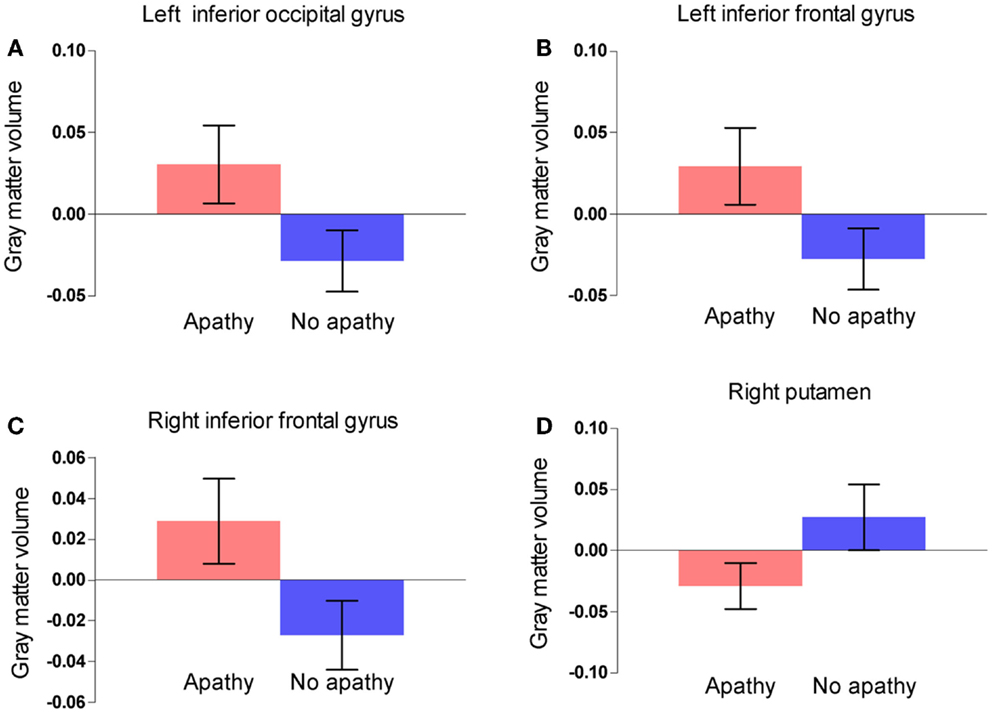
Figure 2. Comparisons of gray matter volume within the clusters between two groups in the left inferior occipital gyrus (A), the right inferior frontal gyrus (B), the left inferior frontal gyrus (C), and the right putamen (D). Error bars show 95% confidence intervals.
When the effects of depressive symptoms were adjusted for SDS scores as a confounding variable and the p-value was set as <0.005 uncorrected, the clusters mentioned above still existed though the number of voxels in the clusters decreased. When we used the same statistical criteria (p < 0.001 with voxel number more than 100), the apathy group showed only significantly smaller gray matter volume in the right precentral gyrus compared to the non-apathy group (Figure 3).
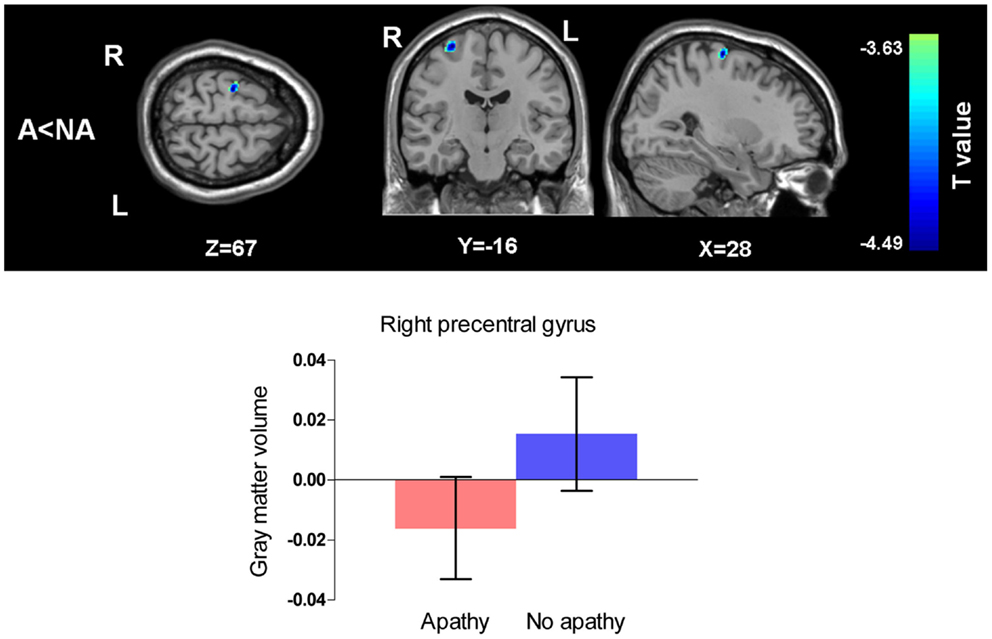
Figure 3. Comparison of gray matter volumes between two groups with SDS scores as a covariate of no interest. (Threshold at p uncorrected <0.001 and voxels more than 100). A, apathy group; NA, non-apathy group. Apathy group had decreased gray matter volume in the right precentral gyrus compared with non-apathy group. Error bars show 95% confidence intervals.
Discussion
The aim of this study was to explore structural brain changes in apathetic healthy participants, particularly in the regions of the prefrontal-basal-ganglia circuit on the basis of the findings in previous lesion studies. We found that gray matter volume changed in three regions within the prefrontal-basal-ganglia circuit in association with apathy symptoms. These findings are in line with the role of this network known to play in motivational functioning. In addition, volume change in the occipital region was also related to apathy. In our apathy group, there were five subjects who also had symptoms of being slightly suspected depressive, while the SDS scores of the rest of people in both groups were in the normal range. (The severity of depression was divided into three levels in our database study, 0–39 normal, 40–59 suspected depressive, and 60–80 possible to be depression.) Since SDS score and apathy score are often correlated with each other, some reports have illustrated that apathy and depression share similar brain mechanisms (Marin et al., 1993; Starkstein et al., 1993). Thus, the regions identified in the first analysis might be partially associated with depression symptoms in addition to apathy symptoms. In the second analysis, on the other hand, we found significant volume reduction in the right precentral gyrus in the apathy group when co-occurring depressive symptoms were controlled. This finding is in line with several lesion and functional studies showing that apathy is a symptom coexisting with depression but it can also occur dissociatively (Levy et al., 1998; Andersson et al., 1999; Alexopoulos et al., 2013).
Reduction in gray matter volumes for several structures, including the nucleus accumbens, right posterior cingulate gyrus, and bilateral inferior frontal gyrus, were reported in apathetic patients with Parkinson’s disease (Kostić and Filippi, 2011; Carriere et al., 2014). Apathetic amyotrophic lateral sclerosis patients also show significant gray matter volume reductions in the occipital lobe and inferior frontal gyrus (Tsujimoto et al., 2011). These findings for neurological diseases are intriguing because the patterns of structural changes associated with apathy are in part opposite to our pattern for healthy participants. These discrepancies suggest that neurodegenerative disease processes in the brain are important for investigating the relationship between neuropsychological symptoms and their anatomical basis. In healthy participants, it may be possible that morphological compensation (i.e., volume increase) occurs in the regions responsible for apathy symptoms. Apathetic symptoms are typically associated with slowed and inefficient processing and integration of information; for example, the interaction between the inferior frontal gyrus and inferior occipital gyrus is mediated by the inferior fronto-occipital fasciculus (Martino et al., 2010). The structural changes in these two cortical regions found in our study might be compensations that enable effective behavior execution in healthy participants. Actually, most of our subjects were still at work or engaged in social activities. The compensatory hypothesis is worthy of examination in future studies. In addition, apathy can be associated with a variety of factors in healthy elderly, such as low income, low instrumental activities of daily living, or cognitive impairment (Adams, 2001; Onyike et al., 2007). Apathy in our healthy subjects may be related to some cognitive impairment because the verbal fluency score was slightly lower in the apathy group although the MMSE score was comparable between the two groups.
On the other hand, volume reduction of the basal ganglia was associated with apathy in healthy participants, as seen in patients with various neuropsychiatric diseases. This is in line with reductions of blood flow and metabolism in the basal ganglia, which is reported in patients with apathy after stroke and Parkinson’s disease (Okada et al., 1997; Isella et al., 2002; Onoda et al., 2010). The putamen is related to action-reward association learning and storage of motor memories (Balleine et al., 2007). When lesions occur in the putamen, they cause disturbances in motor initiation (Miller and Cummings, 2007). Volume reduction in the putamen seems to play a critical role in the appearance of apathy in healthy participants, too. In addition, the inferior frontal gyrus plays a general role in both executive function and inhibitory control (Swick et al., 2008; Zheng et al., 2008; Hampshire et al., 2010). Thus, the increased volume of the inferior frontal gyrus observed here may also contribute to augmented psychomotor or psychosocial inhibition observed in apathetic participants.
Apathy and depression are associated with structural brain changes in several pathological conditions, including Alzheimer’s disease, mild cognitive impairment, and major depression (Marin et al., 1994; Lavretsky et al., 2008). The above mentioned structural changes associated with apathy might be confounded by depression. We found that apathy was associated with the gray matter volume reduction in the right precentral gyrus after adjustment for depressive symptoms. In a previous VBM study, the atrophy in bilateral precentral gyrus has been reported in apathetic patients with Parkinson’s disease, and the high-apathy scores were correlated with low gray matter density (Reijnders et al., 2010). From the point of view of regional cerebral blood flow (rCBF), Kang’s study on AD patients reported that compared with non-apathy group, apathy group showed lower rCBF in bilateral precentral gyrus (Kang et al., 2012). Although depression group also demonstrated the comparable rCBF reduction in the precentral gyrus, the extent of regions with low rCBF in apathy group was larger than depression group, which partly supports our results. In a task-based study of planning performance in patients with schizophrenia, the precentral gyrus of patients with high level of apathy showed lower task-related activation than healthy control (Liemburg et al., 2015). Thus, our preliminary results expand the previous studies and are in line with the notion that apathy and depression have a different neuroanatomical basis. This point was already reported by previous studies in neurodegenerative diseases (Holthoff et al., 2005; Kirsch-Darrow et al., 2006; Naarding et al., 2009) while we got the same view in healthy elderly.
The limitations of our study are its cross-sectional design and relatively small number of subjects. Long-term follow-up studies with a larger number of participants would more confidently explore the causal relationship between brain volume change and apathy. Even though there may be some limitations, the current study identified structural changes associated with well-defined apathy in healthy participants, and the findings contribute to the understanding of the brain mechanisms underlying apathy independent of pathological changes occurring in neuropsychiatric diseases. It is also known that apathy is a prodromal symptom in many degenerative neurological disorders (Delrieu et al., 2014; Mollenhauer, 2015). The current study warrants future study to elucidate the distinctive pathophysiology of apathetic symptoms in patients with brain pathology. In addition, the identified brain structures that changed with apathy, specifically the precentral gyrus, could be plausible targets for therapeutic interventions targeting apathy; for example, treatment by means of repetitive transcranial magnetic stimulation (Oguro et al., 2014). Furthermore, analysis of functional connectivity among brain regions may complement the current findings in the future.
Author Contributions
Conceived and designed the experiments: HY, KO, and SY. Performed the experiments: HY, KO, and SY. Analyzed the data: HY. Contributed reagents/materials/analysis tools: HY, KO, and SY. Wrote the paper including drafting and revising it: HY, KO, and SY.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was financially supported by JSPS KAKENHI Grant Numbers 26870375, 24500483 and ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan). We also thank Lei Gao (Department of Radiology, the First Affiliated Hospital of Nanchang University) for his advice on the data analysis, and Chao-Gan Yan (Department of Child and Adolescent Psychiatry, New York University School of Medicine) for his quite important suggestions in the use of DPARSF software.
References
Adams, K. B. (2001). Depressive symptoms, depletion, or developmental change? withdrawal, apathy, and lack of vigor in the geriatric depression scale. Gerontologist 41, 768–777. doi:10.1093/geront/41.6.768
Alexopoulos, G. S., Hoptman, M. J., Yuen, G., Kanellopoulos, D., Seirup, J. K., Lim, K. O., et al. (2013). Functional connectivity in apathy of late-life depression: a preliminary study. J. Affect. Disord. 149, 398–405. doi:10.1016/j.jad.2012.11.023
Andersson, S., Krogstad, J. M., and Finset, A. (1999). Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol. Med. 29, 447–456. doi:10.1017/S0033291798008046
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. doi:10.1016/j.neuroimage.2007.07.007
Ashburner, J., and Friston, K. J. (2005). Unified segmentation. Neuroimage 26, 839–851. doi:10.1016/j.neuroimage.2005.02.018
Balleine, B. W., Delgado, M. R., and Hikosaka, O. (2007). The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27, 8161–8165. doi:10.1523/JNEUROSCI.1554-07.2007
Baron, J. C. (1994). [Consequences of lesions of the basal ganglia on cerebral metabolic activity: clinical implications]. Rev. Neurol. 150, 599–604.
Brown, R. G., and Pluck, G. (2000). Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci. 23, 412–417. doi:10.1016/S0166-2236(00)01626-X
Carriere, N., Besson, P., Dujardin, K., Duhamel, A., Defebvre, L., Delmaire, C., et al. (2014). Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov. Disord. 29, 897–903. doi:10.1002/mds.25904
D’Antona, R., Baron, J. C., Samson, Y., Serdaru, M., Viader, F., Agid, Y., et al. (1985). Subcortical dementia frontal cortex hypometabolism detected by positron tomography in patients with progressive supranuclear palsy. Brain 108, 785–799. doi:10.1093/brain/108.3.785
Delrieu, J., Desmidt, T., Camus, V., Sourdet, S., Boutoleau-Bretonnière, C., Mullin, E., et al. (2014). Apathy as a feature of prodromal Alzheimer’s disease: an FDG-PET ADNI study. Int. J. Geriatr. Psychiatry 30, 470–477. doi:10.1002/gps.4161
Di Maio, L., Squitieri, F., Napolitano, G., Campanella, G., Trofatter, J. A., and Conneally, P. M. (1993). Suicide risk in Huntington’s disease. J. Med. Genet. 30, 293–295. doi:10.1136/jmg.30.4.293
Diaz, A. P., Schwarzbold, M. L., Thais, M. E., Hohl, A., Bertotti, M. M., Schmoeller, R., et al. (2012). Psychiatric disorders and health-related quality of life after severe traumatic brain injury: a prospective study. J. Neurotrauma 29, 1029–1037. doi:10.1089/neu.2011.2089
Dubois, B., Slachevsky, A., Litvan, I., and Pillon, B. (2000). The FAB: a frontal assessment battery at bedside. Neurology 55, 1621–1626. doi:10.1212/WNL.55.11.1621
Dujardin, K., Sockeel, P., Devos, D., Delliaux, M., Krystkowiak, P., Destée, A., et al. (2007). Characteristics of apathy in Parkinson’s disease. Mov. Disord. 22, 778–784. doi:10.1002/mds.21316
Dureman, I., and Sälde, H. (1959). Psychometric and Experimental Psychological Methods for Clinical Application. Stockholm: Almquist & Wiksell.
Engelborghs, S., Marien, P., Pickut, B. A., Verstraeten, S., and De Deyn, P. P. (2000). Loss of psychic self-activation after paramedian bithalamic infarction. Stroke 31, 1762–1765. doi:10.1161/01.STR.31.7.1762
Eslinger, P. J., and Damasio, A. R. (1985). Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology 35, 1731–1741. doi:10.1212/WNL.35.12.1731
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state: a practical method for grading the state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi:10.1016/0022-3956(75)90026-6
Friston, K. J., Passingham, R. E., Nutt, J. G., Heather, J. D., Sawle, G. V., and Frackowiak, R. S. J. (1989). Localisation in PET images: direct fitting of the intercommissural (AC-PC) line. J. Cereb. Blood Flow Metab. 9, 690–695. doi:10.1038/jcbfm.1989.97
Fukuda, K., and Kobayashi, S. (1973). [A study on a self-rating depression scale (author’s transl)]. Seishin Shinkeigaku Zasshi 75, 673–689.
Grool, A. M., Geerlings, M. I., Sigurdsson, S., Eiriksdottir, G., Jonsson, P. V., Garcia, M. E., et al. (2014). Structural MRI correlates of apathy symptoms in older persons without dementia: AGES-Reykjavik study. Neurology 82, 1628–1635. doi:10.1212/WNL.0000000000000378
Hampshire, A., Chamberlain, S. R., Monti, M. M., Duncan, J., and Owen, A. M. (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50, 1313–1319. doi:10.1016/j.neuroimage.2009.12.109
Holthoff, V. A., Beuthien-Baumann, B., Kalbe, E., Lüdecke, S., Lenz, O., Zündorf, G., et al. (2005). Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biol. Psychiatry 57, 412–421. doi:10.1016/j.biopsych.2004.11.035
Isella, V., Melzi, P., Grimaldi, M., Iurlaro, S., Piolti, R., Ferrarese, C., et al. (2002). Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson’s disease. Mov. Disord. 17, 366–371. doi:10.1002/mds.10041
Kaneko, M. (1990). Dementia and frontal lobe function. Higher Brain Funct. Res. 10, 127–131. doi:10.2496/apr.10.127
Kang, J. Y., Lee, J. S., Kang, H., Lee, H. W., Kim, Y. K., Jeon, H. J., et al. (2012). Regional cerebral blood flow abnormalities associated with apathy and depression in Alzheimer disease. Alzheimer Dis. Assoc. Dis. 26, 217–224. doi:10.1097/WAD.0b013e318231e5fc
Kimura, M., Matsumoto, N., Okahashi, K., Ueda, Y., Satoh, T., Minamimoto, T., et al. (2003). Goal-directed, serial and synchronous activation of neurons in the primate striatum. Neuroreport 14, 799–802. doi:10.1097/00001756-200305060-00004
Kirsch-Darrow, L., Fernandez, H. F., Marsiske, M., Okun, M. S., and Bowers, D. (2006). Dissociating apathy and depression in Parkinson disease. Neurology 67, 33–38. doi:10.1212/01.wnl.0000230572.07791.22
Kobayashi, S., Yamaguchi, S., Kitani, M., Okada, K., and Shimote, K. (1987). Evaluation of practical usefulness of the Okabe’s mini-mental scale in normal aged. Jpn. J. Neuropsychol. 3, 67–72.
Kostić, V. S., and Filippi, M. (2011). Neuroanatomical correlates of depression and apathy in Parkinson’s disease: magnetic resonance imaging studies. J. Neurol. Sci. 310, 61–63. doi:10.1016/j.jns.2011.05.036
Kuzis, G., Sabe, L., Tiberti, C., Dorrego, F., and Starkstein, S. E. (1999). Neuropsychological correlates of apathy and depression in patients with dementia. Neurology 52, 1403–1403. doi:10.1212/WNL.52.7.1403
Laplane, D., Levasseur, M., Pillon, B., Dubois, B., Baulac, M., Mazoyer, B., et al. (1989). Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain 112, 699–725. doi:10.1093/brain/112.3.699
Lavretsky, H., Zheng, L., Weiner, M. W., Mungas, D., Reed, B., Kramer, J. H., et al. (2008). The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int. J. Geriatr. Psychiatry 23, 1040–1050. doi:10.1002/gps.2030
Levy, M. L., Cummings, J. L., Fairbanks, L. A., Masterman, D., Miller, B. L., Craig, A. H., et al. (1998). Apathy is not depression. J. Neuropsychiatry Clin. Neurosci. 10, 314–319. doi:10.1176/jnp.10.3.314
Levy, R., and Dubois, B. (2006). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex 16, 916–928. doi:10.1093/cercor/bhj043
Liemburg, E. J., Dlabac-De Lange, J. J., Bais, L., Knegtering, H., van Osch, M. J., Renken, R. J., et al. (2015). Neural correlates of planning performance in patients with schizophrenia – relationship with apathy. Schizophr. Res. 161, 367–375. doi:10.1016/j.schres.2014.11.028
Litvan, I., Mega, M. S., Cummings, J. L., and Fairbanks, L. (1996). Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 47, 1184–1189. doi:10.1212/WNL.47.5.1184
Marin, R. S., Biedrzycki, R. C., and Firinciogullari, S. (1991). Reliability and validity of the apathy evaluation scale. Psychiatry Res. 38, 143–162. doi:10.1016/0165-1781(91)90040-V
Marin, R. S., Firinciogullari, S., and Biedrzycki, R. C. (1993). The sources of convergence between measures of apathy and depression. J. Affect. Disord. 28, 117–124. doi:10.1016/0165-0327(93)90040-Q
Marin, R. S., Firinciogullari, S., and Biedrzycki, R. C. (1994). Group differences in the relationship between apathy and depression. J. Nerv. Ment. Dis. 182, 235–239. doi:10.1097/00005053-199404000-00008
Martino, J., Brogna, C., Robles, S. G., Vergani, F., and Duffau, H. (2010). Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46, 691–699. doi:10.1016/j.cortex.2009.07.015
Middleton, F. A., and Strick, P. L. (2000). Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 42, 183–200. doi:10.1006/brcg.1999.1099
Miller, B. L., and Cummings, J. L. (eds) (2007). The Human Frontal Lobes: Functions and Disorders. New York: Guilford Press.
Mollenhauer, B. (2015). Prediagnostic presentation of Parkinson’s disease in primary care: a case-control study. Mov. Dis. 14, 57–64. doi:10.1002/mds.26234
Naarding, P., Janzing, J. G., Eling, P., van der Werf, S., and Kremer, B. (2009). Apathy is not depression in Huntington’s disease. J. Neuropsychiatry Clin. Neurosci. 21, 266. doi:10.1176/appi.neuropsych.21.3.266
Oguro, H., Nakagawa, T., Mitaki, S., Ishihara, M., and Onoda, K. (2014). Randomized trial of repetitive transcranial magnetic stimulation for apathy and depression in Parkinson’s disease. J. Neurol Neurophysiol. 5, 242. doi:10.4172/2155-9562.1000242
Okada, K., Kobayashi, S., Yamagata, S., Takahashi, K., and Yamaguchi, S. (1997). Poststroke apathy and regional cerebral blood flow. Stroke 28, 2437–2441. doi:10.1161/01.STR.28.12.2437
Onoda, K., Kuroda, Y., Yamamoto, Y., Abe, S., Oguro, H., Nagai, A., et al. (2010). Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovasc. Dis. 31, 6–11. doi:10.1159/000319771
Onyike, C. U., Sheppard, J. M. E., Tschanz, J. T., Norton, M. C., Green, R. C., Steinberg, M., et al. (2007). Epidemiology of apathy in older adults: the cache county study. Am. J. Geriatr. Psychiatry 15, 365–375. doi:10.1097/01.JGP.0000235689.42910.0d
Politis, A. M., Vozzella, S., Mayer, L. S., Onyike, C. U., Baker, A. S., and Lyketsos, C. G. (2004). A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long-term care. Int. J. Geriatr. Psychiatry 19, 1087–1094. doi:10.1002/gps.1215
Reijnders, J. S., Scholtissen, B., Weber, W. E., Aalten, P., Verhey, F. R., and Leentjens, A. F. (2010). Neuroanatomical correlates of apathy in Parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. 25, 2318–2325. doi:10.1002/mds.23268
Ruff, R. M., Light, R. H., Parker, S. B., and Levin, H. S. (1996). Benton controlled oral word association test: reliability and updated norms. Arch. Clin. Neuropsychol. 11, 329–338. doi:10.1093/arclin/11.4.329
Starkstein, S. E., Fedoroff, J. P., Price, T. R., Leiguarda, R., and Robinson, R. G. (1993). Apathy following cerebrovascular lesions. Stroke 24, 1625–1630. doi:10.1161/01.STR.24.11.1625
Starkstein, S. E., Mayberg, H. S., Preziosi, T., Andrezejewski, P., Leiguarda, R., and Robinson, R. G. (1992). Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 4, 134–139. doi:10.1176/jnp.4.2.134
Stuss, D. T., Van Reekum, R. J. M. K., and Murphy, K. J. (2000). “Differentiation of states and causes of apathy,” in The Neuropsychology of Emotion, ed. J. C. Borod (Oxford: Oxford University Press), 340–363.
Swick, D., Ashley, V., and Turken, U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 9:102. doi:10.1186/1471-2202-9-102
Tsujimoto, M., Senda, J., Ishihara, T., Niimi, Y., Kawai, Y., Atsuta, N., et al. (2011). Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J. Neurol. Sci. 307, 34–40. doi:10.1016/j.jns.2011.05.025
Yan, C. G., and Zang, Y. F. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi:10.3389/fnsys.2010.00013
Yeager, C. A., and Hyer, L. E. E. (2008). Apathy in dementia: relations with depression, functional competence, and quality of life. Psychol. Rep. 102, 718–722. doi:10.2466/pr0.102.3.718-722
Zheng, D., Oka, T., Bokura, H., and Yamaguchi, S. (2008). The key locus of common response inhibition network for no-go and stop signals. J. Cogn. Neurosci. 20, 1434–1442. doi:10.1162/jocn.2008.20100
Keywords: apathy, voxel-based morphometry, gray matter volume, basal ganglia, frontal lobe, precentral gyrus
Citation: Yan H, Onoda K and Yamaguchi S (2015) Gray matter volume changes in the apathetic elderly. Front. Hum. Neurosci. 9:318. doi: 10.3389/fnhum.2015.00318
Received: 23 February 2015; Accepted: 18 May 2015;
Published: 02 June 2015
Edited by:
Richard A. P. Roche, Maynooth University, IrelandReviewed by:
Annalisa Setti, University College Cork, IrelandArun Bokde, Trinity College Dublin, Ireland
Copyright: © 2015 Yan, Onoda and Yamaguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhei Yamaguchi, Department of Neurology, Faculty of Medicine, Shimane University, 89-1, Enya, Izumo, Shimane 6930021, Japan,eWFtYWd1M25AbWVkLnNoaW1hbmUtdS5hYy5qcA==
 Hongjie Yan
Hongjie Yan Keiichi Onoda
Keiichi Onoda Shuhei Yamaguchi
Shuhei Yamaguchi