- 1Section for Experimental Neuropsychiatry, Department for Psychiatry and Psychotherapy, University Medical Center Freiburg, Freiburg, Germany
- 2Department for Neurology, University Medical Center Freiburg, Freiburg, Germany
Immunological mechanisms and therapy approaches in psychotic syndromes were recently supported by the discovery of autoantibody-associated limbic and non-limbic encephalitis. However, how clinical diagnostic procedures in psychiatry should be adapted to these new insights is still unclear. In this study, we analyzed the cerebrospinal fluid (CSF) and neuroimmunological alterations and their association with cerebral MRI (cMRI) and electroencephalographic (EEG) findings. From 2006 to 2013, we acquired 180 CSF samples from psychotic patients. Between 2006 and 2009, CSF examinations were only performed in cases in which organic brain disease was suspected. Since then, this procedure has been integrated into our routine diagnostic workup. CSF basic diagnostics were supplemented by measuring antineuronal antibodies against intracellular synaptic antigens, antibodies against intracellular onconeural antigens, antibodies against neuronal cell surface antigens and thyroid antibodies. In addition, cMRIs and EEGs were conducted. We found white cell counts elevated in 3.4% of the cases, albumin quotient elevated in 21.8%, and protein concentration elevated in 42.2%. Evidence of intrathecal immunoglobulin synthesis was found in 7.2% of the cases. Antibodies measured against neuronal cell surface antigens were positive in 3.2%. Reactivity on antibodies against intracellular onconeural antigens were detected in 3.5%. Serum thyroid antibodies were elevated in 24.7%. Abnormalities were found in 39.5% of cMRIs and in 34.3% of EEGs. The main finding of our study was the high prevalence of CSF and autoantibody abnormalities in 54.4% of psychotic patients. In combination with cMRIs and EEGs, 75.6% showed abnormal findings. Our results are discussed with regard to the concept of immunological encephalopathy. Future studies should analyze the efficacy of immunomodulatory therapies.
Introduction
Psychosis was identified as a possible immunological disease as early as 1930 in the work of Lehmann-Facius among others. He described a highly specific method called “brain–lipoid reaction” to detect immunological signals in psychotic patients. This in turn led to the autoimmune hypothesis of schizophrenia (Roeder, 1939). Immunological concepts were recently supported by autoantibody-associated limbic and non-limbic encephalitis dissembling psychotic syndromes. In this context, antineuronal antibodies against intracellular synaptic antigens, intracellular onconeural antigens, and neuronal cell surface antigens could be distinguished. Neuronal cell surface antigens appear to be particularly associated with psychotic symptoms (Prüss et al., 2010; Dalmau et al., 2011; Vincent et al., 2011). Moreover, steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT) has received an increased level of interest in recent years because it was identified as the cause for classical psychotic and affective disorders in several cases (Castillo et al., 2006).
Role of Cerebrospinal Fluid (CSF) Analysis in Clinical Diagnostics of Psychotic Patients
Cerebrospinal fluid (CSF) investigation is the most precise method of detecting and characterizing central nervous system inflammatory processes, even when cerebral magnetic resonance imaging (cMRI) or electroencephalographic (EEG) results are normal. In most guidelines (e.g., Nice guidelines, http://www.nice.org.uk; German S3-Praxisleitlinie, www.dgppn.de), CSF examinations are not recommended in the diagnostic routine work up of psychotic or affective disorders. However, there have been several recent reports pointing to good response to immune therapy in patients with classical psychiatric manifestations of psychotic or affective disorder without organic symptoms or signs (van Elst et al., 2011; Chang et al., 2013). These observations put the psychiatric clinician in a difficult position because it is hard to judge when to initiate and when to leave CSF analyses in an individual case.
Rationale of Our Study
At the university clinic of psychiatry and psychotherapy Freiburg, traditionally CSF studies have always been done in case of psychotic patients if there were any signs pointing to possible neuroinflammatory features, such as atypical and sudden-onsets, seizures, or suspicious cMRI/EEG findings. Following own experiences with patients with limbic encephalitis presenting like schizophreniform syndromes, since June 2009, we offered CSF analyses as part of our routine work up to all psychotic patients who were admitted for diagnostic purposes. With these data at hand, the aim of this retrospective study was threefold. (1) We wanted to investigate the frequency of CSF and neuroimmunological alterations and their association with cMRI and EEG findings in psychotic syndromes. We hypothesized to find increased prevalences of CSF-basic laboratory abnormalities and autoantibody rates in psychotic patients as well as an association between CSF and cMRI/EEG abnormalities. (2) We wanted to analyze how a change in diagnostic approach might affect the respective immunological findings. Could it be that our new diagnostic approach to offer CSF studies to all psychotic subjects is excessive and produces lots of negative findings? (3) We wanted to analyze on a case by case level, whether the findings of immunological signals have therapeutic implications for our patients.
Participants and Methods
The study received approval from the local ethics committee of the University of Freiburg (EK-Fr 609/14). All patients included gave written informed consent for CSF studies during the clinical diagnostic work up.
CSF Collective
From 2006 to October 2013, we acquired 180 CSF samples from psychotic patients at the Clinic of Psychiatry and Psychotherapy of Freiburg University Hospital. Lumbar punctures (LPs) were generally performed during the initial manifestation of psychotic symptoms. Three clinically defined subgroups were analyzed: (1) patients with a schizophreniform syndrome, (2) patients with a schizoaffective syndrome, and (3) patients with psychotic syndromes in the context of other disorders (Table 1).
Measurement Protocols
From 2006 to June 2009, we obtained 34 CSF analyses following the traditional approach i.e., LP was done only if there were specific symptoms and signs pointing to an organic cause of psychotic symptoms. This corresponds to 9.7 LPs per year. Since June 2009, we have introduced LP as a standard procedure in all psychotic patients with acute de novo psychotic symptoms under our care. We measured 146 cases until October 2013 (34.4 LPs per year).
Immunological assessment
In all cases, paired CSF and serum samples were taken at the same time. CSF diagnostics were performed in the CSF laboratory of the Department of Neurology. CSF white blood count (WBC) and cytological differentiation of CSF sediment were established with manual microscopy (Leica DMRB, Germany) using a Fuchs-Rosenthal counting chamber (Hecht-Assistant, Germany). Basic quantitative protein diagnostics included total CSF protein concentration, albumin, and Ig G, M, and A concentrations in CSF and serum, respectively (ProSpect System, Siemens, Erlangen, Germany). For detection of a blood–brain barrier (BBB) dysfunction, we calculated the age-related albumin quotient since this marker is accepted as the “gold standard” for the estimation of the integrity of the BBB (Reiber and Peter, 2001). For determination of oligoclonal bands (OCBs), we used isoelectric focusing followed by immunofixation (Hydragel Isofocusing, Sebia, France). Intrathecal Ig synthesis was considered significant if intrathecal Ig fraction-values exceeded 10% in the “Reibergram”-analysis (Reiber and Peter, 2001) and/or if OCBs were present exclusively or predominantly in CSF according to the criteria of the European experts' consensus (Andersson et al., 1994). A commercially available immunoblot employing recombinant neuronal antigens as the substrate (Yo, Hu, Ri, Cv2/CRMP5, Ma1, Ma2, SOX1, amphiphysin, and GAD) was used for qualitative detection of antineuronal antibodies against onconeural intracellular or synaptic antigens (ravo Diagnostika, Freiburg, Germany), as has been the case since 2006. CSF antibodies against neuronal cell surface antigens (NMDAR, AMPA-1/2-R, GABA-B-R, and VGKC-complex [LGI1, Caspr2]) were detected using a monospecific cell-based (transfected HEK cells) indirect immunofluorescence assay (Euroimmun, Luebeck, Germany), as has been the case since 2011. Prior to this, samples were sent to the reference laboratory at the Weatherall Institute of Molecular Medicine at the John Radcliffe Hospital (Oxford, United Kingdom) for measuring the serum anti-voltage gated potassium channel (VGKC) complex and anti-N-methyl-D-aspartat-receptor (NMDAR) antibodies. Serum thyroid autoantibodies, including anti-thyroid peroxidase, anti-thyroglobulin, and thyroid-stimulating hormone receptor antibodies, were assessed only in cases of clinical suspicion of thyroid disorder (i.e., abnormalities in thyroid hormones) using electrochemiluminescence immunoassay tests (Roche, Basel, Switzerland). The cMRI and EEG datasets were obtained from clinical records; EEGs were analyzed visually by in-house physicians, while cMRI datasets were analyzed by experienced senior neuroradiologists. Table 1 provides an overview of the number of datasets.
Data Handling and Statistical Analysis
All laboratory data, clinical information and cMRI and EEG datasets were carefully researched and entered into a data bank (SPSS 20, Statistical Package for the Social Sciences). Mainly, descriptive statistics were calculated for the psychotic patient group. For group comparison (respective age) between patients with and without clear-cut antibody abnormalities, we used a Mann–Whitney U-test. Moreover, we performed Pearson correlation for correlation analyses between age and CSF markers.
Results
Demographic Data
Of the 180 examined psychotic patients, 101 were female and 79 were male; the mean age was 34.67 (standard deviation ±14.7) years.
CSF Basic Diagnostics and Immunological Findings
Table 2A summarizes the basic CSF findings. Overall, 3.4% of our psychotic patients displayed increased WBC counts. Seventy-six of the 180 patients or 42.2 % showed an increased protein concentration, 39 patients or 21.8% showed an increased albumin quotient and in 13 patients or 7.2% there were OCBs. Age significantly correlated with the protein concentration (r = 0.185, p = 0.013, N = 180) and the albumin quotient (r = 0.250, p = 0.001, N = 179). There was no significant correlation between age and WBC (r = −0.068, p = 0.368, N = 179) or age and IgG-index (r = −0.047, p = 0.534, N = 180). Table 2B illustrates the specific findings with respect to antineuronal antibodies. In 4 cases or 3.2% we found antibodies against neuronal cell surface antigens. Antibodies against intracellular onconeural antigens were found in 5 cases or 3.5%. Thyroid-stimulating hormone receptor antibodies were increased in 3.1% of investigated cases, thyroid peroxidase antibodies in 17.8% and thyroglobulin antibodies in 15.7%.
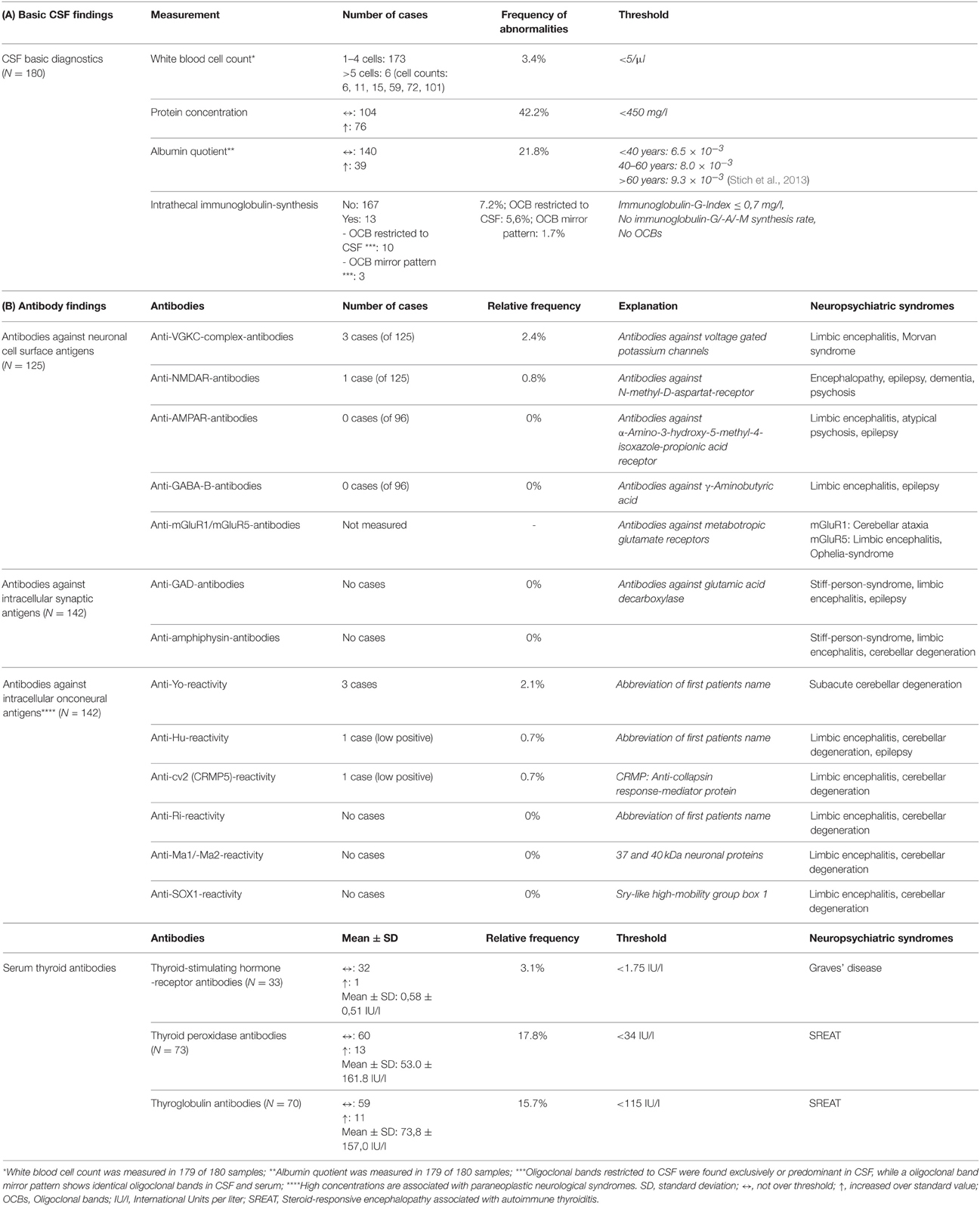
Table 2. (A) Basic CSF findings and (B) Autoantibody results in entire psychotic patient group and associated neuropsychiatric syndromes (http://www.dgn.org/leitlinien/inhalte-nach-kapiteln).
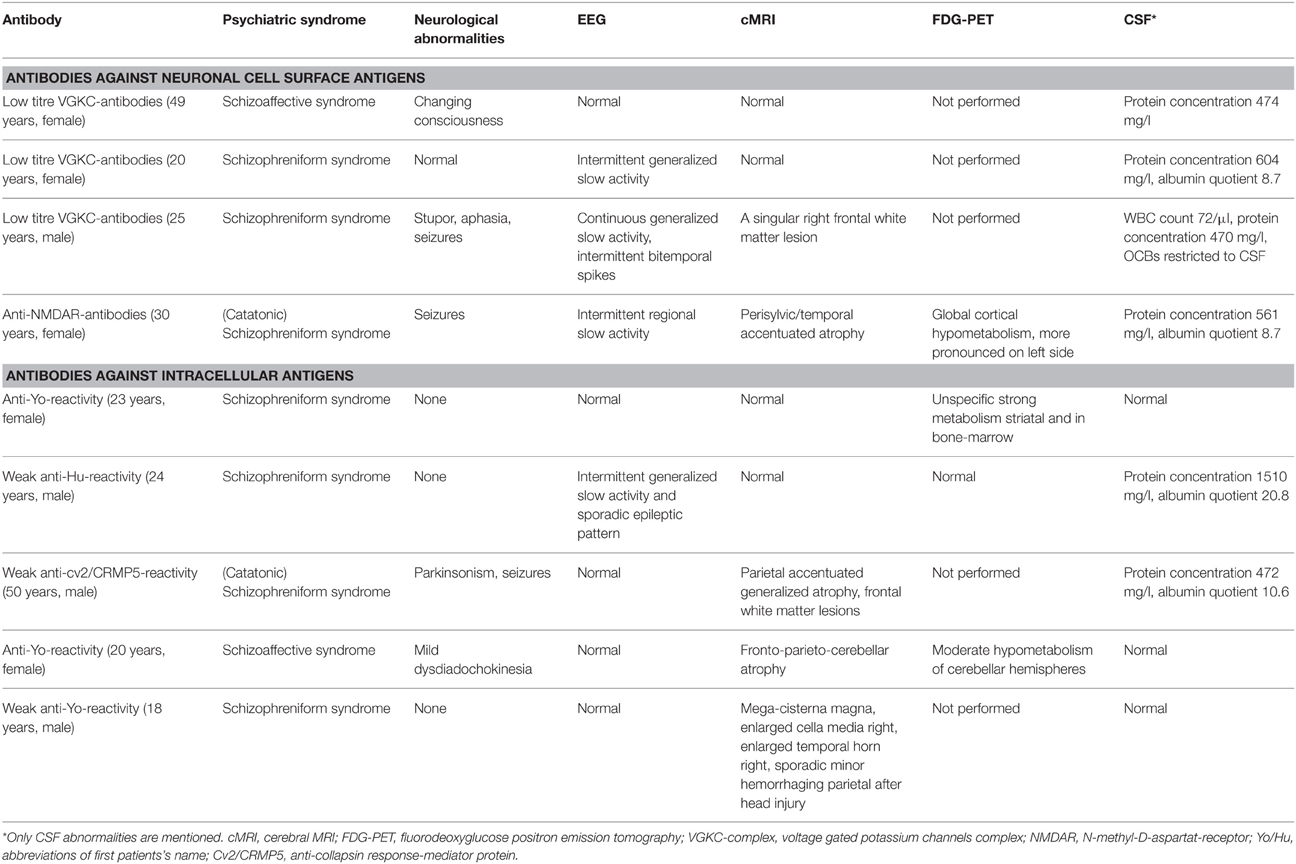
Table 3 specifies all clinical relevant findings of the antibody positive patients. Three of the nine patients with antineural antibodies displayed clear cut additional neurological symptoms (epileptic seizures) in addition to the schizophreniform or schizoaffective syndrome earlier or later in the course of the disease. This was not the case in the other 6 patients. The EEG was abnormal in 4 of 9 patients. The cMRI was completely normal in 4 of 9 cases. However, the abnormalities in the remaining 5 of 9 were unspecific with focal atrophy being the most common finding. An FDG-PET was done in 4 of 9 patients with mixed results.
Patients with clear-cut antibody findings (n = 9) were younger on average compared with antibody negative patients (n = 171), however, these differences were not significant (28.78 ± 12.26 vs. 34.98 ± 14.77; U = 993.5, p = 0.142).
Effect of Diagnostic Protocol on Findings
To analyze how the introduction of a new diagnostic protocol may affect respective results, we compared the cohort obtained between 2006 and June 2009 to the one obtained from data since 2009. Table 4 summarizes respective findings. It illustrates that the introduction of routine CSF studies in 2009 led to a lower detection rate of increased WBC counts from 9.1 to 2.1%. In contrast, the high detection rate of protein abnormalities (increased protein concentration: 47.1% down to 41.1%; albumin quotient: 23.5% down to 21.4%) and intrathecal immunoglobulin synthesis (8.8% down to 6.8%) was not altered in a relevant way and was very high even in routine assessment of CSF in psychotic patients.
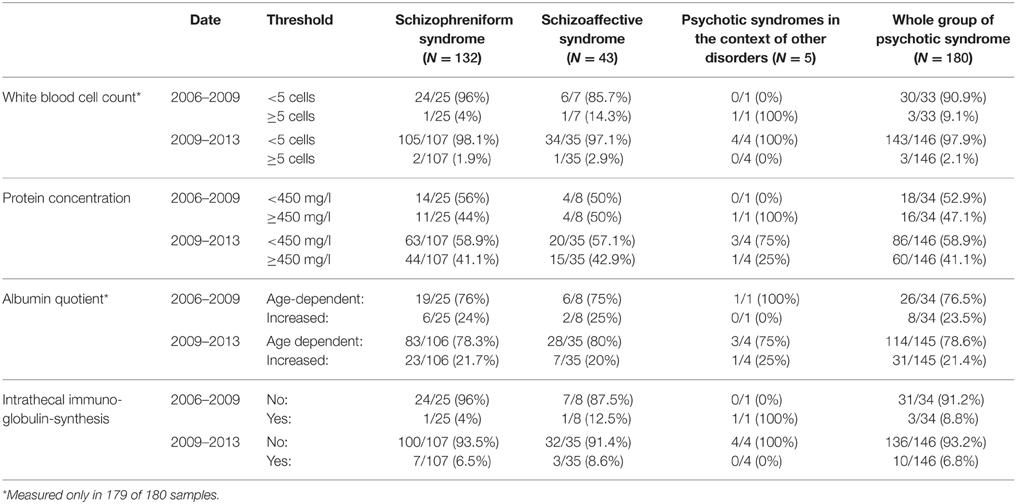
Table 4. CSF-basic diagnostics sorted by date (2006–2009: LP in suspicious cases; 2009–2013: LP as a standard screening procedure) and patient subgroups.
cMRI and EEG
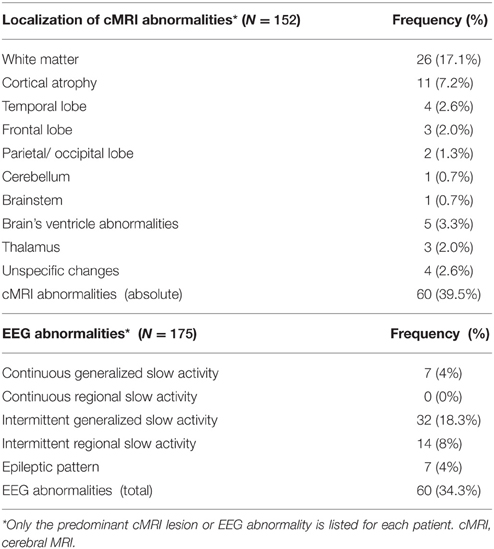
cMRI abnormalities were detected in 39.5% of the cases (60 of 152). Of the available 175 EEG datasets, 60 showed pathological abnormalities (34.3%). Intermittent generalized slow activity was found most frequently (18.3%; Table 5).
Overall Abnormalities
CSF and serum analyses (including antineuronal and anti-thyroid antibodies) showed abnormalities in 98 of the 180 cases (54.4%). cMRI or EEG abnormalities were found in 91 of the 180 psychotic patients (50.6%). Total abnormalities in CSF analysis and cMRI/EEG analysis were detected in 136 of the 180 cases (75.6%).
Discussion
The main finding of our study was the high percentage of CSF and autoantibody abnormalities in 54.4% of all psychotic patients and overall organic abnormalities (including cMRI and EEG) in 75.6%. Before discussing the possible relevance of these findings, we have to stress the shortcomings of our open study.
Limitations
The entire study is open and followed clinical practice. Therefore, while the ecological validity might be high, the sample was generated in a very unsystematic way. The total collective includes a group of patients with suspected organic brain disease identified between 2006 and 2009 and a more systematic screening group since 2009. This includes all psychotic patients who gave their written consent for LP. While the majority of patients agreed to all proposed diagnostic procedures could not clarify retrospectively how many patients rejected CSF studies. Therefore, this sample cannot be regarded as representative. Still, since this issue is rather new to psychiatry, it is difficult to obtain data from more systematic samples. To our knowledge, this is the first study published in the literature looking at a large clinical sample of unselected consecutively diagnosed psychotic patients who were comprehensively investigated using CSF analysis, the measurement of different neuroimmunological markers (autoantibodies), EEG and cMRI. The major shortcoming of this study is the fact that we did not analyze a control group for comparison. However, this is an open clinical observational study, and for ethical reasons, we would not have obtained approval for doing LPs in healthy controls from our ethics committee, due to possible complications. The high rates of organic pathologies might also be influenced by medication effects, comorbidities, or patient history (e.g., EEG findings are very sensitive for such influencing factors). In spite of these limitations, we feel that this study is of clinical significance because we were able to collect a large sample of psychotic patients, who were diagnosed by experienced senior psychiatrists in a tertiary care hospital on a strictly clinical basis. Presently CSF studies do not belong to the recommended diagnostic procedures. However, given the good therapeutic response to immunomodulatory therapies in some patients with immunological encephalopathy the issue of whether these recommendations should be modified and adapted to the latest findings in neuroimmunology is of obvious clinical relevance.
The Effect of More Elaborated Diagnostic Procedures
One might have hypothesized that the change in diagnostic protocol, essentially introducing CSF examination as a routine diagnostic measure, would result in a majority of negative findings. This, however, was not the case. While the prevalence of elevated CSF WBC count went down from 9.1 to 2.1%, the same was not true for all other global CSF basic signals. In particular, we found strong evidence pointing to disturbed BBB function in psychotic patients.
Role of CSF Basic Diagnostics in Psychotic Syndromes
Several studies analyzing CSF in schizophrenia have reported differences, especially in BBB function. Most previous studies are older, and therefore, were assessed under different environmental and technical frameworks (Supplemental Table 1).
WBC Count
In earlier studies, WBC count showed no abnormalities overall in psychotic patients (Vasic et al., 2012). However, in acutely psychotic patients, there were reports of higher rates of activated lymphoid cells and mononuclear phagocytes, which might be indicative of microglial activation, which—according to some reports—normalizes over the course of the disease (Nikkilä et al., 1999, 2001). In our sample, we found elevated WBC counts in 3.4%, which might be evidence of discrete acute autoimmune or infectious inflammatory processes in a small subgroup of psychotic patients.
BBB
Increased BBB permeability with elevated protein concentration and CSF-to-serum albumin quotients have been described in previous studies (Vasic et al., 2012). Bechter et al. for example, reported a moderate BBB dysfunction in 29% of treatment-resistant affective and schizophrenic spectrum disorder patients (Bechter et al., 2010). In particular, albumin, which is produced only in the liver, is accepted to be the “gold standard” for detecting BBB permeability by measuring the serum/CSF quotient (Reiber and Peter, 2001; Vasic et al., 2012). Following older neurophysiological models, a high albumin quotient was interpreted as a “leakage” of BBB. Recent concepts have also discussed a reduced CSF flow caused by low CSF production rate, increased flow resistance in the subarachnoid space, or reduced outflow into venous blood via the arachnoid villi as further causal mechanisms (Reiber, 1994). In our study, we found increased protein concentrations in 42.2% of our patients and an elevated albumin quotient in 21.8%. While several case studies illustrate that immunomodulatory therapy may produce very positive results in single cases, it is not yet clear whether evidence for BBB dysfunction should trigger therapy courses (e.g., with steroids). Future research will have to answer this question.
Intrathecal Immunoglobulin Synthesis
We found an intrathecal humoral immune response, indicating chronic CSF inflammation, in 7.2% of our patients. Due to the chronic and relapsing character of schizophrenia, which is similar to multiple sclerosis, a chronic low-level central nervous system inflammation in a subgroup of psychotic patients was postulated by some authors (Bechter et al., 2010). For example, Bechter postulated the mild encephalitis hypothesis for schizophrenia at least for subgroups of schizophreniform patients (Bechter, 2013). Findings of intrathecal immunoglobulin synthesis may support such notions at least for a small subgroup of psychotic patients.
Antibodies against Neuronal Cell Surface Antigens
Antibodies against neuronal cell surface antigens are particularly associated with psychotic syndromes. Table 2 includes an overview of established autoantibodies and associated neuropsychiatric syndromes. Anti-N-methyl-D-aspartat-receptor (NMDA-R) encephalitis for example begins mainly with an unspecific prodromal period, which is characterized by headache and fever and followed by a psychiatric period of anxiety, paranoia, delusions, short-term memory loss, and disintegration of language to the point of mutism (Prüss et al., 2010; Dalmau et al., 2011; Vincent et al., 2011). Therefore, anti-NMDAR encephalitis is an important differential diagnosis for catatonic schizophrenia. In the course of the disease, patients may suffer from hypoventilation, seizures, and autonomic instability (Prüss et al., 2010; Dalmau et al., 2011). The detection of anti-NMDAR antibodies is clinically important due to the high efficacy of immunomodulatory therapy options, including intravenous corticosteroids, plasma exchange, intravenous immunoglobulins, azathioprine, or monoclonal antibodies (e.g., rituximab) (Peery et al., 2012). In our collective, we found anti-NMDAR-IgG antibody positivity in serum in only one case. In a retrospective study by Steiner et al. a far higher number (9.9%) of low-titer anti-NMDA receptor antibodies were found in schizophrenic patients (Steiner et al., 2013). A recent study by Hammer et al. again showed a high prevalence of these antibodies in schizophrenic patients (8.6%) but, interestingly, an even higher prevalence in healthy controls (10.8%) (Hammer et al., 2014). Steiner and colleagues have in the meantime re-analyzed their sample, now also finding an equal percentage of anti-NMDAR antibodies in healthy controls (Steiner et al., 2014). However, in our clinic in Freiburg, we measured only IgG antibodies, Steiner et al. and Hammer et al. measured also IgA and IgM antibodies (Steiner et al., 2013; Hammer et al., 2014). Looking only on IgG-NMDR-antibodies, we found similar prevalence rates to those of previous studies. In the studies by Steiner et al. and Hammer et al. only the sera of psychotic patients and controls were analyzed (Steiner et al., 2013; Hammer et al., 2014), in our study, we mostly analyzed CSF samples. Looking at the high prevalence rates of NMDAR antibodies in healthy subjects, it may be assumed that positive antibodies in the serum probably must be associated with another mechanism, such as disturbed BBB dysfunction, in NMDAR-antibody carriers to enter a state of immunological encephalopathy (Hammer et al., 2014). Therefore, serum measurement and CSF antibody status, combined with CSF basic diagnostics, might be useful to detect such constellations.
We also found anti-VGKC complex antibodies in 2.4% of our collective (three cases). Anti-VGKC complex encephalitis often leads to limbic encephalopathy and symptoms of seizures, memory loss, and affective symptoms (Reid et al., 2009; Radja and Cavanna, 2013). However, there are cases were such patients at least initially presented with classical psychiatric syndromes, such as acute schizophreniform disorder (van Elst et al., 2011; Gnanavel, 2014).
Antineuronal Antibodies against Intracellular Onconeural Antigens
Interestingly, we also found antineuronal antibodies against intracellular onconeural antigens in 3.5% of our patients (Tables 2B, 3; five cases). In particular, anti-Yo reactivity was detected in three patients (2.1%). Anti-Yo antibodies target Purkinje cells of the cerebellum and are the most common reason for paraneoplastic cerebellar degeneration (Hasadsri et al., 2013; Stich and Rauer, 2014). However, none of our patients had cancer. A recent study by Laadhar et al. showed an association between psychiatric diseases and antibodies against intracellular neuronal antigens. They described confirmed, well-characterized antineuronal antibodies (reproducible in a confirmation test) in five (anti-Yo in three, anti-Ri in two) of 143 patients, and none in the control group (Laadhar et al., 2015). Another large study found anti-Yo antibodies in 0.4% of 1378 schizophrenic patients, but also in 0.6% of healthy controls (Dahm et al., 2014). It was previously assumed that these well-characterized antineuronal antibodies are mostly associated with paraneoplastic neurological syndromes. Low-titre antibodies without neurological symptoms are also known in cancer patients. Our results in combination with the data from Laadhar et al. and Dahm et al. provide the first evidence of the existence of low-titre onconeural antibodies in psychiatric patients (Dahm et al., 2014; Laadhar et al., 2015). Otherwise, high percentages in healthy controls raise the question about the pathophysiological meaning of such findings. In any case, positive antibody findings should result in organic clarification. We recently published the case of a 20-year-old female patient with low-titre anti-Yo positivity with intrathecal synthesis and fronto-parieto-cerebellar atrophy in cMRI and a moderate hypometabolism of the cerebellar hemispheres in the FDG-PET (Endres et al., 2015). Based on such cases, one might speculate that neuronal dysfunction, possibly via cytotoxic T-cell activity, might be a different pathogenetic mechanism leading to neuropsychiatric symptoms in a subgroup of psychotic patients (Kayser et al., 2010). Alternatively, in this constellation, the intracellular antibodies may indicate the presence of a cancer that is not yet detectable with standard diagnostic means. In this constellation, the psychotic syndrome would function like an early heralding paraneoplastic syndrome and might be beneficial in that it could facilitate the early detection of a potentially treatable oncological condition.
Steroid Responsive Encephalopathy Associated with Autoimmune Thyroiditis
In patients with psychotic symptoms and cognitive impairment in the context of autoimmune thyroiditis, a steroid responsive encephalopathy with autoimmune thyroiditis (SREAT) should be considered. For screening, thyroid hormones and particularly thyroid peroxidase and thyroglobulin antibodies should be investigated (Castillo et al., 2006). In our study, thyroid antibodies were elevated in 24.7%, which is remarkably more than the estimated prevalence in the general population (Hollowell et al., 2002). In earlier studies, EEG abnormalities were found in nearly 95% of SREAT patients (most often generalized slowing). cMRI and CSF diagnostics also added to diagnostic certainty. In any case, steroid responsivity is the essential feature for diagnostic assignment (Castillo et al., 2006). Therefore, it is important to offer steroid treatment in suspect cases, since the therapeutic success even in long term and classical psychiatric presentations can be very remarkable.
Psychotic Syndromes—An Immunological Encephalopathy?
The issue of limbic encephalitis and related immunological encephalopathies as a possible cause for schizophreniform or also affective syndromes is still new in psychiatry. Some authors summarize all antibody associated autoimmune encephalopathies within the umbrella concept of immunological encephalopathies and propose a standardized symptomatic immunomodulatory therapeutic approach for all of these diseases including anti-VGKC- and anti-NMDAR-encephalitis as well as SREAT (Friese and Magnus, 2012). Our data illustrate that systematic diagnostic screening in fact result in high rates of diverse abnormal immunological signals. However, the therapeutic implications are still poorly understood.
Implications for Clinical Diagnostic Assessment and Therapy
The issue of adapting clinical diagnostic and therapeutic standards to the advent of concepts of immunological encephalopathy and limbic encephalitis will most likely be very controversial. In many—if not in the majority—of psychiatric clinics, there won't be diagnostic competence for doing routine CSF studies. Additionally, many psychotic patients will not comply with doing CSF studies and in this constellation, currently, we don't do such investigations neither. The question of cost-effectiveness also arises. Does it make sense to diagnose immunological encephalopathy in a patient with psychological symptoms when it is unclear how this patient should then be treated? While such considerations question the rational of offering CSF studies to all patients with acute psychosis, there are also many arguments in favor of such a procedure. The vast majority of our psychotic patients did comply with these investigations and in fact were actively pursuing this road. One likely reason is that the disease model of “psychosis as an inflammatory brain process” was easier to accept for these patients and their families than the concept of schizophrenia. In quite a few of our immunological encephalopathy patients, the response to immune therapy was very good and in fact much better than the response to the classical variant of symptomatic psychopharmacology. These patients were happy to receive a more causal therapy than, for example, only taking symptomatic antipsychotic drugs.
Conclusion
Clinicians must balance the potential benefits of a diagnostic procedure against its medical risks and costs. With the medical risk and financial costs of doing a CSF study being very low, and based on our data one has to judge whether it is worthwhile doing CSF studies in 100 patients in order to detect three with increased WBC counts, about 40 with some evidence of BBB disturbance, seven with OCBs, and seven with autoantibodies against some neuronal cell surface or intracellular antibodies. Given the potentially devastating course of schizophreniform syndromes, the authors of this paper feel that our data strongly point in favor of more comprehensive CSF investigations as routine procedures in psychotic patients. There is still a very large demand for research not only in the diagnostic but also in the linked therapeutic dimension. In case CSF findings like ours were clearly linked to beneficial responses to immunomodulatory treatments, this obviously would change the diagnostic and therapeutic reality in psychiatry in a dramatic way.
Conflict of Interest Statement
Annette Baumgartner: Consulting and lecture fees, grant and research support from Bayer Vital GmbH, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva. Oliver Stich: Consulting and lecture fees, grant and research support from Bayer Vital GmbH, Biogen Idec, Genzyme, Merck Serono, Novartis, Sanofi-Aventis and Teva. Ludger Tebartz van Elst: Advisory boards, lectures, or travel grants within the last three years: Eli Lilly, Janssen-Cilag, Novartis, Shire, UCB, GSK, Servier, Janssen, and Cyberonics. Dominique Endres, Evgeniy Perlov, Tilman Hottenrott, and Rick Dersch declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The study was funded by the Department for Psychiatry and Psychotherapy, University Medical Center Freiburg.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnhum.2015.00476
References
Andersson, M., Alvarez-Cermeño, J., Bernardi, G., Cogato, I., Fredman, P., Frederiksen, J., et al. (1994). Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J. Neurol. Neurosurg. Psychiatr. 57, 897–902.
Bechter, K. (2013). Updating the mild encephalitis hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 42, 71–91. doi: 10.1016/j.pnpbp.2012.06.019
Bechter, K., Reiber, H., Herzog, S., Fuchs, D., Tumani, H., and Maxeiner, H. G. (2010). Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 44, 321–330. doi: 10.1016/j.jpsychires.2009.08.008
Castillo, P., Woodruff, B., Caselli, R., Vernino, S., Lucchinetti, C., Swanson, J., et al. (2006). Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch. Neurol. 63, 197–202. doi: 10.1001/archneur.63.2.197
Chang, Y., Kuo, Y.-H., Wu, P.-C., Yeh, Y.-C., and Chen, H.-C. (2013). The misdiagnosis of steroid-responsive encephalopathy associated with autoimmune thyroiditis as masked depression in an elderly euthyroid woman. Psychosomatics 54, 599–603. doi: 10.1016/j.psym.2013.01.009
Dahm, L., Ott, C., Steiner, J., Stepniak, B., Teegen, B., Saschenbrecker, S., et al. (2014). Seroprevalence of autoantibodies against brain antigens in health and disease. Ann. Neurol. 76, 82–94. doi: 10.1002/ana.24189
Dalmau, J., Lancaster, E., Martinez-Hernandez, E., Rosenfeld, M. R., and Balice-Gordon, R. (2011). Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 10, 63–74. doi: 10.1016/S1474-4422(10)70253-2
Endres, D., Perlov, E., Stich, O., Meyer, P. T., Lützen, N., and Tebartz van Elst, L. (2015). Case report: low-titre anti-Yo reactivity in a female patient with psychotic syndrome and frontoparieto-cerebellar atrophy. BMC Psychiatry 15, 112. doi: 10.1186/s12888-015-0486-x
Friese, M. A., and Magnus, T. (2012). “Antikörper-assoziierte autoimmune enzephalopathien,” in Therapie und Verlauf Neurologischer Erkrankungen, eds T. Brandt, H. C. Diener, and C. Gerloff (Stuttgart: Kohlhammer), 697–704.
Gnanavel, S. (2014). Voltage-gated potassium channel (VGKC) antibody-associated encephalopathy presenting as psychosis: a case report. J. Neuropsychiatry Clin. Neurosci. 26, 34–35. doi: 10.1176/appi.neuropsych.13070157
Hammer, C., Stepniak, B., Schneider, A., Papiol, S., Tantra, M., Begemann, M., et al. (2014). Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol. Psychiatry 19, 1143–1149. doi: 10.1038/mp.2013.110
Hasadsri, L., Lee, J., Wang, B. H., Yekkirala, L., and Wang, M. (2013). Anti-yo associated paraneoplastic cerebellar degeneration in a man with large cell cancer of the lung. Case Rep. Neurol. Med. 2013:725936. doi: 10.1155/2013/725936
Hollowell, J. G., Staehling, N. W., Flanders, W. D., Hannon, W. H., Gunter, E. W., Spencer, C. A., et al. (2002). Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J. Clin. Endocrinol. Metab. 87, 489–499. doi: 10.1210/jcem.87.2.8182
Kayser, M. S., Kohler, C. G., and Dalmau, J. (2010). Psychiatric manifestations of paraneoplastic disorders. Am. J. Psychiatry 167, 1039–1050. doi: 10.1176/appi.ajp.2010.09101547
Laadhar, L., Sidhom, O., Zitouni, M., Sassi, N., Abdelghaffar, W., Lahmar, H., et al. (2015). High prevalence of antineuronal antibodies in tunisian psychiatric inpatients. J. Neuropsychiatry Clin. Neurosci. 27, 54–58. doi: 10.1176/appi.neuropsych.13070153
Nikkilä, H. V., Müller, K., Ahokas, A., Miettinen, K., Rimón, R., and Andersson, L. C. (1999). Accumulation of macrophages in the CSF of schizophrenic patients during acute psychotic episodes. Am. J. Psychiatry 156, 1725–1729.
Nikkilä, H. V., Müller, K., Ahokas, A., Rimón, R., and Andersson, L. C. (2001). Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr. Res. 49, 99–105. doi: 10.1016/S0920-9964(99)00218-2
Peery, H. E., Day, G. S., Dunn, S., Fritzler, M. J., Prüss, H., Souza, C., et al. (2012). Anti-NMDA receptor encephalitis. The disorder, the diagnosis and the immunobiology. Autoimmun. Rev. 11, 863–872. doi: 10.1016/j.autrev.2012.03.001
Prüss, H., Dalmau, J., Arolt, V., and Wandinger, K.-P. (2010). Anti-NMDA-Rezeptor-Enzephalitis. Ein interdisziplinäres Krankheitsbild [Anti-NMDA-receptor encephalitis. An interdisciplinary clinical picture]. Nervenarzt 81, 396–408. doi: 10.1007/s00115-009-2908-9
Radja, G. K., and Cavanna, A. E. (2013). Treatment of VGKC complex antibody-associated limbic encephalitis: a systematic review. J. Neuropsychiatry Clin. Neurosci. 25, 264–271. doi: 10.1176/appi.neuropsych.13020022
Reiber, H. (1994). Flow rate of cerebrospinal fluid (CSF)–a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 122, 189–203. doi: 10.1016/0022-510X(94)90298-4
Reiber, H., and Peter, J. B. (2001). Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 184, 101–122. doi: 10.1016/S0022-510X(00)00501-3
Reid, J. M., Foley, P., and Willison, H. J. (2009). Voltage-gated potassium channel-associated limbic encephalitis in the West of Scotland: case reports and literature review. Scott. Med. J. 54, 27–31. doi: 10.1258/rsmsmj.54.4.27
Roeder, F. (1939). Über die serologische Diagnostik der Schizophrenie aus dem Liquor nach der Methode von Lehmann-Facius. Z. Gesamte Neurol. Psychiatr. 165, 462–467.
Steiner, J., Teegen, B., Schiltz, K., Bernstein, H.-G., Stoecker, W., and Bogerts, B. (2014). Prevalence of N-methyl-D-aspartate receptor autoantibodies in the peripheral blood: healthy control samples revisited. JAMA Psychiatry 71, 838–839. doi: 10.1001/jamapsychiatry.2014.469
Steiner, J., Walter, M., Glanz, W., Sarnyai, Z., Bernstein, H.-G., Vielhaber, S., et al. (2013). Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry 70, 271–278. doi: 10.1001/2013.jamapsychiatry.86
Stich, O., and Rauer, S. (2014). Paraneoplastische neurologische Syndrome und Autoimmunenzephalitiden [Paraneoplastic neurological syndromes and autoimmune encephalitis]. Nervenarzt 85, 485–501. doi: 10.1007/s00115-014-4030-x
Stich, O., Rauer, S., and Kaiser, R. (2013). “Liquordiagnostik,” in Neurologie Compact - Für Klinik und Praxis, eds A. Hufschmidt, C. H. Lücking, and S. Rauer (Baltimore: Thieme), 711–715.
van Elst, L. T., Klöppel, S., and Rauer, S. (2011). Voltage-gated potassium channel/LGI1 antibody-associated encephalopathy may cause brief psychotic disorder. J. Clin. Psychiatry 72, 722–723. doi: 10.4088/JCP.10l06510
Vasic, N., Connemann, B. J., Wolf, R. C., Tumani, H., and Brettschneider, J. (2012). Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur. Arch. Psychiatry Clin. Neurosci. 262, 375–391. doi: 10.1007/s00406-011-0280-9
Keywords: CSF, psychotic syndrome, schizophrenia, antineuronal autoantibodies, immunological encephalopathy
Citation: Endres D, Perlov E, Baumgartner A, Hottenrott T, Dersch R, Stich O and Tebartz van Elst L (2015) Immunological findings in psychotic syndromes: a tertiary care hospital's CSF sample of 180 patients. Front. Hum. Neurosci. 9:476. doi: 10.3389/fnhum.2015.00476
Received: 02 June 2015; Accepted: 17 August 2015;
Published: 10 September 2015.
Edited by:
Jonathan Cavanagh, University of Glasgow, UKReviewed by:
Manousos A. Klados, Max Planck Institute for Human Cognitive & Brain Sciences, GermanyChristian Hammer, École Polytechnique Fédérale de Lausanne, Switzerland
Copyright © 2015 Endres, Perlov, Baumgartner, Hottenrott, Dersch, Stich and Tebartz van Elst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ludger Tebartz van Elst, Section for Experimental Neuropsychiatry, Department for Psychiatry and Psychotherapy, University Medical Center Freiburg, Hauptstr. 5, 79104 Freiburg, Germany,dGViYXJ0enZhbmVsc3RAdW5pa2xpbmlrLWZyZWlidXJnLmRl
 Dominique Endres
Dominique Endres Evgeniy Perlov
Evgeniy Perlov Annette Baumgartner2
Annette Baumgartner2 Tilman Hottenrott
Tilman Hottenrott Rick Dersch
Rick Dersch Oliver Stich
Oliver Stich Ludger Tebartz van Elst
Ludger Tebartz van Elst