- 1Department of Neuroscience, Max Planck Institute for Empirical Aesthetics (MPG), Frankfurt am Main, Germany
- 2Institut für Biomagnetismus und Biosignalanalyse, Universitätsklinikum Münster, Münster, Germany
- 3Departments of Pediatrics and Neuroscience, Albert Einstein College of Medicine, Bronx, NY, United States
- 4Centre for Cognitive Neuroimaging, University of Glasgow, Glasgow, United Kingdom
Editorial on the Research Topic
Brain Oscillations in Human Communication
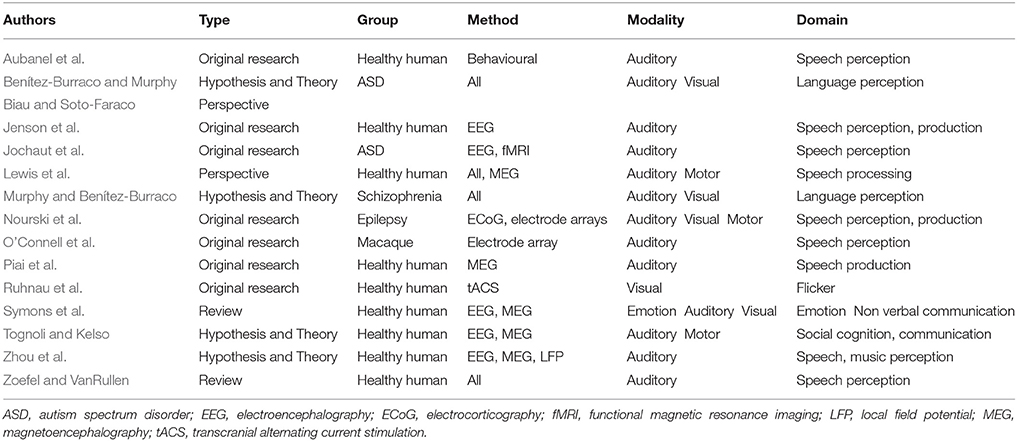
This Research Topic featured 15 articles from a wide range of research areas related to human communication. All contributions focus on rhythmic brain activity as opposed to, for example, event related potentials or functional imaging approaches. Rhythmic brain activity has been shown to be of immense importance for the temporal coordination of neural activity and, consequently, for all aspects of cognition and behaviour (Buzsaki and Draguhn, 2004; Wang, 2010). In this editorial, we summarise the research on rhythmic brain activity in language and communication that appeared in this Research Topic (see Table 1).
Perception of Spoken Language
Low-frequency (delta, theta) neuronal oscillations (and the coupling with gamma-band oscillations) have been shown to have a crucial role in speech perception, particularly in the segmentation of the continuous acoustic stream into linguistically meaningful units (Giraud and Poeppel, 2012; Ding and Simon, 2014; Ding et al., 2016; Keitel et al., in review). By now, many studies have shown that low-frequency neuronal activity in auditory cortex tracks the slow energy fluctuations in the speech acoustics (Luo and Poeppel, 2007; Gross et al., 2013; Rimmele et al., 2015; Keitel et al., 2017). The functional relevance of this neuronal phase alignment, however, and whether it actually reflects the entrainment of endogenous neuronal oscillations to a speech signal (as opposed to event related potentials) are controversial.
O'Connell et al. shed light on the tonotopy of multiscale neuronal entrainment in auditory cortex (A1) by using single cell recordings in macaques. They provide evidence for multiscale entrainment to clicks presented at regular intervals in the gamma and delta range. Particularly, neurons in the region of 11–16 kHz on the tonotopic maps of A1 aligned their excitability to the attended sounds, while the remaining part of A1 showed response suppression. The findings suggest a function of cortical entrainment to a rhythmic stimulation in the selection and processing of attended sounds (O'Connell et al., 2014), which might be at play in speech perception.
A crucial question is which aspects of the speech signal trigger the low-frequency phase alignment to the speech acoustics that are involved in speech comprehension. Aubanel et al. investigated this by isochronously re-timing temporally distorted speech with anchor points either at syllable onsets (linguistic cues) or at amplitude envelope peaks (acoustic cues). They showed that speech comprehension benefits most from linguistically motivated cues, that is, a re-timing at stressed syllable onsets.
The extent to which low-frequency neuronal phase alignment can be modulated by higher-level processes is controversial (Haegens and Golumbic, 2017; Teng et al., 2017). By reviewing previous literature, Zoefel and VanRullen discuss the contribution of high-level processes to the cortical low-frequency entrainment to speech. Particularly, based on high-level modulations of speech processing, as well as the finding of entrainment in the absence of low-level rhythmical cues (Zoefel and VanRullen, 2015, 2016) they suggest that the theta phase alignment indicates oscillatory entrainment and not mere stimulus-driven responses caused by a rhythmic stimulation (cf. Keitel et al., 2014). The authors discuss the functional role of cortical entrainment with respect to speech intelligibility and provide theoretical considerations to integrate stimulus driven effects and top-down modulations of speech processing into a unified model.
Lewis et al. focus on a specific aspect of high-level processes in spoken language comprehension. They review current research on the role of beta-band oscillations in sentence processing. This research suggests a function either in sentence-level top-down predictions about the up-coming linguistic input, or in indicating the maintenance of the current processing mode, relevant for deriving the meaning of a sentence (Lewis and Bastiaansen, 2015; Lewis et al., 2015). In this review, they additionally present preliminary magnetoencephalography (MEG) data, supporting the latter interpretation.
Speech Production and Neuronal Oscillations
Although cortical oscillations have been shown to be involved in sensorimotor coordination (Arnal and Giraud, 2012), which is vital for speech production, the exact function of oscillations in speech production is little understood. Jenson et al. investigated the temporal dynamics of alpha-band activity in the auditory posterior dorsal stream during speech production and perception, using a novel analysis method (combining independent component analysis and event related spectral perturbations) in electroencephalography (EEG) recordings. Together with previous findings by Jenson et al. (2014), these results show the temporal dynamics of the anterior and posterior dorsal stream during speech perception and production. In sum, their findings suggest a crucial role of alpha oscillations in sensorimotor interactions that allow monitoring the speech production through efference copies from the motor system.
The ability to control our speech output and withhold planned speech is critical during communication, as we need to time the turn-taking of the interacting partners (Wilson and Wilson, 2005). In an MEG study, Piai et al. investigated neuronal activity during the withholding of planned speech. They provide evidence for a two-fold mechanism. First, alpha-band desynchronisation in occipital brain areas might indicate the task-specific allocation of attention during the withholding of speech. Second, increased frontal beta-band activity during the withholding of speech most likely indicates the maintenance of the current motor or cognitive state, i.e., maintaining the planned verbal response (Engel and Fries, 2010; Piai et al., 2015; Rimmele et al., in review).
Social Cognition and Communication
In a more natural communication setting, Nourski et al. tested speech production and perception during a conversation between epilepsy patients and an instructor, using electrocorticography (EcoG) recordings. They found no difference in high gamma activity in the auditory core cortex when listening to self-produced speech vs. the speech of others. However, gamma-activity was reduced in non-core areas when participants listened to their own speech. The findings indicate that signals from self-produced speech are differentiated from speech of others at higher non-core auditory processing areas, and high gamma oscillations play a role in these processes (Nourski, 2017).
Tognoli and Kelso approach social cognition and communication beyond the individual brain, by pursuing the hypothesis that social interaction results in the phase-locking and coupling of neuronal activity across brains (e.g., Dumas et al., 2010). They theoretically underpin a neuromarker approach to social cognition, review findings from dual-EEG recordings (Tognoli et al., 2007a,b), and discuss them in the context of previous research on social cognition. In sum, neuromarkers in the alpha, mu, kappa and phi bands seem to be differentially involved in simultaneous action and perception processes (e.g. tango dancing) and the alternating perception and production of social behaviour (e.g., imitating someone).
Oscillations in Non-verbal Communication
For successful interpersonal communication, it is crucial to detect and identify emotional expressions from auditory, visual, and audiovisual information (Jessen and Kotz, 2011; Kotz et al., 2013). Here, Symons et al. review the available literature focussing on oscillatory mechanisms. They conclude that theta- and gamma-band synchronisation most consistently reflect the processing of emotional expressions across sensory modalities (e.g., Knyazev, 2007; Luo et al., 2008). On the other hand, oscillations in the delta-, alpha-, and beta-bands have also been implied in the processing of other's emotions, but their role is less consistent across modalities and tasks.
Another important non-verbal aspect of speech is the use of gestures (Hubbard et al., 2009; Biau and Soto-Faraco, 2013). Based on the previous finding that hand gestures phase-reset ongoing neural oscillations (Biau et al., 2015), Biau and Soto-Faraco discuss the role of beat gestures in audiovisual speech processing. They conclude that beat gestures promote a cross-modal phase reset at important word onsets, which might facilitate the segmentation of the speech stream.
Pathological Changes to Communication in Autism and Schizophrenia
Many psychological and neurological conditions also affect the production or perception of language (e.g., Uhlhaas and Singer, 2006). Jochaut et al. investigated the response to continuous speech in individuals with and without autism spectrum disorder (ASD), using concurrent EEG and fMRI. They report anomalies of theta and gamma oscillations in the left auditory cortex in ASD participants, as well as altered functional connectivity between auditory and other language cortices. Furthermore, the theta/gamma coupling predicted verbal impairment as well as ASD symptoms.
Benítez-Burraco and Murphy take a different approach to language deficits in ASD. In their theoretical article, they propose to relate genetics to the pathophysiology of ASD by studying oscillatory mechanisms for language processing in the autistic brain. They note that candidate genes for ASD are overrepresented among the genes that played a role in the evolution of language and brain oscillations, thereby bringing together these different methodological approaches.
In a second theoretical article, Murphy and Benítez-Burraco (2016) take a similar approach toward understanding the relationship between genes and language deficits in schizophrenia. Here, they suggest that the language deficits in schizophrenia seem to be rooted in the evolutionary processes that brought about modern language. This evolutionary account and the common oscillatory profiles of language deficits in schizophrenia and ASD are further described in a recent article (Murphy and Benítez-Burraco, 2016).
Methodological Considerations and Innovations
This Research Topic also featured predominantly methodological articles that could advance research into communication processes. Ruhnau et al. used a novel combination of concurrent transcranial alternating current stimulation (tACS) and frequency tagging. They carefully tease apart source-level tACS effects and steady-state responses (SSRs) in the MEG, by using a new method to reconstruct sources of SSRs that are unaffected by the strong tACS artifact (Neuling et al., 2015). Frequency tagging is a potentially fruitful approach for studying speech processing (Buiatti et al., 2009), and this proof-of-principle study opens up new possibilities to combine frequency tagging, tACS, and MEG.
Finally, Zhou et al. discuss the implications and limitations of the often used Fourier analysis to study low-frequency neural entrainment. They conclude that true low-frequency entrainment results in a peak in the power spectrum at the fundamental frequency (the lowest frequency produced by an oscillation), and describe how the phenomenon of higher harmonics can be interpreted.
In conclusion, the studies included here review, highlight, and specify the role of distinct cortical oscillations in practically all processes related to human communication.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
JG was supported by the Wellcome Trust (Joint Senior Investigator Grant, No 098433).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arnal, L. H., and Giraud, A. L. (2012). Cortical oscillations and sensory predictions. Trends Cogn. Sci. 16, 390–398. doi: 10.1016/j.tics.2012.05.003
Biau, E., and Soto-Faraco, S. (2013). Beat gestures modulate auditory integration in speech perception. Brain Lang. 124, 143–152. doi: 10.1016/j.bandl.2012.10.008
Biau, E., Torralba, M., Fuentemilla, L., de Diego Balaguer, R., and Soto-Faraco, S. (2015). Speaker's hand gestures modulate speech perception through phase resetting of ongoing neural oscillations. Cortex 68, 76–85. doi: 10.1016/j.cortex.2014.11.018
Buiatti, M., Peña, M., and Dehaene-Lambertz, G. (2009). Investigating the neural correlates of continuous speech computation with frequency-tagged neuroelectric responses. Neuroimage 44, 509–519. doi: 10.1016/j.neuroimage.2008.09.015
Buzsaki, G., and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929. doi: 10.1126/science.1099745
Ding, N., Melloni, L., Zhang, H., Tian, X., and Poeppel, D. (2016). Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164. doi: 10.1038/nn.4186
Ding, N., and Simon, J. Z. (2014). Cortical entrainment to continuous speech: functional roles and interpretations. Front. Hum. Neurosci. 8:311. doi: 10.3389/fnhum.2014.00311
Dumas, G., Nadel, J., Soussignan, R., Martinerie, J., and Garnero, L. (2010). Inter-brain synchronization during social interaction. PLoS ONE 5:e12166. doi: 10.1371/journal.pone.0012166
Engel, A. K., and Fries, P. (2010). Beta-band oscillations–signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Giraud, A. L., and Poeppel, D. (2012). Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci. 15, 511–517. doi: 10.1038/nn.3063
Gross, J., Hoogenboom, N., Thut, G., Schyns, P., Panzeri, S., Belin, P., et al. (2013). Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol. 11:e1001752. doi: 10.1371/journal.pbio.1001752
Haegens, S., and Golumbic, E. Z. (2017). Rhythmic facilitation of sensory processing: a critical review. Neurosci. Biobehav. Rev. pii: S0149-7634(17)30500-6. doi: 10.1016/j.neubiorev.2017.12.002
Hubbard, A. L., Wilson, S. M., Callan, D. E., and Dapretto, M. (2009). Giving speech a hand: Gesture modulates activity in auditory cortex during speech perception. Hum. Brain Mapp. 30, 1028–1037. doi: 10.1002/hbm.20565
Jenson, D., Bowers, A. L., Harkrider, A. W., Thornton, D., Cuellar, M., and Saltuklaroglu, T. (2014). Temporal dynamics of sensorimotor integration in speech perception and production: independent component analysis of EEG data. Front. Psychol. 5:656. doi: 10.3389/fpsyg.2014.00656
Jessen, S., and Kotz, S. A. (2011). The temporal dynamics of processing emotions from vocal, facial, and bodily expressions. Neuroimage 58, 665–674. doi: 10.1016/j.neuroimage.2011.06.035
Keitel, A., Ince, R. A., Gross, J., and Kayser, C. (2017). Auditory cortical delta-entrainment interacts with oscillatory power in multiple fronto-parietal networks. Neuroimage 147, 32–42. doi: 10.1016/j.neuroimage.2016.11.062
Keitel, C., Quigley, C., and Ruhnau, P. (2014). Stimulus-driven brain oscillations in the alpha range: entrainment of intrinsic rhythms or frequency-following response? J. Neurosci. 34, 10137–10140. doi: 10.1523/JNEUROSCI.1904-14.2014
Knyazev, G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 31, 377–395. doi: 10.1016/j.neubiorev.2006.10.004
Kotz, S. A., Kalberlah, C., Bahlmann, J., Friederici, A. D., and Haynes, J. D. (2013). Predicting vocal emotion expressions from the human brain. Hum. Brain Mapp. 34, 1971–1981. doi: 10.1002/hbm.22041
Lewis, A. G., and Bastiaansen, M. (2015). A predictive coding framework for rapid neural dynamics during sentence-level language comprehension. Cortex 68, 155–168. doi: 10.1016/j.cortex.2015.02.014
Lewis, A. G., Wang, L., and Bastiaansen, M. (2015). Fast oscillatory dynamics during language comprehension: Unification versus maintenance and prediction? Brain Lang. 148, 51–63. doi: 10.1016/j.bandl.2015.01.003
Luo, H., and Poeppel, D. (2007). Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron 54, 1001–1010. doi: 10.1016/j.neuron.2007.06.004
Luo, Q., Mitchell, D., Cheng, X., Mondillo, K., Mccaffrey, D., Holroyd, T., et al. (2008). Visual awareness, emotion, and gamma band synchronization. Cereb. Cortex 19, 1896–1904. doi: 10.1093/cercor/bhn216
Murphy, E., and Benítez-Burraco, A. (2016). Language deficits in schizophrenia and autism as related oscillatory connectomopathies: an evolutionary account. Neurosci. Biobehav. Rev. 83, 742-764. doi: 10.1016/j.neubiorev.2016.07.029
Neuling, T., Ruhnau, P., Fuscà, M., Demarchi, G., Herrmann, C. S., and Weisz, N. (2015). Friends, not foes: magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage 118, 406–413. doi: 10.1016/j.neuroimage.2015.06.026
Nourski, K. V. (2017). Auditory processing in the human cortex: An intracranial electrophysiology perspective. Laryngoscope Investig. Otolaryngol. 2, 147–156. doi: 10.1002/lio2.73
O'Connell, M. N., Barczak, A., Schroeder, C. E., and Lakatos, P. (2014). Layer specific sharpening of frequency tuning by selective attention in primary auditory cortex. J. Neurosci. 34, 16496–16508. doi: 10.1523/JNEUROSCI.2055-14.2014
Piai, V., Roelofs, A., Rommers, J., and Maris, E. (2015). Beta oscillations reflect memory and motor aspects of spoken word production. Hum. Brain Mapp. 36, 2767–2780. doi: 10.1002/hbm.22806
Rimmele, J. M., Zion Golumbic, E., Schroger, E., and Poeppel, D. (2015). The effects of selective attention and speech acoustics on neural speech-tracking in a multi-talker scene. Cortex 68, 144–154. doi: 10.1016/j.cortex.2014.12.014
Teng, X., Tian, X., Doelling, K., and Poeppel, D. (2017). Theta band oscillations reflect more than entrainment: behavioral and neural evidence demonstrates an active chunking process. Eur. J. Neurosci. doi: 10.1111/ejn.13742. [Epub ahead of print].
Tognoli, E., Lagarde, J., DeGuzman, G. C., and Kelso, J. A. S. (2007a). The phi complex as a neuromarker of human social coordination. Proc. Natl. Acad. Sci. U.S.A. 104, 8190–8195. doi: 10.1073/pnas.0611453104
Tognoli, E., Magne, C., de Guzman, G. C., and Kelso, J. A. S. (2007b). “Brain rhythms underlying intentional social coordination,” in Society for Neuroscience Itinerary Planner (San Diego, CA: Society for Neuroscience).
Uhlhaas, P. J., and Singer, W. (2006). Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52, 155–168. doi: 10.1016/j.neuron.2006.09.020
Wang, X. J. (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 90, 1195–1268. doi: 10.1152/physrev.00035.2008
Wilson, M., and Wilson, T. P. (2005). An oscillator model of the timing of turn-taking. Psychon. Bull. Rev. 12, 957–968. doi: 10.3758/BF03206432
Zoefel, B., and VanRullen, R. (2015). Selective perceptual phase entrainment to speech rhythm in the absence of spectral energy fluctuations. J. Neurosci. 35, 1954–1964. doi: 10.1523/JNEUROSCI.3484-14.2015
Keywords: speech perception and production, non-verbal Communication, brain rhythms, neurobiology of language, MEG source analysis, communication disorders
Citation: Rimmele JM, Gross J, Molholm S and Keitel A (2018) Editorial: Brain Oscillations in Human Communication. Front. Hum. Neurosci. 12:39. doi: 10.3389/fnhum.2018.00039
Received: 22 January 2018; Accepted: 24 January 2018;
Published: 07 February 2018.
Edited and reviewed by: Mikhail Lebedev, Duke University, United States
Copyright © 2018 Rimmele, Gross, Molholm and Keitel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna M. Rimmele, am9oYW5uYS5yaW1tZWxlQGFlc3RoZXRpY3MubXBnLmRl
Anne Keitel, YW5uZS5rZWl0ZWxAZ2xhc2dvdy5hYy51aw==
 Johanna M. Rimmele
Johanna M. Rimmele Joachim Gross2
Joachim Gross2 Sophie Molholm
Sophie Molholm Anne Keitel
Anne Keitel