- 1Department of Anesthesiology, Washington University Pain Center, Washington University School of Medicine, St. Louis, MO, United States
- 2Departments of Psychiatry, Neuroscience and Biomedical Engineering, Washington University School of Medicine, St. Louis, MO, United States
Deep brain stimulation of the subthalamic nucleus (STN-DBS) is an effective treatment for the motor symptoms of movement disorders including Parkinson's Disease (PD). Despite its therapeutic benefits, STN-DBS has been associated with adverse effects on mood and cognition. Specifically, apathy, which is defined as a loss of motivation, has been reported to emerge or to worsen following STN-DBS. However, it is often challenging to disentangle the effects of STN-DBS per se from concurrent reduction of dopamine replacement therapy, from underlying PD pathology or from disease progression. To this end, pre-clinical models allow for the dissociation of each of these factors, and to establish neural substrates underlying the emergence of motivational symptoms following STN-DBS. Here, we performed a systematic analysis of rodent studies assessing the effects of STN-DBS on reward seeking, reward motivation and reward consumption across a variety of behavioral paradigms. We find that STN-DBS decreases reward seeking in the majority of experiments, and we outline how design of the behavioral task and DBS parameters can influence experimental outcomes. While an early hypothesis posited that DBS acts as a “functional lesion,” an analysis of lesions and inhibition of the STN revealed no consistent pattern on reward-related behavior. Thus, we discuss alternative mechanisms that could contribute to the amotivational effects of STN-DBS. We also argue that optogenetic-assisted circuit dissection could yield important insight into the effects of the STN on motivated behavior in health and disease. Understanding the mechanisms underlying the effects of STN-DBS on motivated behavior-will be critical for optimizing the clinical application of STN-DBS.
Introduction
Deep brain stimulation (DBS) is a surgical therapy whereby electric current is passed through electrodes implanted into specific brain nuclei. DBS applied to the subthalamic nucleus (STN-DBS) has been extensively used to treat motor symptoms of Parkinson Disease (PD) for more than 30 years (Benabid et al., 2009). This neurosurgical treatment is typically applied in patients after years of first-line dopamine replacement therapy (i.e., L-DOPA), which eventually loses its efficacy and starts to induce dyskinesias which further reduce its therapeutic utility (Poewe, 1994; Ahlskog and Muenter, 2001; Obeso et al., 2004). STN-DBS significantly improves PD motor symptoms of tremor, rigidity and akinesia (Limousin et al., 1995; Krack et al., 2003; Fasano et al., 2010) and thus reduces the required dose of dopaminergic agonist or replacement therapy (Moro et al., 1999). Because of its reliable therapeutic efficacy, it has been proposed to apply STN-DBS earlier in the course of PD, before dopaminergic therapy loses efficacy or the emergence of L-Dopa induced dyskinesias (Deuschl et al., 2013; Schuepbach et al., 2013). Moreover, case reports have suggested that STN-DBS may reduce compulsive (Mallet et al., 2002; Fontaine et al., 2004) or addiction-like behaviors (Witjas et al., 2005), which has led to the suggestion that STN-DBS could be applied in patients suffering from obsessive compulsive disorder (Mallet et al., 2008) or to reduce symptoms of substance use disorders (Krack et al., 2010; Rouaud et al., 2010; Pelloux and Baunez, 2013). As a result of earlier intervention with STN-DBS for PD, as well as the increasing indications, the population of patients treated with STN-DBS will expand to more heterogeneous populations.
Along with its therapeutic benefits, neuropsychiatric side effects of STN-DBS have been reported since its first applications. Reported effects range from new onset or worsening of impulsivity, apathy or anhedonia to improvement of pre-existing behavioral symptoms (Chaudhuri and Schapira, 2009; Castrioto et al., 2014). Dissociating the effects of STN-DBS itself from underlying neuropathology and co-occurring pharmacological treatment is critical to understand the etiology of these side effects. One of the most frequently reported side effects of STN-DBS in the clinic is apathy, defined as a loss of motivation or reduction in goal-directed behavior accompanied by flattened affect (Marin, 1996; Levy and Dubois, 2006). Apathy is a core neuropsychiatric symptom of PD, that can be present before STN-DBS and alleviated by dopaminergic agonists (Leentjens et al., 2009). Apathy can be exacerbated following STN-DBS (Drapier et al., 2006; Le Jeune et al., 2009; Wang et al., 2018), which then compromises the quality of life benefits of STN-DBS (Maier et al., 2013, 2016; Martinez-Fernandez et al., 2016). The prevailing explanation for the emergence or worsening of apathy following STN-DBS is a withdrawal-like syndrome due to the reduction of dopaminergic treatment (Thobois et al., 2010; Chagraoui et al., 2018), although there is also evidence supporting a role for STN-DBS itself in this pathogenesis (Le Jeune et al., 2009; Zoon et al., 2019). However, because of the interaction between STN-DBS, pharmacological co-treatments and the progression of PD pathology, it is difficult to determine the underlying causes of motivational symptoms arising after STN-DBS in the clinic.
In this respect, pre-clinical models have the advantage of being able to isolate the contribution of STN-DBS alone to motivation-related behaviors, and to elucidate the neural mechanisms underlying these behaviors. To date, several studies have sought to determine the involvement of STN modulation on motivational processes (for reviews see Temel et al., 2009; Baunez and Gubellini, 2010; Hamani et al., 2017). However, several methodological differences exist between these studies, including the behavioral paradigm used to assess motivation, the parameters of stimulation or the reward used, which has precluded any clear consensus regarding the effects of STN-DBS on motivation and reward processing. To address this controversy, we performed a systematic review of the pre-clinical literature, extracting features of studies focused specifically on reward motivation and consumption behaviors. We identify a consistent pattern of decreased reward seeking, motivation and consumption induced by STN-DBS, which was not evident in studies of STN lesion or inactivation. We also identify several stimulation and experimental parameters that are associated with STN-DBS-induced motivational deficits. Our analysis provides a rationale for using pre-clinical models to dissect the neural mechanisms underlying specific behavioral effects of STN-DBS. This mechanistic understanding will be critical for optimizing STN-DBS as it is applied to expanding patient populations and for increasing clinical indications.
Methods
We systematically analyzed all pre-clinical studies investigating the effect of STN-DBS, STN Lesion, or pharmacological inhibition of the STN on motivation for reward.
Identification of Pertinent Literature
A systematic analysis of the international literature was carried out by selecting articles published in peer-review journals, using PubMed, and BioRxiv databases. The last search was conducted on September 11, 2020. Restrictions were made, limiting the study to academic publications in which the full text was published in English. Search terms were as follows: “subthalamic nucleus” AND “stimulation” AND (“reward” OR “motivation” OR “self-administration” OR “addiction” OR “cocaine” OR “FOOD”); and “subthalamic nucleus” AND (“inactivation” OR “lesion”).
Screening and Eligibility
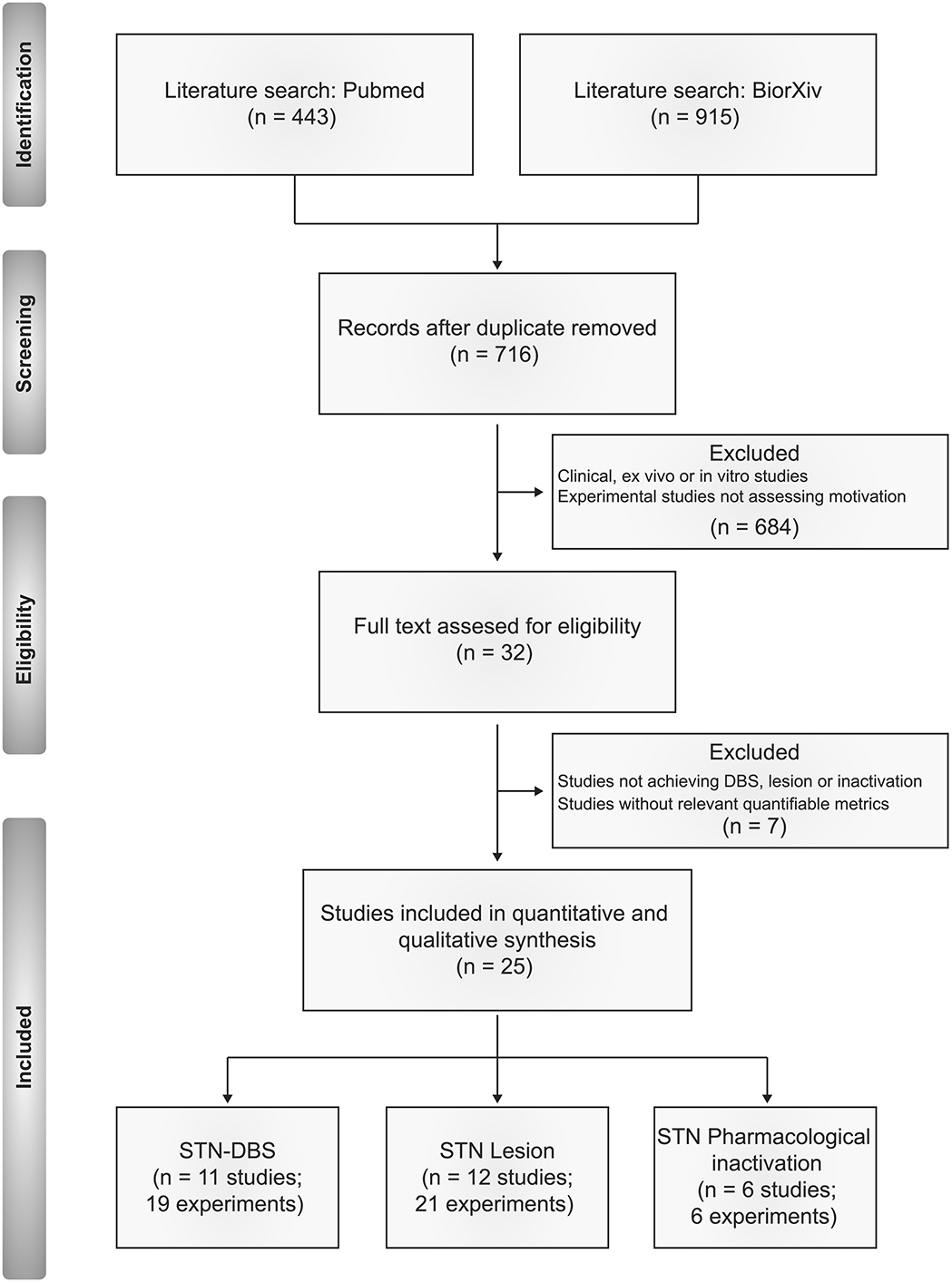
From the list of potential articles produced by systematic research, we selected studies relevant to the topic on the basis of their title and abstract. In brief, we excluded clinical, in vitro and ex vivo investigations, along with experimental studies on rodents not assessing motivation or reward-related behaviors. We then excluded studies applying neuromodulation techniques other than electrical stimulation, lesion or pharmacological inactivation, or studies not providing metrics relevant to the criteria outlined below (Figure 1).
Studies Included
Following this approach, we included 46 relevant experiments across 25 published studies between 1997 and 2020. We summarize the composition of these studies in Figures 2–4.
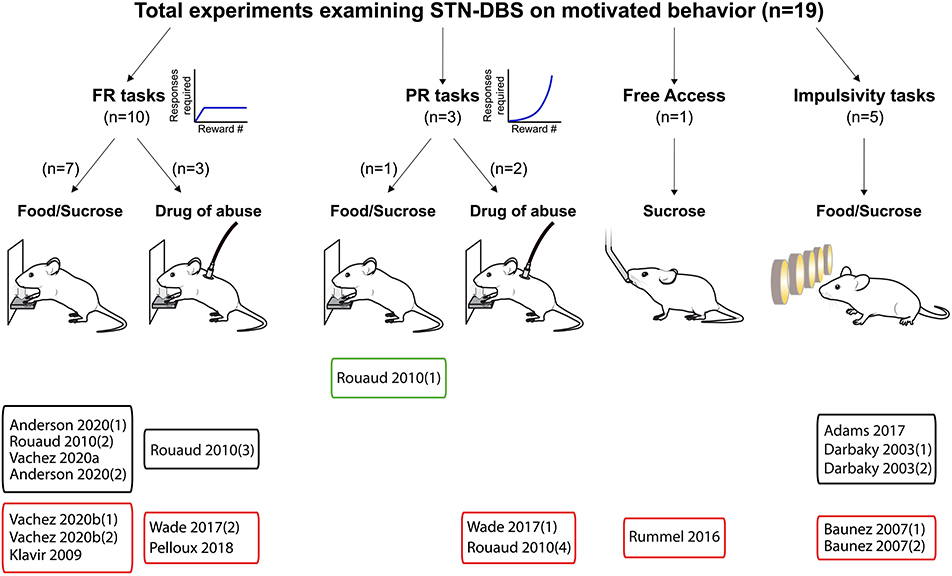
Figure 2. Experiments investigating STN-DBS effects on reward-related behavior. Graphical representation of experiments assessing the effects of STN-DBS on reward-related behavior. Experiments are split according the task and the reward provided, and whether the main effect was an increase (green frames), decrease (red frames) or no change (black frames) in motivational state induced by STN-DBS. FR, Fixed ratio; PR, progressive ratio.
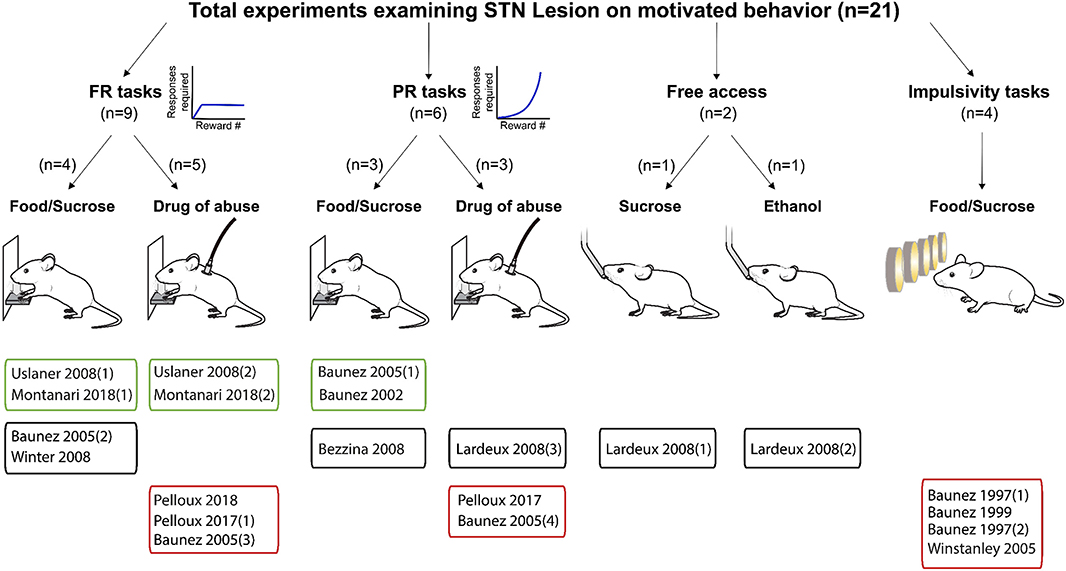
Figure 3. Experiments investigating STN lesion effects on reward related behavior. Graphical representation of experiments assessing the effects of STN lesion on reward-related behavior. Experiments are split according the task, the reward provided and whether the main effect was an increase (green frames), decrease (red frames) or no change (black frames) in motivational state following lesion of the STN. FR, Fixed ratio; PR, progressive ratio.
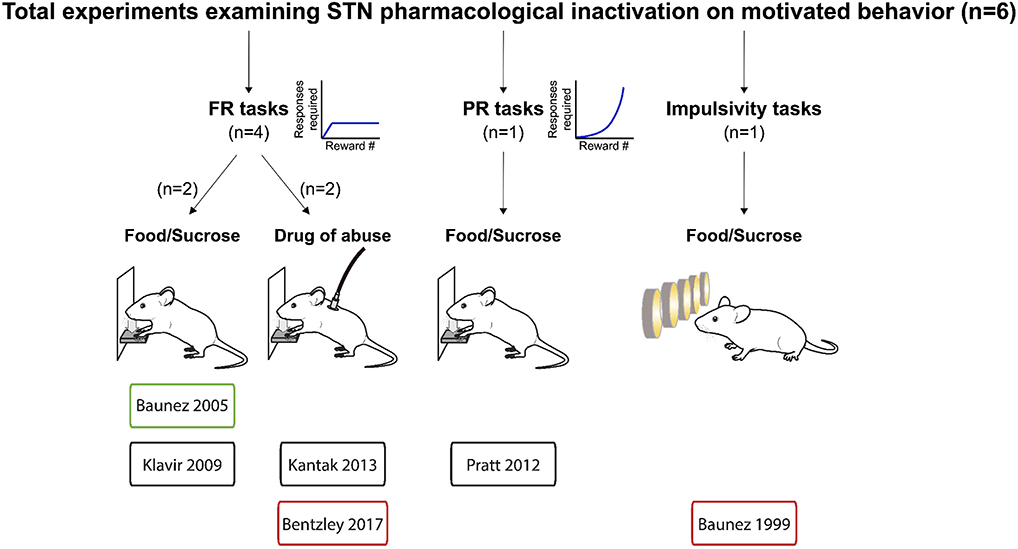
Figure 4. Experiments investigating STN pharmacological inhibition effects on reward related behavior. Graphical representation of experiments assessing the effects of pharmacological inactivation of the STN on reward-related behavior. Experiments are split according the task, the reward provided and whether the main effect was an increase (green frames), decrease (red frames) or no change (black frames) in motivational state following pharmacological inactivation of the STN. FR, Fixed ratio; PR, progressive ratio.
In rodents, assessment of motivation often relies on reward seeking tasks, during which the animal has to perform an operant behavior to receive a reward (Koob and Weiss, 1990). The majority (33/46) of the experiments in our analysis used a standard operant reinforcement task consisting of lever pressing or nose poke to induce reward delivery. Twenty two of the 33 studies used a fixed ratio (FR) paradigm, in which a fixed number of operant responses (lever press or nose poke) is necessary to earn a reward. Most studies (19) used a FR1 paradigm; here, we extracted the number of operant responses and earned rewards to evaluate motivational changes. The remaining 11 operant experiments used the progressive ratio (PR) task, in which the number of required operant responses increases incrementally with each reward earned during the task (Arnold and Roberts, 1997; Bradshaw and Killeen, 2012). The PR task is used to assess motivation by establishing “break-point,” or the number of operant responses the animal is willing to execute in order to obtain the reward (Griffiths et al., 1975). We extracted the break-point, or, if not available, the number of rewards earned. Few additional experiments (n = 3) provided the reward in a free access task, which require no explicit amount of work to obtain a reward. We thus use the consumed reward quantity as an outcome measure.
Finally, we included ten experiments that use tasks designed to assess impulsive behavior in the context of reward seeking. The majority of these studies (n = 8) used variations of the five-choice serial-reaction time task (5-CSRTT) (Robbins, 2002), while single studies using a delay discounting task (Evenden and Ryan, 1996) and rat Iowa Gambling Task (rIGT; van den Bos et al., 2014). In each of these paradigms, the start of the trial is cued, and the animal is required make the choice to complete a trial or not, and to consume the reward if the trial was successful.
The primary outcome of these tasks is to assess impulsivity by using metrics such as pre-mature responses. However, several additional parameters such as the number of non-completed trials (omissions), failures to retrieve the reward, degree of perseverative responding or the latency to execute the operant behavior or reward retrieval can be gleaned from these tasks. Changes in these parameters can reflect altered cognitive processing, motor impairments, attentional deficits or motivational changes. Motivational changes can be inferred with caution by the evolution of the numbers of omissions, especially when coupled with an increase in response latency (Robbins, 2002; Higgins and Silenieks, 2017). In few occasions, perseverative responses have been interpreted as reflecting changes in motivation (Baunez and Robbins, 1997; Baunez et al., 2007), although they are more frequently interpreted as evidence for compulsive behaviors rather than motivation per se (Robbins, 2002; Higgins and Silenieks, 2017). Thus, in order to extract the motivational components of the task of these different studies in a comparable and consistent way, we limited our analysis to the quantification of reward omissions.
The studies in our analysis also varied in terms of type of reward. In 29 of 45 experiments, the delivered reward was palatable food, generally sucrose pellets or solution. While in the remaining 16 experiments, a drug reward (cocaine, heroin or ethanol) was used.
When precise metrics were not provided in the results description, means and SEM were extracted from graphical results section using Engauge Digitizer 12.1 software. For each experiment we calculated the Cohen's d standardized mean difference (mean difference divided by the pooled standard deviation) as an estimate of the effect size (Lee, 2016). Thus, we excluded studies if data were not shown or if the precise number of animals for each experimental group was not provided. We represented each effect size ± 95% confidence intervals on forest plots (Figures 5–7).
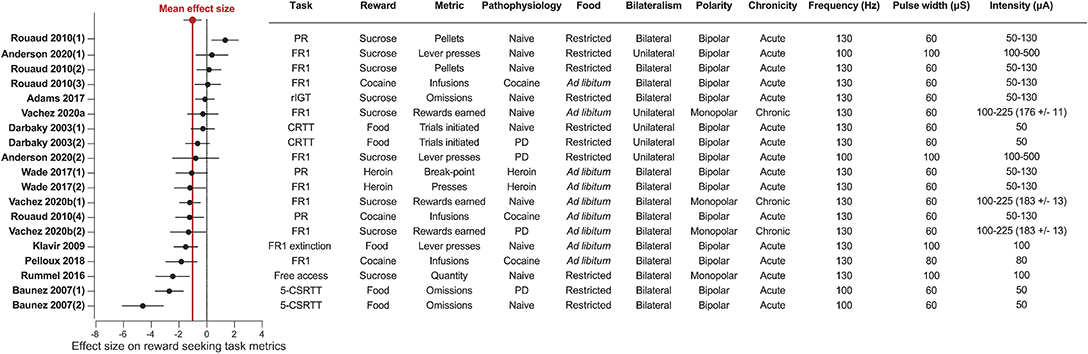
Figure 5. STN-DBS decreases reward related behavior. Forest plot of the Cohen's d standardized mean difference for reward seeking effect of STN-DBS. Mean effect size is depicted by the dashed red line. Key details of the experimental design, and DBS stimulation parameters are summarized in the associated table. All the studies provided water ad libitum. 5-CSRTT, Five choice serial reaction time; CRTT, choice reaction time task; PD, Parkinson's disease; FR, Fixed ratio; PR, progressive ratio; rIGT, rat Iowa Gambling Task.
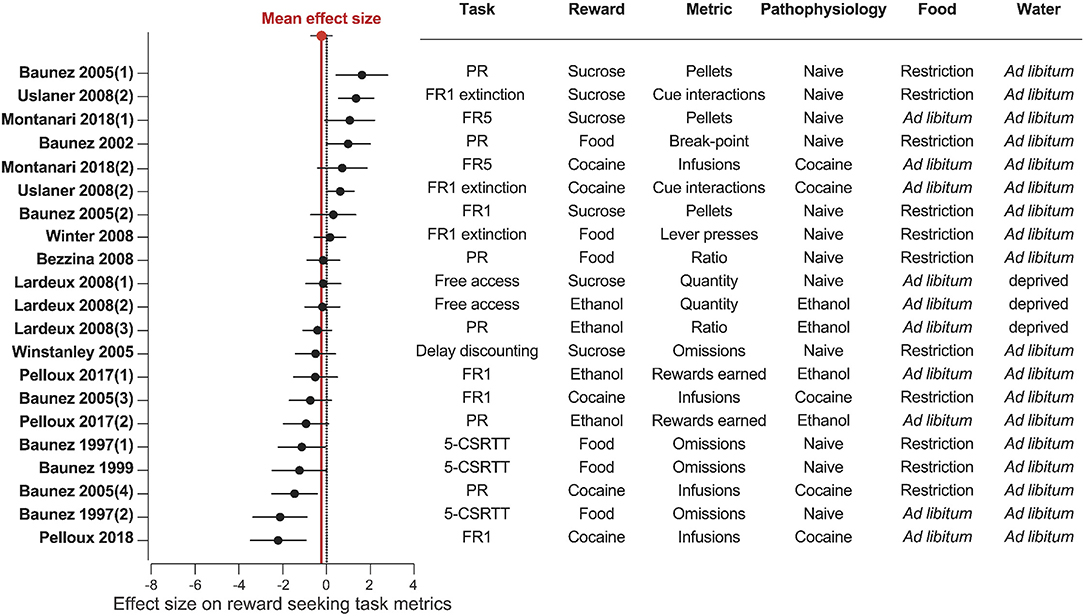
Figure 6. STN lesion does not consistently affect reward related behavior. Forest plot of the Cohen's d standardized mean difference for reward seeking effect of STN lesion, ranked in order of positive to negative effect. Mean effect size is depicted by the dashed red line. 5-CSRTT, five choice serial reaction time; FR, Fixed ratio; PR, progressive ratio.
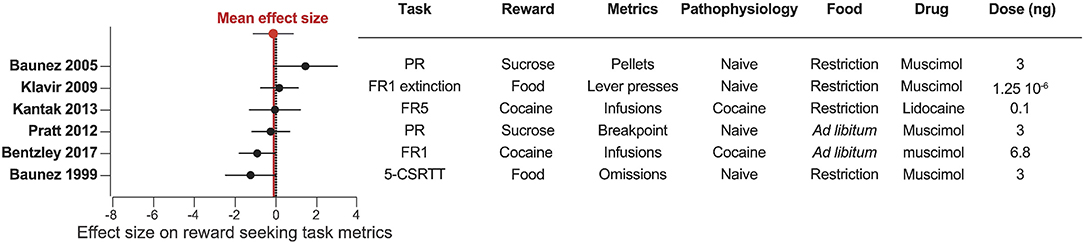
Figure 7. Pharmacological inactivation of the STN does not consistently affect reward related behavior. Forest plot of the Cohen's d standardized mean difference for reward seeking effect of STN pharmacological inactivation, ranked in order of positive to negative effect. Mean effect size is depicted by the dashed red line. 5-CSRTT, five choice serial reaction time test; FR, fixed ratio; PR, progressive ratio.
Results
Our results reveal a consistent effect of STN-DBS decreasing reward motivation and consumption (Figures 2, 5). These results also highlight specific experimental factors related to task design or stimulation parameters that may influence the magnitude of STN-DBS effect on reward-related behavior. Finally, while early hypotheses posited that STN-DBS induces a “functional lesion” of the STN through inactivation via depolarization block (Beurrier et al., 2001; Magarinos-Ascone et al., 2002; Jakobs et al., 2019), our analysis indicates that this decrease in reward seeking is not recapitulated by lesioning or pharmacologically inhibiting the STN (Figures 3, 4, 6, 7).
STN-DBS Decreases Reward Seeking
The systematic analysis of studies using STN-DBS revealed a consistent pattern of decreased reward-seeking, which is summarized in Figures 2, 5. In fact, only a single study reported an increase (30%) in motivation for reward measured by the number of sucrose pellets earned during a PR task (Rouaud et al., 2010). The majority of investigations reported a significant decrease in reward motivation (10/19), while a smaller number found no effect (8/19) (Darbaky et al., 2003; Rouaud et al., 2010; Anderson et al., 2020). In FR or PR operant tasks, STN-DBS consistently decreased intravenous self-administration of addictive drugs (Rouaud et al., 2010; Wade et al., 2017). And while the effects of STN-DBS on motivation for natural rewards is more heterogeneous, the predominant effect of STN-DBS is also a decrease in rewards earned and consumed (Rummel et al., 2016) (Vachez et al., 2020b), but see (Rouaud et al., 2010; Vachez et al., 2020a). A decrease in reward seeking was also evident in extinction tasks, i.e., where STN-DBS operant responses were decreased in the absence of a previously available food pellet (Klavir et al., 2009). Finally, STN-DBS increased the rate of trial omissions in impulsivity tasks (Baunez et al., 2007; Adams et al., 2017), which is one index of decreased reward motivation (Robbins, 2002). In summary, the predominant effect of STN-DBS across these tasks is a reduction in reward seeking and motivation, measured by decreased operant responses, rewards earned, rewards consumed or increased trial omissions. In the following section, we discuss factors that contribute to the variance in results found between studies, which are important to consider when assessing the translational impact of these findings.
Acute vs. Chronic DBS
When interpreting the clinical relevance of STN-DBS in experimental models, one has to keep in mind that patients are stimulated chronically, continuously and that prolonged STN-DBS can drive long term plasticity within the STN or its target nuclei (Shen et al., 2003; Lavian et al., 2013; Chassain et al., 2016). In patients, some therapeutic motor effects of STN-DBS, such as tremor cessation, appear immediately, while it can take several weeks for other symptoms, such as postural instability, to improve (Herrington et al., 2016). The same acute vs. chronic distinction can be made regarding neuropsychiatric symptoms. Some symptoms occur immediately upon STN-DBS onset, such as hypomania, laughing or crying (Krack et al., 2001; Mallet et al., 2007; Wojtecki et al., 2007; Abulseoud et al., 2016), while other symptoms, typically apathy, progressively emerge with chronic stimulation (Drapier et al., 2006; Le Jeune et al., 2009).
In our analysis, only three experiments applied STN-DBS chronically, and two of these experiments showed a significant decrease of sucrose or food self-administration over time (Vachez et al., 2020b). This is in contrast with the absence of motivational deficits during acute STN-DBS during a similar FR1 task or even the increased motivation during a PR task (Rouaud et al., 2010). These differential effects could suggest potential long-term adaptations in the mesolimbic system underlying motivational deficit following chronic STN-DBS. Further investigations specifically using chronic STN-DBS (Melon et al., 2015; Chassain et al., 2016) are needed to understand the long-term effect of STN-DBS on reward-related behavior, and potential plasticity mechanisms underlying these behavioral adaptations.
Unilateral vs. Bilateral DBS
Another important factor to consider when interpreting the effects of STN-DBS on reward seeking is whether the stimulation is applied unilaterally (to a single hemisphere) or bilaterally. One set of studies performed under matched conditions from the same group reported that chronic unilateral STN-DBS during a FR1 task (Vachez et al., 2020a) did not recapitulate the sustained reward seeking deficit that occurred with chronic, bilateral stimulation (Vachez et al., 2020b). With the unilateral STN-DBS, the effect was only transient and lasted no more than 5 days. Overall, the few studies using unilateral STN-DBS do not report robust reward seeking deficits, or report deficits that are only transient (Darbaky et al., 2003; Anderson et al., 2020; Vachez et al., 2020a). In the clinic, bilateral STN-DBS is generally associated with superior reduction in motor symptoms relative to unilateral stimulation (Bastian et al., 2003; Lizarraga et al., 2016), but may also induce more non-motor side-effects (Lee et al., 2011; Sjoberg et al., 2012). Notably, a study within the same clinical center observed apathy following bilateral (Le Jeune et al., 2009) but not unilateral STN-DBS (Vachez et al., 2020a). These observations suggest that preserving function of one STN by applying DBS unilaterally protects against a reward seeking deficits in patients or in animal models. It is also possible that DBS may drive compensatory metabolic or neural circuit changes in the un-stimulated hemisphere that may mitigate reward-seeking deficits induced by STN-DBS. Thus, whether STN-DBS is applied uni- or bilaterally is an important factor to consider when interpreting STN-DBS effects in animal models and its relevance to clinical populations.
Stimulation Polarity
In patients, monopolar electrodes are preferentially used, with the pulse generator within the chest being the ground pole of stimulation (Benabid et al., 2009; Amon and Alesch, 2017). In contrast, most rodent studies use bipolar electrodes. Bipolar stimulation generates a more focal electric field than monopolar electrodes and consequently activates a smaller volume of tissue (Temel et al., 2004; Chaturvedi et al., 2013; Hancu et al., 2019). While there are very few published direct comparisons of bipolar and monopolar stimulation within patients, monopolar stimulation is associated with greater improvement of rigidity, tremor and bradykinesia, but also with a higher incidence of side-effects such as confusion or mania (Deli et al., 2011; Chopra et al., 2012). We found a single study that directly compared monopolar and bipolar STN-DBS (Badstuebner et al., 2017). Consistent with clinical observations, monopolar STN-DBS was also associated with a greater reduction in akinesia, sensorimotor neglect and amphetamine-induced rotation than bipolar DBS in 6-OHDA lesioned rats (Badstuebner et al., 2017). However, monopolar STN-DBS is rarely used in rodent studies, so it is difficult to draw firm conclusions regarding the difference between monopolar and bipolar stimulation on reward-related behavior. Interestingly, the only studies that reported STN-DBS-induced reward seeking deficits in low effort tasks (FR1 or free access consumption) used monopolar electrodes (Rummel et al., 2016; Vachez et al., 2020b). This potential greater decrease in reward motivation with monopolar STN-DBS could be explained by differences in current spread. As we will discuss in subsequent section, although the motor territory of the STN is targeted with DBS, electric current can feasibly spread to associative or limbic territories, or even to adjacent neural structures (Mandat et al., 2006; Tan et al., 2013). Because the electric field induced by monopolar stimulation is more diffuse than with bipolar stimulation, this current spread could be an important driver of decreased reward seeking and motivation.
Pathophysiological and Metabolic State
Finally, the underlying pathophysiology and metabolic state of animals must be carefully considered when interpreting effects of STN-DBS on reward-related behavior. Although STN-DBS has primarily been studied in the context of PD, few studies have directly examined the effects of STN-DBS on reward seeking and motivation in experimental models of PD. In rodents, PD is typically modeled by the selective ablation of dopaminergic neurons with intracranial injections of 6-hydrodopamine (6-OHDA) to mimic the degeneration of dopaminergic neurons observed in PD (Deumens et al., 2002). Dopamine is critical for encoding reward value, action selection and vigor as well as updating behaviors based on past history of prior rewards and punishments [for review, see Berke (2018)]. Therefore, according to the specificity and extent of the dopaminergic lesion, decreased motivation and operant responding for sucrose frequently occurs in 6-OHDA-lesioned animal models independent of STN-DBS (Drui et al., 2014; Favier et al., 2014, 2017; Magnard et al., 2016). Yet, the characteristic striatal dopaminergic denervation in these PD models does not appear to influence the outcome of STN-DBS reward-related behavior. In intact and in 6-OHDA-lesioned rats, STN-DBS induces a similar rate of omission in choice reaction time task (Darbaky et al., 2003; Baunez et al., 2007) and an equivalent decrease of the number of sucrose rewards earned during a FR1 task (Vachez et al., 2020b).
A related factor that definitely affects motivation and thus outcomes of reward seeking tasks is the baseline satiety state of the animal (Berridge, 2004). Some level of food restriction is commonly used to invigorate seeking behaviors and learning in complex tasks such as the 5-CSRTT, and it does so by increasing the motivational value of the reward (Cabeza de Vaca and Carr, 1998; Mosberger et al., 2016). Thus, it is likely that basal food restriction can account for some of the lack of effect of STN-DBS observed in sucrose self-administration studies under low demand conditions [i.e., FR1, Rouaud et al. (2010) and Anderson et al. (2020)], while experiments with this same FR1 task conducted without food restriction have found decreases in reward seeking (Vachez et al., 2020b). The interpretation is that under conditions of basal food restriction, homeostatic drive for calories in sucrose overrides more subtle effects of STN-DBS; when there is no underlying metabolic demand for sucrose, the effects of STN-DBS on incentive motivation are more readily apparent.
Food or Drug Reward
A final and related consideration is whether food or drug reward is used to probe the effects of STN-DNS on reward seeking. Whereas 5/14 experiments reported decreased motivation for sucrose or food reward, 4/5 studies using drug reward found that STN-DBS decreases motivation for the drug. Briefly, bilateral STN-DBS decreases on-going self-administration and escalation of drug taking for both cocaine and heroin (Rouaud et al., 2010; Wade et al., 2017; Pelloux et al., 2018) and decreases relapse to heroin seeking following protracted abstinence (Wade et al., 2017). Importantly, STN-DBS has opposite effects in the same investigation according to the reward; decreasing cocaine self-administration but increasing sucrose taking (Rouaud et al., 2010). Some evidences suggest that the STN encodes reward value (Lardeux et al., 2009); and that sucrose and cocaine elicit activity of different subthalamic neuronal populations (Lardeux et al., 2013). Thus, it is hypothesized that different microcircuits within the STN separately drive motivation for “natural” reward or for addictive drugs. It is therefore possible that these microcircuits could be differentially impacted by STN-DBS, which may explain the more consistent effects of STN-DBS on decreasing motivation for drugs of abuse. Additional work is needed to understand how motivation for drugs abuse is encoded within the STN during different phases of the addiction cycle, which will have important implications for optimizing STN-DBS as a potential therapy for substance use disorders.
Summary
Overall, when applied chronically, bilaterally and with monopolar electrodes to model clinically-relevant conditions, STN-DBS consistently decreases reward seeking behavior. This STN-DBS-induced decrease in reward seeking is consistent across operant tasks but is most evident in satiated rats. Some attempts have been made to harness this feature, by proposing STN-DBS as a potential therapy for addiction (Rouaud et al., 2010; Pelloux and Baunez, 2013; Creed M. C., 2018).
However, this review highlights that STN-DBS has the capacity to decrease seeking for natural rewards as well as for drugs of abuse. A related consideration is that chronic application of STN-DBS leads to the emergence of learned-helplessness behaviors in shuttle-box or forced swim tasks (Temel et al., 2007; Tan et al., 2011; Creed et al., 2013). These results suggest that a general amotivational state may be induced by chronic STN-DBS in rodents, and emphasize the need for a mechanistic understanding of how STN-DBS induces its effects on reward-seeking in order to optimize the therapy for movement or substance-use disorders.
Determining the Neural Mechanisms Underlying the Effects of STN-DBS
STN-DBS Is Not Equivalent to Functional Inactivation
One early hypothesis regarding the mechanism of action of DBS is that stimulation silences local cell bodies (Grill et al., 2004; McIntyre and Anderson, 2016), producing a functional lesion. In PD models and patients, lesioning the STN abolishes pathological hyperactivity and burst firing within the nucleus (Bergman et al., 1994; Hassani et al., 1996; Kreiss et al., 1997; Vila et al., 2000) that is correlated with motor symptoms (Bergman et al., 1990; Guridi et al., 1994, 1996; Wichmann et al., 1994; Henderson et al., 1999; Baron et al., 2002; Kühn et al., 2009; Baunez and Gubellini, 2010). Consistent with this silencing mechanism, STN-DBS inhibits firing of local STN neurons both ex vivo and in vivo (Benazzouz et al., 2000b; Tai et al., 2003; Filali et al., 2004; Welter et al., 2004; Meissner et al., 2005; Shi et al., 2006; Wade et al., 2017). Multiple mechanisms have been proposed to account for this inhibition, including voltage-dependent activation of potassium conductance resulting shunt inhibition (Shin et al., 2007; Florence et al., 2016), inactivation of sodium channels (Beurrier et al., 2001; Magarinos-Ascone et al., 2002) neuronal energy depletion (Lozano et al., 2002) or excitation of pallidal GABAergic terminals to the STN (Filali et al., 2004). While a functional lesion effect has been proposed to account for many of the motor effects of DBS, whether this functional silencing also accounts for adverse psychiatric effects of STN-DBS is less clear. To address this question, we analyzed pre-clinical studies that examined the effect of either electrolytic lesion or pharmacological inactivation of the STN on reward seeking. This inactivation was achieved with muscimol (an agonist of the GABAA receptor which fluxes chloride ions into the cell, thereby hyperpolarizing the membrane) or lidocaine (an antagonist of voltage-gated sodium channels, which are required for action potential firing).
Results of STN lesion studies were more heterogeneous than with DBS, with increases (n = 6), decreases (n = 9), or no change in reward seeking (n = 6) being reported (Figures 3, 6). This heterogeneity cannot be completely explained by the type of reward used; STN-lesions had heterogeneous effects on responding for sucrose and food (Baunez and Robbins, 1997, 1999a; Baunez et al., 2002, 2005; Winstanley et al., 2005; Bezzina et al., 2008; Lardeux and Baunez, 2008; Uslaner et al., 2008; Winter et al., 2008), as well as for addictive drugs (Baunez et al., 2005; Lardeux and Baunez, 2008; Uslaner et al., 2008; Pelloux and Baunez, 2017; Montanari et al., 2018; Pelloux et al., 2018).
The heterogeneity could partially be explained by the behavioral paradigm used. STN lesion did not affect reward intake in free access (Lardeux and Baunez, 2008) or extinction paradigms (Winter et al., 2008) where the cost of responding is low. In impulsivity tasks, STN lesion consistently increased the rate of trial omissions and response latency (Baunez and Robbins, 1997, 1999a; Winstanley et al., 2005), which can be interpreted as decreased motivation (Robbins, 2002; Higgins and Silenieks, 2017) (Figures 3, 6). However, when assayed using classical FR or PR operant tasks, STN lesion either increased (Baunez et al., 2002, 2005; Uslaner et al., 2008; Montanari et al., 2018) or did not change (Baunez et al., 2005; Bezzina et al., 2008; Winter et al., 2008) self-administration of food or sucrose. Decreased cocaine or ethanol taking is the predominantly reported effect of STN lesion (Baunez et al., 2005; Pelloux and Baunez, 2017; Pelloux et al., 2018). However, absence of effect (Bezzina et al., 2008) and even slightly increased drug seeking (Uslaner et al., 2008; Montanari et al., 2018) has also been reported following STN lesion.
Fewer studies have investigated pharmacological inactivation of the STN (Figures 4, 7). Of the six total studies, one experiment reported an increase of food pellets earned during an operant task (Baunez et al., 2005), while three studies reported no effect on food or sucrose pellets (Klavir et al., 2009; Pratt et al., 2012) or cocaine administration (Kantak et al., 2013). Finally, two experiments showed decreased reward seeking with STN inactivation, measured as increased omissions in a food-rewarded 5-CSRTT (Baunez and Robbins, 1999b; Bentzley and Aston-Jones, 2017) or reduced cocaine self-administration (Bentzley and Aston-Jones, 2017). Overall, the mean effect size of STN lesion or inhibition is null, owing to the high variability in experimental outcomes reflecting the heterogeneity of the experimental conditions in terms of task, reward, physiological and metabolic state. These results are in stark contrast to the consistent effects of STN-DBS across studies, and suggest that STN-DBS effects cannot be emulated with lesion or pharmacological inactivation.
STN-DBS Modulates Activity Throughout the Basal Ganglia
The difference in behavioral outcomes between STN-DBS and STN-lesion and inactivation is not entirely surprising. While the predominant effect of DBS on local tissue is depolarization block (Benazzouz et al., 1995; Beurrier et al., 2001; Tai et al., 2003), DBS can modulate distal brain regions through antidromic and orthodromic activation (Fedele and Raiteri, 1999; Li et al., 2007; Kang and Lowery, 2014). These effects are dissociable from effects on stimulated cell bodies, due to lower threshold of activation in fibers relative to cell bodies (Nowak and Bullier, 1998; Dostrovsky et al., 2000; McIntyre et al., 2004). Consequently, STN-DBS and STN-inactivation have distinct effects on activity throughout the basal ganglia (Creed et al., 2012), which is crucial, since coordinated activity in the basal ganglia network is necessary for driving reward seeking behavior (Sesack and Grace, 2010). Another consequence of network modulation by STN-DBS is that it induces striatal dopamine release (Benazzouz et al., 2000a; Bruet et al., 2001; Zhao et al., 2009; Shon et al., 2010; He et al., 2014), which is also not observed with STN lesion (Winter et al., 2008; Walker et al., 2009). Striatal dopamine release signals the difference between expected and experienced reward to drive reward learning, and invigorates action selection including reward seeking (For reviews, see Howe et al., 2013; Berke, 2018). By inappropriately elevating dopamine levels, STN-DBS could increase the noise in the dopamine-mediated reward-prediction error signal, or could induce long-term plasticity in the striatum which could also contribute to impairments in reward seeking behavior (Benazzouz et al., 2000a; Bruet et al., 2001; Gubellini et al., 2006; Carcenac et al., 2015).
STN-DBS Potentially Induces Ectopic Stimulation
An alternative hypothesis to explain the effects of STN-DBS on reward seeking is current spread outside the motor territory of the STN and potentially outside the STN itself. The STN can be divided into three functional territories (Figure 8): motor, associative and limbic, based on its afferent and efferent connections (Lambert et al., 2012; Hamani et al., 2017; Emmi et al., 2020). The caudal and dorsolateral part form the motor STN; it receives inputs from the primary motor cortex and GPe and projects to the GPi and the striatum (Benarroch, 2008). The associative STN lies in the ventral lateral aspect of the rostral nucleus, it receives input from the dorsolateral prefrontal cortex and innervates the SNr (Benarroch, 2008). Activity in the associative territory supports cognitive aspects of motor behavior, including impulsivity and attentional control (Frank, 2006; Alegre et al., 2013; Obeso et al., 2013). Finally, the rostromedial tip of the STN constitutes the limbic territory. This division receives inputs from the medial prefrontal and anterior cingulate cortices and projects to the ventral pallidum and the nucleus accumbens (Cavdar et al., 2018; Emmi et al., 2020). Limbic functions of the STN involve reward encoding and sensory integration to drive appropriate emotional states (Drapier et al., 2008; Lardeux et al., 2009; Eitan et al., 2013; Zenon et al., 2016). Therefore, stimulation of the limbic and/or associative territories is one possible explanation for the effects of STN-DBS on reward-related behavior (Mallet et al., 2007; Zoon et al., 2019). While these subdivisions are established in human and non-human primate, the well-defined topographic segregation is less clear in rodents (Alkemade et al., 2015). In rats, STN neurons with cell bodies localized in a given territory can extend dendrites across the length of the nucleus (Afsharpour, 1985). Thus, the current spread from STN-DBS electrodes, even if well-placed within the motor territory of the STN could also modulate STN neurons in non-motor territories (Figure 8). Beyond this, the STN is embedded within the zona incerta, and sits adjacent to the internal capsule and pallidofugal system (Hamani et al., 2004; Parent and Parent, 2004). These areas would be modulated by current spread outside the STN, and could be relevant for non-motor effects of STN-DBS. As mentioned above, in rodent studies, monopolar stimulation (which induces a larger current spread relative to bipolar stimulating electrodes) is associated with greater deficits in reward seeking (Figure 5). While the effects of monopolar vs. bipolar stimulation on induction of apathy or reward seeking behavior have not been directly compared in the clinic, there is evidence to suggest that high current amplitude and the use of monopolar electrodes are associated with worse psychiatric outcomes (Deli et al., 2011; Chopra et al., 2012). This is consistent with the hypothesis that reward-seeking deficits could be accounted for by current spread to limbic or associative territories of the STN, or to STN-adjacent nuclei and fiber tracts (Tan et al., 2013).
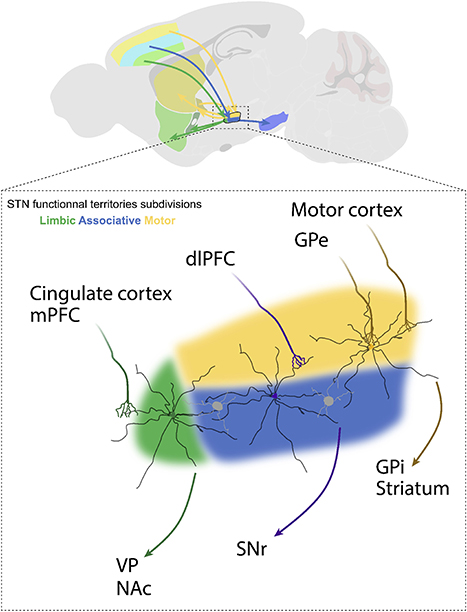
Figure 8. Afferent and efferent connections of STN functional subdivisions. The subthalamic nucleus (STN) is subdivided into a dorsolateral motor territory, a ventromedial associative territory, and a medial limbic territory. Each functional territory receives input from different cortical regions or the external segment of the globus pallidus (GPe), and in turn projects to different downstream structures, including the internal segment of the globus pallidus (GPi), substantia nigra pars reticulata (SNr), nucleus accumbens (NAc) and ventral pallidum (VP). These input-output interactions provide for parallel control of motor, cognitive, and emotional functions. The STN is composed of interneurons and glutamatergic projection neurons whose dendrites may arborize over a distance of up to 500 μm. This is important, because individual STN neurons may physically span into adjacent territories and be effected by DBS applied to these adjacent subdivisions.
Even if it is current spread to STN subterritories and adjacent structures, and not modulation of the motor STN per se that drives reward-seeking deficits, this still does not explain the precise mechanisms underlying these deficits. For example, the reward-related effects could also be due to antidromic activation of afferent structures such as the prefrontal cortex (Irmen et al., 2020), or to modulation of downstream structures such as ventral pallidum or nucleus accumbens (Hahn et al., 2008; Cavdar et al., 2018). Likewise, STN-DBS modulates dopamine tone through polysynaptic outputs of the STN proper leading to stimulation of midbrain dopamine neurons, or through direct activation of dopamine fibers arising from current spread beyond the STN borders (Benazzouz et al., 2000a; Bruet et al., 2001; Tan et al., 2011, 2012; Carcenac et al., 2015). To disentangle these different possibilities, sophisticated approaches to circuit dissection, such as optogenetics, will be required.
Determining the Neural Mechanisms Underlying the Effects of STN-DBS: Future Prospects with Optogenetics
Optogenetics refers to a suite of engineered ion channels that are activated by light in a specific wavelength, and flux ions in response to activation. Channelrhodopsin (ChR2) is non-selective cation channel, that when exposed to blue light (~473 nm), allows sodium and calcium to flow into the cell along their concentration gradients, thereby inducing depolarization and action potentials (Nagel et al., 2003). Conversely, the inhibitory halorhodopsin is a chloride pump that is activated upon stimulation with amber light (~590 nm), increases the intracellular chloride concentration, thereby hyperpolarizing the cell and inhibiting the firing action potentials (Zhang et al., 2007). The location of the injected virus expressing the opsin and placement of the optic fiber for light delivery allows for spatial control of neural activation, while cell-type or projection-specific control of neural populations can be achieved by expressing viruses using intersectional genetic strategies. Finally, the pattern of light stimulation allows for tight temporal control of neural activity (Liewald et al., 2008). Optogenetics has yielded highly valuable insight to functional connectivity and activity within intact or pathological circuits, and could be leveraged to resolve outstanding questions regarding STN-DBS mechanisms.
Optogenetics was first leveraged to elucidate the role of STN in motor processes over 10 years ago, when a seminal study by Gradinaru et al. (2009) targeted the STN with excitatory and inhibitory optogenetic approaches. This investigation first tested the hypothesis that the motor effects of STN-DBS were due to local inhibition. However, optogenetically silencing cell bodies of the STN by activation of halorhodopsin was unable to rescue motor deficits in a 6-OHDA model of PD. Instead, using ChR2 to selectively activate terminal fields of cortical afferents into the STN rescued unilateral motor deficits in the PD model, suggesting a critical role of antidromic activation of “hyperdirect” cortico-STN pathway in the motor effects of DBS (Li et al., 2007; Fraix et al., 2008). Optogenetic activation of STN cell bodies also did not rescue motor deficits, arguing that STN-DBS does not exert its effects through driving action potentials in efferent STN fibers. However, these experiments stimulated at 130 Hz, while kinetics of the variants of ChR2 available at the time were not able to follow such high stimulation frequencies (Gunaydin et al., 2010). More recent studies with mutated opsins [i.e., Chronos, which is capable of following frequencies over 100 Hz (Saran et al., 2018)] have suggested that activation of cell bodies at frequencies relevant to DBS may indeed rescue motor deficits in a PD model (Yu et al., 2020). As with DBS, these investigations demonstrate a frequency-dependence of optogenetic effects, and have elucidated multiple neural mechanisms driving the therapeutic motor effects of STN-DBS in animal models.
The neural mechanisms underlying the potential adverse psychiatric effects of STN-DBS have received considerably less attention (Pan et al., 2014; Yoon et al., 2014, 2016; Tian et al., 2018). In the future, stimulating the STN with fast opsins such as Chronos or ChETA (Gunaydin et al., 2010) in the context of motivation and reward-learning paradigms will provide unique insights about the causality of the STN itself for the effects of STN-DBS on reward-related behavior. A single report has suggested that optogenetic stimulation of STN cell bodies at frequencies >100 Hz can reduce the breakpoint for sucrose, and that this effect is critically dependent on stimulation frequency and pulse width (Tiran-Cappello et al., 2018). However, this report did not distinguish between STN subdivisions, and as discussed above, current spread to the limbic and/or associative STN territories is one prevailing hypothesis for decreased reward seeking following STN-DBS. This hypothesis could be tested by selectively manipulating those functional subterritories. Because of the small size of the STN in rodents such a spatial resolution could be achieved by targeting pathway-specific output structures. For example, a recent study investigated PD-related pain, by injecting ChR2 within the STN and placing optic fibers in different STN output structures, such as substantia nigra reticulata or ventral pallidum to target the motor and limbic STN subterritories, respectively (Luan et al., 2020). Or, akin to the work by Gradinaru et al. (2009), antidromic activation of afferents can be modeled by expressing excitatory opsin in STN-projecting structures and placing fibers above the STN (Sanders and Jaeger, 2016; Sanders, 2017). The medial prefrontal and anterior cingulate cortices constitute major limbic inputs to the STN; thus, manipulations of these pathways could yield important insight into the role of STN-DBS in the context of reward seeking.
The application of optogenetics has also shown promise for the development of novel deep brain stimulation protocols. In the clinic, and in all pre-clinical literature cited here, stimulation is applied at high frequencies (above 50 Hz, Figure 5). However, with optogenetics, specific cell types can be stimulated at precise physiological frequencies, and these physiological activity patterns can be used to drive plasticity within activated circuits. Proof of concept of this approach have been demonstrated for the motor symptoms of PD (Mastro et al., 2017) and for addiction (Pascoli et al., 2011, 2014; Creed et al., 2015; Creed M., 2018). In both of these applications, targeted stimulation of genetically-defined neural circuits was able to reverse behavioral impairments by selectively normalizing circuit function. While this strategy has not yet been demonstrated with STN stimulation, it is possible that selective activation of STN subdivisions at frequencies capable of driving long-term adaptations may induce persistent motor benefits without requiring continuous stimulation that carries with it the potential for adverse motivational effects.
In sum, sophisticated circuit dissection with optogenetics could be used to understand the role of the STN and its functional subterritories in coordinating adaptive motor and reward-related behaviors. With this insight, it may be possible to rationally stimulate the STN in order to achieve sustained motor benefits with lower risk of adverse effects on reward-related behavior. Conversely, it is possible that targeted manipulation of the associative or limbic territories of the STN could be leveraged to optimize or develop novel DBS paradigms to treat symptoms of addiction or obsessive compulsive disorder.
Conclusion
STN-DBS has become a mainstay therapy for movement disorders, and has been proposed to be applied earlier in course of PD, and potentially expanded to other indications, such as obsessive and compulsive disorders or addiction. Clinically, side effects such as depression, impulsivity and apathy have been reported with STN-DBS, which presents a major therapeutic limitation. To prevent side-effects, we must understand their neural underpinnings. In this respect, pre-clinical models have the advantage of being able to dissociate the effects of STN-DBS per se from underlying disease pathology or confounding effects of dopaminergic medications. Here, we focus our review on the specific dimensions of reward motivation, seeking and consumption, which can be clearly defined in operant tasks. When limiting our review to this specific scope, we find that STN-DBS consistently decreases reward motivation, seeking and consumption across a variety of behavioral models. Interestingly, studies that lesioned or inactivated the STN showed no consistent effect on reward-related behavior. Moreover, monopolar stimulation and bilateral stimulation, which both increase the volume of tissue activated, tend to be associated with more severe reward seeking deficits. Together, these observations suggest that reward seeking deficits may not be mediated by local effects within the STN per se, but by modulation of afferent or efferent structures of the limbic territory of the STN, or by current spread to adjacent fiber tracts. To definitively address these questions, optogenetic tools could be used to dissect the STN circuitry and establish links of causality between DBS effects on STN microcircuitry and reward seeking deficits, as has been done for the motor domain of STN-DBS.
A final consideration is that, we focused our review on only the dimension of reward motivation, seeking and consumption in tasks without conflict. Impulsivity, which is another commonly reported effect of STN-DBS is beyond the scope of the current review. However, extensive evidence has implicated the STN in arresting behavior, particularly under conditions of conflict to allow more time to accrue for an optimal decision to be made in rodents (Baunez and Robbins, 1997, 1999b) and patients (Bastin et al., 2014; Benis et al., 2016). This was recently elegantly demonstrated using optogenetic modulation of the STN; STN activation was able to abruptly interrupt reward consumption, while STN-inhibition prevented the ability of novel, salient stimuli to abort reward consumption (Fife et al., 2017). In real-world contexts, reward-related behavior often occurs under conditions of conflict, or with costs associated to reward seeking or consumption. This is particularly relevant in the context of impulse control disorders or addictions, in which reward seeking becomes maladaptive because of its association with adverse consequences. Therefore, future directions for understanding the effect of STN-DBS on reward-related behavior in a translational context will require the application of decision-making tasks that capture dimensions of risk-reward balance, as well as cognitive and motor impulsivity. Understanding the mechanisms underlying the potential adverse psychiatric effects of STN-DBS, and disentangling these from the substrates underlying its beneficial motor effects will be necessary for optimizing its therapeutic potential.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author Contributions
YV performed the literature review. YV and MC wrote the manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institutes on Drug Addiction (R21-DA047127, R01-DA049924), Whitehall Foundation (2017-12-54), NARSAD Young Investigator Award (27197) and Rita Allen Scholar Award in Pain to MC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Sabrina Boulet and Sebastien Carnicella for thoughtful comments on the manuscript.
References
Abulseoud, O. A., Kasasbeh, A., Min, H. K., Fields, J. A., Tye, S. J., Goerss, S., et al. (2016). Stimulation-induced transient nonmotor psychiatric symptoms following subthalamic deep brain stimulation in patients with parkinson's disease: association with clinical outcomes and neuroanatomical correlates. Stereotact. Funct. Neurosurg. 94, 93–101. doi: 10.1159/000445076
Adams, W. K., Vonder Haar, C., Tremblay, M., Cocker, P. J., Silveira, M. M., Kaur, S., et al. (2017). Deep-brain stimulation of the subthalamic nucleus selectively decreases risky choice in risk-preferring rats. eNeuro 4:ENEURO.0094-17.2017. doi: 10.1523/ENEURO.0094-17.2017
Afsharpour, S. (1985). Light microscopic analysis of golgi-impregnated rat subthalamic neurons. J. Comp. Neurol. 236, 1–13. doi: 10.1002/cne.902360102
Ahlskog, J. E., and Muenter, M. D. (2001). Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 16, 448–458. doi: 10.1002/mds.1090
Alegre, M., Lopez-Azcarate, J., Obeso, I., Wilkinson, L., Rodriguez-Oroz, M. C., Valencia, M., et al. (2013). The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in parkinson's disease. Exp. Neurol. 239, 1–12. doi: 10.1016/j.expneurol.2012.08.027
Alkemade, A., Schnitzler, A., and Forstmann, B. U. (2015). Topographic organization of the human and non-human primate subthalamic nucleus. Brain Struct. Funct. 220, 3075–3086. doi: 10.1007/s00429-015-1047-2
Amon, A., and Alesch, F. (2017). Systems for deep brain stimulation: review of technical features. J. Neural. Transm. 124, 1083–1091. doi: 10.1007/s00702-017-1751-6
Anderson, C., Sheppard, D., and Dorval, A. D. (2020). Parkinsonism and subthalamic deep brain stimulation dysregulate behavioral motivation in a rodent model. Brain Res. 1736:146776. doi: 10.1016/j.brainres.2020.146776
Arnold, J. M., and Roberts, D. C. (1997). A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol. Biochem. Behav. 57, 441–447. doi: 10.1016/S0091-3057(96)00445-5
Badstuebner, K., Gimsa, U., Weber, I., Tuchscherer, A., and Gimsa, J. (2017). Deep brain stimulation of hemiparkinsonian rats with unipolar and bipolar electrodes for up to 6 weeks: behavioral testing of freely moving animals. Parkinsons Dis. 2017:5693589. doi: 10.1155/2017/5693589
Baron, M. S., Wichmann, T., Ma, D., and DeLong, M. R. (2002). Effects of transient focal inactivation of the basal ganglia in parkinsonian primates. J. Neurosci. 22, 592–599. doi: 10.1523/JNEUROSCI.22-02-00592.2002
Bastian, A. J., Kelly, V. E., Revilla, F. J., Perlmutter, J. S., and Mink, J. W. (2003). Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov. Disord. 18, 1000–1007. doi: 10.1002/mds.10493
Bastin, J., Polosan, M., Benis, D., Goetz, L., Bhattacharjee, M., Piallat, B., et al. (2014). Inhibitory control and error monitoring by human subthalamic neurons. Transl. Psychiatry 4:e439. doi: 10.1038/tp.2014.73
Baunez, C., Amalric, M., and Robbins, T. W. (2002). Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J. Neurosci. 22, 562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002
Baunez, C., Christakou, A., Chudasama, Y., Forni, C., and Robbins, T. W. (2007). Bilateral high-frequency stimulation of the subthalamic nucleus on attentional performance: transient deleterious effects and enhanced motivation in both intact and parkinsonian rats. Eur. J. Neurosci. 25, 1187–1194. doi: 10.1111/j.1460-9568.2007.05373.x
Baunez, C., Dias, C., Cador, M., and Amalric, M. (2005). The subthalamic nucleus exerts opposite control on cocaine and “natural” rewards. Nat. Neurosci. 8, 484–489. doi: 10.1038/nn1429
Baunez, C., and Gubellini, P. (2010). Effects of GPi and STN inactivation on physiological, motor, cognitive and motivational processes in animal models of Parkinson's disease. Prog. Brain Res. 183, 235–258. doi: 10.1016/S0079-6123(10)83012-2
Baunez, C., and Robbins, T. W. (1997). Bilateral lesions of the subthalamic nucleus induce multiple deficits in an attentional task in rats. Eur. J. Neurosci. 9, 2086–2099. doi: 10.1111/j.1460-9568.1997.tb01376.x
Baunez, C., and Robbins, T. W. (1999a). Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience 92, 1343–1356. doi: 10.1016/S0306-4522(99)00065-2
Baunez, C., and Robbins, T. W. (1999b). Effects of transient inactivation of the subthalamic nucleus by local muscimol and APV infusions on performance on the five-choice serial reaction time task in rats. Psychopharmacology 141, 57–65. doi: 10.1007/s002130050806
Benabid, A. L., Chabardes, S., Mitrofanis, J., and Pollak, P. (2009). Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 8, 67–81. doi: 10.1016/S1474-4422(08)70291-6
Benarroch, E. E. (2008). Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology 70, 1991–1995. doi: 10.1212/01.wnl.0000313022.39329.65
Benazzouz, A., Gao, D., Ni, Z., and Benabid, A. L. (2000a). High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport 11, 1593–1596. doi: 10.1097/00001756-200005150-00044
Benazzouz, A., Piallat, B., Ni, Z. G., Koudsie, A., Pollak, P., and Benabid, A. L. (2000b). Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson's disease. Cell Transplant 9, 215–221. doi: 10.1177/096368970000900207
Benazzouz, A., Piallat, B., Pollak, P., and Benabid, A. L. (1995). Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci. Lett. 189, 77–80. doi: 10.1016/0304-3940(95)11455-6
Benis, D., David, O., Piallat, B., Kibleur, A., Goetz, L., Bhattacharjee, M., et al. (2016). Response inhibition rapidly increases single-neuron responses in the subthalamic nucleus of patients with Parkinson's disease. Cortex 84, 111–123. doi: 10.1016/j.cortex.2016.09.006
Bentzley, B. S., and Aston-Jones, G. (2017). Inhibiting subthalamic nucleus decreases cocaine demand and relapse: therapeutic potential. Addict. Biol. 22, 946–957. doi: 10.1111/adb.12380
Bergman, H., Wichmann, T., and DeLong, M. R. (1990). Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438. doi: 10.1126/science.2402638
Bergman, H., Wichmann, T., Karmon, B., and DeLong, M. R. (1994). The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 72, 507–520. doi: 10.1152/jn.1994.72.2.507
Berke, J. D. (2018). What does dopamine mean? Nat. Neurosci. 21, 787–793. doi: 10.1038/s41593-018-0152-y
Berridge, K. C. (2004). Motivation concepts in behavioral neuroscience. Physiol. Behav. 81, 179–209. doi: 10.1016/j.physbeh.2004.02.004
Beurrier, C., Bioulac, B., Audin, J., and Hammond, C. (2001). High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J. Neurophysiol. 85, 1351–1356. doi: 10.1152/jn.2001.85.4.1351
Bezzina, G., Boon, F. S., Hampson, C. L., Cheung, T. H., Body, S., Bradshaw, C. M., et al. (2008). Effect of quinolinic acid-induced lesions of the subthalamic nucleus on performance on a progressive-ratio schedule of reinforcement: a quantitative analysis. Behav. Brain Res. 195, 223–230. doi: 10.1016/j.bbr.2008.09.005
Bradshaw, C. M., and Killeen, P. R. (2012). A theory of behaviour on progressive ratio schedules, with applications in behavioural pharmacology. Psychopharmacology 222, 549–564. doi: 10.1007/s00213-012-2771-4
Bruet, N., Windels, F., Bertrand, A., Feuerstein, C., Poupard, A., and Savasta, M. (2001). High frequency stimulation of the subthalamic nucleus increases the extracellular contents of striatal dopamine in normal and partially dopaminergic denervated rats. J. Neuropathol. Exp. Neurol. 60, 15–24. doi: 10.1093/jnen/60.1.15
Cabeza de Vaca, S., and Carr, K. D. (1998). Food restriction enhances the central rewarding effect of abused drugs. J. Neurosci. 18, 7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998
Carcenac, C., Favier, M., Vachez, Y., Lacombe, E., Carnicella, S., Savasta, M., et al. (2015). Subthalamic deep brain stimulation differently alters striatal dopaminergic receptor levels in rats. Mov. Disord. 30, 1739–1749. doi: 10.1002/mds.26146
Castrioto, A., Lhommee, E., Moro, E., and Krack, P. (2014). Mood and behavioural effects of subthalamic stimulation in Parkinson's disease. Lancet Neurol. 13, 287–305. doi: 10.1016/S1474-4422(13)70294-1
Cavdar, S., Özgür, M., Çakmak, Y. Ö., Kuvvet, Y., Kunt, S. K., and Saglam, G. (2018). Afferent projections of the subthalamic nucleus in the rat: emphasis on bilateral and interhemispheric connections. Acta Neurobiol. Exp. 78, 251–263. doi: 10.21307/ane-2018-023
Chagraoui, A., Boukhzar, L., Thibaut, F., Anouar, Y., and Maltete, D. (2018). The pathophysiological mechanisms of motivational deficits in Parkinson's disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 81, 138–152. doi: 10.1016/j.pnpbp.2017.10.022
Chassain, C., Melon, C., Salin, P., Vitale, F., Couraud, S., Durif, F., et al. (2016). Metabolic, synaptic and behavioral impact of 5-week chronic deep brain stimulation in hemiparkinsonian rats. J. Neurochem. 136, 1004–1016. doi: 10.1111/jnc.13438
Chaturvedi, A., Lujan, J. L., and McIntyre, C. C. (2013). Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J. Neural Eng. 10, 056023. doi: 10.1088/1741-2560/10/5/056023
Chaudhuri, K. R., and Schapira, A. H. (2009). Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474. doi: 10.1016/S1474-4422(09)70068-7
Chopra, A., Tye, S. J., Lee, K. H., Sampson, S., Matsumoto, J., Adams, A., et al. (2012). Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson's disease: a review of the literature. J. Neuropsychiatry Clin. Neurosci. 24, 102–110. doi: 10.1176/appi.neuropsych.10070109
Creed, M. (2018). Current and emerging neuromodulation therapies for addiction: insight from pre-clinical studies. Curr. Opin. Neurobiol. 49, 168–174. doi: 10.1016/j.conb.2018.02.015
Creed, M., Pascoli, V. J., and Lüscher, C. (2015). Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347:569–564. doi: 10.1126/science.1260776
Creed, M. C. (2018). Toward a targeted treatment for addiction. Science 357:464–465. doi: 10.1126/science.aao1197
Creed, M. C., Hamani, C., and Nobrega, J. N. (2012). Early gene mapping after deep brain stimulation in a rat model of tardive dyskinesia: comparison with transient local inactivation. Eur. Neuropsychopharmacol. 22, 506–517. doi: 10.1016/j.euroneuro.2011.11.004
Creed, M. C., Hamani, C., and Nobrega, J. N. (2013). Effects of repeated deep brain stimulation on depressive- and anxiety-like behavior in rats: comparing entopeduncular and subthalamic nuclei. Brain Stimul. 6, 506–514. doi: 10.1016/j.brs.2012.09.012
Darbaky, Y., Forni, C., Amalric, M., and Baunez, C. (2003). High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur. J. Neurosci. 18, 951–956. doi: 10.1046/j.1460-9568.2003.02803.x
Deli, G., Balas, I., Nagy, F., Balazs, E., Janszky, J., Komoly, S., et al. (2011). Comparison of the efficacy of unipolar and bipolar electrode configuration during subthalamic deep brain stimulation. Parkinsonism Relat. Disord. 17, 50–54. doi: 10.1016/j.parkreldis.2010.10.012
Deumens, R., Blokland, A., and Prickaerts, J. (2002). Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 175, 303–317. doi: 10.1006/exnr.2002.7891
Deuschl, G., Schupbach, M., Knudsen, K., Pinsker, M. O., Cornu, P., Rau, J., et al. (2013). Stimulation of the subthalamic nucleus at an earlier disease stage of Parkinson's disease: concept and standards of the EARLYSTIM-study. Parkinsonism Relat. Disord. 19, 56–61. doi: 10.1016/j.parkreldis.2012.07.004
Dostrovsky, J. O., Levy, R., Wu, J. P., Hutchison, W. D., Tasker, R. R., and Lozano, A. M. (2000). Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J. Neurophysiol. 84, 570–574. doi: 10.1152/jn.2000.84.1.570
Drapier, D., Drapier, S., Sauleau, P., Haegelen, C., Raoul, S., Biseul, I., et al. (2006). Does subthalamic nucleus stimulation induce apathy in Parkinson's disease? J. Neurol. 253, 1083–1091. doi: 10.1007/s00415-006-0177-0
Drapier, D., Peron, J., Leray, E., Sauleau, P., Biseul, I., Drapier, S., et al. (2008). Emotion recognition impairment and apathy after subthalamic nucleus stimulation in Parkinson's disease have separate neural substrates. Neuropsychologia 46, 2796–2801. doi: 10.1016/j.neuropsychologia.2008.05.006
Drui, G., Carnicella, S., Carcenac, C., Favier, M., Bertrand, A., Boulet, S., et al. (2014). Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson's disease. Mol. Psychiatry 19, 358–367. doi: 10.1038/mp.2013.3
Eitan, R., Shamir, R. R., Linetsky, E., Rosenbluh, O., Moshel, S., Ben-Hur, T., et al. (2013). Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Front. Syst. Neurosci. 7:69. doi: 10.3389/fnsys.2013.00069
Emmi, A., Antonini, A., Macchi, V., Porzionato, A., and De Caro, R. (2020). Anatomy and Connectivity of the subthalamic nucleus in humans and non-human primates. Front. Neuroanat. 14:13. doi: 10.3389/fnana.2020.00013
Evenden, J. L., and Ryan, C. N. (1996). The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128, 161–170. doi: 10.1007/s002130050121
Fasano, A., Romito, L. M., Daniele, A., Piano, C., Zinno, M., Bentivoglio, A. R., et al. (2010). Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain 133, 2664–2676. doi: 10.1093/brain/awq221
Favier, M., Carcenac, C., Drui, G., Vachez, Y., Boulet, S., Savasta, M., et al. (2017). Implication of dorsostriatal D3 receptors in motivational processes: a potential target for neuropsychiatric symptoms in Parkinson's disease. Sci. Rep. 7:41589. doi: 10.1038/srep41589
Favier, M., Duran, T., Carcenac, C., Drui, G., Savasta, M., and Carnicella, S. (2014). Pramipexole reverses Parkinson's disease-related motivational deficits in rats. Mov. Disord. 29, 912–920. doi: 10.1002/mds.25837
Fedele, E., and Raiteri, M. (1999). In vivo studies of the cerebral glutamate receptor/NO/cGMP pathway. Prog. Neurobiol. 58, 89–120. doi: 10.1016/S0301-0082(98)00077-X
Fife, K. H., Gutierrez-Reed, N. A., Zell, V., Bailly, J., Lewis, C. M., Aron, A. R., et al. (2017). Causal role for the subthalamic nucleus in interrupting behavior. eLife 6:e27689. doi: 10.7554/eLife.27689.016
Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M., and Dostrovsky, J. O. (2004). Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp. Brain Res. 156, 274–281. doi: 10.1007/s00221-003-1784-y
Florence, G., Sameshima, K., Fonoff, E. T., and Hamani, C. (2016). Deep brain stimulation: more complex than the inhibition of cells and excitation of fibers. Neuroscientist 22, 332–345. doi: 10.1177/1073858415591964
Fontaine, D., Mattei, V., Borg, M., von Langsdorff, D., Magnie, M.-N., Chanalet, S., et al. (2004). Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease. Case report. J. Neurosurg. 100, 1084–1086. doi: 10.3171/jns.2004.100.6.1084
Fraix, V., Pollak, P., Vercueil, L., Benabid, A. L., and Mauguiere, F. (2008). Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson's disease. Clin. Neurophysiol. 119, 2513–2518. doi: 10.1016/j.clinph.2008.07.217
Frank, M. J. (2006). Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 19, 1120–1136. doi: 10.1016/j.neunet.2006.03.006
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., and Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359. doi: 10.1126/science.1167093
Griffiths, R. R., Findley, J. D., Brady, J. V., Dolan-Gutcher, K., and Robinson, W. W. (1975). Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacologia 43, 81–83. doi: 10.1007/BF00437619
Grill, W. M., Snyder, A. N., and Miocinovic, S. (2004). Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport 15, 1137–1140. doi: 10.1097/00001756-200405190-00011
Gubellini, P., Eusebio, A., Oueslati, A., Melon, C., Kerkerian-Le Goff, L., and Salin, P. (2006). Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: effects on motor behaviour and striatal glutamate transmission. Eur. J. Neurosci. 24, 1802–1814. doi: 10.1111/j.1460-9568.2006.05047.x
Gunaydin, L. A., Yizhar, O., Berndt, A., Sohal, V. S., Deisseroth, K., and Hegemann, P. (2010). Ultrafast optogenetic control. Nat. Neurosci 13, 387–392. doi: 10.1038/nn.2495
Guridi, J., Herrero, M. T., Luquin, M. R., Guillen, J., Ruberg, M., Laguna, J., et al. (1996). Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain 119(Pt 5), 1717–1727. doi: 10.1093/brain/119.5.1717
Guridi, J., Herrero, M. T., Luquin, R., Guillen, J., and Obeso, J. A. (1994). Subthalamotomy improves MPTP-induced parkinsonism in monkeys. Stereotact. Funct. Neurosurg. 62, 98–102. doi: 10.1159/000098603
Hahn, P. J., Russo, G. S., Hashimoto, T., Miocinovic, S., Xu, W., McIntyre, C. C., et al. (2008). Pallidal burst activity during therapeutic deep brain stimulation. Exp. Neurol. 211, 243–251. doi: 10.1016/j.expneurol.2008.01.032
Hamani, C., Florence, G., Heinsen, H., Plantinga, B. R., Temel, Y., Uludag, K., et al. (2017). Subthalamic nucleus deep brain stimulation: basic concepts and novel perspectives. eNeuro 4:ENEURO.0140-17.2017. doi: 10.1523/ENEURO.0140-17.2017
Hamani, C., Saint-Cyr, J. A., Fraser, J., Kaplitt, M., and Lozano, A. M. (2004). The subthalamic nucleus in the context of movement disorders. Brain 127, 4–20. doi: 10.1093/brain/awh029
Hancu, I., Boutet, A., Fiveland, E., Ranjan, M., Prusik, J., Dimarzio, M., et al. (2019). On the (non-)equivalency of monopolar and bipolar settings for deep brain stimulation fMRI studies of Parkinson's disease patients. J. Magn. Reson. Imaging 49, 1736–1749. doi: 10.1002/jmri.26321
Hassani, O. K., Mouroux, M., and Feger, J. (1996). Increased subthalamic neuronal activity after nigral dopaminergic lesion independent of disinhibition via the globus pallidus. Neuroscience 72, 105–115. doi: 10.1016/0306-4522(95)00535-8
He, Z., Jiang, Y., Xu, H., Jiang, H., Jia, W., Sun, P., et al. (2014). High frequency stimulation of subthalamic nucleus results in behavioral recovery by increasing striatal dopamine release in 6-hydroxydopamine lesioned rat. Behav. Brain Res. 263, 108–114. doi: 10.1016/j.bbr.2014.01.014
Henderson, J. M., Annett, L. E., Ryan, L. J., Chiang, W., Hidaka, S., Torres, E. M., et al. (1999). Subthalamic nucleus lesions induce deficits as well as benefits in the hemiparkinsonian rat. Eur. J. Neurosci. 11, 2749–2757. doi: 10.1046/j.1460-9568.1999.00692.x
Herrington, T. M., Cheng, J. J., and Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. J. Neurophysiol. 115, 19–38. doi: 10.1152/jn.00281.2015
Higgins, G. A., and Silenieks, L. B. (2017). Rodent test of attention and impulsivity: the 5-choice serial reaction time task. Curr. Protoc. Pharmacol. 78, 5.49.1–5.49.34. doi: 10.1002/cpph.27
Howe, M. W., Tierney, P. L., Sandberg, S. G., Phillips, P. E., and Graybiel, A. M. (2013). Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500, 575–579. doi: 10.1038/nature12475
Irmen, F., Horn, A., Mosley, P., Perry, A., Petry-Schmelzer, J. N., Dafsari, H. S., et al. (2020). Left prefrontal connectivity links subthalamic stimulation with depressive symptoms. Ann. Neurol. 87, 962–975. doi: 10.1002/ana.25734
Jakobs, M., Fomenko, A., Lozano, A. M., and Kiening, K. L. (2019). Cellular, molecular, and clinical mechanisms of action of deep brain stimulation-a systematic review on established indications and outlook on future developments. EMBO Mol. Med. 11:e9575. doi: 10.15252/emmm.201809575
Kang, G., and Lowery, M. M. (2014). Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson's disease: a simulation study. Front. Comput. Neurosci. 8:32. doi: 10.3389/fncom.2014.00032
Kantak, K. M., Yager, L. M., and Brisotti, M. F. (2013). Impact of medial orbital cortex and medial subthalamic nucleus inactivation, individually and together, on the maintenance of cocaine self-administration behavior in rats. Behav. Brain Res. 238, 1–9. doi: 10.1016/j.bbr.2012.10.021
Klavir, O., Flash, S., Winter, C., and Joel, D. (2009). High frequency stimulation and pharmacological inactivation of the subthalamic nucleus reduces “compulsive” lever-pressing in rats. Exp. Neurol. 215, 101–109. doi: 10.1016/j.expneurol.2008.09.017
Koob, G. F., and Weiss, F. (1990). Pharmacology of drug self-administration. Alcohol 7, 193–197. doi: 10.1016/0741-8329(90)90004-V
Krack, P., Batir, A., Van Blercom, N., Chabardes, S., Fraix, V., Ardouin, C., et al. (2003). Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N. Engl. J. Med. 349, 1925–1934. doi: 10.1056/NEJMoa035275
Krack, P., Hariz, M. I., Baunez, C., Guridi, J., and Obeso, J. A. (2010). Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 33, 474–484. doi: 10.1016/j.tins.2010.07.002
Krack, P., Kumar, R., Ardouin, C., Dowsey, P. L., McVicker, J. M., Benabid, A. L., et al. (2001). Mirthful laughter induced by subthalamic nucleus stimulation. Mov. Disord. 16, 867–875. doi: 10.1002/mds.1174
Kreiss, D. S., Mastropietro, C. W., Rawji, S. S., and Walters, J. R. (1997). The response of subthalamic nucleus neurons to dopamine receptor stimulation in a rodent model of Parkinson's disease. J. Neurosci. 17, 6807–6819. doi: 10.1523/JNEUROSCI.17-17-06807.1997
Kühn, A. A., Tsui, A., Aziz, T., Ray, N., Brücke, C., Kupsch, A., et al. (2009). Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp. Neurol. 215, 380–387. doi: 10.1016/j.expneurol.2008.11.008
Lambert, C., Zrinzo, L., Nagy, Z., Lutti, A., Hariz, M., Foltynie, T., et al. (2012). Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage 60, 83–94. doi: 10.1016/j.neuroimage.2011.11.082
Lardeux, S., and Baunez, C. (2008). Alcohol preference influences the subthalamic nucleus control on motivation for alcohol in rats. Neuropsychopharmacology 33, 634–642. doi: 10.1038/sj.npp.1301432
Lardeux, S., Paleressompoulle, D., Pernaud, R., Cador, M., and Baunez, C. (2013). Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. J. Neurophysiol. 110, 1497–1510. doi: 10.1152/jn.00160.2013
Lardeux, S., Pernaud, R., Paleressompoulle, D., and Baunez, C. (2009). Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. J. Neurophysiol. 102, 2526–2537. doi: 10.1152/jn.91009.2008
Lavian, H., Ben-Porat, H., and Korngreen, A. (2013). High and low frequency stimulation of the subthalamic nucleus induce prolonged changes in subthalamic and globus pallidus neurons. Front. Syst. Neurosci. 7:73. doi: 10.3389/fnsys.2013.00073
Le Jeune, F., Drapier, D., Bourguignon, A., Peron, J., Mesbah, H., Drapier, S., et al. (2009). Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology 73, 1746–1751. doi: 10.1212/WNL.0b013e3181c34b34
Lee, D. K. (2016). Alternatives to P value: confidence interval and effect size. Korean J. Anesthesiol. 69, 555–562. doi: 10.4097/kjae.2016.69.6.555
Lee, E. M., Kurundkar, A., Cutter, G. R., Huang, H., Guthrie, B. L., Watts, R. L., et al. (2011). Comparison of weight changes following unilateral and staged bilateral STN DBS for advanced PD. Brain Behav. 1, 12–18. doi: 10.1002/brb3.9
Leentjens, A. F., Koester, J., Fruh, B., Shephard, D. T., Barone, P., and Houben, J. J. (2009). The effect of pramipexole on mood and motivational symptoms in Parkinson's disease: a meta-analysis of placebo-controlled studies. Clin. Ther. 31, 89–98. doi: 10.1016/j.clinthera.2009.01.012
Levy, R., and Dubois, B. (2006). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex 16, 916–928. doi: 10.1093/cercor/bhj043
Li, S., Arbuthnott, G. W., Jutras, M. J., Goldberg, J. A., and Jaeger, D. (2007). Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J. Neurophysiol. 98, 3525–3537. doi: 10.1152/jn.00808.2007
Liewald, J. F., Brauner, M., Stephens, G. J., Bouhours, M., Schultheis, C., Zhen, M., et al. (2008). Optogenetic analysis of synaptic function. Nat. Methods 5, 895–902. doi: 10.1038/nmeth.1252
Limousin, P., Pollak, P., Benazzouz, A., Hoffmann, D., Broussolle, E., Perret, J. E., et al. (1995). Bilateral subthalamic nucleus stimulation for severe Parkinson's disease. Mov. Disord. 10, 672–674. doi: 10.1002/mds.870100523
Lizarraga, K. J., Jagid, J. R., and Luca, C. C. (2016). Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation on gait kinematics in Parkinson's disease: a randomized, blinded study. J. Neurol. 263, 1652–1656. doi: 10.1007/s00415-016-8191-3
Lozano, A. M., Dostrovsky, J., Chen, R., and Ashby, P. (2002). Deep brain stimulation for Parkinson's disease: disrupting the disruption. Lancet Neurol. 1, 225–231. doi: 10.1016/S1474-4422(02)00101-1
Luan, Y., Tang, D., Wu, H., Gu, W., Wu, Y., Cao, J.-L., et al. (2020). Reversal of hyperactive subthalamic circuits differentially mitigates pain hypersensitivity phenotypes in parkinsonian mice. Proc. Natl. Acad. Sci. U.S.A. 117, 10045–10054. doi: 10.1073/pnas.1916263117
Magarinos-Ascone, C., Pazo, J. H., Macadar, O., and Buno, W. (2002). High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience 115, 1109–1117. doi: 10.1016/S0306-4522(02)00538-9
Magnard, R., Vachez, Y., Carcenac, C., Krack, P., David, O., Savasta, M., et al. (2016). What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson's disease? Transl. Psychiatry 6:e753. doi: 10.1038/tp.2016.17
Maier, F., Lewis, C. J., Horstkoetter, N., Eggers, C., Dembek, T. A., Visser-Vandewalle, V., et al. (2016). Subjective perceived outcome of subthalamic deep brain stimulation in Parkinson's disease one year after surgery. Parkinsonism Relat. Disord. 24, 41–47. doi: 10.1016/j.parkreldis.2016.01.019
Maier, F., Lewis, C. J., Horstkoetter, N., Eggers, C., Kalbe, E., Maarouf, M., et al. (2013). Patients' expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson's disease: a mixed-method approach. J. Neurol. Neurosurg. Psychiatr. 84, 1273–1281. doi: 10.1136/jnnp-2012-303670
Mallet, L., Mesnage, V., Houeto, J.-L., Pelissolo, A., Yelnik, J., Behar, C., et al. (2002). Compulsions, Parkinson's disease, and stimulation. Lancet 360, 1302–1304. doi: 10.1016/S0140-6736(02)11339-0
Mallet, L., Polosan, M., Jaafari, N., Baup, N., Welter, M.-L., Fontaine, D., et al. (2008). Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 359, 2121–2134. doi: 10.1056/NEJMoa0708514
Mallet, L., Schupbach, M., N'Diaye, K., Remy, P., Bardinet, E., Czernecki, V., et al. (2007). Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc. Natl. Acad. Sci. U.S.A. 104, 10661–10666. doi: 10.1073/pnas.0610849104
Mandat, T. S., Hurwitz, T., and Honey, C. R. (2006). Hypomania as an adverse effect of subthalamic nucleus stimulation: report of two cases. Acta Neurochir. 148, 895–897. doi: 10.1007/s00701-006-0795-4
Marin, R. S. (1996). Apathy: concept, syndrome, neural mechanisms, and treatment. Semin. Clin. Neuropsychiatry 1, 304–314.
Martinez-Fernandez, R., Pelissier, P., Quesada, J. L., Klinger, H., Lhommee, E., Schmitt, E., et al. (2016). Postoperative apathy can neutralise benefits in quality of life after subthalamic stimulation for Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 87, 311–318. doi: 10.1136/jnnp-2014-310189
Mastro, K. J., Zitelli, K. T., Willard, A. M., Leblanc, K. H., Kravitz, A. V., and Gittis, A. H. (2017). Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine depleted mice. Nat. Neurosci. 20, 815–823. doi: 10.1038/nn.4559
McIntyre, C. C., and Anderson, R. W. (2016). Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J. Neurochem. 139(Suppl. 1), 338–345. doi: 10.1111/jnc.13649
McIntyre, C. C., Grill, W. M., Sherman, D. L., and Thakor, N. V. (2004). Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J. Neurophysiol. 91, 1457–1469. doi: 10.1152/jn.00989.2003
Meissner, W., Leblois, A., Hansel, D., Bioulac, B., Gross, C. E., Benazzouz, A., et al. (2005). Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128, 2372–2382. doi: 10.1093/brain/awh616
Melon, C., Chassain, C., Bielicki, G., Renou, J. P., Kerkerian-Le Goff, L., Salin, P., et al. (2015). Progressive brain metabolic changes under deep brain stimulation of subthalamic nucleus in parkinsonian rats. J. Neurochem. 132, 703–712. doi: 10.1111/jnc.13015
Montanari, C., Giorla, E., Pelloux, Y., and Baunez, C. (2018). Subthalamic nucleus mediates the modulation on cocaine self-administration induced by ultrasonic vocalization playback in rats. Addict. Biol. 25:e12710. doi: 10.1111/adb.12710
Moro, E., Scerrati, M., Romito, L. M., Roselli, R., Tonali, P., and Albanese, A. (1999). Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson's disease. Neurology 53, 85–90. doi: 10.1212/WNL.53.1.85
Mosberger, A. C., de Clauser, L., Kasper, H., and Schwab, M. E. (2016). Motivational state, reward value, and Pavlovian cues differentially affect skilled forelimb grasping in rats. Learn. Mem. 23, 289–302. doi: 10.1101/lm.039537.115
Nagel, G., Szellas, T., Huhn, W., Kateriya, S., Adeishvili, N., Berthold, P., et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 100, 13940–13945. doi: 10.1073/pnas.1936192100
Nowak, L. G., and Bullier, J. (1998). Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp. Brain Res. 118, 489–500. doi: 10.1007/s002210050305
Obeso, I., Wilkinson, L., Rodriguez-Oroz, M. C., Obeso, J. A., and Jahanshahi, M. (2013). Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson's disease. Exp. Brain Res. 226, 451–462. doi: 10.1007/s00221-013-3457-9
Obeso, J. A., Rodriguez-Oroz, M., Marin, C., Alonso, F., Zamarbide, I., Lanciego, J. L., et al. (2004). The origin of motor fluctuations in Parkinson's disease: importance of dopaminergic innervation and basal ganglia circuits. Neurology 62, S17–30. doi: 10.1212/WNL.62.1_suppl_1.S17
Pan, M.-K., Tai, C.-H., Liu, W.-C., Pei, J.-C., Lai, W.-S., and Kuo, C.-C. (2014). Deranged NMDAergic cortico-subthalamic transmission underlies parkinsonian motor deficits. J. Clin. Invest. 124, 4629–4641. doi: 10.1172/JCI75587
Parent, M., and Parent, A. (2004). The pallidofugal motor fiber system in primates. Parkinsonism Relat. Disord. 10, 203–211. doi: 10.1016/j.parkreldis.2004.02.007
Pascoli, V., Terrier, J., Espallergues, J., Valjent, E., O'Connor, E. C., and Lüscher, C. (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464. doi: 10.1038/nature13257
Pascoli, V., Turiault, M., and Lüscher, C. (2011). Reversal of cocaine-evoked synaptic potentiation resets drug-adaptive behaviour. Nature 481, 71–75. doi: 10.1038/nature10709
Pelloux, Y., and Baunez, C. (2013). Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr. Opin. Neurobiol. 23, 713–720. doi: 10.1016/j.conb.2013.02.016
Pelloux, Y., and Baunez, C. (2017). Targeting the subthalamic nucleus in a preclinical model of alcohol use disorder. Psychopharmacology 234, 2127–2137. doi: 10.1007/s00213-017-4618-5
Pelloux, Y., Degoulet, M., Tiran-Cappello, A., Cohen, C., Lardeux, S., George, O., et al. (2018). Subthalamic nucleus high frequency stimulation prevents and reverses escalated cocaine use. Mol. Psychiatry. 23, 2266–2276. doi: 10.1038/s41380-018-0080-y
Poewe, W. H. (1994). Clinical aspects of motor fluctuations in Parkinson's disease. Neurology 44, S6–S9.
Pratt, W. E., Choi, E., and Guy, E. G. (2012). An examination of the effects of subthalamic nucleus inhibition or mu-opioid receptor stimulation on food-directed motivation in the non-deprived rat. Behav. Brain Res. 230, 365–373. doi: 10.1016/j.bbr.2012.02.031
Robbins, T. W. (2002). The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163, 362–380. doi: 10.1007/s00213-002-1154-7
Rouaud, T., Lardeux, S., Panayotis, N., Paleressompoulle, D., Cador, M., and Baunez, C. (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc. Natl. Acad. Sci. U.S.A. 107, 1196–1200. doi: 10.1073/pnas.0908189107
Rummel, J., Voget, M., Hadar, R., Ewing, S., Sohr, R., Klein, J., et al. (2016). Testing different paradigms to optimize antidepressant deep brain stimulation in different rat models of depression. J. Psychiatr. Res. 81, 36–45. doi: 10.1016/j.jpsychires.2016.06.016
Sanders, T. H. (2017). Stimulation of cortico-subthalamic projections amplifies resting motor circuit activity and leads to increased locomotion in dopamine-depleted mice. Front. Integr. Neurosci. 11:24. doi: 10.3389/fnint.2017.00024
Sanders, T. H., and Jaeger, D. (2016). Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol. Dis. 95, 225–237. doi: 10.1016/j.nbd.2016.07.021
Saran, S., Gupta, N., and Roy, S. (2018). Theoretical analysis of low-power fast optogenetic control of firing of chronos-expressing neurons. Neurophotonics 5:025009. doi: 10.1117/1.NPh.5.2.025009
Schuepbach, W. M., Rau, J., Knudsen, K., Volkmann, J., Krack, P., Timmermann, L., et al. (2013). Neurostimulation for Parkinson's disease with early motor complications. N. Engl. J. Med. 368, 610–622. doi: 10.1056/NEJMoa1205158
Sesack, S. R., and Grace, A. A. (2010). Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. doi: 10.1038/npp.2009.93
Shen, K. Z., Zhu, Z. T., Munhall, A., and Johnson, S. W. (2003). Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse 50, 314–319. doi: 10.1002/syn.10274
Shi, L. H., Luo, F., Woodward, D. J., and Chang, J. Y. (2006). Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test. Synapse 59, 445–457. doi: 10.1002/syn.20261
Shin, D. S., Samoilova, M., Cotic, M., Zhang, L., Brotchie, J. M., and Carlen, P. L. (2007). High frequency stimulation or elevated K+ depresses neuronal activity in the rat entopeduncular nucleus. Neuroscience 149, 68–86. doi: 10.1016/j.neuroscience.2007.06.055
Shon, Y. M., Lee, K. H., Goerss, S. J., Kim, I. Y., Kimble, C., Van Gompel, J. J., et al. (2010). High frequency stimulation of the subthalamic nucleus evokes striatal dopamine release in a large animal model of human DBS neurosurgery. Neurosci. Lett. 475, 136–140. doi: 10.1016/j.neulet.2010.03.060
Sjoberg, R. L., Lidman, E., Haggstrom, B., Hariz, M. I., Linder, J., Fredricks, A., et al. (2012). Verbal fluency in patients receiving bilateral versus left-sided deep brain stimulation of the subthalamic nucleus for Parkinson's disease. J. Int. Neuropsychol. Soc. 18, 606–611. doi: 10.1017/S1355617711001925
Tai, C. H., Boraud, T., Bezard, E., Bioulac, B., Gross, C., and Benazzouz, A. (2003). Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J. 17, 1820–1830. doi: 10.1096/fj.03-0163com
Tan, S. K., Hartung, H., Schievink, S., Sharp, T., and Temel, Y. (2013). High-frequency stimulation of the substantia nigra induces serotonin-dependent depression-like behavior in animal models. Biol. Psychiatry 73, e1–3. doi: 10.1016/j.biopsych.2012.07.032
Tan, S. K., Hartung, H., Visser-Vandewalle, V., Steinbusch, H. W., Temel, Y., and Sharp, T. (2012). A combined in vivo neurochemical and electrophysiological analysis of the effect of high-frequency stimulation of the subthalamic nucleus on 5-HT transmission. Exp. Neurol. 233, 145–153. doi: 10.1016/j.expneurol.2011.08.027
Tan, S. K., Janssen, M. L., Jahanshahi, A., Chouliaras, L., Visser-Vandewalle, V., Lim, L. W., et al. (2011). High frequency stimulation of the subthalamic nucleus increases c-fos immunoreactivity in the dorsal raphe nucleus and afferent brain regions. J. Psychiatr. Res. 45, 1307–1315. doi: 10.1016/j.jpsychires.2011.04.011
Temel, Y., Boothman, L. J., Blokland, A., Magill, P. J., Steinbusch, H. W., Visser-Vandewalle, V., et al. (2007). Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc. Natl. Acad. Sci. U.S.A. 104, 17087–17092. doi: 10.1073/pnas.0704144104
Temel, Y., Tan, S., Vlamings, R., Sesia, T., Lim, L. W., Lardeux, S., et al. (2009). Cognitive and limbic effects of deep brain stimulation in preclinical studies. Front. Biosci. 14, 1891–1901. doi: 10.2741/3349
Temel, Y., Visser-Vandewalle, V., van der Wolf, M., Spincemaille, G. H., Desbonnet, L., Hoogland, G., et al. (2004). Monopolar versus bipolar high frequency stimulation in the rat subthalamic nucleus: differences in histological damage. Neurosci. Lett. 367, 92–96. doi: 10.1016/j.neulet.2004.05.087
Thobois, S., Ardouin, C., Lhommee, E., Klinger, H., Lagrange, C., Xie, J., et al. (2010). Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain 133, 1111–1127. doi: 10.1093/brain/awq032
Tian, J., Yan, Y., Xi, W., Zhou, R., Lou, H., Duan, S., et al. (2018). Optogenetic Stimulation of GABAergic Neurons in the Globus Pallidus Produces Hyperkinesia. Front. Behav. Neurosci. 12:185. doi: 10.3389/fnbeh.2018.00185
Tiran-Cappello, A., Pelloux, Y., Brocard, C., Degoulet, M., and Baunez, C. (2018). A glimpse at deep brain stimulation mechanisms using subthalamic nucleus optogenetic manipulations. bioRxiv bioRxiv:2018.06.11.147166. doi: 10.1101/450767
Uslaner, J. M., Dell'Orco, J. M., Pevzner, A., and Robinson, T. E. (2008). The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology 33, 2352–2361. doi: 10.1038/sj.npp.1301653
Vachez, Y., Bahout, M., Magnard, R., David, P.-M., Carcenac, C., Robert, G., et al. (2020a). Bilateral but not unilateral subthalamic stimulation promotes apathy: a translational study in rodents and Parkinson's disease patients. bioRxiv bioRxiv:2020.06.11.147116. doi: 10.1101/2020.06.11.147116
Vachez, Y., Carcenac, C., Magnard, R., Kerkerian-Le Goff, L., Salin, P., Savasta, M., et al. (2020b). Subthalamic nucleus stimulation impairs motivation: implication for apathy in Parkinson's disease. Mov. Disord. 35, 616–628. doi: 10.1002/mds.27953
van den Bos, R., Koot, S., and de Visser, L. (2014). A rodent version of the iowa gambling task: 7 years of progress. Front. Psychol. 5:203. doi: 10.3389/fpsyg.2014.00203
Vila, M., Perier, C., Feger, J., Yelnik, J., Faucheux, B., Ruberg, M., et al. (2000). Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur. J. Neurosci. 12, 337–344. doi: 10.1046/j.1460-9568.2000.00901.x
Wade, C. L., Kallupi, M., Hernandez, D. O., Breysse, E., de Guglielmo, G., Crawford, E., et al. (2017). High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacology 42, 1850–1859. doi: 10.1038/npp.2016.270
Walker, R. H., Koch, R. J., Moore, C., and Meshul, C. K. (2009). Subthalamic nucleus stimulation and lesioning have distinct state-dependent effects upon striatal dopamine metabolism. Synapse 63, 136–146. doi: 10.1002/syn.20592
Wang, Y., Li, Y., Zhang, X., and Xie, A. (2018). Apathy following bilateral deep brain stimulation of subthalamic nucleus in Parkinson's disease: a meta-analysis. Parkinsons Dis. 2018:9756468. doi: 10.1155/2018/9756468
Welter, M. L., Houeto, J. L., Bonnet, A. M., Bejjani, P. B., Mesnage, V., Dormont, D., et al. (2004). Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch. Neurol. 61, 89–96. doi: 10.1001/archneur.61.1.89
Wichmann, T., Bergman, H., and DeLong, M. R. (1994). The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 72, 521–530. doi: 10.1152/jn.1994.72.2.521
Winstanley, C. A., Baunez, C., Theobald, D. E., and Robbins, T. W. (2005). Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur. J. Neurosci. 21, 3107–3116. doi: 10.1111/j.1460-9568.2005.04143.x
Winter, C., Flash, S., Klavir, O., Klein, J., Sohr, R., and Joel, D. (2008). The role of the subthalamic nucleus in “compulsive” behavior in rats. Eur. J. Neurosci. 27, 1902–1911. doi: 10.1111/j.1460-9568.2008.06148.x
Witjas, T., Baunez, C., Henry, J. M., Delfini, M., Regis, J., Cherif, A. A., et al. (2005). Addiction in Parkinson's disease: impact of subthalamic nucleus deep brain stimulation. Mov. Disord. 20, 1052–1055. doi: 10.1002/mds.20501
Wojtecki, L., Nickel, J., Timmermann, L., Maarouf, M., Sudmeyer, M., Schneider, F., et al. (2007). Pathological crying induced by deep brain stimulation. Mov. Disord. 22, 1314–1316. doi: 10.1002/mds.21266
Yoon, H. H., Min, J., Hwang, E., Lee, C. J., Suh, J.-K. F., Hwang, O., et al. (2016). Optogenetic Inhibition of the subthalamic nucleus reduces levodopa-induced dyskinesias in a rat model of Parkinson's disease. Stereotact. Funct. Neurosurg. 94, 41–53. doi: 10.1159/000442891
Yoon, H. H., Park, J. H., Kim, Y. H., Min, J., Hwang, E., Lee, C. J., et al. (2014). Optogenetic inactivation of the subthalamic nucleus improves forelimb akinesia in a rat model of Parkinson disease. Neurosurgery 74, 533–40. doi: 10.1227/NEU.0000000000000297
Yu, C., Cassar, I. R., Sambangi, J., and Grill, W. M. (2020). Frequency-specific optogenetic deep brain stimulation of subthalamic nucleus improves Parkinsonian motor behaviors. J. Neurosci. 40, 4323–4334. doi: 10.1523/JNEUROSCI.3071-19.2020
Zenon, A., Duclos, Y., Carron, R., Witjas, T., Baunez, C., Regis, J., et al. (2016). The human subthalamic nucleus encodes the subjective value of reward and the cost of effort during decision-making. Brain 139, 1830–1843. doi: 10.1093/brain/aww075
Zhang, F., Wang, L.-P., Brauner, M., Liewald, J. F., Kay, K., Watzke, N., et al. (2007). Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639. doi: 10.1038/nature05744
Zhao, X. D., Cao, Y. Q., Liu, H. H., Li, F. Q., You, B. M., and Zhou, X. P. (2009). Long term high frequency stimulation of STN increases dopamine in the corpus striatum of hemiparkinsonian rhesus monkey. Brain Res. 1286, 230–238. doi: 10.1016/j.brainres.2009.06.069
Keywords: deep brain stimulation (DBS), subthalamic nucleus (STN), reward, motivation, rodent, operant
Citation: Vachez YM and Creed MC (2020) Deep Brain Stimulation of the Subthalamic Nucleus Modulates Reward-Related Behavior: A Systematic Review. Front. Hum. Neurosci. 14:578564. doi: 10.3389/fnhum.2020.578564
Received: 30 June 2020; Accepted: 29 October 2020;
Published: 20 November 2020.
Edited by:
Adolfo Ramirez-Zamora, University of Florida Health, United StatesReviewed by:
Michael Beyeler, University of California, Santa Barbara, United StatesHasan Onur Keles, Ankara University, Turkey
Copyright © 2020 Vachez and Creed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meaghan C. Creed, bWVhZ2hhbi5jcmVlZEB3dXN0bC5lZHU=
 Yvan M. Vachez
Yvan M. Vachez Meaghan C. Creed
Meaghan C. Creed