- 1Department of Electronics and Communication Engineering, Rajiv Gandhi University, Arunachal Pradesh, India
- 2Department of Electronics and Communication, Indian Institute of Information Technology, Sri City, India
- 3School of Electronics Engineering, VIT-AP University, Amrawati, India
The electroencephalogram (EEG) serves as an essential tool in exploring brain activity and holds particular importance in the field of mental health research. This review paper examines the application of artificial intelligence (AI), encompassing machine learning (ML) and deep learning (DL), for classifying schizophrenia (SCZ) through EEG. It includes a thorough literature review that addresses the difficulties, methodologies, and discoveries in this field. ML approaches utilize conventional models like Support Vector Machines and Decision Trees, which are interpretable and effective with smaller data sets. In contrast, DL techniques, which use neural networks such as convolutional neural networks (CNNs) and long short-term memory networks (LSTMs), are more adaptable to intricate EEG patterns but require significant data and computational power. Both ML and DL face challenges concerning data quality and ethical issues. This paper underscores the importance of integrating various techniques to enhance schizophrenia diagnosis and highlights AI’s potential role in this process. It also acknowledges the necessity for collaborative and ethically informed approaches in the automated classification of SCZ using AI.
1 Introduction
The electroencephalogram (EEG), commonly known as brain waves, is a crucial tool that shows the electrical activity in the brain (Rangayyan, 2015). It facilitates our comprehension of distinct brain regions such as the cerebrum, cerebellum, brain stem, and thalamus. The cerebrum has two hemispheres and a complex outer layer called the cerebral cortex, composed of intricate neuron arrangements. Below the cortex, nerve fibers project and form connections with other brain regions and the peripheral nervous system. The EEG is generated by cortical potentials resulting from interactions among cell bodies and dendrites of pyramidal neurons (Cooper et al., 2014). The scalp acts as a medium for capturing signals from the brain’s intrinsic processes, cognitive activities, and responses to external stimuli, detected by surface electrodes. Scalp EEG provides a consolidated representation of neural activity across various cerebral areas. In medical environments, multiple EEG channels are concurrently recorded from diverse scalp locations to compare activities in various regions (Hughes, 1983; Nahm et al., 1999). The International Federation of Societies for Electroencephalography and Clinical Neurophysiology recommends the 10–20 system for electrode placement (Cooper et al., 2014), ensuring equal spacing for symmetrical positioning and method for placing electrodes during EEG recordings is presented in Figure 1. For specific cases like monitoring sleep or detecting seizures, a more extended recording with multiple channels might be needed. Specialized techniques, like needle electrodes or recording from an exposed part of the cortex, enhance EEG capabilities (Tatum and William, 2021). Different techniques, such as rest or exposure to stimuli like light or sound, help record the EEG in various states (Sarma and Barma, 2009).
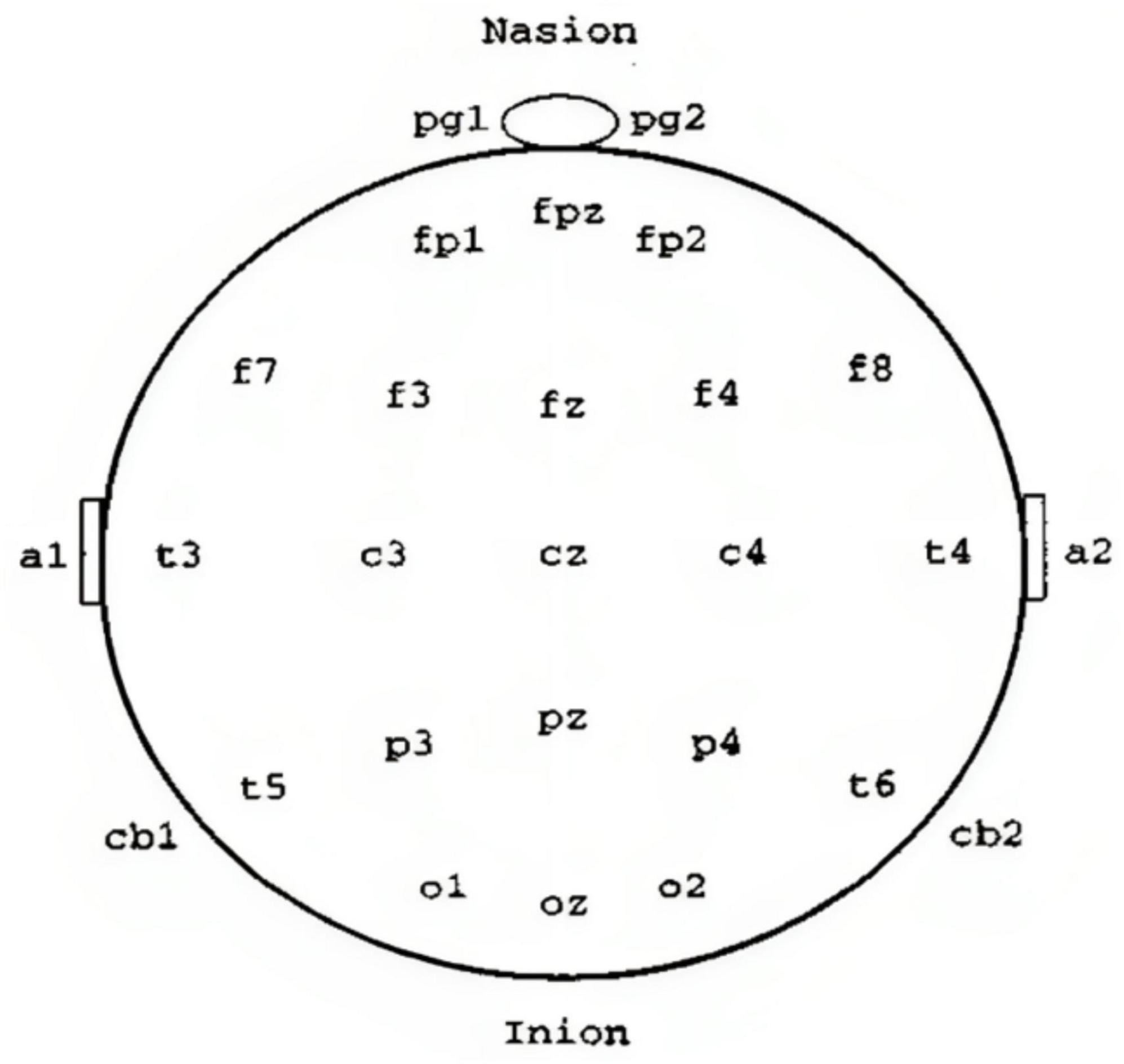
Figure 1. The 10–20 system guides EEG electrode placement (Cooper et al., 2014).
Electroencephalogram signals demonstrate distinct rhythmic or periodic patterns, with frequency bands characterized by specific terms. The terminology for EEG frequency bands includes Delta (δ) with a frequency range of 0.5 to 4 Hz, Theta (θ) spanning from 4 to 8 Hz, Alpha (α) ranging between 8 and 13 Hz, and Beta (β) exceeding 13 Hz (Babiloni et al., 2020). Each band represents a different spectrum of electrical activity in the brain, and these classifications are fundamental in EEG analysis, providing insights into various cognitive states and neurological conditions. EEG rhythms intricately reflect a range of physiological and cognitive processes (Miller, 2007). The alpha rhythm, a prevailing resting pattern in the brain, is frequently observed in relaxed adults, particularly in the occipital area with synchronized activity on both sides (Niedermeyer, 1997). During tasks such as listening or mental arithmetic with closed eyes, strong alpha waves emerge, diminishing when the eyes open in response to visual stimuli. As individuals progress through distinct sleep stages, the alpha wave transitions to slower rhythms. Theta waves appear during the initial stages of sleep, while delta waves become prominent in deeper sleep. High-frequency beta waves characterize background activity in individuals experiencing heightened intensity or anxiety. Any deviation from the anticipated rhythm in a specific state may suggest abnormality (Dang-Vu et al., 2008). For instance, the presence of abnormal slow waves like delta or theta during wakefulness is considered atypical. Abnormal slow waves in corresponding regions can be induced by focal brain injuries or tumors. Additionally, a one-sided depression (left-right asymmetry) in rhythm may indicate disruptions in cortical pathways, while the presence of spikes and sharp waves could signal the existence of epileptogenic regions in specific parts of the brain (Yang et al., 2021).
Electroencephalogram can be used to detect and study various mental health conditions, including epilepsy (Cascella et al., 2009), schizophrenia, bipolar disorder, major depressive disorder (Yasin et al., 2021), ADHD (Lenartowicz and Loo, 2014), anxiety disorders (Tolin et al., 2020), sleep disorders (Peter-Derex et al., 2021), neurodevelopmental disorders (Lau-Zhu et al., 2019), traumatic brain injury (Rapp et al., 2015), and dementia (Custodio et al., 2020). It provides insights into brain activity and abnormalities associated with these conditions, assisting in diagnosis and treatment planning. However, EEG is typically used alongside other clinical assessments for a comprehensive evaluation. Expert interpretation by professionals in neurology or psychiatry is crucial. Timely medication and consultations with doctors can be crucial for saving the lives of patients. However, schizophrenia is a serious and chronic mental health disorder that affects a person’s thinking, emotions, and behavior (Aggernaes, 1994). People with schizophrenia often experience a distorted perception of reality, which can include hallucinations (seeing or hearing things that others don’t), delusions (false beliefs), disorganized thinking, and impaired social functioning. The exact cause of schizophrenia is not known, but it is likely to result from a combination of genetic, biological, and environmental factors (Tandon et al., 2008).
The 11th revision of the International Classification of Diseases (ICD-11), approved by the World Health Assembly, includes a section on “Schizophrenia Spectrum and Other Primary Psychiatric Disorders.” This part of the ICD-11 covers various mental health conditions related to schizophrenia and other primary psychiatric disorders. Schizophrenia is a serious mental illness affecting about 20 million people worldwide (He et al., 2020). Diagnosis is usually based on observed symptoms like hallucinations and disordered speech, along with persistent disengagement from work or social activities. Unlike some physical illnesses, there are no clear biological markers for schizophrenia (Marder and Galderisi, 2017). Brain activity is affected, but other mental illnesses like bipolar disorder or ADHD also influence baseline brain activity (Newson and Thiagarajan, 2019). It’s common for schizophrenia to be confused with other disorders like depression or bipolar disorder, emphasizing the challenge of accurately identifying mental health conditions (Pearlson, 2015). However, a comprehensive diagnosis involves combining EEG findings with clinical assessments, interviews, and possibly other neuroimaging techniques. Trained professionals interpret EEG results within the broader context of an individual’s clinical presentation. Ongoing research aims to enhance our understanding of schizophrenia and improve diagnostic accuracy through neuroimaging methods.
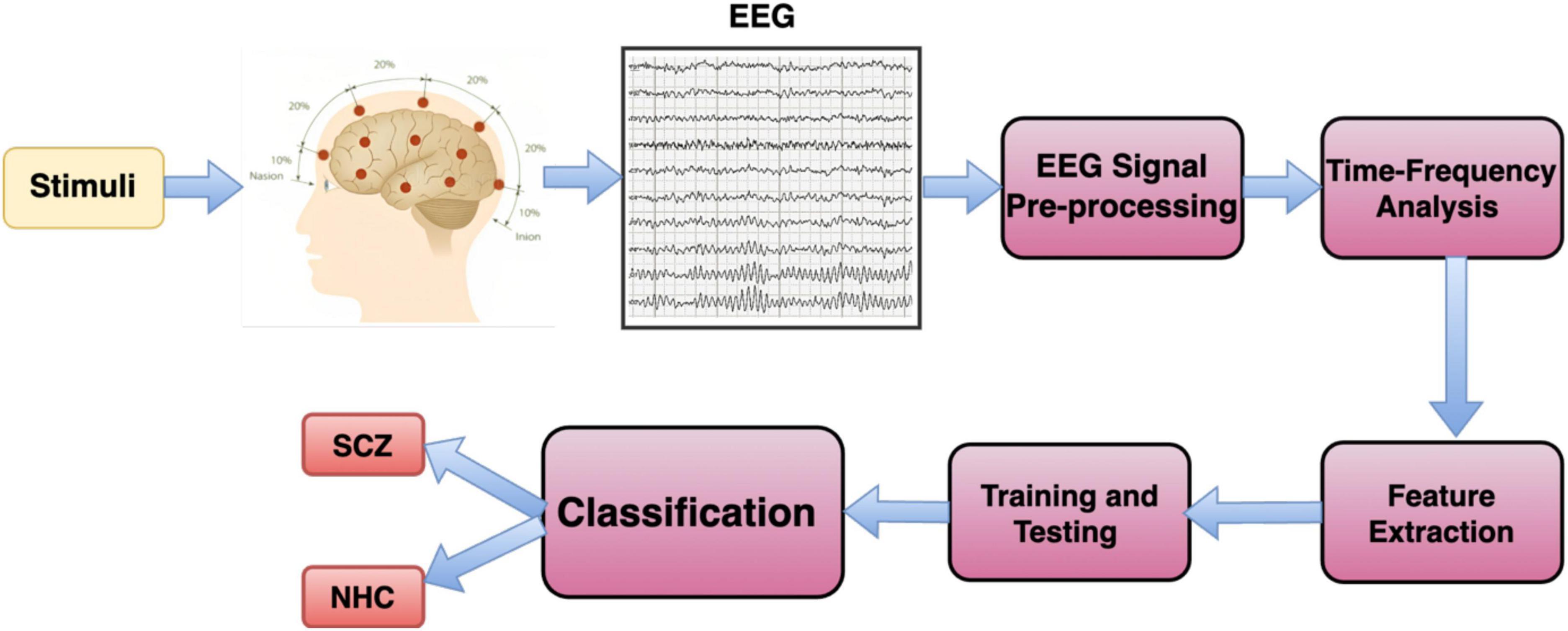
Recently, artificial intelligence (AI) has been used in many areas, including student engagement, virtual reality therapy, text sorting, cybersecurity, detecting and managing diseases, elderly care, analyzing biological data, addressing pandemics, and improving healthcare (Shaffi et al., 2023). In healthcare, AI can help prevent diseases and improve the quality of life. It’s especially useful for accurately diagnosing diseases. AI, particularly machine learning and deep learning, can analyze complex medical data more accurately and quickly, thanks to faster GPUs (graphics processing units) and available datasets (Madiajagan and Sridhar Raj, 2019). A Block diagram for AI-based Schizophrenia classification is illustrated in Figure 2.
AI research in this area involves using both traditional machine learning (ML) and deep learning (DL) methods. The algorithm for diagnosing Schizophrenia (SCZ) using AI includes several steps: preprocessing, feature extraction and selection, and classification. When it comes to diagnosing SCZ through EEG signals, feature extraction is particularly crucial (Sadeghi et al., 2022). In traditional ML, the features extracted from EEG signals are typically divided into four categories: time, frequency, time-frequency, and non-linear fields. These categories help in analyzing different aspects of the EEG signals for a more comprehensive understanding in the diagnosis process. Creating features manually from EEG signals has limitations in uncovering complex characteristics within the data, hindering optimal performance (Khosla et al., 2020). Additionally, selecting effective feature extraction methods for different EEG data structures is challenging, time-consuming, and may not perform well with large datasets, reducing their effectiveness. The deep learning (DL) model demonstrated the capability to manage large datasets, although it required considerably more time for both training and testing in comparison to machine learning (ML) methods (Janiesch et al., 2021).
2 Machine learning vs. deep learning for EEG
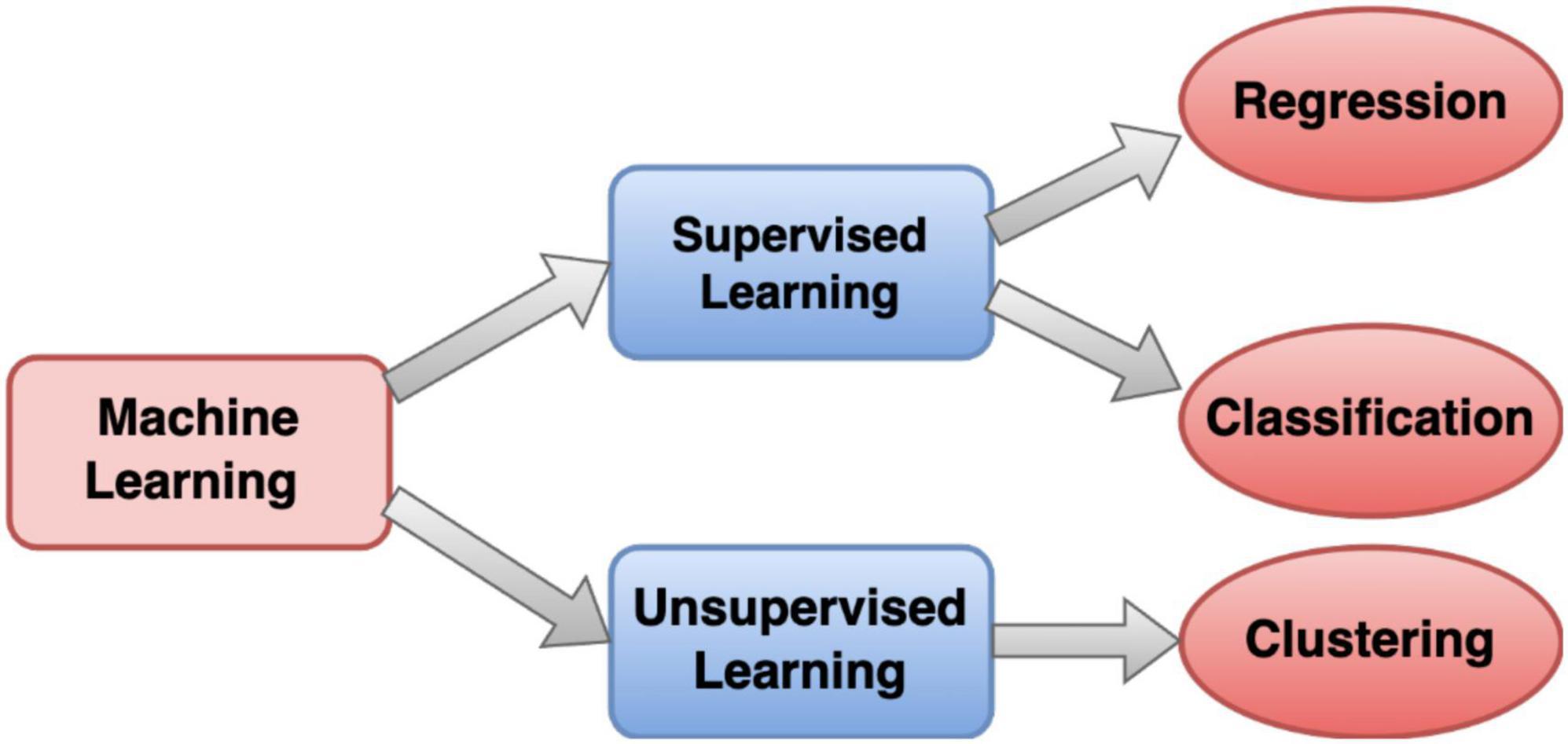
In the realm of EEG analysis using traditional machine learning, a systematic two-step process is employed. Firstly, features are manually extracted from EEG signals, encompassing characteristics like statistical measures or power spectral density. The quality of this feature engineering profoundly impacts the performance of subsequent machine learning models. Common algorithms utilized for EEG classification include Support Vector Machines (SVM), Decision Trees (DT), Random Forests (RF), Logistic Regression (LR), k-Nearest Neighbor (kNN), Naïve Bayes (NB), Boosted tree (BT), Adaboost and other related algorithms (Chen et al., 2014; Rahul et al., 2021; de Miras et al., 2023; Kumar et al., 2023). A pictorial form of machine learning categorization is shown in Figure 3. One notable advantage lies in the interpretability of these models, as the explicitly defined features allow users to comprehend the factors influencing the model’s decisions. Additionally, traditional machine learning models may demonstrate robustness with smaller datasets, making them suitable for scenarios where data availability is limited (Qayyum et al., 2021; Baygin et al., 2023).
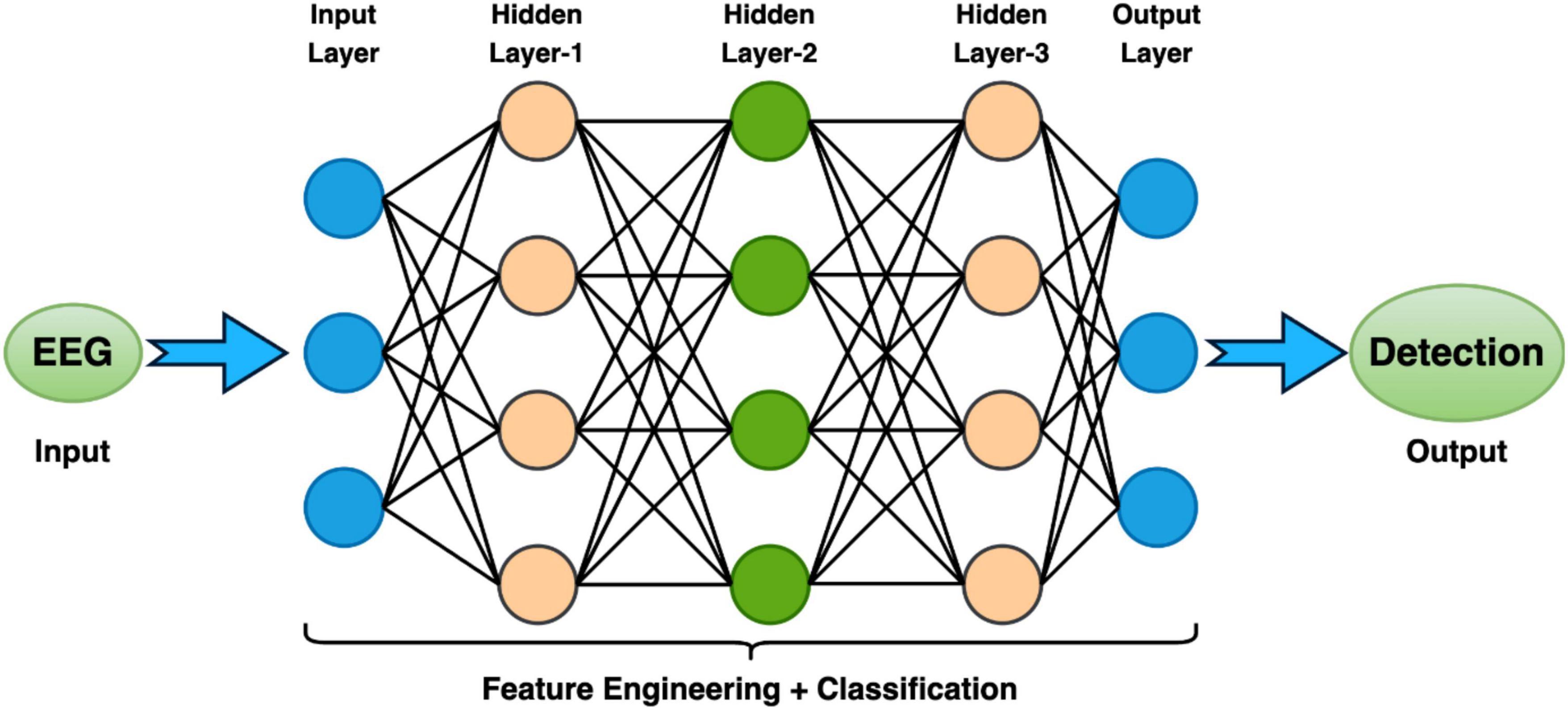
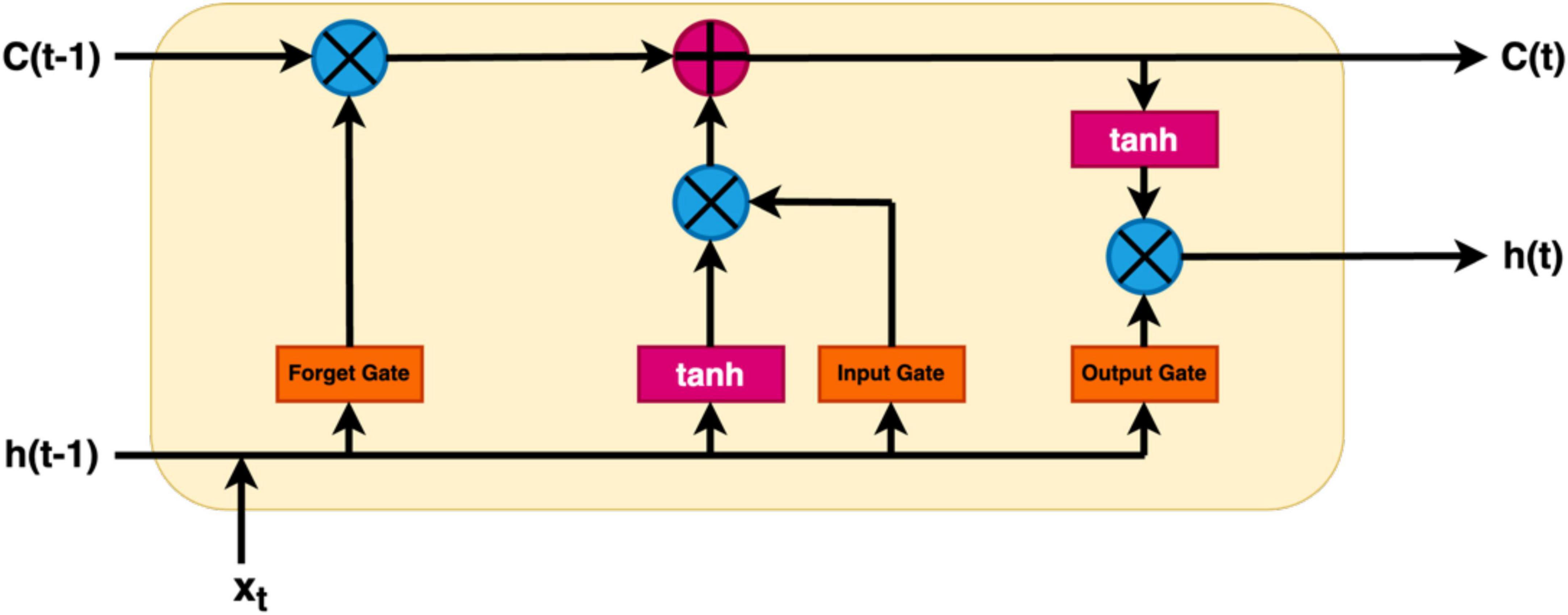
In the context of EEG analysis, deep learning adopts a distinctive approach by training artificial neural networks to directly glean hierarchical representations from raw EEG data. This obviates the need for manual feature extraction, enabling the model to autonomously discern intricate patterns. Notably, Convolutional Neural Networks (CNNs), Deep Neural Network (DNN), Transfer Learning Models (TLM), Deep Belief Network (DBM), Recurrent Neural Networks (RNNs), Long Short-Term Memory (LSTM), Bi-directional-LSTM (Bi-LSTM) and others related model within the deep learning paradigm excel in learning spatial and temporal dependencies from raw EEG signals, making them adept at tasks like image classification and sequential data analysis, respectively (Tan et al., 2017; Merlin Praveena et al., 2022; Rahul and Sharma, 2022; Chaitanya et al., 2023). However, deep learning models often necessitate substantial datasets for effective generalization, and data augmentation techniques may be required to address data scarcity. While these models can achieve high performance, they are often perceived as “black boxes” due to the complexity of the learned representations, posing challenges in understanding their internal workings. Furthermore, the computational demands for training deep learning models, particularly large neural networks, are significant, requiring powerful GPUs. Choosing between deep learning and machine learning for EEG analysis involves careful consideration of several factors. Deep learning is advantageous for handling raw and complex EEG data, where automatic feature learning is beneficial, while machine learning may suffice for datasets with well-defined features (Li et al., 2020). Computational resources play a crucial role, as deep learning, especially with large neural networks, demands substantial computing power. If interpretability is paramount, as in medical applications, machine learning’s transparent nature may be preferred over the perceived nature of deep learning models. The specific EEG analysis task, whether it involves classification, segmentation, or anomaly detection, also influences the choice of methodology. Ultimately, practitioners weigh these factors to determine the most suitable approach based on their data characteristics, available resources, interpretability needs, and analysis objectives. A Deep Learning model consists of an input layer, hidden layers, and an output layer is demonstrated in Figure 4. Additionally, a module of LSTM network is shown in Figure 5.
3 Literature search
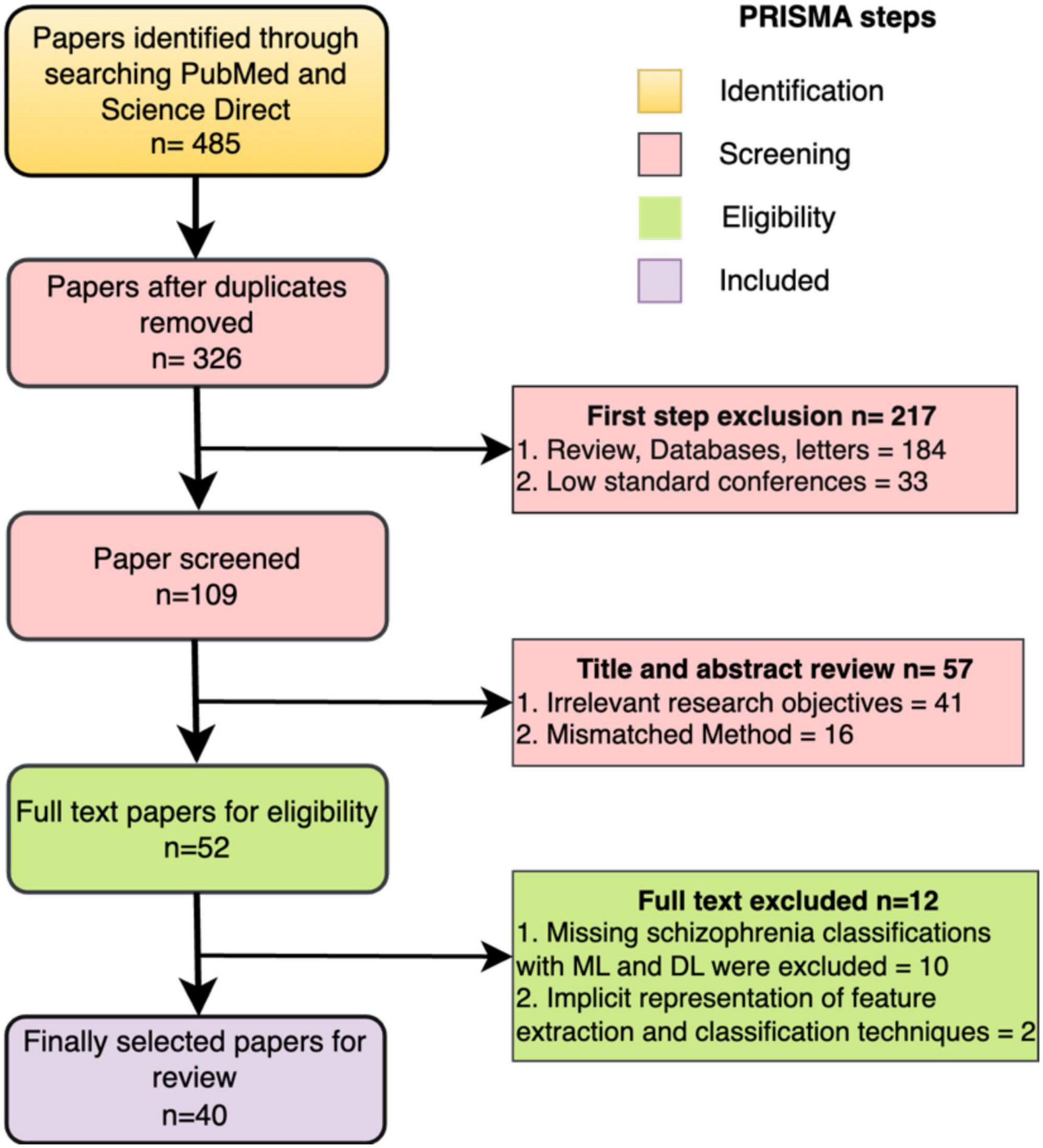
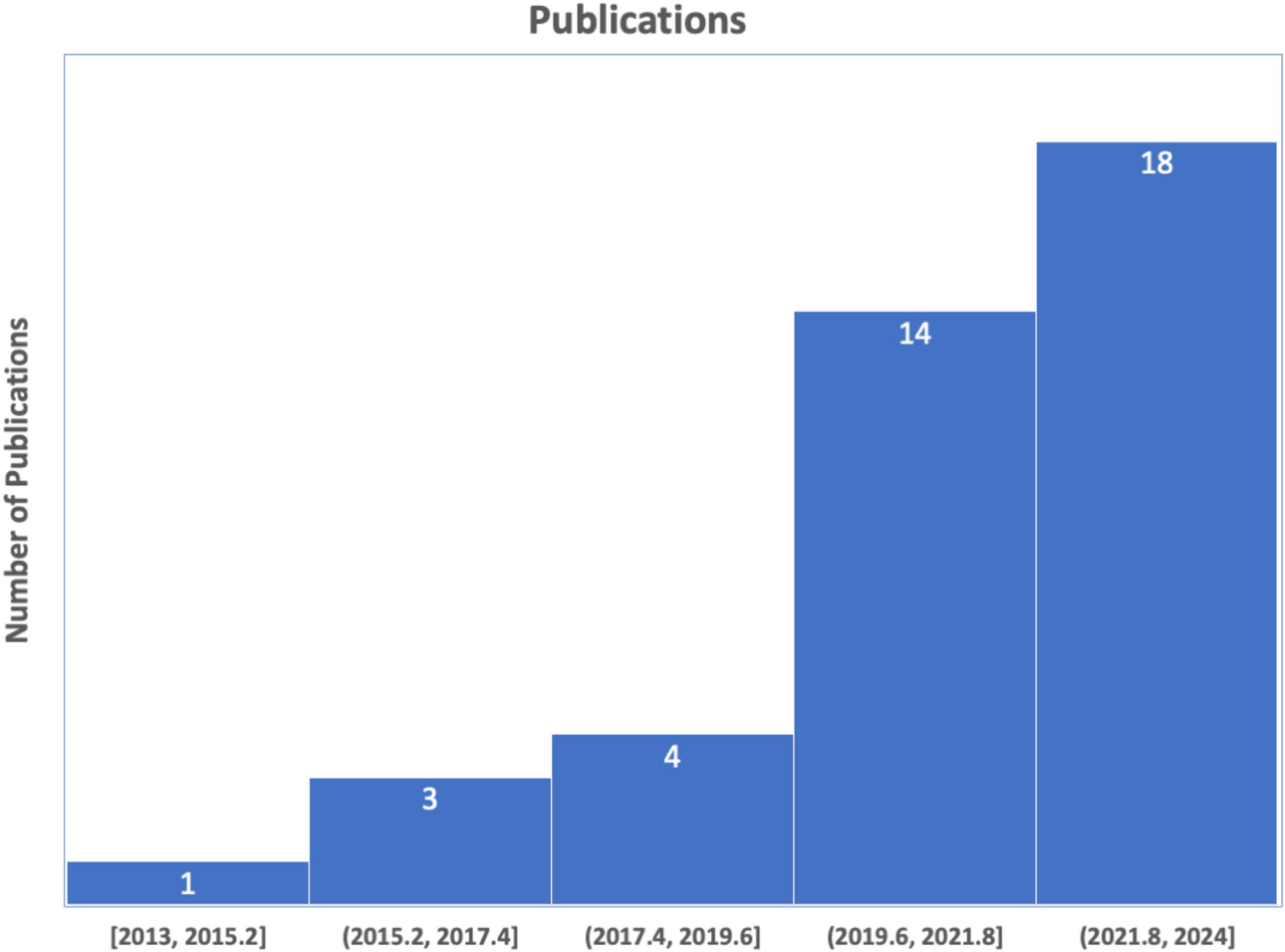
We conducted a comprehensive literature search following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Moher et al., 2009), as illustrated in Figure 6. This search specifically focused on the study concerning the classification of schizophrenia using EEG. We began by carefully articulating the study issue of schizophrenia to generate a targeted route for our investigation. We compiled an exhaustive list of keywords and search terms linked to schizophrenia classification using EEG, including variations to ensure inclusivity. In the initial phase of the identification process, PubMed and Science Direct were searched using the terms “Automatic detection of Schizophrenia,” “Automatic classification of Schizophrenia,” “Automatic classification of Schizophrenia using Machine learning,” and “Automatic classification of Schizophrenia using Deep learning,” covering the years from 2013 to November 2023, yielding a total of 485 articles. The subsequent screening phase involved excluding items not in English, as well as reviews, databases, or letters. Further screening based on each article’s title and abstract identified 57 with unrelated study objectives and unmatched techniques, leaving 52 items for thorough inspection. Additionally, 10 publications were excluded for not containing schizophrenia classifications with ML and DL, and 2 studies lacked clear representations of feature extraction methods or classification algorithms. After identifying, reviewing, and verifying that 40 papers met the inclusion criteria, the procedure was successfully concluded. The studies reviewed were distributed based on their year of publication. The databases utilized in the 40 studies are outlined in Figure 7.
4 Schizophrenia classification using machine learning
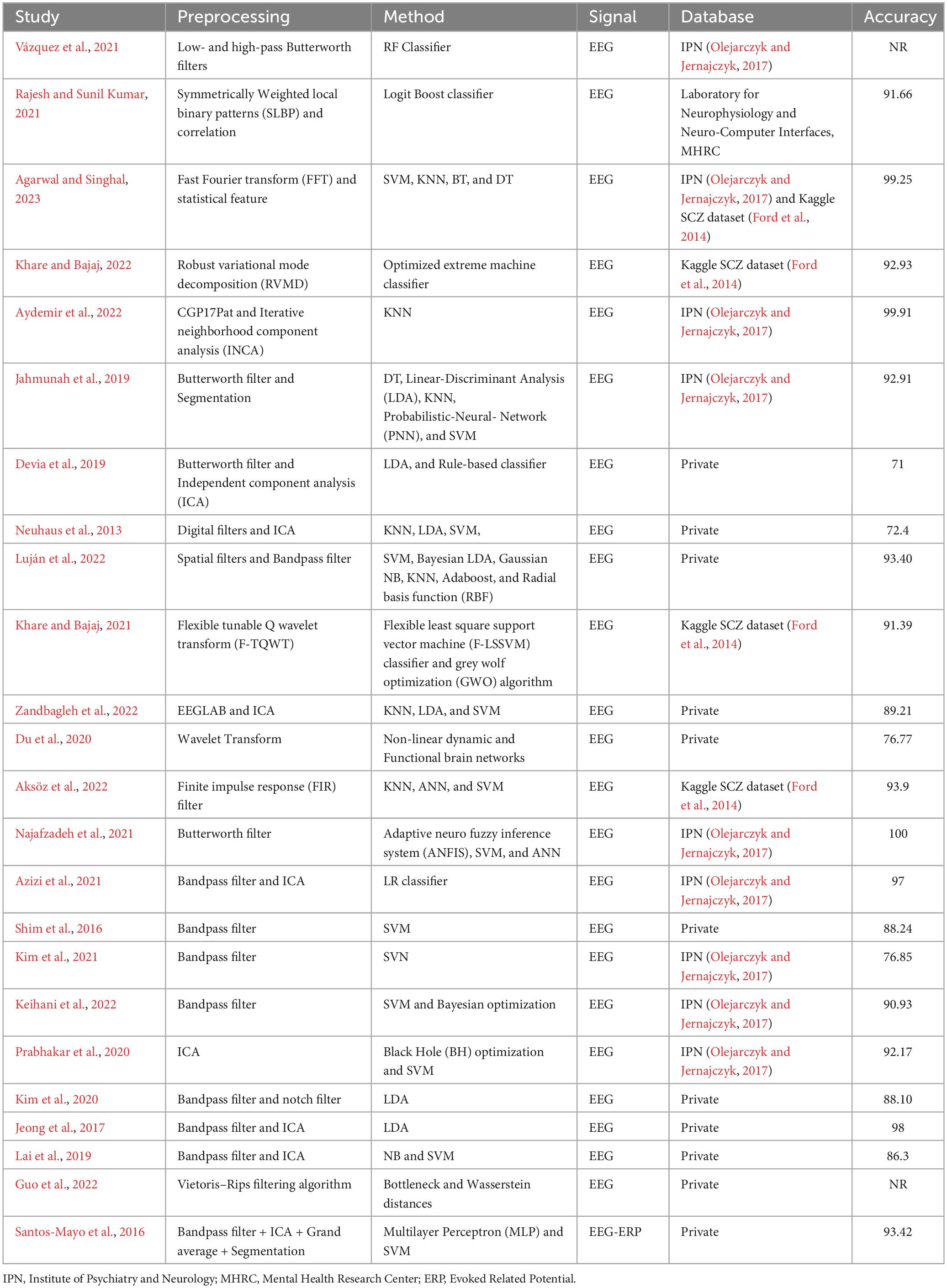
Detecting schizophrenia using machine learning (ML) is a promising area of research with significant potential for improving early diagnosis and intervention. ML techniques can analyze various data sources, including biomedical signals, to identify patterns and markers associated with schizophrenia. In this study, the details of papers reviewed are summarized in Table 1, where out of 40 papers, 24 specifically addressed Schizophrenia classification using machine learning.
The Table 1 presents a diverse array of methodologies applied in EEG signal analysis, offering insights into various preprocessing techniques and classification methods. Notably, reference (Agarwal and Singhal, 2023) employs a robust strategy that integrates FFT and statistical features with SVM, KNN, BT, and DT classifiers, achieving an impressive accuracy of 99.25% across both the IPN and Kaggle SCZ datasets. Conversely, reference (Devia et al., 2019) reports a lower accuracy of 71% on private EEG dataset, attributed to the use of a Butterworth filter and ICA in conjunction with a small dataset. The integration of spatial filters and a bandpass filter in reference (Luján et al., 2022), utilizing six different classifiers where RBF stands out, results in a notable accuracy of 93.40% on private data. The method described in Khare and Bajaj (2022), which uses RVMD for preprocessing and optimized extreme machine learning on the Kaggle SCZ dataset, achieved lower accuracy but outperformed (Devia et al., 2019). The approach proposed in Aydemir et al. (2022), employing CGP17Pat for feature extraction and KNN for classification, obtained a very high accuracy of 99.91%, though it did not surpass the results achieved by Najafzadeh et al. (2021). These studies employ diverse databases, including IPN, Kaggle SCZ, and private datasets, showcasing the adaptability of methods across various contexts. Various preprocessing techniques, such as wavelet transform (Du et al., 2020), FIR filter (Aksöz et al., 2022), and adaptive neuro-fuzzy inference system (ANFIS) (Najafzadeh et al., 2021), highlight the richness and diversity of approaches in EEG signal analysis. Noteworthy is the study in Najafzadeh et al. (2021), which achieves a perfect accuracy of 100% using a Butterworth filter alongside ANFIS, SVM, and ANN. The methods in Shim et al. (2016); (Jeong et al., 2017; Lai et al., 2019; Kim et al., 2020, 2021; Azizi et al., 2021; Keihani et al., 2022), and (Santos-Mayo et al., 2016), which use a bandpass filter in combination with other preprocessing techniques, fail to achieve the impressive accuracy seen in Aydemir et al. (2022); (Agarwal and Singhal, 2023), and (Najafzadeh et al., 2021). The studies in Vázquez et al. (2021) and (Guo et al., 2022), employing Butterworth and Vietoris–Rips filtering for preprocessing and RF classifier and Bottleneck and Wasserstein distances, respectively, for classification of SCZ, did not report their results in terms of accuracy. The methods in Jahmunah et al. (2019); (Khare and Bajaj, 2021; Rajesh and Sunil Kumar, 2021; Luján et al., 2022; Zandbagleh et al., 2022), and (Aksöz et al., 2022) achieved moderate accuracy with different preprocessing techniques. However, the approaches in Neuhaus et al. (2013) and (Du et al., 2020) secure lower accuracies but still surpass the performance of Devia et al. (2019). The reported accuracies highlight the significant role of preprocessing and classification choices in EEG signal processing effectiveness. These studies underscore the need for context-specific, tailored approaches and the importance of balancing preprocessing and classification methods for optimal results.
5 Schizophrenia classification using deep learning
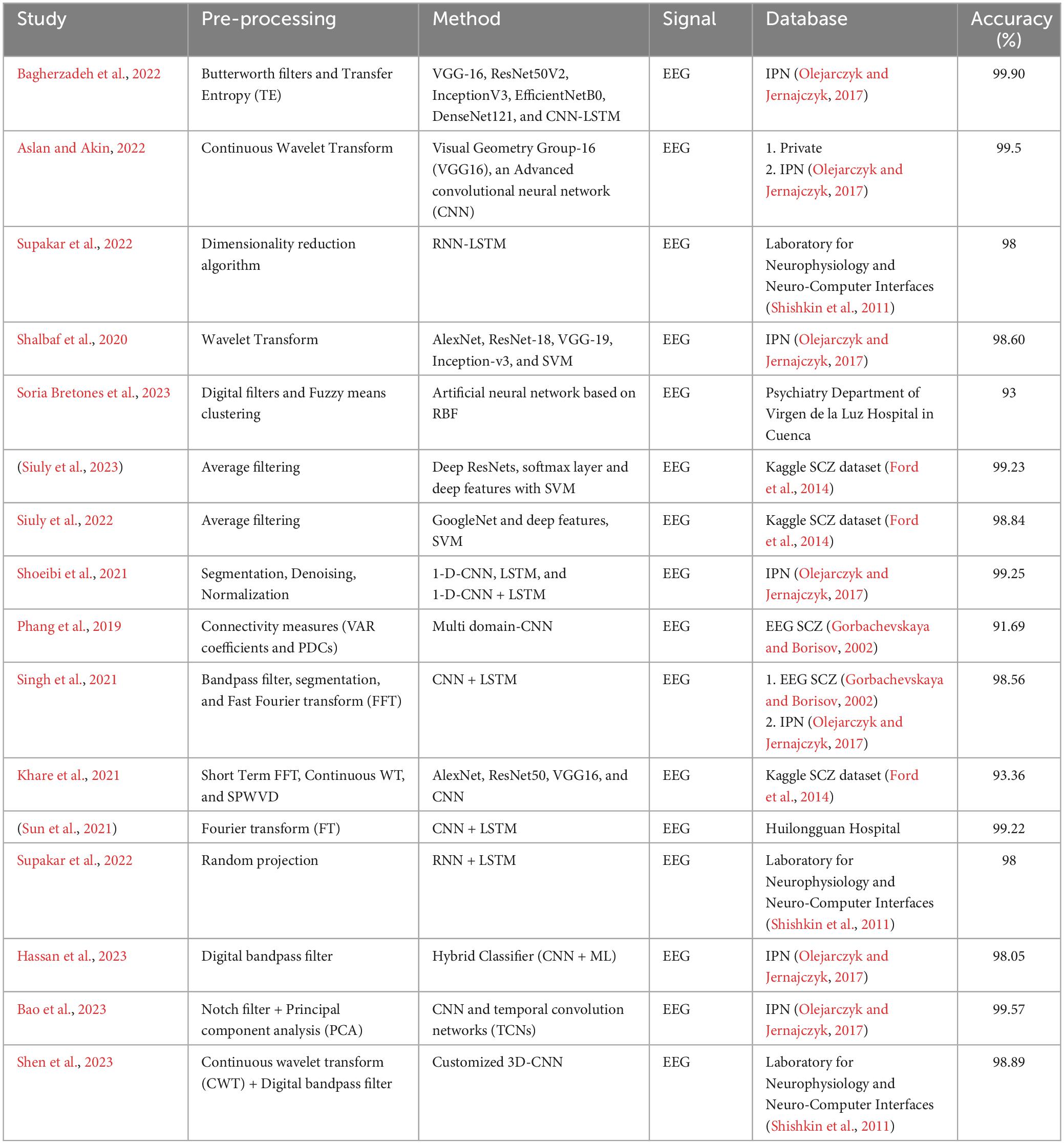
The deployment of these sophisticated neural networks enables the creation of a dynamic and adaptive system that can discern subtle and complex patterns indicative of schizophrenia. This process is vital in enhancing the accuracy of detection, as it allows the model to uncover intricate relationships and features that might be challenging for traditional methods to identify. The amalgamation of neural network capabilities with deep learning principles positions these models as powerful tools in the quest for more effective and nuanced approaches to schizophrenia detection. In this section, the details of the papers reviewed are summarized in Table 2, where out of 40 papers, 16 specifically addressed schizophrenia classification using deep learning.
Table 2 provides a comprehensive summary of various studies that have used deep learning methods for the detection of SCZ using EEG. The method presented in Bagherzadeh et al. (2022) employ Butterworth filters and Transfer Entropy (TE) in conjunction with various deep learning architectures, including VGG-16, ResNet50V2, InceptionV3, EfficientNetB0, DenseNet121, and CNN-LSTM, achieving an impressive accuracy of 99.90% on EEG signals from the IPN database. Aslan and Akin (2022) employs CWT and advanced CNN and VGG16, achieving a high accuracy of 99.5% on both private and IPN EEG data. Supakar et al. (2022) employ a dimensionality reduction algorithm in conjunction with RNN-LSTM, achieving an accuracy of 98% on Laboratory for Neurophysiology and Neuro-Computer Interfaces dataset. Shalbaf et al. (2020) utilize Wavelet Transform with AlexNet, ResNet-18, VGG-19, Inception-v3, and SVM, achieving an accuracy of 98.60% on the IPN dataset. Soria Bretones et al. (2023) introduce digital filters and fuzzy means clustering combined with an artificial neural network based on radial basis function (RBF), achieving an accuracy of 93% on EEG data from the Psychiatry Department of Virgen de la Luz Hospital in Cuenca. Siuly et al. (2023) and (Siuly et al., 2022) apply average filtering with different deep learning architectures, such as deep ResNets, GoogleNet, and SVM, achieving high accuracies of 99.23% and 98.84%, respectively, on the Kaggle SCZ dataset. Shoeibi et al. (2021) employ segmentation, denoising, and normalization techniques with 1-D-CNN, LSTM, and 1-D-CNN + LSTM, achieving an accuracy of 99.25% on EEG signals from the IPN database. Phang et al. (2019) utilize connectivity measures, specifically VAR coefficients and PDCs, with a multi-domain CNN, achieving an accuracy of 91.69% on EEG SCZ data. Singh et al. (2021) combine bandpass filtering, segmentation, and FFT with CNN + LSTM, achieving an accuracy of 98.56% on EEG data from both EEG SCZ and IPN databases. Lastly (Khare et al., 2021) apply STFT, CWT, and SPWVD with various CNN architectures (AlexNet, ResNet50, VGG16), achieving an accuracy of 93.36% on the Kaggle SCZ dataset. Sun et al. (2021) achieved 99.22% accuracy using Fourier Transform with CNN + LSTM, while (Supakar et al., 2022) reported 98% with Random Projection and RNN-LSTM. Hassan et al. (2023) combined a Digital Bandpass Filter with a CNN + ML hybrid, reaching 98.05% accuracy. Notably, (Bao et al., 2023) attained the highest accuracy of 99.57% using Notch Filter, PCA, CNN, and TCNs. Shen et al. (2023) employed Continuous Wavelet Transform and a 3D-CNN, achieving 98.89%. The highest accuracy was reported by Bagherzadeh et al. (2022), achieving an impressive 99.90% and the other hand, the lowest accuracy was noted in the study (Phang et al., 2019), with an accuracy of 91.69%. These varied accuracies highlight the influence of specific preprocessing techniques and neural network architectures on the effectiveness of detecting schizophrenia in EEG.
6 EEG datasets for schizophrenia detection
Electroencephalogram signals can be readily obtained from high-quality open datasets accessible to the public. These datasets, which are easily obtainable, open, and shareable, significantly aid independent researchers in developing new algorithms or findings and conducting performance comparisons in studies related to Schizophrenia diagnosis. There are multiple EEG datasets specifically designed for predicting SCZ. However, ethical concerns prevent most of this data from being shared in the public domain, limiting the reproducibility of related works. Some private datasets, including those used in studies (Neuhaus et al., 2013; Santos-Mayo et al., 2016; Shim et al., 2016; Jeong et al., 2017; Devia et al., 2019; Lai et al., 2019; Du et al., 2020; Kim et al., 2020; Aslan and Akin, 2022; Bagherzadeh et al., 2022; Guo et al., 2022; Luján et al., 2022; Zandbagleh et al., 2022) utilized experimental EEG datasets where researchers devised experimental paradigms. Participants provided written informed consent, and approval was obtained from the Institutional Review Board (IRB). The scarcity of publicly available datasets poses a major challenge for researchers exploring diseases and conducting disease prediction and medical pattern detection tasks. Nevertheless, in recent years, several EEG datasets for SCZ diagnosis have become accessible in an open environment, gaining significant attention. In this research domain, there are a few popular publicly available datasets for binary classification of SCZ. The initial dataset is sourced from the Institute of Psychiatry and Neurology in Warsaw, Poland, and is openly accessible in the RepOD dataset (Olejarczyk and Jernajczyk, 2017). The second dataset originates from the Neurophysiology and Neuro-Computer Interfaces laboratory at the Mental Health Research Center (MHRC), Russia, and is publicly available. The third and less-explored SCZ EEG dataset is collected under a project of the National Institute of Mental Health (NIMH; R01MH058262) and is publicly available on the Kaggle platform (Ford et al., 2014).
7 Challenges in classification of schizophrenia using ML and DL
Machine learning (ML) faces challenges in classifying schizophrenia due to diverse and limited datasets, hindering the development of generalized models. The complexity of schizophrenia symptoms makes it hard to choose relevant features and interpret model results, affecting our understanding of clinical significance. Imbalanced datasets, ethical concerns, and the need for collaboration between machine learning experts and clinicians further complicate building accurate and ethical classification models. Overcoming these challenges requires improving data quality, fostering collaboration, and addressing ethical considerations to integrate machine learning effectively into clinical practices for schizophrenia diagnosis. Traditional ML distinguishes itself through a multi-stage approach involving pre-processing, feature selection, and extraction. In ML, the need for predefined feature engineering is prevalent, and the models are characterized by task-specific features (Cortes-Briones et al., 2022). This approach may struggle with adaptability to various data types or applications, and the learning process often lacks autonomy. While ML provides a structured and interpretable framework, its reliance on explicit feature engineering can limit its ability to handle complex relationships within the data. Despite these limitations, ML remains a valuable tool in various domains, contributing insights and predictions based on well-defined features and structured algorithms (Bzdok and Meyer-Lindenberg, 2018).
Several challenges are associated with deep learning (DL) models. One significant hurdle is the substantial need for extensive training data to construct effective DL models. Transfer learning, a strategy leveraging data from related tasks, can partially address this issue, improving the model’s performance. However, it does not entirely replace the requirement for original data (Sharma et al., 2023). Another challenge involves dealing with unbalanced data, which is common in biological datasets where negative samples often outnumber positive ones. Training DL models on skewed data may lead to unexpected outcomes, and the impact of imbalanced data on model performance has been extensively studied (Johnson and Khoshgoftaar, 2019). Additionally, uncertainty scaling is crucial in healthcare applications to assess the accuracy of ML and DL-based diagnoses, preventing overconfident predictions. Catastrophic forgetting is another issue, occurring when new information disrupts previously learned knowledge in simple DL models. To mitigate this problem, training a new model from scratch with both old and new data is a recommended solution. DL models also face the risk of overfitting during training due to numerous interrelated parameters, impairing their overall effectiveness. Inadequate training data further contributes to overfitting, causing the learned distribution to deviate from the true distribution. Additionally, the vanishing gradient problem arises in DL, especially during backpropagation, when weights may not update effectively, leading to termination of the neural network training process (Li et al., 2019).
The key highlights of this study are summarized as follows:
1. This study provides a detailed review of DL and ML methodologies applied in the detection of SCZ.
2. This study evaluates the shortcomings of current DL and ML methodologies, proposing possible solutions.
3. This study offers a comprehensive analysis of all relevant parameters from existing studies that use DL and ML techniques, specifically within the comparison and discussion sections.
4. It also explores the future of integrating EEG and neuroimaging data to diagnose SCZ through AI algorithms.
8 Conclusion
This paper finds into the application of artificial intelligence, specifically machine learning (ML) and deep learning (DL), in the classification of SCZ using EEG signals. The review highlights that traditional ML models like Support Vector Machines and Decision Trees are interpretable and robust with smaller datasets, while DL methods using neural networks demand more data and computational resources but adapt well to complex EEG patterns. The review encompasses a thorough examination of methodologies, challenges, and findings in this realm. Both approaches face challenges in data quality, interpretability, and ethics. The diverse techniques highlighted in reviewed studies emphasize the need for a balanced, context-specific approach. While AI holds promise for advancing SCZ diagnosis, interdisciplinary collaboration and ethical considerations are crucial for its effective integration into mental health.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JR: Investigation, Writing−original draft. DS: Data curation, Visualization, Writing−original draft. LS: Conceptualization, Formal Analysis, Supervision, Validation, Writing−review and editing. UN: Funding acquisition, Resources, Supervision, Writing−review and editing. AS: Project administration, Supervision, Writing−review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, M., and Singhal, A. (2023). Fusion of pattern-based and statistical features for Schizophrenia detection from EEG signals. Med. Eng. Phys. 112:103949. doi: 10.1016/j.medengphy.2023.103949
Aksöz, A., Akyüz, D., Bayir, F., Yildiz, N. C., Orhanbulucu, F., Latifoğlu, F., et al. (2022). Analysis and classification of schizophrenia using event related potential signals. Comput. Sci. 2022, 32–36. doi: 10.1186/s40345-022-00258-4
Aslan, Z., and Akin, M. (2022). A deep learning approach in automated detection of schizophrenia using scalogram images of EEG signals. Phys. Eng. Sci. Med. 45, 83–96. doi: 10.1007/s13246-021-01083-2
Aydemir, E., Dogan, S., Baygin, M., Ooi, C., Barua, P., Tuncer, T., et al. (2022). CGP17Pat: Automated schizophrenia detection based on a cyclic group of prime order patterns using EEG Signals. Healthcare 10:643. doi: 10.3390/healthcare10040643
Azizi, S., Hier, D., and Wunsch, D. (2021). Schizophrenia classification using resting state EEG functional connectivity: Source level outperforms sensor level. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 1770–1773. doi: 10.1109/EMBC46164.2021.9630713
Babiloni, C., Barry, R., Başar, E., Blinowska, K., Cichocki, A., Drinkenburg, W., et al. (2020). International Federation of Clinical Neurophysiology (IFCN) - EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin. Neurophysiol. 131, 285–307. doi: 10.1016/j.clinph.2019.06.234
Bagherzadeh, S., Shahabi, M., and Shalbaf, A. (2022). Detection of schizophrenia using hybrid of deep learning and brain effective connectivity image from electroencephalogram signal. Comput. Biol. Med. 146:105570. doi: 10.1016/j.compbiomed.2022.105570
Bao, X., Wang, S., Zheng, L., Housden, R., Hajnal, J., and Rhode, K. A. (2023). Novel Ultrasound Robot with Force/torque Measurement and Control for Safe and Efficient Scanning. IEEE Trans. Instrum. Meas. 72, 1–12. doi: 10.1109/TIM.2023.3239925
Baygin, M., Barua, P., Chakraborty, S., Tuncer, I., Dogan, S., Palmer, E., et al. (2023). CCPNet136: automated detection of schizophrenia using carbon chain pattern and iterative TQWT technique with EEG signals. Physiol. Meas. 44, acb03c. doi: 10.1088/1361-6579/acb03c
Bzdok, D., and Meyer-Lindenberg, A. (2018). Machine learning for precision psychiatry: opportunities and challenges. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 223–230. doi: 10.1016/j.bpsc.2017.11.007
Cascella, N., Schretlen, D., and Sawa, A. (2009). Schizophrenia and epilepsy: is there a shared susceptibility? Neurosci. Res. 63, 227–235. doi: 10.1016/j.neures.2009.01.002
Chaitanya, M., Sharma, L., Rahul, J., Sharma, D., and Roy, A. (2023). Artificial intelligence based approach for categorization of COVID-19 ECG images in presence of other cardiovascular disorders. Biomed. Phys. Eng. Express. 9, acbd53. doi: 10.1088/2057-1976/acbd53
Chen, W., Wang, Y., Cao, G., Chen, G., and Gu, Q. (2014). A random forest model based classification scheme for neonatal amplitude-integrated EEG. Biomed. Eng. Online 13 Suppl 2, S4. doi: 10.1186/1475-925X-13-S2-S4
Cortes-Briones, J., Tapia-Rivas, N., D’Souza, D., and Estevez, P. (2022). Going deep into schizophrenia with artificial intelligence. Schizophr. Res. 245, 122–140. doi: 10.1016/j.schres.2021.05.018
Custodio, N., Duque, L., Montesinos, R., Alva-Diaz, C., Mellado, M., and Slachevsky, A. (2020). Systematic review of the diagnostic validity of brief cognitive screenings for early dementia detection in spanish-speaking adults in Latin America. Front. Aging Neurosci. 12:270. doi: 10.3389/fnagi.2020.00270
Dang-Vu, T. T., Schabus, M., Desseilles, M., Albouy, G., Boly, M., Darsaud, A., et al. (2008). Spontaneous neural activity during human slow wave sleep. Proc. Natl. Acad. Sci. U. S. A. 105, 15160–15165. doi: 10.1073/pnas.0801819105
de Miras, J. R., Ibáñez-Molina, A. J., Soriano, M. F., and Iglesias-Parro, S. (2023). Schizophrenia classification using machine learning on resting state EEG signal. Biomed. Signal Process. Control 79:104233.
Devia, C., Mayol-Troncoso, R., Parrini, J., Orellana, G., Ruiz, A., Maldonado, P., et al. (2019). EEG classification during scene free-viewing for schizophrenia detection. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 1193–1199. doi: 10.1109/TNSRE.2019.2913799
Du, X., Li, J., Xiong, D., Pan, Z., Wu, F., Ning, Y., et al. (2020). [Research on electroencephalogram specifics in patients with schizophrenia under cognitive load]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 37, 45–53. doi: 10.7507/1001-5515.201810007
Ford, J., Palzes, V., Roach, B., and Mathalon, D. (2014). Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr. Bull. 40, 804–812. doi: 10.1093/schbul/sbt072
Gorbachevskaya, N. N., and Borisov, S. (2002). EEG data of healthy adolescents and adolescents with symptoms of schizophrenia. Avaliable online at: http://brain.bio.msu.ru/eeg_schizophrenia.htm
Guo, G., Zhao, Y., Liu, C., Fu, Y., Xi, X., Jin, L., et al. (2022). Method for persistent topological features extraction of schizophrenia patients’ electroencephalography signal based on persistent homology. Front. Comput. Neurosci. 16:1024205. doi: 10.3389/fncom.2022.1024205
Hassan, F., Hussain, S. F., and Qaisar, S. M. (2023). Fusion of multivariate EEG signals for schizophrenia detection using CNN and machine learning techniques. Inf. Fus. 92, 466–478.
He, H., Liu, Q., Li, N., Guo, L., Gao, F., Bai, L., et al. (2020). Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: results from the Global Burden of Disease Study 2017. Epidemiol. Psychiatr. Sci. 29, e91. doi: 10.1017/S2045796019000891
Hughes, J. R. (1983). A review of the positive spike phenomenon: recent studies. EEG Evok. Pot. Psychiatry Behav. Neurol. 1983, 295–324.
Jahmunah, V., Lih, O. S., Rajinikanth, V., Ciaccio, E., Hao Cheong, K., and Arunkumar, N. (2019). Automated detection of schizophrenia using nonlinear signal processing methods. Artif. Intell. Med. 100:101698. doi: 10.1016/j.artmed.2019.07.006
Janiesch, C., Zschech, P., and Heinrich, K. (2021). Machine learning and deep learning. Electron. Markets 31, 685–695.
Jeong, J., Wendimagegn, T., Chang, E., Chun, Y., Park, J., Kim, H., et al. (2017). Classifying schizotypy using an audiovisual emotion perception test and scalp electroencephalography. Front. Hum. Neurosci. 11:450. doi: 10.3389/fnhum.2017.00450
Johnson, J. M., and Khoshgoftaar, T. M. (2019). Survey on deep learning with class imbalance. J. Big Data 6, 1–54.
Keihani, A., Sajadi, S., Hasani, M., and Ferrarelli, F. (2022). Bayesian optimization of machine learning classification of resting-state EEG microstates in schizophrenia: a proof-of-concept preliminary study based on secondary analysis. Brain Sci. 12:1497. doi: 10.3390/brainsci12111497
Khare, S. K., Bajaj, V., and Acharya, U. R. (2021). SPWVD-CNN for automated detection of schizophrenia patients using EEG signals. IEEE Trans. Instrument. Measure. 70, 1–9.
Khare, S., and Bajaj, V. (2021). A self-learned decomposition and classification model for schizophrenia diagnosis. Comput. Methods Programs Biomed. 211:106450. doi: 10.1016/j.cmpb.2021.106450
Khare, S., and Bajaj, V. (2022). A hybrid decision support system for automatic detection of Schizophrenia using EEG signals. Comput. Biol. Med. 141:105028. doi: 10.1016/j.compbiomed.2021.105028
Khosla, A., Khandnor, P., and Chand, T. (2020). A comparative analysis of signal processing and classification methods for different applications based on EEG signals. Biocybern. Biomed. Eng. 2, 649–690.
Kim, J., Lee, H., and Lee, S. (2020). EEG source network for the diagnosis of schizophrenia and the identification of subtypes based on symptom severity-a machine learning approach. J. Clin. Med. 9:3934. doi: 10.3390/jcm9123934
Kim, K., Duc, N., Choi, M., and Lee, B. (2021). EEG microstate features for schizophrenia classification. PLoS One 16:e0251842. doi: 10.1371/journal.pone.0251842
Kumar, T. S., Rajesh, K. N. V., Maheswari, S., Kanhangad, V., and Acharya, U. R. (2023). Automated Schizophrenia detection using local descriptors with EEG signals. Eng. Applic. Artif. Intell. 117:105602.
Lai, H., Feng, J., Wang, Y., Deng, W., Zeng, J., Li, T., et al. (2019). [Resting-state electroencephalogram classification of patients with schizophrenia or depression]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 36, 916–923. doi: 10.7507/1001-5515.201812041
Lau-Zhu, A., Lau, M., and McLoughlin, G. (2019). Mobile EEG in research on neurodevelopmental disorders: Opportunities and challenges. Dev. Cogn. Neurosci. 36:100635. doi: 10.1016/j.dcn.2019.100635
Lenartowicz, A., and Loo, S. K. (2014). Use of EEG to diagnose ADHD. Curr. Psychiatry Rep. 16:498. doi: 10.1007/s11920-014-0498-0
Li, G., Ha Lee, C., Jung, J. J., Youn, Y. C., and Camacho, D. (2020). Deep learning for EEG data analytics: A survey. Concurr. Comput. 18, e5199.
Li, Y., Huang, C., Ding, L., Li, Z., Pan, Y., and Gao, X. (2019). Deep learning in bioinformatics: Introduction, application, and perspective in the big data era. Methods 166, 4–21. doi: 10.1016/j.ymeth.2019.04.008
Luján, M., Mateo Sotos, J., Torres, A., Santos, J., Quevedo, O., and Borja, A. (2022). Mental disorder diagnosis from EEG signals employing automated leaning procedures based on radial basis functions. J. Med. Biol. Eng. 42, 853–859. doi: 10.1007/s40846-022-00758-9
Madiajagan, M., and Sridhar Raj, S. (2019). Parallel computing, graphics processing unit (GPU) and new hardware for deep learning in computational intelligence research. Deep learning and parallel computing environment for bioengineering systems. London: Academic Press.
Marder, S., and Galderisi, S. (2017). The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16, 14–24. doi: 10.1002/wps.20385
Merlin Praveena, D., Angelin Sarah, D., and George, S. T. (2022). Deep learning techniques for EEG signal applications–a review. IETE J. Res. 68, 3030–3037.
Miller, R. (2007). Theory of the normal waking EEG: from single neurones to waveforms in the alpha, beta and gamma frequency ranges. Int. J. Psychophysiol. 64, 18–23. doi: 10.1016/j.ijpsycho.2006.07.009
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi: 10.1136/bmj.b2535
Nahm, W., Stockmanns, G., Petersen, J., Gehring, H., Konecny, E., Kochs, H., et al. (1999). Concept for an intelligent anaesthesia EEG monitor. Med. Inform. Internet Med. 24, 1–9. doi: 10.1080/146392399298492
Najafzadeh, H., Esmaeili, M., Farhang, S., Sarbaz, Y., and Rasta, S. (2021). Automatic classification of schizophrenia patients using resting-state EEG signals. Phys. Eng. Sci. Med. 44, 855–870. doi: 10.1007/s13246-021-01038-7
Neuhaus, A., Popescu, F., Bates, J., Goldberg, T., and Malhotra, A. (2013). Single-subject classification of schizophrenia using event-related potentials obtained during auditory and visual oddball paradigms. Eur Arch Psychiatry Clin Neurosci. 263, 241–247. doi: 10.1007/s00406-012-0326-7
Newson, J., and Thiagarajan, T. C. (2019). EEG frequency bands in psychiatric disorders: A review of resting state studies. Front Hum Neurosci. 12:521. doi: 10.3389/fnhum.2018.00521
Niedermeyer, E. (1997). Alpha rhythms as physiological and abnormal phenomena. Int. J. Psychophysiol. 26, 31–49. doi: 10.1016/s0167-8760(97)00754-x
Olejarczyk, E., and Jernajczyk, W. (2017). Graph-based analysis of brain connectivity in schizophrenia. PLoS One 12:e0188629. doi: 10.1371/journal.pone.0188629
Pearlson, G. (2015). Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu. Rev. Clin. Psychol. 11, 251–281. doi: 10.1146/annurev-clinpsy-032814-112915
Peter-Derex, L., Berthomier, C., Taillard, J., Berthomier, P., Bouet, R., Mattout, J., et al. (2021). Automatic analysis of single-channel sleep EEG in a large spectrum of sleep disorders. J. Clin. Sleep Med. 17, 393–402. doi: 10.5664/jcsm.8864
Phang, C., Noman, F., Hussain, H., Ting, C., and Ombao, H. A. (2019). Multi-domain connectome convolutional neural network for identifying schizophrenia from EEG connectivity patterns. IEEE J. Biomed. Health Inform. 24, 1333–1343. doi: 10.1109/JBHI.2019.2941222
Prabhakar, S., Rajaguru, H., and Kim, S. (2020). Schizophrenia EEG signal classification based on swarm intelligence computing. Comput. Intell. Neurosci. 2020:8853835. doi: 10.1155/2020/8853835
Qayyum, A., Qadir, J., Bilal, M., and Al-Fuqaha, A. (2021). Secure and robust machine learning for healthcare: A survey. IEEE Rev. Biomed. Eng. 14, 156–180. doi: 10.1109/RBME.2020.3013489
Rahul, J., and Sharma, L. D. (2022). Automatic cardiac arrhythmia classification based on hybrid 1-D CNN and Bi-LSTM model. Biocyber. Biomed. Eng. 42, 312–324.
Rahul, J., Sora, M., Dev Sharma, L., and Kumar Bohat, V. (2021). An improved cardiac arrhythmia classification using an RR interval-based approach. Biocyber. Biomed. Eng. 41, 656–666.
Rajesh, K., and Sunil Kumar, T. (2021). Schizophrenia Detection in Adolescents from EEG Signals using Symmetrically weighted Local Binary Patterns. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 963–966. doi: 10.1109/EMBC46164.2021.9630232
Rapp, P. E., Keyser, D. O., Albano, A., Hernandez, R., Gibson, D. B., Zambon, R. A., et al. (2015). Traumatic brain injury detection using electrophysiological methods. Front. Hum. Neurosci. 9:11. doi: 10.3389/fnhum.2015.00011
Sadeghi, D., Shoeibi, A., Ghassemi, N., Moridian, P., Khadem, A., Alizadehsani, R., et al. (2022). An overview of artificial intelligence techniques for diagnosis of Schizophrenia based on magnetic resonance imaging modalities: Methods, challenges, and future works. Comput. Biol. Med. 146:105554. doi: 10.1016/j.compbiomed.2022.105554
Santos-Mayo, L., San-Jose-Revuelta, L., and Arribas, J. I. A. (2016). Computer-aided diagnosis system With EEG Based on the P3b wave during an auditory odd-ball task in schizophrenia. IEEE Trans. Biomed. Eng. 64, 395–407. doi: 10.1109/TBME.2016.2558824
Sarma, P., and Barma, S. (2009). Review on stimuli presentation for affect analysis based on EEG. IEEE Access 8, 51991–51995.
Shaffi, N., Mahmud, M., Hajamohideen, F., Subramanian, K., and Shamim Kaiser, M. (2023). “Machine Learning and Deep Learning Methods for the Detection of Schizophrenia Using Magnetic Resonance Images and EEG Signals: An Overview of the Recent Advancements,” in Information and Communication Technology for Competitive Strategies (ICTCS 2022). ICTCS 2022. Lecture Notes in Networks and Systems, eds A. Joshi, M. Mahmud, and R. G. Ragel (Singapore: Springer).
Shalbaf, A., Bagherzadeh, S., and Maghsoudi, A. (2020). Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys. Eng. Sci. Med. 43, 1229–1239. doi: 10.1007/s13246-020-00925-9
Sharma, M., Patel, R., Garg, A., SanTan, R., and Acharya, U. (2023). Automated detection of schizophrenia using deep learning: a review for the last decade. Physiol. Meas. 44, acb24d. doi: 10.1088/1361-6579/acb24d
Shen, M., Wen, P., Song, B., and Li, Y. (2023). Automatic identification of schizophrenia based on EEG signals using dynamic functional connectivity analysis and 3D convolutional neural network. Comput. Biol. Med. 160, 107022. doi: 10.1016/j.compbiomed.2023.107022
Shim, M., Hwang, H., Kim, D., Lee, S., and Im, C. (2016). Machine-learning-based diagnosis of schizophrenia using combined sensor-level and source-level EEG features. Schizophr. Res. 176, 314–319. doi: 10.1016/j.schres.2016.05.007
Shishkin, S., Ganin, I., and Kaplan, A. (2011). Event-related potentials in a moving matrix modification of the P300 brain-computer interface paradigm. Neurosci. Lett. 496, 95–99. doi: 10.1016/j.neulet.2011.03.089
Shoeibi, A., Sadeghi, D., Moridian, P., Ghassemi, N., Heras, J., Alizadehsani, R., et al. (2021). Automatic Diagnosis of Schizophrenia in EEG Signals Using CNN-LSTM Models. Front. Neuroinform. 15:777977. doi: 10.3389/fninf.2021.777977
Singh, K., Singh, S., and Malhotra, J. (2021). Spectral features based convolutional neural network for accurate and prompt identification of schizophrenic patients. Proc. Inst. Mech. Eng. H. 235, 167–184. doi: 10.1177/0954411920966937
Siuly, S., Guo, Y., Alcin, O., Li, Y., Wen, P., and Wang, H. (2023). Exploring deep residual network based features for automatic schizophrenia detection from EEG. Phys. Eng. Sci. Med. 46, 561–574. doi: 10.1007/s13246-023-01225-8
Siuly, S., Li, Y., Wen, P., and Alcin, O. (2022). SchizoGoogLeNet: The GoogLeNet-Based Deep Feature Extraction Design for Automatic Detection of Schizophrenia. Comput. Intell. Neurosci. 2022:1992596. doi: 10.1155/2022/1992596
Soria Bretones, C., Roncero Parra, C., Cascón, J., Borja, A., and Mateo Sotos, J. (2023). Automatic identification of schizophrenia employing EEG records analyzed with deep learning algorithms. Schizophr. Res. 261, 36–46. doi: 10.1016/j.schres.2023.09.010
Sun, J., Cao, R., Zhou, M., Hussain, W., Wang, B., Xue, J., et al. (2021). A hybrid deep neural network for classification of schizophrenia using EEG Data. Sci. Rep. 1: 4706.
Supakar, R., Satvaya, P., and Chakrabarti, P. (2022). A deep learning based model using RNN-LSTM for the Detection of Schizophrenia from EEG data. Comput. Biol. Med. 151:106225. doi: 10.1016/j.compbiomed.2022.106225
Tan, C., Sun, F., Zhang, W., Chen, J., and Liu, C. (2017). “Multimodal classification with deep convolutional-recurrent neural networks for electroencephalography,” in Neural Information Processing: 24th International Conference, ICONIP 2017, Guangzhou, China, November 14-18, 2017, Proceedings, Part II 24, (Springer International Publishing).
Tandon, R., Keshavan, M., and Nasrallah, H. (2008). Schizophrenia, just the facts what we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 102, 1–18. doi: 10.1016/j.schres.2008.04.011
Tatum, I., and William, O. (2021). Handbook of EEG interpretation. Berlin: Springer Publishing Company.
Tolin, D. F., Davies, C. D., Moskow, D. M., and Hofmann, S. G. (2020). Biofeedback and neurofeedback for anxiety disorders: A quantitative and qualitative systematic review. Adv. Exp. Med. Biol. 1191, 265–289. doi: 10.1007/978-981-32-9705-0_16
Vázquez, M., Maghsoudi, A., and Mariño, I. (2021). An interpretable machine learning method for the detection of schizophrenia using EEG signals. Front. Syst. Neurosci. 15:652662. doi: 10.3389/fnsys.2021.652662
Yang, D., Shin, Y. I., and Hong, K. S. (2021). systemic review on transcranial electrical stimulation parameters and EEG/fNIRS features for brain diseases. Front. Neurosci. 15:629323. doi: 10.3389/fnins.2021.629323
Yasin, S., Hussain, S., Aslan, S., Raza, I., Muzammel, M., and Othmani, A. (2021). EEG based Major Depressive disorder and Bipolar disorder detection using Neural Networks: A review. Comput. Methods Programs Biomed. 202:106007. doi: 10.1016/j.cmpb.2021.106007
Keywords: EEG, Schizophrenia (SCZ), AI, machine learning, deep learning, classification
Citation: Rahul J, Sharma D, Sharma LD, Nanda U and Sarkar AK (2024) A systematic review of EEG based automated schizophrenia classification through machine learning and deep learning. Front. Hum. Neurosci. 18:1347082. doi: 10.3389/fnhum.2024.1347082
Received: 30 November 2023; Accepted: 26 January 2024;
Published: 14 February 2024.
Edited by:
Sunil Kumar Telagamsetti, University of Gävle, SwedenReviewed by:
Zulay R. Lugo, University Hospital of Caracas, VenezuelaAukul Pandey, Delhi Technological University, India
Trilochan Panigrahi, National Institute of Technology, Goa, India
Copyright © 2024 Rahul, Sharma, Sharma, Nanda and Sarkar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Umakanta Nanda, dWtfbmFuZGFAeWFob28uY28uaW4=
 Jagdeep Rahul
Jagdeep Rahul Diksha Sharma2
Diksha Sharma2 Lakhan Dev Sharma
Lakhan Dev Sharma Umakanta Nanda
Umakanta Nanda Achintya Kumar Sarkar
Achintya Kumar Sarkar