- 1Independent Internal Medicine-Pediatrics Physician, Educator, and Researcher, Colorado Springs, CO, United States
- 2DAN Women & Babies Program, Department of Newborn & Developmental Pediatrics, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
- 3Department of Pediatrics, University of Toronto, Toronto, ON, Canada
- 4CVI Center, Perkins School for the Blind, Educational Programs, Watertown, MA, United States
- 5Institute on Human Development and Disability, University of Washington, Seattle, WA, United States
- 6Department of Psychiatry & Behavioral Science, University of Washington School of Medicine, Seattle, WA, United States
- 7Department of Social and Behavioral Sciences, Yale School of Public Health, New Haven, CT, United States
- 8SeeLab, Smith-Kettlewell Eye Research Institute, San Francisco, CA, United States
- 9Translational Clinical Eye Research Centre, Alder Hey Children’s Hospital, Liverpool, United Kingdom
- 10Department of Ophthalmology, Boston Children’s Hospital and Harvard Medical School, Boston, MA, United States
Cerebral/Cortical visual impairment (CVI), a brain-based visual condition, is a leading cause of childhood blindness and low vision but remains underdiagnosed in individuals with Down syndrome (DS). This report presents three case studies of adolescents with DS and CVI (DS + CVI), illustrating how CVI can manifest alongside the developmental, cognitive, behavioral, and social profiles of individuals with DS. We describe comprehensive ophthalmological evaluations, assessment for visual perceptual deficits with a screener questionnaire, functional vision assessments, and neuroimaging (when available). Through a detailed retrospective examination of these cases, we explore the complex interplay between CVI and DS, emphasizing how CVI-related challenges—such as difficulties with visual attention, spatial perception, processing, and navigation—are often misattributed to DS alone or to other more commonly recognized co-occurring conditions in DS. Diagnostic overshadowing, coupled with a lack of standardized screening tools, has led to delayed diagnoses and missed opportunities for intervention. Our findings highlight the importance of recognizing CVI in individuals with DS using reliable tools for assessment of functional vision to better appreciate the effect on their diverse developmental outcomes, and to incorporate CVI into our understanding of the DS phenotype. These case reports underscore the need for further research to determine the prevalence and impact of CVI in DS and advocate for the development of tailored screening protocols and evidence-based interventions to support individuals with DS + CVI across the lifespan.
Introduction
Cerebral/Cortical visual impairment (CVI) is a brain-based visual impairment and the leading cause of childhood blindness and low vision in the United States (Chang et al., 2024), with a rising global prevalence (Teoh et al., 2021; Pehere et al., 2019). CVI is often linked to early brain injuries, including perinatal hypoxia, neonatal complications, and genetic conditions such as Down syndrome (DS) (Lehman et al., 2024; Pehere et al., 2018; Wilton et al., 2021). While CVI is increasingly recognized in other neurodevelopmental conditions, it remains under-identified in DS despite its potential impact on development (Wilton et al., 2021; Chokron and Dutton, 2016; Dale and Sonksen, 2002; Hatton et al., 1997; Mosca et al., 2015).
DS is a complex neurogenetic condition caused by the duplication of all or part of chromosome 21, making it the most common chromosomal anomaly (Mai et al., 2019). Developmental outcomes in DS vary widely (Frank and Esbensen, 2015; Winders et al., 2018), shaped by a dynamic interplay of co-occurring medical (Bull et al., 2022; Baksh et al., 2023; Amr, 2018; Breslin et al., 2014; Laws and Hall, 2014; Rako et al., 2021), ophthalmologic (Jain et al., 2023), and neurodevelopmental conditions, including autism spectrum disorder (ASD) (Richards et al., 2015; Fidler et al., 2022) and attention-deficit/hyperactivity disorder (ADHD) (Ekstein et al., 2011). Advances in research have deepened our understanding of these conditions (Antonarakis et al., 2020), and evidence-based care guidelines have improved life expectancy and quality of life (Bull et al., 2022; Tsou et al., 2020). Our understanding of the strengths and challenges in DS continues to evolve (Fidler, 2005; Schworer et al., 2022). Recognizing variability in medical, behavioral, social, and cognitive profiles is crucial for developing tailored interventions (Fidler and Nadel, 2007; Onnivello et al., 2023a). Despite improvements in care, individuals with DS exhibit a broad range of developmental and cognitive trajectories (Baumer et al., 2024; Channell et al., 2021), suggesting that additional unrecognized factors contribute to these differences.
We propose that CVI is one such overlooked factor. Currently, CVI is absent from DS screening guidelines despite its potential to significantly affect developmental, cognitive, and social outcomes.
This retrospective case report examines three adolescent females with DS and CVI (DS + CVI), analyzing long-term medical, developmental, and behavioral data. Clinical records were reviewed, including ophthalmological evaluations, neuroimaging (when available), structured screening tools for visual perceptual deficits, and functional vision assessments. We reconstruct each diagnostic trajectory, emphasizing early neonatal, medical, and developmental factors contributing to delayed CVI recognition. We highlight diagnostic overshadowing, where CVI-related difficulties—such as impaired visual attention, processing, and navigation—were misattributed to DS or other co-occurring conditions. By analyzing these cases, we illustrate how CVI manifests in DS and advocate for systematic CVI screening and integration into routine clinical care to improve developmental outcomes.
Case reports
Case 1
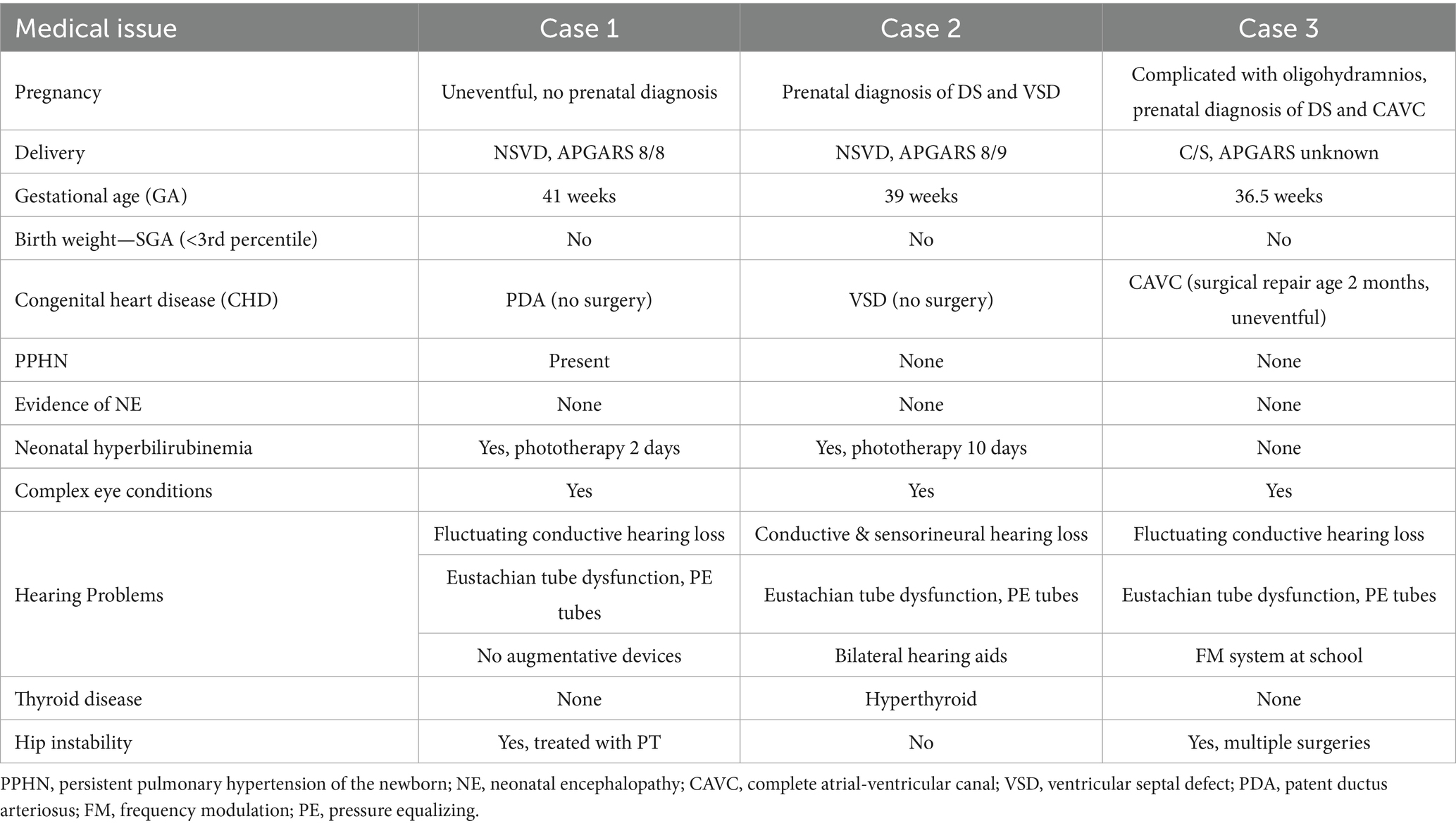
Born at term, this female infant had APGAR scores of 8 and 8 at one and 5 min. She required oxygen supplementation for mild respiratory distress during a 10-day stay in the neonatal intensive care unit (NICU), during which time she was treated briefly with phototherapy for transient hyperbilirubinemia. A hemodynamically stable patent ductus arteriosus (PDA) was identified but resolved spontaneously. No significant perinatal complications were recorded (Table 1).
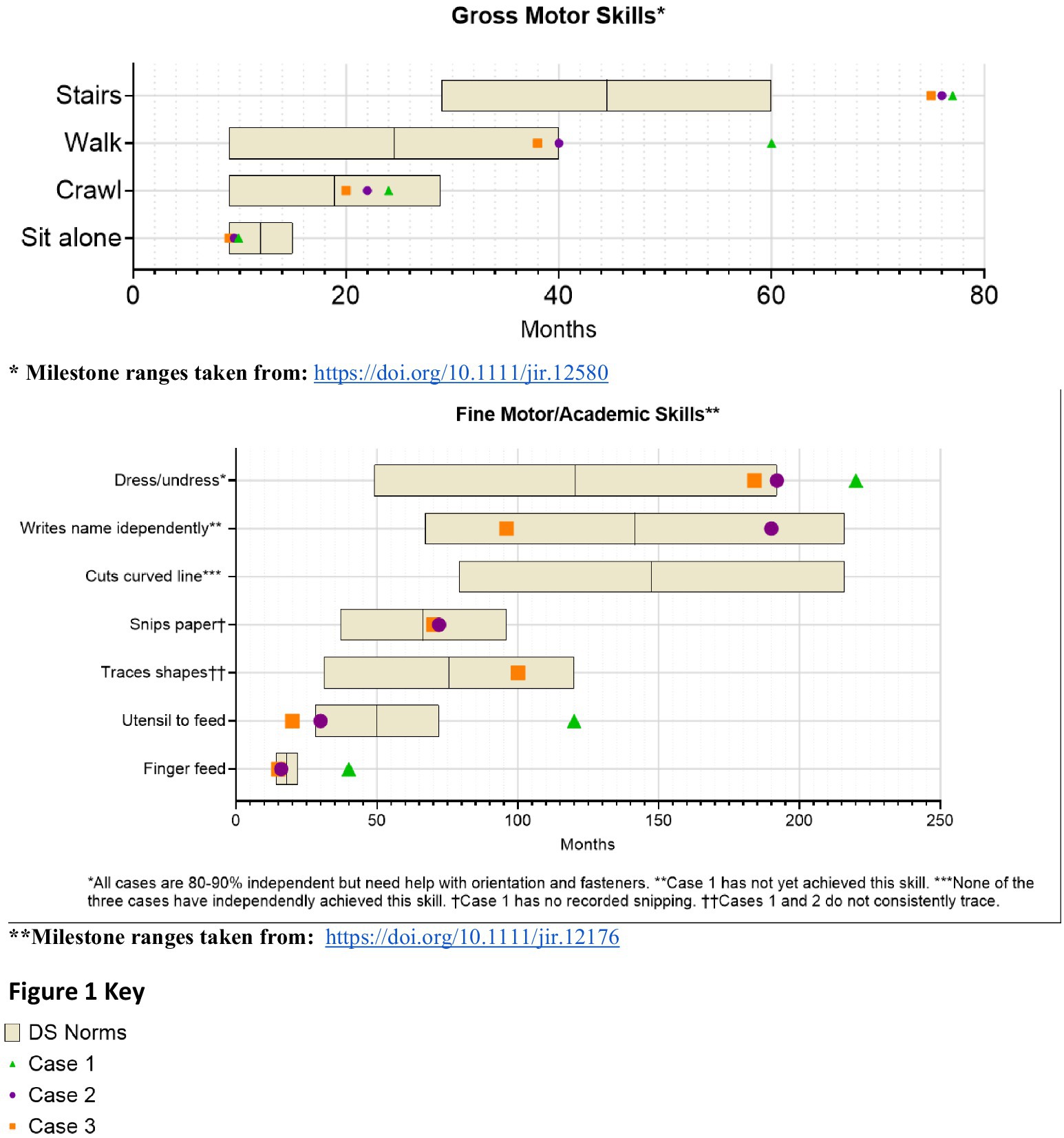
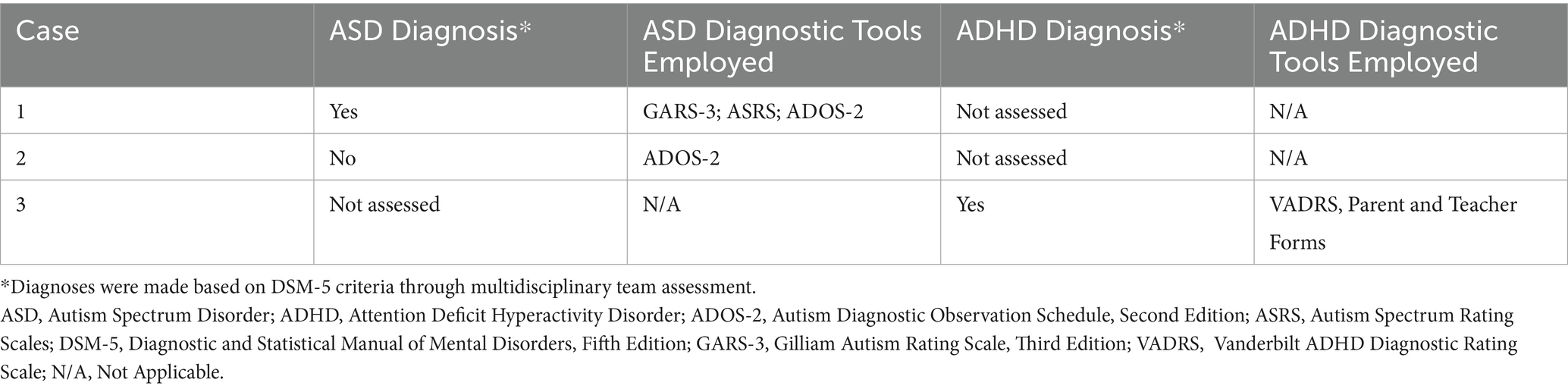
From infancy, delays in visual-motor integration were apparent, and inconsistent reaching behaviors and limited eye-hand coordination persisted; at age 3 year, she was not using hands and eyes together. Gross motor skills showed progressive delays (Figure 1). Hip instability contributed to persistent difficulty with gait. Early assessments confirmed delays across cognitive, language, and motor domains, with persistent fine motor challenges such as difficulty reaching, holding objects or writing. Socially, she engaged well in near-gaze interactions but displayed variable joint attention. Language development slowed, particularly after stopping sign language, but she does rely primarily on spoken language, often using scripted phrases and echolalia. At age 14, she underwent a multidisciplinary ASD evaluation and was diagnosed with autism (Table 2).
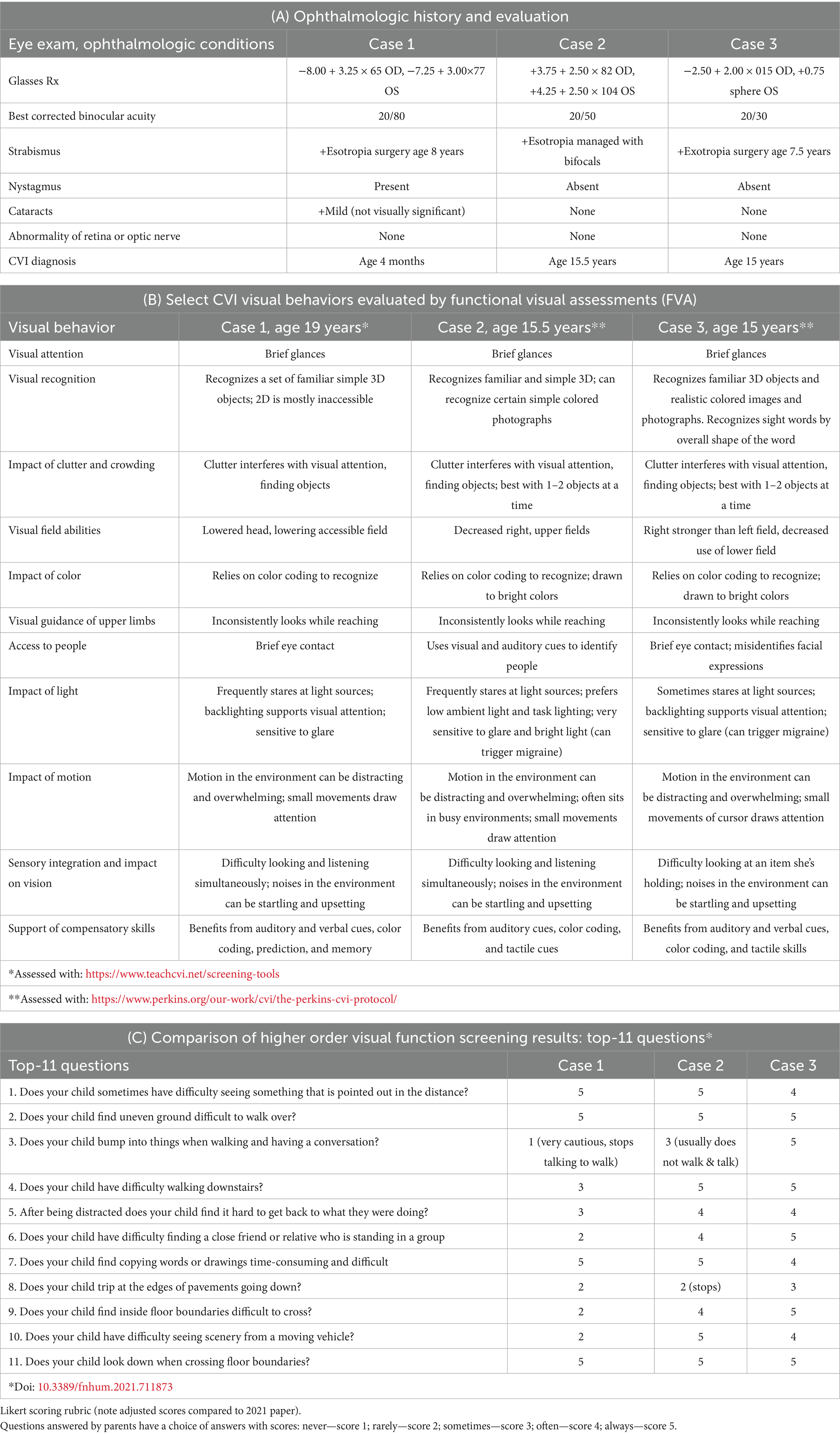
Ophthalmologic evaluations revealed significant nystagmus by 2 months (Table 3A). She has been treated for strabismus with surgery at age 8 and though she wears corrective lenses for her high myopia, her most recent best-corrected binocular visual acuity (VA) is limited at 20/80 (monocular testing was difficult). She was diagnosed with CVI at 4 months due to lack of visual fixation not explained by ocular findings. She received vision services through school, but her parents and school were led to believe that her CVI had resolved by middle school. Reevaluation at age 19 using the TeachCVI Screener #2 (TeachCVI Project, 2017; Martin et al., 2024) was prompted by ongoing functional vision concerns and confirmed persistent CVI-related deficits (Table 3B). While MRI was not performed, functional assessments revealed significant challenges with clutter, motion, and depth perception. Behavioral patterns such as mirror aversion, difficulty visually following another’s pointing finger to distant objects, and sensitivity to loud sounds became more pronounced by adolescence.
Presently, focused CVI accommodations, including the use of contrasting backgrounds, color coded visual schedules, and clutter reduction, have enhanced her ability to navigate both academic and social environments. Behavioral interventions emphasizing multisensory learning have further supported her development, although she struggles to process information from visual, auditory, and motor channels at the same time. Academic skills are minimal and life skills have been emphasized at school.
Case 2
This female infant was born at term after a pregnancy complicated by a prenatal diagnosis of ventricular septal defect (VSD), with APGARS of 8 and 9 at one and 5 min. Her neonatal course included transient tachypnea of the newborn (TTN) and mild hyperbilirubinemia, requiring a 7-day NICU stay for observation and treatment. She stabilized quickly and was discharged without complications to continue phototherapy as an outpatient for a total of 10 days (Table 1).
Developmental milestones revealed early gross motor skills in normal limits for DS, with progressive delays (Figure 1). Visuomotor challenges became evident during infancy, with limited reaching and grasping. While she initially learned baby signs, verbal language development slowed after sign language use was discontinued, leading to delays in communication noted by age 3. Early assessments confirmed global developmental delays. Socially, she was interactive but struggled with joint attention and sustained eye contact. She later displayed strong phonics-based reading skills but struggled to read multiple words on a page and had difficulty tracing and writing. Additional medical conditions include mild hearing loss, hyperthyroidism, obstructive sleep apnea, and migraines (Table 1). By age 10, concerns about social behavior and repetitive movements led to an ASD evaluation, which ruled out the diagnosis (Table 2).
Ophthalmologic findings included hyperopia (Table 3A). Esotropia was managed with bifocals. At the most recent follow up, best corrected binocular VA is 20/50 (monocular testing was difficult). Behavioral observations highlighted difficulty navigating stairs and hesitating at thresholds, and heightened sensitivity to sound and motion which interfered with group activities and transitions.
Persistent functional vision concerns prompted CVI evaluation at age 15.5. Functional visual assessments using The Perkins CVI Protocol (CVI Center at Perkins School for the Blind, 2024) confirmed significant deficits in visual attention, clutter, motion, glare, and depth perception (Table 3B). Brain MRI showed increased susceptibility in the basal ganglia regions (Table 4).
Currently, CVI-specific interventions, such as the use of slant boards, dimmed overhead lighting with task lighting, realistic photographs, a 3D schedule system, and multisensory learning aids have improved her academic engagement. Structured routines, deaf-blind supports, and cane use have reduced her anxiety and improved her participation in group settings.
Case 3
Born preterm at 36.5 weeks via cesarean section due to decreased amniotic fluid, this female infant was vigorous at birth (APGARS unknown). Prenatal imaging identified a complete atrioventricular canal (CAVC), which required surgical repair at 2 months. Her neonatal course was otherwise uneventful, with a brief 2-day NICU stay for monitoring.
From infancy, developmental delays were apparent, particularly in visuomotor skills. Developmental milestones revealed early gross motor skills in normal limits for DS, with progressive delays (Figure 1). Language development progressed steadily due to consistent use of multimodal communication methods. Early assessment confirmed significant cognitive, motor, and language delays. Socially, she demonstrated a preference for humor and verbal engagement, often incorporating lines from songs or movies into conversations. Additional medical conditions included congenital heart disease requiring repair, hip instability requiring surgery, obstructive sleep apnea, fluctuating hearing loss, and migraines. Behavioral challenges included difficulty navigating crowded environments, difficulty with transitions, and noncompliance and aggressive behavior in a busy classroom. She was asked to leave her inclusive school setting after 4th grade, after aggressive behaviors including hitting, yelling, and pushing over tables accelerated. At age 13, she was diagnosed with ADHD, which improved with pharmacological treatment.
Ophthalmologic evaluations revealed exotropia with significant myopic astigmatism OD and mild hyperopia OS indicating significant anisometropia but no amblyopia. Exotropia was surgically corrected at age 7.5. At the most recent follow up, her best corrected binocular VA is 20/30 (20/40 monocular VA).
CVI evaluation at age 15 confirmed significant visual processing deficits, including clutter and navigation difficulties. Brain MRI findings indicated increased susceptibility in the globus pallidus, and functional assessments corroborated CVI-related impairments.
Today, CVI-specific accommodations, including the use of backlighting, clutter-free environments, realistic photographs, and cane use have improved her ability to navigate both academic and social contexts.
Diagnostic assessments across the three cases
The diagnosis of CVI in the three cases was established through a systematic and multidisciplinary approach, incorporating birth history, comprehensive eye examinations, assessment with semi-structured question inventory screener, functional vision assessments, and neuroimaging findings where available (see discussion in Pilling et al., 2023). We used the validated Top-11 CVI Screener and HFVQI-51 semi-structured interview (Chandna et al., 2024), completed by parents of the cases, to assess higher visual function deficits. Table 3 provides a comparative analysis of CVI-related behaviors across the cases, illustrating distinct yet overlapping profiles.
Diagnostic assessments revealed a shared constellation of challenges consistent with CVI (Fazzi et al., 2007; Boot et al., 2010; Philip and Dutton, 2014; Sakki et al., 2018), including visual perceptual deficits and visually guided motor behavior delays, leading to challenges with functional vision. All three cases showed both visual function deficits (decreased VA, decreased visual fields, oculomotor impairment), as well as functional vision deficits of the ventral stream (e.g., route finding and orientation, recognition) and dorsal stream (e.g., visually guided movement, motion perception) (Chang et al., 2024). All three had difficulty integrating sensory information (auditory, visual, tactile at one time), to varying degrees. This sensory sensitivity occasionally led to episodes of visual overwhelm, manifesting as shutdowns or vasovagal reactions, sometimes triggering migraines. Compensatory behaviors, such as trailing hands along walls or hesitating before navigating unfamiliar spaces, reflected attempts to adapt to a visually unpredictable world.
Notably, all three cases scored in the high-severity and spectrum of CVI, exceeding normative thresholds established for neurotypical individuals and aligning with findings from other CVI cohorts without DS. The mean severity scores for each case were 2.4, 3.27, and 3.45 (numerical scale: never = 1; rarely, sometimes, often, always = 5) and significantly higher than the mean severity score from normative data (see below). Because of high severity scores on the Top-11 screener, all three cases were evaluated with the detailed HVFQI-51, a structured 51 question inventory (Chandna et al., 2021). Severity scores (SD) for HVFQI-51 for the three cases were: 3.39 (0.18); 3.05 (0.77); 3.04 (0.49), values considerably different from the normative cohort (1.43 S.D. 0.49 cohort n = 120) (Chandna et al., 2024).
Although there were similarities in functional visual profiles, the three cases had unique variations and varying levels of severity. These findings align with prior reports emphasizing the heterogeneity of CVI-related sensitivities (Philip and Dutton, 2014).
Neuroimaging findings by MRI scans did not show clear lesions in afferent visual pathways, but did reveal differences in basal ganglia structures, specifically the globus pallidus and substantia nigra. EEG findings in both cases indicated nonspecific slowing.
Discussion
The findings across the three cases emphasize the intricate interplay of developmental, behavioral, and functional challenges associated with CVI in individuals with DS. Each of the three cases had progressive developmental delays compared to DS norms, as visualized in Figure 1, yet each case had a unique developmental trajectory, underscoring the individualized effect of CVI in DS amidst the varied factors influencing overall development. These cases demonstrate the confusing overlap between DS and CVI, ASD, and ADHD (Chokron and Dutton, 2023), as well as the challenge of distinguishing ASD in VI (Molinaro et al., 2020). Functionally, challenges with visual perception and visually guided motor behavior impacted activities of daily living, navigation, leisure activities, and independence. Because of diagnostic overshadowing and lack of awareness of CVI, the complex challenges faced by the three cases were easily attributed to DS alone, concurrent medical conditions, or co-occurring neurodevelopmental conditions like ASD and ADHD, highlighting current challenges with diagnosing CVI in DS.
Clear vision is fundamental to achieving optimal developmental and functional outcomes in individuals with DS, particularly given their strengths as social learners and visual processors (Rosenbaum et al., 2024; Rosenbaum and Gorter, 2012). A heightened awareness of DS + CVI is important as early identification of CVI in DS may lead to early intervention and individualized accommodations that might improve the trajectory of visual development and potentially impact overall development, fostering greater independence and well-being for individuals with DS + CVI across their lifespan.
All three cases had complex ocular conditions common in DS (Jain et al., 2023) and were followed by ophthalmology from birth, but CVI was missed in two cases until the teen years, due to lack of awareness, as well as the lack of clearly defined CVI risk factors, screening guidelines, and diagnostic criteria that would prompt a CVI evaluation in DS. These cases share some common elements in their birth histories, clinical ophthalmologic exams, and imaging studies that may serve as a basis for further study of factors increasing vulnerability to CVI in DS.
One key observation is that none of the cases had classic risk factors for CVI at birth (Chang et al., 2024), but all three birth histories included medical events common for babies with DS, such as brief NICU stays, oxygen supplementation, and treatment for hyperbilirubinemia. While these medical factors are not traditionally recognized as CVI risk factors, emerging evidence outside the DS population suggests that even mild hypoxia (James et al., 2019; Nagy et al., 2021; Li et al., 2022) and mild hyperbilirubinemia in term infants (Amin et al., 2019; Hou et al., 2014) may have subtle but lasting effects on visual pathways in the brain, and both are common in neonates with DS.
In addition, these cases had clinical ophthalmologic findings of subnormal visual acuity (VA) and visual perceptual deficits that persisted despite traditional corrective measures of their complex ocular conditions like strabismus and nystagmus. Complex ocular conditions are common in DS (Santoro et al., 2024) but also occur in CVI (Fazzi et al., 2007), and the fact that the complicated visual profiles of these cases did not fully account for the observed functional vision deficits in these cases is a hallmark feature of brain-based visual impairment. Subnormal VA is common in DS, and may serve as a clinical finding that should prompt further questioning and evaluation for CVI.
MRI findings in two cases align with established neuroimaging patterns in DS alone, and include basal ganglia anomalies, which are more prevalent in DS than in the general population (Santoro et al., 2024; Takashima and Becker, 1985; Lee et al., 2013). While linked to depth perception, motor coordination, and contextual learning challenges in non-DS populations (Milardi et al., 2019; Paprocka et al., 2020), their functional significance in DS remains unclear.
These cases suggest that a CVI diagnosis benefits individuals with DS + CVI, their families, and their care teams. According to parents, the CVI diagnosis validated their concerns about their teen’s vision, provided them with a new framework for understanding their teens’ behavior, increased their empathy and patience, and provided new tools to support their loved ones. Anecdotally, therapeutic interventions targeting each teen’s CVI-specific needs have improved functionality, reduced anxiety, and increased engagement in learning environments in all three cases. However, further research on CVI accommodations would help clarify the effectiveness of interventions.
Despite its retrospective nature (reliance on parental recall for early developmental milestones and behaviors, incompleteness of records, and variability of diagnostic tools), this case report provides compelling evidence for further research into CVI’s prevalence, spectrum, and impact within the DS population. The cases presented are teenagers, which may limit the generalizability of findings to younger children, and a case study by nature will not capture the breadth of presentations of CVI in DS.
However, together with recent publications describing nuanced profiles of development in DS (Onnivello et al., 2023a; Onnivello et al., 2023b), cognition in DS (Channell et al., 2021) and autism symptoms in DS (Fidler et al., 2022), these cases raise the possibility that CVI and ocular visual impairments may influence the broader DS phenotype in ways previously unrecognized. To help clarify the role of visual function deficits in DS, prospective studies of development in DS which consider the impact of vision and studies that identify and quantify higher visual function deficits in the larger DS population are necessary. A detailed multidisciplinary assessment of ASD and/or ADHD-like challenges in DS, particularly in familiar versus novel environments, is essential to accurately differentiate between vision-related behaviors and those associated with the neurodevelopmental disorders. Modifications to existing diagnostic tools to account for visual impairments may improve accuracy in these populations (Williams et al., 2014). Incorporating CVI assessments within the already established ophthalmology screening protocols recommended by the AAP may improve early identification, facilitate timely interventions, and enhance developmental outcomes.
Future research should focus on refining CVI-specific screening tools tailored to the DS population and validating interventions for CVI and the long-term impact of CVI accommodations on functional outcomes and quality of life. Additionally, conducting cohort studies involving individuals with DS both with and without CVI, using standardized CVI semi-structured questionnaire interviews such as the Top-11 CVI Screener and HFVQI-51 could provide critical insights into the prevalence, spectrum and severity of HVFDs in this population. These studies should include neuroimaging analyses, including functional MRI (fMRI) (Hirsch et al., 2015), to identify potential biomarkers, anatomical, and neurological differences that may contribute to visual processing challenges, alongside looking at early neonatal, medical, and developmental characteristics contributing to CVI + DS versus DS alone.
Data availability statement
The datasets presented in this article are not readily available because they are the property of SeeLab, Smith-Kettlewell Research Institute. Permission must be granted by AC, via the corresponding author. Requests to access the datasets should be directed to YXJ2aW5kLmNoYW5kbmFAYWxkZXJoZXkubmhzLnVr.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
EB: Conceptualization, Investigation, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. RB: Conceptualization, Writing – original draft, Writing –review & editing. IW: Conceptualization, Writing – original draft, Writing – review & editing. KL: Conceptualization, Writing – review & editing. EM: Writing – review & editing. HM: Visualization, Writing – review & editing. MW: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. SV: Investigation, Writing – review & editing. AC: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. GH: Conceptualization, Supervision, Writing – review & editing.
Group members of DSMIG DS+CVI Workgroup
Elizabeth Boatwright, Independent Internal Medicine-Pediatrics Physician, Educator, and Researcher, Colorado Springs, CO, United States; Rudaina Banihani, DAN Women & Babies Program, Department of Newborn & Developmental Pediatrics, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, Department of Pediatrics, University of Toronto, Toronto, ON, Canada; Ilse Willems, CVI Center, Perkins School for the Blind, Watertown, MA, United States; Kathleen Lehman, Institute on Human Development and Disability, University of Washington, Seattle, WA, United States, Department of Psychiatry & Behavioral Science, University of Washington School of Medicine, Seattle, WA, United States; Arvind Chandna, SeeLab, Smith-Kettlewell Eye Research Institute, San Francisco, CA, United States, Translational Clinical Eye Research Centre, Alder Hey Children’s Hospital, Liverpool, United Kingdom; Gena Heidary, Department of Ophthalmology, Boston Children’s Hospital and Harvard Medical School, Boston, MA, United States.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the families who have graciously shared their experiences, perspectives, and medical records, contributing to the evolving understanding of Cerebral/Cortical visual impairment in Down syndrome. Additionally, the authors appreciate the dedication and expertise of the healthcare providers involved in the care of children with Down syndrome, including the Down Syndrome Medical Interest Group (DSMIG). In particular, we acknowledge the multi-disciplinary expertise contributing to the DS + CVI Workgroup of DSMIG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amin, S., Smith, T., and Timler, G. (2019). Developmental influence of unconjugated hyperbilirubinemia and neurobehavioral disorders. Pediatr. Res. 85, 191–197. doi: 10.1038/s41390-018-0216-4
Amr, N. (2018). Thyroid disorders in subjects with Down syndrome: an update. Acta Biomed. 89, 132–139. doi: 10.23750/abm.v89i1.7120
Antonarakis, S., Skotko, B., Rafii, M., Strydom, A., Pape, S., Bianchi, D., et al. (2020). Down syndrome. Nat. Rev. Dis. Primers 6:9. doi: 10.1038/s41572-019-0143-7
Baksh, R., Pape, S., Chan, L., Aslam, A., Gulliford, M., Strydom, A., et al. (2023). Multiple morbidity across the lifespan in people with Down syndrome or intellectual disabilities: a population-based cohort study using electronic health records. Lancet Public Health 8, e453–e462. doi: 10.1016/S2468-2667(23)00057-9
Baumer, N., DePillis, R., Pawlowski, K., Zhang, B., and Mazumdar, M. (2024). Developmental milestones for children with Down syndrome. Pediatrics 154:e2023065402. doi: 10.1542/peds.2023-065402
Boot, F., Pel, J., van der Steen, J., and Evenhuis, H. (2010). Cerebral visual impairment: which perceptive visual dysfunctions can be expected in children with brain damage? A systematic review. Res. Dev. Disabil. 31, 1149–1159. doi: 10.1016/j.ridd.2010.08.001
Breslin, J., Spanò, G., Bootzin, R., Anand, P., Nadel, L., and Edgin, J. (2014). Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev. Med. Child Neurol. 56, 657–664. doi: 10.1111/dmcn.12376
Bull, M., Trotter, T., Santoro, S., Christensen, C., Grout, R., Burke, L. W., et al. (2022). Health supervision for children and adolescents with Down syndrome. Pediatrics 149:e2022057010. doi: 10.1542/peds.2022-057010
Chandna, A., Ghahghaei, S., Foster, S., and Kumar, R. (2021). Higher visual function deficits in children with cerebral visual impairment and good visual acuity. Front. Hum. Neurosci. 15:711873. doi: 10.3389/fnhum.2021.711873
Chandna, A., Wong, M., Veitzman, S., Menjivar, E., and Kulkarni, A. (2024). Higher visual function deficits are independent of visual acuity measures in children with cerebral visual impairment. Front. Hum. Neurosci. 18:1451257. doi: 10.3389/fnhum.2024.1451257
Chang, M., and Merabet, L.CVI Working Group (2024). Special Commentary: Cerebral/Cortical visual impairment working definition: a report from the National Institutes of Health CVI Workshop. Ophthalmology 131, 1359–1365. doi: 10.1016/j.ophtha.2024.09.017
Channell, M., Mattie, L., Hamilton, D., Capone, G., Mahone, E., Sherman, S., et al. (2021). Capturing cognitive and behavioral variability among individuals with Down syndrome: a latent profile analysis. J. Neurodev. Disord. 13:16. doi: 10.1186/s11689-021-09365-2
Chokron, S., and Dutton, G. (2016). Impact of cerebral visual impairments on motor skills: implications for developmental coordination disorders. Front. Psychol. 7:1471. doi: 10.3389/fpsyg.2016.01471
Chokron, S., and Dutton, G. (2023). From vision to cognition: potential contributions of cerebral visual impairment to neurodevelopmental disorders. J. Neural Transm. 130, 409–424. doi: 10.1007/s00702-022-02572-8
CVI Center at Perkins School for the Blind . (2024). The Perkins CVI Protocol. Available online at: https://www.perkins.org/our-work/cvi/the-perkins-cvi-protocol/ (Accessed January 19, 2025).
Dale, N., and Sonksen, P. (2002). Developmental outcome, including setback, in young children with severe visual impairment. Dev. Med. Child Neurol. 44, 613–622. doi: 10.1017/s0012162201002651
Ekstein, S., Glick, B., Weill, M., Kay, B., and Berger, I. (2011). Down syndrome and attention-deficit/hyperactivity disorder (ADHD). J. Child Neurol. 26, 1290–1295. doi: 10.1177/0883073811405201
Fazzi, E., Signorini, S., Bova, S., La Piana, R., Ondei, P., Bertone, C., et al. (2007). Spectrum of visual disorders in children with cerebral visual impairment. J. Child Neurol. 22, 294–301. doi: 10.1177/08830738070220030801
Fidler, D. (2005). The emerging Down syndrome behavioral phenotype in early childhood: implications for practice. Infants Young Child. 18, 86–103. doi: 10.1097/00001163-200504000-00003
Fidler, D., and Nadel, L. (2007). Education and children with Down syndrome: neuroscience, development, and intervention. Ment. Retard. Dev. Disabil. Res. Rev. 13, 262–271. doi: 10.1002/mrdd.20166
Fidler, D., Prince, M., Van Deusen, K., Esbensen, A., Thurman, A., Abbeduto, L., et al. (2022). Latent profiles of autism symptoms in children and adolescents with Down syndrome. J. Intellect. Disabil. Res. 66, 265–281. doi: 10.1111/jir.12910
Frank, K., and Esbensen, A. (2015). Fine motor and self-care milestones for individuals with Down syndrome using a retrospective chart review. J. Intellect. Disabil. Res. 59, 719–729. doi: 10.1111/jir.12176
Hatton, D., Bailey, D. Jr., Burchinal, M., and Ferrell, K. (1997). Developmental growth curves of preschool children with vision impairments. Child Dev. 68, 788–806. doi: 10.1111/j.1467-8624.1997.tb01962.x
Hirsch, G., Bauer, C., and Merabet, L. (2015). Using structural and functional brain imaging to uncover how the brain adapts to blindness. J. Psychiatry Brain Funct. 2:5. doi: 10.7243/2055-3447-2-7
Hou, C., Norcia, A. M., Madan, A., and Good, W. V. (2014). Visuocortical function in infants with a history of neonatal jaundice. Invest. Ophthalmol. Vis. Sci. 55, 6443–6449. doi: 10.1167/iovs.14-14261
Jain, A., Boyd, N., Paulsen, K., Vogel, B., Nguyen, L., and Santoro, J. (2023). Ophthalmologic and neuro-ophthalmologic findings in children with Down syndrome. Am. J. Med. Genet. C 193:e32068. doi: 10.1002/ajmg.c.32068
James, M., Connor, C. M. O., Cullinane, A., Murray, D., and Boylan, G. (2019). Ophthalmic outcomes following neonatal hypoxic-ischaemic encephalopathy; oculomotor, biometric and refractive data in early childhood. Eye 33, 1152–1157. doi: 10.1038/s41433-019-0390-6
Laws, G., and Hall, A. (2014). Early hearing loss and language abilities in children with Down syndrome. Int. J. Lang. Commun. Disord. 49, 333–342. doi: 10.1111/1460-6984.12077
Lee, K., Lee, K., and Weon, Y. (2013). Asymptomatic moyamoya syndrome, atlantoaxial subluxation and basal ganglia calcification in a child with Down syndrome. Korean J. Pediatr. 56, 540–543. doi: 10.3345/kjp.2013.56.12.540
Lehman, S., Yin, L., and Chang, M.American Academy of Pediatrics, Section on Ophthalmology, Council on Children with Disabilities; American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology; American Association of Certified Orthoptists (2024). Diagnosis and care of children with Cerebral/Cortical visual impairment: clinical report. Pediatrics 154:e2024068465. doi: 10.1542/peds.2024-068465
Li, Y., Wisnowski, J., Chalak, L., Mathur, A., McKinstry, R., Licona, G., et al. (2022). Mild hypoxic-ischemic encephalopathy (HIE): timing and pattern of MRI brain injury. Pediatr. Res. 92, 1731–1736. doi: 10.1038/s41390-022-02026-7
Mai, C., Isenburg, J., Canfield, M., Meyer, R., Correa, A., Alverson, C., et al. (2019). National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 111, 1420–1435. doi: 10.1002/bdr2.1589
Martin, J., Bradley, C., Kran, B., and Ross, N. (2024). Rasch analysis and targeting assessment of the teach-CVI survey tool in a cohort of CVI patients. Front. Ophthalmol. 4:1495000. doi: 10.3389/fopht.2024.1495000
Milardi, D., Quartarone, A., Bramanti, A., Anastasi, G., Bertino, S., Basile, G., et al. (2019). The cortico-basal ganglia-cerebellar network: past, present and future perspectives. Front. Syst. Neurosci. 13:61. doi: 10.3389/fnsys.2019.00061
Molinaro, A., Micheletti, S., Rossi, A., Gitti, F., Galli, J., Merabet, L., et al. (2020). Autistic-like features in visually impaired children: a review of literature and directions for future research. Brain Sci. 10:507. doi: 10.3390/brainsci10080507
Mosca, R., Kritzinger, A., and van der Linde, J. (2015). Language and communication development in preschool children with visual impairment: a systematic review. S. Afr. J. Commun. Disord. 62, e1–e10. doi: 10.4102/sajcd.v62i1.119
Nagy, E., Self, J., Williams, C., and Vollmer, B. (2021). Disorders of vision in neonatal hypoxic-ischaemic. Arch. Dis. Child Fetal Neonatal. Ed. 106, 357–362. doi: 10.1136/archdischild-2020-318998
Onnivello, S., Schworer, E. K., Daunhauer, L. A., and Fidler, D. J. (2023b). Acquisition of cognitive and communication milestones in infants with Down syndrome. J. Intellect. Disabil. Res. 67, 239–253. doi: 10.1111/jir.12893
Onnivello, S., Schworer, E., Prince, M., Daunhauer, L., and Fidler, D. (2023a). Early developmental profiles among infants with Down syndrome. J. Intellect. Disabil. Res. 67, 228–238. doi: 10.1111/jir.12997
Paprocka, J., Machnikowska-Sokołowska, M., Gruszczyńska, K., and Emich-Widera, E. (2020). Neuroimaging of basal ganglia in neurometabolic diseases in children. Brain Sci. 10:849. doi: 10.3390/brainsci10110849
Pehere, N., Chougule, P., and Dutton, G. (2018). Cerebral visual impairment in children: causes and associated ophthalmological problems. Indian J. Ophthalmol. 66, 812–815. doi: 10.4103/ijo.IJO_1274_17
Pehere, N., Narasaiah, A., and Dutton, G. (2019). Cerebral visual impairment is a major cause of profound visual impairment in children aged less than 3 years: a study from tertiary eye care center in South India. Indian J. Ophthalmol. 67, 1544–1547. doi: 10.4103/ijo.IJO_1850_18
Philip, S., and Dutton, G. (2014). Identifying and characterising cerebral visual impairment in children: a review. Clin. Exp. Optom. 97, 196–208. doi: 10.1111/cxo.12155
Pilling, R., Allen, L., Bowman, R., Ravenscroft, J., Saunders, K., and Williams, C. (2023). Clinical assessment, investigation, diagnosis and initial management of cerebral visual impairment: a consensus practice guide. Eye 37, 1958–1965. doi: 10.1038/s41433-022-02261-6
Rako, K., Ranade, S., and Allen, A. (2021). Orthopaedic management in Down syndrome. J. Pediatr. Soc. North Am. 3, 283–217. doi: 10.55275/JPOSNA-2021-283
Richards, C., Jones, C., Groves, L., Moss, J., and Oliver, C. (2015). Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry 2, 909–916. doi: 10.1016/S2215-0366(15)00376-4
Rosenbaum, P., and Gorter, J. (2012). The ‘F-words’ in childhood disability: I swear this is how we should think! Child Care Health Dev. 38, 457–463. doi: 10.1111/j.1365-2214.2011.01338.x
Rosenbaum, P., Imms, C., Miller, L., Hughes, D., and Cross, A. (2024). Perspectives in childhood-onset disabilities: integrating 21st-century concepts to expand our horizons. Disabil. Rehabil. 47, 2682–2692. doi: 10.1080/09638288.2024.2394647
Sakki, H., Dale, N., Sargent, J., Perez-Roche, T., and Bowman, R. (2018). Is there consensus in defining childhood cerebral visual impairment? A systematic review of terminology and definitions. Br. J. Ophthalmol. 102, 424–432. doi: 10.1136/bjophthalmol-2017-310694
Santoro, J., Khoshnood, M., Jafarpour, S., Nguyen, L., Boyd, N., Vogel, B., et al. (2024). Neuroimaging abnormalities associated with immunotherapy responsiveness in Down syndrome regression disorder. Ann. Clin. Transl. Neurol. 11, 1034–1045. doi: 10.1002/acn3.52023
Schworer, E., Fidler, D., Kaur, M., Needham, A., Prince, M., and Daunhauer, L. (2022). Infant precursors of executive function in Down syndrome. J. Intellect. Disabil. Res. 66, 108–120. doi: 10.1111/jir.12824
Takashima, S., and Becker, L. (1985). Basal ganglia calcification in Down’s syndrome. J. Neurol. Neurosurg. Psychiatry 48, 61–64. doi: 10.1136/jnnp.48.1.61
TeachCVI Project (2017). Screening list for children with a suspicion of cerebral visual impairment (CVI): screening list CVI 2. Available online at: https://www.teachcvi.net/screening-tools
Teoh, L., Solebo, A., and Rahi, J.British Childhood Visual Impairment and Blindness Study Interest Group (2021). Visual impairment, severe visual impairment, and blindness in children in Britain (BCVIS2): a national observational study. Lancet Child Adolesc. Health 5, 190–200. doi: 10.1016/S2352-4642(20)30366-7
Tsou, A., Bulova, P., Capone, G., Chicoine, B., and Gelaro, B.Harville TO, et al. (2020). Medical care of adults with Down syndrome: a clinical guideline. JAMA 324, 1543–1556. doi: 10.1001/jama.2020.17024
Williams, M., Fink, C., Zamora, I., and Borchert, M. (2014). Autism assessment in children with optic nerve hypoplasia and other vision impairments. Dev. Med. Child. Neurol. 56, 66–72. doi: 10.1111/dmcn.12264
Wilton, G., Woodhouse, R., Vinuela-Navarro, V., England, R., and Woodhouse, J. (2021). Behavioural features of cerebral visual impairment are common in children with Down syndrome. Front. Hum. Neurosci. 15:673342. doi: 10.3389/fnhum.2021.673342
Keywords: Cerebral/Cortical visual impairment (CVI), Down syndrome (DS)/Trisomy 21, Down syndrome and Cerebral/Cortical visual impairment (DS + CVI), visual attention, Top-11 Higher Visual Function Question Inventory (HVFQI), functional vision assessment (FVA), case report, visual perception
Citation: Boatwright E, Banihani R, Willems I, Lehman K, Mazel E, Mark H, Wong M, Vietzman S, Chandna A and Heidary G (2025) Cerebral/Cortical visual impairment (CVI) in Down syndrome: a case series. Front. Hum. Neurosci. 19:1563420. doi: 10.3389/fnhum.2025.1563420
Edited by:
Jeff C. Rabin, University of the Incarnate Word, United StatesReviewed by:
Linda Lawrence, Independent Researcher, Salina, KS, United StatesNicola McDowell, Massey University, New Zealand
Copyright © 2025 Boatwright, Banihani, Willems, Lehman, Mazel, Mark, Wong, Vietzman, Chandna and Heidary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Boatwright, Ym9hdHdyaWdodHMyQG1hYy5jb20=
 Elizabeth Boatwright
Elizabeth Boatwright Rudaina Banihani
Rudaina Banihani Ilse Willems
Ilse Willems Kathleen Lehman
Kathleen Lehman Ellen Mazel
Ellen Mazel Hannah Mark
Hannah Mark Mike Wong
Mike Wong Silvia Vietzman
Silvia Vietzman Arvind Chandna
Arvind Chandna Gena Heidary
Gena Heidary