- 1Department of Psychology, University of the Bundeswehr Munich, Neubiberg, Germany
- 2Department of Psychology, University of Messina, Messina, Italy
- 3Department of Psychology, University of Groningen, Groningen, Netherlands
Editorial on the Research Topic
Transcranial electrical stimulation (tACS, tDCS, tRNS) in basic and clinical neuroscience: current progress and future directions
Transcranial electrical stimulation (tES) as a non-invasive brain stimulation technique has been used to study brain physiology for many years now (Nitsche and Paulus, 2000; Antal et al., 2017). Within this period, rapid advancement in understanding its mechanisms of action (Liu et al., 2018; Jackson et al., 2016; Yavari et al., 2018) and optimization of neuromodulatory effects have taken place (Agboada et al., 2019, 2020; Mosayebi Samani et al., 2019a,b; Wischnewski et al., 2019), with evidence from healthy and clinical populations (Alizadehgoradel et al., 2024; Ney et al., 2021; Vicario and Nitsche, 2013). The tES methods, including transcranial direct current, alternating current, and random noise stimulation (tDCS, tACS, and tRNS), operate via the application of weak currents through electrodes on the scalp with the aim of influencing brain physiology (Antal et al., 2017). So far, tDCS and tACS have been employed to enhance performance in cognitive and behavioral tasks (Fröhlich et al., 2015; Reinhart et al., 2017), as well as treat neuropsychiatric disorders such as depression, Alzheimer's, Parkinson's, stroke, schizophrenia, and many more in clinical trials (Lefaucheur et al., 2017; Elyamany et al., 2021). While progress has been significant, challenges remain, including inter-subject variability, sub-optimal stimulation parameters, and lack of long-term effects (Bland and Sale, 2019; Ammann et al., 2017; Strube et al., 2016; Wiethoff et al., 2014). This Research Topic focused on tES progress and how it may shape future behavioral and cognitive applications as well as therapeutic use.
Mechanisms of tES: current progress
The basic physiological mechanisms of tES have been established in animal (Ranieri et al., 2012; Rahman et al., 2013; Krause et al., 2019; Wischnewski et al., 2024), human (Nitsche et al., 2005; Mosayebi-Samani et al., 2023; Woods et al., 2016), and computational models (Bikson et al., 2015; Bonaiuto and Bestmann, 2015). However, the exact mechanisms by which these effects lead to behavioral modulation are still lacking. In this Research Topic, four potential mechanisms of tACS-induced after-effects were discussed by Agboada et al.: spike-timing, spike-phase coupling, homeostatic, and state-dependent plasticity. Further, the tACS study by Carrasco-Gómez et al. reported stimulation-induced plasticity that agrees with the theories discussed by Agboada et al.. Three papers - Chen et al., Muccio et al., and Wu et al., reported plasticity induced by tDCS. However, as revealed by Wu et al., the different mechanisms by which different tES techniques operate mean their combinations might not always result in the desired after-effects.
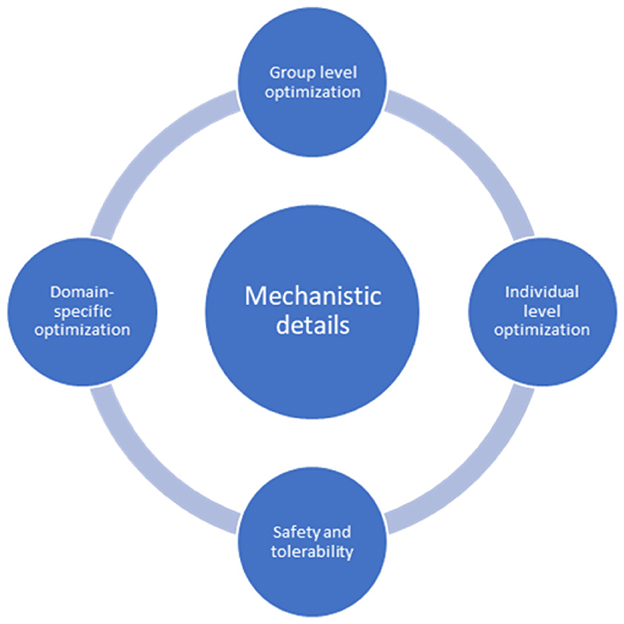
At the center of future tES studies is the continuous investigation of the mechanistic processes underlying observed after-effects. When optimizing tES at the individual and group levels, domain-specific aims must inform safety and tolerability considerations (Figure 1).
Clinical applications and the future of tES
In this Research Topic, Chen et al. explored the rehabilitative effects of tDCS and exergames on smartphone addiction combined with electroencephalography. TDCS improved executive control and decision-making abilities and increased P300 amplitudes in the frontal, central, and parietal cortical regions. These changes were stable over a 4-week follow-up period. Similarly, using functional neuroimaging to test the simultaneous and cumulative effects of tDCS in multiple sclerosis patients, Muccio et al. found that tDCS acutely enhanced metabolic activity, which persisted post-stimulation. At follow-up after 20 sessions of home-based tDCS with an adaptive cognitive task, the authors reported sustained after-effects of the stimulation. These studies emphasize the importance of neurophysiological evidence of tES effects, which offers mechanistic details about the stimulation efficacy. Currently, only a handful of clinical trials have measured neurophysiological and clinical measurement outcomes. Clinical studies with tES should therefore utilize a multi-modal paradigm to correlate brain and behavioral/clinical changes. Furthermore, in a pre-registered clinical trial, Xue et al. presented a protocol for assessing the effects of tDCS in patients with post-operative delirium after elective hip fracture surgery. They plan to recruit 160 patients over the age of 65 years. Using functional near-infrared spectroscopy for evaluating brain metabolic changes before and after tDCS, the authors will explore the efficacy of the stimulation in lowering post-operative delirium.
The future of tES lies in the optimization of stimulation parameters at the individual and group levels through different experimental and computational approaches (Zrenner and Ziemann, 2024). One potential individualized approach to modulate alpha oscillations applied by Carrasco-Gómez et al. used MEG to optimize tACS frequency. Also, for clinical use, tES must induce long-term after-effects (Agboada et al.). This is particularly relevant since the relatively low side-effects of tES compared to pharmacological alternatives could enforce its long-term therapeutic application (Matsumoto and Ugawa, 2017). This means reporting adverse side-effects and tES tolerability by each study to collect relevant information on how stimulation interacts with specific domains (Bikson et al., 2016). For example, Bjekić et al. compared the subjective rating of tES side-effects among healthy participants. Almost all participants (more than 95%) reported less discomfort across all tES conditions; however, when compared with sham, tACS showed slightly lower levels of discomfort than tDCS and oscillatory tDCS.
This Research Topic's collection offers a snapshot of the progress in understanding and optimizing tES in both basic and clinical neuroscience. By exploring the mechanisms of action, safety, tolerability, and clinical applications, this Research Topic highlights the potential of tES to modulate brain activity and improve outcomes in cognitive, behavioral, and neurological domains. The insights presented here, ranging from behavioral experiments in healthy human subjects to clinical studies provide a comprehensive framework for advancing scientific knowledge and translating it into practical strategies for therapeutic interventions. As we continue to refine tES protocols, personalize stimulation parameters, and investigate long-term after-effects, the research presented in this topic is essential for shaping the future of tES research and its clinical application.
Author contributions
DA: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – original draft. CV: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft. MW: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. DA was supported by NextGenerationEU – dtec.bw. CV was supported by the Ministero Istruzione Università e Ricerca (PRIN 2022, NextGenerationEU. Project Code: 2022L3AALJ). MW was supported by the Research School of Behavioral and Cognitive Neurosciences (University of Groningen) seed grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agboada, D., Mosayebi Samani, M., Jamil, A., Kuo, M. F., and Nitsche, M. A. (2019). Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci. Rep. 9:18185. doi: 10.1038/s41598-019-54621-0
Agboada, D., Mosayebi-Samani, M., Kuo, M. F., and Nitsche, M. A. (2020). Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal transcranial direct current stimulation - better effects with intensified protocols? Brain Stimul. 13, 987–997. doi: 10.1016/j.brs.2020.04.009
Alizadehgoradel, J., Molaei, B., Barzegar Jalali, K., Pouresmali, A., Sharifi, K., Hallajian, A. H., et al. (2024). Targeting the prefrontal-supplementary motor network in obsessive-compulsive disorder with intensified electrical stimulation in two dosages: a randomized, controlled trial. Transl. Psychiatry 14:78. doi: 10.1038/s41398-024-02736-y
Ammann, C., Lindquist, M. A., and Celnik, P. A. (2017). Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul. 10, 757–763. doi: 10.1016/j.brs.2017.04.003
Antal, A., Alekseichuk, I., Bikson, M., Brockmöller, J., Brunoni, A. R., Chen, R., et al. (2017). Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 128, 1774–1809. doi: 10.1016/j.clinph.2017.06.001
Bikson, M., Grossman, P., Thomas, C., Zannou, A. L., Jiang, J., Adnan, T., et al. (2016). Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9, 641–661. doi: 10.1016/j.brs.2016.06.004
Bikson, M., Truong, D. Q., Mourdoukoutas, A. P., Aboseria, M., Khadka, N., Adair, D., et al. (2015). Modeling sequence and quasi-uniform assumption in computational neurostimulation. Prog. Brain. Res. 222, 1–23. doi: 10.1016/bs.pbr.2015.08.005
Bland, N. S., and Sale, M. V. (2019). Current challenges: the ups and downs of tACS. Exp. Brain Res. 237, 3071–3088. doi: 10.1007/s00221-019-05666-0
Bonaiuto, J. J., and Bestmann, S. (2015). Understanding the nonlinear physiological and behavioral effects of tDCS through computational neurostimulation. Prog. Brain. Res. 222, 75–103. doi: 10.1016/bs.pbr.2015.06.013
Elyamany, O., Leicht, G., Herrmann, C. S., and Mulert, C. (2021). Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 271, 135–156. doi: 10.1007/s00406-020-01209-9
Fröhlich, F., Sellers, K. K., and Cordle, A. L. (2015). Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev. Neurother. 15, 145–167. doi: 10.1586/14737175.2015.992782
Jackson, M. P., Rahman, A., Lafon, B., Kronberg, G., Ling, D., Parra, L. C., et al. (2016). Animal models of transcranial direct current stimulation: methods and mechanisms. Clin. Neurophysiol. 127, 3425–3454. doi: 10.1016/j.clinph.2016.08.016
Krause, M. R., Vieira, P. G., Csorba, B. A., Pilly, P. K., and Pack, C. C. (2019). Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc. Natl. Acad. Sci. 116, 5747–5755. doi: 10.1073/pnas.1815958116
Lefaucheur, J. P., Antal, A., Ayache, S. S., Benninger, D. H., Brunelin, J., Cogiamanian, F., et al. (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92. doi: 10.1016/j.clinph.2016.10.087
Liu, A., Vöröslakos, M., Kronberg, G., Henin, S., Krause, M. R., Huang, Y., et al. (2018). Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 9:5092. doi: 10.1038/s41467-018-07233-7
Matsumoto, H., and Ugawa, Y. (2017). Adverse events of tDCS and tACS: a review. Clin. Neurophysiol. Pract. 2, 19–25. doi: 10.1016/j.cnp.2016.12.003
Mosayebi Samani, M., Agboada, D., Jamil, A., Kuo, M. F., and Nitsche, M. A. (2019a). Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119, 350–361. doi: 10.1016/j.cortex.2019.04.016
Mosayebi Samani, M., Agboada, D., Kuo, M. F., and Nitsche, M. A. (2019b). Probing the relevance of repeated cathodal transcranial direct current stimulation over the primary motor cortex for prolongation of after-effects. J. Physiol. 598, 805–816. doi: 10.1113/JP278857
Mosayebi-Samani, M., Agboada, D., Mutanen, T. P., Haueisen, J., Kuo, M.-F., Nitsche, M. A., et al. (2023). Transferability of cathodal tDCS effects from the primary motor to the prefrontal cortex: a multimodal TMS-EEG study. Brain Stimul. 16, 515–539. doi: 10.1016/j.brs.2023.02.010
Ney, L. J., Vicario, C. M., Nitsche, M. A., and Felmingham, K. L. (2021). Timing matters: transcranial direct current stimulation after extinction learning impairs subsequent fear extinction retention. Neurobiol. Learn. Mem. 177:107356. doi: 10.1016/j.nlm.2020.107356
Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527(Pt 3), 633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
Nitsche, M. A., Seeber, A., Frommann, K., Klein, C. C., Rochford, C., Nitsche, M. S., et al. (2005). Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. 568(Pt 1), 291–303. doi: 10.1113/jphysiol.2005.092429
Rahman, A., Reato, D., Arlotti, M., Gasca, F., Datta, A., Parra, L. C., et al. (2013). Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J. Physiol. 591, 2563–2578. doi: 10.1113/jphysiol.2012.247171
Ranieri, F., Podda, M. V., Riccardi, E., Frisullo, G., Dileone, M., Profice, P., et al. (2012). Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J. Neurophysiol. 107, 1868–1880. doi: 10.1152/jn.00319.2011
Reinhart, R. M. G., Cosman, J. D., Fukuda, K., and Woodman, G. F. (2017). Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Attent. Percept. Psychophys. 79, 3–23. doi: 10.3758/s13414-016-1224-2
Strube, W., Bunse, T., Nitsche, M. A., Nikolaeva, A., Palm, U., Padberg, F., et al. (2016). Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiol. Rep. 4:e12884. doi: 10.14814/phy2.12884
Vicario, C. M., and Nitsche, M. A. (2013). Transcranial direct current stimulation: a remediation tool for the treatment of childhood congenital dyslexia? Front. Hum. Neurosci. 7:139. doi: 10.3389/fnhum.2013.00139
Wiethoff, S., Hamada, M., and Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 7, 468–475. doi: 10.1016/j.brs.2014.02.003
Wischnewski, M., Engelhardt, M., Salehinejad, M. A., Schutter, D. J. L. G., Kuo, M.-F., Nitsche, M. A., et al. (2019). NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb. Cortex 29, 2924–2931. doi: 10.1093/cercor/bhy160
Wischnewski, M., Tran, H., Zhao, Z., Shirinpour, S., Haigh, Z. J., Rotteveel, J., et al. (2024). Induced neural phase precession through exogenous electric fields. Nat. Commun. 15:1687. doi: 10.1038/s41467-024-45898-5
Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1048. doi: 10.1016/j.clinph.2015.11.012
Yavari, F., Jamil, A., Mosayebi Samani, M., Vidor, L. P., and Nitsche, M. A. (2018). Basic and functional effects of transcranial electrical stimulation (tES)-an introduction. Neurosci. Biobehav. Rev. 85, 81–92. doi: 10.1016/j.neubiorev.2017.06.015
Keywords: neuroplasticity, tACS, tDCS, tRNS, long-term effects, clinical trials
Citation: Agboada D, Vicario CM and Wischnewski M (2025) Editorial: Transcranial electrical stimulation (tACS, tDCS, tRNS) in basic and clinical neuroscience: current progress and future directions. Front. Hum. Neurosci. 19:1640565. doi: 10.3389/fnhum.2025.1640565
Received: 03 June 2025; Accepted: 05 June 2025;
Published: 20 June 2025.
Edited and reviewed by: Mingzhou Ding, University of Florida, United States
Copyright © 2025 Agboada, Vicario and Wischnewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Desmond Agboada, ZGVzbW9uZC5hZ2JvYWRhQHVuaWJ3LmRl; ZGVzbW9uZC5hZ2JvYWRhQGdtYWlsLmNvbQ==; Carmelo Mario Vicario, Y2FybWVsb21hcmlvLnZpY2FyaW9AdW5pbWUuaXQ=; Miles Wischnewski, bS53aXNjaG5ld3NraUBydWcubmw=
 Desmond Agboada
Desmond Agboada Carmelo Mario Vicario
Carmelo Mario Vicario Miles Wischnewski
Miles Wischnewski