- 1 Laboratory of Entomology, Wageningen University and Research Centre, Wageningen, Netherlands
- 2 Enza Zaden R&D B.V., Enkhuizen, Netherlands
Malaria is caused by Plasmodium parasites which are transmitted by mosquitoes. Until recently, human malaria was considered to be caused by human-specific Plasmodium species. Studies on Plasmodium parasites in non-human primates (NHPs), however, have identified parasite species in gorillas and chimpanzees that are closely related to human Plasmodium species. Moreover, P. knowlesi, long known as a parasite of monkeys, frequently infects humans. The requirements for such a cross-species exchange and especially the role of mosquitoes in this process are discussed, as the latter may act as bridge vectors of Plasmodium species between different primates. Little is known about the mosquito species that would bite both humans and NHPs and if so, whether humans and NHPs share the same Plasmodium vectors. To understand the vector-host interactions that can lead to an increased Plasmodium transmission between species, studies are required that reveal the nature of these interactions. Studying the potential role of NHPs as a Plasmodium reservoir for humans will contribute to the ongoing efforts of human malaria elimination, and will help to focus on critical areas that should be considered in achieving this goal.
Non-Human Primate Plasmodium Prevalence
Non-human primates (NHPs) often serve as reservoir of pathogens of human diseases. The yellow fever virus (Ellis and Barrett, 2008), Chikungunya virus (McCrae et al., 1971; Labadie et al., 2010), and the protozoa Giardia lamblia that cause diarrhea (Kowalewski et al., 2011), are all examples of pathogens that originate from NHPs and are infectious to humans. Malaria is caused by parasites of the genus Plasmodium that can infect a wide range of vertebrate hosts from reptiles, birds to mammals. Only five Plasmodium species are known to cause malaria in humans: Plasmodium falciparum, P. knowlesi, P. malariae, P. ovale, and P. vivax. Of these five species, P. falciparum is most common and most lethal (WHO, 2011).
Plasmodium parasites are, however, also found in other primates than humans. Plasmodium reichenowi was the first Plasmodium species identified in NHPs, isolated from an infected chimpanzee (Coatney et al., 1971; Rayner et al., 2011). Other NHP Plasmodium species have been identified (Coatney et al., 1971; Rayner et al., 2011) and their identity can be studied in more detail since the development of molecular tools. A recent study in which mitochondrial, apicoplast, and nuclear gene sequences from 2700 fecal samples from wild-living African apes have been analyzed, showed that Plasmodium species are widely distributed in NHPs and with high prevalence (Liu et al., 2010). The detected parasites could be classified into six discrete major clades, all belonging to the subgenus, termed Laverania, which discriminates them from more divergent Plasmodium species (Liu et al., 2010; Rayner et al., 2011). Interestingly, some of these Laverania clades were only associated with chimpanzee (Pan troglodytes) samples and others only with samples from western lowland gorilla’s (Gorilla gorilla).
Although infections with Plasmodium parasites appear to be host-specific, chimpanzees, and gorillas were nevertheless found to be infected with mitochondrial DNA similar to that of human P. vivax (Liu et al., 2010). Chimpanzees were also found to be infected with P. ovale and P. malariae, which may have contributed to the perseverance of these human malaria parasites in Africa (Duval et al., 2009, 2010; Hayakawa et al., 2009; Krief et al., 2010; Duval and Ariey, 2012). Several studies performed between 1940 and 1956 showed that these species can be transmitted from apes to humans (Rodhain and Dellaert, 1955a,b; Garnham et al., 1956; Rayner et al., 2011). P. falciparum has not been found in wild NHPs but has been detected in captive chimpanzees and bonobos (Liu et al., 2010; Rayner et al., 2011). Interestingly, mitochondrial sequence analysis has revealed that western lowland gorillas probably served as the source of human P. falciparum (Liu et al., 2010) and P. falciparum diverged between 112,000 and 1,036,000 years ago (Baron et al., 2011).
Little is known about the ability of NHP malaria parasites to infect humans, except for the fifth human Plasmodium parasite, P. knowlesi, which was long known only as a parasite of monkeys. The parasite was extensively studied for basic immunological, chemotherapeutic, and biological relationships between malaria parasites and their primate hosts (Collins, 2012). A study in 2004 in Malaysian Borneo revealed that 58% of blood samples from people that had been diagnosed with P. malariae, were actually infected with P. knowlesi after detection by PCR (Singh et al., 2004). These findings have led people to consider P. knowlesi to be the fifth human malaria parasite (White, 2008; Collins, 2012). This example indicates that care should be taken when screening for Plasmodium by standard blood smears or rapid diagnostic tests, which will not reveal if a person is infected with ape Plasmodium.
In recent years the diversity and origin of malaria parasites has been studied in detail using advanced molecular techniques (Krief et al., 2010; Liu et al., 2010; Baron et al., 2011; Prugnolle et al., 2011). In several reviews on this topic the potential risk of a parasite exchange between NHPs and humans has been identified (Kevin, 2009; Rayner et al., 2011; Duval and Ariey, 2012). The authors of these studies suggest that: (a) Humans living near wild-ape communities should be tested for zoonotic infections using molecular approaches capable of differentiating between human and zoonotic parasites, (b) Wild NHPs should be screened for P. vivax, P. malariae, and P. ovale, because the evidence for cross-transmission is the strongest for these Plasmodium species, and (c). Mosquito species should be identified that could transmit Plasmodium parasites between NHPs and humans. Techniques for screening for Plasmodium parasites in humans and NHPs have advanced in recent years and have been applied in some of the studies on NHP Plasmodium mentioned above. The identification of mosquito species that are likely to transmit Plasmodium parasites between NHPs and humans has received little attention and will be discussed below.
The Role of Mosquitoes
Mosquitoes play a key role in the transmission of Plasmodium parasites between humans. Mosquito species characteristics will therefore influence the Plasmodium transmission between humans and NHPs. Transmission studies with malaria vectors have mainly focused on those mosquito species that transmit Plasmodium between humans. Mosquito species that are likely to facilitate a cross-species exchange of Plasmodium have occasionally been studied for their capability of transmitting P. knowlesi in Asia (Collins, 2012). Such studies have not been done in Africa, and consequently knowledge about the role of African mosquitoes as Plasmodium bridge vectors is lacking (Figure 1).
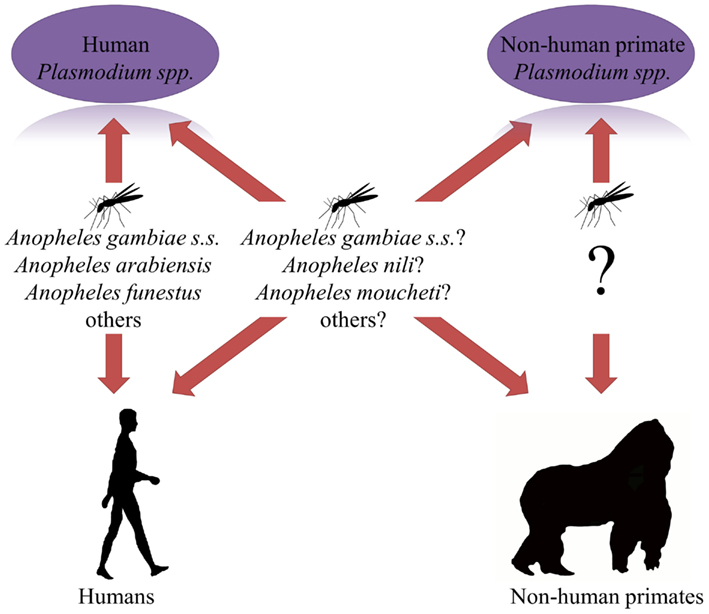
Figure 1. Interactions between mosquitoes, their hosts, and human and non-human primate Plasmodium species in Africa. Question marks indicate unidentified vectors.
Mosquito Susceptibility
About 30 of the approximately 450 species of the insect genus Anopheles are vectors of human malaria (White, 1982). The susceptibility of a mosquito to malaria parasites has a great influence on the effectiveness of a mosquito species as a malaria vector. Little is known about the susceptibility of mosquitoes to the different species of NHP Plasmodia, mainly because these Plasmodium parasites are not available as a laboratory culture. Experiments with a chimpanzee infected with P. reichenowi showed that the malaria mosquito species Anopheles quadrimaculatus, A. stephensi, A. maculatus, A. dirus, and A. culicifacies could be infected with this parasite species. However, A. gambiae s.s. and A. albimanus were refractory to the infection (Blacklock and Adler, 1922; Collins et al., 1986), which suggests that not all vectors of P. falciparum can equally transmit NHP Plasmodium.
Studies that nowadays would be considered unethical have shown that mosquitoes infected with NHP Plasmodia can transfer the parasites to humans and cause illness (Coatney et al., 1961; Schmidt et al., 1961; Bennett and Warren, 1965; Contacos et al., 1970). Current molecular tools should make it possible to identify mosquitoes in the field that are infected with NHP Plasmodium parasites. Detection of parasites in wild apes and humans living near wild-ape communities will support the potential of these mosquitoes as a bridge vector.
Mosquito Habitat
Anopheles gambiae s.s. and A. funestus are important African vectors of human malaria partly because they prefer semi-open and open areas and these are the areas that are often more populated by humans. In forested areas other mosquito species become important as malaria vectors for humans. A. nili, for example can breed in shaded streams and plays a role in the transmission of malaria in localized rainforest areas (Carnevale et al., 1992; Guerra et al., 2006). A. moucheti is considered a forest species and is often reported to be the main vector of human malaria in rainforest areas of Africa (Ollomo et al., 1997; Fontenille et al., 2003; Guerra et al., 2006; Antonio-Nkondjio et al., 2008).
Although studies on mosquitoes that transmit human malaria in rainforest areas are scarce, A. nili, A. moucheti, and other forest Anopheles spp. are possible candidates for malaria parasite transmission from NHPs to humans or vice versa (Figure 1). Deforestation, mining, and agriculture may lead to more open areas, thereby giving other mosquito species, like A. gambiae s.s. and A. funestus, which are known to be effective vectors of human malaria (Gillies and Coetzee, 1987), the opportunity to populate these regions. These human activities will also lead to closer contact between NHPs and human beings, thereby increasing the risk of disease transmission.
Identification of mosquito species that act as malaria vectors in rainforest areas is essential when trying to eliminate or eradicate human malaria, because these species may possibly transfer Plasmodium from NHPs to humans or vice versa. In addition to this, the prevalence of NHP malaria parasites in forest mosquitoes and humans living in these areas should be investigated. This will help to focus on critical areas that should receive additional interventions when trying to eliminate or eradicate malaria.
Host Preference
Host selection by mosquitoes drives the transmission of Plasmodium parasites between humans, and will play a key role in the frequency and efficiency of an exchange of Plasmodium parasite between humans and NHPs. Yet, next to nothing is currently known about the mosquito species that could facilitate such an exchange (Figure 1; Rayner et al., 2011).
Identification of mosquito blood meals by multiplex PCR (Garros et al., 2011) may lead to identification of mosquito species that feed on both humans and NHPs. Collected mosquitoes may also be used to determine Plasmodium infections in mosquitoes, but the number of mosquitoes needed to confirm such infections is high given the relatively low natural infection rate of human Plasmodium infections in mosquitoes (Marchand et al., 2011). Blood-fed mosquitoes can be caught nearby human dwellings with resting boxes, clay pots, or pitfall traps (Service, 1993; Odiere et al., 2007; Qiu et al., 2007). However, the collection of blood-fed mosquitoes in areas where NHPs are naturally abundant may require specific arrangements.
To find their blood host, mosquitoes use physical cues like heat, moisture, vision in addition to chemical cues like carbon dioxide and body odor (Takken and Knols, 1999). Mosquito species with a certain host preference use body odor components to distinguish between different host species (Costantini et al., 1998; Takken and Knols, 1999; Pates et al., 2001). A. gambiae s.s. is one of the most important and best studied vectors of human malaria. It is a highly efficient vector because of its restricted, anthropophilic, host range, and preference to feed indoors (Costantini et al., 1998; Takken and Knols, 1999). Interestingly, its sister species, A. quadriannulatus and A. arabiensis, have a wider host range that is more zoophilic (White et al., 1980; Hunt et al., 1998; Torr et al., 2008) or opportunistic (Costantini et al., 1996, 1998), respectively. Volatiles released by the human skin provide essential cues that guide A. gambiae s.s. to its human host (Smallegange and Takken, 2010). Recently, it was found that the human skin microbiota plays a critically important role in producing these volatiles and thereby in mediating mosquito-host interactions (Figure 2; Verhulst et al., 2009, 2010, 2011).
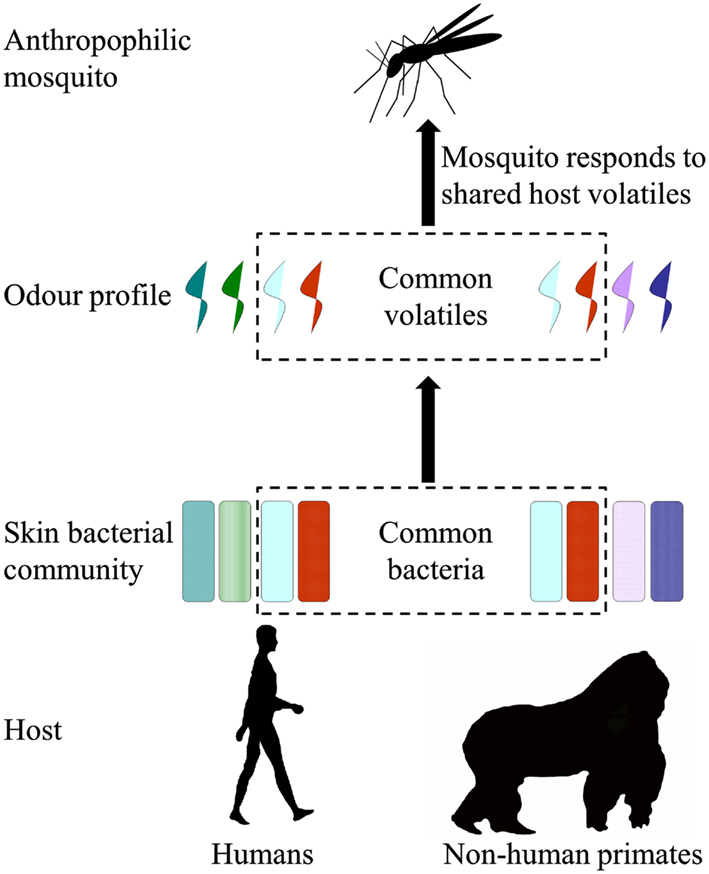
Figure 2. Mosquito-host interactions and the possible response of anthropophilic mosquitoes to volatiles shared by humans and non-human primates.
The volatiles that are released from the human skin have been studied intensively and this resulted in the identification of more than 350 compounds (Bernier et al., 1999, 2000; Curran et al., 2005; Penn et al., 2007; Gallagher et al., 2008). Less is known about the volatiles released by NHPs. Skin glands and their associated bacteria determine which volatiles are produced and therefore play an important role in the attractiveness of human sweat to mosquitoes (Smallegange et al., 2011). Mosquitoes also vary in their attraction to different parts of the human body (Dekker et al., 1998). The type of skin glands and associated bacteria determine the difference between, for example, foot odor (eccrine glands) and axillary odor (apocrine glands). In most mammals, eccrine glands are limited to the friction surfaces of the hands, feet, and tail. By contrast, the eccrine glands of great apes and humans are distributed over the general body surface (Smallegange et al., 2011). Also the extensive aggregation of both apocrine and eccrine glands in the axillae is only found in humans and great apes (Folk and Semken, 1991; Smallegange et al., 2011).
While the distribution of sweat glands is relatively similar between humans and great apes, little is known about the body odor profiles of great apes and other NHPs and how these compare to human body odor. It also remains to be investigated if the similarities or differences between ape and human odor are mediated by their skin microbiota (Smallegange et al., 2011). Mosquitoes caught near NHPs in sanctuaries in Africa may be used in the laboratory to understand how mosquitoes are attracted to NHPs. It is not known if anthropophilic mosquitoes like A. gambiae s.s. are also attracted to odorants from NHPs and thereby may bite both humans and NHPs (Figure 2). Studying the bacteria that play a role in the attractiveness of humans and NHPs to mosquitoes may lead to the identification of volatiles that specifically attract or repel mosquitoes that may form a bridge between humans and NHPs. Next, these volatiles may be used to trap mosquitoes near wild populations of NHPs to identify potential vectors and the malaria parasites they carry (Figure 3). Studying their host-seeking behavior in the laboratory will also reveal which mosquito species would readily bite both humans and NHPs and how restricted this host preference is.
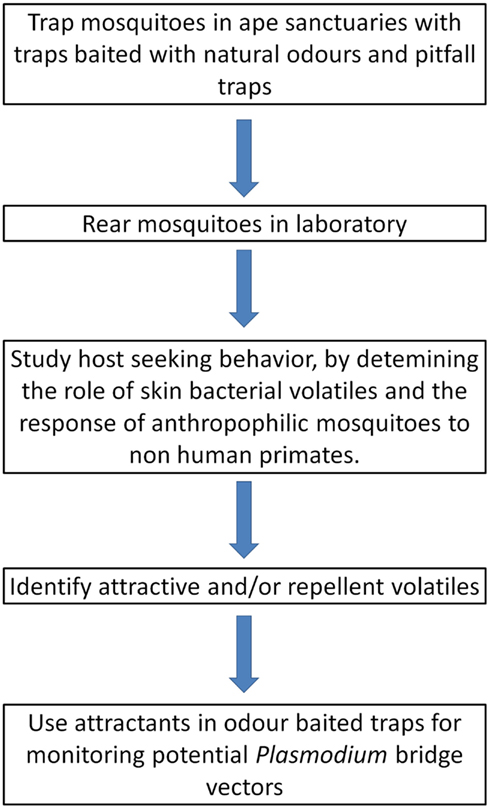
Figure 3. Study design for the identification of mosquito species that may act as bridge vectors of Plasmodium.
Conclusion
In 2007 the Bill and Melinda Gates Foundation, followed by the World Health Organization (WHO) and the Roll Back Malaria (RBM) Partnership declared that the paradigm of malaria control and elimination has been extended to encompass an ultimate goal of malaria eradication (Roberts and Enserink, 2007; Roll Back Malaria Partnership, 2008; Alonso et al., 2011). Since that declaration the discussion on the probability of malaria eradication has intensified (Roberts and Enserink, 2007; Greenwood, 2008; Ferguson et al., 2010; Kappe et al., 2010; Alonso et al., 2011; The malERA Consultative Group on Vector Control, 2011). However, in 2010 there were still an estimated 216 million human cases of malaria and 655,000 deaths and the consequences of malaria may decrease gross domestic product by as much as 1.3% (WHO, 2011).
The possibility that NHPs act as a reservoir for human Plasmodium or are a source of NHP Plasmodium that may affect human health should be investigated before malaria eradication is considered. Current vector control efforts are focused on human habitats, mainly because the most important vector of human malaria A. gambiae s.s. is highly anthropophilic and found only around human settlements (Costantini et al., 1998; Takken and Knols, 1999; Pates et al., 2001). In the process of malaria elimination in any country, emphasis is often laid on monitoring parasite prevalence in humans. The case of P. knowlesi has shown that conventional methods of screening for Plasmodium parasites will not reveal human infections with NHP Plasmodia and therefore humans living near wild-ape communities will need more detailed testing to detect these parasites (Kevin, 2009; Rayner et al., 2011).
The significance of NHPs as a potential source for human infection will largely depend on the vector species that transmit Plasmodium between apes, and whether their behavior facilitates transmission to humans. By understanding how mosquito species are attracted to NHPs, and using this knowledge to identify the vectors that may transmit Plasmodium between apes and humans, we can understand the zoonotic potential of NHP Plasmodium parasites. Identification of the bacterial volatiles that specifically attract mosquitoes that may form a bridge between humans and NHPs may lead to the development of selective odor-baited mosquito traps (Mukabana et al., 2012). These traps can be used for monitoring or mass trapping of mosquito species that facilitate such a cross-species exchange of Plasmodium parasites (Figure 3). Detailed screening for Plasmodium parasites and trapping of vectors in areas where humans and NHPs coexist will help to focus on critical areas that should receive additional interventions when attempting to eliminate or eradicate malaria.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Julian C. Rayner (Wellcome Trust Sanger Institute, Cambridge, UK) for his helpful participation in discussions on this topic. The research of Dr. Niels O. Verhulst is funded by a grant from the Earth and Life Science Foundation (ALW) of the Netherlands Organization for Scientific Research (NWO).
References
Alonso, P. L., Brown, G., Arevalo-Herrera, M., Binka, F., Chitnis, C., Collins, F., Doumbo, O. K., Greenwood, B., Hall, B. F., Levine, M. M., Mendis, K., Newman, R. D., Plowe, C. V., Rodríguez, M. H., Sinden, R., Slutsker, L., and Tanner, M. (2011). A research agenda to underpin malaria eradication. PLoS Med. 8, e1000406. doi:10.1371/journal.pmed.1000406
Antonio-Nkondjio, C., Ndo, C., Kengne, P., Mukwaya, L., Awono-Ambene, P., Fontenille, D., and Simard, F. (2008). Population structure of the malaria vector Anopheles moucheti in the equatorial forest region of Africa. Malar. J. 7, 120.
Baron, J., Higgins, J., and Dzik, W. (2011). A revised timeline for the origin of Plasmodium falciparum as a human pathogen. J. Mol. Evol. 73, 297–304.
Bennett, G. F., and Warren, M. (1965). Transmission of a new strain of Plasmodium cynomolgi to man. J. Parasitol. 51, 79–80.
Bernier, U. R., Booth, M. M., and Yost, R. A. (1999). Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal. Chem. 71, 1–7.
Bernier, U. R., Kline, D. L., Barnard, D. R., Schreck, C. E., and Yost, R. A. (2000). Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for yellow fever mosquito (Aedes aegypti). Anal. Chem. 72, 747–756.
Blacklock, B., and Adler, S. (1922). A parasite resembling Plasmodium falciparum in a chimpanzee. Ann. Trop. Med. Parasitol. 16, 99–106.
Carnevale, P., Le Goff, G., Toto, J. C., and Robert, V. (1992). Anopheles nili as the main vector of human malaria in villages of Southern Cameroon. Med. Vet. Entomol. 6, 135–138.
Coatney, G. R., Collins, W. E., Warren, M., and Contacos, P. G. (1971). The Primate Malarias. Washington, DC: Government Printing Office.
Coatney, G. R., Elder, H. A., Contacos, P. G., Getz, M. E., Greenland, R., Rossan, R. N., and Schmidt, L. H. (1961). Transmission of the M strain of Plasmodium cynomolgi to man. Am. J. Trop. Med. Hyg. 10, 673–678.
Collins, W. E. (2012). Plasmodium knowlesi: a malaria parasite of monkeys and humans. Annu. Rev. Entomol. 57, 107–121.
Collins, W. E., Skinner, J. C., Pappaioanou, M., Broderson, J. R., and Mehaffey, P. (1986). The sporogonic cycle of Plasmodium reichenowi. J. Parasitol. 72, 292–298.
Contacos, P. G., Coatney, G. R., Orihel, T. C., Collins, W. E., Chin, W., and Jeter, M. H. (1970). Transmission of Plasmodium schwetzi from the chimpanzee to man by mosquito bite. Am. J. Trop. Med. Hyg. 19, 190–195.
Costantini, C., Gibson, G., Sagnon, N., Della Torre, A., Brady, J., and Coluzzi, M. (1996). Mosquito responses to carbon dioxide in a West African Sudan Savanna village. Med. Vet. Entomol. 10, 220–227.
Costantini, C., Sagnon, N., Della Torre, A., Diallo, M., Brady, J., Gibson, G., and Coluzzi, M. (1998). Odor-mediated host preferences of West-African mosquitoes, with particular reference to malaria vectors. Am. J. Trop. Med. Hyg. 58, 56–63.
Curran, A. M., Rabin, S. I., Prada, P. A., and Furton, K. G. (2005). Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 31, 0098–0331.
Dekker, T., Takken, W., Knols, B. G. J., Bouman, E., Laak, S., Bever, A., and Huisman, P. W. T. (1998). Selection of biting sites on a human host by Anopheles gambiae s.s., An. arabiensis and An. quadriannulatus. Entomol. Exp. Appl. 87, 295–300.
Duval, L., and Ariey, F. (2012). Ape Plasmodium parasites as a source of human outbreaks. Clin. Microbiol. Infect. 18, 528–532.
Duval, L., Fourment, M., Nerrienet, E., Rousset, D., Sadeuh, S. A., Goodman, S. M., Andriaholinirina, N. V., Randrianarivelojosia, M., Paul, R. E., Robert, V., Ayala, F. J., and Ariey, F. (2010). African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc. Natl. Acad. Sci. U.S.A. 107, 10561–10566.
Duval, L., Nerrienet, E., Rousset, D., Sadeuh Mba, S. A., Houze, S., Fourment, M., Le Bras, J., Robert, V., and Ariey, F. (2009). Chimpanzee malaria parasites related to Plasmodium ovale in Africa. PLoS ONE 4, e5520. doi:10.1371/journal.pone.0005520
Ellis, B. R., and Barrett, A. D. T. (2008). The enigma of yellow fever in East Africa. Rev. Med. Virol. 18, 331–346.
Ferguson, H. M., Dornhaus, A., Beeche, A., Borgemeister, C., Gottlieb, M., Mulla, M. S., Gimnig, J. E., Fish, D., and Killeen, G. F. (2010). Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 7, e1000303. doi:10.1371/journal.pmed.1000303
Folk, G. E., and Semken, A. (1991). The evolution of sweat glands. Int. J. Biometeorol. 35, 180–186.
Fontenille, D., Cohuet, A., Awono-Ambene, P. H., Antonio-Nkondjio, C., Wondji, C., Kengne, P., Dia, I., Boccolini, D., Duchemin, J. B., Rajaonarivelo, V., Dabire, R., Adja-Akre, M., Ceainu, C., Le Goff, G., and Simard, F. (2003). Systematics and biology of Anopheles vectors of Plasmodium in Africa, recent data. Med. Trop. (Mars.) 63, 247–253.
Gallagher, M., Wysocki, C. J., Leyden, J. J., Spielman, A. I., Sun, X., and Preti, G. (2008). Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 159, 780–791.
Garnham, P. C., Lainson, R., and Gunders, A. E. (1956). Some observations on malaria parasites in a chimpanzee, with particular reference to the persistence of Plasmodium reichenowi and Plasmodium vivax. Ann. Soc. Belg. Med. Trop. 36, 811–821.
Garros, C., Gardès, L., Allène, X., Rakotoarivony, I., Viennet, E., Rossi, S., and Balenghien, T. (2011). Adaptation of a species-specific multiplex PCR assay for the identification of blood meal source in Culicoides (Ceratopogonidae: Diptera): applications on Palaearctic biting midge species, vectors of Orbiviruses. Infect. Genet. Evol. 11, 1103–1110.
Gillies, M. T., and Coetzee, M. (1987). A Supplement to the Anophelinae of Africa South of the Sahara. Johannesburg: The South African Institute for Medical Research.
Greenwood, B. M. (2008). Control to elimination: implications for malaria research. Trends Parasitol. 24, 449–454.
Guerra, C. A., Snow, R. W., and Hay, S. I. (2006). A global assessment of closed forests, deforestation and malaria risk. Ann. Trop. Med. Parasitol. 100, 189–204.
Hayakawa, T., Arisue, N., Udono, T., Hirai, H., Sattabongkot, J., Toyama, T., Tsuboi, T., Horii, T., and Tanabe, K. (2009). Identification of Plasmodium malariae, a human malaria parasite, in imported chimpanzees. PLoS ONE 4, e7412. doi:10.1371/journal.pone.0007412
Hunt, R. H., Coetzee, M., and Fettene, M. (1998). The Anopheles gambiae complex: a new species from Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 92, 231–235.
Kappe, S. H. I., Vaughan, A. M., Boddey, J. A., and Cowman, A. F. (2010). That was then but this is now: malaria research in the time of an eradication agenda. Science 328, 862–866.
Kowalewski, M. M., Salzer, J. S., Deutsch, J. C., Raño, M., Kuhlenschmidt, M. S., and Gillespie, T. R. (2011). Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: patterns of zoonotic protozoa infection relative to degree of human–primate contact. Am. J. Primatol. 73, 75–83.
Krief, S., Escalante, A. A., Pacheco, M. A., Mugisha, L., André, C., Halbwax, M., Fischer, A., Krief, J.-M., Kasenene, J. M., Crandfield, M., Cornejo, O. E., Chavatte, J.-M., Lin, C., Letourneur, F., Grüner, A. C., McCutchan, T. F., Rénia, L., and Snounou, G. (2010). On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from bonobos. PLoS Pathog. 6, e1000765. doi:10.1371/journal.ppat.1000765
Labadie, K., Larcher, T., Joubert, C., Mannioui, A., Delache, B., Brochard, P., Guigand, L., Dubreil, L., Lebon, P., Verrier, B., De Lamballerie, X., Suhrbier, A., Cherel, Y., Le Grand, R., and Roques, P. (2010). Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 120, 894–906.
Liu, W., Li, Y., Learn, G. H., Rudicell, R. S., Robertson, J. D., Keele, B. F., Ndjango, J.-B. N., Sanz, C. M., Morgan, D. B., Locatelli, S., Gonder, M. K., Kranzusch, P. J., Walsh, P. D., Delaporte, E., Mpoudi-Ngole, E., Georgiev, A. V., Muller, M. N., Shaw, G. M., Peeters, M., Sharp, P. M., Rayner, J. C., and Hahn, B. H. (2010). Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature 467, 420–425.
Marchand, R. P., Culleton, R., Maeno, Y., Quang, N. T., and Nakazawa, S. (2011). Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, Southern Vietnam. Emerging Infect. Dis. 17, 1232–1239.
McCrae, A. W. R., Henderson, B. E., Kirya, B. G., and Sempala, S. D. K. (1971). Chikungunya virus in the entebbe area of Uganda: isolations and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 65, 152–168.
Mukabana, W., Mweresa, C., Otieno, B., Omusula, P., Smallegange, R. C., van Loon, J. J. A., and Takken, W. (2012). A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J. Chem. Ecol. 38, 235–244.
Odiere, M., Bayoh, M. N., Gimnig, J., Vulule, J., Irungu, L., and Walker, E. (2007). Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in Western Kenya with clay pots. J. Med. Entomol. 44, 14–22.
Ollomo, B., Karch, S., Bureau, P., Elissa, N., Georges, A. J., and Millet, P. (1997). Lack of malaria parasite transmission between apes and humans in Gabon. Am. J. Trop. Med. Hyg. 56, 440–445.
Pates, H. V., Takken, W., Stuke, K., and Curtis, C. F. (2001). Differential behaviour of Anopheles gambiae sensu stricto (Diptera: Culicidae) to human and cow odours in the laboratory. Bull. Entomol. Res. 91, 289–296.
Penn, D. J., Oberzaucher, E., Grammer, K., Fischer, G., Soini, H. A., Wiesler, D., Novotny, M. V., Dixon, S. J., Xu, Y., and Brereton, R. G. (2007). Individual and gender fingerprints in human body odour. J. R. Soc. Interface 4, 331–340.
Prugnolle, F., Durand, P., Ollomo, B., Duval, L., Ariey, F., Arnathau, C., Gonzalez, J.-P., Leroy, E., and Renaud, F. (2011). A fresh look at the origin of Plasmodium falciparum, the most malignant malaria agent. PLoS Pathog. 7, e1001283. doi:10.1371/journal.ppat.1001283
Qiu, Y. T., Spitzen, J., Smallegange, R. C., and Knols, B. (2007). “Monitoring systems for adult insect pests and disease vectors,” in Emerging Pests and Vector-Borne Diseases in Europe, eds W. Takken, and B. Knols (Wageningen: Wageningen Academic Publishers), 329–353.
Rayner, J. C., Liu, W., Peeters, M., Sharp, P. M., and Hahn, B. H. (2011). A plethora of Plasmodium species in wild apes: a source of human infection? Trends Parasitol. 27, 222–229.
Roberts, L., and Enserink, M. (2007). Malaria: did they really say eradication? Science 318, 1544–1545.
Rodhain, J., and Dellaert, R. (1955a). Study of Plasmodium schwetzi E. Brumpt. II. Transmission of Plasmodium schwetzi to man. Ann. Soc. Belg. Med. Trop. 35, 73–76.
Rodhain, J., and Dellaert, R. (1955b). Study of Plasmodium schwetzi E. Brumpt. III. Transmission of Plasmodium schwetzi to man. Ann. Soc. Belg. Med. Trop. 35, 757–777.
Roll Back Malaria Partnership. (2008). The Global Malaria Action Plan for a Malaria Free World, Geneva. Available at: http://www.rollbackmalaria.org/gmap/gmap.pdf. [Accessed 19 January 2012; Online].
Schmidt, L. H., Greenland, R., and Genther, C. S. (1961). The transmission of Plasmodium cynomolgi to man. Am. J. Trop. Med. Hyg. 10, 679–688.
Singh, B., Sung, L. K., Matusop, A., Radhakrishnan, A., Shamsul, S. S. G., Cox-Singh, J., Thomas, A., and Conway, D. J. (2004). A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363, 1017–1024.
Smallegange, R. C., and Takken, W. (2010). “Host-seeking behaviour of mosquitoes – responses to olfactory stimuli in the laboratory,” in Olfaction in Vector-Host Interactions, eds W. Takken, and B. Knols (Wageningen: Wageningen Academic Publishers), 143–180.
Smallegange, R. C., Verhulst, N. O., and Takken, W. (2011). Sweaty skin: an invitation to bite? Trends Parasitol. 27, 143–148.
Takken, W., and Knols, B. G. J. (1999). Odor-mediated behavior of afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131–157.
The malERA Consultative Group on Vector Control. (2011). A research agenda for malaria eradication: vector control. PLoS Med. 8, e1000401. doi:10.1371/journal.pmed.1000401
Torr, S. J., Della Torre, A., Calzetta, M., Costantini, C., and Vale, G. A. (2008). Towards a fuller understanding of mosquito behaviour: use of electrocuting grids to compare the odour-orientated responses of Anopheles arabiensis and An. quadriannulatus in the field. Med. Vet. Entomol. 22, 93–108.
Verhulst, N. O., Beijleveld, H., Knols, B. G. J., Takken, W., Schraa, G., Bouwmeester, H. J., and Smallegange, R. C. (2009). Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 8, 302.
Verhulst, N. O., Qiu, Y. T., Beijleveld, H., Maliepaard, C., Knights, D., Schulz, S., Berg-Lyons, D., Lauber, C. L., Verduijn, W., Haasnoot, G. W., Mumm, R., Bouwmeester, H. J., Claas, F. H. J., Dicke, M., Loon, J. J. A. V., Takken, W., Knight, R., and Smallegange, R. C. (2011). Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS ONE 6, e28991. doi:10.1371/journal.pone.0028991
Verhulst, N. O., Takken, W., Dicke, M., Schraa, G., and Smallegange, R. C. (2010). Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol. Ecol. 74, 1–9.
White, G. B., Tessfaye, F., Boreham, P. F. L., and Lemma, G. (1980). Malaria vector capacity of Anopheles arabiensis and An. quadriannulatus in Ethiopia: chromosomal interpretation after 6 years storage of field preparations. Trans. R. Soc. Trop. Med. Hyg. 74, 683–684.
Keywords: apes, cross-species transmission, host preference, mosquito behavior, Plasmodium
Citation: Verhulst NO, Smallegange RC and Takken W (2012) Mosquitoes as potential bridge vectors of malaria parasites from non-human primates to humans. Front. Physio. 3:197. doi: 10.3389/fphys.2012.00197
Received: 31 January 2012; Accepted: 22 May 2012;
Published online: 11 June 2012.
Edited by:
Rubén Bueno-Marí, University of Valencia, SpainReviewed by:
Beatrice Hahn, University of Pennsylvania, USAWolfgang Richard Mukabana, International Centre of Insect Physiology and Ecology, Kenya
Stephen Rich, University of Massachusetts, USA
Copyright: © 2012 Verhulst, Smallegange and Takken. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Niels O. Verhulst, Laboratory of Entomology, Wageningen University and Research Centre, P.O. Box 8031, 6700 EH Wageningen, Netherlands. e-mail:bmllbHMudmVyaHVsc3RAd3VyLm5s

 Renate C. Smallegange1,2 and Willem Takken1
Renate C. Smallegange1,2 and Willem Takken1