- UCLA Cardiac Arrhythmia Center, UCLA Health System/David Geffen School of Medicine at UCLA, University of California, Los Angeles, Los Angeles, CA, USA
Atrial fibrillation (AF) is the most common arrhythmia prompting clinical presentation, is associated with significant morbidity and mortality. The incidence and prevalence of this arrhythmia is expected to grow significantly in the coming decades. Of the available pharmacologic and non-pharmacologic treatment options, the fastest growing and most intensely studied is catheter-based ablation therapy for AF. Given the varying success rates for AF ablation, the increasingly complex factors that need to be taken into account when deciding to proceed with ablation, as well as varying definitions of procedural success, accurate detection of arrhythmia recurrence and its burden is of significance. Detecting and monitoring AF recurrence following catheter ablation is therefore an important consideration. Multiple studies have demonstrated the close relationship between the intensity of rhythm monitoring with wearable ambulatory cardiac monitors, or implantable cardiac rhythm monitors and the detection of arrhythmia recurrence. Other studies have employed algorithms dependent on intensive monitoring and arrhythmia detection in the decision tree on whether to proceed with repeat ablation or medical therapy. In this review, we discuss these considerations, types of monitoring devices, and implications for monitoring AF recurrence following catheter ablation.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia prompting presentation for clinical care (January et al., 2014). In patients over 40 years of age, the prevalence of AF is 2.3%; 6% in patients 65 years and over (Feinberg et al., 1995); and rising to 10% in those greater than 80 years (Lloyd-Jones et al., 2010) with these values expected to rise significantly in the coming decades (Go et al., 2001). Although, AF has been associated with increased mortality in the long-term (Benjamin et al., 1998), the most devastating non-fatal complication of AF remains cerebrovascular accidents (CVA) (Flegel et al., 1987; Wolf et al., 1991). To this end, the importance of anticoagulation in risk reduction of CVA, is strongly emphasized, and widely recognized (Gage et al., 2001; Wyse et al., 2002; January et al., 2014). Despite AF being one of the earliest recognized arrhythmias, treatment options for AF remain limited.
In the past two decades, few new pharmacologic options have become available, all with limited efficacy and potential toxicity. Following the recognition of pulmonary vein triggers (Haissaguerre et al., 1998), catheter ablation of AF continues to gain acceptance as a viable treatment option (Tung et al., 2012), with its main indication being the elimination of symptomatic AF, when pharmacologic agents are contraindicated or have failed (January et al., 2014).
As the decision to proceed with AF ablation depends on a number of factors including age, comorbidities, patient preference, contraindications to anticoagulation, and left atrial dimensions/fibrosis, the determination of success following ablation similarly depends on similar factors (Kircher et al., 2012), since AF is typically not considered cured following initial catheter ablation. As a result, the methods employed in detecting and monitoring AF recurrence following catheter ablation have a significant impact on the determination of “success.” Measures used to define success include complete elimination or reduction of AF burden, and the elimination or reduction of symptoms. Without adequate monitoring, the complete absence of symptoms may not equate absence of AF, and further, evaluating reduction or elimination of AF is not possible with incomplete monitoring.
Objectives of Monitoring after Atrial Fibrillation Ablation
Success rates for catheter ablation of AF, defined typically as freedom from AF, have ranged from 60 to 90%, depending on the study, and the number of procedures performed (January et al., 2014). At present, the indication for catheter ablation of AF is symptom control in patients for whom pharmacologic options are contraindicated or have failed (Fuster et al., 2011; January et al., 2014). As such, it stands to reason that patient-reported symptoms should be the primary factor used for determination of outcome/success following ablation. This would certainly simplify follow up after catheter ablation for AF. In addition, the indication for therapeutic anticoagulation is individualized, and is based on CVA risk factors, most commonly, the CHADS2 (Gage et al., 2001) and/or CHADS2-VASc (Camm et al., 2010) scores, and is independent of the outcome following catheter ablation of AF.
However, a number of important considerations should be entertained. First, symptom reporting by patients is known to be an inaccurate estimation of true AF burden, even in patients who have experienced symptomatic episodes of AF. In a study of patients with paroxysmal or persistent AF, with implanted pacemakers for other indications, AF was detected in 88% of patients over 19 ± 11 months of follow up, with over a third of patients with long duration episodes of AF (>48 h) being asymptomatic (Israel et al., 2004). In the same study, the authors also observed poor correlation between reported symptoms and AF, with up to 40% of patients reporting symptoms consistent with AF, but no such documentation on device interrogations. In another study, up to 45% of patients with AF recurrences documented by an implanted loop recorder, reported no symptoms of AF (Tondo et al., 2013). Further, following catheter ablation for AF, the perception of symptoms may also change, either due to modulation of atrial tissue and/or innervation, or a placebo effect. In a study tracking the burden of silent AF with continuous 7-day Holter monitoring before and serially following ablation for AF, the percentage of silent episodes of AF increased significantly at 3, 6, and 12 months following catheter ablation (Hindricks et al., 2005). Intensive monitoring early (60 days) after AF ablation may be useful in identifying patients at risk of recurrence in the long-term (12-months) (Pokushalov et al., 2012), such that targeted strategies for management of such patients can be applied early, and potentially before symptoms develop. Pokushalov and colleagues employed such a strategy, and identified patients with recurrences at 3 months following AF ablation, randomizing them to medical therapy, or if possible triggers were identified with an implantable monitor, to repeat ablation (Pokushalov et al., 2011). The rate of AF recurrence at 12 months was significantly greater in patients randomized to medical therapy than those randomized to repeat ablation.
Clinical factors associated with arrhythmia recurrences may be related to the length of time post-ablation, and hence duration of follow up. In one study (Kim et al., 2014) total ablation time correlated with recurrences at 6 months. However, at 1- and 2-year follow up, other factors such as the presence of hypertension, left atrial size, left atrial appendage (LAA) emptying fraction under 20%, and decreased LAA emptying velocity were correlated with AF recurrence. This suggests that follow up monitoring and arrhythmia detection for certain patients may need to be extended past the typical 3-, 6-, and 12-month periods.
Lastly, given the variable reported success rates following catheter ablation for AF, continued research on technologies and strategies to improve AF ablation will rely heavily on accurate demonstration of no AF recurrence. It is well recognized that for patients with asymptomatic AF, the intensity of follow up is correlated with the incidence of recurrences (Roche et al., 2002). As patient symptoms may change following ablation, identification of such episodes, necessitating continued AAD use or other management strategies would be important. Accurate documentation of true AF burden following catheter ablation may also be very useful in discussions with patients. Demonstrating the impact of AF ablation or lack thereof may be useful in providing objective data to patients, and reported symptoms with AF recurrences. Such data may help patients participate in clinical decision-making regarding their arrhythmia.
Atrial Arrhythmia Recurrences Following Catheter Ablation of Atrial Fibrillation
Although AF recurrences following catheter ablation are most commonly due to PV reconnection (Callans et al., 2004) or incomplete ablation lines (Del Greco et al., 2008), other atrial arrhythmias may occur after AF ablation. Focal and micro-reentrant atrial tachycardias, macro-reentrant atrial flutters, and premature ventricular contractions have been reported following catheter ablation (Gerstenfeld et al., 2004; Mesas et al., 2004; Deisenhofer et al., 2006; Fiala et al., 2007; Sawhney et al., 2011; Patel et al., 2014). These arrhythmias are often complex due atrial scars created by prior ablation, and may involve multiple macro-reentrant circuits. Ablation of the critical isthmus of one circuit may shift the arrhythmia to another circuit. Of particular importance is the characterization of the recurrent arrhythmia, as this is useful in pre-procedural planning. Patients may also have more than one arrhythmia induced at electrophysiology study, and as a result, the determination of the clinical arrhythmia is crucial. Focal atrial tachycardias from pulmonary veins or other regions may be differentiated from macro-reentrant tachycardias with monitoring (Gerstenfeld et al., 2007; Chang et al., 2011; Wasmer et al., 2012), and a variety of approaches may be devised prior to electrophysiology study.
Existing Devices for Rhythm Monitoring
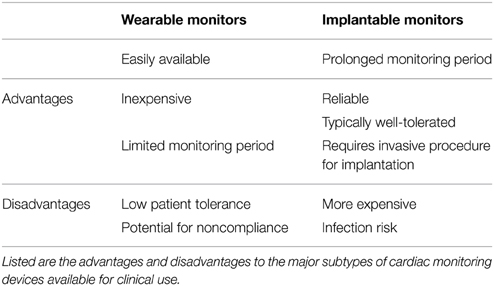
There are a variety of different cardiac rhythm monitoring devices available (Table 1) clinically for patient use today. They however fall into two major subtypes: wearable non-invasive and implanted devices.
Wearable Cardiac Rhythm Monitors
Although multiple wearable devices exist, they can be classified by length of time for which they are worn, the number of leads, and whether real-time monitoring is performed or not. Holter monitors can be typically worn for 24 h, 7 or 30 days or for any time interval provided the device is capable of recording for that length of time. Advantages and limitations are specific to each device selected (Table 2), with the relative ease of use of short-term devices counterbalanced by low sensitivity and specificity of such devices for arrhythmia detection. Devices worn for longer periods of time, 7–30 days have the advantage of being more sensitive and specific in determining arrhythmia recurrence, however, these gains are countered by relative discomfort experienced by patients, with increasing noncompliance with wearing such devices continuously. A number of studies have assessed the diagnostic utility of different durations of monitoring (Kottkamp et al., 2004; Senatore et al., 2005; Dagres et al., 2010). Kottkamp et al. demonstrated that compared to the conventional 24-h monitoring, 7-day ECG recordings detected more arrhythmias in the early post-ablation period, and at 6 and 12 months. The yield of a longer monitoring period was underscored by the study from Senatore et al., where daily and symptom-triggered recordings transmitted trans-telephonically (for 3 months beginning 1 month post-ablation) was compared to 24-h Holter and ECG recordings. In this study, arrhythmia detection by trans-telephonic monitoring was twice that in conventional Holter and ECG recordings. Dagres and colleagues evaluated over 200 consecutive patients with 7-day Holter monitoring 6 months post-ablation. By evaluating the data collected at increasing 24-h intervals, they showed that any Holter monitoring period of less than 5 days would have significantly underestimated the rates of recurrence.
Overcoming some of the limitations of Holter recordings, the ZIO Patch system offers improved ease of use for patients, as it is a wearable adhesive patch without interconnecting wires, and can provide continuous monitoring for 14 days. Additionally patients can shower with this system in place, but not with the standard Holter system. In a small study comparing 14-day ZIO Patch to a 24-h Holter, not surprisingly, the patch system performed better at detecting arrhythmias (Barrett et al., 2014). Other patch devices and electrodes are currently under development (Lobodzinski, 2013), with the goal of miniaturizing the devices and electrodes used for monitoring.
Implantable Cardiac Rhythm Monitors
Subcutaneous implantable cardiac rhythm monitors (ICRMs) improve the sensitivity and specificity of cardiac rhythm monitoring, and overcome the limitation on patient non-compliance seen with long-term wearable devices (Table 2). If placed so that there is no interference from myopotentials, or movement of the monitor within the device pocket, implanted monitors with AF recognition algorithms are a powerful means of rhythm monitoring. In the XPECT trial, Hindricks et al. demonstrated that compared to a specialized Holter recording, ICRMs exhibited a sensitivity, specificity, positive predictive value, and negative predictive value of 96.1, 85.4, 79.3, and 97.4%, respectively, for identifying patients with any AF (Hindricks et al., 2010). However, implanted monitors are limited to only a small subset of patients who may have other indications for intensive monitoring, and have not been widely adopted for routine clinical use following AF ablation. Outside of research studies, the role that routine use of ICRMs may play in post-AF ablation rhythm management remains unclear. The recently introduced Medtronic Reveal LINQ may offer additional advantages due to its small size, reduced procedural requirements for implantation, and smaller size on the chest wall. Rather than a small incision and blunt dissection to create a pocket for the device, the LINQ is implanted by a syringe injector with an incision <1 cm. Whether the availability of such a small device and the ease of implantation will increase its use for monitoring of arrhythmias following catheter ablation outside of research studies remains to be seen. Apart from subcutaneous ICRMs, implanted permanent pacemakers (PPMs) or cardioverter-defibrillators (ICDs) with an atrial lead can also be used for monitoring recurrence following AF ablation. Due to larger memory capabilities, these devices can store more details and for longer periods than standard subcutaneous ICRMs. Another added advantage is the ability to assess the cycle lengths of AF in greater detail, as well as improved discrimination of AF from other atrial arrhythmias that may mimic AF. These devices however, have a focused applicability for patients with AF. Only patients with AF and an additional indication for pacing such as sick sinus syndrome in the case of PPMs, and cardiomyopathy or ventricular arrhythmias in the case of ICDs, will qualify for these devices.
Our Approach to Monitoring Recurrence Following Catheter Ablation of Atrial Fibrillation
Routine monitoring following catheter ablation of AF is usually at the discretion of the physician and/or institution. At our center, patients typically undergo 7- to 14-day ambulatory telemetry monitoring during the initial evaluation and prior to catheter ablation to document AF burden. In addition to quantifying the severity of AF and correlation with symptoms, identification of other arrhythmias (narrow complex tachycardias such as atrioventricular nodal reentrant, atrioventricular reciprocating, and focal or macro-reentrant atrial tachycardias or flutters) that degenerate into AF may occur, necessitating a different ablation strategy. Following catheter ablation, patients are seen for a 30-day outpatient follow up visit during which a 12-lead ECG is performed. At 3, 6, and 12 months post-ablation, patients undergo 7-day Holter monitoring to assess AF recurrence, burden, identification of other arrhythmias, and correlation with any reported symptoms. In patients with implanted devices (pacemakers and defibrillators with an atrial lead, or loop recorders) device interrogation is typically performed to assess for atrial high rate episodes, device mode switches, and overall atrial tachycardia/AF burden.
Implications for Monitoring AF Recurrences
The role of implanted monitoring devices strictly for monitoring recurrences following catheter ablation is unclear at present. Outside of research studies, routine use of ICRMs has not been reported in the literature. Routine clinical used of extended Holter monitoring for 7 days (at least 5 days) is supported by multiple clinical studies (Kottkamp et al., 2004; Piorkowski et al., 2005; Senatore et al., 2005; Dagres et al., 2010). A recent provocative study (Charitos et al., 2014) used computer simulations to predict the diagnostic utility of various durations of Holter monitoring, and the frequency with which they would need to be performed in a 12-year period. The authors reviewed the rhythm histories from 647 patients with AF and ICRMs, and used computational simulations to assess arrhythmia detection for 24-h, 7-, 14-, and 30-day monitors. The authors observed that the frequency with which any monitor needed to be performed depended on the rate and temporal aggregation of AF recurrences as well as the duration of monitoring, in the population of interest. They found that to achieve a >95% sensitivity in detecting AF recurrences, more frequent monitoring with shorter duration monitors (greater than twelve 24-h; six 7-day; four 14-day; and three 30-day monitors) were needed. When the sensitivity over the monitored time was assessed, more frequent but shorter monitoring durations were more time effective. The implication of this study is that patients are under-monitored for arrhythmia recurrence after catheter ablation, if they do not have an implanted device.
Conclusion
The population burden of AF is expected to increase dramatically in the next decades. It will become increasingly important to adequately assess the success rates of non-pharmacologic and pharmacologic therapies to improve patient care. With the advent of smaller wearable monitoring devices, and with significant improvements in the size, implantation procedures for ICRMs, the landscape of monitoring AF recurrence following not only catheter ablation procedures, but also with pharmacologic therapies will likely significantly change.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barrett, P. M., Komatireddy, R., Haaser, S., Topol, S., Sheard, J., Encinas, J., et al. (2014). Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 127, 95. e11–97. doi: 10.1016/j.amjmed.2013.10.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Benjamin, E. J., Wolf, P. A., D'agostino, R. B., Silbershatz, H., Kannel, W. B., and Levy, D. (1998). Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation 98, 946–952. doi: 10.1161/01.CIR.98.10.946
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Callans, D. J., Gerstenfeld, E. P., Dixit, S., Zado, E., Vanderhoff, M., Ren, J. F., et al. (2004). Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 15, 1050–1055. doi: 10.1046/j.1540-8167.2004.04052.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Camm, A. J., Kirchhof, P., Lip, G. Y., Schotten, U., Savelieva, I., Ernst, S., et al. (2010). Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31, 2369–2429. doi: 10.1093/eurheartj/ehq278
Chang, S. L., Tsao, H. M., Lin, Y. J., Lo, L. W., Hu, Y. F., Tuan, T. C., et al. (2011). Differentiating macroreentrant from focal atrial tachycardias occurred after circumferential pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 22, 748–755. doi: 10.1111/j.1540-8167.2010.02002.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Charitos, E. I., Ziegler, P. D., Stierle, U., Robinson, D. R., Graf, B., Sievers, H. H., et al. (2014). How often should we monitor for reliable detection of atrial fibrillation recurrence? Efficiency considerations and implications for study design. PLoS ONE 9:e89022. doi: 10.1371/journal.pone.0089022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dagres, N., Kottkamp, H., Piorkowski, C., Weis, S., Arya, A., Sommer, P., et al. (2010).:Influence of the duration of Holter monitoring on the detection of arrhythmia recurrences after catheter ablation of atrial fibrillation: implications for patient follow-up. Int. J. Cardiol. 139, 305–306. doi: 10.1016/j.ijcard.2008.10.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deisenhofer, I., Estner, H., Zrenner, B., Schreieck, J., Weyerbrock, S., Hessling, G., et al. (2006). Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation. Europace 8, 573–582. doi: 10.1093/europace/eul077
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Del Greco, M., Cristoforetti, A., Marini, M., and Ravelli, F. (2008). Image fusion shows the role of incomplete ablation lines in creating a substrate for left atrial flutter occurring after atrial fibrillation ablation. Heart Rhythm 5, 163–164. doi: 10.1016/j.hrthm.2007.09.021
Feinberg, W. M., Blackshear, J. L., Laupacis, A., Kronmal, R., and Hart, R. G. (1995). Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 155, 469–473. doi: 10.1001/archinte.1995.00430050045005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fiala, M., Chovancik, J., Moravec, R., Wojnarova, D., Szymeczek, H., Neuwirth, R., et al. (2007). [Recurrent arrhythmias after catheter ablation of originally paroxysmal atrial fibrillation and results of repeat ablation]. Vnitr. Lek. 53, 1248–1254.
Flegel, K. M., Shipley, M. J., and Rose, G. (1987). Risk of stroke in non-rheumatic atrial fibrillation. Lancet 1, 526–529. doi: 10.1016/S0140-6736(87)90174-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fuster, V., Ryden, L. E., Cannom, D. S., Crijns, H. J., Curtis, A. B., Ellenbogen, K. A., et al. (2011). 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 57, e101–e198. doi: 10.1016/j.jacc.2010.09.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gage, B. F., Waterman, A. D., Shannon, W., Boechler, M., Rich, M. W., and Radford, M. J. (2001). Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285, 2864–2870. doi: 10.1001/jama.285.22.2864
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gerstenfeld, E. P., Callans, D. J., Dixit, S., Russo, A. M., Nayak, H., Lin, D., et al. (2004). Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 110, 1351–1357. doi: 10.1161/01.CIR.0000141369.50476.D3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gerstenfeld, E. P., Dixit, S., Bala, R., Callans, D. J., Lin, D., Sauer, W., et al. (2007). Surface electrocardiogram characteristics of atrial tachycardias occurring after pulmonary vein isolation. Heart Rhythm 4, 1136–1143. doi: 10.1016/j.hrthm.2007.05.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Go, A. S., Hylek, E. M., Phillips, K. A., Chang, Y., Henault, L. E., Selby, J. V., et al. (2001). Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285, 2370–2375. doi: 10.1001/jama.285.18.2370
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haissaguerre, M., Jais, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659–666. doi: 10.1056/NEJM199809033391003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hindricks, G., Piorkowski, C., Tanner, H., Kobza, R., Gerds-Li, J. H., Carbucicchio, C., et al. (2005). Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 112, 307–313. doi: 10.1161/CIRCULATIONAHA.104.518837
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hindricks, G., Pokushalov, E., Urban, L., Taborsky, M., Kuck, K. H., Lebedev, D., et al. (2010). Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ. Arrhythm. Electrophysiol. 3, 141–147. doi: 10.1161/CIRCEP.109.877852
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Israel, C. W., Gronefeld, G., Ehrlich, J. R., Li, Y. G., and Hohnloser, S. H. (2004). Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J. Am. Coll. Cardiol. 43, 47–52. doi: 10.1016/j.jacc.2003.08.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
January, C. T., Wann, L. S., Alpert, J. S., Calkins, H., Cigarroa, J. E., Cleveland, J. C. Jr., et al. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 64, e1–e76. doi: 10.1016/j.jacc.2014.03.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, M. N., Lee, J. J., Kim, S. A., Kim, Y. H., Choi, J. I., Park, S. M., et al. (2014). The difference of predictors for recurrence after catheter ablation of non-paroxysmal atrial fibrillation according to follow-up period. Int. Heart J. 55, 312–318. doi: 10.1536/ihj.13-370
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kircher, S., Hindricks, G., and Sommer, P. (2012). Long-term success and follow-up after atrial fibrillation ablation. Curr. Cardiol. Rev. 8, 354–361. doi: 10.2174/157340312803760758
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kottkamp, H., Tanner, H., Kobza, R., Schirdewahn, P., Dorszewski, A., Gerds-Li, J. H., et al. (2004). Time courses and quantitative analysis of atrial fibrillation episode number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification: early or delayed cure? J. Am. Coll. Cardiol. 44, 869–877. doi: 10.1016/j.jacc.2004.04.049
Lloyd-Jones, D., Adams, R. J., Brown, T. M., Carnethon, M., Dai, S., De Simone, G., et al. (2010). Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121, 948–954. doi: 10.1161/CIRCULATIONAHA.109.192666
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lobodzinski, S. S. (2013). ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog. Cardiovasc. Dis. 56, 224–229. doi: 10.1016/j.pcad.2013.08.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mesas, C. E., Pappone, C., Lang, C. C., Gugliotta, F., Tomita, T., Vicedomini, G., et al. (2004). Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J. Am. Coll. Cardiol. 44, 1071–1079. doi: 10.1016/j.jacc.2004.05.072
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Patel, P. J., Ahlemeyer, L., Freas, M., Cooper, J. M., Marchlinski, F. E., Callans, D. J., et al. (2014). Outflow tract premature ventricular depolarizations after atrial fibrillation ablation may reflect autonomic influences. J. Interv. Card. Electrophysiol. 41, 187–192. doi: 10.1007/s10840-014-9914-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piorkowski, C., Kottkamp, H., Tanner, H., Kobza, R., Nielsen, J. C., Arya, A., et al. (2005). Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 16, 1286–1292. doi: 10.1111/j.1540-8167.2005.00245.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pokushalov, E., Romanov, A., Corbucci, G., Artyomenko, S., Turov, A., Shirokova, N., et al. (2011). Use of an implantable monitor to detect arrhythmia recurrences and select patients for early repeat catheter ablation for atrial fibrillation: a pilot study. Circ. Arrhythm. Electrophysiol. 4, 823–831. doi: 10.1161/CIRCEP.111.964809
Pokushalov, E., Romanov, A., Corbucci, G., Bairamova, S., Losik, D., Turov, A., et al. (2012). Does atrial fibrillation burden measured by continuous monitoring during the blanking period predict the response to ablation at 12-month follow-up? Heart Rhythm 9, 1375–1379. doi: 10.1016/j.hrthm.2012.03.047
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Roche, F., Gaspoz, J. M., Da Costa, A., Isaaz, K., Duverney, D., Pichot, V., et al. (2002). Frequent and prolonged asymptomatic episodes of paroxysmal atrial fibrillation revealed by automatic long-term event recorders in patients with a negative 24-hour Holter. Pacing Clin. Electrophysiol. 25, 1587–1593. doi: 10.1046/j.1460-9592.2002.01587.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sawhney, N., Anand, K., Robertson, C. E., Wurdeman, T., Anousheh, R., and Feld, G. K. (2011). Recovery of mitral isthmus conduction leads to the development of macro-reentrant tachycardia after left atrial linear ablation for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 4, 832–837. doi: 10.1161/CIRCEP.111.964817
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Senatore, G., Stabile, G., Bertaglia, E., Donnici, G., De Simone, A., Zoppo, F., et al. (2005). Role of transtelephonic electrocardiographic monitoring in detecting short-term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J. Am. Coll. Cardiol. 45, 873–876. doi: 10.1016/j.jacc.2004.11.050
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tondo, C., Tritto, M., Landolina, M., Pg, D. E. G., Bencardino, G., Moltrasio, M., et al. (2013). Rhythm-symptom correlation in patients on continuous monitoring after catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 25, 154–160. doi: 10.1111/jce.12292
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tung, R., Buch, E., and Shivkumar, K. (2012). Catheter ablation of atrial fibrillation. Circulation 126, 223–229. doi: 10.1161/CIRCULATIONAHA.111.048421
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wasmer, K., Monnig, G., Bittner, A., Dechering, D., Zellerhoff, S., Milberg, P., et al. (2012). Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm 9, 1660–1666. doi: 10.1016/j.hrthm.2012.06.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wolf, P. A., Abbott, R. D., and Kannel, W. B. (1991). Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–988. doi: 10.1161/01.STR.22.8.983
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wyse, D. G., Waldo, A. L., Dimarco, J. P., Domanski, M. J., Rosenberg, Y., Schron, E. B., et al. (2002). A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 347, 1825–1833. doi: 10.1056/NEJMoa021328
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: atrial fibrillation, catheter ablation, monitoring, ambulatory, rhythm monitoring, arrhythmias, cardiac
Citation: Ajijola OA, Boyle NG and Shivkumar K (2015) Detecting and monitoring arrhythmia recurrence following catheter ablation of atrial fibrillation. Front. Physiol. 6:90. doi: 10.3389/fphys.2015.00090
Received: 30 January 2015; Paper pending published: 24 February 2015;
Accepted: 05 March 2015; Published: 27 March 2015.
Edited by:
Rahul Doshi, Keck School of Medicine of USC, USAReviewed by:
Flavia Ravelli, University of Trento, ItalyKrzysztof R. Grzeda, The Jackson Laboratory for Genomic Medicine, USA
Copyright © 2015 Ajijola, Boyle and Shivkumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olujimi A. Ajijola, UCLA Cardiac Arrhythmia Center, UCLA Health System/David Geffen School of Medicine at UCLA, University of California-Los Angeles, 100 Medical Plaza, Suite 660, Westwood Blvd, Los Angeles, CA 90095-1679, USAb2FqaWpvbGFAbWVkbmV0LnVjbGEuZWR1
 Olujimi A. Ajijola
Olujimi A. Ajijola Noel G. Boyle
Noel G. Boyle