- The Department of Pharmacology and Experimental Therapeutics, The Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA
To counter the development of salt-sensitive hypertension, multiple brain G-protein-coupled receptor (GPCR) systems are activated to facilitate sympathoinhibition, sodium homeostasis, and normotension. Currently there is a paucity of knowledge regarding the role of down-stream GPCR-activated Gα-subunit proteins in these critically important physiological regulatory responses required for long-term blood pressure regulation. We have determined that brain Gαi2-proteins mediate natriuretic and sympathoinhibitory responses produced by acute pharmacological (exogenous central nociceptin/orphanin FQ receptor (NOP) and α2-adrenoceptor activation) and physiological challenges to sodium homeostasis (intravenous volume expansion and 1 M sodium load) in conscious Sprague–Dawley rats. We have demonstrated that in salt-resistant rat phenotypes, high dietary salt intake evokes site-specific up-regulation of hypothalamic paraventricular nucleus (PVN) Gαi2-proteins. Further, we established that PVN Gαi2 protein up-regulation prevents the development of renal nerve-dependent sympathetically mediated salt-sensitive hypertension in Sprague–Dawley and Dahl salt-resistant rats. Additionally, failure to up-regulate PVN Gαi2 proteins during high salt-intake contributes to the pathophysiology of Dahl salt-sensitive (DSS) hypertension. Collectively, our data demonstrate that brain, and likely PVN specific, Gαi2 protein pathways represent a central molecular pathway mediating sympathoinhibitory renal-nerve dependent responses evoked to maintain sodium homeostasis and a salt-resistant phenotype. Further, impairment of this endogenous “anti-hypertensive” mechanism contributes to the pathophysiology of salt-sensitive hypertension.
Selectivity of Central Gα Proteins
G-proteins are a family of heterotrimeric proteins composed of α, β, and γ subunits. Following ligand binding at a transmembrane G-protein coupled receptor (GPCR), signal transduction is initiated by α-subunit mediated exchange of GDP for GTP and the dissociation into activated α and βγ complexes that initiate downstream signal transduction to ultimately evoke a biological response. This review is focused on delineating the central actions of Gα proteins in cardiovascular and fluid and electrolyte homeostasis. There are four major subtypes of Gα proteins, Gαi/o, Gαs, Gαz, and Gαq (Figure 1), discussed in this review, with a subsequent focus on Gαi2 proteins. The Gαi/o and Gαz classes of Gα proteins principally inhibit adenylate cyclase, thereby resulting in reduced cAMP levels. Additionally, via associated βγ subunits Gαi/o proteins also activate potassium channels. In contrast, Gαs proteins stimulate the activity of adenylate cyclase to drive increased cAMP production. The final subtype investigated in these studies, Gαq, does not impact cAMP levels, and instead activates phospholipase C (PLC), resulting in increased levels of intracellular inositol triphosphate (IP3) and modulation of calcium release. It has been demonstrated in cell culture systems that selective recruitment and/or availability of Gα-subunit proteins plays a critical role in determining the intracellular signaling responses to GPCR activation following ligand binding (Nasman et al., 2001). The specific intracellular actions evoked by selective action of individual Gα proteins has been elegantly elucidated, in part, via studies conducted on the nociceptin/orphanin FQ receptor (NOP). Activation of the NOP GPCR results in signal transduction via Gαi1−3, Gαo, Gαz, and Gαq proteins to initiate multiple cellular responses, including activation of potassium channels and inhibition of calcium channels, adenylyl cyclase, and MAPK phosphorylation—all of which modulate neurotransmitter release and neuronal activity (Reinscheid et al., 1995; Chan et al., 1998; Hawes et al., 1998; Jeong and Ikeda, 1998; Yung et al., 1999; Tso and Wong, 2006). In vitro studies on the α2-adrenoceptor, an extensively studied key GPCR in cardiovascular regulation, have demonstrated signal transduction via Gαi1−3, Gαo, Gαs, Gαz, and Gαq subunit proteins which principally modulate adenylate cyclase activity (Remaury et al., 1993; Nasman et al., 2001; Hein, 2006). However, despite the elucidation of signal transduction pathways in vitro for the NOP and α2-adrenoceptor (among others), there are essentially no data at present correlating intracellular Gα protein pathways to the physiological responses elicited by central GPCR activation in vivo.
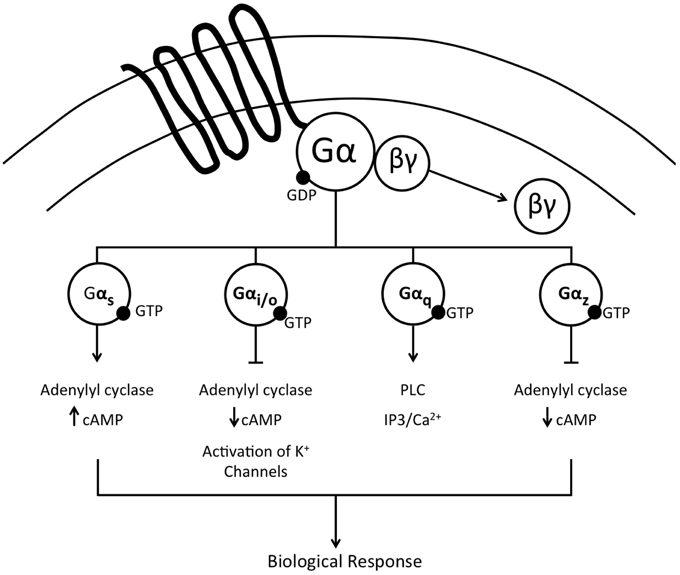
Figure 1. Schematic representation of the major Gα protein signal transduction pathways activated following ligand binding at a G-protein coupled receptor.
Brain Gα Proteins and Pharmacological GPCR Activation
To investigate the potential of selective physiological actions of central Gα-proteins following ligand binding at a GPCR in vivo, we elected to use the highly selective GPCR agonist nociceptin/orphanin FQ (N/OFQ) (Reinscheid et al., 1995; Calo et al., 2005; Krowicki and Kapusta, 2006). N/OFQ, an opioid-like peptide, produces highly reproducible cardiovascular depressor and diuretic responses when administered centrally to conscious rats via actions on the NOP receptor (Kapusta et al., 1997, 1999; Kapusta and Kenigs, 1999; Kakiya et al., 2000). Prior in vitro studies suggested that central NOP receptors signal through Gαi1, Gαi2, Gαi3 (Hawes et al., 1998), GαoA, B (Tso and Wong, 2006), Gαz (Chan et al., 1998; Jeong and Ikeda, 1998), and Gαq (Yung et al., 1999) transduction pathways. We therefore examined how inhibition of central Gαi/Gαo proteins via pertussis toxin (Katada and Ui, 1982; Gullapalli and Ramarao, 2002) or targeted selective down-regulation of central Gαz or Gαq proteins via intracerebroventricular (i.c.v.) oligodeoxynucleotide (ODN) pre-treatment (Rossi et al., 1995; Standifer et al., 1996; Silva et al., 2000; Hadjimarkou et al., 2002) impacted the characteristic cardiovascular, renal excretory, and plasma vasopressin (AVP) responses to i.c.v. injection of N/OFQ in conscious Sprague–Dawley rats. In control saline vehicle or scrambled ODN pre-treatment groups, i.c.v. administration of N/OFQ evoked bradycardia, hypotension, and diuresis. The hypotension and bradycardia, but not diuresis, to N/OFQ was abolished in pertussis toxin-pretreated rats in which the actions of brain Gαi/Gαo proteins were inhibited. In contrast, i.c.v. ODN pretreatment, which significantly and specifically down-regulated central Gαz or Gαq protein expression as previously reported (Rossi et al., 1995; Standifer et al., 1996; Silva et al., 2000; Hadjimarkou et al., 2002) had a significant effect on the diuresis to central N/OFQ without impacting the cardiovascular responses. Owing to the known role of N/OFQ in suppressing AVP release, reducing water reabsorption in the collecting ducts of the kidneys and promoting diuresis, we determined that the effects of central Gαz or Gαq down-regulation on N/OFQ diuresis were mediated by blunting (Gαz) or augmenting (Gαq) N/OFQ evoked suppression of AVP release (Wainford et al., 2008). These studies demonstrate that in conscious Sprague–Dawley rats the GPCR ligand N/OFQ acts centrally via the NOP receptor to activate individual Gα protein signaling pathways that control cardiovascular (Gαi/Gαo) vs. renal excretory (Gαz or Gαq) function.
Extending our finding that central Gαz/Gαq protein pathways play a pivotal role in modulating (inhibiting vs. stimulating, respectively) AVP release and thereby diuresis to central N/OFQ (Wainford et al., 2008), we examined the role of brain Gαz/Gαq proteins in the regulation of AVP during a physiological challenge. During high salt intake, Dahl salt-sensitive (DSS) rats exhibit elevated plasma AVP (Bayorh et al., 1998; Gunnet et al., 2008). In contrast, in salt-resistant rat phenotypes [i.e., Sprague–Dawley (SD) and Dahl salt-resistant (DSR) rats], increased dietary salt intake does not alter circulating AVP levels (Serino et al., 2001; Wainford and Kapusta, 2009). A key finding of these investigations is that in response to a high salt diet, SD and DSR rats down-regulate Gαq protein levels within the hypothalamic PVN, a major central site involved in AVP synthesis and release (Landgraf et al., 1990; Nielsen et al., 1993; Grindstaff and Cunningham, 2001). This site-specific down-regulation of PVN Gαq proteins was correlated with the ability of SD and DSR rats to maintain unchanged plasma AVP levels and fluid/electrolyte balance in the face of a high salt challenge (Wainford and Kapusta, 2010). In contrast, DSS rats do not exhibit such site-specific down-regulation of Gαq proteins, which may contribute to excess AVP release in this rat phenotype. Owing to the impact of PVN Gαq proteins on the regulation of AVP release, we speculate that within the PVN, the changes in Gαq protein expression are predominantly localized to the magnocellular neurons. Of physiological relevance, ODN-mediated down-regulation of brain Gαq proteins restored plasma AVP in salt-challenged DSS rats to levels observed prior to consuming a high salt diet (Wainford and Kapusta, 2010). Moreover, diuresis stimulated by pharmacological or physiological manipulations that are known to evoke this response exclusively or in part via suppression of AVP release (Kondo et al., 1993; Kakiya et al., 2000; Krowicki and Kapusta, 2006; Ruginsk et al., 2007) was completely or partially restored (Wainford and Kapusta, 2010). Therefore, the down-regulation of PVN Gαq proteins plays a critical counter-regulatory role in preventing AVP hypersecretion by reducing the ability of endogenous GPCR ligands to trigger AVP release, and may represent a new therapeutic target in pathophysiological states featuring AVP dysregulation (e.g., heart failure, salt-sensitive hypertension). However, the potential impact of PVN Gαz/Gαq proteins in other pathophysiological states featuring AVP excess remains to be determined and is not the focus of the remainder of this review.
Our prior studies also established a role for brain Gαi/Gαo proteins to mediate hypotension and bradycardia in response to central activation of the NOP receptor (Wainford et al., 2008), but did not determine which specific Gαi/Gαo protein is responsible for the observed effects. To address this issue and provide clarity as to the individual roles of brain Gαi/Gαo proteins in cardiovascular regulation, conscious Sprague–Dawley rats were administered gaunabenz, a selective α2-adrenoceptor agonist (Degoute, 2007). The α2-adrenoceptor is intimately involved in the central control of cardiovascular function and fluid and electrolyte homeostasis (Ruffolo et al., 1991; Nasman et al., 2001). In vitro studies have demonstrated that immediately following post-ligand binding, α2-adrenoceptors activate downstream Gαi(1–3), Gαo, and Gαs protein signal transduction pathways (Remaury et al., 1993; Eason and Liggett, 1995). Ligand-binding at central α2-adrenoceptors in conscious rats evokes bradycardia, hypotension, sympathoinhibition (Grisk and Dibona, 1998; Huang and Leenen, 1998), diuresis and natriuresis (Gellai and Edwards, 1988; Menegaz et al., 2001). Using a highly targeted ODN approach to selectivity down-regulate central Gαi1, Gαi2, Gαi3, Gαo, or Gαs proteins (Rossi et al., 1995; Standifer et al., 1996; Silva et al., 2000; Hadjimarkou et al., 2002), we determined that the hypotensive and natriuretic responses to selective central α2-adrenoceptor activation are abolished by Gαi2 down-regulation, or are converted to a pressor response by Gαs down-regulation. We speculate these responses reflect altered sympathetic outflow to multiple organ systems (e.g., renal, splanchnic). In contrast, the profound α2-agonist-stimulated bradycardia and diuresis was unaltered by prior down-regulation of individual brain Gαi(1–3), Gαs, or Gαo proteins (Wainford and Kapusta, 2012). These data suggest potential redundancy of Gα protein pathways to regulate heart rate. In light of our prior findings about AVP regulation (described above), we speculate the diuretic response to central α2-adrenoceptor activation is regulated by Gαz/Gαq due to a significant component of this response being mediated by suppression of AVP release (Brooks et al., 1986; Cabral et al., 1998). Together, these studies provide new insight into the intracellular mechanism of action of brain α2-adrenoceptors in vivo and document that the physiological responses evoked by receptor activation are greatly influenced by the availability and/or brain protein expression levels of individual downstream Gα proteins (Wainford and Kapusta, 2012). Further, these data provide compelling in vivo support for the pharmacological paradigm of functional selectivity (Patel et al., 2010) as predicted from in vitro studies and mathematical models (Nasman et al., 2001; Hein, 2006).
Brain Gαi2 Proteins and Acute Physiological GPCR Activation
Maintenance of fluid and electrolyte homeostasis in response to acute increases in sodium intake occurs via complex integrated neural, humoral, and hemodynamic pathways to regulate the renal excretion of sodium. If these endogenous pathways are impaired, salt-sensitive hypertension can develop. A key component of the renal handling of sodium and water, and thus blood pressure, in response to alterations in plasma sodium, is the regulation of sympathetic outflow and renal sympathetic nerve traffic (Guyton, 1991; Lohmeier et al., 1999; DiBona, 2004, 2005; May et al., 2009), which is directly influenced by multiple brain GPCR systems (e.g., the α2-adrenoreceptor) (Ruffolo et al., 1991; Nasman et al., 2001). However, the central mechanisms regulating sympathetic outflow (global and renal specific) to facilitate natriuresis during elevations in plasma sodium content remain unclear (DiBona, 2005; Kompanowska-Jezierska et al., 2008; Bie, 2009). Consequently, we elected to examine the role of endogenous brain Gαi2 protein-gated signal transduction pathways in mediating the sympathetic and natriuretic responses to acute physiological challenges [i.v. volume expansion (VE) and i.v. sodium load] that impact the renal handling of sodium, in part, by suppressing central sympathetic outflow (Haselton et al., 1994; Kapusta and Obih, 1995; DiBona and Kopp, 1997; Singer et al., 1998; Manunta et al., 2011). Selective ODN-mediated down-regulation of brain Gαi2 proteins (1) abolished the renal sympathoinhibitory response and attenuated the natriuresis to VE (Kapusta et al., 2012) and (2) abolished the global sympathoinhibitory response and attenuated the natriuresis to a 1 M sodium load (Wainford et al., 2013) via a mechanism requiring intact renal sympathetic nerves. Demonstrating the central nature of this effect, it is notable that kidney function was maintained following down-regulation of brain Gαi2 proteins, as demonstrated by the natriuretic and diuretic responses to i.v. bolus administration of furosemide in Gαi2 ODN-treated rats (Kapusta et al., 2012). The results generated from these physiological studies demonstrate that central Gαi2 proteins are involved in mediating the renal excretory responses to these acute challenges to sodium homeostasis via an inhibitory influence on sympathetic outflow, specifically renal sympathetic nerve activity. This conclusion is supported by evidence that down-regulation of brain Gαi2 proteins prevented the suppression of global sympathetic outflow [assessed by circulating norepinephrine (NE) levels] during a sodium load (Wainford et al., 2013). Moreover, in rats implanted with a renal nerve recording electrode, brain Gαi2 protein down-regulation abolished isotonic saline volume expansion (5% body weight) evoked suppression of renal sympathetic nerve activity (Kapusta et al., 2012). Bilateral renal denervation to remove the influence of the renal sympathetic nerves on kidney function prevented Gαi2 protein down-regulation from altering the natriuretic response to either the i.v. VE or 1 M sodium load (Kapusta et al., 2012; Wainford et al., 2013). Linking our data generated following acute exogenous central GPCR activation (described above) to these physiological studies provides evidence that central α2-adrenoceptors are the principal brain GPCR involved in producing renal sympathoinhibition, and consequently natriuresis, in response to acute VE (Patel, 1991). Therefore, we postulate that endogenous α2-adrenoceptor mediated activation of brain Gαi2 protein-gated pathways represents a key mechanism by which sympathoinhibition and natriuresis occurs in vivo in response to acute challenges to sodium homeostasis. Collectively, these data report a central molecular mechanism that plays a key role in modulating the level of central sympathetic outflow, blood pressure, and natriuresis in response to acute pharmacological or physiological stimuli (Figure 2). Further, these data establish that central Gαi2 proteins impact sympathetic outflow predominantly via regulation of the renal sympathetic nerves. The present studies extend our knowledge of the CNS regulatory mechanisms influencing the kidney to maintain sodium homeostasis. Given the intimate association between fluid and electrolyte homeostasis and the long-term control of arterial pressure, we speculate that Gαi2-protein mediated suppression of central sympathetic outflow may represent a new component in integrated autonomic regulatory processes that govern the long-term regulation of systemic arterial blood pressure. The importance of enhancing our understanding of the underlying cellular signaling pathways involved in the neural control of sodium excretion in health and disease is highlighted by the multiple pathophysiological disease states that exhibit sodium retention, including heart failure and certain models of hypertension, particularly salt-sensitive hypertension (Bayorh et al., 1998; Lastra et al., 2010).
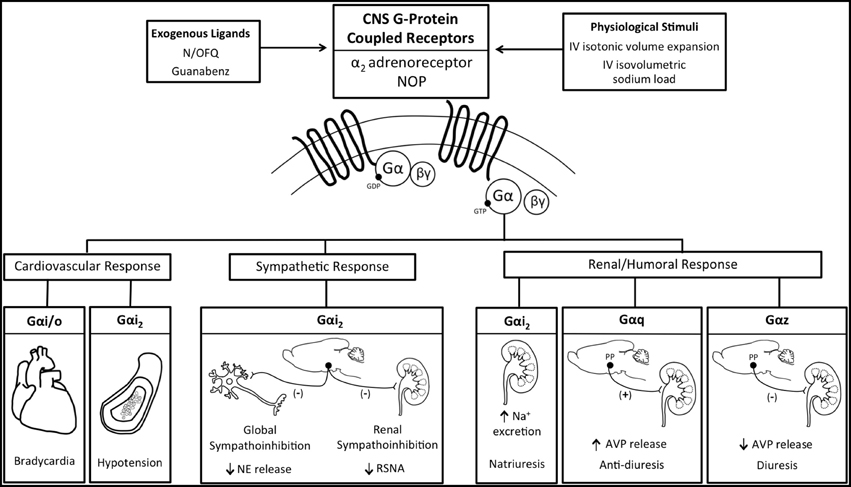
Figure 2. Schematic representation of the functional selectivity of G-protein coupled receptor activated Gα protein signal transduction pathways in mediating the physiological responses evoked by central exogenous administration of N/OFQ and Guanbenz or the physiological stimuli of an iv volume expansion or sodium load. AVP, vasopressin; NE, Norepinephrine; NOP, nociceptin/orphanin FQ receptor; RSNA, renal sympathetic nerve activity.
Brain Gαi2 Proteins and Salt-resistance
Due to the dramatic impact of hypertension on human health and the prevalence of salt-sensitive hypertension in approximately 50% of hypertensive patients (Meneton et al., 2005; Franco and Oparil, 2006; Whelton et al., 2012; Kotchen et al., 2013), we elected to study the potential role of central Gαi2 proteins in the pathophysiology of salt-sensitive hypertension. It is well established in normotensive salt-resistant subjects that neural (renal sympathetic) and humoral (angiotensin-aldosterone) sodium-retaining mechanisms are suppressed to counter the influence of dietary salt-intake on central sympathetic outflow and systemic cardiovascular hemodynamics (Lohmeier et al., 1999; Brooks et al., 2005; Osborn et al., 2008). Consequently, we examined the role of central Gαi2 proteins in facilitating sodium homeostasis during a 7-day dietary sodium restriction or supplementation in the Sprague–Dawley rat. In control scrambled ODN-treated rats, 7-days of altered dietary sodium intake did not alter blood pressure and evoked a site-specific PVN decrease (sodium deficiency) or increase (sodium excess) in PVN Gαi2 proteins (Kapusta et al., 2012). The sodium-stimulated changes in Gαi2 protein expression were restricted to the PVN and did not occur in other hypothalamic regions (e.g., SON, posterior hypothalamus). Further, dietary sodium restriction or excess did not alter expression levels of Gαi1, Gαi3, or Gαo proteins in any brain site examined. The observation of sodium-evoked site and subunit-specific changes in PVN Gαi2 protein expression is of considerable pathophysiological interest as this brain site plays a key role in responding to alterations in body fluid sodium concentration/osmolality (Toney et al., 2003) and the central control of sympathetic outflow, sodium homeostasis, and blood pressure (Leenen et al., 2002; Kenney et al., 2003; He et al., 2005; Carmichael and Wainford, 2015). In rats adapted to a high-sodium diet (0.9% NaCl drinking water), acute ODN-mediated down-regulation of central Gαi2 proteins resulted in the development of sodium retention, global sympathoexcitation (i.e., increased circulating NE), and moderately elevated blood pressure. These findings provide further support for the long established Guytonion hypothesis of the intimate connection between fluid and electrolyte homeostasis and the long-term regulation of blood pressure (Guyton, 1991). Additionally, in accordance with recent hypotheses (Rodriguez-Iturbe and Vaziri, 2007; Fujita and Fujita, 2013; Stocker et al., 2013), our data indicate that enhanced central sympathetic outflow plays a role in these regulatory processes. The PVN as a potential locus of Gαi2protein mediated regulation of sympathetic outflow is not unexpected, due to the critical role of the PVN in mediating sympathoinhibition (Coote, 1995; Akine et al., 2003; Frithiof et al., 2009), particularly in response to increased plasma and/or cerebrospinal fluid sodium in salt-resistant subjects (Brooks et al., 2005; DiBona, 2005; He et al., 2005). These data demonstrate that PVN Gαi2 protein pathways play a role in maintaining fluid and electrolyte balance during alterations in sodium intake by controlling the influence of the sympathetic nervous system on natriuresis and suggest a role for these proteins in long-term blood pressure regulation (Kapusta et al., 2012).
Currently, the central molecular mechanisms that mediate sympathoinhibition in response to excess dietary sodium intake as found in the typical western diet [Center for Disease Control and Prevention (CDC), 2010] remain to be fully elucidated. Based on our prior observations, we hypothesized that in salt-resistant phenotypes (e.g., Sprague–Dawley rat) subjected to a chronic elevation in dietary sodium intake, brain Gαi2 protein signaling pathways will be augmented to maximize inhibition of central sympathetic outflow to the kidneys via the renal sympathetic nerves and thereby facilitate sodium excretion. Further, we predict that blockade of this central Gαi2 protein sympathoinhibitory pathway will trigger sodium and water retention and the development of salt-sensitive hypertension. To chronically down-regulate central Gαi2 proteins, our acute ODN administration protocol was adapted to utilize an alzet osmotic minipump connected to an indwelling i.c.v. cannula to deliver a continuous infusion of a control scrambled or Gαi2 ODN (Kapusta et al., 2013). During ODN infusion, naïve or previously bilaterally renal denervated male Sprague–Dawley rats were randomly assigned to receive a normal salt (0.4%) or high-salt (8.0%) diet for 21-days. In control scrambled ODN-infused rats, salt-loading which did not alter blood pressure, evoked a site-specific increase in hypothalamic paraventricular nucleus (PVN) Gαi2 protein levels and suppression of circulating norepinephrine content and plasma renin activity. To demonstrate the functional significance of sodium-evoked PVN specific increase in Gαi2 proteins, we examined how chronic ODN-mediated down-regulation of brain Gαi2 protein levels impacted blood pressure and neural/humoral sodium retaining mechanisms during high salt intake. In these studies, salt-loaded rats continuously infused i.c.v. with a Gαi2 ODN developed salt-sensitive hypertension (Kapusta et al., 2013). The development of salt-sensitive hypertension, featuring an impaired pressure-natriuresis response, during CNS Gαi2 protein down-regulation involves global sympathoexcitation that we postulate contributes to increased systemic arterial pressure over time through (1) increased vascular contractility and (2) enhanced renal tubular sodium reabsorption (DiBona and Kopp, 1997; Lohmeier et al., 1999). In contrast to an inhibitory influence on sympathetic activity and NE release, Gαi2 protein pathways do not impact the suppression of the renin-angiotensin system (as assessed by reduced plasma renin activity) during chronic increases in dietary sodium intake (Kapusta et al., 2013). In separate studies using the technique of chronic bilateral renal denervation, which remove the influence of the renal sympathetic nerves on kidney function, we were able to prevent the development of central Gαi2 ODN-induced salt-sensitive hypertension. In renal denervated animals, we did not observe global sympathoexcitation or resetting of the chronic pressure-natriuresis curve in response to elevated in dietary sodium intake (Wainford et al., 2013). These data demonstrate the physiological importance of PVN Gαi2 proteins as a sodium-activated “anti-hypertensive” central pathway which regulates the renal handling of sodium via the renal sympathetic nerves to prevent the development of salt-sensitive hypertension (Figure 3). Our findings support the inclusion of a role for enhanced central sympathetic outflow in the current modeling of the pathophysiology of hypertension that is based on the classical Guytonian theory of pressure natriuresis (Guyton, 1991). Further, our data highlight central Gαi2 proteins as a candidate mechanism that facilitates the communication between the central nervous system and the kidneys to maintain long-term blood pressure regulation.
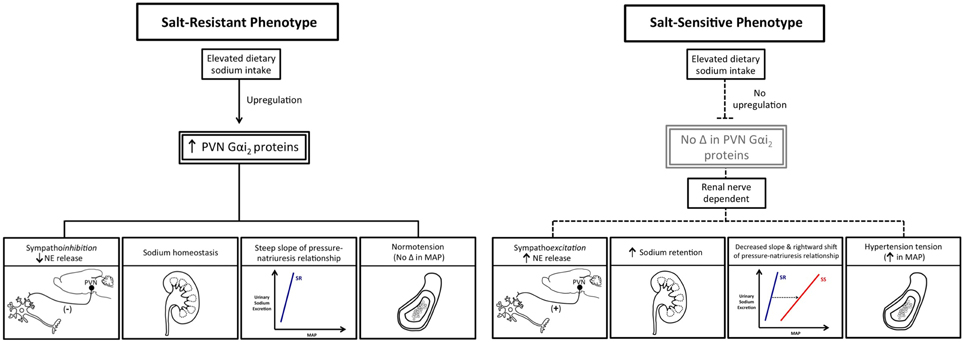
Figure 3. Schematic representation of the differential impact of elevated dietary sodium intake on PVN Gαi2 protein expression and the consequences of this on central sympathetic outflow, sodium homeostasis, and blood pressure in salt-resistant vs. salt-sensitive phenotypes. MAP, mean arterial pressure; NE, Norepinephrine; PVN, paraventricular nucleus.
Brain Gαi2 Proteins and Salt-sensitivity
Among multiple proposed mechanisms, impaired sympathetic nervous system activity has been hypothesized to play a critical role in the development of salt-sensitive hypertension (Brooks et al., 2005; Osborn et al., 2009; Fujita and Fujita, 2013), a theory gaining traction following reports of increased sympathetic nervous system activity in animal models of salt-sensitive hypertension (Kandlikar and Fink, 2011; Foss et al., 2013) and salt-sensitive humans (Yatabe et al., 2010). Further, increasing attention is focusing on the role of the renal sympathetic nerves in blood pressure regulation following reports that renal nerve ablation attenuates hypertension in human subjects (Esler et al., 2012; Krum et al., 2014). Despite these recent advances, it is widely acknowledged that the central nervous system mechanisms that modulate central sympathetic outflow to the kidney to facilitate sodium homeostasis and blood pressure regulation require further investigation (Brooks et al., 2005; Ellison and Brooks, 2011; Stocker et al., 2013). Based on our prior findings, generated solely in the Sprague–Dawley rat phenotype, we hypothesized that increased dietary sodium intake upregulates PVN Gαi2 proteins to suppress central sympathetic outflow, particularly to the kidneys, to maintain fluid and electrolyte balance and normotension in multiple salt-resistant phenotypes. Extending this hypothesis, we predicted that failure to up-regulate PVN Gαi2 proteins in response to increased salt intake contributes to the pathophysiology of salt-sensitive hypertension.
Utilizing the Dahl rat phenotypes as an established model of salt-resistance vs. salt-sensitivity, we observed that high dietary sodium intake evoked a site-specific up-regulation of PVN Gαi2 protein levels in the DSR and Brown Norway rat phenotypes (Wainford et al., 2015). These findings extend our prior studies in Sprague–Dawley rats (Kapusta et al., 2012, 2013) and demonstrate that PVN Gαi2 protein up-regulation in response to increased salt-intake occurs in multiple rat phenotypes that exhibit salt-resistance. In contrast, in the DSS rat, which developed salt-sensitive hypertension, there was a failure to up-regulate PVN Gαi2 proteins (Wainford et al., 2015). This difference would suggest a molecular mechanism that contributes to DSS rat hypertension. During high salt-intake, central ODN-mediated Gαi2 protein down-regulation evoked sympathetically mediated salt-sensitive hypertension in the DSR rat and exacerbated the magnitude of salt-sensitive hypertension observed in the DSS phenotype. As determined via radiotelemetry, down-regulation of CNS Gαi2proteins evoked an elevation in blood pressure in the DSR rat upon high-salt challenge of approximately 20 mmHg in a 3-day period, followed by a prolonged and persistent increase in blood pressure over time. In the DSS rat, CNS Gαi2protein down-regulation also evoked an enhanced pressor response to salt-intake, after which the rate of development of hypertension was comparable between scrambled and Gαi2 treated animals. This rapid elevation in blood pressure following salt-intake and brain Gαi2protein down-regulation reflects a reduction in the slope of the blood pressure-sodium excretion relationship, indicative of increased short-term salt-sensitivity of blood pressure (Wainford et al., 2015). This would reset the pressure-natriuresis set-point to a higher level within several days, as observed in the current studies, and as hypothesized by current models of the development of salt-sensitive hypertension (Van Vliet et al., 2006; McLoone et al., 2009; Fujita and Fujita, 2013).
Chronic removal of the influence of the renal sympathetic nerves abolished central Gαi2 ODN-induced sympathetically mediated salt-sensitive hypertension in DSR rats and attenuated the development of salt-sensitive hypertension in DSS rats. These data support a direct role of renal sympathetic innervation of the kidney in the development of salt-sensitive hypertension (Wainford et al., 2015). Based on our prior studies, we speculate in intact animals in which the influence of brain Gαi2 was removed, there was a failure to suppress sympathetic outflow to the kidneys. This would have the consequence of increasing both renal NE release and NE-mediated sodium reabsorption leading to the observed sodium retention to drive the pathophysiology of salt-sensitivity. To confirm that impaired up-regulation of central Gαi2 proteins impacts salt-sensitivity in the DSS rat, we conducted studies using an 8-congenic DSS rat, which has chromosome 8 encoding the GNAI2 gene from the Brown Norway rat (Mattson et al., 2008). During high salt-intake, the 8-congenic DSS rat exhibited up-regulation of PVN Gαi2 proteins as well as attenuation of sodium evoked hypertension, sodium retention and sympathoexcitation (Wainford et al., 2015). As anticipated, because of the multifactorial nature of DSS hypertension, restoration of sodium-stimulated PVN Gαi2 protein up-regulation attenuated, but did not abolish, the hypertension. Collectively, these studies report that sodium evoked up-regulation of PVN Gαi2 proteins represents a conserved central molecular mechanism that is required to suppress renal sympathetic outflow to maintain sodium homeostasis and normotension in multiple salt-resistant rat phenotypes. Additionally, studies conducted in the DSS and 8-congenic DSS rat provide the first direct evidence that impairment of PVN Gαi2 signal transduction pathways contributes to the pathophysiology of salt-sensitive hypertension (Figure 3).
Future Directions and Significance
Despite the insight our recent studies have provided over the last several years into the impact of central Gαi2proteins on the salt-sensitivity of blood pressure, several questions remain to be addressed. A critical issue remains the time course of up-regulation of hypothalamic PVN Gαi2 proteins in response to increases in dietary sodium intake. At present, all data generated by our laboratory represents end-point assessment after 21-days of increased dietary intake (Kapusta et al., 2013; Wainford et al., 2015). Our physiological data suggests up-regulation of Gαi2 proteins is an early event but this is yet to be confirmed. Further, identification of the mechanism driving the increased expression of hypothalamic PVN Gαi2 proteins in response to altered dietary sodium intake remains to be established. Despite our failure to detect changes in either plasma sodium or osmolality in SD, DSR, or Brown Norway rats during increased sodium intake in end-point measurements made on day-21 of high salt-intake (Kapusta et al., 2013; Wainford et al., 2015), we speculate minute changes in plasma sodium/osmolality following sodium ingestion (i.e., after a meal) are sensed by osmoreceptor/sodium-sensitive receptors located in the hypothalamic PVN, the circumventricular organs (Brooks et al., 2005; Stocker et al., 2013) or the renal afferent nerve terminals (DiBona and Kopp, 1997) and trigger the observed alterations in protein expression. An alternative potential mechanism driving the increase in PVN Gαi2 protein expression during salt intake may be sodium-evoked alterations in PVN neurotransmitter release (e.g., altered intra-PVN levels of glutamate and/or GABA) and that the complex inhibitory and excitatory actions evoked by these neurotransmitters may evoke altered PVN Gαi2 levels. The current studies strongly suggest the PVN as the locus of the observed actions of central Gαi2 proteins, however future microinjection studies, designed to down-regulate Gαi2 proteins site-specifically within the PVN are required to definitively establish the PVN as a brain site in which Gαi2 protein pathways act to produce sympathoinhibitory influences on the renal handling of sodium and water to counter the development of salt-sensitive hypertension. We speculate, given the profound impact of alterations in PVN Gαi2 on sympathetic outflow, that the observed changes in Gαi2 expression in response to increased salt intake occur in the parvocellular sympathetic-regulatory neurons, not the magnocellular neurons. An additional issue not addressed by our current work is the cellular mechanism(s) by which central Gαi2 proteins modulate sympathetic outflow, sodium homeostasis, and blood pressure. We postulate that central Gαi2 proteins influence these physiological parameters through alteration of neuronal firing patterns, via actions to inhibit adenylate cyclase activity and/or Ca2+ channels in sympathetic neurons, to evoke changes in the activity of the CNS to impact central sympathetic outflow. However, electrophysiological recordings conducted in conscious animals and in brain slice preparation studies are required to establish the underlying cellular mechanisms involved in these responses.
Our observation of an increase in PVN Gαi2 protein expression in multiple salt-resistant rat phenotypes in response to elevated sodium intake, and the absence of this response in the DSS rat, is of high physiological significance because of the pivotal role that the PVN plays in the neural network that influences sympathetic outflow and blood pressure regulation. Debate exists regarding the underlying cause of salt-sensitive hypertension, which could result from either over-activity of the sympathetic nervous system (Osborn et al., 2009) or excessive salt reabsorption at the level of the kidneys (Montani and Van Vliet, 2009). Our studies, as well as those of Mu et al. (2011); Mu et al., suggest that the underlying pathogenesis of salt-sensitive hypertension involves integration of both the central nervous system and the kidneys and we demonstrate that this communication is regulated by the renal sympathetic nerves via a CNS Gαi2 protein pathway. The potential translational impact of our findings of a role of Gαi2 proteins in blood pressure regulation is suggested by reports of single nucleotide polymorphisms (SNPs) in the human GNAI2 gene. SNPs in the GNAI2 gene are associated with increased hypertension risk in Caucasian Italians and in the Millennium Genome Project for Hypertension in Japan (Menzaghi et al., 2006; Kohara et al., 2008), providing the first evidence of the potential utility of GNAI2 SNPs as a biomarker of hypertension risk. However, at present the association between human GNAI2 SNPs and the salt-sensitivity of blood pressure remains to be investigated. Our findings of a direct role of the renal sympathetic nerves in the pathophysiology of salt-sensitive hypertension provides renewed support for the decades of evidence that have documented a role of the renal sympathetic nerves in the pathogenesis of hypertension in multiple animal models (DiBona and Esler, 2010) and in human hypertension (Schlaich et al., 2009). The potential clinical applicability of these data is provided by the recent renal denervation studies in humans that have resulted in a persistent reduction in blood pressure (Esler et al., 2012; Krum et al., 2014). We acknowledge that the clinical efficacy of renal nerve ablation in resistant hypertensive patients remains to be definitively established following recent studies, which failed to report reduced blood pressure in resistant hypertensive patients (Bhatt et al., 2014; Bakris et al., 2015). However, based upon the successful renal ablation trials, it has been speculated that the success of this clinical procedure reflects, in part, reduced hypothalamic-mediated sympathetic outflow (DiBona and Esler, 2010)—a hypothesis congruent with our current data. Collectively, our data suggest a novel molecular/cellular target (brain Gαi2 proteins) at which new therapies can be directed to alter systemic cardiovascular parameters (e.g., antihypertensive medications) and/or renal excretory function (natriuretic compounds) to treat the multiple disease states that feature sympathoexcitation and the impaired renal handling of sodium, such as salt-sensitive hypertension or congestive heart failure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
RW is supported by NIH grants R01HL107330 and K02HL112718.
Abbreviations
AVP, vasopressin; CNS, central nervous system; DSR, Dahl salt-resistant; DSS, Dahl salt-sensitive; GPCR, G-protein coupled receptor; i.c.v., intracerebroventricular; PVN, paraventricular nucleus; M, molar; NE, norepinephrine; N/OFQ, nociceptin/orphanin FQ; NOP, nociceptin/orphanin FQ receptor; SD, Sprague–Dawley; VE, volume expansion.
References
Akine, A., Montanaro, M., and Allen, A. M. (2003). Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton. Neurosci. 108, 17–21. doi: 10.1016/j.autneu.2003.08.009
Bakris, G. L., Townsend, R. R., Flack, J. M., Brar, S., Cohen, S. A., D'Agostino, R., et al. (2015). 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the SYMPLICITY HTN-3 trial. J. Am. Coll. Cardiol. 65, 1314–1321. doi: 10.1016/j.jacc.2015.01.037
Bayorh, M. A., Ogbolu, E. C., Williams, E., Thierry-Palmer, M., Sanford, G., Emmett, N., et al. (1998). Possible mechanisms of salt-induced hypertension in Dahl salt-sensitive rats. Physiol. Behav. 65, 563–568. doi: 10.1016/s0031-9384(98)00194-2
Bhatt, D. L., Kandzari, D. E., O'Neill, W. W., D'Agostino, R., Flack, J. M., Katzen, B. T., et al. (2014). A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 370, 1393–1401. doi: 10.1056/NEJMoa1402670
Bie, P. (2009). Blood volume, blood pressure and total body sodium: internal signalling and output control. Acta. Physiol. 195, 187–196. doi: 10.1111/j.1748-1716.2008.01932.x
Brooks, D. P., Share, L., and Crofton, J. T. (1986). Central adrenergic control of vasopressin release. Neuroendocrinology 42, 416–420. doi: 10.1159/000124480
Brooks, V. L., Haywood, J. R., and Johnson, A. K. (2005). Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin. Exp. Pharmacol. Physiol. 32, 426–432. doi: 10.1111/j.1440-1681.2005.04206.x
Cabral, A. D., Kapusta, D. R., Kenigs, V. A., and Varner, K. J. (1998). Central alpha2-receptor mechanisms contribute to enhanced renal responses during ketamine-xylazine anesthesia. Am. J. Physiol. 275, R1867–R1874.
Calo, G., Guerrini, R., Rizzi, A., Salvadori, S., Burmeister, M., Kapusta, D. R., et al. (2005). UFP-101, a peptide antagonist selective for the nociceptin/orphanin FQ receptor. CNS Drug. Rev. 11, 97–112. doi: 10.1111/j.1527-3458.2005.tb00264.x
Carmichael, C. Y., and Wainford, R. D. (2015). Hypothalamic signaling mechanisms in hypertension. Curr. Hypertens. Rep. 17, 39. doi: 10.1007/s11906-015-0550-4
Center for Disease Control Prevention (CDC). (2010). Sodium intake among adults – United States, 2005–2006. Morb. Mortal. Wkly. Rep. 59, 746–749.
Chan, J. S. C., Yung, L. Y., Lee, J. W. M., Wi, Y. L., Pei, G., and Wong, Y. H. (1998). Pertussis toxin-insensitive signaling of the ORL1 receptor: coupling to Gz and G16 proteins. J. Neurochem. 71, 2203–2210. doi: 10.1046/j.1471-4159.1998.71052203.x
Coote, J. H. (1995). Cardiovascular functions of the paraventricular nucleus of the hypothalamus. Biol. Signals 4, 142–149. doi: 10.1159/000109434
Degoute, C. S. (2007). Controlled hypotension: a guide to drug choice. Drugs 67, 1053–1076. doi: 10.2165/00003495-200767070-00007
DiBona, G. F. (2004). The sympathetic nervous system and hypertension. Hypertension 43, 147–150. doi: 10.1161/01.HYP.0000113047.47711.fa
DiBona, G. F. (2005). Neural control of the kidney. Am. J. Physiol. 289, R633–R641. doi: 10.1152/ajpregu.00258.2005
DiBona, G. F., and Esler, M. (2010). Translational Physiology: the antihypertensive effect of renal denervation. Am. J. Physiol. 298, R245–R253. doi: 10.1152/ajpregu.00647.2009
Eason, M. G., and Liggett, S. B. (1995). Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the α2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J. Biol. Chem. 270, 24753–24760. doi: 10.1074/jbc.270.42.24753
Ellison, D. H., and Brooks, V. L. (2011). Renal nerves, WNK4, glucocorticoids, and salt transport. Cell Metab. 13, 619–620. doi: 10.1016/j.cmet.2011.05.007
Esler, M. D., Krum, H., Schlaich, M., Schmieder, R. E., Böhm, M., Sobotka, P. A., et al. (2012). Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126, 2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880
Foss, J. D., Fink, G. D., and Osborn, J. W. (2013). Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61, 806–811. doi: 10.1161/HYPERTENSIONAHA.111.00474
Franco, V., and Oparil, S. (2006). Salt Sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J. Med. Coll. Nutr. 25, 247S–255S. doi: 10.1080/07315724.2006.10719574
Frithiof, R., Ramchandra, R., Hood, S., May, C., and Rundgren, M. (2009). Hypothalamic paraventricular nucleus mediates sodium induced changes in cardiovascular and renal function in conscious sheep. Am. J. Physiol. 297, R185–R193. doi: 10.1152/ajpregu.00058.2008
Fujita, M., and Fujita, T. (2013). The role of the CNS in salt-sensitive hypertension. Curr. Hypertens. Rep. 15, 390–394. doi: 10.1007/s11906-013-0358-z
Gellai, M., and Edwards, R. M. (1988). Mechanism of alpha 2-adrenoceptor agonist-induced diuresis. Am. J. Physiol. 255, F317–F323.
Grindstaff, R. R., and Cunningham, J. T. (2001). Cardiovascular regulation of vasopressin neurons in the supraoptic nucleus. Exp. Neurol. 171, 219–226. doi: 10.1006/exnr.2001.7745
Grisk, O., and Dibona, G. F. (1998). Influence of arterial baroreceptors and intracerebroventricular guanabenz on synchronized renal nerve activity. Acta. Physiol. Scand. 163, 209–218. doi: 10.1046/j.1365-201x.1998.00357.x
Gullapalli, S., and Ramarao, P. (2002). Role of L-type Ca2+ channels in pertussis toxin induced antagonism of U50, 488H analgesia and hypothermia. Brain. Res. 946, 191–191. doi: 10.1016/S0006-8993(02)02880-9
Gunnet, J. W., Wines, P., Xiang, M., Rybczynski, P., Andrade-Gordon, P., de Garavilla, L., et al. (2008). Pharmacological characterization of RWJ-676070, a dual vasopressin V(1A)/V(2) receptor antagonist. Eur. J. Pharmacol. 590, 333–342. doi: 10.1016/j.ejphar.2008.06.010
Guyton, A. C. (1991). Blood-pressure control: special role of the kidneys and body fluids. Science 252, 1813–1116. doi: 10.1126/science.2063193
Hadjimarkou, M. M., Silva, R. M., Rossi, G. C., Pasternak, G. W., and Bodnar, R. J. (2002). Feeding induced by food depravation is differentially reduced by G-protein α-subunit antisense probes in rats. Brain Res. 955, 45–54. doi: 10.1016/S0006-8993(02)03361-9
Haselton, J. R., Goering, J., and Patel, K. P. (1994). Parvocellular neurons in the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J. Auton. Nerv. Syst. 50, 1–11. doi: 10.1016/0165-1838(94)90117-1
Hawes, B. E., Fried, S., Yao, X., Weig, B., and Graziano, M. P. (1998). Nociceptin (ORL-1) and μ-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J. Neurochem. 71, 1024–1033. doi: 10.1046/j.1471-4159.1998.71031024.x
He, F. J., Markandu, N. D., Sagnella, G. A., de Wardener, H. E., and MacGregor, G. A. (2005). Plasma sodium: ignored and underestimated. Hypertension 45, 98–102. doi: 10.1161/01.HYP.0000149431.79450.a2
Hein, L. (2006). Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 326, 541–551. doi: 10.1007/s00441-006-0285-2
Huang, B. S., and Leenen, F. H. (1998). Both brain angiotensin II and ‘ouabain’ contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32, 1028–1033. doi: 10.1161/01.HYP.32.6.1028
Jeong, S. W., and Ikeda, S. R. G. (1998). Protein α subunit Gαz couples neurotransmitter receptors to ion channels in sympathetic neurons. Neuron 21, 1201–1212. doi: 10.1016/S0896-6273(00)80636-4
Kakiya, S., Murase, T., Aimra, H., Yokoi, H., Iwasaki, Y., Miura, Y., et al. (2000). Role of endogenous nociceptin in the regulation of arginine vasopressin release in conscious rats. Endocrinology 141, 4466–4471. doi: 10.1210/endo.141.12.7809
Kandlikar, S. S., and Fink, G. D. (2011). Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am. J. Physiol. 301, H1965–H1973. doi: 10.1152/ajpheart.00086.2011
Kapusta, D. R., Chang, J. K., and Kenigs, V. A. (1999). Central administration of [Phe1Ψ (CH2-NH)Gly2]nociceptin(1–13)-NH2 and orphanin FQ/nociceptin (OFQ/N) produce similar cardiovascular and renal responses in conscious rats. J. Pharmacol. Exp. Ther. 289, 173–180.
Kapusta, D. R., and Kenigs, V. A. (1999). Cardiovascular and renal responses produced by central orphanin FQ/nociceptin occur independent of renal nerves. Am. J. Physiol. 77, R987–R995.
Kapusta, D. R., and Obih, J. C. (1995). Central kappa opioids blunt the renal excretory responses to volume expansion by a renal nerve-dependent mechanism. J. Pharmacol. Exp. Ther. 273, 199–205
Kapusta, D. R., Pascale, C. L., Kuwabara, J. T., and Wainford, R. D. (2013). CNS Gαi2–subunit proteins maintain salt-resistance via a renal nerve dependent sympathoinhibitory pathway. Hypertension 61, 368–375. doi: 10.1161/HYPERTENSIONAHA.111.00014
Kapusta, D. R., Pascale, C. L., and Wainford, R. D. (2012). Brain heterotrimeric Gαi2-subunit protein-gated pathways mediate central sympathoinhibition to maintain fluid and electrolyte homeostasis during stress. FASEB J. 26, 2776–2787. doi: 10.1096/fj.11-196550
Kapusta, D. R., Sezen, S. F., Chang, J. K., Lippton, H., and Kenigs, V. A. (1997). Diuretic and antinaturetic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci. 60, PL15–PL21.
Katada, T., and Ui, M. (1982). Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc. Natl. Acad. Sci. U.S.A. 79, 3129–3133. doi: 10.1073/pnas.79.10.3129
Kenney, M. J., Weiss, M. L., and Haywood, J. R. (2003). The paraventricular nucleus: an important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta. Physiol. Scand. 177, 7–15. doi: 10.1046/j.1365-201x.2003.01042.x
Kohara, K., Tabara, Y., Nakura, J., Imai, Y., Ohkubo, T., Hata, A., et al. (2008). Identification of hypertension-susceptibility genes and pathways by a systemic multiple candidate gene approach: the millennium genome project for hypertension. Hypertens. Res. 31, 203–212. doi: 10.1291/hypres.31.203
Kompanowska-Jezierska, E., Wolff, H., Kuczeriszka, M., Gramsbergen, J. B., Walkowska, A., Johns, E. J., et al. (2008). Renal nerves and nNOS: roles in natriuresis of acute isovolumetric sodium loading in conscious rats. Am. J. Physiol. 294, R1130–R1139. doi: 10.1152/ajpregu.00908.2007
Kondo, K., Murase, T., Otake, K., Ito, M., Kurimoto, F., and Oiso, Y. (1993). Galanin as a physiological neurotransmitter in hemodynamic control of arginine vasopressin release in rats. Neuroendocrinology 57, 224–229. doi: 10.1159/000126363
Kotchen, T. A., and Cowley, A. W. Jr, Frohlich, E. D. (2013). Salt in health and disease—a delicate balance. N. Engl. J. Med. 368, 1229–1237. doi: 10.1056/nejmra1212606
Krowicki, Z. K., and Kapusta, D. R. (2006). Tonic nociceptinergic inputs to neurons in the hypothalamic paraventricular nucleus contribute to sympathetic vasomotor tone and water and electrolyte homeostasis in conscious rats. J. Pharmacol. Exp. Ther. 317, 446–453. doi: 10.1124/jpet.105.094441
Krum, H., Schlaich, M. P., Sobotka, P. A., Bohm, M., Mahfoud, F., Rocha-Singh, K., et al. (2014). Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 282, 622–629. doi: 10.1016/S0140-6736(13)62192-3
Landgraf, R., Malkinson, T., Horn, T., Veale, W. L., Lederis, K., and Pittman, Q. J. (1990). Release of vasopressin and oxytocin by paraventricular stimulation in rats. Am. J. Physiol. 258, R155–R159.
Lastra, G., Dhuper, S., Johnson, M. S., and Sowers, J. R. (2010). Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat. Rev. Cardiol. 7, 577–584. doi: 10.1038/nrcardio.2010.123
Leenen, F. H., Ruzicka, M., and Huang, B. S. (2002). The brain and salt sensitive hypertension. Curr. Hypertens. Rep. 4, 129–135. doi: 10.1007/s11906-002-0037-y
Lohmeier, T. E., Hildebrandt, W., and Hood, W. A. (1999). Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension 33, 487–492. doi: 10.1161/01.HYP.33.1.487
Manunta, P., Hamlyn, J. M., Simonini, M., Messaggio, E., Lanzani, C., Bracale, M., et al. (2011). Endogenous ouabain and the renin-angiotensin-aldosterone system: distinct effects on Na handling and blood pressure in human hypertension. J. Hypertens. 29, 349–356. doi: 10.1097/hjh.0b013e32833ea821
Mattson, D. L., Dwinell, M. R., Greene, A. S., Kwitek, A. E., Roman, R. J., Jacob, H. J., et al. (2008). Chromosome substitution revels the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am. J. Physiol. Renal Physiol. 295, F837–F842. doi: 10.1152/ajprenal.90341.2008
May, C. N., Frithiof, R., Hood, S. G., McAllen, R. M., McKinley, M. J., and Ramchandra, R. (2009). Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp. Physiol. 95, 34–40. doi: 10.1113/expphysiol.2008.046342
McLoone, V. I., Ringwood, J. V., and Van Vliet, B. N. (2009). A multi-component model of the dynamics of salt-induced hypertension in Dahl-S rats. BMC Physiol. 9:20. doi: 10.1186/1472-6793-9-20
Menegaz, R. G., Kapusta, D. R., Mauad, H., and de Melo Cabral, A. (2001). Activation of alpha(2)-receptors in the rostral ventrolateral medulla evokes natriuresis by a renal nerve mechanism. Am. J. Physiol. 281, R98–R107.
Meneton, P., Jeunemaitre, X., de Wardener, H. E., and MacGregor, G. A. (2005). Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular disease. Physiol. Rev. 85, 679–715. doi: 10.1152/physrev.00056.2003
Menzaghi, C., Paroni, G., De Bonis, C., Soccio, T., Marucci, A., Bacci, S., et al. (2006). The-318 C>G single-nucleotide polymorphism in GNAI2 gene promoter region impairs transcriptional activity through specific binding of Sp1 transcription factor and is associated with high blood pressure in Caucasians from Italy. J. Am. Soc. Nephrol. 17, S115–S119. doi: 10.1681/asn.2005121340
Montani, J. P., and Van Vliet, B. N. (2009). Understanding the contribution of Guyton's large circulatory model to long-term control of arterial pressure. Exp. Physiol. 94, 382–388. doi: 10.1113/expphysiol.2008.043299
Mu, S., Shimosawa, T., Ogura, S., Wang, H., Uetake, Y., Kawakami-Mori, F., et al. (2011). Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat. Med. 17, 573–580. doi: 10.1038/nm0811-1020
Nasman, J., Kukkonen, J. P., Ammoun, S., and Akerman, K. E. (2001). Role of G protein availability in differential signaling by alpha 2-adrenoceptors. Biochem. Pharmacol. 62, 913–922. doi: 10.1016/s0006-2952(01)00730-4
Nielsen, S., DiGiovanni, S. R., Christensen, E. I., Knepper, M. A., and Harris, H. W. (1993). Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. U.S.A. 90, 11663–11667. doi: 10.1073/pnas.90.24.11663
Osborn, J. W., Averina, V. A., and Fink, G. D. (2009). Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp. Physiol. 94, 389–396. doi: 10.1113/expphysiol.2008.043281
Osborn, J. W., Collister, J. P., and Guzman, P. (2008). Effect of peripheral sympathetic nerve dysfunction on salt sensitivity of arterial pressure. Clin. Exp. Pharmacol. Physiol. 35, 273–279. doi: 10.1111/j.1440-1681.2007.04827.x
Patel, C. B., Noor, N., and Rockman, H. A. (2010). Functional selectivity in adrenergic and angiotensin signalling systems. Mol. Pharmacol. 78, 983–992. doi: 10.1124/mol.110.067066
Patel, K. P. (1991). Central alpha-2 adrenergic mechanisms in the renal nerve mediated natriuresis and diuresis produced by acute volume expansion. J. Auton. Nerv. Syst. 36, 47–54. doi: 10.1016/0165-1838(91)90129-q
Reinscheid, R. K., Nothacker, H. P., Bourson, A., Ardati, A., Henningsen, R. A., Bunzow, J. R., et al. (1995). Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794. doi: 10.1126/science.270.5237.792
Remaury, A., Larrouy, D., Daviaud, D., Rouot, B., and Paris, H. (1993). Coupling of the alpha 2-adrenergic receptor to the inhibitory G-protein Gi and adenylate cyclase in HT29 cells. Biochem. J. 292, 283–288.
Rodriguez-Iturbe, B., and Vaziri, N. D. (2007). Salt-sensitive hypertension: update on novel findings. Nephrol. Dial. Transplant. 22, 992–995. doi: 10.1093/ndt/gfl757
Rossi, G. C., Standifer, K. M., and Pasternak, G. W. (1995). Differential blockade of morphine, and morphine-6 beta-glucuronide analgesia by antisense oligodeoxynucleotides directed against MOR-1 and G-protein alpha subunits in rats. Neurosci. Lett. 198, 99–102. doi: 10.1016/0304-3940(95)11977-5
Ruffolo, R. R. Jr. Nichols, A. J., Stadel, J. M., and Hieble, J. P. (1991). Structure and function of alpha-adrenoceptors. Pharmacol. Rev. 43, 475–505
Ruginsk, S. G., Oliveira, F. R., Margatho, L. O., Vivas, L., Elias, L. L., and Antunes-Rodrigues, J. (2007). Glucocorticoid modulation of neuronal activity and hormone secretion induced by blood volume expansion. Exp. Neurol. 206, 192–200. doi: 10.1016/j.expneurol.2007.04.012
Schlaich, M., Sobotka, P. A., Krum, H., Lambert, E., and Esler, M. D. (2009). Renal sympathetic ablation for the treatment of uncontrolled hypertension. New Engl. J. Med. 361, 932–934. doi: 10.1056/NEJMc0904179
Serino, R., Ueta, Y., Hanamiya, M., Nomura, M., Yamamoto, Y., Yamaguchi, K. I., et al. (2001). Increased levels of hypothalamic neuronal nitric oxide synthase and vasopressin in salt-loaded Dahl rat. Auton. Neurosci. 87, 225–235. doi: 10.1016/S1566-0702(00)00279-4
Silva, R. M., Rossi, G. C., Mathis, J. P., Standifer, K. M., Pasternak, G. W., and Bodnar, R. J. (2000). Morphine and morphinw-6-beta-glucaronide-induced feeding are differentially reduced by G-protein alpha-subunit antisense in rats. Brain Res. 876, 62–75. doi: 10.1016/S0006-8993(00)02621-4
Singer, D. R., Markandu, N. D., Buckley, M. G., Miller, M. A., Sagnella, G. A., and MacGregor, G. A. (1998). Contrasting endocrine responses to acute oral compared with intravenous sodium loading in normal humans. Am. J. Physiol. 244, F111–F119.
Standifer, K. M., Rossi, G. C., and Pasternak, G. W. (1996). Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol. Pharmacol. 50, 293–298.
Stocker, S. D., Monahan, K. D., and Browning, K. N. (2013). Neurogenic and sympathoexcitatory actions of NaCl in Hypertension. Curr. Hypertens. Rep. 5, 538–546. doi: 10.1007/s11906-013-0385-9
Toney, G. M., Chen, Q. H., Cato, M. J., and Stocker, S. D. (2003). Central osmotic regulation of sympathetic nerve activity. Acta. Physiol. Scand. 177, 43–55. doi: 10.1046/j.1365-201X.2003.01046.x
Tso, W., and Wong, Y. H. (2006). Opioid receptor-like (ORL1) receptor utilizes both GoA and GoB for signal transduction. Prot. Pep. Lett. 13, 437–441. doi: 10.2174/092986606776819547
Van Vliet, B. N., Chafe, L. L., Halfyard, S. J., and Leonard, A. M. (2006). Distinct rapid and slow phases of salt-induced hypertension in Dahl salt-sensitive rats. J. Hypertens. 24, 1599–1606. doi: 10.1097/01.hjh.0000239296.25260.e0
Wainford, R. D., Carmichael, C. Y., Pascale, C. L., and Kuwabara, J. T. (2015). Gαi2-protein mediated signal transduction: a CNS molecular mechanism countering the development of sodium-dependent hypertension. Hypertension 65, 178–186. doi: 10.1161/HYPERTENSIONAHA.114.04463
Wainford, R. D., and Kapusta, D. R. (2009). Chronic high-NaCl intake prolongs the cardiorenal responses to central N/OFQ and produces regional changes in the endogenous brain NOP receptor system. Am. J. Physiol. 296, R280–R288. doi: 10.1152/ajpregu.00096.2008
Wainford, R. D., and Kapusta, D. R. (2010). Hypothalamic paraventricular nucleus G alpha q subunit protein pathways mediate vasopressin dysregulation and fluid retention in salt-sensitive rats. Endocrinology 151, 5403–5414. doi: 10.1210/en.2010-0345
Wainford, R. D., and Kapusta, D. R. (2012). Functional selectivity of central Gα-subunit proteins in mediating the cardiovascular and renal excretory responses evoked by central α(2)-adrenoceptor activation in vivo. Br. J. Pharmacol. 166, 210–220. doi: 10.1111/j.1476-5381.2011.01662.x
Wainford, R. D., Kurtz, K., and Kapusta, D. R. (2008). Central G-alpha subunit protein-mediated control of cardiovascular function, urine output, and vasopressin secretion in conscious Sprague-Dawley rats. Am. J. Physiol. 295, R535–R542. doi: 10.1152/ajpregu.00043.2008
Wainford, R. D., Pascale, C. L., and Kuwabara, J. T. (2013). Brain Gαi2–subunit protein-gated pathways are required to mediate the centrally evoked sympathoinhibitory mechanisms activated to maintain sodium homeostasis. J. Hypertens. 31, 747–757. doi: 10.1097/HJH.0b013e32835ebd54
Whelton, P. K., Appel, L. J., Sacco, R. L., Anderson, C. A., Antman, E. M., Campbell, N., et al. (2012). Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 126, 2880–2889. doi: 10.1161/CIR.0b013e318279acbf
Yatabe, M. S., Yatabe, J., Yoneda, M., Watanabe, T., Otsuki, M., Felder, R. A., et al. (2010). Salt sensitivity is associated with insulin release, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am. J. Clin. Nutr. 91, 77–82. doi: 10.3945/ajcn.2009.29028
Keywords: central Gαi2 proteins, blood pressure regulation, central G-protein coupled receptors, renal sympathetic nerves, sympathetic nervous system, salt-sensitive hypertension, sodium homeostasis
Citation: Carmichael CY and Wainford RD (2015) Brain Gαi2-subunit proteins and the prevention of salt sensitive hypertension. Front. Physiol. 6:233. doi: 10.3389/fphys.2015.00233
Received: 29 May 2015; Accepted: 03 August 2015;
Published: 19 August 2015.
Edited by:
Alexander John Rouch, Oklahoma State University-Center for Health Sciences, USAReviewed by:
Olaf Grisk, University of Greifswald, GermanyKathleen S. Curtis, Oklahoma State University-Center for Health Sciences, USA
Copyright © 2015 Carmichael and Wainford. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard D. Wainford, Department of Pharmacology and Experimental Therapeutics, The Whitaker Cardiovascular Institute, Boston University School of Medicine, 72 East Concord St., Boston, MA 02118, USA,cndhaW5mQGJ1LmVkdQ==
 Casey Y. Carmichael
Casey Y. Carmichael Richard D. Wainford
Richard D. Wainford