- 1Department of Biochemistry, Brody School of Medicine at East Carolina University, Greenville, NC, USA
- 2Department of Chemistry, East Carolina University, Greenville, NC, USA
A commentary on
Effect of Skeletal Muscle Native Tropomyosin on the Interaction of Amoeba Actin with Heavy Meromyosin
by Eisenberg, E., and Weihing, R. R. (1970). Nature 228, 1092–1093. doi: 10.1038/2281092a0
Troponin-tropomyosin inhibits skeletal and cardiac muscle contraction at low Ca2+. Binding of rigor-type myosin S1 to actin-tropomyosin-troponin, particularly at saturating Ca2+, produces activation of myosin ATPase activity in excess of that seen in the absence of the regulatory proteins. The binding energy of S1 can overcome the inhibitory activity of troponin (Bremel et al., 1972) and may allow tropomyosin to move deep into the groove of actin. That particular arrangement of actin, tropomyosin, and troponin is a much better activator of ATP hydrolysis than actin alone. That active configuration of actin was called state 2 in the Hill model (Hill et al., 1980) and later named the M state because of its requirement for tight myosin binding.
Eisenberg and Weihing found evidence that troponin itself can stabilize the active state of actin in the absence of high affinity S1 binding (Eisenberg and Weihing, 1970). They showed that troponin-tropomyosin enhanced the ability of amoeba actin to activate myosin S1 ATPase activity at high Ca2+. That observation is often overlooked but may be an important clue to managing some muscle disorders. Actin filaments containing the hypertrophic cardiomyopathy associated Δ14 mutation of TnT also enhanced S1 ATPase rates 2-3-fold higher than actin filaments without bound regulatory proteins (Gafurov et al., 2004). Because small changes in the structure of actin or troponin allow this increased activation to occur, the troponin complex must have a latent ability to enhance actin activation of myosin ATPase activity. The 14 C-terminal residues of TnT attenuate the ability of troponin to enhance actin activation. Troponin containing Δ14 TnT might act by stabilizing tropomyosin in the M state position of the actin groove under saturating Ca2+ conditions.
The inactive state of actin-tropomyosin-troponin (state 1 or the B state) occurs at low free Ca2+ when the inhibitory region of TnI is bound to actin. Because of associations among the regulatory proteins, tropomyosin is stabilized outside of the actin groove and there is little stimulation of myosin ATPase activity. Removal of the 14 C-terminal residues of TnT prevents formation of the B state. Compared with wild type actin filaments in EGTA, those containing Δ14 TnT exhibit less cooperativity in equilibrium binding of myosin S1 (Gafurov et al., 2004), and they do not exhibit the acrylodan tropomyosin fluorescence increase under conditions favoring the inactive state (Borrego-Diaz and Chalovich, 2010; Franklin et al., 2012).
Ca2+ binding to TnC opens a hydrophobic patch to which the switch region of TnI can bind (Herzberg et al., 1986). Under this condition, TnI is detached from actin and tropomyosin is situated in the actin groove. Several lines of evidence indicate that the major state formed with Ca2+ is a second inactive state with tropomyosin partially in the actin groove (Trybus and Taylor, 1980; McKillop and Geeves, 1991; Lehman et al., 2001; Kimura et al., 2002; Pirani et al., 2005; Poole et al., 2006). Full movement into the groove to form the active M state requires rigor S1 binding or a structural change in troponin. In the Hill model of regulation, Ca2+ binding to troponin was thought to create an inactive state 1 with bound Ca2+. State 1 with bound Ca2+ may be equivalent to the state intermediate between the B and M states that is called the C state (because of its link to Ca2+). The level of activation of ATPase activity at saturating Ca2+ is determined by the amount of M state formed in its equilibrium with the C state. The major state formed with Δ14 TnT containing actin filaments at low Ca2+ is likely to be the C state as the B state cannot form. The C state is also stabilized by a hypertrophic cardiomyopathy causing mutation, R146G TnI. The R146G mutation in TnI gives relative stabilization to the C state at low Ca2+ and the C state is highly stabilized at saturating Ca2+(Mathur et al., 2009). An analysis of ATPase rates of R146G TnI containing actin filaments supported the idea that the C state is ineffective in stimulating myosin ATPase activity.
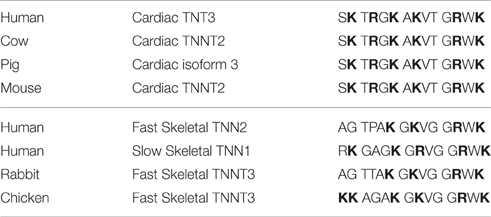
Studying natural mutations and modifications of troponin has given muscle researchers insights into the regulation of contraction. Long term deviations from the normal distribution of B, C, and M states of regulated actin seem to lead to progressive cardiac dysfunction. The last 14 residues of human cardiac TnT are critical for controlling the equilibria among the B, C, and M states of regulated actin; they stabilize the B state at low Ca2+ and destabilize the M state at saturating Ca2+. Table 1 compares the C-terminal sequences of several forms of troponin T. Note the conservation of the four terminal residues and the pattern of basic residues (bold). The regularly spaced basic residues suggest the possibility of acidic target sites for controlling both the B and M states.
The C-terminal region of TnT might function by directly affecting movement of tropomyosin on the actin surface. The C-terminal region could destabilize the active M state at saturating Ca2+ by interfering with tropomyosin movement into the actin groove. At low Ca2+, the C-terminal region of TnT could participate in holding tropomyosin away from the actin groove. The C-terminal 14 residues of TnT could also potentially alter the pathway of transmission of information from Ca2+ binding to TnC through the events leading to tropomyosin repositioning. Deciphering the mechanisms of action of the C-terminal region of TnT may lead to new therapies for cardiac disorders.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
Funded by NIH grant AR44504 and the Brody Brothers Grant to JC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Borrego-Diaz, E., and Chalovich, J. M. (2010). Kinetics of regulated actin transitions measured by probes on tropomyosin. Biophys. J. 98, 2601–2609. doi: 10.1016/j.bpj.2010.02.030
Bremel, R. D., Murray, J. M., and Weber, A. (1972). Manifestations of cooperative behavior in the regulated actin filament during actin-activated ATP hydrolysis in the presence of calcium. Cold Spring Harb. Symp. Quant. Biol. (Basel) 37, 267–275.
Eisenberg, E., and Weihing, R. R. (1970). Effect of skeletal muscle native tropomyosin on the interaction of amoeba actin with heavy meromyosin. Nature 228, 1092–1093. doi: 10.1038/2281092a0
Franklin, A. J., Baxley, T., Kobayashi, T., and Chalovich, J. M. (2012). The C-terminus of troponin T is essential for maintaining the inactive state of regulated actin. Biophys. J. 102, 2536–2544. doi: 10.1016/j.bpj.2012.04.037
Gafurov, B., Fredricksen, S., Cai, A., Brenner, B., Chase, P. B., and Chalovich, J. M. (2004). The Δ14 mutation of human cardiac troponin T enhances ATPase activity and alters the cooperative binding of S1-ADP to regulated actin. Biochemistry 43, 15276–15285. doi: 10.1021/bi048646h
Herzberg, O., Moult, J., and James, M. N. (1986). A model for the Ca2+-induced conformational transition of troponin C. A trigger for muscle contraction. J. Biol.Chem. 261, 2638–2644.
Hill, T. L., Eisenberg, E., and Greene, L. E. (1980). Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc. Natl. Acad. Sci. U.S.A. 77, 3186–3190.
Kimura, C., Maeda, K., Maeda, Y., and Miki, M. (2002). Ca2+S1-induced movement of troponin T on reconstituted skeletal muscle thin filaments observed by fluorescence energy transfer spectroscopy. J. Biochem. 132, 93–102. doi: 10.1093/oxfordjournals.jbchem.a003204
Lehman, W., Rosol, M., Tobacman, L. S., and Craig, R. (2001). Troponin organization on relaxed and activated thin filaments revealed by electron microscopy and three-dimensional reconstruction. J. Mol. Biol. 307, 739–744. doi: 10.1006/jmbi.2001.4514
Mathur, M. C., Kobayashi, T., and Chalovich, J. M. (2009). Some cardiomyopathy causing troponin I mutations stabilize a functional intermediate actin state. Biophys. J. 96, 2237–2244. doi: 10.1016/j.bpj.2008.12.3909
McKillop, D. F. A., and Geeves, M. A. (1991). Regulation of the actomyosin subfragment 1 interaction by troponin/tropomyosin. Evidence for control of a specific isomerization between two actoùmyosin subfragment 1 states. Biochem. J. 279, 711–718.
Pirani, A., Xu, C., Hatch, V., Craig, R., Tobacman, L. S., and Lehman, W. (2005). Single particle analysis of relaxed and activated muscle thin filaments. J. Mol. Biol. 346, 761–772. doi: 10.1016/j.jmb.2004.12.013
Poole, K. J. V., Lorenz, M., Evans, G., Rosenbaum, G., Pirani, A., Craig, R., et al. (2006). A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J. Struct. Biol. 155, 273–284. doi: 10.1016/j.jsb.2006.02.020
Keywords: troponin, tropomyosin, cardiomyopathy, troponin T, mutations
Citation: Chalovich JM and Johnson D (2016) Commentary: Effect of Skeletal Muscle Native Tropomyosin on the Interaction of Amoeba Actin with Heavy Meromyosin. Front. Physiol. 7:377. doi: 10.3389/fphys.2016.00377
Received: 28 June 2016; Accepted: 15 August 2016;
Published: 31 August 2016.
Edited by:
Jose Renato Pinto, Florida State University, USAReviewed by:
Aldrin V. Gomes, University of California, Davis, USADarshan Trivedi, Stanford University, USA
Copyright © 2016 Chalovich and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph M. Chalovich, Y2hhbG92aWNoakBlY3UuZWR1
 Joseph M. Chalovich
Joseph M. Chalovich Dylan Johnson
Dylan Johnson