- Laboratory of Integrative Cardiovascular and Metabolic Physiology, Division of Physiology, Department of Medicine, University of Fribourg, Fribourg, Switzerland
The body's ability to rapidly and appropriately regulate blood pressure in response to changing physiological demand is a key feature of a healthy cardiovascular system. Passively tilting the body, thereby changing central blood volume, is a well-recognized and controlled method of evaluating this ability. However, such studies usually involve single tilt angles, or intermittent tilting separated by supine, resting periods; valuable information concerning the adaptive capacity of the regulatory systems involved is therefore currently lacking. Furthermore, despite increasing recognition that men and women differ in the magnitude of their haemodynamic response to such stimuli, little is known about the degree to which gender differences in body composition and anthropometry influence these regulatory pathways, or indeed if these differences are apparent in response to graded, incremental tilting. In the present study we measured, in 23 young, healthy adults (13 men, 10 women), the continuous beat-to-beat haemodynamic response to graded, incremental tilting (0°, 20°, 40°, 60°, and back to 40°) with each tilt angle lasting 16 min. On average, we observed increases in heart rate (+41%), blood pressure (+10%), and total peripheral resistance (+16%) in response to tilting. However, whilst men showed an immediate decrease in cardiac output upon tilting (−8.9%) cardiac output in women did not change significantly from supine values. Interestingly, the decrease in stroke volume observed in women was significantly less than that observed in men (−22 vs. −36%, p < 0.05); although the present study could not determine if this difference was due to gender per se or due to differences in body size (in particular height) between the two gender groups. Such disparities in the magnitude of autonomic response may indicate (in the case of our gradual incremental tilt procedure) a better buffering capacity to progressive changes in central blood volume in women; which warrants further investigation, particularly in light of the well-recognized differences in cardiovascular disease risk between men and women.
Introduction
The appropriate and effective regulation of blood pressure, in order to maintain homeostasis despite changing physiological requirements, is a fundamental aspect of cardiovascular health. It has long been recognized that young women have, in general, lower resting blood pressure than young men, concomitant with a higher prevalence of orthostatic intolerance (Robertson, 1999; Waters et al., 2002; Fu et al., 2004). However, it has only recently been recognized that men and women regulate blood pressure through different physiological mechanisms—with young women generally showing enhanced parasympathetic (vagal) input to cardiac regulation, compared to the predominance of sympathetic vascular regulation observed in men (Evans et al., 2001; Hart et al., 2009, 2012; Kim et al., 2011; Joyner et al., 2015; Reulecke et al., 2016).
Passive tilting of the body (typically head-up), is widely used to investigate cardiovascular reflexes and regulatory capacity by altering blood distribution from the center to the periphery. However, whilst a number of studies have now explored gender differences using a single tilt angle (typically between 60° and 80°) compared to supine (Fu et al., 2005; Ndayisaba et al., 2015; Kangas et al., 2016; Patel et al., 2016; Reulecke et al., 2016), fewer studies have investigated these differences in response to graded, incremental tilting (i.e., no return to supine between each tilt angle (Shoemaker et al., 2001). Such studies are warranted as they may provide important information regarding response thresholds and possible gender differences in such thresholds.
Furthermore, it is important to note that little information is available concerning the role that anthropometric differences may play in the relationship between gender and blood pressure regulation. Indeed, we know that women typically have a smaller body size than men, with a higher proportion of fat to fat-free mass, which have been suggested to influence stroke volume and peripheral blood flow, respectively (Joyner et al., 2015). However, whether or not the variability in blood pressure regulation observed between men and women lies more in differences in body size/composition than in gender per se has not been fully explored.
With these limitations in our current understanding of the relationship between gender and blood pressure regulation, we aimed to assess the haemodynamic response to graded, incremental head-up tilting in a group of healthy, young adults, and to determine the degree to which any apparent gender differences may be related to differences in anthropometry and body composition.
Materials and Methods
Subjects
23 healthy, young adults (10 women, 13 men; age 25.0 ± 0.5 y) participated in the present study. This sample size was derived using an online power calculator (http://www.statisticalsolutions.net/pssTtest_calc.php) and based on the following: (i) resting values from previous studies of our local young adult population; (ii) haemodynamic response observed by others using a similar study protocol (Shoemaker et al., 2001); and (iii) an error probably (α) of 0.05 and power (1-β) of 0.80. These calculations indicated a necessary sample size of 8–10 subjects in each gender group. All subjects were Caucasian, non-obese (BMI < 26 kg/m2) and weight-stable, with less than 3% body weight variation in the 6 months preceding the study. Smokers, pregnant, or breast-feeding women, individuals taking medication, and those with any blood pressure abnormalities or cardiometabolic disease were excluded. Women were only tested during the follicular phase of their menstrual cycle. Prior to the day of testing, participants visited the laboratory in order to complete a questionnaire regarding their lifestyle (including physical activity level, caffeine intake and sleep quality) and medical history, and to familiarize themselves with the experimental procedure and equipment. All participants were requested to avoid physical activity, caffeine, and dietary supplements in the 24 h prior to testing. This study was carried out with the approval of the Fribourg state ethical review board, and all subjects gave written informed consent in accordance with the Declaration of Helsinki.
Anthropometric and Body Composition Measurements
Before anthropometric measurement, subjects were requested to empty their bladder. Body weight, height, and sitting height were measured to the nearest 0.1 kg and 1 mm respectively, using a mechanical column scale with integrated stadiometer (Seca model 709, Hamburg, Germany). Body composition was then assessed using a multi-frequency bioelectrical impedance analysis (Inbody 720, Biospace Co., Ltd, Seoul, Korea), and waist circumference and abdominal fat percentage by bioelectrical impedance analysis using ViScan (Tanita Corporation, Tokya, Japan); which has been shown to be accurate both for the measurement of waist circumference (Schutz et al., 2012) and for predicting total abdominal fat when validated against Magnetic Resonance Imaging techniques (Browning et al., 2010; Thomas et al., 2010). Body surface area was calculated according to Mosteller's formula (Mosteller, 1987).
Experimental Design
All experiments were conducted in the morning (08:30) after a 12 h overnight fast, in a quiet room at a comfortable ambient temperature (21–23°C). Monitoring electrodes were placed on the subject's neck and trunk (as described below) and the subject was then asked to lie supine on a clinical tilt table (Model 2900-00; CNSystems, Medizintechnik, Graz, Austria) with integrated foot support. Subjects were positioned such that their feet were in contact with the foot support, with straps placed to secure the subject at the level of the hips. In order to minimize skeletal muscle work and movement artifacts, subjects were encouraged to avoid movement and remain relaxed throughout the test. During the whole experiment, the participants were able to watch calm movies or documentaries to prevent sleeping and avoid boredom.
After a baseline measurement period of 40–45 min in the supine position, the subjects were passively tilted in increasing increments of 20 degrees (i.e., supine, 20°, 40°, 60°), remaining at each head-up tilt (HUT) angle for 16 min. The motorized tilt table achieved each 20° of tilt within 4–5 s. The subjects were then returned to an angle of 40° for a further 16 min, allowing us to determine the reversibility of the haemodynamic changes observed. Incremental graded tilting was utilized so as to compare the steady state changes in cardiovascular parameters consecutive to a slow gradual tilt and thereby the temporal adaptation of the body to progressive fluid shift (from thorax to lower limbs). Furthermore, by avoiding large changes in tilt angle (such as could occur if each tilt were separated by a supine period, or a single large tilt angle were used) we aimed to avoid issues of over-stimulation, whereby subtle changes/adaptations may be masked. It should also be noted that: (i) a return to 40° at the end of the experimental protocol (rather than supine or 20°) was chosen after a pilot study found that returning directly from 60° to supine was not well tolerated by the subjects (discomfort and altered sensation of body position in the horizontal plan); and (ii) we chose to monitor the variables for 16 min at each tilt angle to ensure we were monitoring a steady-state response and to minimize movement artifacts.
Continuous Haemodynamic Monitoring
Beat-to-beat cardiovascular recordings were performed using a Task Force Monitor (TFM) (CNSystems), with data sampled at a rate of 1000 Hz, as previously described (Girona et al., 2014; Miles-Chan et al., 2015). Briefly, ECG/Impedance electrodes were positioned together with upper arm and finger BP cuffs. Electrode strips were placed at the neck and thoracic regions, the latter specifically midclavicular at the xiphoid process level (Standard electrode kit, CNSystems). BP was monitored continuously using the Penaz principle from either the index or the middle finger of the right hand and was calibrated to oscillometric brachial BP measurements on the contralateral arm. During the experiment, the right hand and finger cuff were maintained at heart level, on an adjustable side board attached to the tilt table, in order to avoid hydrostatic pressure artifacts.
Impedance cardiography measurements, in which the changes in thoracic impedance are converted to reflect changes in thoracic fluid content over time, were performed based on the original Kubicek approach (Kubicek et al., 1966, 1970) but using an improved estimate of thoracic volume (Fortin et al., 2006), which allows calculation of cardiac stroke volume (SV). Heart rate (HR) was calculated from the appropriate RR-Interval (RRI). Cardiac output (CO) was computed as the product of SV and HR. Mean arterial BP (MAP) was calculated from diastolic BP (DBP) and systolic BP (SBP) as follows: MAP = DBP + ⅓(SBP − DBP). Total peripheral resistance (TPR) was calculated as MAP/CO. Indices of SV (SI), CO (CI), and TPR (TPRI) were calculated by dividing each of these variables by body surface area.
Autonomic Assessment
Sympathovagal balance was evaluated by power spectrum analysis of HR and BP variability (Hilz and Dütsch, 2006). High frequency (HF: 0.15–0.40 Hz) power components of RRI (HF_RRI) and low-frequency (LF: 0.04–0.15 Hz) power components of DBP (LF_DBP) and SBP (LF_SBP) were assessed. Baroreflex sensitivity (BRS) was assessed using the sequence method from spontaneous fluctuations in HR and BP, as previously described (Bertinieri et al., 1985).
Data and Statistical Analysis
All data are presented as Mean ± SEM unless otherwise stated, and were analyzed using the computer software STATISTIX 8 (Analytical Software, St. Paul, Minnesota, USA). Differences in baseline haemodynamic and anthropometric characteristics between genders were assessed by one-way ANOVA, and lifestyle parameters assessed by chi-square test. The mean of the last 4 min at each tilt (and the corresponding delta from baseline) were analyzed by repeated-measures ANOVA followed by Dunnett's multiple comparison tests. Relationships between the response of these haemodynamic variables and anthropometric characteristics were initially assessed by Pearson's correlation. Subsequently, positively-correlated anthropometric characteristics were entered into a stepwise linear regression (forward selection) analysis to determine the best predictive model for each haemodynamic variable.
Results
Anthropometry, Body Composition, and Lifestyle
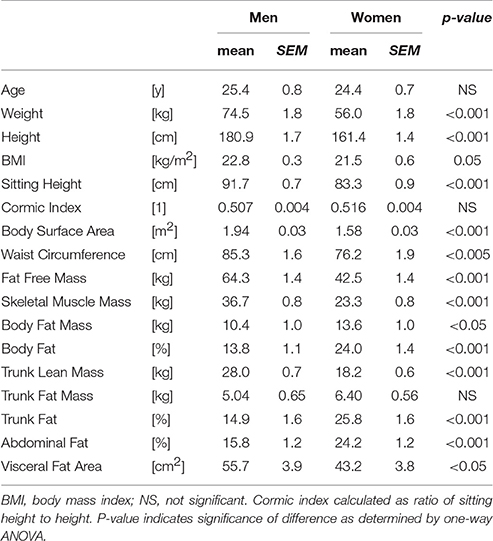
The anthropometric and body composition characteristics of the study population are shown in Table 1. On average, the women were shorter and lighter than the men, which was reflected in a lower average body surface area. However, the ratio of sitting height to height (Cormic index) did not differ between the two genders, and the small difference in BMI was at the limit of statistical significance (p = 0.05). Women showed a greater total body fat mass (both in terms of absolute mass and percentage fat), and a higher percentage of body fat in the trunk. However, in contrast both visceral fat area and waist circumference were lower in women than men. In terms of lifestyle parameters (assessed by questionnaire), there were no differences between men and women in terms of physical fitness levels, caffeine intake, or sleep quality.
Resting Cardiovascular Parameters
The haemodynamic characteristics of the study population at rest and in response to the graded tilt are summarized in Figures 1, 2. During the baseline supine period, heart rate was higher (Figure 1A; p < 0.05), and mean blood pressure lower (Figure 1B; p < 0.001) in women compared to men. This difference in blood pressure could be observed in both SBP (Figure 2A; p < 0.001) and DBP (Figure 2C; p < 0.005). Stroke volume was lower in women at rest (Figure 1C; p < 0.01), however there was no significant difference between the two genders at rest when adjusted for body surface area (i.e., stroke index, SI; Figure 1D). There were also no significant differences between men and women in terms of either CO (Figure 1E), CO adjusted for body surface area (cardiac index, CI; Figure 1F), TPR (Figure 1G), or BRS (Figure 2F). However, the mean TPR index (TPRI; Figure 1H) at rest was higher in men than women (p < 0.05).
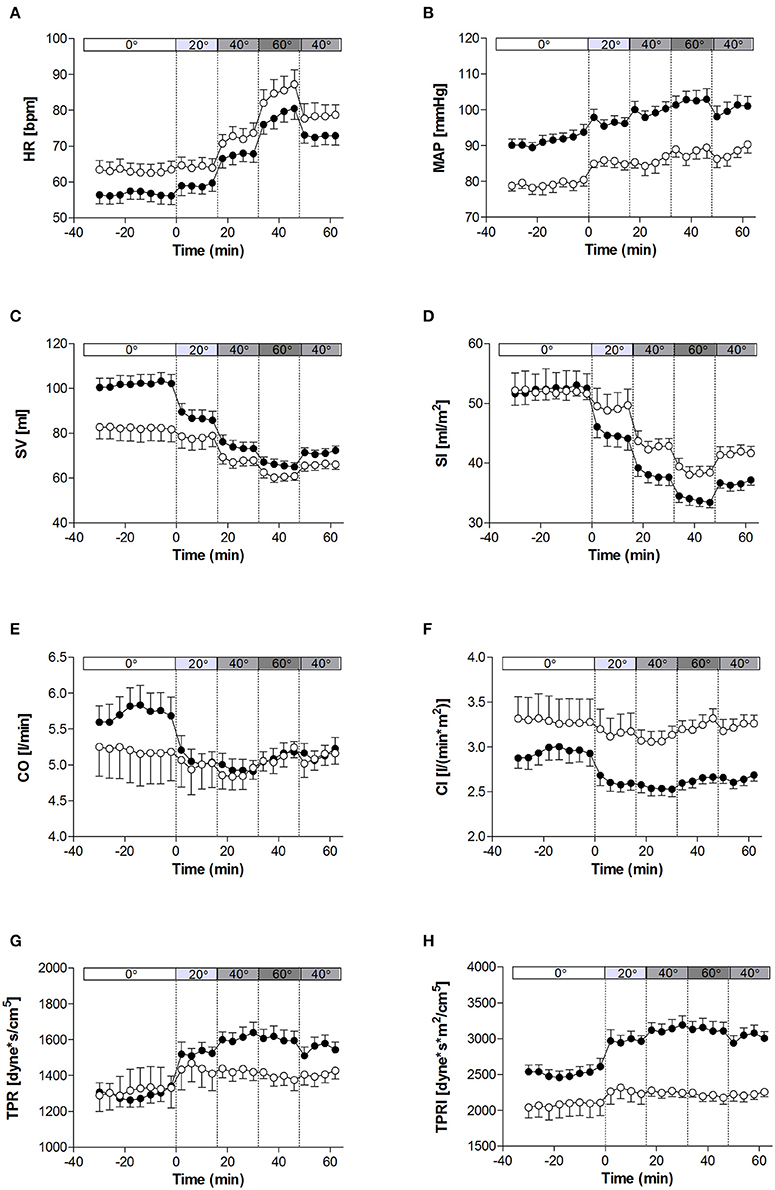
Figure 1. Hemodynamic measures at rest and in response to graded, incremental tilt in healthy young adults. (A) HR, heart rate; (B) MAP, mean arterial blood pressure; (C) SV, stroke volume; (D) SI, stroke index; (E) CO, cardiac output; (F) CI, cardiac index; (G) TPR, total peripheral resistance; (H) TPRI, total peripheral resistance index. Values are mean ± SEM of 4 min of measurement. Men (n = 13) are represented by closed circles; women (n = 10) are represented by open circles. Tilt angle is indicated in the bar above each panel (i.e., 0°, 20°, 40°, 60°, and back to 40°).
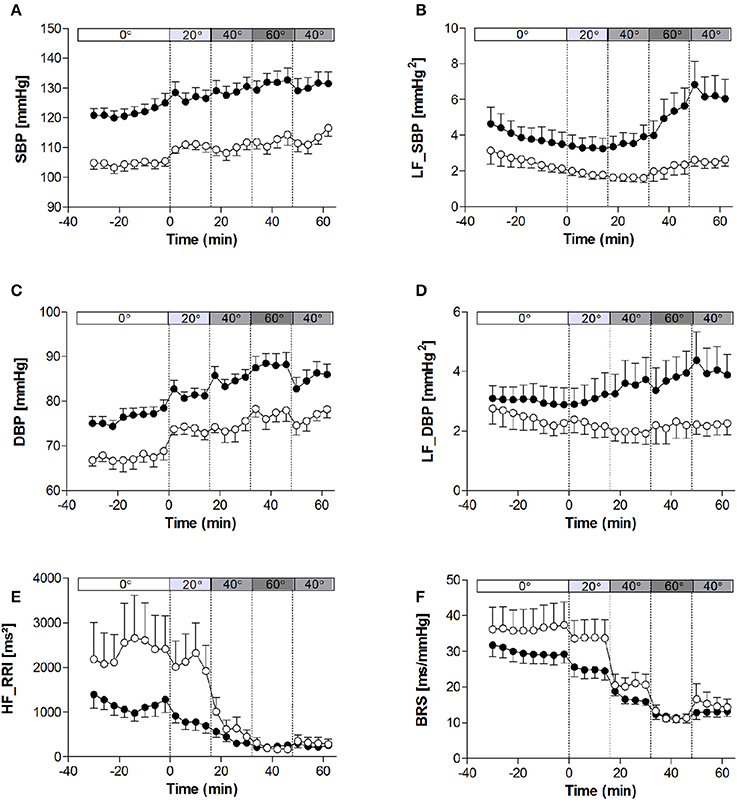
Figure 2. Heart rate variability, blood pressure variability, and baroreflex sensitivity at rest and in response to graded, incremental tilt in healthy young adults. (A) SBP, systolic blood pressure; (B) LF_SBP, low-frequency power component of SBP; (C) DBP, diastolic blood pressure; (D) LF_DBP, low-frequency power component of DBP; (E) HF_RRI, high frequency power component of R-R interval; (F) BRS, baroreflex sensitivity. Values are mean ± SEM of 4 min of measurement. Men (n = 13) are represented by closed circles; women (n = 10) are represented by open circles. Tilt angle is indicated in the bar above each panel (i.e., 0°, 20°, 40°, 60°, and back to 40°).
Cardiovascular Response to Tilting
When comparing the last 4 min at each tilt angle, HR, MAP, SBP, and DBP all increased in response to the graded, incremental tilt (all p < 0.001), with no differential effect of gender. SV and SI showed significant effects due to both tilt angle (p < 0.001) and gender (p < 0.001), with significant decreases observed in men at 20° but in women, despite a downward trend seen at 20°, this difference only reached statistical significance after the 40° tilt. Overall, the magnitude of this decrease in SV (from supine to 60°) was greater in men than women (men: −37.2 ± 4.2 ml (−36%); women: −20.9 ± 4.8 ml (−22%); p < 0.05), but this difference did not persist when SV was adjusted for body surface area (SI). No significant correlation was observed between change in HR and change in SV across the tilting protocol (Figure 3A). Furthermore, CO and CI immediately and significantly decreased in men following the 20° tilt (p < 0.001), but in women did not differ significantly from supine values at any of the tilt angles. The same pattern was observed in terms of the high-frequency power component of RRI (HF_RRI; Figure 2E), and was mirrored by changes in TPR and TPRI; which both increased in men, but did not significantly differ from supine in women. The low-frequency components of SBP and DBP (Figures 2B,D, respectively) were both significantly lower in women than men throughout the tilting protocol. However, the only significant change noted in response to the tilting itself was a significant increase in LF_SBP in men during the 60° tilt relative to supine. Lastly, tilting caused a significant decrease in BRS (p < 0.001), with significant decreases observed in men at 20° but in women only from the 40° tilt onward.
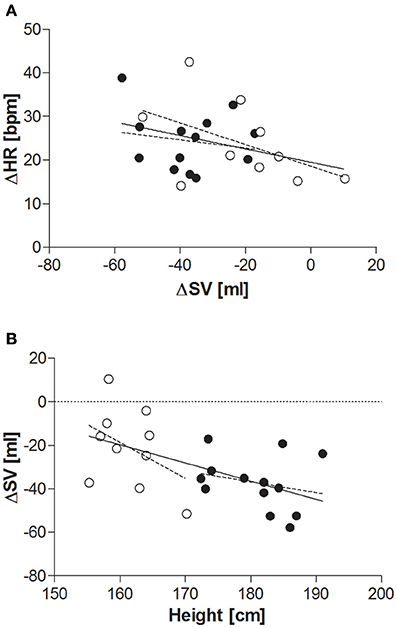
Figure 3. (A) Relationship between change in SV and change in HR (supine to 60° tilt). Men (n = 13) are represented by closed circles; women (n = 10) are represented by open circles. HR, heart rate (beats per minute); ΔHR, change in HR between supine and 60° head-up tilt; SV, stroke volume (ml); ΔSV, change in SV between supine and 60° head-up tilt. Pearson's correlation: all subjects: r = −0.339, p = 0.1; men: r = −0.177, p = 0.6; women: r = −0.489, p = 0.2. (B) Relationship between height and overall change in SV supine to 60° tilt. Men (n = 13) are represented by closed circles; women (n = 10) are represented by open circles. SV, stroke volume; ΔSV, change in SV between supine and 60° head-up tilt. Pearson's correlation: all subjects: r = −0.552, p < 0.01; men: r = −0.243, p = 0.4; women: r = −0.410, p = 0.2.
Whilst MAP during the second 40° tilt period matched that of the first, CO and HR remained elevated and BRS remained depressed in both men and women during this second (post 60°) period.
Correlations
Resting blood pressure was significantly positively correlated with weight, height, sitting height, body surface area, waist circumference, fat free mass, skeletal muscle mass, and trunk lean mass, and negatively correlated with percentage body fat (Table 2). However, following stepwise linear regression to remove auto-correlates, only height remained significantly predictive of MAP (Table 3).
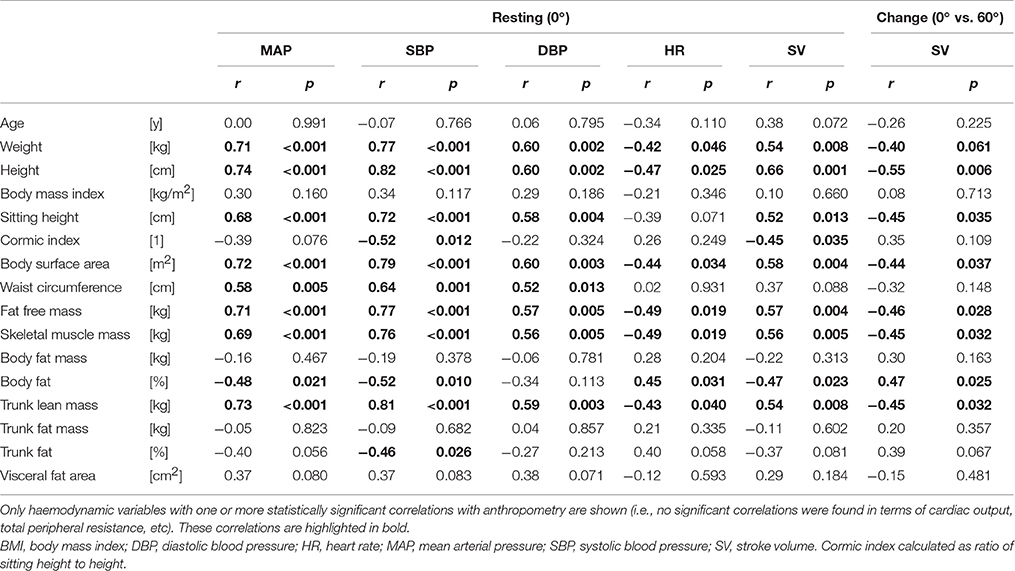
Table 2. Correlations (Pearson) between anthropometric characteristics and haemodynamic variables (at rest and in response to head-up tilt).
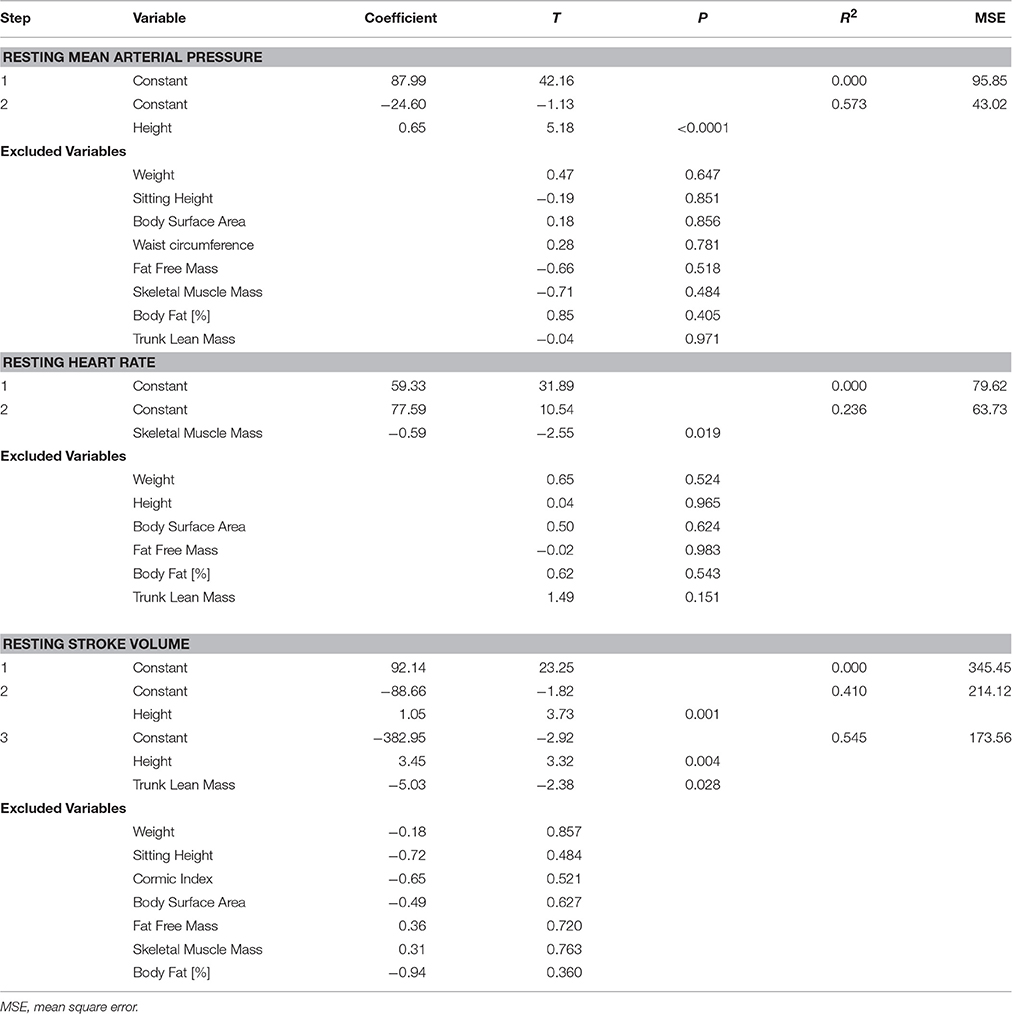
Table 3. Stepwise linear regression analysis (forward selection) of resting mean arterial pressure, heart rate, and stroke volume.
Resting heart rate was significantly, negatively correlated with weight, height, body surface area, fat free mass, skeletal muscle mass, and trunk lean mass (Table 2); with only skeletal muscle mass remaining significant following stepwise linear regression (Table 3). Conversely, resting (supine) SV was positively correlated with weight, height, sitting height, body surface area, fat free mass, skeletal muscle mass, and trunk lean mass, and negatively correlated with percentage body fat, and Cormic index (Table 2); with height and trunk lean mass remaining significantly predictive following stepwise linear regression (Table 3).
Whilst none of the measured anthropometric or body composition variables were found to be predictive of change in HR or blood pressure during the graded incremental tilt, significant correlations were observed between a number of these variables and change in steady-state SV (Table 2)—with height the primary predictive variable, and body surface area also contributing to the resulting stepwise model (Table 4). However, the relationship between change in steady-state SV and height did not reach statistical significance when analyzing data within (rather than across) the two gender groups (Figure 3B).
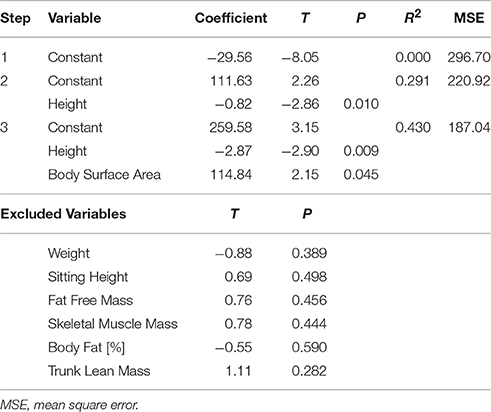
Table 4. Stepwise linear regression analysis (forward selection) of overall change in stroke volume (0°−60°).
No significant correlations were found between either CO or TPR and any of the measured anthropometric or body composition variables at rest, nor in response to the graded tilt.
Discussion
The results of the present study offer a clear demonstration of the ability of healthy young individuals to respond appropriately to changing blood distribution so as to maintain blood pressure and blood supply. This can be seen by the comparatively large decreases observed at 60° tilt in SV (on average −36% and −22% for men and women, respectively) as compared to the change in MAP (+10%), with the decrease in central blood volume being largely buffered by a large increase in heart rate (+41%; +24 beats/min), and augmented TPR (+16%; +21% in men, +10% in women) such that CO remained fairly constant. However, despite HR and SV being closely, inversely related (Cote et al., 2012) no significant correlation between change in each of these variables over the tilting protocol was found—which is most likely due to the relatively small sample size of the present study (as discussed below), but could also be related to an apparent heterogeneity in the relationship between these two variables (Goswami et al., 2009), or intrinsic variability in haemodynamic adjustments occurring within the heart and lungs (Guazzi et al., 1995). Indeed, the change in CO observed during the 60° tilt (average: −1.7 ± 3.9%) was less than that observed by others (10–23%) (Tuckman and Shillingford, 1966; Hainsworth and Al-Shamma, 1988; Fu et al., 2005; Bundgaard-Nielsen et al., 2009). This apparent discrepancy is most likely due to the incremental nature of the tilting protocol used in the present study as opposed to the discontinuous protocols (i.e., each tilt separated by a supine rest) used in the latter studies, and hence our findings may be more representative of the adaptive capacity of the haemodynamic regulatory mechanisms involved.
Under resting, supine conditions, the women in the present study showed a higher average heart rate, and lower average blood pressure, stroke volume, and total peripheral resistance than their male counterparts; although these differences were most likely due to differences in skeletal muscle mass and height between the two groups rather than an effect of gender per se. Similarly, differences in the magnitude of changes in steady-state CO and TPR between men and women could be largely accounted for by differences in body surface area (i.e., when considering CI and TPRI).
A number of differences in the time-course of the haemodynamic response also appear to exist between the genders which cannot be explained by anthropometry alone. In particular, the majority of measured variables changed immediately upon tilting in men (i.e., between supine and 20°), but not until 40° in women. These changes are in parallel to BRS and vagal tone (as represented by HF_RRI) which remained elevated in women during the 20° but dropped dramatically at 40°. Taken together, these results indicate an asymmetry in the time course of parasympathetic response to graded tilt between men and women. The relative lack of change in the LF components of SBP and DBP observed in women while supine and across all tilt angles may be due to sex hormones, in particular estrogen, which is known to be sympatho-inhibitory, and inversely related to muscle sympathetic nerve activity (Joyner et al., 2015). In contrast, men displayed elevated sympathetic activity (as assessed by the LF power components of SBP and DBP), in line with a previous study showing significantly higher values of LF and total power in men than women when supine or standing upright (Barantke et al., 2008). Similarly, this agrees with the recent study of Reulecke et al. (2016), who investigated the effect of gender on the time-course of autonomic regulation during an orthostatic challenge (70°), and found that men showed an instantaneous increase in sympathetic activity which persisted across the entire orthostatic phase, and at a much higher level than that observed in women. The results of the present study therefore appear to support the suggestion of Kim et al. (2011) that, in comparison to men, women may rely more on baroreflex control of heart rate than on control of sympathetic vasomotor tone.
It should be noted, however, that there were some key limitations of this study, and as such these potential gender differences should be interpreted with caution. Firstly, the sample size of the present study, whilst in line with others investigating the effect of gender on the haemodynamic response tilting (Shoemaker et al., 2001; Fu et al., 2005; Patel et al., 2016; Reulecke et al., 2016), is not sufficient to draw any conclusions regarding the relative contribution of gender per se above that of gender-related differences in body size and/or composition. Further investigation is therefore warranted to confirm these results in a larger number of participants and across a larger range of body morphology. Secondly, whilst impedance cardiography has shown good correlations with both Doppler (Cybulski et al., 1993) and invasive techniques (Fortin et al., 2006) for the determination of SV, this assessment does require estimation of the electrical participating thoracic volume (a function of the subject's gender, height, and weight), and is therefore prone to error. Additional error may arise from small changes in the shape of the thorax or position of the heart that occur with changes in body posture.
Interestingly, there was a strong relationship observed between change in SV in response to tilting and height; with height and BMI together accounting for >40% of variability in delta SV. However, the present study could not determine if the difference in SV response observed between men and women was reflective of differences in body size (in particular height) between the two gender groups or due to gender per se. It would therefore be of interest to determine whether this relationship can also be observed within genders and/or across a larger range of heights, particularly given the well-established epidemiological link between short stature and risk for coronary heart disease (Paajanen et al., 2010). Furthermore, the smaller decreases in SV observed in women compared to men during the incremental head-up tilt—even when adjusted for differences in body size (i.e., stroke index)—are of particular interest, as they appear to be at odds with reports of an increased prevalence of orthostatic intolerance in young women (Robertson, 1999; Waters et al., 2002; Fu et al., 2004). This finding may indicate a better buffering capacity in response to slow progressive changes in central blood volume in women (as occurred with incremental tilting), and warrants further investigation, particularly in light of the well-recognized differences in cardiovascular disease risk between men and women (Hart et al., 2012).
Author Contributions
Both authors (DS and JM-C) performed the experiment and analyzed the data; JM-C wrote the manuscript; both authors (DS and JM-C) read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Abdul Dulloo and Jean-Pierre Montani for their useful comments and suggestions regarding both the design of the study and the preparation on the manuscript.
References
Barantke, M., Krauss, T., Ortak, J., Lieb, W., Reppel, M., Burgdorf, C., et al. (2008). Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J. Cardiovasc. Electrophysiol. 19, 1296–1303. doi: 10.1111/j.1540-8167.2008.01257.x
Bertinieri, G., di Rienzo, M., Cavallazzi, A., Ferrari, A. U., Pedotti, A., and Mancia, G. (1985). A new approach to analysis of the arterial baroreflex. J. Hypertens. Suppl. 3, S79–S81.
Browning, L. M., Mugridge, O., Chatfield, M. D., Dixon, A. K., Aitken, S. W., Joubert, I., et al. (2010). Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity 18, 2385–2391. doi: 10.1038/oby.2010.71.
Bundgaard-Nielsen, M., Sørensen, H., Dalsgaard, M., Rasmussen, P., and Secher, N. H. (2009). Relationship between stroke volume, cardiac output and filling of the heart during tilt. Acta Anaesthesiol. Scand. 53, 1324–1328. doi: 10.1111/j.1399-6576.2009.02062.x
Cote, A. T., Phillips, A. A., Bredin, S. S., and Warburton, D. E. (2012). A questionable association of stroke volume and arterial pulse pressure under gravitational stress. J. Trauma Acute Care Surg. 72, 708–712. doi: 10.1097/TA.0b013e31822dd202
Cybulski, G., Miskiewicz, Z., Szulc, J., Torbicki, A., and Pasierski, T. (1993). A comparison between the automatized impedance cardiography and pulsed-wave Doppler echocardiography methods for measurements of stroke volume (SV) and systolic time intervals (STI). J. Physiol. Pharmacol. 44, 251–258.
Evans, J. M., Ziegler, M. G., Patwardhan, A. R., Ott, J. B., Kim, C. S., Leonelli, F. M., et al. (2001). Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. (1985) 91, 2611–2618.
Fortin, J., Habenbacher, W., Heller, A., Hacker, A., Grüllenberger, R., Innerhofer, J., et al. (2006). Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput. Biol. Med. 36, 1185–1203. doi: 10.1016/j.compbiomed.2005.06.001
Fu, Q., Arbab-Zadeh, A., Perhonen, M. A., Zhang, R., Zuckerman, J. H., and Levine, B. D. (2004). Hemodynamics of orthostatic intolerance: implications for gender differences. Am. J. Physiol. Heart Circ. Physiol. 286, H449–H457. doi: 10.1152/ajpheart.00735.2002
Fu, Q., Witkowski, S., Okazaki, K., and Levine, B. D. (2005). Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R109–R116. doi: 10.1152/ajpregu.00013.2005
Girona, M., Grasser, E. K., Dulloo, A. G., and Montani, J. P. (2014). Cardiovascular and metabolic responses to tap water ingestion in young humans: does the water temperature matter? Acta Physiol. (Oxf). 211, 358–370. doi: 10.1111/apha.12290
Goswami, N., Roessler, A., Lackner, H. K., Schneditz, D., Grasser, E., and Hinghofer-Szalkay, H. G. (2009). Heart rate and stroke volume response patterns to augmented orthostatic stress. Clin. Auton. Res. 19, 157–165. doi: 10.1007/s10286-009-0001-x
Guazzi, M., Pepi, M., Maltagliati, A., Celeste, F., Muratori, M., and Tamborini, G. (1995). How the two sides of the heart adapt to graded impedance to venous return with head-up tilting. J. Am. Coll. Cardiol. 26, 1732–1740. doi: 10.1016/0735-1097(95)00370-3
Hainsworth, R., and Al-Shamma, Y. M. (1988). Cardiovascular responses to upright tilting in healthy subjects. Clin. Sci. 74, 17–22.
Hart, E. C., Charkoudian, N., Wallin, B. G., Curry, T. B., Eisenach, J. H., and Joyner, M. J. (2009). Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53, 571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391
Hart, E. C., Joyner, M. J., Wallin, B. G., and Charkoudian, N. (2012). Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J. Physiol. 590, 2069–2079. doi: 10.1113/jphysiol.2011.224642
Hilz, M. J., and Dütsch, M. (2006). Quantitative studies of autonomic function. Muscle Nerve 33, 6–20. doi: 10.1002/mus.20365
Joyner, M. J., Barnes, J. N., Hart, E. C., Wallin, B. G., and Charkoudian, N. (2015). Neural control of the circulation: how sex and age differences interact in humans. Compr. Physiol. 5, 193–215. doi: 10.1002/cphy.c140005
Kangas, P., Tahvanainen, A., Tikkakoski, A., Koskela, J., Uitto, M., Viik, J., et al. (2016). Increased cardiac workload in the upright posture in men: noninvasive hemodynamics in men versus women. J. Am. Heart Assoc. 5:e002883. doi: 10.1161/JAHA.115.002883
Kim, A., Deo, S. H., Vianna, L. C., Balanos, G. M., Hartwich, D., Fisher, J. P., et al. (2011). Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am. J. Physiol. Heart Circ. Physiol. 301, H2454–H2465. doi: 10.1152/ajpheart.00772.2011
Kubicek, W. G., From, A. H., Patterson, R. P., Witsoe, D. A., Castaneda, A., Lillehei, R. C., et al. (1970). Impedance cardiography as a noninvasive means to monitor cardiac function. J. Assoc. Adv. Med. Instrum. 4, 79–84.
Kubicek, W. G., Karnegis, J. N., Patterson, R. P., Witsoe, D. A., and Mattson, R. H. (1966). Development and evaluation of an impedance cardiac output system. Aerosp. Med. 37, 1208–1212.
Miles-Chan, J. L., Charrière, N., Grasser, E. K., Montani, J. P., and Dulloo, A. G. (2015). The blood pressure-elevating effect of Red Bull energy drink is mimicked by caffeine but through different hemodynamic pathways. Physiol. Rep. 3:e12290. doi: 10.14814/phy2.12290.
Mosteller, R. D. (1987). Simplified calculation of body-surface area. N. Engl. J. Med. 317, 1098. doi: 10.1056/NEJM198710223171717
Ndayisaba, J. P., Fanciulli, A., Granata, R., Duerr, S., Hintringer, F., Goebel, G., et al. (2015). Sex and age effects on cardiovascular autonomic function in healthy adults. Clin. Auton. Res. 25, 317–326. doi: 10.1007/s10286-015-0310-1
Paajanen, T. A., Oksala, N. K., Kuukasjärvi, P., and Karhunen, P. J. (2010). Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur. Heart J. 31, 1802–1809. doi: 10.1093/eurheartj/ehq155
Patel, K., Rössler, A., Lackner, H. K., Trozic, I., Laing, C., Lorr, D., et al. (2016). Effect of postural changes on cardiovascular parameters across gender. Medicine (Baltimore) 95:e4149. doi: 10.1097/MD.0000000000004149
Reulecke, S., Charleston-Villalobos, S., Voss, A., González-Camarena, R., González-Hermosillo, J., Gaitán-González, M. J., et al. (2016). Men and women should be separately investigated in studies of orthostatic challenge due to different gender-related dynamics of autonomic response. Physiol. Meas. 37, 314–332. doi: 10.1088/0967-3334/37/3/314
Robertson, D. (1999). The epidemic of orthostatic tachycardia and orthostatic intolerance. Am. J. Med. Sci. 317, 75–77.
Schutz, Y., Sarafian, D., Miles, J. L., Montani, J. P., and Dulloo, A. G. (2012). Non-contact assessment of waist circumference: will tape measurements become progressively obsolete? Eur. J. Clin. Nutr. 66, 269–272. doi: 10.1038/ejcn.2011.183
Shoemaker, J. K., Hogeman, C. S., Khan, M., Kimmerly, D. S., and Sinoway, L. I. (2001). Gender affects sympathetic and hemodynamic response to postural stress. Am. J. Physiol. Heart Circ. Physiol. 281, H2028–H2035.
Thomas, E. L., Collins, A. L., McCarthy, J., Fitzpatrick, J., Durighel, G., Goldstone, A. P., et al. (2010). Estimation of abdominal fat compartments by bioelectrical impedance: the validity of the ViScan measurement system in comparison with MRI. Eur. J. Clin. Nutr. 64, 525–533. doi: 10.1038/ejcn.2010.18
Tuckman, J., and Shillingford, J. (1966). Effect of different degrees of tilt on cardiac output, heart rate, and blood pressure in normal man. Br. Heart J. 28, 32–39.
Keywords: head-up tilt, gender, haemodynamics, blood pressure regulation, sex difference
Citation: Sarafian D and Miles-Chan JL (2017) The Influence of Gender and Anthropometry on Haemodynamic Status at Rest and in Response to Graded Incremental Head-Up Tilt in Young, Healthy Adults. Front. Physiol. 7:656. doi: 10.3389/fphys.2016.00656
Received: 06 September 2016; Accepted: 14 December 2016;
Published: 04 January 2017.
Edited by:
Elisabeth Lambert, Baker IDI Heart and Diabetes Institute, AustraliaReviewed by:
Joyce McClendon Evans, University of Kentucky, USAMark Butlin, Macquarie University, Australia
Copyright © 2017 Sarafian and Miles-Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer L. Miles-Chan, amVubmlmZXJseW5uLm1pbGVzQHVuaWZyLmNo
 Delphine Sarafian
Delphine Sarafian Jennifer L. Miles-Chan
Jennifer L. Miles-Chan