- 1Department of Anaesthesiology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, China
- 2Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China
- 3Key Laboratory for Protein Sciences of Ministry of Education, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China
The mechanosensitive channel of small conductance (MscS) is a bacterial membrane pore that senses membrane tension and protects cells from lysis by releasing osmolytes. MscS is a homoheptameric channel with a cytoplasmic domain with seven portals and a β-barrel opening to the cytoplasm. TtMscS, an MscS channel from Thermoanaerobacter tengcongensis, is an anion-selective channel. A previous study from our laboratory has defined the crucial role of β-barrel in the anion selectivity of TtMscS (Zhang et al., 2012). However, the mechanistic details by which the β-barrel determines anion selectivity remain unclear. Here, using mutagenesis and patch-clamp recordings, we investigated the function and structural correlations between β-barrels and the anion selectivity of TtMscS at the atomic level. Our results indicated that mutation of V274, a residue at the center of the inner wall of the β-barrel in TtMscS, caused the anion selectivity of TtMscS reverse to cation selectivity. Moreover, the electrostatic potential (T272) and physical size (L276) of residues in the inner wall of β-barrel also determine the anion selectivity of TtMscS. In summary, the present study confirmed that the β-barrel region of TtMscS acts as a “selective filter” that renders TtMscS anion selectivity.
Introduction
Mechanosensitive (MS) channels are membrane pores that are universally present in prokaryotes and eukaryotes (Martinac et al., 2008; Kung et al., 2010). These channels sense membrane tension and consequently accelerate the passage of osmolytes from areas of high concentration to those of low concentration (Kung, 2005). In bacteria, MS channels can be activated by turgor pressure. Like an “emergency valve,” when bacteria experience hypoosmotic shock, such as during rain, the channels open and release osmolytes along with water, thus alleviating cell turgor and protecting cells from lysis (Martinac et al., 1987). Additionally, a recent study has suggested that MS channels play a crucial role in the calcium regulation of bacteria with life-threatening hypoosmotic conditions (Cox et al., 2013), thereby ensuring bacterial survival across a wide range of external osmolarity levels. In mammals, MS channels have been implicated in numerous physiological processes, including touch, pain sensation, hearing, blood pressure control, micturition, tissue growth, cell volume regulation, and turgor control (Arnadóttir and Chalfie, 2010; Ranade et al., 2015).
The mechanosensitive channel of small conductance (MscS) is a homoheptameric channel that belongs to a family of pressure-sensitive channels. MscS channels are small conductance channels that open at a slight pressure of tens of mmHg; this pressure may result from an osmotic difference of only a few milliosmoles through the membrane (Martinac et al., 1987; Kung et al., 2010). This characteristic allows bacteria to detect circumstantial osmotic changes more efficiency.
Two crystal structures of EcMscS (the MscS channel from Escherichia coli) have been solved. One is a wild-type EcMscS structure that presumably represents a closed state of MscS (Bass et al., 2002), and the other is EcMscS with an A106V mutation. This mutant has a substantial rotational rearrangement of the transmembrane (TM) region and an increased pore size; it is considered to reflect an open state of MscS (Wang et al., 2008). The MscS channels include two functional domains: a TM domain and a cytoplasmic domain. The TM domain enables the sensors to sense the tension exerted on the membrane or depolarization of the membrane potential (Bass et al., 2002; Bezanilla and Perozo, 2002). The cytoplasmic domain, with seven portals and a β-barrel (a distal pore at the bottom of MscS) opening to the cytoplasm, is conserved in the MscS family (Perozo and Rees, 2003; Schumann et al., 2004). However, the function of the cytoplasmic domain is unclear. Recent biophysical studies have suggested that the cytoplasmic vestibule undergoes significant conformational changes when this channel opens (Miller et al., 2003). In addition, the cytoplasmic domain has been reported to be involved in the adaptation and ion-selective process of the channel (Sotomayor et al., 2007; Gamini et al., 2011; Cox et al., 2013).
The crystal structure and functions of TtMscS, an MscS channel from Thermoanaerobacter tengcongensis (T. tengc), has recently been reported by our team (Zhang et al., 2012). Although, TtMscS has only 27% similarity to EcMscS, their overall structural folds are similar. The entire structure of TtMscS closely resembles the EcMscS structure of the closed state except for two distinct cytoplasmic regions: the side portals and the bottom β-barrel. Sequence alignment combined with structural analysis revealed that three bulky residues, Phe157, Met243, and Trp246, lead to a much smaller portal for TtMscS than EcMscS. In contrast, the β-barrel in TtMscS is much larger than that in EcMscS. In addition, the pattern of electrostatic surface potentials inside the β-barrel pore and around the β-barrel region between TtMscS and EcMscS are opposite. The larger pore size and distinctive surface potential distribution indicate that the β-barrel is a dominant pathway for ion entry in TtMscS (Zhang et al., 2012). However, the mechanistic details of the β-barrel structure to anion selection of TtMscS are still unclear.
In this study, on the basis of structural analysis, we introduced several mutations at key residues in the inner wall of the β-barrel pore, aiming to change the physical size and electrostatic potential of the side chains of these key residues. Our electrophysiological results showed that key point mutations in the β-barrel attenuated or reversed the anion selectivity of TtMscS. In conclusion, the present study defines several residues that are crucial for the anion selectivity of TtMscS and supports the hypothesis that the β-barrel of TtMscS acts as a “selective filter” that confers TtMscS anion selectivity.
Materials and Methods
Protein Expression and Purification
The gene encoding the MscS channel from T. tengcongensis (TtMscS) was cloned into pET-21, a vector with an N-terminal 6 × His tag followed by a tobacco etch virus (TEV) protease cleavage site. The channel was expressed in BL21 cell cultures by induction with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at A600 ~ 1.0. The cells were cultured at 23°C for 18 h and collected. The collected bacteria were homogenized in lysis buffer (containing: 50 mM Tris · HCl and 200 mM NaCl; PH: 8.0) and lysed by sonication. The suspension was centrifuged (15,000 × g) for 15 min to remove cell debris. The supernatant was collected and centrifuged by ultracentrifugation (100,000 × g) for 1 h to obtain the membrane fraction (sediment). The membrane fraction was suspended in buffer A (containing 25 mM Tris · HCl, 20 mM imidazole and 500 mM NaCl; PH: 8.0), and Triton X-100 (0.5%, vol/vol) and PMSF (1 mM) were then added. The suspension was incubated at 4°C with slow stirring overnight. Then, the sample was subjected to ultracentrifugation (100,000 × g) for 30 min. The supernatant was harvested and loaded onto a Ni-NTA column (GE Healthcare) pre-equilibrated with buffer A containing 0.1% Triton X-100. Next, the column was washed with buffer A by adding 20 mM imidazole and 0.02% n-dodecyl-β-D-maltopyranoside (DDM) to detach any non-specific binding. The channel protein was washed with buffer B (containing 25 mM Tris·HCl, 300 mM imidazole, 500 mM NaCl, and 0.02% DDM, PH: 8.0), and the N-terminal 6 × His-tag was removed by TEV protease. The channel protein was concentrated and further purified by gel filtration (Superdex-200, GE Healthcare) with elution buffer (25 mM Tris·HCl, 200 mM NaCl, 5 mM DTT and 0.02% DDM; PH: 8.0). To confirm the purity of the protein, the peak fraction was collected and separated by SDS-PAGE and stained with Coomassie blue. The purified proteins were stored at 4°C for no more than 1 week for further functional studies.
Preparation of Giant Liposomes and Electrical Recording
All lipids used in this study were obtained from Avanti Polar Lipids. The wild-type TtMscS channel and mutant proteins were reconstituted into lipid vesicles composed of 1-pal-mitoyl-2-oleoyl-phosphatidylethanolamine (POPE, 7.5 mg/mL) and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG, 2.5 mg/mL) by using a previously described method (Li et al., 2007). To ensure single channel recording, the protein concentrations of lipids were all 150 μg/ml. Before electrophysiological analysis, the lipids were subjected to regular dehydrate and hydrate processes to obtain giant liposomes. We performed patch-clamp recording in asymmetrical solution with conditioning in 150 mM KCl, 500 mM sucrose, 5 mM K-HEPES (pH 7.0) in the bath solution, and 15 mM KCl, 500 mM sucrose, 5 mM K-HEPES (pH 7.0) in the pipette solution (the 500 sucrose provides osmotic protection, prevents giant liposomes from bursting and stabilizes the gigaohm seal formation). When measuring the preference for other anions, KCl was exchanged to the corresponding saline solution (KNO3, KF, KBr). Membrane voltages were controlled. After gigaohm seal (with a constant resistance of 3–8 GΩ) formation, the current was recorded with an Axopatch 200B amplifier with a Digidata 1322A analog-to-digital converter (Axon Instruments). Single channel currents were recorded in Clampex 10.2 software, filtered at 2 kHz, and digitized at 5 kHz. The data were analyzed in Clampfit 10.2 software, and the current amplitudes were measured by the difference between the cursor aligned at the baseline and peak of the current traces. The mechanical pressure was measured with a pressure monitor (PM015D, WPI).
Estimate of the Ion Conductance and Ion Permeability Ratio of TtMscS Channel
We plotted single-channel currents against voltage to acquire I–V curves. Ion conductance was estimated by the slope of the I–V curves. The permeability ratios were calculated using the following versions of the Goldman–Hodgkin–Katz (GHK) equation:
where X is a monovalent anion. [X]o/[K+]o and [X]i/[K+]i are ion concentration on extracellular (cis-side) and intracellular sides (trans-side), respectively.
Estimate of the Free Energy of Activation of the TtMscS Channel
We estimated the free energy of activation of the TtMscS channel protein by using a previously described method (Kloda and Martinac, 2001). Briefly, the open probability of the TtMscS channel plotted against negative pressure was fitted to a Boltzmann distribution function given by:
where N is the number of channels in the patch, and α is the sensitivity constant to negative pressure; p is the negative pressure applied to the pipette, and p1/2 is the negative pressure applied when the open probability of the channel is 0.5 (Po = 0.5). Po and Po max represent the open probability and maximum open probability of the channel, respectively.
A two-state Boltzmann model was used to fit the gating of MS channels, with the change of area tΔA as the dominant energy term (Sukharev et al., 1999). The free energy ΔG is a linear function of membrane tension, as shown in the following equation:
where ΔGo is the difference in free energy between the closed and opened conformations of MS channels with the absence of the externally membrane tension. ΔA is the difference in membrane area occupied by open and closed channels at a given membrane tension, and tΔA is the work for the gating of MS channels gated at the open probability of 0 < Po < 1.
Because the Boltzmann function for the open probability of MS channels can be written as
By using Laplace's law
where r is the radius of curvature of the liposome patch shaped by the negative pressure applied to the pipette. Thus, when the open probability Po = 0.5 (i.e., p = p1/2 and t = t1/2) the free energy difference ΔGo = 0. Consequently, t1/2 = ΔGo/ΔA and p1/2 = 2ΔGo/rΔA, whereas α = rΔA/2kT. The expression of the free energy for the activation of TtMscS channel in this study is given by
Statistical Analyses
All data presented in this paper were expressed as the mean ± SEM. The ion selectivity (PCl/PK) was estimated by using the Goldman–Hodgkin–Katz (GHK) equation. The PX/PK ratios of TtMscS in different anion solutions (Figures 2C, 5C) were analyzed by using unpaired t-tests. The conductance (Figure 2D) and PCl/PK ratios of mutations (Figures 4D, 6B,D) were analyzed using one-way ANOVA, and post-hoc LSD tests were performed.
Results
TtMscS Is a Mechano-Sensitive and Anion-Selective Channel
We cloned the TtMscS gene into the pET-21a vector. The TtMscS channel was overexpressed in BL21 competent cells. Through gel filtration chromatography, we acquired high-purity TtMscS protein (32 kDa) (Figure 1A). To detect the activity and physiological functions of TtMscS, we reconstituted high-purity protein into lipid vesicles (giant liposome) and tested the electrophysiological properties by using a patch-clamp recording system. In patch-clamp recording experiments, the TtMscS channel opened under negative pressure, thus reflecting a perfect mechanosensitive property (Figure 1B). The open probability plotted against negative pipette pressure was fitted to a Boltzmann distribution function (Figure 1D). The sensitivity parameter (1/α) of TtMscS to pressure was 4.8 ± 0.9 mmHg, and P1/2, the pressure at the point when the channel open probability was 0.5, was 79.7 ± 5.2 mmHg (mean ± SEM, n = 3). Using ΓMSC = α P1/2 = ΔGo/kT, we obtained the free energy of the channel activation ΔGo = 17.5 ± 2.9 kT (see section Materials and Methods). The ion selectivity was measured with a reversal potential under asymmetric KCl solutions (15 mM KCl in pipette and 150 mM in the bath). TtMscS exhibited an average reversal potential of −26.7 ± 0.9 mV (Figure 1C, mean ± SEM, n = 7). According to the GHK equation, we calculated the permeability ratio of TtMscS for chloride vs. potassium PCl/PK ~ 4:1. The results suggested that TtMscS is a mechano-sensitive, anion-selective ion channel.
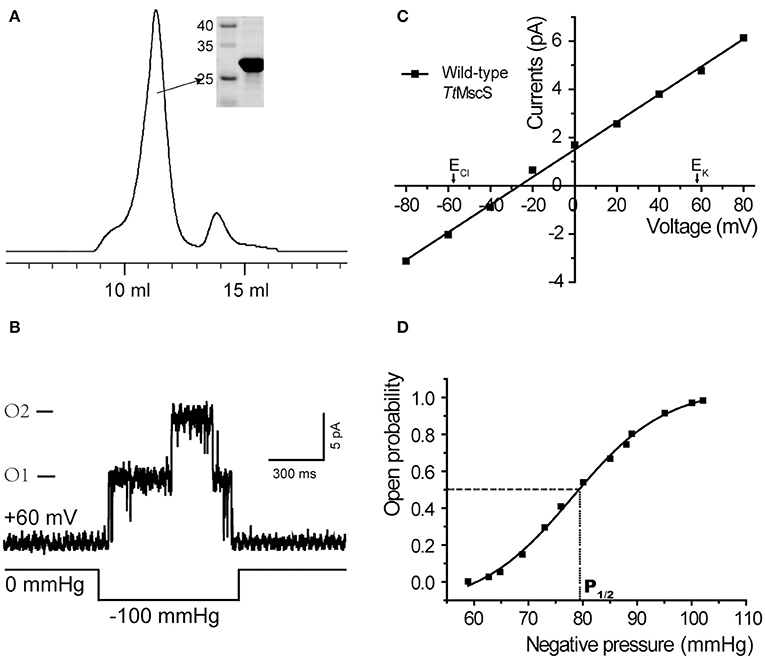
Figure 1. TtMscS channels display strong mechano-sensitivity and anion selectivity. (A) Representative trace of TtMscS in gel filtration chromatography: volume to peak was 11–12 ml; purity of protein was identified by SDS-PAGE gel was stained with Coomassie blue. (B) Single-channel traces of TtMscS were recorded in asymmetric KCl solutions (bath/pipette: 150/15 mM) with a holding voltage of +60 mV; single or dual channels opened under negative pressure applied to the patch pipette. (C) I–V curve for wild-type TtMscS recorded in asymmetric KCl solutions (bath/pipette: 150/15 mM). According to the GHK equation, the reversal potential of an ideal anion- or cation-selective channel was −58 or +58 mV. The reversal potential for TtMscS was −26.7 ± 0.9 mV (mean ± SEM, n = 7). (D) Open probability of TtMscS vs. negative pressure applied to the patch pipette fitted by the Boltzmann distribution function. P1/2 represents negative pressure at the point at which the open probability is 0.5.
TtMscS Showed Different Degrees of Preference for Different Anions
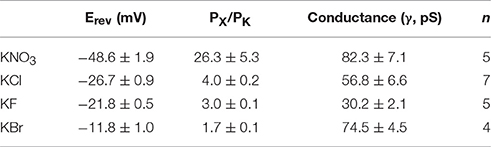
We demonstrated that TtMscS showed a preference for Cl− over K+. Therefore, we further investigated the preference of TtMscS for other anions. To determine the anion preference of TtMscS for various anions (, F−, Br−), we exchanged other anions (, F−, Br−) in asymmetric solutions and contrasted the differences of corresponding reversal potentials. Our results demonstrated that TtMscS showed selective preferences for various anions, namely, > Cl− ≈ F− > Br− [Figures 2B, C, Table 1, unpaired t-test, t(1, 10) = 5.047, p = 0.001 for KNO3 vs. KCl comparison; t(1, 10) = 2.906, p = 0.2 for KCl vs. KF comparison; t(1, 7) = 13.533, p = 0.033 for KF vs. KBr comparison]. Notably, TtMscS showed much stronger selectivity for (Erev = −48.6 ± 1.9 mV, PCl/PK ~ 26:1, n = 5) than other anions, very similarly to the anion preference of the GABA receptor (PNa/PK/PCl ~ 0:0.03:1; Wotring et al., 2003).
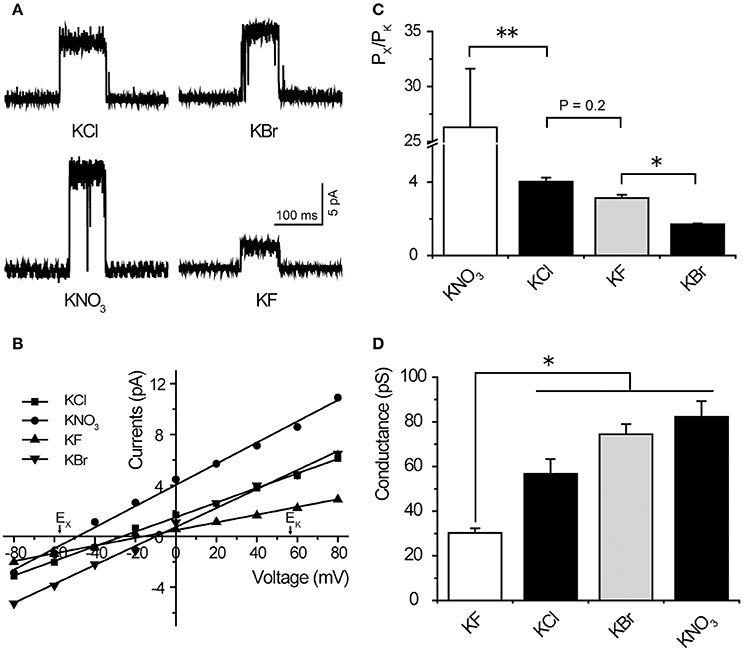
Figure 2. TtMscS showed different preferences for different anions. (A) Single-channel traces of TtMscS in different anion solutions (KNO3, KCl, KF, KBr) at +60 mV. (B) I–V curves for TtMscS recorded in asymmetric anion solutions (KNO3, KCl, KF, KBr) (bath/pipette: 150/15 mM). The reversal potentials were −48.6 ± 1.9 mV (mean ± SEM, n = 5), −21.8 ± 1.5 mV (mean ± SEM, n = 5), and −11.8 ± 1.0 mV (mean ± SEM, n = 5) for KNO3, KF and KBr, respectively. (C) Ion selectivity (PCl/PK) of TtMscS in different asymmetric anion solutions (KNO3, KCl, KF, KBr). (D) Ion conductance of TtMscS in different asymmetric anion solutions (KNO3, KCl, KF, KBr). *p < 0.05, **p < 0.01.
Ion conductance is a basic property of ion channels (Bezanilla, 2008). We compared the ion conductance of TtMscS performed with various anions (, Cl−, F−, Br−). Unexpectedly, TtMscS expressed much lower conductance to F− than other anions (including Cl−, Br−, and ) that are larger than F− [Figures 2A,D, Table 1, one-way ANOVA, F(3, 17) = 14.069, p < 0.001 for Figure 2D]. This result suggested that the conductance of ion channels is determined not only by the physical size of the ions but also by the interaction between ions and ion channels. The lowest conductance for F− may be due to the large binding energy of F− with the TtMscS channel.
β-Barrel Region May Confers the Anion Selectivity of TtMscS
TtMscS is a homoheptameric complex, each monomer of which consists of 286 amino acids. Like other MscS channels, the TtMscS channel has two characteristic domains: a transmembrane domain and a cytoplasmic domain. The cytoplasmic domain comprises two distinct openings: the portals and the β-barrel (Figures 3A,B). Despite the similar overall structural fold between TtMscS and EcMscS, the portal and the β-barrel of these are strikingly different. Our previous results have indicated that the β-barrel may underlie the structural foundation for the anion-selective property of TtMscS (Zhang et al., 2012), but the mechanistic details by which the β-barrel determines anion selectivity are still vague. Therefore, we further analyzed the crystal structure of TtMscS and found three distinct residues—T272, V274, and L276—located in the inner wall of the β-barrel pore, and the V274 residue strikingly pointed at the center of the β-barrel (Figures 3C,D). On the basis of this structural information, we designed a series of mutations and tested their anion preferences by using a giant liposome patch-clamp system.
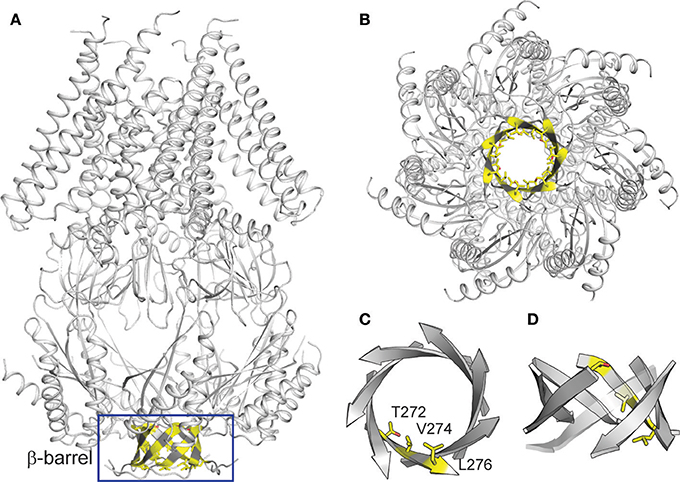
Figure 3. Structural analysis of TtMscS. (A) overall structure of TtMscS homoheptamer with a β-barrel at the distal end of TtMscS. (B) Crystal structure of TtMscS viewed from the bottom, with residues T272, V274, and L276 in the inner wall of β-barrel shown as sticks. (C,D) Amplified structure of the β-barrel viewed from the bottom (C) and cytoplasmic sides (D). Three residues (T272, V274, L276) in one protomer are shown as sticks.
Residue V274 Plays a Crucial Role in the Ion Selection of TtMscS
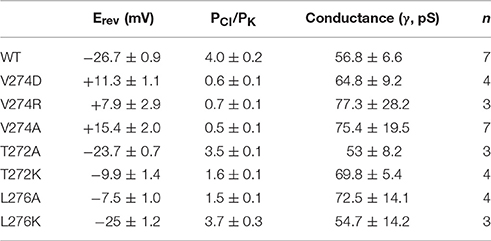
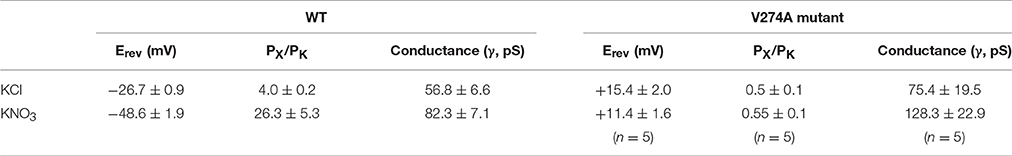
Considering electrostatic potential, we mutated the V274 residue into a negatively charged aspartic acid (V274D) and tested it by using the patch-clamp system in asymmetric KCl solutions (15 mM KCl in pipette and 150 mM in the bath). Surprisingly, the reversal potential shifted from −26.7 ± 0.9 to +11.3 ± 1.1 mV (mean ± SEM, n = 4), thus indicating that the V274D mutation reversed the anion selectivity of TtMscS to slight cation selectivity (Figures 4A,D). However, when we mutated the V274 residue to a positively charged arginine (V274R), the reversal potential did not exhibit a striking change compared with that of the V274D mutant (Figures 4B,D, shifted to +7.9 ± 2.9 mV, mean ± SEM, n = 3). We mutated V274 to alanine, which has a smaller side chain (V274A). The V274A mutation shifted the reversal potential to +15.4 ± 2.0 mV (Figures 4C,D, mean ± SEM, n = 7). Together, the three mutations at V274 neutralized the anion selectivity of TtMscS [Figure 4D, Table 2, one-way ANOVA, F(3, 18) = 135.801, p < 0.001] thus indicating that the V274 residue plays an essential role in the anion selectivity of TtMscS.
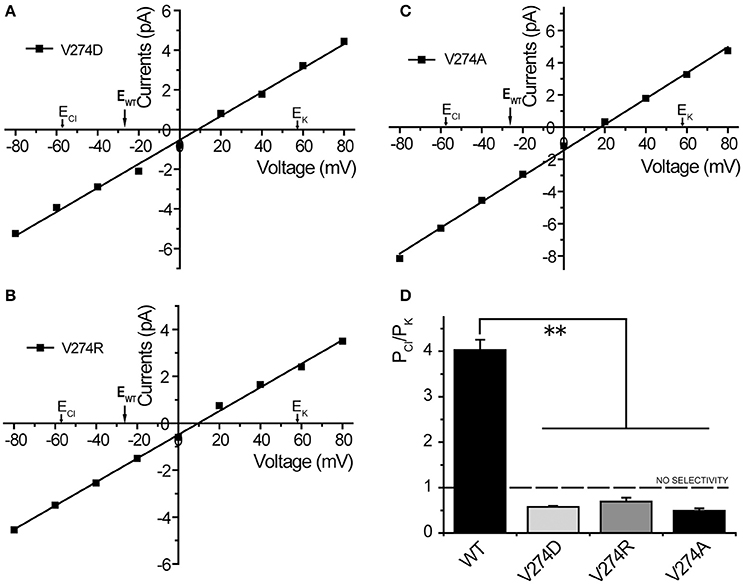
Figure 4. Residue V274 inside the β-barrel plays a vital role in the anion selectivity of TtMscS. (A–C) I–V curves for mutations V274D, V274R, and V274A. The reversal potentials were +11.3 ± 1.1 mV (mean ± SEM, n = 4), +7.9 ± 2.9 mV (mean ± SEM, n = 3), and +15.4 ± 2.0 mV (mean ± SEM, n = 7) for V274D, V274R, and V274A, respectively. The arrow (EWT) represents the reversal potential position of wild-type TtMscS. (D) Summary of ion selectivity (PCl/PK) for wild-type TtMscS (WT), V274D, V274R, and V274A mutations. **p < 0.01.
As shown in Figures 2B,C, wild-type TtMscS displayed much stronger selectivity for than for the other monovalent anions (Cl−, Br−, and F−). To determine whether V274 residue is also critical for the selectivity of TtMscS channel, we further tested the reversal potential changes of V274A mutation in asymmetric KNO3 solutions. Similar to the situation in KCl solution, V274A reversed the reversal potential from −48.6 ± 1.9 mV (wild type) to +11.4 ± 1.6 (mean ± SEM, n = 5; V274A), which indicated that V274A mutation also neutralized the selectivity of TtMscS channel (Figures 5A–C, Table 3).
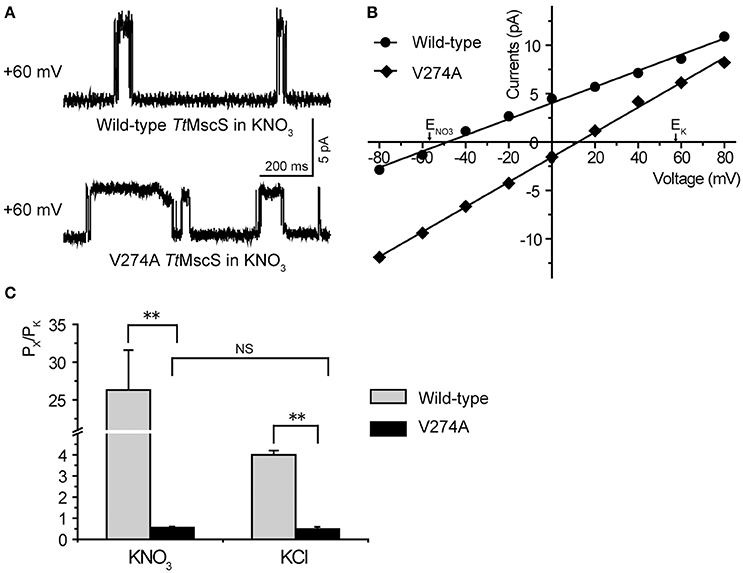
Figure 5. V274 residue is critical for the selectivity of TtMscS channel. (A) Single-channel traces of wild-type TtMscS and V274A mutation in KNO3 solution at +60 mV. (B) I–V curves for TtMscS and V274A mutation recorded at asymmetric KNO3 solution. The reversal potentials of V274A mutation was +11.4 ± 1.6 mV (mean ± SEM, n = 5). (C) The summary of ion selectivity (PX/PK: PNO3/PK or PCl/PK) for wild-type TtMscS and V274A mutation in KNO3 and KCl solutions. NS: no significant difference. **p < 0.01.
Collectivity, those results strongly support that V274 residue plays a vital role in the process of monovalent anions selectivity, including selectivity of Cl−, , and maybe as well as Br−, F−, in TtMscS channel.
The Electrostatic Potential of Residue T272 and the Physical Size of Residue L276 Affect the Anion Selectivity of TtMscS
Our structural analysis showed that T272 and L276 are two additional residues located in the inner wall of the β-barrel. To identify the roles of these two residues in the anion selectivity of TtMscS, we mutated T272 and L276 to alanine (small physical size, T272A and L276A) or lysine (positively charged, T272K and L276K), respectively. The electrophysiological results showed that the reversal potential of the T272A mutant, compared with that of wild-type TtMscS, did not exhibit substantial changes (Figures 6A,B, Table 2, Erev = −23.7 ± 0.7 mV, mean ± SEM, n = 3). However, the reversal potential of the T272K mutant shifted to −9.9 ± 1.4 mv (mean ± SEM, n = 4), thus indicating a significant attenuation of anion selection [Figures 6A,B, Table 2, one-way ANOVA, F(2, 11) = 37.443, p < 0.001 for Figure 6C]. In addition, the results for the L276 mutant were opposite from those of the V274 mutant. L276A rather than L276K markedly changed the reversal potential [Figures 6C,D, Table 2, for L276A, Erev = −7.5 ± 1.0 mV, mean ± SEM, n = 4; for L276K, Erev = −25.0 ± 1.2 mV, mean ± SEM, n = 3, one-way ANOVA, F(2, 11) = 37.881, p < 0.001 for Figure 6D]. From the above comparison, we concluded that the electrostatic potential of T272 and the physical size of L276 to notably influence the anion selection of TtMscS.
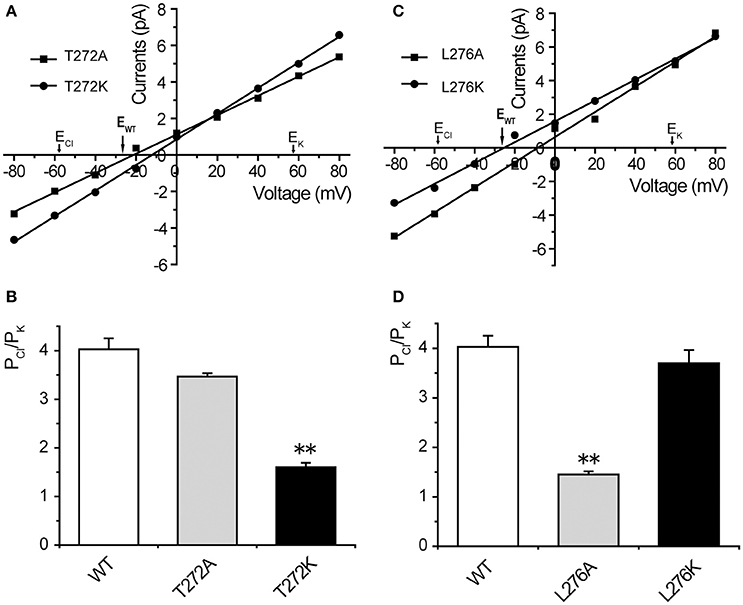
Figure 6. T272 and L276 residues co-determined the anion selectivity of TtMscS. (A) I–V curves for T272A and T272K. The reversal potentials were −23.7 ± 0.7 mV (mean ± SEM, n = 3) and −9.9 ± 1.4 mV (mean ± SEM, n = 4) for T272A and T272K, respectively. The arrow (EWT) indicates the reversal potential position of wild-type TtMscS. (B) Summary of ion selectivity (PCl/PK) for wild-type TtMscS (WT), T272A, and T272K mutations. (C) I–V curves for L276A and L276K. The reversal potentials were −7.5 ± 1.0 mV (mean ± SEM, n = 4) and −25.0 ± 1.2 mV (mean ± SEM, n = 3) for L276A and L276K, respectively. The arrow (EWT) indicates the reversal potential of wild-type TtMscS. (D) Summary of ion selectivity (PCl/PK) for wild-type TtMscS (WT), L276A and L276K mutations. **p < 0.01.
Discussion
Ion selectivity is a basic property of ion channels and is crucial for their physiological function (Roux et al., 2011). In the present study, a single mutation and patch-clamp recording system were used to investigate the mechanism of anion selectivity of TtMscS. Our results suggested that TtMscS is a mechano-sensitive and anion-selective channel. The preference ratio was PCl/PK ~ 4:1, and the free energy of the channel activation was ΔGo = 17.5 ± 2.9 kT. Second, TtMscS showed varying preferences for different anions (the degree order was > Cl− ≈ F− > Br−), especially for , for which TtMscS exhibited a more marked preference as compared with other anions (Erev = −48.6 ± 1.9, PNO3/PK ~ 26:1). A conductance comparison suggested that TtMscS had much lower conductance to F− than to other anions (Cl−, Br−, ) larger than F−. Third, mutations at the center of the β-barrel (V274) reversed the reversal potentials and thus reversed the ion preference (from anion preference to cation preference) of TtMscS. Finally, changes in electrostatic potential (T272K) and physical size (L276A) of residues in the inner wall of β-barrel both attenuated the anion preference of TtMscS. Together, our results indicated that all central pointed residues located in the inner wall of the β-barrel play a crucial role in the anion selectivity of TtMscS. Generally, functional properties are based on structural domains. The present results suggested that the β-barrel of TtMscS acts as a “selective filter” that confers TtMscS its anion selection.
Mechanisms underlying the anion selectivity of ion channels have been reported previously. However, the location of selective filters varies substantially among anion selective channels. ClC chloride channels belong to a large Cl− channel family found in organisms including bacteria and animals (Dutzler, 2006). In addition to Cl−, ClC chloride channels are selective for other small monovalent anions (Br−, I−, , and SCN−; Miller, 2006). Structural studies have indicated that an appropriate hourglass-shaped ion pathway and a partial positive binding site together determine the anion selectivity of ClC channels (Dutzler et al., 2002, 2003; Dutzler, 2007). This finding might describe principles of anion selectivity for anion-selective ion channels, despite differences between ion channels and pumps. In mammals, GABA receptors are ligand-gated chloride channels, which are inhibitory neurotransmitter receptors critical for maintaining appropriate neuronal activity and synaptic transmission (Wotring et al., 2003; Sigel and Steinmann, 2012). However, the mechanism underlying the chloride selectivity of GABA receptors is unclear. Glutamate-gated chloride channel α (GluCl) is a glutamate-gated chloride selective channel that belongs to the same family of GABA receptors (Cys-loop family). A structural analysis has demonstrated that the electropositive concave pocket at the bottom of GluCl channel is crucial for the anion selective property (Hibbs and Gouaux, 2011).
Unlike voltage-gated K+, Na+, Ca2+, and Cl− channels, whose selectivity arises from specific residues in the TM domain, the selectivity of MscS channels is defined by the physical size and charge distribution within the cytoplasmic vestibular domain (Gamini et al., 2011). Quantitative studies have reported that the cytoplasmic equatorial portals act as selective-filters and gateways for ion permeation in MscS channels (Perozo and Rees, 2003; Martinac et al., 2008; Gamini et al., 2011; Cox et al., 2013). However, the structural comparison showed that TtMscS has smaller portals but a larger β-barrel than EcMscS (Zhang et al., 2012). In addition, the size and charge distribution of the cylindrical β-barrel pore in TtMscS is highly similar to the bottom electropositive concave pocket of the GluCl channel (Hibbs and Gouaux, 2011; Zhang et al., 2012). Moreover, the β-barrel is a common structural motif in outer membrane proteins (OMPs) which have been described in the outer membranes of bacteria (Schulz, 2002) and mitochondria (Heins et al., 1994; Hoogenboom et al., 2007). Voltage-gated anion channels (VDAC), which are outer membrane proteins of mitochondria characterized by a transmembrane β-barrel structure, show a slight anion selectivity (Heins et al., 1994). All of these findings suggest that the β-barrel of TtMscS might reasonably be capable of ion permeation and ion selection. In the present study, a single residue mutation at the center of the β-barrel (V274) neutralized and even reversed the anion selectivity of TtMscS, thus suggesting a crucial role for residue locates at the center of β-barrel for the anion selectivity of TtMscS. Mutations of the other two extrusive residues at the inner wall of the β-barrel both attenuated anion selectivity, thus further verifying the crucial role of the β-barrel in the anion selectivity. The presented results suggest that the β-barrel acts as a “selective filter” module at the distal of anion selection of TtMscS. Complex proteins comprise a series of functional domains that endow the characteristic properties of TtMscS and confers this channel with a moderate degree of anion selectivity.
The MS channel responds to mechanical tension along the plane of the membrane. Studies have shown that the movement of transmembrane helices plays a critical role in the gating process of MscS channels (Martinac, 2004; Sotomayor and Schulten, 2004), suggesting that TM1 and TM2 may be responsible for gating the channel (Böttcher et al., 2015). In addition, the interaction of charged and polar residues in the TM1-TM2 helices with lipid headgroups can also affect the gating of MS channels (Sotomayor and Schulten, 2004). In the present study, the results showed that the TtMscS channel was open under negative pressure, but the negative pressure corresponding to the 1/2 channel open probability (P1/2) of TtMscS was much larger than that for EcMscS (−79.7 vs. −36 mmHg), even though both proteins belong to the same family and have very similar structures. The different pressure sensibilities between TtmscS and EcMscS may arise from the distinct arrangement of amino acid residues in the TM domain.
In summary, our results provide new insight into the molecular mechanism of the ion permeability and selectivity of the TtMscS channel. Our results indicate that both the electrostatic potential and the physical size of side chains inside the “selective filter” determine the anion selectivity of TtMscS, and thus may aid in understanding the mechanism of substrate selectivity of OMPs and the anion selectivity of chloride channels in eukaryotes.
Author Contributions
YS, BZ, and FG: Performed all the experiments. BZ, MY, and YL: Initiated the project. All the authors contributed to data analysis and paper writing.
Funding
This work was supported by the National Natural Science Foundation (31200771 to FG, 31171011, 31371066, 31671049 to YL), the Ministry of Science and Technology (2013CB91060101 to YL), the Science and Technology Commission of Shanghai Municipality (16411967400 to ZL), and the “Personalized Medicines-Molecular Signature-based Drug Discovery and Development,” Strategic Priority Research Program of the Chinese Academy of Sciences, (Grant No. XDA12040214).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arnadóttir, J., and Chalfie, M. (2010). Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39, 111–137. doi: 10.1146/annurev.biophys.37.032807.125836
Bass, R. B., Strop, P., Barclay, M., and Rees, D. C. (2002). Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587. doi: 10.1126/science.1077945
Bezanilla, F. (2008). Ion channels: from conductance to structure. Neuron 60, 456–468. doi: 10.1016/j.neuron.2008.10.035
Bezanilla, F., and Perozo, E. (2002). Structural biology. Force and voltage sensors in one structure. Science 298, 1562–1563. doi: 10.1126/science.1079369
Böttcher, B., Prazak, V., Rasmussen, A., Black, S. S., and Rasmussen, T. (2015). The structure of YnaI implies structural and mechanistic conservation in the MscS family of mechanosensitive channels. Structure 23, 1705–1714. doi: 10.1016/j.str.2015.06.023
Cox, C. D., Nomura, T., Ziegler, C. S., Campbell, A. K., Wann, K. T., and Martinac, B. (2013). Selectivity mechanism of the mechanosensitive channel MscS revealed by probing channel subconducting states. Nat. Commun. 4, 2137. doi: 10.1038/ncomms3137
Dutzler, R. (2006). The ClC family of chloride channels and transporters. Curr. Opin. Struct. Biol. 16, 439–446. doi: 10.1016/j.sbi.2006.06.002
Dutzler, R. (2007). A structural perspective on ClC channel and transporter function. FEBS Lett. 581, 2839–2844. doi: 10.1016/j.febslet.2007.04.016
Dutzler, R., Campbell, E. B., and MacKinnon, R. (2003). Gating the selectivity filter in ClC chloride channels. Science 300, 108–112. doi: 10.1126/science.1082708
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R. (2002). X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294. doi: 10.1038/415287a
Gamini, R., Sotomayor, M., Chipot, C., and Schulten, K. (2011). Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys. J. 101, 80–89. doi: 10.1016/j.bpj.2011.05.042
Heins, L., Mentzel, H., Schmid, A., Benz, R., and Schmitz, U. K. (1994). Biochemical, molecular, and functional characterization of porin isoforms from potato mitochondria. J. Biol. Chem. 269, 26402–26410.
Hibbs, R. E., and Gouaux, E. (2011). Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60. doi: 10.1038/nature10139
Hoogenboom, B. W., Suda, K., Engel, A., and Fotiadis, D. (2007). The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J. Mol. Biol. 370, 246–255. doi: 10.1016/j.jmb.2007.04.073
Kloda, A., and Martinac, B. (2001). Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 20, 1888–1896. doi: 10.1093/emboj/20.8.1888
Kung, C. (2005). A possible unifying principle for mechanosensation. Nature 436, 647–654. doi: 10.1038/nature03896
Kung, C., Martinac, B., and Sukharev, S. (2010). Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313–329. doi: 10.1146/annurev.micro.112408.134106
Li, Y., Berke, I., Chen, L., and Jiang, Y. (2007). Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J. Gen. Physiol. 129, 109–120. doi: 10.1085/jgp.200609655
Martinac, B. (2004). Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117, 2449–2460. doi: 10.1242/jcs.01232
Martinac, B., Buechner, M., Delcour, A. H., Adler, J., and Kung, C. (1987). Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 84, 2297–2301. doi: 10.1073/pnas.84.8.2297
Martinac, B., Saimi, Y., and Kung, C. (2008). Ion channels in microbes. Physiol. Rev. 88, 1449–1490. doi: 10.1152/physrev.00005.2008
Miller, C. (2006). ClC chloride channels viewed through a transporter lens. Nature 440, 484–489. doi: 10.1038/nature04713
Miller, S., Bartlett, W., Chandrasekaran, S., Simpson, S., Edwards, M., and Booth, I. R. (2003). Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22, 36–46. doi: 10.1093/emboj/cdg011
Perozo, E., and Rees, D. C. (2003). Structure and mechanism in prokaryotic mechanosensitive channels. Curr. Opin. Struct. Biol. 13, 432–442. doi: 10.1016/S0959-440X(03)00106-4
Ranade, S. S., Syeda, R., and Patapoutian, A. (2015). Mechanically activated ion channels. Neuron 87, 1162–1179. doi: 10.1016/j.neuron.2015.08.032
Roux, B., Berneche, S., Egwolf, B., Lev, B., Noskov, S. Y., Rowley, C. N., et al. (2011). Ion selectivity in channels and transporters. J. Gen. Physiol. 137, 415–426. doi: 10.1085/jgp.201010577
Schulz, G. E. (2002). The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 1565, 308–317. doi: 10.1016/S0005-2736(02)00577-1
Schumann, U., Edwards, M. D., Li, C., and Booth, I. R. (2004). The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 572, 233–237. doi: 10.1016/j.febslet.2004.07.045
Sigel, E., and Steinmann, M. E. (2012). Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 287, 40224–40231. doi: 10.1074/jbc.R112.386664
Sotomayor, M., and Schulten, K. (2004). Molecular dynamics study of gating in the mechanosensitive channel of small conductance MscS. Biophys. J. 87, 3050–3065. doi: 10.1529/biophysj.104.046045
Sotomayor, M., Vasquez, V., Perozo, E., and Schulten, K. (2007). Ion conduction through MscS as determined by electrophysiology and simulation. Biophys. J. 92, 886–902. doi: 10.1529/biophysj.106.095232
Sukharev, S. I., Sigurdson, W. J., Kung, C., and Sachs, F. (1999). Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540. doi: 10.1085/jgp.113.4.525
Wang, W., Black, S. S., Edwards, M. D., Miller, S., Morrison, E. L., Bartlett, W., et al. (2008). The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science 321, 1179–1183. doi: 10.1126/science.1159262
Wotring, V. E., Miller, T. S., and Weiss, D. S. (2003). Mutations at the GABA receptor selectivity filter: a possible role for effective charges. J. Physiol. 548(Pt 2), 527–540. doi: 10.1113/jphysiol.2002.032045
Keywords: TtMscS, anion selectivity, β-barrel, selective filter, patch-clamp, single mutation
Citation: Song Y, Zhang B, Guo F, Yang M, Li Y and Liu Z-Q (2017) Identification of Intracellular β-Barrel Residues Involved in Ion Selectivity in the Mechanosensitive Channel of Thermoanaerobacter tengcongensis. Front. Physiol. 8:832. doi: 10.3389/fphys.2017.00832
Received: 19 June 2017; Accepted: 06 October 2017;
Published: 25 October 2017.
Edited by:
Christoph Fahlke, Forschungszentrum Jülich, GermanyReviewed by:
Delany Torres-Salazar, National Institutes of Health (NIH), United StatesMarcelo Catalan, Arturo Prat University, Chile
Copyright © 2017 Song, Zhang, Guo, Yang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maojun Yang, bWFvanVueWFuZ0B0c2luZ2h1YS5lZHUuY24=
Yang Li, bGl5YW5nQHNpbW0uYWMuY24=
Zhi-Qiang Liu, ZHJsaXV6aHFAaG90bWFpbC5jb20=
†These authors have contributed equally to this work.
 Yingcai Song1†
Yingcai Song1† Fei Guo
Fei Guo Yang Li
Yang Li