- 1Department of Physiotherapy, College of Medicine, University of Malawi, Blantyre, Malawi
- 2Center for Exercise Science and Sports Medicine, University of the Witwatersrand, Johannesburg, South Africa
- 3Physiology Unit, Department of Biomedical Sciences, College of Medicine, Blantyre, Malawi
Low bone mineral density is becoming more common among people living with HIV following the use of current antiretroviral therapy drugs such as tenofovir. Although pharmacological therapies used to treat low bone mineral density are associated with adverse effects and may increase the pill burden in people living with HIV who are already burdened by antiretroviral therapy drugs, non-pharmacological strategies to prevent and treat reduced bone mineral density resulting from antiretroviral therapy drugs in people living with HIV have not been fully explored. Despite evidence that exercise is effective in increasing bone mineral density, effects of exercise on low bone mineral density resulting from antiretroviral therapy drugs in HIV infected individuals are still unknown. This review highlights gaps in the strategies used to manage reduced bone mineral density resulting from antiretroviral therapy drugs and focuses on exercise as an alternative or adjunctive strategy.
Introduction
Despite benefits of increased survival, use of the current antiretroviral therapy (ART) drugs in HIV patients are associated with reduced bone mineral density(BMD) (Purdy et al., 2008; Haskelberg et al., 2012; Alonge et al., 2013; Dave et al., 2015; Escota et al., 2015; Chitu-Tisu et al., 2016; Matovu et al., 2016; Mirembe et al., 2016). Reduced BMD is characterized by osteopaenia and osteoporosis which predispose people living with HIV(PLWHIV) to future fall related fractures thereby increasing the risk for morbidity and mortality. The prevalence of osteoporosis and osteopenia in people living with HIV and receiving ART is estimated to be over three times higher than that in HIV uninfected individuals (Brown and Qaqish, 2006). However, despite the widespread use of ART drugs among people living with HIV (World Health Organisation, 2015a), there is no consensus on effective strategies to manage reduced bone mineral density resulting from ART (Matovu et al., 2016).
Effective strategies to prevent and treat reduced bone mineral density in PLWHIV and receiving ART have no clear directions. Pharmacological strategies to manage reduced BMD resulting from ART include medications such as bisphosphonates, teriparatide and denosumab as well as providing vitamin D and calcium supplements (Ali et al., 2014). However these pharmacological therapies (Gallagher and Sai, 2010; Harris and Brown, 2012; Ali et al., 2014) are associated with adverse effects such as tumors, infection, nasopharyngitis, osteosarcoma as well as bronchitis (Kendler et al., 2010; Harris and Brown, 2012; Ali et al., 2014) which limit their recommendation for use in HIV infected individuals (Harris and Brown, 2012). In addition, the current cost of treating bone loss using pharmacological therapies such as bisphosphonates is prohibitive (Matovu et al., 2016). Further, pharmacological therapies may increase the pill burden in people living with HIV who are already burdened by ART. Physical activities such as jogging, walking, dancing and weight lifting are also recommended as non-pharmacological strategies for preventing and treating bone loss (Howe et al., 2011), but the effectiveness of physical activity in increasing bone mass resulting from ART in people living with HIV has not been fully elucidated.
Although there is growing evidence that physical activity and exercise increases bone mineral density in both adult men and women (Ryan et al., 2004; Cheung and Giangregorio, 2012; Mosti et al., 2014; Multanen et al., 2015), the effects of exercise on loss of bone mass resulting from antiretroviral drugs in PLWHIV remains unexplored (Grace et al., 2015). Exercise programmes differ in terms of frequency, intensity, duration and type. Among many studies, there is heterogeneity in the type, intensity, frequency and duration of exercise interventions to increase bone mineral density (Bolam et al., 2013) with most trials conducted in either women or adult men (Howe et al., 2011; Mosti et al., 2013, 2014) despite evidence of increases in bone loss among young men as well (Watts et al., 2012). Although reduced bone mineral density is common among PLWHIV following the use of antiretroviral drugs (Purdy et al., 2008; Haskelberg et al., 2012; Alonge et al., 2013; Dave et al., 2015; Escota et al., 2015; Chitu-Tisu et al., 2016; Matovu et al., 2016; Mirembe et al., 2016) knowledge on effects of exercises in increasing bone mineral density in this patient group is still lacking.
This review will highlight knowledge gaps in strategies to manage bone loss resulting from ART. The review will focus on challenges of pharmacological strategies used in treating reduced bone mineral density resulting from ART and the effects of exercises in increasing bone mineral density.
Methods
From April to September 2017, online databases such as EMBASE, Google Scholar, MEDLINE, PubMed, Scopus and The Cochrane Library were searched with no period restriction using key words: bone mineral density, antiretroviral therapy, exercise, people living with HIV and progressive resistance exercise. Published articles with potentially relevant titles and abstracts were retrieved. Articles were included in the review if they were (i) investigating the prevalence of low bone mineral density resulting from ART; (ii) examining strategies that are used to treat reduced bone mineral density or (iii) investigating the effects of exercise in increasing bone mineral density. A total of 109 articles met the inclusion criteria and were included in the review. All included publications were reviewed in their entirety.
Physiology of Bone Mineral Density
Bone is a connective tissue in which the matrix is made up of collagen fibers and minerals. Collagen is a protein that provides the bone's flexible framework (Sharp, 2011). The minerals contribute to the bone mineral density or bone mineral content and give the bone its strength and hardness. Collagen allows bones to bend in order to withstand stress while bone mineral density give bones strength to support the body's other tissues (Sharp, 2011). Bone mineral density in the matrix contributes to the support and protection functions of the skeleton. As such, bone mineral density is a surrogate for bone strength and is used to predict fracture risk in an individual. Reduced bone mineral density is characterized by osteopenia and osteoporosis and can predispose an individual to future fall related fractures.
Bone grows through modeling and remodeling processes (Creager, 1992; Harada and Rodan, 2003; Seeman and Delmas, 2006; Marieb and Hoehn, 2007; Pavy-Le Traon et al., 2007; Guadalupe-Grau et al., 2009; Sharp, 2011; Kruger and Nell, 2017). The modeling process involves osteoblast cells that perform bone formation by laying down new bone (Sharp, 2011). On the other hand, remodeling is a continuous process which involves bone resorption. In resorption osteoclasts are attracted to areas needing repair and move in to remove damaged bone (Seeman and Delmas, 2006; Sharp, 2011) thus some bone tissue is added along one surface while reabsorption occurs at another surface. The remodeling process occurs throughout one's life. It is estimated that 5–7% of bone mass is remodeled every week and approximately 0.5 gram of calcium is deposited or reabsorbed by the adult skeleton daily (Marieb and Hoehn, 2007). Any imbalances between modeling and remodeling lead to reduced load bearing capacity as well as loss of bone mineral density which in turn increase the risk for fractures (Sharp, 2011). Therefore, increased bone mineral density may reduce the incidence of fractures.
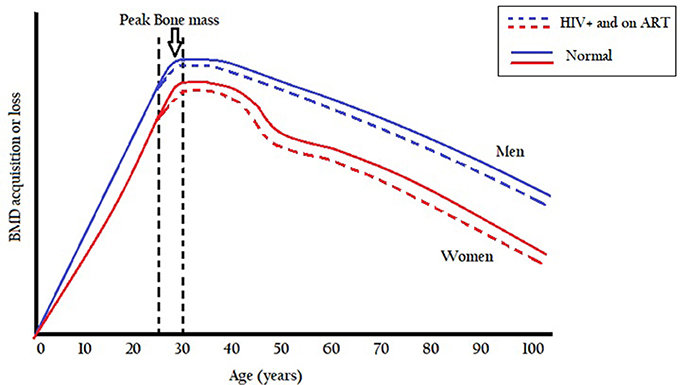
Bone mass formation is normally in excess of reabsorption with increasing age and peaks between ages 25 and 30 years, and thereafter bone mass starts to decrease leading to lower bone mineral density (Seeman and Delmas, 2006). Modeling and remodeling processes during growth are aimed at establishing peak bone mass so as to maintain bone strength in adulthood (Seeman and Delmas, 2006). During childhood growth spurt, bone mineral density accumulates with the bone growing both in size and strength (Kruger and Nell, 2017). After the growth spurt, usually during the pre- and post-adolescent period, bone formation continues until a peak bone mass is reached between ages 25 and 30 years. The age of attainment of peak bone mineral density is site-specific with gains of about 5–12% in bone mineral density observed after 30 years old in other individuals (Lorentzon et al., 2005). After the third decade, bone mineral density is maintained for about 10 years before it starts to decline at a rate of about 0.3–0.5% per year in both males and females (Baxter-Jones et al., 2011; Kruger and Nell, 2017). At ages between 45 and 55 women lose more bone mineral than men after which the rate of bone loss is gradual and the same in both sexes (Figure 1). A rapid loss of bone mineral density in women between ages 45 and 55 years is possibly due to a decrease in estrogen production as the menstrual cycle ceases during this period (Kruger and Nell, 2017).
Although bone remodeling is a normal and natural process, some factors are thought to disrupt the remodeling process (Kruger and Nell, 2017) and thereby reduce or increase the rate of bone mineral density. Factors such as; physical inactivity, low body weight, nutritional deficiencies especially of calcium and vitamin D, depression, smoking, heavy alcohol use, corticoids and other medications, including more recently some antiretroviral drugs (Purdy et al., 2008; Harris and Brown, 2012; Haskelberg et al., 2012; Alonge et al., 2013; Ameet et al., 2014; Dave et al., 2015; Escota et al., 2015; Chitu-Tisu et al., 2016; Matovu et al., 2016; Mirembe et al., 2016) are associated with reduced bone mineral density. Although some factors are not modifiable, physical activity can be changed to stimulate greater accumulation of peak bone mass (Michelson et al., 1996; Guadalupe-Grau et al., 2009; Welz et al., 2010; Pinto Neto et al., 2011; Mdodo et al., 2015; Kruger and Nell, 2017). Since bone modeling and remodeling depends, in part, on mechanical stress, bone strength is enhanced or reduced in response to increased or reduced mechanical loading. Hence an individual's physical activity lifestyle can play a role in either increasing or reducing bone mineral density.
Bone Mineral Density and Art
Despite benefits of increased survival, anti-retroviral therapy has been shown to significantly contribute to loss of bone mass (Purdy et al., 2008; Harris and Brown, 2012; Haskelberg et al., 2012; Alonge et al., 2013; Ameet et al., 2014; Dave et al., 2015; Escota et al., 2015; Chitu-Tisu et al., 2016; Matovu et al., 2016; Mirembe et al., 2016). Consequently, medical comorbidities such as osteoporosis and fragility fractures resulting from low bone mineral density are on the rise (Battalora et al., 2014). Emerging evidence indicates that HIV infection is strongly associated with a fivefold increased risk for hip fractures independent of age, gender and comorbidities (Güerri-Fernandez et al., 2013). Güerri-Fernandez et al. reported an increased risk for hip fractures (hazard ratio, 6.2) among HIV infected patients compared to a non-HIV infected general population (Güerri-Fernandez et al., 2013). This risk is higher compared to the risk of lung cancer (hazard ratio, 3.6) and a combined risk of cardiovascular and pulmonary diseases (odds ratio, 1.58) among people living with HIV (Kirk et al., 2007; Schouten et al., 2014). Therefore, the increased risk for hip fractures could consequently increase the risk for mortality and morbidity in people living with HIV.
Initiation of antiretroviral therapy has been shown to increase bone loss in people living with HIV irrespective of regimen (Yin and Overton, 2011; Grant and Cotter, 2016). Reports show a decrease of about 2–6% in bone mineral density in the first two years of ART initiation regardless of the regimen (Duvivier et al., 2009). Although mechanisms underlying bone loss resulting from ART are unclear, it has been suggested by Duvivier (Duvivier et al., 2009) and Borderi (Borderi and Pierluigi, 2013) that HIV infection of osteoblasts may be related to a negative balance of bone remodeling thereby leading to a reduction in bone mineral density in people living with HIV (Duvivier et al., 2009; Borderi and Pierluigi, 2013). Despite suggestions that the likelihood of HIV infection of osteoblasts is very low due to low expression of CD4 (Nachera et al., 2001; Borderi et al., 2009), recent evidence suggests that higher levels of C-C chemokine receptor 5 (CCR5) may affect the functional regulation of osteoclasts thereby leading to bone loss (Lee et al., 2017).
While decreases in bone mineral density occur at initiation of ART irrespective of regimen, more bone loss is associated with tenofovir-containing regimens than other regimens (McComsey et al., 2011; Brown et al., 2015; Grant and Cotter, 2016; Matovu et al., 2016). Tenofovir leads to approximately 1–3% greater bone mineral loss compared to non tenofovir-containing regimens (Grant and Cotter, 2016). McComsey et al. (2011) compared the effects of tenofovir vs. other ART regimens on bone mass in PLWHIV. They observed greater decreases in spine and hip BMD in participants treated with tenofovir than those treated with other regimens (McComsey et al., 2011). This could suggest that tenofovir has an independent effect on bone regardless of host, viral and immunological factors. Despite evidence that tenofovir significantly contributes to loss of bone mass (McComsey et al., 2011; Brown et al., 2015; Grant and Cotter, 2016; Matovu et al., 2016), most first line ART treatment regimens recommended by World Health Organization (WHO) in resource-limited settings, contain tenofovir (World Health Organisation, 2013, 2015b). This makes reduced bone mineral density highly likely among people living with HIV in most resource-limited settings yet strategies to minimize bone loss in PLWHIV in these settings are currently lacking (Matovu et al., 2016).
Although ART contributes to bone loss in people living with HIV, other factors may play a role as well. Traditional factors such as physical activity, lower body mass index, female sex, older age, nutritional deficiencies of calcium and vitamin D, depression, smoking and alcohol use are believed to contribute to loss of bone mass in the general population (Michelson et al., 1996; Guadalupe-Grau et al., 2009; Welz et al., 2010; Pinto Neto et al., 2011; Mdodo et al., 2015). While there is a controversy on traditional risk factors contributing to reduced bone mineral density in PLWHIV(Michelson et al., 1996), other authors have demonstrated that risk factors for reduced bone mineral density in HIV are similar to other populations (Bonjoch et al., 2010). Other reports indicate that poverty may also contribute to low bone mineral density (Navarro et al., 2009) suggesting that PLWHIV in resource-limited settings could be at a higher risk for loss of bone mineral mass, often the setting of highest HIV burden.
Evidence is emerging that HIV severity also contributes to reduced bone mineral density in HIV infected individuals receiving ART (Ofotokun et al., 2012, 2016; Battalora et al., 2014). A study by Grant et al. demonstrated that with ART initiation, HIV infected individuals with a low CD4 cell count (<50 cells/mm3) had greater bone loss than those with a higher CD4 cell count (>500 cells/mm3) (Grant et al., 2013). This indicates that chronic inflammation induced by HIV may impact bone metabolism. However, despite regional increases in HIV inflammation (Kaul et al., 2011, 2015), data on effective strategies to manage bone loss in people living with HIV lack clear guidelines.
Management of Bone Loss Resulting from Art
Pharmacological Therapies
Treatment strategies for reduced bone mineral density resulting from anti-retroviral therapy have no clear directions. Pharmacological strategies to manage bone loss resulting from antiretroviral therapy include providing vitamin D and calcium supplements as well as pharmacological therapies such as Bisphosphonates, Teriparatide and Denosumab (Ali et al., 2014). This section will discuss limitations of vitamin D and calcium supplements as well as pharmacological therapies in treating reduced bone mineral density in people living with HIV.
Although vitamin D deficiency has been implicated in the pathogenesis of bone loss in people living with HIV (Harris and Brown, 2012), there are no clear recommendations for vitamin D and calcium supplements to treat low bone mineral density in this population (Lima et al., 2011). A study by Dao reported a 70.3% vitamin D deficiency in a cohort of 672 HIV infected participants (Dao et al., 2011). Factors such as African American race and exposure to ART drugs were found to be associated with increased risk to vitamin D deficiency (Dao et al., 2011). This could suggest that vitamin D deficiencies could be higher among the Afro American race, moreover it is in Africa where the use of ART is becoming common as a result of high prevalence rates of HIV. A review by Harris and Brown (2012) recommends higher doses of vitamin D in people living with HIV exhibiting bone loss to maintain targeted levels of bone mass (Harris and Brown, 2012). However, due to their small effect on fracture risk reduction, vitamin D and calcium supplements are best used as additional therapies with other osteoporotic drugs (Gallagher and Sai, 2010) and their sole use is not advised.
Among osteoporotic drug treatments, beneficial effects of Denosumab in managing reduced bone mineral density in people living with HIV are not clear (Ali et al., 2014). Denosumab is a long acting monoclonal antibody that blocks bone resorption (Ali et al., 2014). Denosumab decreases osteoclastogenesis and is recommended for use in persons with a history of osteoporotic fractures or those who are intolerant to other osteoporotic therapies (Harris and Brown, 2012). However, long term use of denosumab in treating bone loss leads to atypical fractures (Sellmeyer, 2010). Although seemingly effective, use of Denosumab brings adverse effects such as, tumors, infection, nasopharyngitis, back pain, bronchitis and arthralgia (Kendler et al., 2010; Harris and Brown, 2012). These adverse effects are of particular concern to people living with HIV considering that they are already at an increased risk for infection.
Although available in resource limited settings and could be an alternative to denosumab for treating bone loss, bisphosphonates have a number of side effects which are a cause of concern in people living with HIV. Bisphosphonates, available as alendronate, ibandronate, risedronate, and zoledronic acid are said to decrease fracture risks in some parts of the body by between 25 and 50% in the general populations (Dennis et al., 1996; Ettinger et al., 1999; Harris et al., 1999; Black et al., 2007). However, despite improvements in bone mineral density among HIV infected individuals following use of alendronate and zoledronic acid, side effects such as difficulty swallowing, esophageal inflammation, dyspepsia, and gastric ulcer are also observed (Harris and Brown, 2012). In addition, bisphosphonates induce atypical femoral fractures and may not be used for more than 5 years (Fleming et al., 2001; Ali et al., 2014). This raises concerns of the long term effects of using bisphosphonates for managing reduced bone mineral density in HIV infected individuals who are currently living longer as a result of ART.
While teriparatide is recommended in individuals where bisphosphates have failed, its recommendation for use to treat bone loss in people living with HIV is still controversial. Some reports indicate that teriparatide has a risk for osteosarcoma (Ali et al., 2014), albeit rare, which may limit its recommendation for use in HIV infected individuals. Additionally, a review by Harris and Brown (2012) concluded that data on safety and efficacy of teriparatide in people living with HIV is lacking and requires further investigation (Harris and Brown, 2012).
Apart from the many challenges associated with pharmacological therapies in treating bone loss in HIV infected individuals, compliance and adherence issues have also been associated with pharmacological therapies (Brown, 2013). A retrospective study by Fan et al. (2013) which assessed the level of compliance with drugs prescribed for bone loss for seven years concluded that most patients do not continue to take the medication as prescribed (Fan et al., 2013). It has also been observed that half of patients treated with bisphosphates discontinue with treatment after 4 months (Solomon et al., 2005; Fan et al., 2013). Since pharmacological therapies are associated with a number of side effects and adherence problems which may limit their use among HIV infected individuals on ART (Fleming et al., 2001; Solomon et al., 2005; Kendler et al., 2010; Sellmeyer, 2010; Harris and Brown, 2012; Brown, 2013; Fan et al., 2013; Ali et al., 2014), exercise based interventions could be an attractive alternative.
Physical Activity
Guidelines for good bone health include physical activity and exercise as a major component in preventing bone loss (Body et al., 2011; Sharp, 2011; Borderi and Pierluigi, 2013; Cosman et al., 2014). Physical activity has been suggested as a non-pharmacological strategy that can be used to increase bone mineral density even in people living with HIV (Sharp, 2011; Borderi and Pierluigi, 2013). Among others, physical activities such as jogging, walking, dancing and weight lifting are shown to be beneficial in preventing and treating low bone mineral density (Howe et al., 2011). However, evidence that physical activity is related to higher bone mass is often inappropriately interpreted as evidence that any activity will improve bone mass (Beck et al., 2016).
Contrary to reports that all physical activity could be important in increasing bone mineral density (Body et al., 2011), weight bearing physical activities with high force, yield a notable increase in bone mineral density (Howe et al., 2011). This could suggest that the type and intensity of the physical activity has an additive effect on bone density. Although weight bearing physical activities are recommended to improve BMD, appropriate parameters for frequency, intensity, duration and type of physical activity to increase bone mineral density especially among HIV infected individuals has not been fully explored (Schambelan et al., 2002; Cosman et al., 2014).
Exercise and Bone Mineral Density
Type and Design of Exercises
There is growing evidence that exercise increases bone mineral density (Ryan et al., 2004; Cheung and Giangregorio, 2012; Mosti et al., 2014; Multanen et al., 2015). However, not all types of exercises provide notable stimulus to bone (Guadalupe-Grau et al., 2009; Xu et al., 2016). Aerobic exercises such as swimming, walking and cycling provide insignificant improvement to bone mineral density (Martyn-St James and Carroll, 2008; Rector et al., 2008; Ma et al., 2013). Simply prescribing these exercises in isolation is insufficient to optimize bone health. Bone respond positively to impact activities and high intensity progressive resistance training (Beck et al., 2016; Bolam et al., 2016). For example, a Cochrane review (Howe et al., 2011) on the effects of exercise on bone mineral density in postmenopausal women reported that exercises such as jumping, jogging, or dancing results in a between group difference in favor of exercise at the hip (1.55%) but not at the lumbar spine (−1.22%). Similarly, exercises such as walking showed between group improvement with exercise at the lumbar spine (0.85%) but not at the femoral neck (−1.20%) (Howe et al., 2011). Yet progressive resistance exercises resulted in significant between group differences in favor of exercise at both the femoral neck (1.03%) and lumbar spine (0.86%) (Howe et al., 2011). Results from this review suggest that progressive resistance exercises may be effective in increasing bone mineral density. However, despite reports of increases in bone loss due to ART (Yin and Overton, 2011; Grant and Cotter, 2016), the impact of progressive resistance exercise in increasing bone mineral density in PLWHIV has not been fully investigated nor promoted.
Although progressive resistance exercises have been shown to increase bone mass in the general population (Fairfield et al., 2001; Ryan et al., 2004; Ahola et al., 2009; Bailey and Brooke-Wavell, 2010; Ciccolo et al., 2010; Morseth et al., 2010; Howe et al., 2011; Kukuljan et al., 2011; Marques et al., 2011; Cheung and Giangregorio, 2012; Langsetmo et al., 2012; Watts et al., 2012; Allison et al., 2013; Kelley et al., 2013a,b; Mosti et al., 2013, 2014; Behringer et al., 2014; Kemmler and von Stengel, 2014; Hinton et al., 2015; Hui et al., 2015; Multanen et al., 2015; Kemmler et al., 2016), there is heterogeneity in the type, intensity, frequency and duration of exercise interventions to increase bone mineral density among many studies (Bolam et al., 2013). In a longitudinal randomized trial, Allison et al. (2013) investigated the influence of 12 months high impact exercises on bone mineral density in 50 men. Results of the study revealed an increase of 1.2% in bone mineral density. Similarly, Bailey and Brooke-Wavell (2010) demonstrated a significant increase in BMD after 6 months of exercise in 65 women compared to 20 non-exercising women. However, in both studies, there was an increase in dropout rates with increasing number of exercise days indicating that long exercise durations could lead to exercise adherence problems.
Adherence to the recommended exercise regimen is key to the success of any exercise intervention. The World Health Organization defines adherence as “the extent to which a person's behavior—taking medication, following a diet, and/or executing lifestyle changes—corresponds with agreed recommendations from health care provider” (World Health Organisation, 2003). In exercise, adherence refers to complying with an exercise design for a specified period of time. It involves maintaining the frequency, intensity, duration and type of a given or prescribed exercise. There are reports that adherence to exercise falls below the desirable level among people living with HIV (Petróczi et al., 2010). However, reports indicate that exercise interventions yield higher adherence rates compared to pharmacological interventions in treating low bone mineral density (Kelley and Kelley, 2013).
Among the different types of exercise interventions, compliance is higher with progressive resistance exercises than aerobic exercises(Vancampfort et al., 2017) with an adherence rate of over 80% in randomized controlled trials (Aitken et al., 2015). Reports also indicate that facility based exercises with shorter durations (Kelley and Kelley, 2013) such as maximal strength exercises (Mosti et al., 2013, 2014) have increased adherence. In addition, adherence is increased in exercise programmes that are individualized and supervised by qualified professionals (Hong et al., 2008; Jordan et al., 2010; Tønnesen et al., 2016). It is important therefore to design shorter, supervised, individualized and facility based progressive resistant exercise programmes targeting BMD in order to increase adherence.
Progressive Resistance Exercises
Progressive resistance exercises have proven to be beneficial among people living with HIV. A systematic review by O'Brien et al, found no significant differences in CD4 count and viral load in HIV infected individuals before and after participating in progressive resistance exercises for at least three times per week for at least 6 weeks demonstrating that exercises are safe for people living with HIV (O'Brien et al., 2017). Significant improvements in cardiorespiratory fitness, strength, body composition and body weight following participation in progressive resistance exercises have also been reported among people living with HIV (O'Brien et al., 2008, 2017; Neto et al., 2015). Although the benefits of progressive resistance exercise in people living with HIV are wide, effects of such exercises on bone mineral density in this population have not been fully evaluated (Grace et al., 2015). Only one study by Santos et al. (2015) investigating effects of progressive resistance exercise on BMD in PLWHIV was identified from the literature. In this study, Santos et al. (2015), demonstrated that a shorter exercise duration of 12 weeks was appropriate to impact significant bone increases in 20 individuals living with HIV.
However, the study by Santos et al. (2015) has some methodological shortcomings. The exercise design used lacked other basic elements of an appropriate exercise programme to elicit improvements (Slade and Keating, 2011). The trial neither used non HIV infected controls nor local reference data for BMD for comparison. In addition, different types of exercises were used raising concerns of which of the exercises had a greater impact on bone mineral density. The effectiveness of a 12 weeks' exercise duration to improve BMD is supported by evidence from studies by Mosti et al. (2013, 2014), who demonstrated the effects of a 12 weeks progressive resistance exercises in increasing BMD in young women and postmenopausal women (Mosti et al., 2013, 2014). This evidence indicate that shorter exercise durations could as well impact bone metabolism, thereby minimizing exercise adherence problems.
Progressive resistance exercises have been proven to be safe and beneficial in improving metabolic outcomes among PLWHIV (O'Brien et al., 2008; Fillipas et al., 2010; Grace et al., 2015; Neto et al., 2015). However, there is still lack of knowledge on the optimal mode of frequency, duration and intensity of progressive resistance exercise on BMD in people living with HIV (Fillipas et al., 2010; Grace, 2016) which requires investigation.
Conclusion
Most pharmacological strategies used to treat bone loss are associated with a number of adverse effects which limit their recommendation for use in PLWHIV. In addition, compliance and adherence issues associated with pharmacological strategies in treating bone loss may limit their use among PLWHIV who are already burdened by ART. Exercise based interventions such as progressive resistance exercises seem to be an attractive safe and effective alternative strategy that could be used to manage bone loss resulting from ART in PLWHIV. Although progressive resistance exercises are effective in increasing BMD, there is lack of knowledge on the optimal frequency, intensity and duration of the exercise to impact bone which need further investigations. In addition, effects of progressive resistance exercises in increasing BMD in PLWHIV have not been fully investigated. Only one study examining the effects of progressive resistance exercise in increasing BMD among PLWHIV and receiving ART was identified from the literature. Future studies investigating the effects of progressive resistance exercises in increasing BMD in PLWHIV should adopt trial designs with clear descriptions of exercise frequency, intensity and duration.
Author Contributions
EC, DC, and FL made significant contributions to the conception and design of the work. EC drafted the work. DC and FL critically revised the work for important intellectual content. All authors had final approval of the version to be published, and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Wellcome Trust (UK) (Grant No: 087547/Z/08/Z), the Carnegie Corporation of New York (Grant No:B 8606.R02), Sida (Grant No:54100029). The statements made and views expressed are solely the responsibility of the author. EC is a cohort 7 CARTA PhD fellow.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahola, R., Korpelainen, R., Vainionpää, A., Leppäluoto, J., and Jämsä, T. (2009). Time - course changes of exercise and its association with 12 month bone changes. BMC Musculoskelet. Disord. 10:138. doi: 10.1186/1471-2474-10-138
Aitken, D., Buchbinder, R., Jones, G., and Winzenberg, T. (2015). Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Aust. Fam. Physic. 44, 39–42. Available online at: https://search.informit.com.au/documentSummary;dn=905348623633626;res=IELHEA
Ali, M. K., Magee, M. J., Dave, J. A., Ofotokun, I., Tungsiripat, M., Jones, T. K., et al. (2014). HIV and metabolic, body, and bone disorders: what we know from low- and middle-income countries. J. Acquir. Immune Defic. Syndr. 67, S27–S39. doi: 10.1097/QAI.0000000000000256
Allison, S. J., Folland, J. P., Rennie, W. J., Summers, G. D., and Brooke-Wavell, K. (2013). High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone 53, 321–328. doi: 10.1016/j.bone.2012.12.045
Alonge, T., Okoje-Adesomoju, V., Atalabi, O., Obamuyide, H., Olaleye, D., and Adewole, I. (2013). Prevalence of abnormal bone mineral density in HIV-positive patients in ibadan, Nigeria. J. West Afr. Coll. Surg. 3, 1–14.
Ameet, D., Milind, K., and Sachin, D. (2014). Prevalence of low bone mineral density among HIV patients on long-term suppressive antiretroviral therapy in resource limited setting of western India. J. Int. AIDS Soc. 17:19567. doi: 10.7448/IAS.17.4.19567
Bailey, C. A., and Brooke-Wavell, K. (2010). Optimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral intervention. Bone 46, 1043–1049. doi: 10.1016/j.bone.2009.12.001
Battalora, L., Young, B., and Overton, E. (2014). Bone, fractures, antiretroviral therapy and HIV. Curr. Infect. Dis. Rep. 16, 1–10. doi: 10.1007/s11908-014-0393-1
Baxter-Jones, A. D. G., Faulkner, R. A., Forwood, M. R., Mirwald, R. L., and Bailey, D. A. (2011). Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J. Bone Miner. Res. 26, 1729–1739. doi: 10.1002/jbmr.412
Beck, B. R., Daly, R. M., Fiatarone, M. A., and Taaffe, D. R. (2016). Exercise and sports science australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J. Sci. Med. Sport 1403, 1–8. doi: 10.1016/j.jsams.2016.10.001
Behringer, M., Gruetzner, S., McCourt, M., and Mester, J. (2014). Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis. J. Bone Miner. Res. 29, 467–478. doi: 10.1002/jbmr.2036
Black, D. M., Delmas, P. D., Eastell, R., Reid, I. R., Boonen, S., Cauley, J. A., et al. (2007). Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 356, 1809–1822. doi: 10.1056/NEJMoa067312
Body, J. J., Bergmann, P., Boonen, S., Boutsen, Y., Bruyere, O., Devogelaer, J. P., et al. (2011). Non-pharmacological management of osteoporosis: a consensus of the Belgian bone club. Osteoporos. Int. 22, 2769–2788. doi: 10.1007/s00198-011-1545-x
Bolam, K. A., Skinner, T. L., Jenkins, D. G., Galvão, D. A., and Taaffe, D. R. (2016). The osteogenic effect of impact-loading and resistance exercise on bone mineral density in middle-aged and older men: a pilot study. Gerontology 62, 22–32. doi: 10.1159/000435837
Bolam, K. A., Van Uffelen, J. G. Z., and Taaffe, D. R. (2013). The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos. Int. 24, 2749–2762. doi: 10.1007/s00198-013-2346-1
Bonjoch, A., Figueras, M., Estany, C., Perez-Alvarez, N., Rosales, J., del Rio, L., et al. (2010). High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS 24, 2827–2833. doi: 10.1097/QAD.0b013e328340a28d
Borderi, M., Gibellini, D., Vescini, F., Crignis, E., Cimatti, D, Biagetti, C., et al. (2009). Metabolic bone disease in HIV infection. AIDS 23, 1297–1310. doi: 10.1097/QAD.0b013e32832ce85a
Borderi, M., and Pierluigi, V. (2013). How to monitor bone disease in HIV infection. HAART HIV Correl. Pathol. Other Infect. 182–189. Available online at: http://www.mnlpublimed.com/public/HA18-A04.pdf
Brown, T. T. (2013). Challenges in the management of osteoporosis and vitamin D deficiency in HIV infection. Top. Antivir. Med. 21, 115–118. Available online at: https://europepmc.org/abstract/MED/23981599
Brown, T. T., Moser, C., Currier, J. S., Ribaudo, H. J., Rothenberg, J., Kelesidis, T., et al. (2015). Changes in bone mineral density after initiation of antiretroviral treatment with Tenofovir Disoproxil Fumarate/Emtricitabine Plus Atazanavir/Ritonavir, Darunavir/Ritonavir, or Raltegravir. J. Infect. Dis. 212, 1241–1249. doi: 10.1093/infdis/jiv194
Brown, T. T., and Qaqish, R. B. (2006). Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 20, 2165–2174. doi: 10.1097/QAD.0b013e32801022eb
Cheung, A. M., and Giangregorio, L. (2012). Mechanical stimuli and bone health : what is the evidence ? Curr. Opin. Rheumatol. 24, 561–566. doi: 10.1097/BOR.0b013e3283570238
Chitu-Tisu, C. E., Barbu, E. C., Lazar, M., Ion, D. A., and Badarau, I. A. (2016). Low bone mineral density and associated risk factors in HIV-infected patients. GERMS 6, 50–59. doi: 10.11599/germs.2016.1089
Ciccolo, J. T., Carr, L. J., Krupel, K. L., and Longval, J. L. (2010). The role of resistance training in the prevention and treatment of chronic disease. Am. J. Lifestyle Med. 4, 293–308. doi: 10.1177/1559827609354034
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician's guide to prevention and treatment of osteoporosis. Osteoporos. Int. 25, 2359–2381. doi: 10.1007/s00198-014-2794-2
Dao, C. N., Patel, P., Overton, E. T., Rhame, F., Pals, S. L., Johnson, C., et al. (2011). Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the us general population. Clin. Infect. Dis. 52, 396–405. doi: 10.1093/cid/ciq158
Dave, J. A., Cohen, K., Micklesfield, L. K., Maartens, G., and Levitt, N. S. (2015). Antiretroviral therapy, especially efavirenz, is associated with low bone mineral density in HIV-infected South Africans. PLoS ONE 10:e0144286. doi: 10.1371/journal.pone.0144286
Dennis, M., Steven, R., David, B., and Jane, A. (1996). Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348, 1535–1541.
Duvivier, C., Kolta, S., Assoumou, L., Ghosn, J., Rozenberg, S., Murphy, R. L., et al. (2009). Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 23, 817–824. doi: 10.1097/QAD.0b013e328328f789
Escota, G. V., Mondy, K., Bush, T., Conley, L., Brooks, J. T., Önen, N., et al. (2015). High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res. Hum. Retroviruses 31, 59–67. doi: 10.1089/aid.2015.0158
Ettinger, B., Black, D. M., Mitlak, B. H., Knickerbocker, R. K., Nickelsen, T., Genant, H. K., et al. (1999). Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with Raloxifene. JAMA 282, 637–645. doi: 10.1001/jama.282.7.637
Fairfield, W. P., Finkelstein, J. S., Klibanski, A., and Grinspoon, S. K. (2001). Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J. Clin. Endocrinol. Metab. 86, 2020–2026. doi: 10.1210/jc.86.5.2020
Fan, T., Zhang, Q., and Sen, S. S. (2013). Persistence with weekly and monthly bisphosphonates among postmenopausal women: analysis of a US pharmacy claims administrative database. Clinicoecon. Outcomes Res. 5, 589–595. doi: 10.2147/CEOR.S39076
Fillipas, S., Cherry, C. L., Cicuttini, F., Smirneos, L., and Holland, A. E. (2010). The effects of exercise training on metabolic and morphological outcomes for people living with HIV: a systematic review of randomised controlled trials. HIV Clin. Trials 11, 270–282. doi: 10.1310/hct1105-270
Fleming, B., Greenfield, S., Engelgau, M., Pogach, L., Clauser, S., and Parrot, M. (2001). The diabetes quality improvement project. Diab. Care 24, 1815–1820. doi: 10.2337/diacare.24.10.1815
Gallagher, J. C., and Sai, A. J. (2010). Vitamin D insufficiency, deficiency, and bone health. J. Clin. Endocrinol. Metab. 95, 2630–2633. doi: 10.1210/jc.2010-0918
Grace, J. M. (2016). “Exercise therapy for acquired immune deficiency syndrome (AIDS) patients,” in Fitness Medicine (London, UK: InTechOpen), 129–45.
Grace, J. M., Semple, S. J., and Combrink, S. (2015). Exercise therapy for human immunodeficiency virus/AIDS patients: guidelines for clinical exercise therapists. J. Exerc. Sci. Fit. 13, 49–56. doi: 10.1016/j.jesf.2014.10.003
Grant, P. M., and Cotter, A. G. (2016). Tenofovir and bone health. Curr. Opin. HIV AIDS 11, 326–332. doi: 10.1097/COH.0000000000000248
Grant, P. M., Kitch, D., McComsey, G. A., Dube, M. P., Haubrich, R., Huang, J., et al. (2013). Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin. Infect. Dis. 57, 1483–1488. doi: 10.1093/cid/cit538
Guadalupe-Grau, A., Fuentes, T., Guerra, B., and Calbet, J. A. (2009). Exercise and bone mass in adults. Sport Med. 39, 439–468. doi: 10.2165/00007256-200939060-00002
Güerri-Fernandez, R., Vestergaard, P., Carbonell, C., Knobel, H., Avilés, F. F., Castro, A. S., et al. (2013). HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J. Bone Miner. Res. 28, 1259–1263. doi: 10.1002/jbmr.1874
Harada, S., and Rodan, G. A. (2003). Control of osteoblast function and regulation of bone mass. Nature 423, 349–355. doi: 10.1038/nature01660
Harris, S. T., Watts, N. B., McKeever, C. D., Hangartner, T., Keller, M., Brown, J., et al. (1999). Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282, 1344–1352. doi: 10.1001/jama.282.14.1344
Harris, V., and Brown, T. (2012). Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies J. Infect. Dis. 205, 391–398. doi: 10.1093/infdis/jis199
Haskelberg, H., Hoy, J. F., Amin, J., Ebeling, P. R., Emery, S., Carr, A., et al. (2012). Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS ONE 7, 1–9. doi: 10.1371/journal.pone.0038377
Hinton, P. S., Nigh, P., and Thyfault, J. (2015). Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: a 12-month randomized, clinical trial. Bone 79, 203–212. doi: 10.1016/j.bone.2015.06.008
Hong, S.-Y., Hughes, S., and Prohaska, T. (2008). Factors affecting exercise attendance and completion in sedentary older adults: a meta-analytic approach. J. Phys. Act. Health 5, 385–397. doi: 10.1123/jpah.5.3.385
Howe, T. E., Shea, B., Dawson, L. J., Downie, F., Murray, A., Ross, C., et al. (2011). Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011:CD000333. doi: 10.1002/14651858.CD000333.pub2
Hui, S. S.-C., Xie, Y. J., Woo, J., and Kwok, T. C.-Y. (2015). Effects of tai chi and walking exercises on weight loss, metabolic syndrome parameters, and bone mineral density: a cluster randomized controlled trial. Evid. Based Complement. Alternat. Med. 2015:976123. doi: 10.1155/2015/976123
Jordan, J., Holden, M., Mason, E., and Foster, N. (2010). Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults (Review). Cochrane Database Syst. Rev. 2010:CD005956. doi: 10.1002/14651858.CD005956.pub2
Kaul, R., Cohen, C. R., Chege, D., Yi, T. J., Tharao, W., McKinnon, L. R., et al. (2011). Biological factors that may contribute to regional and racial disparities in HIV prevalence. Am. J. Reprod. Immunol. 65, 317–324. doi: 10.1111/j.1600-0897.2010.00962.x
Kaul, R., Prodger, J., Joag, V., Shannon, B., Yegorov, S., Galiwango, R., et al. (2015). Inflammation and HIV Transmission in Sub-Saharan Africa. Curr. HIV/AIDS Rep. 12, 216–222. doi: 10.1007/s11904-015-0269-5
Kelley, G. A., and Kelley, K. S. (2013). Dropouts and compliance in exercise interventions targeting bone mineral density in adults: a meta-analysis of randomized controlled trials. J. Osteoporos. 2013:250423. doi: 10.1155/2013/250423
Kelley, G. A., Kelley, K. S., and Kohrt, W. M. (2013a). Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 53, 103–111. doi: 10.1016/j.bone.2012.11.031
Kelley, G. A., Kelley, K. S., and Kohrt, W. M. (2013b). Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2013:741639. doi: 10.1155/2013/741639
Kemmler, W., Engelke, K., and von Stengel, S. (2016). Long-term exercise and bone mineral density changes in postmenopausal women-are there periods of reduced effectiveness? J. Bone Miner. Res. 31, 215–222. doi: 10.1002/jbmr.2608
Kemmler, W., and von Stengel, S. (2014). Dose-response effect of exercise frequency on bone mineral density in post-menopausal, osteopenic women. Scand. J. Med. Sci. Sports 24, 526–534. doi: 10.1111/sms.12024
Kendler, D. L., Roux, C., Benhamou, C. L., Brown, J. P., Lillestol, M., Siddhanti, S., et al. (2010). Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 25, 72–81. doi: 10.1359/jbmr.090716
Kirk, G. D., Merlo, C., Driscoll, P. O., Mehta, S. H., Galai, N., Vlahov, D., et al. (2007). HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin. Infect. Dis. 45, 103–110. doi: 10.1086/518606
Kruger, M. J., and Nell, T. A. (2017). Bone mineral density in people living with HIV: a narrative review of the literature. AIDS Res. Ther. 14, 1–17. doi: 10.1186/s12981-017-0162-y
Kukuljan, S., Nowson, C. A., Sanders, K. M., Nicholson, G. C., Seibel, M. J., Salmon, J., et al. (2011). Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J. Clin. Endocrinol. Metab. 96, 955–963. doi: 10.1210/jc.2010-2284
Langsetmo, L., Hitchcock, C., Kingwell, E., Davison, K., Berger, C., Forsmo, S., et al. (2012). Physical activity, body mass index and bone mineral density— associations in a prospective population-based cohort of women and men: the canadian multicentre osteoporosis study (CaMos). Bone 50, 401–408. doi: 10.1016/j.bone.2011.11.009
Lee, J., Hoshino, A., Inoue, K., Saitou, T., Uehara, S., Kobayashi, Y., et al. (2017). The HIV co-receptor CCR5 regulates osteoclast function. Nat. Commun. 8:2226. doi: 10.1038/s41467-017-02368-5
Lima, A. L., de Oliveira, P. R., Palpler, P. G., Marcolino, F. M. D., Meirelles, E., Sugawara, A., et al. (2011). Osteopenia and osteoporosis in people living with HIV : multiprofessional approach. HIV/AIDS (Auckl) 3, 117–124. doi: 10.2147/HIV.S6617
Lorentzon, M., Mellstrom, D., and Ohlsson, C. (2005). Age of attainment of peak bone mass is site specific in Swedish men–the GOOD study. J. Bone Miner. Res. 20, 1223–1227. doi: 10.1359/JBMR.050306
Ma, D., Wu, L., and He, Z. (2013). Effects of walking on the preservation of bone mineral density in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Menopause 20, 1216–1226. doi: 10.1097/GME.0000000000000100
Marieb, E. N., and Hoehn, K. (2007). Human Anatomy & Physiology (San Francisco, CA: Pearson Benjamin Cummings), 175–201.
Marques, E. A., Wanderley, F., Machado, L., Sousa, F., Viana, J. L., Moreira-Gonçalves, D., et al. (2011). Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp. Gerontol. 46, 524–532. doi: 10.1016/j.exger.2011.02.005
Martyn-St James, M., and Carroll, S. (2008). Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 1143, 521–531. doi: 10.1016/j.bone.2008.05.012
Matovu, F. K., Wattanachanya, L., Beksinsk, M., Pettifor, J. M., and Ruxrungtham, K. (2016). Bone health and HIV in resource-limited settings: a scoping review. Curr. Opin. HIV AIDS 11, 306–325. doi: 10.1097/COH.0000000000000274
McComsey, G. A., Kitch, D., Daar, E. S., Tierney, C., Jahed, N. C., Tebas, P., et al. (2011). Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS clinical trials group A5224s, a substudy of ACTG. J. Infect. Dis. 203, 1791–1801. doi: 10.1093/infdis/jir188
Mdodo, R., Frazier, E. L., Dube, S. R., Mattson, C. L., Sutton, M. Y., Brooks, J. T., et al. (2015). Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann. Intern. Med. 162, 335–344. doi: 10.7326/M14-0954
Michelson, D., Stratakis, C., Hill, L., Reynolds, J., Galliven, E., Chrousos, G., et al. (1996). Bone mineral density in women with depression. N. Engl. J. Med. 335, 1176–1181. doi: 10.1056/NEJM199610173351602
Mirembe, B. G., Kelly, C. W., Mgodi, N., Greenspan, S., Dai, J. Y., Mayo, A., et al. (2016). Bone mineral density changes among young, healthy African women receiving oral tenofovir for HIV preexposure prophylaxis. J. Acquir. Immune Defic. Syndr. 71, 287–294. doi: 10.1097/QAI.0000000000000858
Morseth, B., Emaus, N., Wilsgaard, T., Jacobsen, B. K., and Jørgensen, L. (2010). Leisure time physical activity in adulthood is positively associated with bone mineral density 22 years later. The Tromso study. Eur. J. Epidemiol. 25, 325–331. doi: 10.1007/s10654-010-9450-8
Mosti, M., Carlsen, T., Aas, E., Hoff, J., Stunes, A., and Syversen, U. (2014). Maximal strength training improves bone mineral density and neuromuscular performance in young adult women. J. Strength Cond. Res. 28, 2935–2945. doi: 10.1519/JSC.0000000000000493
Mosti, M., Nils, K., Stunes, A., Hoff, J., and Syversen, U. (2013). Maximal strength training in postmenopausal women with osteoporosis or osteopenia. J. Strength Cond. Res. 27, 2879–2886. doi: 10.1519/JSC.0b013e318280d4e2
Multanen, J., Nieminen, M. T., Häkkinen, A., Kujala, U. M., Jämsä, T., Kautiainen, H., et al. (2015). Effects of high - impact training on bone and articular cartilage: 12 - month randomised controlled quantitative MRI study. J. Bone Miner. Res. 29, 192–201. doi: 10.1002/jbmr.2015
Nachera, M., Serrano, S., Gonza, A., Marin, M. L., Vilella, R., Hinarejos, P., et al. (2001). Osteoblasts in HIV-infected patients : HIV-1 infection and cell function. AIDS 15, 2239–2243. doi: 10.1097/00002030-200111230-00004
Navarro, M. C., Sosa, M., Saavedra, P., Lainez, P., Marrero, M., Torres, M., et al. (2009). Poverty is a risk factor for osteoporotic fractures. Osteoporos. Int. 20, 393–398. doi: 10.1007/s00198-008-0697-9
Neto, M. G., Conceico, C. S., Carvalho, V. O., and Brites, C. (2015). Effects of combined aerobic and resistance exercise on exercise capacity, muscle strength and quality of life in HIV-infected patients: a systematic review and meta-analysis. PLoS ONE 10:e0138066. doi: 10.1371/journal.pone.0138066
O'Brien, K. K., Tynan, A., Nixon, S. A., and Glazier, R. H. (2017). Effectiveness of progressive resistive exercise (PRE) in the context of HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect. Dis. 17:268. doi: 10.1186/s12879-017-2342-8
O'Brien, K., Tynan, A.-M., Nixon, S., and Glazier, R. H. (2008). Effects of progressive resistive exercise in adults living with HIV/AIDS: systematic review and meta-analysis of randomized trials. AIDS Care 20, 631–653. doi: 10.1080/09540120701661708
Ofotokun, I., McIntosh, E., and Weitzmann, M. N. (2012). HIV: inflammation and bone. Curr. HIV/AIDS Rep. 9, 16–25. doi: 10.1007/s11904-011-0099-z
Ofotokun, I., Titanji, K., Vunnava, A., Roser-Page, S., Vikulina, T., Villinger, F., et al. (2016). Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 30, 405–414. doi: 10.1097/QAD.0000000000000918
Pavy-Le Traon, A., Heer, M., Narici, M. V., Rittweger, J., and Vernikos, J. (2007). From space to earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur. J. Appl. Physiol. 101, 143–194. doi: 10.1007/s00421-007-0474-z
Petróczi, A., Hawkins, K., Jones, G., Naughton, D. P., Bank, W., Thomas, S., et al. (2010). HIV patient characteristics that affect adherence to exercise programmes: an observational study. Open AIDS J. 44, 148–155. doi: 10.2174/1874613601004010148
Pinto Neto, L. F. S., Ragi-Eis, S., Vieira, N. F. R., Soprani, M., Neves, M. B., Ribeiro-Rodrigues, R., et al. (2011). Low bone mass prevalence, therapy type, and clinical risk factors in an HIV-infected brazilian population. J. Clin. Densitom. 14, 434–439. doi: 10.1016/j.jocd.2011.06.004
Purdy, J., Gafni, R., Reynolds, J., Zeichner, S., and Hazra, R. (2008). Decreased bone mineral density with off - label use of Tenofovir in HIV - infected children and adolescents. J. Pediatr. 152, 582–584. doi: 10.1016/j.jpeds.2007.12.020
Rector, R. S., Rogers, R., Ruebel, M., and Hinton, P. S. (2008). Participation in road cycling vs running is associated with lower bone mineral density in men. Metabolism 1157, 226–232. doi: 10.1016/j.metabol.2007.09.005
Ryan, A. S., Ivey, F. M., Hurlbut, D. E., Martel, G. F., Lemmer, J. T., Sorkin, J. D., et al. (2004). Regional bone mineral density after resistive training in young and older men and women. Scand. J. Med. Sci. Sport 14, 16–23. doi: 10.1111/j.1600-0838.2003.00328.x
Santos, W. R., Paes, P. P., Ferreira - Silva, I. A., Santos, A. P., Vercese, N., et al. (2015). Impact of strength training on bone mineral density in patients infected with HIV exhibiting lipodystrophy. J. Strength. Cond. Res. 29, 3466–3471. doi: 10.1519/JSC.0000000000001001
Schambelan, M., Benson, C. A., Carr, A., Currier, J. S., Dubé, M. P., Gerber, J. G., et al. (2002). Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an international AIDS society – USA panel. AIDS J. Acquir. Immune Defic. Syndr. 31, 257–275. doi: 10.1097/00126334-200211010-00001
Schouten, J., Wit, F. W., Stolte, I. G., Kootstra, N. A., Van Der Valk, M., Geerlings, S. E., et al. (2014). Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between hiv-infected and uninfected individuals: the age H IV cohort study. Clin. Infect. Dis. 59, 1787–1797. doi: 10.1093/cid/ciu701
Seeman, E., and Delmas, P. D. (2006). Bone quality–the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261. doi: 10.1056/NEJMra053077
Sellmeyer, D. E. (2010). Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 304, 1480–1484. doi: 10.1001/jama.2010.1360
Sharp, M. (2011). HIV and bone health. BETA 23, 28–37. Available online at: http://sfaf.org/hiv-info/hot-topics/beta/2011-beta-winterspring-bone-health.pdf
Slade, S. C., and Keating, J. L. (2011). Exercise prescription: a case for standardised reporting. Br. J. Sports Med. 46, 1110–1113. doi: 10.1136/bjsports-2011-090290
Solomon, D. H., Avorn, J., Katz, J. N., Finkelstein, J. S., Arnold, M., Polinski, J. M., et al. (2005). Compliance with osteoporosis medications. Arch. Intern. Med. 165, 2414–2419. doi: 10.1001/archinte.165.20.2414
Tønnesen, R., Schwarz, P., and Hovind, P. H. (2016). Physical exercise associated with improved BMD independently of sex and vitamin D levels in young adults. Eur. J. Appl. Physiol. 116, 1297–1304. doi: 10.1007/s00421-016-3383-1
Vancampfort, D., Mugisha, J., Richards, J., De Hert, M., Lazzarotto, A. R., Schuch, F. B., et al. (2017). Dropout from physical activity interventions in people living with HIV: a systematic review and meta-analysis. AIDS Care 29, 636–643. doi: 10.1080/09540121.2016.1248347
Watts, N. B., Adler, R. A., Bilezikian, J. P., Drake, M. T., Eastell, R., Orwoll, E. S. F. J., et al. (2012). Osteoporosis in men: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 97, 1802–1822. doi: 10.1210/jc.2011-3045
Welz, T., Childs, K., Ibrahim, F., Poulton, M., Taylor, C. B., Moniz, C. F., et al. (2010). Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS 24, 1923–1928. doi: 10.1097/QAD.0b013e32833c3281
World Health Organisation (2015a). Global Health Sector Response To HIV , 2000 – 2015: Focus on Innovations. Progress Report.
World Health Organisation (2003). Adherence to Long-Term Therapies: Evidence for Action. World Health Organisation.
World Health Organisation (2013). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Guidelines, 1–269.
World Health Organisation (2015b). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: What's New in Policy Brief, 1–16.
Xu, J., Lombardi, G., Jiao, W., and Banfi, G. (2016). Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sport Med. 46, 1165–1182. doi: 10.1007/s40279-016-0494-0
Keywords: bone mineral density (BMD), antiretroviral therapy (ART), people living with HIV (PLWHIV), exercise, progressive resistance exercise (PRE), osteopenia, osteoporosis
Citation: Chisati EM, Constantinou D and Lampiao F (2018) Management of Reduced Bone Mineral Density in HIV: Pharmacological Challenges and the Role of Exercise. Front. Physiol. 9:1074. doi: 10.3389/fphys.2018.01074
Received: 22 April 2018; Accepted: 18 July 2018;
Published: 07 August 2018.
Edited by:
Hassane Zouhal, University of Rennes 2 – Upper Brittany, FranceReviewed by:
P. Bryant Chase, Florida State University, United StatesChristelle Jaffré, University of Picardie Jules Verne, France
Copyright © 2018 Chisati, Constantinou and Lampiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enock M. Chisati, ZWNoaXNhdGlAbWVkY29sLm13
 Enock M. Chisati
Enock M. Chisati Demitri Constantinou
Demitri Constantinou Fanuel Lampiao
Fanuel Lampiao