- 1Physiology Section, Atmosphere and Ocean Research Institute, The University of Tokyo, Kashiwa, Japan
- 2Ushimado Marine Institute, Faculty of Science, Okayama University, Setouchi, Japan
- 3Mouse Genomics Resource Laboratory, National Institute of Genetics, Mishima, Japan
Body fluid regulation, or osmoregulation, continues to be a major topic in comparative physiology, and teleost fishes have been the subject of intensive research. Great progress has been made in understanding the osmoregulatory mechanisms including drinking behavior in teleosts and mammals. Mudskipper gobies can bridge the gap from aquatic to terrestrial habitats by their amphibious behavior, but the studies are yet emerging. In this review, we introduce this unique teleost as a model to study osmoregulatory behaviors, particularly amphibious behaviors regulated by the central action of hormones. Regarding drinking behavior of mammals, a thirst sensation is aroused by angiotensin II (Ang II) through direct actions on the forebrain circumventricular structures, which predominantly motivates them to search for water and take it into the mouth for drinking. By contrast, aquatic teleosts can drink water that is constantly present in their mouth only by reflex swallowing, and Ang II induces swallowing by acting on the hindbrain circumventricular organ without inducing thirst. In mudskippers, however, through the loss of buccal water by swallowing, which appears to induce buccal drying on land, Ang II motivates these fishes to move to water for drinking. Thus, mudskippers revealed a unique thirst regulation by sensory detection in the buccal cavity. In addition, the neurohypophysial hormones, isotocin (IT) and vasotocin (VT), promote migration to water via IT receptors in mudskippers. VT is also dipsogenic and the neurons in the forebrain may mediate their thirst. VT regulates social behaviors as well as osmoregulation. The VT-induced migration appears to be a submissive response of subordinate mudskippers to escape from competitive and dehydrating land. Together with implications of VT in aggression, mudskippers may bridge the multiple functions of neurohypophysial hormones. Interestingly, cortisol, an important hormone for seawater adaptation and stress response in teleosts, also stimulates the migration toward water, mediated possibly via the mineralocorticoid receptor. The corticosteroid system that is responsive to external stressors can accelerate emergence of migration to alternative habitats. In this review, we suggest this unique teleost as an important model to deepen insights into the behavioral roles of these hormones in relation to osmoregulation.
Evolution of Body Fluid Regulation From Fishes to Tetrapods
Ionic concentration, osmolality, and volume of body fluids are important internal parameters that are tightly controlled in vertebrates by the ingestion and excretion of water and ions (Bourque, 2008). As vertebrates expanded their habitats from aquatic to terrestrial environments, terrestrial adaptation requires critical changes in the osmoregulatory and cardiovascular systems to counter both dehydration and gravity (Leow, 2015). To cope with dehydration, they drink water and reduce evaporative water loss from the body surface by a developed body integument consisting of layers of keratinized skin cells. In addition, the kidney of endothermic mammals and birds is equipped with juxtamedullary nephrons that can produce hyperosmotic urine, which is an adaptation to reduce water loss from excretion.
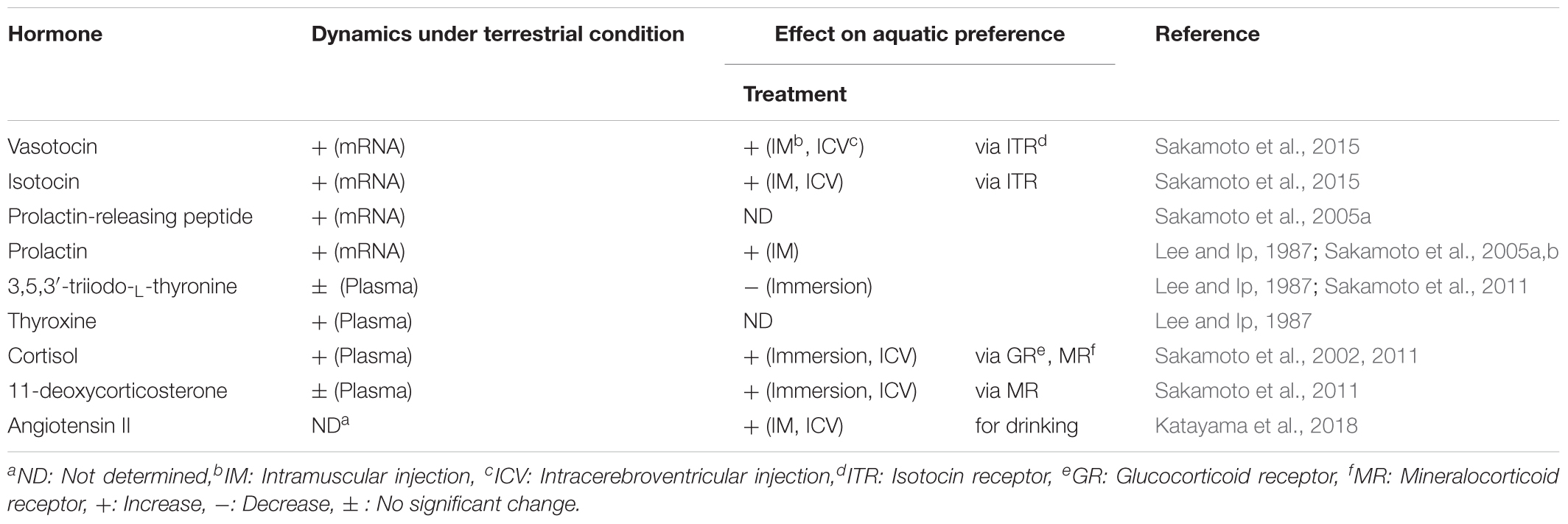
Similar to terrestrial tetrapods, teleost fishes are osmotic and ionic regulators and the ionic composition of their body fluis is similar to those of tetrapods, whose plasma osmolality is approximately one third of seawater regardless of the salinity that they inhabit (Evans, 2008). Their osmoregulatory ability might have allowed them to flourish in a wide range of aquatic environments including freshwater, seawater, and in particular cases allowed survival and success even on land (Takei, 2015). Marine teleosts exposed to severe dehydration drink seawater to cope with this problem (Hirano et al., 1972; Kobayashi et al., 1983; Perrott et al., 1992; Takei, 2015). After drinking, seawater is desalinated in the esophagus, and then water is absorbed together with NaCl in the intestine after isotonic dilution (Parmelee and Renfro, 1983; Nagashima and Ando, 1994; Takei et al., 2016). High amount of HCO3- is secreted into intestinal luminal fluid so that Ca2+ and Mg2+ are removed by precipitation in the form of carbonate aggregates (Wilson et al., 2002; Kurita et al., 2008; Grosell, 2011). The excess monovalent ions such as Na+ and Cl- are excreted from the branchial or cutaneous ionocytes (Uchida et al., 1996; Sakamoto et al., 2000; Seo et al., 2015) and divalent ions such as Ca2+, Mg2+, and SO42- are excreted from the kidney (Watanabe and Takei, 2011). In freshwater teleosts, uptake of environmental ions through the gill is activated for hyperosmoregulation (Takei et al., 2014). This action is mediated by ion transporters such as Na+-K+-ATPase (NKA) and Ca2+-ATPase (Hoenderop et al., 2005; Hwang et al., 2011). In these studies, species differences in osmoregulatory mechanisms and hormonal function have been found (Takei et al., 2014). Further, the osmoregulatory mechanisms are flexible in euryhaline or migratory species such as eels and salmonids, which experience drastic salinity changes during their life cycle and have to switch ion and water regulation to opposite directions via active transport (Figure 1A). Studies on these teleosts have highlighted pivotal roles of various hormones in adaptation to fluctuating environmental salinities (McCormick, 2001; Takei and McCormick, 2012).
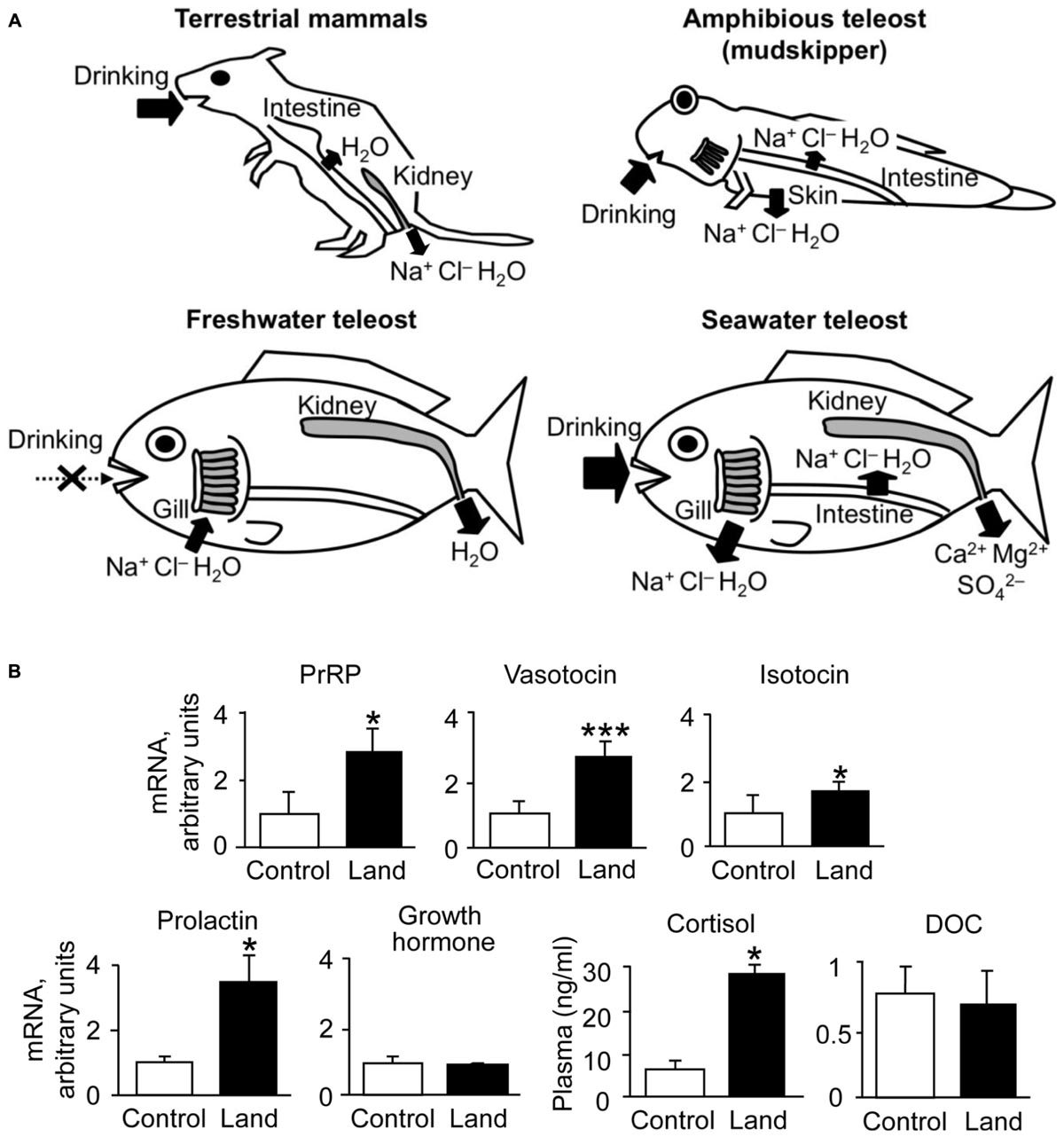
FIGURE 1. Environmental adaptations in vertebrates. (A) Osmoregulatory mechanisms in mammals and teleosts. Arrows show active and passive transport of ions and/or water. The osmoregulatory mechanisms are flexible in euryhaline species such as catadromous eels and anadromous salmonids, which switch ion and water regulation to opposite directions via active transport. In addition to aquatic teleosts, the amphibious and euryhaline mudskipper, which invades land in its lifecycle, is used for the study of osmoregulation. (B) Dynamics of osmoregulatory hormones in terrestrial adaptation of mudskippers. Cortisol and DOC are shown as plasma concentrations (Sakamoto et al., 2002, 2011), and the other hormones are shown as the expression of their genes in the brain of mudskippers in controls (in one-third seawater) or on land (n = 4–8) (Sakamoto et al., 2005a, 2015). Data are shown as the means ± SE. ∗p < 0.05, ∗∗∗p < 0.001 with t-test or Mann–Whitney U-test. PrRP, prolactin-releasing peptide; DOC, 11-deoxycorticosterone.
Ample studies have clarified functions of osmoregulatory and cardiovascular hormones in terrestrial tetrapods and aquatic teleosts (McCormick and Bradshaw, 2006; Bourque, 2008; Mével et al., 2012; Takei et al., 2014; Leow, 2015). In teleosts, however, considerably less research effort is directed at determining their role in behaviors. In addition, it is little known how their functions are conserved or have evolved among diverse taxa through evolutionary time. An exception is drinking behavior induced by angiotensin II (Ang II). In mammals, circulating Ang II is a major factor in the increased thirst and sodium appetite of hypovolemia (Fitzsimons, 1998). These effects play important roles in sustaining the blood volume and blood pressure and would certainly have been evolutionarily advantageous. With regard to thirst, Ang II act on the thirst center to motivate terrestrial mammals to seek for and ingest water. Ingestion of water rapidly satiates thirst sensation by sensory detection of water in the gastrointestinal tract (Zimmerman et al., 2016). Ang II also acts in concert with vasopressin (VP) to decrease the loss of water (Fitzsimons, 1998). In aquatic teleosts, Ang II and neurohypophysial hormones similarly regulate drinking (Takei et al., 1979; Balment and Carrick, 1985; Perrott et al., 1992; Kozaka et al., 2003; Watanabe et al., 2007; Fuentes and Eddy, 2012). However, as we often found differences in the response to osmoregulatory hormones among teleost species (Kobayashi et al., 1983), a comparative approach may benefit deeper understanding on the function of osmoregulatory hormones, which will not be readily available when studying mammals exclusively.
Amphibious Mudskipper as a Unique Model for Studying Osmoregulatory Behavior
Mudskipper fishes including Periophthalmus modestus are euryhaline species that can tolerate salinities ranging from 0 to 40 parts per thousand (ppt). They often experience rapid changes in salinity each day with tide in the estuary and so their osmoregulatory mechanisms are highly flexible. Furthermore, they spend the greater time of their lives out of water to feed and to escape from aquatic predators. They have acquired behavioral and physiological adaptations to amphibious lives (Clayton, 1993; Graham, 1997; Sakamoto and Ando, 2002; Sakamoto et al., 2005a). The roles of endocrine systems in their amphibious features have been investigated (Table 1). Because of the unique amphibious behavior (i.e., migration between terrestrial and aquatic areas), mudskippers may serve as a valuable experimental model to investigate the central actions of osmoregulatory hormones and to provide new insights into the evolution of hormonal actions during transition from aquatic to terrestrial lifestyle.
Which actions of osmoregulatory hormones have been conserved and/or exploited in this teleost? Among the accumulated data, we will focus on three topics in this review. First, we discuss the role of Ang II in drinking behavior. The drinking behavior of mudskippers is composed of migration to water, taking water into the mouth, and swallowing, which may most likely be associated with thirst. The second topic is the interaction between osmoregulation and social behavior, both of which are regulated by the neurohypophysial hormones, vasotocin (VT) and isotocin (IT). Finally, we introduce the role of corticosteroids in the amphibious behavior. Aldosterone is a major mineralocorticoid in mammals, but only minimally represented in teleosts. In teleosts, cortisol acts as mineralocorticoid as well as glucocorticoid (Takahashi and Sakamoto, 2013). Thus, cortisol action on the amphibious behavior has been investigated. We expect that this review will arouse further interest in the functional evolution of osmoregulatory hormones not only for fish endocrinologists but also for those working on other animals.
Angiotensin II and Thirst-Motivated Migration
Comparative studies using various vertebrates such as teleosts, amphibians, and mammals suggest that adaptation to life on dry land with a full influence of the gravitational force necessitates an elaborate renin-angiotensin system to be evolved (Nishimura, 1978; Leow, 2015). In mammals, the renin-angiotensin cascade is initiated by the release of renin from the juxtaglomerular cells in the renal afferent arteriole. Renin is released by hypovolemia and subsequent decreases in perfusion pressure at the arteriole (Kobayashi and Takei, 1996; Nishimura, 2017). The principal action of the active principle, Ang II, is to restore blood volume by retaining NaCl and water. Ang II stimulates secretion of VP and aldosterone, thereby further contributing to volume retention. Indeed, loss of function of the renin-angiotensin or VP system resulted in a hypotensive phenotype (Doan et al., 2001; Fujiwara and Bichet, 2005). Since inhibitors of the renin-angiotensin system attenuate hypovolemia-induced drinking, plasma Ang II is closely related to extracellular dehydration (Kobayashi and Takei, 1996). Unlike in mammals, plasma Ang II levels increase by hyperosmotic stimulus (cellular dehydration) as well as by hypovolemic stimulus in teleosts (Nishimura, 1978; Tierney et al., 1995; Takei, 2000). Transfer from fresh water to seawater results in a small and transient increase in plasma Ang II concentration in parallel with plasma osmolality (Okawara et al., 1987). Thus, Ang II functions as a fast-acting hormone in response to fluctuation of environmental salinity (Takei et al., 2014). The dipsogenic effect of Ang II has been examined extensively in various vertebrates including teleost and elasmobranch fishes (Kobayashi et al., 1983; Perrott et al., 1992; Anderson et al., 2001; Fuentes and Eddy, 2012). Ang II is the most potent dipsogenic hormone thus far known in many vertebrate species (Fitzsimons, 1998; Takei, 2000; McKinley and Johnson, 2004).
Thirst is defined as a conscious sensation of a need for water and a desire to drink (Fitzsimons, 1979). In terrestrial animals such as mammals, thirst is followed by a search for water, and its motivation or consciousness is generated in the hypothalamic area and the medial thalamic-cortex network (Denton et al., 1999; Gizowski and Bourque, 2018). Thirst is induced by an increase in systemic Ang II concentration (Kobayashi et al., 1979; Fitzsimons, 1998; Takei, 2000; McKinley and Johnson, 2004). In mammals and birds, systemic Ang II binds to the sensory circumventricular organs (CVOs) in the forebrain that lack the blood-brain barrier to induce drinking behaviors (Simpson and Routtenberg, 1973; Kobayashi and Takei, 1996) (Figure 2A). Angiotensin type 1 receptors (AT1) are present in high density in the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO) which are known as forebrain CVOs for Ang II-induced thirst (Johnson and Buggy, 1978; Fitzsimons, 1998; McKinley, 2003). Although Ang II also binds to type 2 receptors (AT2), AT2 receptors are sparse at these regions (Rowe et al., 1992). AT1 antagonist losartan inhibited Ang II-induced drinking, but AT2 receptor antagonist PD-123177 did not have any inhibitory action (Timmermans et al., 1993; Goodfriend et al., 1996). Thus, Ang II-induced drinking behavior is mediated through AT1 receptors (Fitzsimons, 1998). It is believed that amphibians such as terrestrial toads and arboreal frogs do not normally drink but instead obtain water by absorption through the ventral skin (Jørgensen, 1997; Bentley, 2002). Interestingly, Ang II induces such water-acquiring behavior called “cutaneous drinking”, in which the pelvic patch is pressed against a moist surface (Hoff and Hillyard, 1991; Propper et al., 1995; Maejima et al., 2010). The cutaneous drinking behavior seems to be regulated via AT1 in the forebrain where CVOs probably localize (Duvernoy and Risold, 2007; Maejima et al., 2010; Uchiyama, 2015), suggesting conserved neural basis of thirst throughout tetrapods. In aquatic teleost fishes, however, none of the regions in the forebrain appear to be involved in elicitation of drinking, since removal of the whole forebrain in eels did not affect the drinking induced by seawater exposure (Hirano et al., 1972) or by injection of Ang II (Takei et al., 1979). These stimuli may act on the hindbrain to initiate swallowing reflex in aquatic teleosts, which complete drinking only by swallowing of buccal water without a search for water (Figure 2C). The area postrema (AP) in the hindbrain is proposed to be the primary site of systemic Ang II action, since Evans blue injected into the blood stained this hindbrain CVO (Mukuda et al., 2005) and lesioning of the AP impaired Ang II-induced drinking in eels (Nobata et al., 2013). The AP neurons send cholinergic fibers to the glossopharyngeal-vagal motor complex (Ito et al., 2006), which in turn control the upper esophageal sphincter (UES) muscle (Mukuda and Ando, 2003; Nobata et al., 2013). The UES muscle is the first gate of the alimentary tract and its relaxation leads to initiation of swallowing.
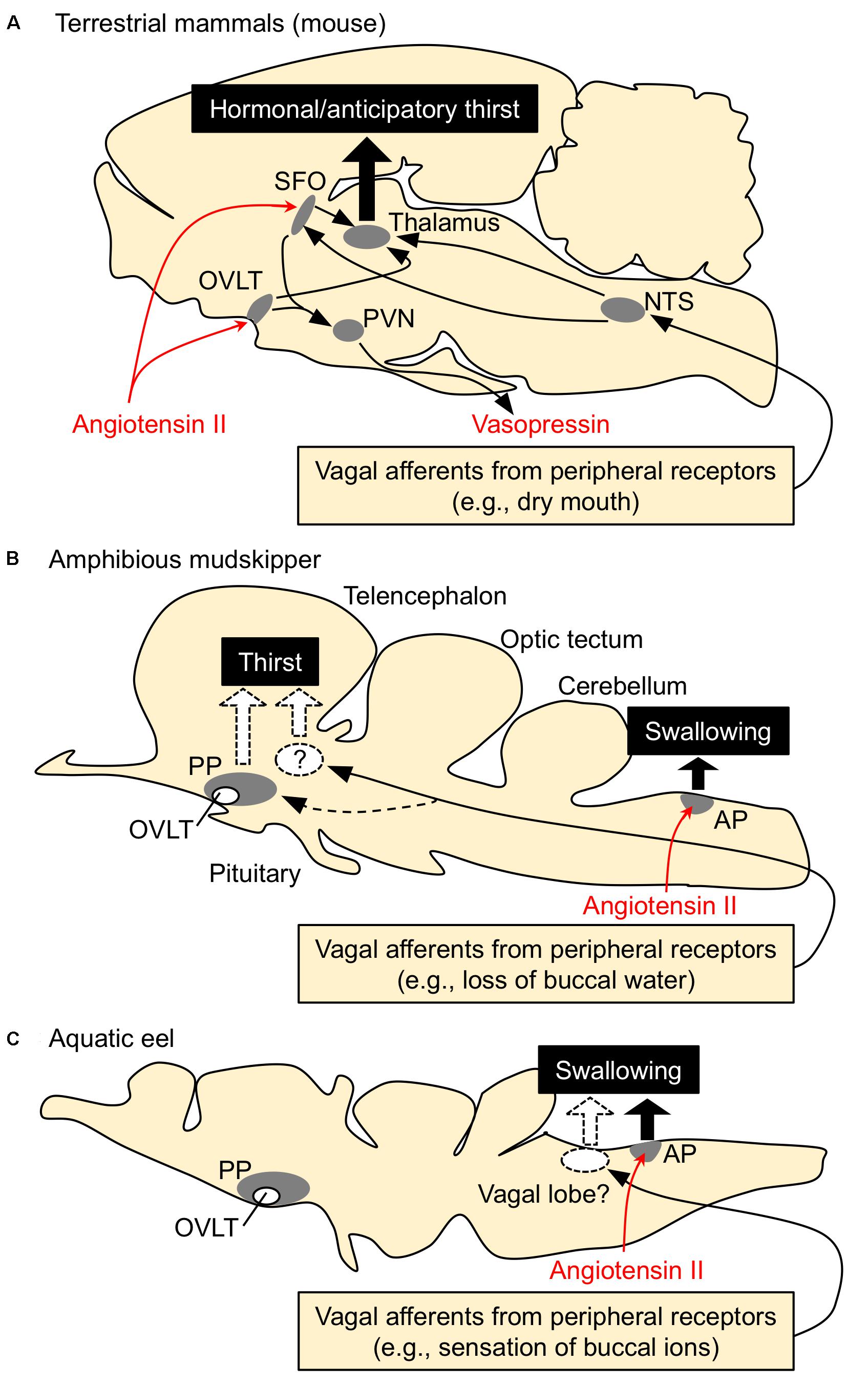
FIGURE 2. Schematic drawing for the regulatory mechanisms of drinking behavior in terrestrial mammals, amphibious mudskippers, and aquatic eels. Systemic angiotensin II acts on the circumventricular organs (CVOs) that are outside the blood-brain barrier. Among CVOs, the area postrema (AP) and the organum vasculosum of the lamina terminalis (OVLT) exist in mice, mudskippers, and eels, but the subfornical organ (SFO) is identified only in tetrapods. (A) Thirst-inducing mechanisms in mice. Systemic angiotensin II is perceived by the neurons in the SFO and OVLT. The signal is transmitted to the thalamus for thirst inducement, and to the paraventricular (PVN) and supraoptic (SON) nuclei for vasopressin secretion. The generation of thirst seems to involve activation of the cortex, which might be mediated by relay neurons in the medial parts of the thalamus. Signals from peripheral receptors (e.g., dry mouth, buccal food) also reach thirst-regulating regions (e.g., SFO) via visceral afferents that course through spinal or vagal pathways. This thirst is evoked before any changes in blood parameters and thus noted as “anticipatory thirst”. NTS, nucleus tractus solitarius. (B,C) Mechanisms of drinking in mudskippers and eels. The AP neurons receive systemic angiotensin II and induce swallowing, possibly through the glossopharyngeal-vagal motor complex in the medulla oblongata. In mudskippers (B), sensory detection of loss of buccal water motivates mudskippers to refill water possibly through the vagal afferents, suggesting generation of thirst. Possible thirst center, which regulates migration to water for drinking, has not been identified yet, but vasotocin neurons in the parvocellular preoptic nucleus (PP) might be involved in the neural basis. In eels (C), sensory detection of an increase in Cl- concentration in buccal water induces swallowing of water as an anticipatory drinking. This local stimulus is sensed by afferent fibers of vagus and glossopharyngeal nerves, while the forebrain and AP are not involved in the anticipatory drinking. Regulation of drinking behavior by the vagal afferents appears to be conserved among vertebrates. In contrast, an involvement of the forebrain in the eel drinking has not been implicated.
From the comparative point of view, it is intriguing to examine whether amphibious mudskippers have the mechanism inducing thirst as a motivation for drinking. Our recent study showed that peripheral or central administration of Ang II motivates the fish to move to water and to increase the volume of water ingested (Katayama et al., 2018). An OVLT-like structure has been found histologically in the parvocellular preoptic nucleus (PP) of the mudskipper (Hamasaki et al., 2016), but our histochemical study did not support the direct action of Ang II on this region. AT1 receptors have been cloned in teleosts including mudskippers (Nishimura, 2017) although no expression study has demonstrated a calcium signal in the recombinant receptors (Russell et al., 2001). AT1-like mRNA was not detected in the OVLT-like region of mudskippers, while many nuclei in the AP expressed the mRNA (Katayama et al., 2018). AT2 receptors have been cloned in teleosts (Nishimura, 2017), but AT2 mRNA was not detected in the eel brain (Wong and Takei, 2013). Thus, AT1, not AT2, appears to mediate Ang II-induced drinking behavior also in teleosts. In mudskippers, Ang II, through its action on the AP, induced swallowing of buccal water, which is stored on land, and the loss of buccal water motivated mudskippers to move to water (Figure 2B; Katayama et al., 2018). Although regulation of the renin-angiotensin system has not been examined when mudskippers are on land, Ang II appears to induce drinking naturally in teleosts since an inhibitor of the renin-angiotensin system (captopril) attenuates spontaneous drinking (Okawara et al., 1987; Perrott et al., 1992; Kobayashi and Takei, 1996). In addition to the osmoregulatory problem, the effect of gravity cannot be nullified in terrestrial environments. Blood pressure of mudskippers is maintained during the transition from submersion to emersion unlike other teleost species, in spite of the influence of gravity (Ishimatsu et al., 1999). Since Ang II contributes to the maintenance of cardiovascular homeostasis in teleosts (Mével et al., 2012; Nishimura, 2017), its relative importance may have been enhanced for cardiovascular regulation as well as for osmoregulation in amphibious mudskippers. In a series of their drinking behaviors, the migration to water stimulated by loss of buccal water is equivalent to the drinking behavior in tetrapods evoked by local stimuli (e.g., dry mouth). Such drinking has been revealed as “anticipatory thirst” in mice because it operates before blood osmolality fluctuates (Berridge, 2004; Zimmerman et al., 2016). In mudskippers, the buccal cavity is filled with water before they exit to land and this behavior appears not to be involved in blood osmolality and hormones. Therefore, the thirst by local sensation may contribute to an anticipatory mechanism to prevent potential dehydration on land.
Although migratory behavior induced by local sensation has not been demonstrated in aquatic fishes, it is well recognized that eels detect an increase in Cl- concentration in buccal water, which enhances swallowing of water (Hirano, 1974). This local stimulus is sensed by afferent fibers of vagus and/or glossopharyngeal nerves (Mayer-Gostan and Hirano, 1976), whereas the forebrain and AP appear not to be involved in the sensory detection because the lesioning of these regions did not attenuate swallowing induced by seawater exposure in the eel (Figure 2C; Hirano et al., 1972; Nobata and Takei, 2011). This “chloride response” could prevent future dehydration in hyperosmotic marine environments, and thus was referred to as an anticipatory drinking (Hirano, 1974). The sensation of ions in the buccal cavity of aquatic fishes may be similar to the sensation of buccal water underlying the thirst of tetrapods and mudskippers. In basal vertebrates such as river lamprey (Lampetra fluviatilis), the transfer from seawater to fresh water rapidly decreased drinking rate without a change in plasma osmolality (Rankin, 2002). Thus, the mechanism of anticipatory drinking by local sensation appears to be widely distributed among vertebrates. Mudskippers, which evolved from the aquatic teleosts to invade the terrestrial environment (You et al., 2014; Ord and Cooke, 2016), have developed the thirst-inducing mechanism by local sensation, in addition to the hormonal/anticipatory regulation of swallowing at the medulla oblongata observed in totally aquatic fishes (Figures 2B,C). Phylogenetically distant vertebrates (ray-finned fish and tetrapods) appear to have acquired the thirst sensation that elicits a series of drinking behaviors when they are exposed to a desiccating environment (Katayama et al., 2018). From the evolutionary point of view, it is intriguing to examine possible thirst mechanisms of amphibious lungfish, which belong to the class Sarcopterygii and are recognized as the closest living relatives of tetrapods (Brinkmann et al., 2004). Since ancestral vertebrates should not have experienced terrestrial environments, thirst may have evolved multiple times during the course of terrestrialization in vertebrates.
In mammals, the input signal for anticipatory thirst was shown to be relayed to the SFO neurons that monitor blood factors such as Ang II (Figure 2A; Zimmerman et al., 2016). The SFO orchestrates a motivation for drinking by engaging the medial thalamic-cortex network (Gizowski and Bourque, 2018). Given the complex mechanisms of thirst in mammals, the mudskipper with a simpler brain architecture might be a useful model to investigate the mechanisms of anticipatory thirst by local sensation. In addition to the osmoregulatory purpose, the thirst response also prevents the gills from desiccation, and maintains the branchial respiration in mudskippers (Tamura et al., 1976; Sayer, 2005). Thus, maintaining the moistness of the gill could be one of the selection pressures for the development of thirst. Buccal water is also used for sucking of food when mudskippers eat on land (Michel et al., 2015), and thus water in the cavity decreases after feeding. Because the protrusion and retraction of this water mass is essential for intra-oral transport of prey on land, eating appears to be a potent stimulus for thirst development by local sensation. Many mammalian species drink primarily during meals (Fitzsimons and Le Magnen, 1969; Oatley and Toates, 1969; Berridge, 2004). Food consumption rapidly activated SFO neurons in mammals, beginning at the onset of feeding before any changes in blood parameters occurred (Zimmerman et al., 2016). Activation of SFO neurons during eating was unaffected by angiotensin blockers. Thus, sensory detection of buccal food and its consequent activation of SFO neurons through an angiotensin-independent pathway are indicated for prandial thirst in mammals. In teleosts, however, it has not been examined whether prandial drinking functions in anticipatory fashion to prevent food ingestion-dependent alterations in blood composition. More fish studies on anticipatory drinking, as well as on fast-acting Ang II actions, will be required to know the comprehensive mechanisms for “fast” adaptive response to hyperosmotic environments. Given that anticipatory drinking triggered by local sensation is conserved among vertebrates, comparison of drinking behavior in mudskippers with anticipatory thirst in mammals might provide an answer to the question of why the anticipatory response evolved (Krashes, 2016).
Neurohypophysial Hormones for Osmoregulation and Social Behaviors
The neurohypophysial hormones, VP and oxytocin (OXT), regulate fluid homeostasis, which requires a tight control of both NaCl and water in mammals (Johnson and Thunhorst, 1997; McKinley et al., 2004). Particularly, antidiuretic VP is a fast- and short-acting hormone that is indispensable for fluid retention in terrestrial environments. VP neurons in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus react to increases in plasma osmolality by releasing the antidiuretic hormone (Nielsen et al., 1995; Bourque, 2008; Sands et al., 2011; Watts, 2015). The VP neurons are downstream targets of the angiotensinergic neurons innervating the SFO and OVLT (Figure 2A; Ferguson, 2009). Systemic Ang II enhances secretion of VP into the circulation through these neural pathways (Zimmerman et al., 2017). Thus, the Ang II-VP system enhances water retention by the kidney. In contrast, OXT decreases ingestive behaviors, including drinking, salt intake, and feeding (Arletti et al., 1990; Blevins et al., 2003; Stricker and Stricker, 2011; Ryan et al., 2017), and increases renal NaCl excretion after a salt load (Balment et al., 1980; Verbalis et al., 1991; Conrad et al., 1993). In addition to systemic osmoregulation, it has recently been suggested that VP neurons and OXT-receptor-expressing neurons anticipate future osmotic fluctuation by drinking, cues predicting water (e.g., visual cue), feeding, or sleeping (Gizowski et al., 2016; Mandelblat-Cerf et al., 2017; Ryan et al., 2017). For example, the neural activity of VP neurons in the PVN and SON, and thereby VP secretion rapidly fell during drinking before any change in blood parameters occurred (Stricker and Stricker, 2011; Mandelblat-Cerf et al., 2017).
In teleosts, neurohypophysial hormones serve for adaptation to a desiccating seawater environment. Transfer of trout to seawater downregulated transcription of VT (the teleost homolog of VP) in the magnocellular preoptic nucleus (PM) of trout (Hyodo and Urano, 1991). In flounders, however, transfer from seawater to fresh water decreased plasma VT concentration (Bond et al., 2002), whereas transfer from fresh water to seawater increased plasma VT levels and VT mRNAs in the hypothalamus (Balment et al., 2006). Thus, VT responses to environmental osmotic challenges and its physiological functions appear to differ among species. IT (the teleost homolog of OXT), as well as VT, has important functions in teleost osmoregulation. VT and/or IT regulate secretion of extra univalent ions in the gill and opercular epithelium (Guibbolini and Avella, 2003; Martos-Sitcha et al., 2015b). These hormones also regulate water transport via aquaporin-1 paralogs, which contribute to water absorption in the intestine for seawater adaptation (Martos-Sitcha et al., 2015a). IT mRNA levels in the hypothalamus increased after transfer to hypersaline media but not to hyposaline media (Martos-Sitcha et al., 2014). These results suggest that IT and its receptor are important for seawater adaptation. In addition to these osmoregulatory functions, VT and IT neurons localized throughout the hypothalamic regions project not only into the pituitary but also into multiple extra-hypothalamic regions, and are known to mediate social behaviors (Holmqvist and Ekstrom, 1995; Thompson and Walton, 2004; Goodson, 2005; Godwin and Thompson, 2012; Lindeyer et al., 2015). Even in mammals, however, little is known about a possible link between osmoregulation and social behaviors, both of which are controlled by the neurohypophysial hormones.
The amphibious behavior in mudskippers may reflect many functions of neurohypophysial hormones that bridge osmoregulation and social behaviors. Mudskippers moved to water when treated with VT or IT either peripherally or centrally (Sakamoto et al., 2015). Migration to water induced by both VT and IT was inhibited by the OXT receptor blocker (H-9405), which specifically induces IT-receptor blockade in teleosts (Watanabe et al., 2007). Expression studies of VT type 1a receptor (V1a) and IT receptors of teleosts in mammalian cell lines indicate that V1a is nearly specific to VT, whereas the sensitivity of the IT receptor to IT is 3-10 times higher than that to VT (Mahlmann et al., 1994; Hausmann et al., 1995; Warne, 2001; Yamaguchi et al., 2012). Thus, neurons expressing IT receptors may regulate amphibious behavior for osmoregulation. However, other VT/IT receptors, especially VT type 2 receptor (V2), might be implicated in the aquatic preference. The V2-type receptor, which is localized in the hypothalamus and osmoregulatory organs, is involved in body fluid homeostasis in teleosts (Konno et al., 2010a,b; Lema, 2010; Martos-Sitcha et al., 2014). When VT or IT was intracerebroventricularly injected, the drinking rate of mudskippers was enhanced by VT, but not by IT (Katayama et al., 2018). Considering the affinities of VT/IT to their receptors, the VT-specific regulation of drinking appears to be mediated by VT receptors in the mudskipper brain. In mammals, VP neurons that innervate the OVLT play an important role in the above-mentioned “anticipatory thirst” (Gizowski et al., 2016). In mudskippers, immunoreactive VT fibers were found in the PP including the OVLT-like region (Hamasaki et al., 2016). These findings suggest that VT may transmit the signal to the neural pathway of thirst-motivated behavior (Figure 2B; Katayama et al., 2018). In eels, however, peripherally injected VT reduced drinking rate, but IT increased it (Ando et al., 2000; Nobata and Ando, 2013). Thus, the role of neurohypophysial hormones in regulation of drinking has not been established in teleosts. When mudskippers were dehydrated under terrestrial condition, brain mRNA levels of pro-VT markedly increased while a moderate increase was seen in pro-IT mRNA levels (Figure 1B). Given the relatively wide distribution of VT-positive fibers throughout the brain, increased VT under terrestrial condition may naturally stimulate drinking and migration to water (Sakamoto et al., 2015). Nuclei involved in the amphibious behavior were not identified, but brain regions where both VT and IT fibers are localized (e.g., the tuberal nuclei of the hypothalamus, medulla oblongata) may include nuclei expressing IT receptors to regulate this behavior.
In addition to the osmoregulatory function, regulation of social behavior by the VT system has been extensively studied in teleosts (Huffman et al., 2015; Yokoi et al., 2015; Loveland and Fernald, 2017; Perrone and Silva, 2018). Central administration of VT in some species indicates that VT neurons mediate aggression, although the directionality (stimulation/inhibition) varies across species (Godwin and Thompson, 2012; Kagawa et al., 2013). In mudskippers, VT specifically regulated general aggressive behavior and/or social communication (i.e., fin display, operculum display, replacement, attacking, chasing, and biting) (Figure 3A). In particular, VT-injected males showed significantly higher frequencies of fin display, operculum display and attack than vehicle-injected males. The former two types of behaviors are less aggressive than the latter one, and it is suggested that the VT might modulate social communication as well as aggression in mudskippers. Pro-VT mRNA levels in the whole brain of subordinate, however, were higher than in that of dominant (Figure 3B). In several freshwater teleosts, aggressive dominant males have high VT expression in the PM, whereas the submissive subordinate males have high VT expression in the PP (Larson et al., 2006; Greenwood et al., 2008; Kagawa, 2013). Together with prolonged aquatic stay by VT-injected mudskippers, VT in the PP may play a characteristic role in promoting migration into water for submissive subordinates relative to aggressive dominants (Kagawa et al., 2013). VT expression in the PP is involved in the hormonal stress response in the European eel and the rainbow trout (Olivereau and Olivereau, 1990; Gilchriest et al., 2000). However, the migration into water by VT-injected mudskippers cannot be fully explained by a physiological stress response, since there was no difference in plasma cortisol levels (Kagawa et al., 2013). In the mudskipper brain, VT fibers were localized in the preoptic and ventromedial telencephalic areas (Sakamoto et al., 2015). These regions include V1a-expressing neurons in teleosts (Kline et al., 2011; Huffman et al., 2012; Lema et al., 2015). With regard to V1a receptor subtypes, telencephalic V1a1 levels were higher in subordinates compared to dominants, and levels of V1a2 in the telencephalon of dominant males correlated with aggression in killifish (Lema et al., 2015). In cichlid fish, the ventromedial telencephalic area was the site of high density expression for both of these receptors (Loveland and Fernald, 2017). These findings suggest that VT neurons projecting to the ventromedial telencephalic area and/or preoptic area act via V1a2 in the dominant mudskipper to elicit general aggressive behavior, and that the VT neurons act via V1a1 in the subordinate to inhibit general aggressive behavior (Figure 3C). As described above, VT neurons projecting to the tuberal nuclei of the hypothalamus and medulla oblongata may act at least in part via IT receptors to stay in the aquatic habitat forced by the dominant fish (Figure 3C). Given the suite of processes mediated by neurohypophysial hormones, migration of subordinate mudskippers into water reflects a unique interaction between the hormonal regulations of social and osmoregulatory behaviors from competitive and dehydrating land to aquatic environments in the amphibious teleost. A few studies on teleosts have shown that IT controls the reproductive behavior and/or spawning act (Gonçalves and Oliveira, 2011). Mudskippers spawn in their mudflat burrows filled with water, and secure embryonic development within the hypoxic burrows by transporting mouthfuls of air (Ishimatsu et al., 2007). Thus, the unique link between osmoregulation and reproduction regulated by IT should be possible.
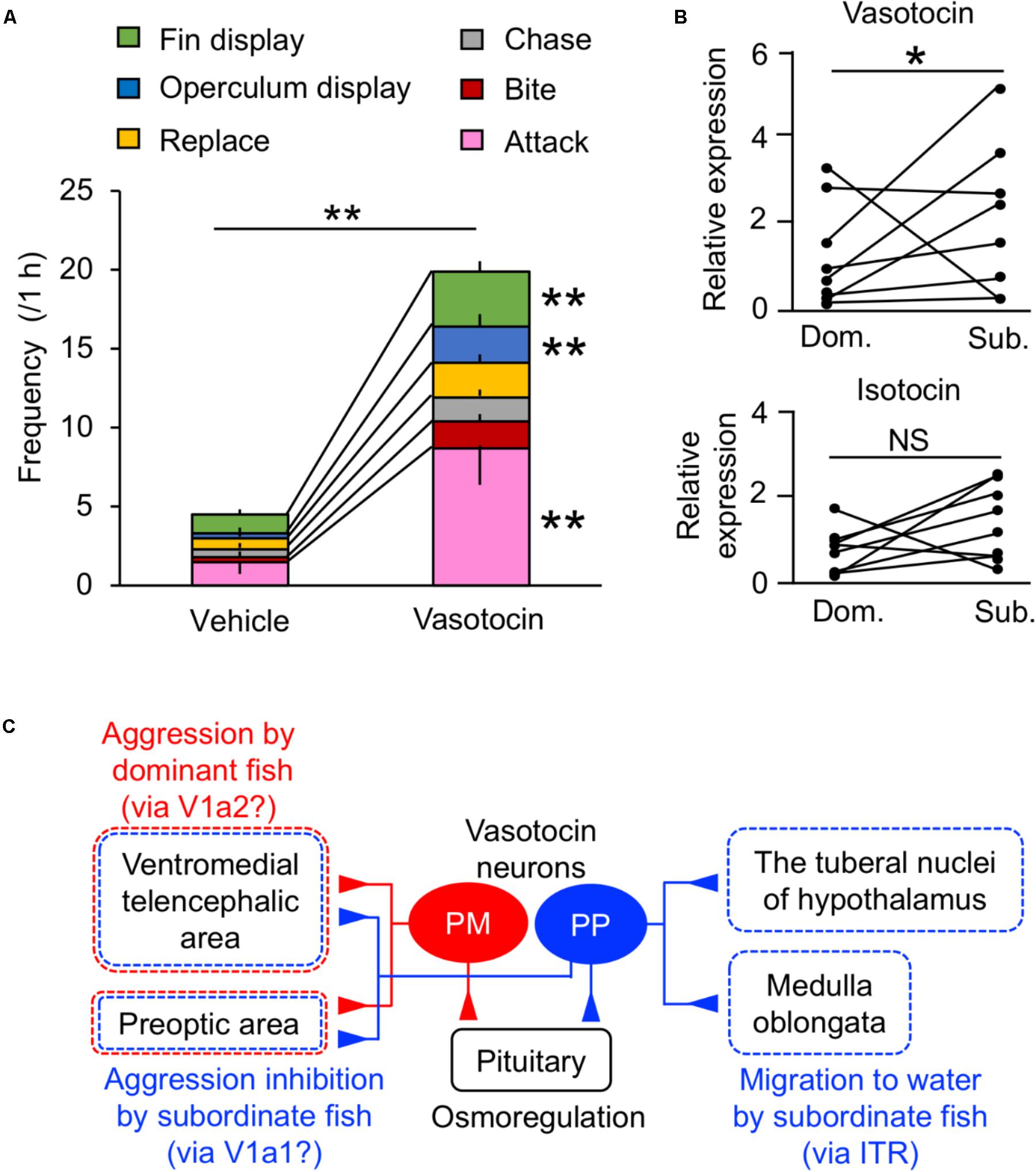
FIGURE 3. Behavioral actions of vasotocin in mudskippers. (A) The frequency of each type of aggressive behavior after injection of vasotocin (500 pg/g-bw) or vehicle in mudskippers (n = 6). A size-matched pair of males was used for behavioral observation in a tank with aquatic and terrestrial areas. Data are shown as the means ± SE. ∗∗p < 0.005 with t-test. (B) The expression of vasotocin and isotocin mRNAs in dominant (Dom.) and subordinate (Sub.) mudskippers (n = 7). Upon introduction in an experimental tank with aquatic and terrestrial areas, a pair of males can be classified as aggressive dominant or submissive subordinate based on the frequency of their aggressive behaviors, which is significantly higher in dominant male. Points of each pair are connected. ∗p < 0.05 with Mann–Whitney U-test. NS, not significant. The original data for (A) and (B) are published in Kagawa et al. (2013). (C) A model illustrating the potential influence of vasotocin neurons on the regulation of aggressive behavior. The cell bodies of vasotocin are localized in the magnocellular (PM) and the parvocellular (PP) preoptic nucleus. Vasotocin may act via V1a-type receptors (V1a1/V1a2) in the ventromedial telencephalic area and the preoptic area to regulate general aggressive behavior. PM cells and V1a2 may mediate aggression by dominant males, while PP cells and V1a1 may mediate submissive behaviors by subordinate males. Since stimulation of migration to water by both vasotocin and isotocin is inhibited by the blocker of isotocin receptor (ITR), brain regions where both vasotocin and isotocin fibers are localized, such as the tuberal nuclei of the hypothalamus and the medulla oblongata, may regulate the amphibious behavior via ITR. The main receptors for specific behaviors are given in parentheses. Broken lines show possible brain regions involved in each behavior.
Corticosteroids for Ion Regulation and Stress Response
Corticosteroids function as glucocorticoids and mineralocorticoids in vertebrates. Glucocorticoids regulate metabolism and growth, while mineralocorticoids regulate the body fluid osmolality. In tetrapods, these functions are achieved by two distinct hormones: cortisol/corticosterone (glucocorticoids) and aldosterone (mineralocorticoid). Glucocorticoids and mineralocorticoids activate their receptors – the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), respectively (Figure 4A). In the mammalian brain, the GR and MR are both highly expressed in the hippocampus and in several hypothalamic nuclei such as PVN and arcuate nucleus. Co-localization of these receptors has been found in most neurons of the nuclei. These findings suggest that the expression balance of GR/MR within the nucleus is critical for many physiological and short-term behavioral responses, such as regulation of salt intake, mood, appetite, and exploratory behavior (Rozeboom et al., 2007; Kawata et al., 2008; Geerling and Loewy, 2009; Sakamoto et al., 2012). The CVOs such as the SFO and the AP are considered to be involved in the synergistic action of Ang II and mineralocorticoid on salt appetite (Epstein, 1982; Fluharty and Epstein, 1983; Alhadeff and Betley, 2017), since the neurons in those brain areas express both angiotensin receptor (Gehlert et al., 1991; Tsutsumi and Saavedra, 1991; Song et al., 1992) and MR (Coirini et al., 1983).
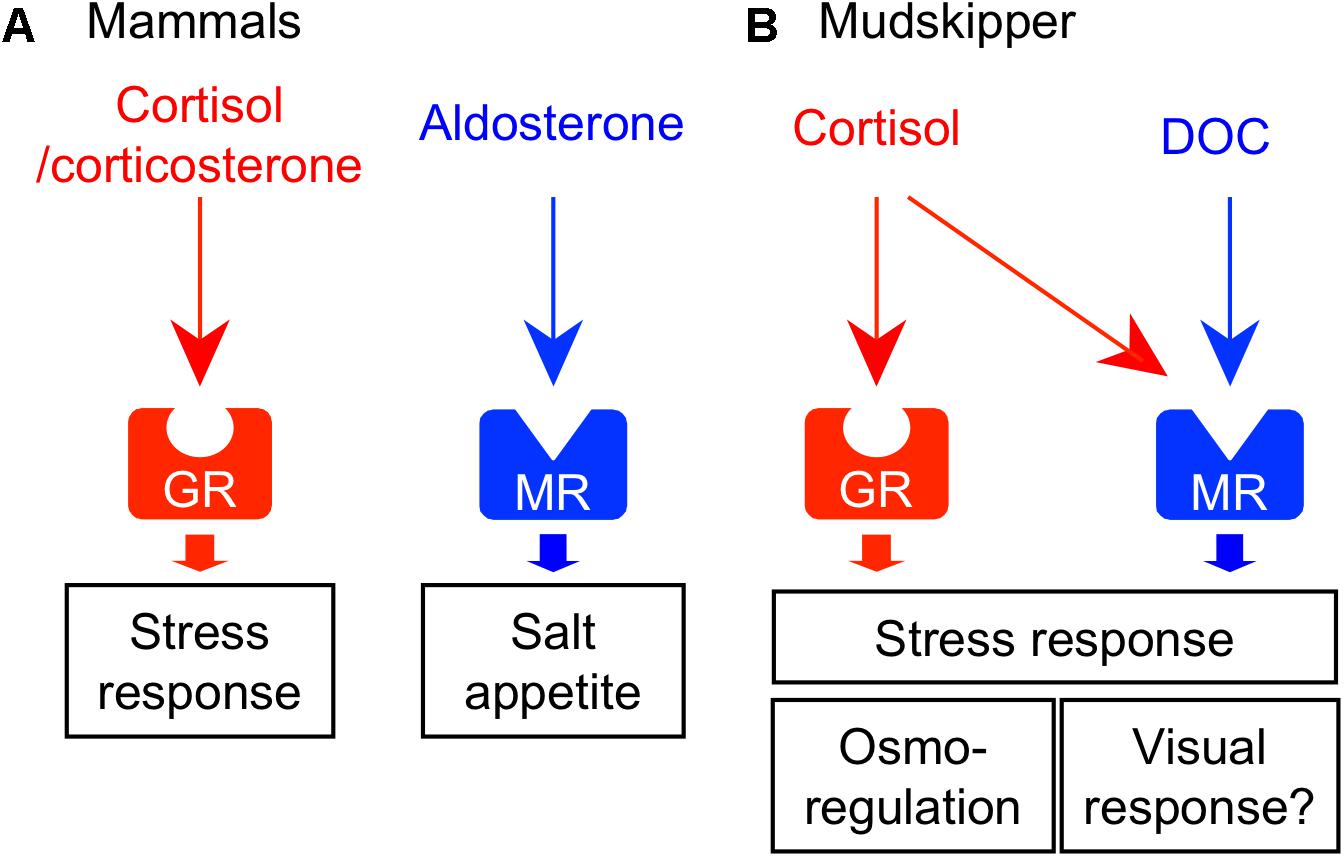
FIGURE 4. Receptor-ligand interactions of corticosteroids and their central actions. (A) Corticosteroid system in mammals. Glucocorticoid (cortisol/corticosterone) and mineralocorticoid (aldosterone) activate their respective receptors – glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). These corticosteroids act on the various brain regions and regulate homeostatic behaviors such as stress response and salt appetite. (B) Corticosteroid system in mudskippers. Cortisol-GR system mediates both glucocorticoid and mineralocorticoid functions. 11-deoxycorticosterone (DOC) is the preferred ligand for MR. In mudskippers, both MR and GR regulate the amphibious behavior related to a stress response. The aquatic preferences induced by GR and MR signaling may be related to osmoregulation and visual response, respectively.
As mentioned earlier, cortisol functions not only as glucocorticoid but also as mineralocorticoid in teleosts (Mommsen et al., 1999). Two different GR coding genes (GR1 and GR2) and one MR gene have been found in this fish group (Bury et al., 2003; Greenwood et al., 2003). Cortisol interacts with MR as well as with GRs, but cortisol-MR axis appears not to be important for osmoregulation unlike in mammals (Prunet et al., 2006; Stolte et al., 2008) (Figure 4B). Indeed, a constitutive MR-knockout medaka can grow and adapt to seawater, as well as to fresh water (Sakamoto et al., 2016). By contrast, inhibition of the GR by RU-486 prevented killifish from seawater adaptation (Shaw et al., 2007). Many studies using euryhaline teleosts indicate that cortisol-GR system plays important roles in both seawater and freshwater adaptation as a slow-acting hormone (McCormick, 2001; Takahashi and Sakamoto, 2013; Takei et al., 2014). In many teleosts, plasma cortisol and GR transcripts in osmoregulatory organs changed after transfer to seawater or fresh water although the directionality varied across species (McCormick, 2001; Takahashi and Sakamoto, 2013). In mudskippers (Sakamoto et al., 2002), plasma cortisol concentrations markedly increased when the teleosts were dehydrated under terrestrial condition (Figure 1B). Cortisol stimulated epithelial apoptosis in the mudskipper esophagus so that NaCl was desalinated from ingested seawater. Cortisol also induced cell proliferation to reduce permeability for freshwater adaptation (Takahashi et al., 2006). The dual functions of cortisol in teleosts may stem from the distinct action on multiple GR isoforms with different sensitivities to cortisol for transactivation and transrepression activities (Prunet et al., 2006; Stolte et al., 2008). In the gills of some teleosts, cortisol stimulated the differentiation of ionocytes into seawater type or freshwater type, and elevated the activity and/or transcription of key transporters in ionocytes such as NKA, Na+-K+-2Cl- cotransporter type 1, and cystic fibrosis transmembrane conductance regulator (Deane et al., 2000; McCormick, 2001; Aruna et al., 2012; Takahashi and Sakamoto, 2013). These actions increased ion excretion for seawater adaptation and ion uptake for freshwater adaptation, respectively. Cortisol also elevated the NKA activity and aquaporin expression in the intestine, thereby increasing water absorption across the epithelia to maintain water balance in a dehydrating seawater environment (Veillette et al., 1995; Veillette and Young, 2005; Cutler et al., 2007). These studies suggest that the hypo- and hyper-osmoregulatory action of cortisol-GR system is well conserved among euryhaline teleosts (Takei et al., 2014).
In teleosts, the circulation of aldosterone, present in extremely low levels, is unlikely to have actions on the GRs or MRs (Prunet et al., 2006). However, 11-deoxycorticosterone (DOC) is a circulating corticosteroid that is present in significant concentrations, and can activate MRs but not GRs in teleosts (Sturm et al., 2005; Prunet et al., 2006; Milla et al., 2008; Stolte et al., 2008). In expression studies in mammalian cell lines, transactivation of the teleost MR is 10 times more sensitive to DOC than to cortisol, whereas the teleost GR is specific to cortisol (Sturm et al., 2005; Prunet et al., 2006; Stolte et al., 2008). In agreement with the presence of the ligand for MRs in the plasma, the teleost MR mRNA was found in many tissues (Greenwood et al., 2003; Sturm et al., 2005; Arterbery et al., 2010). The expressions of MR mRNA are relatively modest in osmoregulatory organs involved in ionoregulation (e.g., gill), but considerably higher in the brains of most teleosts examined (e.g., Sakamoto et al., 2016). These recent finding suggested that MR system may carry out some behavioral functions in teleosts. In fact, mudskippers migrated into water when treated with DOC and cortisol (Sakamoto et al., 2011). Cortisol may act as an endogenous ligand for the brain MRs to stimulate the migration to water naturally, since plasma cortisol, rather than DOC, increased in mudskippers under terrestrial condition (Figure 1B). However, MRs can be insensitive to cortisol activation in vivo because 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) catalyzes the conversion of cortisol to the MR-inactive cortisone (Funder et al., 1988). Without the expression of 11β-HSD2, MRs probably function as cortisol receptors. Hence, study on localization of 11β-HSD2 in the teleost brain is further required. The aquatic preference in 10 ppt seawater, possibly stimulated by the brain MR signaling, may reflect the induction of salt appetite as shown by aldosterone in mammals (Alhadeff and Betley, 2017). Thus, it is of interest to examine synergistic effects of Ang II and corticosteroids to evaluate salinity preference of mudskippers using an aquarium test system. In contrast, the GR signaling may also contribute to the aquatic preference because the cortisol-stimulated behavior was not completely inhibited by the specific GR blocker, RU-486. Since the cortisol-GR system is implicated in excretion of extra ions by elevating the NKA activity in teleosts (McCormick, 2001; Takahashi and Sakamoto, 2013), mudskippers may migrate to water for ion excretion through the skin under the pectoral fin (Sakamoto et al., 2000, 2002). The distinct function of MR and GR signaling should be investigated in the osmoregulatory behavior of mudskippers.
In addition to the osmoregulatory function, GRs and MRs appear to regulate stress responses in the teleost brain (Takahashi and Sakamoto, 2013; Myers et al., 2014; Sakamoto et al., 2018). In teleosts, GRs and MRs are localized in key components of the stress axis, such as the forebrain pallial area, the corticotrophin-releasing hormone cells in the preoptic nucleus, and the adrenocorticotropic-hormone cells in the pituitary pars distalis (Stolte et al., 2008; Kikuchi et al., 2015; Sakamoto et al., 2016). The aquatic preference of mudskippers, stimulated by the brain MR/GR signaling, may also be a stress response, since the dehydrated mudskipper under terrestrial condition appears to be stressed (Figure 4; Sakamoto et al., 2002). Such a system that is responsive to external stressors can also mediate the start of migration from river to ocean in salmon (Clements and Schreck, 2004; Flores et al., 2012). The expression of GRs and MRs mRNA were observed in most of the PM and PP, known to produce VT and IT (Teitsma et al., 1998), and the cortisol-GR system regulated VT and IT release from the hypothalamus-pituitary complex (Kalamarz-Kubiak et al., 2015). Thus, future studies should focus on the “cross-talk” among these hormones in the brain to clarify the link between osmoregulation and stress response, both of which are primarily regulated by the neurohypophysial hormones and corticosteroids. Furthermore, GR mutant adult zebrafish became immobile with reduced exploratory behavior when placed into an unfamiliar aquarium (Ziv et al., 2013). The mutant did not habituate to this stressor upon repeated exposure. Addition of the antidepressant fluoxetine or visual interactions with a wild type fish restored normal behavior. Thus, GR signaling appears to contribute to mood regulation, as well as to the stress response. In contrast, MR-knockout medaka failed to track moving dots although the swimming motility of the mutant increased (Sakamoto et al., 2016). Thus, MR is required for normal activity of locomotion in response to visual motion stimuli. Vision is more important in terrestrial lifestyle than in aquatic one, and sophisticated vision might have promoted land invasion in vertebrates (MacIver et al., 2017). Mudskippers with their unique vision system (Takiyama et al., 2016) will be a good model to analyze the evolution of corticosteroids-regulated vision response.
Conclusion and Perspectives
In this review, we summarized the role of Ang II, neurohypophysial hormones, and corticosteroids in the regulation of amphibious behavior in mudskippers. Mudskippers migrate to water for drinking and for escape from dominant conspecifics and stressful situations (Figure 5). The analyses of their drinking patterns suggest that the neural basis of amphibious behavior is connected to a water detection system in the buccal cavity, which is related to induction of thirst. Direct action of systemic Ang II on the OVLT-like structure and the Ang-II/neurohypophysial hormone axis in the forebrain have not yet been investigated in teleosts including mudskippers. In future research, the target site(s) of systemic Ang II other than those along the lamina terminalis might be identified in the mudskipper forebrain. VT also regulates amphibious behavior related to aggression/submission as well as to drinking, suggesting their distinct functions in each site of the brain. Cortisol may bind to the MR in the brain to elicit a preference for aquatic habitation, which reveals a conserved central action of mineralocorticoid signaling throughout vertebrates (Sakamoto et al., 2018).
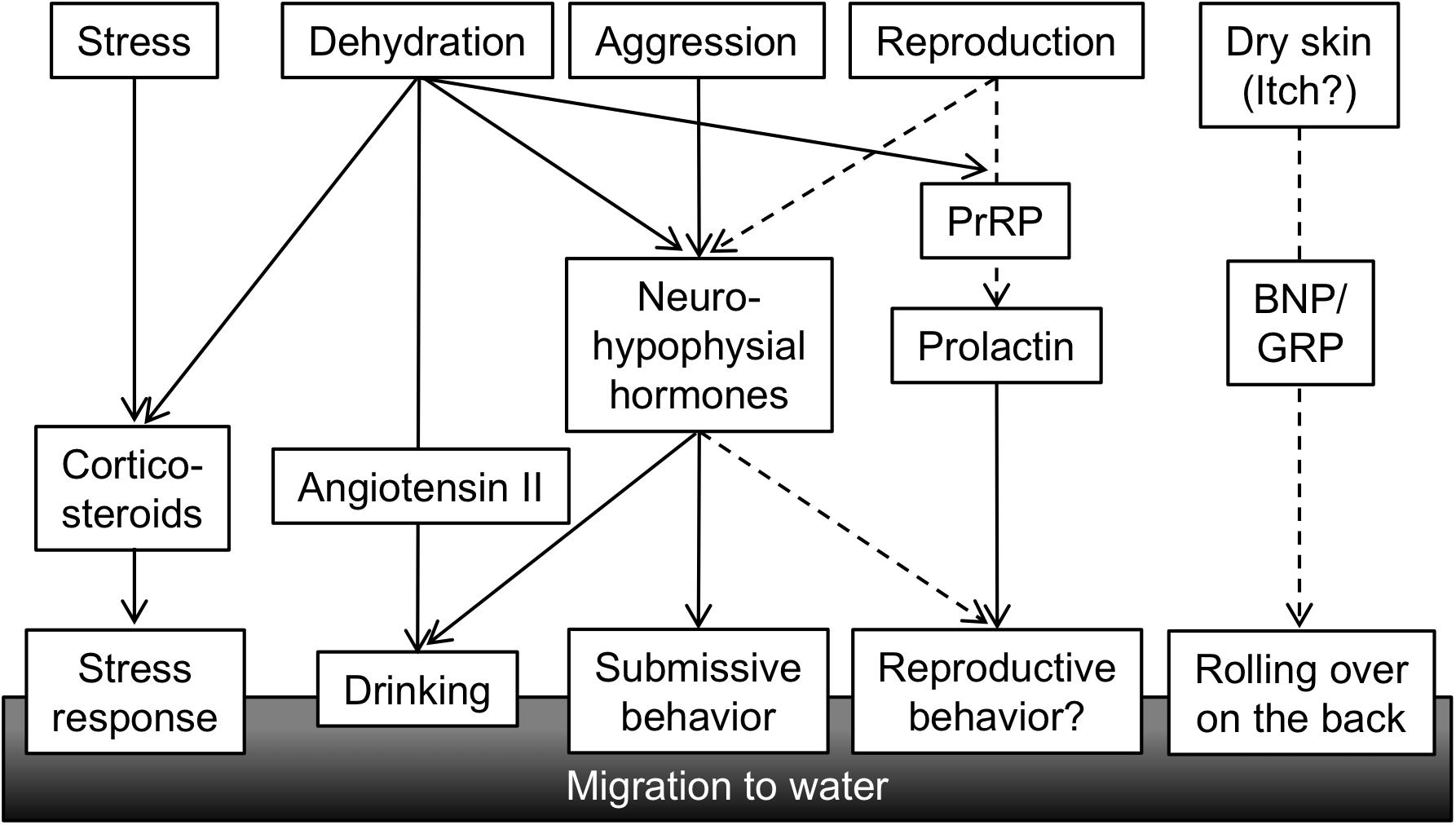
FIGURE 5. A conceptual diagram of hormones regulating amphibious behavior of mudskippers. Various stimuli related to the amphibious lifestyle of mudskipper activate secretion of hormones, which in turn leads to behavioral and physiological outputs. Solid lines showing functional associations are based on published data, while broken lines are speculation. PrRP, prolactin-releasing peptide; BNP, B-type natriuretic peptide; GRP, gastrin-releasing peptide.
It remains to be discovered how other behaviors regulated by osmoregulatory hormones have evolved during vertebrate terrestrialization, and these research gaps will need to be addressed in future study (Figure 5). As described above in regard to IT function, mudskippers spawn in their mudflat burrows that are filled with water, and they secure embryonic development of their young within the hypoxic burrows by transporting mouthfuls of air (Ishimatsu et al., 2007). These unique reproductive behaviors including both migration and parental care might be regulated by osmoregulatory hormones such as IT and prolactin (PRL). PRL plays a critical role in freshwater adaptation in teleost fishes (Manzon, 2002; Sakamoto and McCormick, 2006). PRL reduces ion and water permeability of osmoregulatory surfaces in fresh water, and increases ion uptake (Hirano, 1986; Manzon, 2002; Sakamoto et al., 2005b; Shu et al., 2016). Further, many of the reproductive functions of PRL appear to be conserved throughout the vertebrates (Whittington and Wilson, 2013). Migration plays an important role in the reproductive cycle of many vertebrates. For example, PRL injection induced migration from land to water in salamanders (Moriya, 1982). In the amphibious behavior of mudskippers, the PRL-releasing peptide/PRL axis induced a preference for aquatic habitation (Sakamoto et al., 2005b). This action resembles the migration to water of salamanders for spawning. PRL transcription and secretion were promoted by PRL-releasing peptide (Sakamoto et al., 2003; Fujimoto et al., 2006). In mudskippers, mRNA levels of PRL-releasing peptide and of PRL are similarly regulated. The mRNA levels in the brain-pituitary axis increased during both terrestrial and freshwater acclimation (Sakamoto et al., 2005a), although the dynamics of PRL mRNA during spawning has not been examined in mudskippers. During the chum salmon maturation process, PRL mRNA levels increased with the onset of anadromy (Onuma et al., 2010), whereas PRL may have no role in the reproductive migration of catadromous eels (Sudo et al., 2013). Thus, the role of PRL in reproductive migration appears to depend on environmental conditions where the teleosts live, and may have become important in the amphibious lifestyles of mudskippers. In teleosts, like in mammals, PRL also regulates reproductive development and brood care behavior as well as migration (Whittington and Wilson, 2013). Mudskippers showing such various reproductive traits can be fascinating models to explore hormonal function in behaviors related to both osmoregulation and reproduction.
Furthermore, in mudskippers, aquatic preference and rolling behavior on wet land are notable for moistening the dorsal skin (Ip et al., 1991). Since natriuretic peptides and gastrin-releasing peptide are currently known as key molecules to transmit itch sensation to the central nervous system in rodents (Sun and Chen, 2007; Mishra and Hoon, 2013; Liu et al., 2014), further analyses of these peptides may elucidate relationships between habitats and itch sensation by dry skin, as well as the unknown evolution of the itch sensation in vertebrates. As mentioned already, the “cross-talk” among these hormones in mudskippers may explain the coordination of amphibious behavior and other physiological regulation throughout vertebrate species.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported in part by JSPS KAKENHI Grant No. JP 16J01114.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Susumu Hyodo of the University of Tokyo and Dr. Christopher A. Loretz of the State University of New York at Buffalo for critical reading of this manuscript. We also thank Dr. T. Mukuda of Tottori University, Dr. M. Kusakabe of the Shizuoka University, Dr. N. Kagawa of Kindai University, and Drs. H. Sakamoto, N. Tsutsui, Y. Kobayashi, and H. Takahashi of Okayama University for help with this research on the mudskipper.
Abbreviations
11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; Ang II, angiotensin II; AP, area postrema; AT1, angiotensin type 1 receptor; AT2, angiotensin type 2 receptor; BNP, B-type natriuretic peptide; CVOs, circumventricular organs; DOC, 11-deoxycorticosterone; GR, glucocorticoid receptor; GRP, gastrin-releasing peptide; IT, isotocin; ITR, isotocin receptor; MR, mineralocorticoid receptor; NKA, Na+-K+-ATPase; NTS, nucleus tractus solitarius; OVLT, organum vasculosum of the lamina terminalis; OXT, oxytocin; PM, magnocellular preoptic nucleus; PP, parvocellular preoptic nucleus; ppt, parts per thousand; PRL, prolactin; PrRP, prolactin-releasing peptide; PVN, paraventricular nuclei of the hypothalamus; SFO, subfornical organ; SON, supraoptic nuclei of the hypothalamus; UES, upper esophageal sphincter; VP, vasopressin; VT, vasotocin; V1a, vasopressin/vasotocin type 1a receptor; V2, vasopressin/vasotocin type 2 receptor.
References
Alhadeff, A. L., and Betley, J. N. (2017). Pass the salt: the central control of sodium intake. Nat. Neurosci. 20, 130–131. doi: 10.1038/nn.4485
Anderson, W. G., Takei, Y., and Hazon, N. (2001). The dipsogenic effect of the renin–angiotensin system in elasmobranch fish. Gen. Comp. Endocrinol. 124, 300–307. doi: 10.1006/gcen.2001.7712
Ando, M., Fujii, Y., Kadota, T., Kozaka, T., Mukuda, T., Takase, I., et al. (2000). Some factors affecting drinking behavior and their interactions in seawater-acclimated eels. Anguilla japonica. Zool. Sci. 17, 171–178. doi: 10.2108/zsj.17.171
Arletti, R., Benelli, A., and Bertolini, A. (1990). Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830. doi: 10.1016/0031-9384(90)90234-U
Arterbery, A. S., Deitcher, D. L., and Bass, A. H. (2010). Corticosteroid receptor expression in a teleost fish that displays alternative male reproductive tactics. Gen. Comp. Endocrinol. 165, 83–90. doi: 10.1016/j.ygcen.2009.06.004
Aruna, A., Nagarajan, G., and Chang, C. F. (2012). Involvement of corticotrophin-releasing hormone and corticosteroid receptors in the brain–pituitary–gill of tilapia during the course of seawater acclimation. J. Neuroendocrinol. 24, 818–830. doi: 10.1111/j.1365-2826.2012.02282.x
Balment, R., Brimble, M., and Forsling, M. (1980). Release of oxytocin induced by salt loading and its influence on renal excretion in the male rat. J. Physiol. 308, 439–449. doi: 10.1113/jphysiol.1980.sp013481
Balment, R. J., and Carrick, S. (1985). Endogenous renin-angiotensin system and drinking behavior in flounder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 248, R157–R160. doi: 10.1152/ajpregu.1985.248.2.R157
Balment, R. J., Lu, W., Weybourne, E., and Warne, J. M. (2006). Arginine vasotocin a key hormone in fish physiology and behaviour: a review with insights from mammalian models. Gen. Comp. Endocrinol. 147, 9–16. doi: 10.1016/j.ygcen.2005.12.022
Bentley, P. J. (2002). Endocrines and Osmoregulation: A Comparative Account in Vertebrates. Heidelberg: Springer. doi: 10.1007/978-3-662-05014-9
Berridge, K. C. (2004). Motivation concepts in behavioral neuroscience. Physiol. Behav. 81, 179–209. doi: 10.1016/j.physbeh.2004.02.004
Blevins, J. E., Eakin, T. J., Murphy, J. A., Schwartz, M. W., and Baskin, D. G. (2003). Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 993, 30–41. doi: 10.1016/j.brainres.2003.08.036
Bond, H., Winter, M., Warne, J., McCrohan, C., and Balment, R. (2002). Plasma concentrations of arginine vasotocin and urotensin II are reduced following transfer of the euryhaline flounder (Platichthys flesus) from seawater to fresh water. Gen. Comp. Endocrinol. 125, 113–120. doi: 10.1006/gcen.2001.7736
Bourque, C. W. (2008). Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 9, 519–531. doi: 10.1038/nrn2400
Brinkmann, H., Venkatesh, B., Brenner, S., and Meyer, A. (2004). Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc. Natl. Acad. Sci. U.S.A. 101, 4900–4905. doi: 10.1073/pnas.0400609101
Bury, N., Sturm, A., Le Rouzic, P., Lethimonier, C., Ducouret, B., Guiguen, Y., et al. (2003). Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 31, 141–156. doi: 10.1677/jme.0.0310141
Clements, S., and Schreck, C. B. (2004). Central administration of corticotropin-releasing hormone alters downstream movement in an artificial stream in juvenile chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 137, 1–8. doi: 10.1016/j.ygcen.2004.02.004
Coirini, H., Marusic, E. T., De Nicola, A. F., Rainbow, T. C., and McEwen, B. S. (1983). Identification of mineralocorticoid binding sites in rat brain by competition studies and density gradient centrifugation. Neuroendocrinology 37, 354–360. doi: 10.1159/000123575
Conrad, K. P., Gellai, M., North, W. G., and Valtin, H. (1993). Influence of oxytocin on renal hemodynamics and sodium excretion. Ann. N. Y. Acad. Sci. 689, 346–362. doi: 10.1111/j.1749-6632.1993.tb55559.x
Cutler, C., Phillips, C., Hazon, N., and Cramb, G. (2007). Cortisol regulates eel (Anguilla anguilla) aquaporin 3 (AQP3) mRNA expression levels in gill. Gen. Comp. Endocrinol. 152, 310–313. doi: 10.1016/j.ygcen.2007.01.031
Deane, E. E., Kelly, S. P., and Woo, N. Y. (2000). Hypercortisolemia does not affect the branchial osmoregulatory responses of the marine teleost Sparus sarba. Life Sci. 66, 1435–1444. doi: 10.1016/S0024-3205(00)00454-9
Denton, D., Shade, R., Zamarippa, F., Egan, G., Blair-West, J., McKinley, M., et al. (1999). Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc. Natl. Acad. Sci. U.S.A. 96, 5304–5309. doi: 10.1073/pnas.96.9.5304
Doan, T. N., Gletsu, N., Cole, J., and Bernstein, K. E. (2001). Genetic manipulation of the renin-angiotensin system. Curr. Opin. Nephrol. Hypertens. 10, 483–491. doi: 10.1097/00041552-200107000-00002
Duvernoy, H. M., and Risold, P. Y. (2007). The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res. Rev. 56, 119–147. doi: 10.1016/j.brainresrev.2007.06.002
Epstein, A. N. (1982). Mineralocorticoids and cerebral angiotensin may act together to produce sodium appetite. Peptides 3, 493–494. doi: 10.1016/0196-9781(82)90113-9
Evans, D. H. (2008). Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R704–R713. doi: 10.1152/ajpregu.90337.2008
Ferguson, A. V. (2009). Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89, 370–376. doi: 10.1159/000211202
Fitzsimons, J. T. (1979). The Physiology of Thirst and Sodium Appetite. Cambridge: Cambridge Univ. Press, 1–31.
Fitzsimons, J. T. (1998). Angiotensin, thirst, and sodium appetite. Physiol. Rev. 78, 583–686. doi: 10.1152/physrev.1998.78.3.583
Fitzsimons, T., and Le Magnen, J. (1969). Eating as a regulatory control of drinking in the rat. J. Comp. Physiol. Psychol. 67, 273–283. doi: 10.1037/h0026772
Flores, A.-M., Shrimpton, J., Patterson, D., Hills, J., Cooke, S., Yada, T., et al. (2012). Physiological and molecular endocrine changes in maturing wild sockeye salmon, Oncorhynchus nerka, during ocean and river migration. J. Comp. Physiol. B 182, 77–90. doi: 10.1007/s00360-011-0600-4
Fluharty, S. J., and Epstein, A. N. (1983). Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. synergistic interaction with systemic mineralocorticoids. Behav. Neurosci. 97, 746–758. doi: 10.1037/0735-7044.97.5.746
Fuentes, J., and Eddy, F. (2012). “Drinking in marine, euryhaline and freshwater,” in Ionic Regulation in Animals: A Tribute to Professor WTW Potts, eds W. T. W. Potts, N. Hazon, and B. Eddy (Berlin: Springer), 135–149.
Fujimoto, M., Sakamoto, T., Kanetoh, T., Osaka, M., and Moriyama, S. (2006). Prolactin-releasing peptide is essential to maintain the prolactin level and osmotic balance in freshwater teleost fish. Peptides 27, 1104–1109. doi: 10.1016/j.peptides.2005.06.034
Fujiwara, T. M., and Bichet, D. G. (2005). Molecular biology of hereditary diabetes insipidus. J. Am. Soc. Nephrol. 16, 2836–2846. doi: 10.1681/ASN.2005040371
Funder, J. W., Pearce, P. T., Smith, R., and Smith, A. I. (1988). Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242, 583–585. doi: 10.1126/science.2845584
Geerling, J. C., and Loewy, A. D. (2009). Aldosterone in the brain. Am. J. Physiol. Renal Physiol. 297, F559–F576. doi: 10.1152/ajprenal.90399.2008
Gehlert, D. R., Gackenheimer, S. L., and Schober, D. A. (1991). Autoradiographic localization of subtypes of angiotensin II antagonist binding in the rat brain. Neuroscience 44, 501–514. doi: 10.1016/0306-4522(91)90073-W
Gilchriest, B., Tipping, D., Hake, L., Levy, A., and Baker, B. (2000). The effects of acute and chronic stresses on vasotocin gene transcripts in the brain of the rainbow trout (Oncorhynchus mykiss). J. Neuroendocrinol. 12, 795–801. doi: 10.1046/j.1365-2826.2000.00522.x
Gizowski, C., and Bourque, C. W. (2018). The neural basis of homeostatic and anticipatory thirst. Nat. Rev. Nephrol. 14, 11–25. doi: 10.1038/nrneph.2017.149
Gizowski, C., Zaelzer, C., and Bourque, C. W. (2016). Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature 537, 685–688. doi: 10.1038/nature19756
Godwin, J., and Thompson, R. (2012). Nonapeptides and social behavior in fishes. Horm. Behav. 61, 230–238. doi: 10.1016/j.yhbeh.2011.12.016
Gonçalves, D. M., and Oliveira, R. F. (2011). “Hormones and sexual behavior of teleost fishes,” in Hormones and Reproduction of Vertebrates: Fishes, eds D. Norris and K. H. Lopez (New York, NY: Elsevier), 119–147.
Goodfriend, T. L., Elliott, M. E., and Catt, K. J. (1996). Angiotensin receptors and their antagonists. N. Engl. J. Med. 334, 1649–1655. doi: 10.1056/NEJM199606203342507
Goodson, J. L. (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. doi: 10.1016/j.yhbeh.2005.02.003
Graham, J. B. (1997). Air-Breathing Fishes: Evolution, Diversity, and Adaptation. Cambridge, MA: Academic Press.
Greenwood, A. K., Butler, P. C., White, R. B., DeMarco, U., Pearce, D., and Fernald, R. D. (2003). Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144, 4226–4236. doi: 10.1210/en.2003-0566
Greenwood, A. K., Wark, A. R., Fernald, R. D., and Hofmann, H. A. (2008). Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc. Biol. Sci. 275, 2393–2402. doi: 10.1098/rspb.2008.0622
Grosell, M. (2011). Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiol. 202, 421–434. doi: 10.1111/j.1748-1716.2010.02241.x
Guibbolini, M., and Avella, M. (2003). Neurohypophysial hormone regulation of Cl-secretion: physiological evidence for V1-type receptors in sea bass gill respiratory cells in culture. J. Endocrinol. 176, 111–119. doi: 10.1677/joe.0.1760111
Hamasaki, S., Mukuda, T., Kaidoh, T., Yoshida, M., and Uematsu, K. (2016). Impact of dehydration on the forebrain preoptic recess walls in the mudskipper, Periophthalmus modestus: a possible locus for the center of thirst. J. Comp. Physiol. B 186, 891–905. doi: 10.1007/s00360-016-1005-1
Hausmann, H., Meyerhof, W., Zwiers, H., Lederis, K., and Richter, D. (1995). Teleost isotocin receptor: structure, functional expression, mRNA distribution and phylogeny. FEBS Lett. 370, 227–230. doi: 10.1016/0014-5793(95)00832-T
Hirano, T. (1974). Some factors regulating water intake by the eel, Anguilla japonica. J. Exp. Biol. 61, 737–747.
Hirano, T., Satou, M., and Utida, S. (1972). Central nervous system control of osmoregulation in the eel (Anguilla japonica). Comp. Biochem. Physiol. A Physiol. 43, 537–544. doi: 10.1016/0300-9629(72)90241-1
Hoenderop, J. G., Nilius, B., and Bindels, R. J. (2005). Calcium absorption across epithelia. Physiol. Rev. 85, 373–422. doi: 10.1152/physrev.00003.2004
Hoff, K. V. S., and Hillyard, S. D. (1991). Angiotensin II stimulates cutaneous drinking in the toad Bufo punctatus. Physiol. Zool. 64, 1165–1172. doi: 10.1086/physzool.64.5.30156238
Holmqvist, B. I., and Ekstrom, P. (1995). Hypophysiotrophic systems in the brain of the atlantic salmon. neuronal innervation of the pituitary and the origin of pituitary dopamine and nonapeptides identified by means of combined carbocyanine tract tracing and immunocytochemistry. J. Chem. Neuroanat. 8, 125–145. doi: 10.1016/0891-0618(94)00041-Q
Huffman, L. S., Hinz, F. I., Wojcik, S., Aubin-Horth, N., and Hofmann, H. A. (2015). Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni. Gen. Comp. Endocrinol. 212, 106–113. doi: 10.1016/j.ygcen.2014.03.004
Huffman, L. S., O’Connell, L. A., Kenkel, C. D., Kline, R. J., Khan, I. A., and Hofmann, H. A. (2012). Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J. Chem. Neuroanat. 44, 86–97. doi: 10.1016/j.jchemneu.2012.05.002
Hwang, P. P., Lee, T. H., and Lin, L. Y. (2011). Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R28–R47. doi: 10.1152/ajpregu.00047.2011
Hyodo, S., and Urano, A. (1991). Changes in expression of provasotocin and proisotocin genes during adaptation to hyper- and hypo-osmotic environments in rainbow trout. J. Comp. Physiol. B 161, 549–556. doi: 10.1007/BF00260744
Ip, Y. K., Chew, S. F., and Tang, P. C. (1991). Evaporation and the turning behavior of the mudskipper, Boleophthalmus boddaerti. Zoolog. Sci. 8, 621–623.
Ishimatsu, A., Aguilar, N. M., Ogawa, K., Hishida, Y., Takeda, T., Oikawa, S., et al. (1999). Arterial blood gas levels and cardiovascular function during varying environmental conditions in a mudskipper, Periophthalmodon schlosseri. J. Exp. Biol. 202, 1753–1762.
Ishimatsu, A., Yoshida, Y., Itoki, N., Takeda, T., Lee, H. J., and Graham, J. B. (2007). Mudskippers brood their eggs in air but submerge them for hatching. J. Exp. Biol. 210, 3946–3954. doi: 10.1242/jeb.010686
Ito, S., Mukuda, T., and Ando, M. (2006). Catecholamines inhibit neuronal activity in the glossopharyngeal–vagal motor complex of the Japanese eel: significance for controlling swallowing water. J. Exp. Zool. A Ecol. Gen. Physiol. 305, 499–506. doi: 10.1002/jez.a.282
Johnson, A. K., and Buggy, J. (1978). Periventricular preoptic-hypothalamus is vital for thirst and normal water economy. Am. J. Physiol. 234, R122–R129. doi: 10.1152/ajpregu.1978.234.3.R122
Johnson, A. K., and Thunhorst, R. L. (1997). The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front. Neuroendocrinol. 18, 292–353. doi: 10.1006/frne.1997.0153
Jørgensen, C. B. (1997). 200 years of amphibian water economy: from Robert Townson to the present. Biol. Rev. Camb. Philos. Soc. 72, 153–237. doi: 10.1017/S0006323196004963
Kagawa, N. (2013). Social rank-dependent expression of arginine vasotocin in distinct preoptic regions in male Oryzias latipes. J. Fish Biol. 82, 354–363. doi: 10.1111/j.1095-8649.2012.03490.x
Kagawa, N., Nishiyama, Y., Kato, K., Takahashi, H., Kobayashi, Y., Sakamoto, H., et al. (2013). Potential roles of arginine-vasotocin in the regulation of aggressive behavior in the mudskipper (Periophthalmus modestus). Gen. Comp. Endocrinol. 194, 257–263. doi: 10.1016/j.ygcen.2013.09.023
Kalamarz-Kubiak, H., Kleszczyńska, A., and Kulczykowska, E. (2015). Cortisol stimulates arginine vasotocin and isotocin release from the hypothalamo-pituitary complex of round goby (Neogobius melanostomus): probable mechanisms of action. J. Exp. Zool. A Ecol. Gen. Physiol. 323, 616–626. doi: 10.1002/jez.1952
Katayama, Y., Sakamoto, T., Saito, K., Tsuchimochi, H., Kaiya, H., Watanabe, T., et al. (2018). Drinking by amphibious fish: convergent evolution of thirst mechanisms during vertebrate terrestrialization. Sci. Rep. 8:625. doi: 10.1038/s41598-017-18611-4
Kawata, M., Nishi, M., Matsuda, K., Sakamoto, H., Kaku, N., Masugi-Tokita, M., et al. (2008). Steroid receptor signalling in the brain–lessons learned from molecular imaging. J. Neuroendocrinol. 20, 673–676. doi: 10.1111/j.1365-2826.2008.01727.x
Kikuchi, Y., Hosono, K., Yamashita, J., Kawabata, Y., and Okubo, K. (2015). Glucocorticoid receptor exhibits sexually dimorphic expression in the medaka brain. Gen. Comp. Endocrinol. 223, 47–53. doi: 10.1016/j.ygcen.2015.09.031
Kline, R. J., O’Connell, L. A., Hofmann, H. A., Holt, G. J., and Khan, I. A. (2011). The distribution of an AVT V1a receptor in the brain of a sex changing fish, Epinephelus adscensionis. J. Chem. Neuroanat. 42, 72–88. doi: 10.1016/j.jchemneu.2011.06.005
Kobayashi, H., and Takei, Y. (1996). The Renin-Angiotensin System: Comparative Aspects. Berlin: Springer Science & Business Media. doi: 10.1007/978-3-642-61164-3
Kobayashi, H., Uemura, H., Takei, Y., Itatsu, N., Ozawa, M., and Ichinohe, K. (1983). Drinking induced by angiotensin II in fishes. Gen. Comp. Endocrinol. 49, 295–306. doi: 10.1016/0016-6480(83)90147-8
Kobayashi, H., Uemura, H., Wada, M., and Takei, Y. (1979). Ecological adaptation of angiotensin-induced thirst mechanism in tetrapods. Gen. Comp. Endocrinol. 38, 93–104. doi: 10.1016/0016-6480(79)90093-5
Konno, N., Hyodo, S., Yamaguchi, Y., Matsuda, K., and Uchiyama, M. (2010a). Vasotocin/V2-type receptor/aquaporin axis exists in African lungfish kidney but is functional only in terrestrial condition. Endocrinology 151, 1089–1096. doi: 10.1210/en.2009-1070en.2009-1070
Konno, N., Kurosawa, M., Kaiya, H., Miyazato, M., Matsuda, K., and Uchiyama, M. (2010b). Molecular cloning and characterization of V2-type receptor in two ray-finned fish, gray bichir, Polypterus senegalus and medaka, Oryzias latipes. Peptides 31, 1273–1279. doi: 10.1016/j.peptides.2010.04.014
Kozaka, T., Fujii, Y., and Ando, M. (2003). Central effects of various ligands on drinking behavior in eels acclimated to seawater. J. Exp. Biol. 206, 687–692. doi: 10.1242/jeb.00146
Krashes, M. J. (2016). Physiology: forecast for water balance. Nature 537, 626–627. doi: 10.1038/537626a
Kurita, Y., Nakada, T., Kato, A., Doi, H., Mistry, A. C., Chang, M. H., et al. (2008). Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1402–R1412. doi: 10.1152/ajpregu.00759.2007
Larson, E. T., O’Malley, D. M., and Melloni, R. H. Jr. (2006). Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav. Brain Res. 167, 94–102. doi: 10.1016/j.bbr.2005.08.020
Lee, C. G. L., and Ip, Y. K. (1987). Environmental effect on plasma thyroxine (T4), 3, 5, 3′-triido-L-thyronine (T3), prolactin and cyclic adenosine 3′, 5′-monophosphate (cAMP) content in the mudskippers Periophthalmus chrysospilos and Boleophthalmus boddaerti. Comp. Biochem. Physiol. A Physiol. 87, 1009–1014. doi: 10.1016/0300-9629(87)90028-4
Lema, S. C. (2010). Identification of multiple vasotocin receptor cDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol. Cell. Endocrinol. 321, 215–230. doi: 10.1016/j.mce.2010.02.015
Lema, S. C., Sanders, K. E., and Walti, K. A. (2015). Arginine vasotocin, isotocin and nonapeptide receptor gene expression link to social status and aggression in sex-dependent patterns. J. Neuroendocrinol. 27, 142–157. doi: 10.1111/jne.12239
Leow, M. K. S. (2015). Environmental origins of hypertension: phylogeny, ontogeny and epigenetics. Hypertens. Res. 38, 299–307. doi: 10.1038/hr.2015.7
Lindeyer, C. M., Langen, E. M., Swaney, W. T., and Reader, S. M. (2015). Nonapeptide influences on social behaviour: effects of vasotocin and isotocin on shoaling and interaction in zebrafish. Behaviour 152, 897–915. doi: 10.1163/1568539X-00003261
Liu, X. Y., Wan, L., Huo, F.-Q., Barry, D. M., Li, H., Zhao, Z.-Q., et al. (2014). B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol. Pain 10:4. doi: 10.1186/1744-8069-10-4
Loveland, J. L., and Fernald, R. D. (2017). Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behav. Brain Res. 317, 188–203. doi: 10.1016/j.bbr.2016.09.008
MacIver, M. A., Schmitz, L., Mugan, U., Murphey, T. D., and Mobley, C. D. (2017). Massive increase in visual range preceded the origin of terrestrial vertebrates. Proc. Natl. Acad. Sci. U.S.A. 114, E2375–E2384. doi: 10.1073/pnas.1615563114
Maejima, S., Konno, N., Matsuda, K., and Uchiyama, M. (2010). Central angiotensin II stimulates cutaneous water intake behavior via an angiotensin II type-1 receptor pathway in the Japanese tree frog Hyla japonica. Horm. Behav. 58, 457–464. doi: 10.1016/j.yhbeh.2010.05.007
Mahlmann, S., Meyerhof, W., Hausmann, H., Heierhorst, J., Schonrock, C., Zwiers, H., et al. (1994). Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc. Natl. Acad. Sci. U.S.A. 91, 1342–1345. doi: 10.1073/pnas.91.4.1342
Mandelblat-Cerf, Y., Kim, A., Burgess, C. R., Subramanian, S., Tannous, B. A., Lowell, B. B., et al. (2017). Bidirectional anticipation of future osmotic challenges by vasopressin neurons. Neuron 93, 57–65. doi: 10.1016/j.neuron.2016.11.021
Manzon, L. A. (2002). The role of prolactin in fish osmoregulation: a review. Gen. Comp. Endocrinol. 125, 291–310. doi: 10.1006/gcen.2001.7746
Martos-Sitcha, J. A., Campinho, M. A., Mancera, J. M., Martinez-Rodriguez, G., and Fuentes, J. (2015a). Vasotocin and isotocin regulate aquaporin 1 function in the sea bream. J. Exp. Biol. 218, 684–693. doi: 10.1242/jeb.114546
Martos-Sitcha, J. A., MartinezRodriguez, G., Mancera, J. M., and Fuentes, J. (2015b). AVT and IT regulate ion transport across the opercular epithelium of killifish (Fundulus heteroclitus) and gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 182, 93–101. doi: 10.1016/j.cbpa.2014.12.027
Martos-Sitcha, J. A., Fuentes, J., Mancera, J. M., and Martinez-Rodriguez, G. (2014). Variations in the expression of vasotocin and isotocin receptor genes in the gilthead sea bream Sparus aurata during different osmotic challenges. Gen. Comp. Endocrinol. 197, 5–17. doi: 10.1016/j.ygcen.2013.11.026
Mayer-Gostan, N., and Hirano, T. (1976). The effects of transecting the IXth and Xth cranial nerves on hydromineral balance in the eel Anguilla anguilla. J. Exp. Biol. 64, 461–475.
McCormick, S. D. (2001). Endocrine control of osmoregulation in teleost fish. Am. Zool. 41, 781–794. doi: 10.1093/icb/41.4.781
McCormick, S. D., and Bradshaw, D. (2006). Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 147, 3–8. doi: 10.1016/j.ygcen.2005.12.009
McKinley, M., Cairns, M., Denton, D., Egan, G., Mathai, M., Uschakov, A., et al. (2004). Physiological and pathophysiological influences on thirst. Physiol. Behav. 81, 795–803. doi: 10.1016/j.physbeh.2004.04.055
McKinley, M. J. (2003). The Sensory Circumventricular Organs of the Mammalian Brain: Subfornical Organ, OVLT and Area Postrema. Berlin: Springer Science & Business Media. doi: 10.1007/978-3-642-55532-9
McKinley, M. J., and Johnson, A. K. (2004). The physiological regulation of thirst and fluid intake. News Physiol. Sci. 19, 1–6. doi: 10.1152/nips.01470.2003
Mével, L., Lancien, F., Mimassi, N., and Conlon, J. M. (2012). Brain neuropeptides in central ventilatory and cardiovascular regulation in trout. Front. Endocrinol. 3:124. doi: 10.3389/fendo.2012.00124
Michel, K. B., Heiss, E., Aerts, P., and Van Wassenbergh, S. (2015). A fish that uses its hydrodynamic tongue to feed on land. Proc. R. Soc. B Biol. Sci. 282:20150057. doi: 10.1098/rspb.2015.0057
Milla, S., Terrien, X., Sturm, A., Ibrahim, F., Giton, F., Fiet, J., et al. (2008). Plasma 11-deoxycorticosterone (DOC) and mineralocorticoid receptor testicular expression during rainbow trout Oncorhynchus mykiss spermiation: implication with 17alpha, 20beta-dihydroxyprogesterone on the milt fluidity? Reprod. Biol. Endocrinol. 6:19. doi: 10.1186/1477-7827-6-19
Mishra, S. K., and Hoon, M. A. (2013). The cells and circuitry for itch responses in mice. Science 340, 968–971. doi: 10.1126/science.1233765
Mommsen, T. P., Vijayan, M. M., and Moon, T. W. (1999). Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 9, 211–268. doi: 10.1023/A:1008924418720
Moriya, T. (1982). Prolactin induces increase in the specific gravity of salamander, Hynobius retardatus, that raises adaptability to water. J. Exp. Zool. 223, 83–88. doi: 10.1002/jez.1402230114
Mukuda, T., and Ando, M. (2003). Medullary motor neurones associated with drinking behaviour of Japanese eels. J. Fish Biol. 62, 1–12. doi: 10.1046/j.1095-8649.2003.00002.x
Mukuda, T., Matsunaga, Y., Kawamoto, K., Yamaguchi, K. I., and Ando, M. (2005). “Blood-contacting neurons” in the brain of the Japanese eel Anguilla japonica. J. Exp. Zool. A Comp. Exp. Biol. 303, 366–376. doi: 10.1002/jez.a.134
Myers, B., McKlveen, J. M., and Herman, J. P. (2014). Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front. Neuroendocrinol. 35:180–196. doi: 10.1016/j.yfrne.2013.12.003
Nagashima, K., and Ando, M. (1994). Characterization of esophageal desalination in the seawater eel, Anguilla japonica. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 164, 47–54. doi: 10.1007/BF00714570
Nielsen, S., Chou, C. L., Marples, D., Christensen, E. I., Kishore, B. K., and Knepper, M. A. (1995). Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 92, 1013–1017. doi: 10.1073/pnas.92.4.1013
Nishimura, H. (1978). Physiological evolution of the renin-angiotensin system. Jpn. Heart J. 19, 806–822. doi: 10.1536/ihj.19.806
Nishimura, H. (2017). Renin-angiotensin system in vertebrates: phylogenetic view of structure and function. Anat. Sci. Int. 92, 215–247. doi: 10.1007/s12565-016-0372-8
Nobata, S., and Ando, M. (2013). “Regulation of drinking,” in Eel Physiology, eds F. Trischitta, Y. Takei, and P. Sébert (Boca Raton, FL: CRC Press), 225–248. doi: 10.1201/b15365-9
Nobata, S., Ando, M., and Takei, Y. (2013). Hormonal control of drinking behavior in teleost fishes; insights from studies using eels. Gen. Comp. Endocrinol. 192, 214–221. doi: 10.1016/j.ygcen.2013.05.009
Nobata, S., and Takei, Y. (2011). The area postrema in hindbrain is a central player for regulation of drinking behavior in Japanese eels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1569–R1577. doi: 10.1152/ajpregu.00056.2011
Oatley, K., and Toates, F. (1969). The passage of food through the gut of rats and its uptake of fluid. Psychono. Sci. 16, 225–226. doi: 10.3758/BF03332656
Okawara, Y., Karakida, T., Aihara, M., Yamaguchi, K. I., and Kobayashi, H. (1987). Involvement of angiotensin II in water intake in the Japanese eel, Anguilla japonica: endocrinology. Zool. Sci. 4, 523–528.
Olivereau, M., and Olivereau, J. (1990). Effect of pharmacological adrenalectomy on corticotropin-releasing factor-like and arginine vasotocin immunoreactivities in the brain and pituitary of the eel: immunocytochemical study. Gen. Comp. Endocrinol. 80, 199–215. doi: 10.1016/0016-6480(90)90165-I
Onuma, T. A., Ban, M., Makino, K., Katsumata, H., Hu, W., Ando, H., et al. (2010). Changes in gene expression for GH/PRL/SL family hormones in the pituitaries of homing chum salmon during ocean migration through upstream migration. Gen. Comp. Endocrinol. 166, 537–548. doi: 10.1016/j.ygcen.2010.01.015
Ord, T. J., and Cooke, G. M. (2016). Repeated evolution of amphibious behavior in fish and its implications for the colonisation of novel environments. Evolution 70, 1747–1759. doi: 10.1111/evo.12971
Parmelee, J. T., and Renfro, J. L. (1983). Esophageal desalination of seawater in flounder: role of active sodium transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 245, R888–R893. doi: 10.1152/ajpregu.1983.245.6.R888
Perrone, R., and Silva, A. C. (2018). Status-dependent vasotocin modulation of dominance and subordination in the weakly electric fish Gymnotus omarorum. Front. Behav. Neurosci. 12:1. doi: 10.3389/fnbeh.2018.00001
Perrott, M., Grierson, C., Hazon, N., and Balment, R. (1992). Drinking behaviour in sea water and fresh water teleosts, the role of the renin-angiotensin system. Fish Physiol. Biochem. 10, 161–168. doi: 10.1007/BF00004527
Propper, C. R., Hillyard, S. D., and Johnson, W. E. (1995). Central angiotensin II induces thirst-related responses in an amphibian. Horm. Behav. 29, 74–84. doi: 10.1006/hbeh.1995.1006
Prunet, P., Sturm, A., and Milla, S. (2006). Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen. Comp. Endocrinol. 147, 17–23. doi: 10.1016/j.ygcen.2006.01.015
Rowe, B. P., Saylor, D. L., and Speth, R. C. (1992). Analysis of angiotensin II receptor subtypes in individual rat brain nuclei. Neuroendocrinology 55, 563–573. doi: 10.1159/000126177
Rozeboom, A. M., Akil, H., and Seasholtz, A. F. (2007). Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc. Natl. Acad. Sci. U.S.A. 104, 4688–4693. doi: 10.1073/pnas.0606067104
Russell, M. J., Klemmer, A. M., and Olson, K. R. (2001). Angiotensin signaling and receptor types in teleost fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 128, 41–51. doi: 10.1016/S1095-6433(00)00296-8
Ryan, P. J., Ross, S. I., Campos, C. A., Derkach, V. A., and Palmiter, R. D. (2017). Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 20, 1722–1733. doi: 10.1038/s41593-017-0014-z
Sakamoto, H., Takahashi, H., Matsuda, K., Nishi, M., Takanami, K., Ogoshi, M., et al. (2012). Rapid signaling of steroid hormones in the vertebrate nervous system. Front. Biosci. 17:996–1019. doi: 10.2741/3970
Sakamoto, T., Agustsson, T., Moriyama, S., Itoh, T., Takahashi, A., Kawauchi, H., et al. (2003). Intra-arterial injection of prolactin-releasing peptide elevates prolactin gene expression and plasma prolactin levels in rainbow trout. J. Comp. Physiol. B 173, 333–337. doi: 10.1007/s00360-003-0340-1
Sakamoto, T., Amano, M., Hyodo, S., Moriyama, S., Takahashi, A., Kawauchi, H., et al. (2005a). Expression of prolactin-releasing peptide and prolactin in the euryhaline mudskippers (Periophthalmus modestus): prolactin-releasing peptide as a primary regulator of prolactin. J. Mol. Endocrinol. 34, 825–834.
Sakamoto, T., Oda, A., Narita, K., Takahashi, H., Oda, T., Fujiwara, J., et al. (2005b). Prolactin: fishy tales of its primary regulator and function. Ann. N. Y. Acad. Sci. 1040, 184–188.
Sakamoto, T., and Ando, M. (2002). Calcium ion triggers rapid morphological oscillation of chloride cells in the mudskipper, Periophthalmus modestus. J. Comp. Physiol. B 172, 435–439. doi: 10.1007/s00360-002-0272-1
Sakamoto, T., Hyodo, S., and Takagi, W. (2018). A possible principal function of corticosteroid signaling that is conserved in vertebrate evolution: lessons from receptor-knockout small fish. J. Steroid Biochem. Mol. Biol. doi: 10.1016/j.jsbmb.2018.02.011 [Epub ahead of print].
Sakamoto, T., and McCormick, S. D. (2006). Prolactin and growth hormone in fish osmoregulation. Gen. Comp. Endocrinol. 147, 24–30. doi: 10.1016/j.ygcen.2005.10.008
Sakamoto, T., Mori, C., Minami, S., Takahashi, H., Abe, T., Ojima, D., et al. (2011). Corticosteroids stimulate the amphibious behavior in mudskipper: potential role of mineralocorticoid receptors in teleost fish. Physiol. Behav. 104, 923–928. doi: 10.1016/j.physbeh.2011.06.002
Sakamoto, T., Nishiyama, Y., Ikeda, A., Takahashi, H., Hyodo, S., Kagawa, N., et al. (2015). Neurohypophysial hormones regulate amphibious behaviour in the mudskipper goby. PLoS One 10:e0134605. doi: 10.1371/journal.pone.0134605
Sakamoto, T., Yasunaga, H., Yokota, S., and Ando, M. (2002). Differential display of skin mRNAs regulated under varying environmental conditions in a mudskipper. J. Comp. Physiol. B 172, 447–453. doi: 10.1007/s00360-002-0274-z
Sakamoto, T., Yokota, S., and Ando, M. (2000). Rapid morphological oscillation of mitochondrion-rich cell in estuarine mudskipper following salinity changes. J. Exp. Zool. 286, 666–669. doi: 10.1002/(SICI)1097-010X(20000501)286:6<666::AID-JEZ14>3.0.CO;2-G
Sakamoto, T., Yoshiki, M., Takahashi, H., Yoshida, M., Ogino, Y., Ikeuchi, T., et al. (2016). Principal function of mineralocorticoid signaling suggested by constitutive knockout of the mineralocorticoid receptor in medaka fish. Sci. Rep. 6:37991. doi: 10.1038/srep37991
Sands, J. M., Blount, M. A., and Klein, J. D. (2011). Regulation of renal urea transport by vasopressin. Trans. Am. Clin. Climatol. Assoc. 122, 82–92.
Sayer, M. D. J. (2005). Adaptations of amphibious fish for surviving life out of water. Fish Fish. 6, 186–211. doi: 10.1111/j.1467-2979.2005.00193.x
Seo, M. Y., Kuroki, M., Okamura, A., Tsukamoto, K., Watanabe, S., and Kaneko, T. (2015). Occurrence of larval and adult types of ion-secreting ionocytes in Japanese eel Anguilla japonica. Ichthyol. Res. 62, 487–494. doi: 10.1007/s10228-015-0463-x
Shaw, J. R., Gabor, K., Hand, E., Lankowski, A., Durant, L., Thibodeau, R., et al. (2007). Role of glucocorticoid receptor in acclimation of killifish (Fundulus heteroclitus) to seawater and effects of arsenic. Am. J. Physio. Regul. Integr. Comp. Physiol. 292, R1052–R1060. doi: 10.1152/ajpregu.00328.2006
Shu, Y., Lou, Q., Dai, Z., Dai, X., He, J., Hu, W., et al. (2016). The basal function of teleost prolactin as a key regulator on ion uptake identified with zebrafish knockout models. Sci. Rep. 6:18597. doi: 10.1038/srep18597
Simpson, J. B., and Routtenberg, A. (1973). Subfornical organ: site of drinking elicitation by angiotensin II. Science 181, 1172–1175. doi: 10.1126/science.181.4105.1172
Song, K., Allen, A. M., Paxinos, G., and Mendelsohn, F. A. (1992). Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J. Comp. Neurol. 316, 467–484. doi: 10.1002/cne.903160407
Stolte, E. H., de Mazon, A. F., Leon-Koosterziel, K. M., Jesiak, M., Bury, N. R., Sturm, A., et al. (2008). Corticosteroid receptors involved in stress regulation in common carp, Cyprinus carpio. J. Endocrinol. 198, 403–417. doi: 10.1677/JOE-08-0100
Stricker, E. M., and Stricker, M. L. (2011). Pre-systemic controls of fluid intake and vasopressin secretion. Physiol. Behav. 103, 86–88. doi: 10.1016/j.physbeh.2010.11.019
Sturm, A., Bury, N., Dengreville, L., Fagart, J., Flouriot, G., Rafestin-Oblin, M., et al. (2005). 11-deoxycorticosterone is a potent agonist of the rainbow trout (Oncorhynchus mykiss) mineralocorticoid receptor. Endocrinology 146, 47–55. doi: 10.1210/en.2004-0128
Sudo, R., Suetake, H., Suzuki, Y., Aoyama, J., and Tsukamoto, K. (2013). Profiles of mRNA expression for prolactin, growth hormone, and somatolactin in Japanese eels, Anguilla japonica: the effect of salinity, silvering and seasonal change. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164, 10–16. doi: 10.1016/j.cbpa.2012.09.019
Sun, Y. G., and Chen, Z. F. (2007). A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703. doi: 10.1038/nature06029
Takahashi, H., and Sakamoto, T. (2013). The role of ‘mineralocorticoids’ in teleost fish: relative importance of glucocorticoid signaling in the osmoregulation and ‘central’ actions of mineralocorticoid receptor. Gen. Comp. Endocrinol. 181, 223–228. doi: 10.1016/j.ygcen.2012.11.016
Takahashi, H., Takahashi, A., and Sakamoto, T. (2006). In vivo effects of thyroid hormone, corticosteroids and prolactin on cell proliferation and apoptosis in the anterior intestine of the euryhaline mudskipper (Periophthalmus modestus). Life Sci. 79, 1873–1880. doi: 10.1016/j.lfs.2006.06.021
Takei, Y. (2000). Comparative physiology of body fluid regulation in vertebrates with special reference to thirst regulation. Jpn. J. Physiol. 50, 171–186. doi: 10.2170/jjphysiol.50.171
Takei, Y. (2015). From aquatic to terrestrial life: evolution of the mechanisms for water acquisition. Zool. Sci. 32, 1–7. doi: 10.2108/zs140142
Takei, Y., Hirano, T., and Kobayashi, H. (1979). Angiotensin and water intake in the Japanese eel, Anguilla japonica. Gen. Comp. Endocrinol. 38, 466–475. doi: 10.1016/0016-6480(79)90155-2
Takei, Y., Hiroi, J., Takahashi, H., and Sakamoto, T. (2014). Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physio. Regul. Integr. Comp. Physiol. 307, R778–R792. doi: 10.1152/ajpregu.00104.2014
Takei, Y., and McCormick, S. D. (2012). “Hormonal control of fish euryhalinity,” in Fish physiology, eds S. D. McCormick, A. P. Farrell, and C. J. Brauner (New York, NY: Elsevier), 69–123.
Takei, Y., Wong, M. K. S., Pipil, S., Ozaki, H., Suzuki, Y., Iwasaki, W., et al. (2016). Molecular mechanisms underlying active desalination and low water permeability in the esophagus of eels acclimated to seawater. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R231–R244. doi: 10.1152/ajpregu.00465.2016
Takiyama, T., Hamasaki, S., and Yoshida, M. (2016). Comparison of the visual capabilities of an amphibious and an aquatic goby that inhabit tidal mudflats. Brain Behav. Evol. 87, 39–50. doi: 10.1159/000443923
Tamura, S. O., Morii, H., and Yuzuriha, M. (1976). Respiration of the amphibious fishes Periophthalmus cantonensis and Boleophthalmus chinensis in water and on land. J. Exp. Biol. 65, 97–107.
Teitsma, C. A., Anglade, I., Toutirais, G., Muñoz-Cueto, J. A., Saligaut, D., Ducouret, B., et al. (1998). Immunohistochemical localization of glucocorticoid receptors in the forebrain of the rainbow trout (Oncorhynchus mykiss). J. Comp. Neurol. 401, 395–410. doi: 10.1002/(SICI)1096-9861(19981123)401:3<395::AID-CNE7>3.0.CO;2-P
Thompson, R. R., and Walton, J. C. (2004). Peptide effects on social behavior: effects of vasotocin and isotocin on social approach behavior in male goldfish (Carassius auratus). Behav. Neurosci. 118, 620–626. doi: 10.1037/0735-7044.118.3.620
Tierney, M., Luke, G., Cramb, G., and Hazon, N. (1995). The role of the renin-angiotensin system in the control of blood pressure and drinking in the European eel, Anguilla anguilla. Gen. Comp. Endocrinol. 100, 39–48. doi: 10.1006/gcen.1995.1130
Timmermans, P. B., Wong, P. C., Chiu, A. T., Herblin, W. F., Benfield, P., Carini, D., et al. (1993). Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 45, 205–251.
Tsutsumi, K., and Saavedra, J. M. (1991). Quantitative autoradiography reveals different angiotensin II receptor subtypes in selected rat brain nuclei. J. Neurochem. 56, 348–351. doi: 10.1111/j.1471-4159.1991.tb02602.x
Uchida, K., Kaneko, T., Yamauchi, K., and Hirano, T. (1996). Morphometrical analysis chloride cell activity in the gill filaments and lamellae and changes in Na+, K+-ATPase activity during seawater adaptation in chum salmon fry. J. Exp. Zool. A Ecol. Genet. Physiol. 276, 193–200. doi: 10.1002/(SICI)1097-010X(19961015)276:3<193::AID-JEZ3>3.0.CO;2-I
Uchiyama, M. (2015). “Angiotensin II and water balance in amphibians,” in Sodium and Water Homeostasis, eds K. A. Hyndman and T. L. Pannabecker (Berlin: Springer), 73–90. doi: 10.1007/978-1-4939-3213-9_4
Veillette, P. A., Sundell, K., and Specker, J. L. (1995). Cortisol mediates the increase in intestinal fluid absorption in Atlantic salmon during parr-smolt transformation. Gen. Comp. Endocrinol. 97, 250–258. doi: 10.1006/gcen.1995.1024
Veillette, P. A., and Young, G. (2005). Tissue culture of sockeye salmon intestine: functional response of Na+-K+-ATPase to cortisol. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1598–R1605. doi: 10.1152/ajpregu.00741.2004
Verbalis, J. G., Mangione, M. P., and Stricker, E. M. (1991). Oxytocin produces natriuresis in rats at physiological plasma concentrations. Endocrinology 128, 1317–1322. doi: 10.1210/endo-128-3-1317
Warne, J. M. (2001). Cloning and characterization of an arginine vasotocin receptor from the euryhaline flounder Platichthys flesus. Gen. Comp. Endocrinol. 122, 312–319. doi: 10.1006/gcen.2001.7644
Watanabe, T., and Takei, Y. (2011). Molecular physiology and functional morphology of SO42– excretion by the kidney of seawater-adapted eels. J. Exp. Biol. 214, 1783–1790. doi: 10.1242/jeb.051789
Watanabe, Y., Sakihara, T., Mukuda, T., and Ando, M. (2007). Antagonistic effects of vasotocin and isotocin on the upper esophageal sphincter muscle of the eel acclimated to seawater. J. Comp. Physiol. B 177, 867–873. doi: 10.1007/s00360-007-0184-1
Watts, A. G. (2015). 60 years of neuroendocrinology: the structure of the neuroendocrine hypothalamus: the neuroanatomical legacy of Geoffrey Harris. J. Endocrinol. 226, T25–T39. doi: 10.1530/JOE-15-0157
Whittington, C. M., and Wilson, A. B. (2013). The role of prolactin in fish reproduction. Gen. Comp. Endocrinol. 191, 123–136. doi: 10.1016/j.ygcen.2013.05.027
Wilson, R. W., Wilson, J. M., and Grosell, M. (2002). Intestinal bicarbonate secretion by marine teleost fish—why and how? Biochim. Biophys. Acta 1566, 182–193. doi: 10.1016/S0005-2736(02)00600-4
Wong, M. K. S., and Takei, Y. (2013). Angiotensin AT2 receptor activates the cyclic-AMP signaling pathway in eel. Mol. Cell. Endocrinol. 365, 292–302. doi: 10.1016/j.mce.2012.11.009
Yamaguchi, Y., Kaiya, H., Konno, N., Iwata, E., Miyazato, M., Uchiyama, M., et al. (2012). The fifth neurohypophysial hormone receptor is structurally related to the V2-type receptor but functionally similar to V1-type receptors. Gen. Comp. Endocrinol. 178, 519–528. doi: 10.1016/j.ygcen.2012.07.008
Yokoi, S., Okuyama, T., Kamei, Y., Naruse, K., Taniguchi, Y., Ansai, S., et al. (2015). An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLoS genet. 11:e1005009. doi: 10.1371/journal.pgen.1005009
You, X., Bian, C., Zan, Q., Xu, X., Liu, X., Chen, J., et al. (2014). Mudskipper genomes provide insights into the terrestrial adaptation of amphibious fishes. Nat. Commun. 5:5594. doi: 10.1038/ncomms6594
Zimmerman, C. A., Leib, D. E., and Knight, Z. A. (2017). Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 18, 459–469. doi: 10.1038/nrn.2017.71
Zimmerman, C. A., Lin, Y.-C., Leib, D. E., Guo, L., Huey, E. L., Daly, G. E., et al. (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684. doi: 10.1038/nature18950
Keywords: amphibious behavior, osmoregulation, angiotensin II, neurohypophysial hormones, corticosteroids, thirst, social behavior, mudskipper
Citation: Katayama Y, Sakamoto T, Takanami K and Takei Y (2018) The Amphibious Mudskipper: A Unique Model Bridging the Gap of Central Actions of Osmoregulatory Hormones Between Terrestrial and Aquatic Vertebrates. Front. Physiol. 9:1112. doi: 10.3389/fphys.2018.01112
Received: 04 June 2018; Accepted: 25 July 2018;
Published: 14 August 2018.
Edited by:
Carlos Rosas, Universidad Nacional Autónoma de México, MexicoReviewed by:
Juan Fuentes, Centro de Ciências do Mar (CCMAR), PortugalSteffen Madsen, University of Southern Denmark, Denmark
Hon Jung Liew, Universiti Malaysia Terengganu, Malaysia
Fernando Diaz, Centro de Investigación Científica y de Educación Superior de Ensenada, Mexico
Copyright © 2018 Katayama, Sakamoto, Takanami and Takei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukitoshi Katayama, a2F0YXlhbWEzZ0Bhb3JpLnUtdG9reW8uYWMuanA=
 Yukitoshi Katayama
Yukitoshi Katayama Tatsuya Sakamoto
Tatsuya Sakamoto Keiko Takanami
Keiko Takanami Yoshio Takei
Yoshio Takei