- 1Department of Neuroscience, Psychology, Drug Sciences and Child Health (NEUROFARBA), University of Florence, Florence, Italy
- 2Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy
Hypertrophic cardiomyopathy (HCM) is the most common mendelian heart disease, with a prevalence of 1/500. HCM is a primary cause of sudden death, due to an heightened risk of ventricular tachyarrhythmias that often occur in young asymptomatic patients. HCM can slowly progress toward heart failure, either with preserved or reduced ejection fraction, due to worsening of diastolic function. Accumulation of intra-myocardial fibrosis and replacement scars underlies heart failure progression and represents a substrate for sustained arrhythmias in end-stage patients. However, arrhythmias and mechanical abnormalities may occur in hearts with little or no fibrosis, prompting toward functional pathomechanisms. By studying viable cardiomyocytes and trabeculae isolated from inter-ventricular septum samples of non-failing HCM patients with symptomatic obstruction who underwent myectomy operations, we identified that specific abnormalities of intracellular Ca2+ handling are associated with increased cellular arrhytmogenesis and diastolic dysfunction. In HCM cardiomyocytes, diastolic Ca2+ concentration is increased both in the cytosol and in the sarcoplasmic reticulum and the rate of Ca2+ transient decay is slower, while the amplitude of Ca2+-release is preserved. Ca2+ overload is the consequence of an increased Ca2+ entry via L-type Ca2+-current [due to prolongation the action potential (AP) plateau], combined with a reduced rate of Ca2+-extrusion through the Na+/Ca2+ exchanger [due to increased cytosolic (Na+)] and a lower expression of SERCA. Increased late Na+ current (INaL) plays a major role, as it causes both AP prolongation and Na+ overload. Intracellular Ca2+ overload determines an higher frequency of Ca2+ waves leading to delayed-afterdepolarizations (DADs) and premature contractions, but is also linked with the increased diastolic tension and slower relaxation of HCM myocardium. Sustained increase of intracellular [Ca2+] goes hand-in-hand with the increased activation of Ca2+/calmodulin-dependent protein-kinase-II (CaMKII) and augmented phosphorylation of its targets, including Ca2+ handling proteins. In transgenic HCM mouse models, we found that Ca2+ overload, CaMKII and increased INaL drive myocardial remodeling since the earliest stages of disease and underlie the development of hypertrophy, diastolic dysfunction and the arrhythmogenic substrate. In conclusion, diastolic dysfunction and arrhythmogenesis in human HCM myocardium are driven by functional alterations at cellular and molecular level that may be targets of innovative therapies.
Introduction: Arrhythmic Substrate in HCM, from Tissue to the Single Myocyte
Hypertrophic cardiomyopathy (HCM) is the most common monogenic inheritable heart disease (Maron et al., 2000; Gersh et al., 2011; Authors/Task Force Members et al., 2014), with a prevalence of 1:500. HCM is a leading cause of sudden cardiac death in the young (Maron et al., 2000) and a prevalent cause of heart failure and stroke in all age groups (Maron et al., 2003). Mutations in genes coding for sarcomeric proteins are found in over 60% of patients with HCM, the most commonly involved genes being MYH7 (β-myosin heavy-chain) and MYBPC3 (cardiac myosin-binding protein-C). Increased ventricular arrhythmogenesis is one of the main pathophysiological features of this disease (Olivotto et al., 2009) and is responsible for the heightened risk of lethal arrhythmias in HCM patients. Despite being only mildly symptomatic in about 2/3 of patients (Maron et al., 2016), HCM can slowly progress toward heart failure, either with preserved or reduced ejection fraction, due to worsening of diastolic and/or systolic function (Olivotto et al., 2012). Accumulation of intra-myocardial fibrosis and replacement scars underlies heart failure progression and represents a substrate for sustained arrhythmias (Galati et al., 2016). Late gadolinium enhancement (LGE) at cardiac magnetic resonance, an index of cardiac fibrosis, is widely used to stratify the severity of disease progression (Authors/Task Force Members et al., 2014) and to help defining the risk of lethal arrhythmias and terminal heart failure (Chan et al., 2014). However, LGE only identifies extensive replacement scars and well predicts the risk of arrhythmias only at the advanced stages of the disease (Chan et al., 2014). Replacement fibrosis in HCM appears to be related with local myocardial ischemia, caused by microvascular dysfunction (Sotgia et al., 2008), as myocardial tissue in regions with severe microvascular ischemia is slowly replaced by collagen. The degree of microvascular dysfunction, as assessed by positron emission tomography with labeled ammonia measuring the reduction of local myocardial blood flow, is related with patient outcome, including the risk of arrhythmias at advanced disease stages (Cecchi et al., 2003). Replacement fibrosis and microvascular ischemia are strongly related with the risk of ventricular arrhythmias because they create a stable substrate for reentry circuits, the main drivers of sustained ventricular arrhythmias (Pogwizd and Corr, 1987). In order for a reentry circuit to be established, an area of conduction block adjacent to a region of slower, unidirectional conduction is needed: indeed, in HCM myocardium, patchy replacement fibrosis generates areas of no-conduction, while the surrounding ischemic regions (due to microvascular dysfunction) cause slower, altered conduction, and transient local alterations of repolarization (Hurtado-de-Mendoza et al., 2017). Following these observation, the simplest conclusion would be that structural left ventricular remodeling at macroscopic myocardial level, featuring replacement fibrosis and microvascular dysfunction, is the main determinant of arrhythmias in HCM. However, a clear relationship between fibrosis (measured with LGE), microvascular dysfunction (measured by PET) and arrhythmic risk is observed only in the minority of patients (10–15%) that experience a slow progression toward end-stage HCM, an highly arrhythmogenic condition not unlike terminal ischemic heart failure, thus requiring aggressive preventive strategies (Priori et al., 2015). On the contrary, the majority of sudden cardiac death events due to lethal ventricular arrhythmias occur in patients at earlier stages of disease progression, often in the absence of marked structural abnormalities besides left-ventricular (LV) hypertrophy, also in very young patients (Maurizi et al., 2018). In early stages of hypertrophic cardiomyopathies, replacement scars are absent and only microscopic intramyocardial fibrosis is present, and its extent can be assessed by CMR using T1-mapping (Dass et al., 2012) or extracellular volume (ECV) measurements with gadolinium contrast, both altered even before the onset of hypertrophy in HCM-mutation carriers (Ho et al., 2013). However, the link between the degree of ECV expansion and the risk of arrhythmias in early stage HCM is still uncertain (Avanesov et al., 2017). Therefore, arrhythmias in HCM cannot be the sole consequence of the substrate for re-entry circuits at tissue level. On the contrary, the main determinants of arrhythmogenesis in HCM are to be found within the affected cardiomyocytes, a consequence of the alterations of ion currents and intracellular Ca2+ handling that occur as a part of HCM-related ventricular cardiomyocyte remodeling (Coppini et al., 2013; Coppini et al., 2017; Ferrantini et al., 2017; Ferrantini et al., 2018). Indeed, from a pathophysiological standpoint, the vast majority of ventricular tachycardia episodes begin with one or more premature ectopic ventricular beats (Ulus et al., 2013). Premature ventricular activations are essential to initiate the abnormal rotating conduction of the re-entry circuits in the presence of an appropriate substrate. Even in the presence of extended structural alterations, such as large scars and diffuse interstitial fibrosis, ectopic premature activations initiating in abnormal cardiomyocytes could be essential triggers to initiate the re-entry that is then maintained by the structural substrate. Interestingly, ectopic activity is the primary event producing local unidirectional block, which is an essential prerequisite for the establishment of a re-entry circuit. Ectopic ventricular beats are very common in HCM patients (Adabag et al., 2005) and originate from the premature spontaneous premature activation of a group of adjacent synchronized ventricular cardiomyocytes within the ventricular mass, which is then propagated to the whole surrounding ventricular mass, often in a chaotic and unpredictable manner (Sato et al., 2009). Early- and delayed- afterdepolarizations are the cellular arrhythmic events that may result into the spontaneous generation of premature action potentials in the affected cardiomyocytes. The following part of this review will illustrate how an increased likelihood of early and delayed after-depolarizations in HCM cardiomyocytes is a consequence of specific alterations of ion currents and intracellular Ca2+-handling.
Materials and Methods
Most of the results presented in the figures were previously published in Coppini et al. (2013) and in Ferrantini et al. (2018). Previously unpublished results are presented in Figures 3, 4. Cardiomyocytes and intact trabeculae were freshly isolated as previously described (Coppini et al., 2013; Ferrantini et al., 2018), using surgical upper inter-ventricular septum samples excised from HCM patients who underwent myectomy operation for the relief of severe symptoms due to obstruction of the LV outflow tract. Notably, all recruited patients had preserved systolic function but impaired diastole and most of them have a history of documented non-sustained ventricular tachycardia at Holter monitoring. The experimental protocols were approved by the ethical committee of Careggi University-Hospital of Florence (2006/0024713, renewed May 2009; 2013/0035305). In single cardiomyocytes, APs and Ca2+ transients were simultaneously measured using combined patch-clamp and Ca2+-fluorescence measurements, during stimulation at different frequencies. Cell capacitance was measured in voltage-clamp mode. Isometric force was measured from intact trabeculae during electrical field stimulation. Cells/trabeculae were exposed to test drugs for at least 5 min prior to recordings. Number of cells/trabeculae for each comparison, as well as the statistical tests used, are indicated in the respective figure legends.
Remodeling of Ion Currents in HCM Cardiomyocytes
Hypertrophic cardiomyopathy (HCM) cellular pathophysiology is characterized by the interplay between primary alterations (direct consequences of causal sarcomeric mutations altering myofilament function) and a large number of secondary myocardial modifications, comprising cellular electrophysiological remodeling (changes in transmembrane currents) and alterations of intracellular Ca2+ handling [Ca2+ transients and diastolic (Ca2+)] (Coppini et al., 2013; Coppini et al., 2017; Ferrantini et al., 2017; Ferrantini et al., 2018). Due to the profound electrophysiological differences between mouse and human cardiomyocytes, studies conducted on transgenic rodent models did not help to identify the features if ion-current remodeling occurring in HCM myocardium (van der Velden et al., 2015). To overcome this limitation, we investigated the abnormalities of electrical function, Ca2+ handling and contraction of human HCM myocardium as coexisting contributing factors for contractile dysfunction and arrhythmias in this disease (Coppini et al., 2013). We used isolated myocytes and intact trabeculae from fresh myocardial samples from the interventricular septum of HCM patients undergoing surgical myectomy operation for refractory symptoms related to severe obstruction of the left ventricular outflow tract, compared with septal samples from non-hypertrophic surgical patients (Coppini et al., 2013; Ferrantini et al., 2018). Overall, we performed patch-clamp and ion-fluorescence studies in over 200 cardiomyocytes from 43 HCM patients (Coppini et al., 2013; Ferrantini et al., 2018). Results of patch clamp studies in isolated ventricular cardiomyocytes showed that the duration of action potentials (APD), recorded at various pacing rates, was significantly prolonged in cardiomyocytes from HCM cardiomyocytes, with regards to controls (Figure 1A). As expected, prolongation of APD was associated with prolonged QTc in patients from the HCM cohort (average QTc = 470 ms). Of note, a recent large multi-center observational study on HCM patients suggested that mild QT prolongation is a common observation in those patients (Johnson et al., 2011). Prolonged APD was the main determinant of the increased risk of early afterdepolarisations (EADs) (Antzelevitch and Belardinelli, 2006), that is, spontaneous depolarisations occurring before the end of the repolarization phase: the occurrence of this type of cellular arrhythmias was 6-fold more frequent in HCM vs. control cardiomyocytes (Figure 1B). Interestingly, the frequency of EADs and the degree of APD prolongation went hand in hand with the incidence of ventricular arrhythmias in patients: patients whose cells show markedly prolonged APDs had a higher rate of documented non-sustained ventricular tachycardia at 24 h ECG (Coppini et al., 2013; Figure 1C). APD prolongation in HCM cardiomyocytes is caused by a combination of decreased repolarizing potassium currents and increased depolarizing (Ca2+ and Na+) currents: transient outward K+ current (Ito), inward-rectifier current (IK1), as well as delayed rectifier K+ currents were significantly reduced, while both L-Type Ca2+ current (ICaL) and late Na+ current (INaL) were increased in HCM cells, as compared to control cardiomyocytes (Coppini et al., 2013; Figures 1D–H). Pathological changes of the density of ion currents in HCM cardiomyocytes were caused by different mechanisms. The reduced density of potassium currents in HCM cardiomyocytes was the consequence of the lower levels of expression of K+ channel genes (Figures 1D,E), as observed in several human and animal models of cardiac hypertrophy and heart failure (Ravens and Cerbai, 2008), including heart failure with preserved ejection fraction (HFpEF) (Cho et al., 2017). In line with other models of LV hypertrophy and diastolic dysfunction (Cho et al., 2017), in human HCM myocardium the ion-channel genes with the most severely depressed expression were Ito and IK1 (Coppini et al., 2013): this might be a consequence of the increased activity of Ca2+/calmodulin-dependent protein-kinase II (CaMKII) in HCM cardiomyocytes (Coppini et al., 2013), which is able to down-regulate Ito and IK1 currents by reducing the expression of functional channels (Wagner et al., 2009). Moreover, the down-regulation of IK1 (Kir2.1) may also be related with the increased expression of micro-RNA miR-1 (Yang et al., 2007) observed in HCM specimens (Coppini et al., 2013). The small increase of ICaL density in HCM cardiomyocytes is likely determined by the slightly higher expression of CACNA1.2 gene and CaV1.2 protein (Figures 1F–H). In addition, the inactivation kinetics of ICaL is markedly slower in HCM vs. control cardiomyocytes (Coppini et al., 2013; Ferrantini et al., 2018; Figure 1G), contributing to prolong ICaL activation during the AP plateau, thus delaying repolarization. Interestingly, we found that the slower inactivation of ICaL observed in HCM cardiomyocytes may be related with the increased phosphorylation of L-type Ca2+ channel β-subunit by CaMKII (Hudmon et al., 2005), observed in HCM myocardium (Figure 3). Finally, INaL was consistently increased in all studied HCM myocytes: INaL integral (estimating the total Na+ flow upon a single current activation) was 2–3 times larger in HCM as compared with control cells (Figure 1J). As INaL is the slowly inactivating or non-inactivating component of Na+ current that remains active as a depolarizing current during the AP plateau, it directly contributes to prolong APD in HCM cardiomyocytes, as previously shown in human and animal models of cardiac hypertrophy and heart failure (Maltsev et al., 1998; Pieske and Houser, 2003; Pogwizd et al., 2003). The aforementioned changes in Ca2+, late Na+ and K+ current densities were quantitatively introduced into validated mathematical models of human ventricular myocyte (Grandi et al., 2010; Coppini et al., 2013; Passini et al., 2016): these studies confirmed that the observed ion current changes are sufficient to explain the prolongation of APD in human HCM cardiomyocytes. Also, modeling studies suggested that increased INaL plays a pivotal role as determinant of APD prolongation and EADs in HCM (Passini et al., 2016), as repolarization reserve is extremely reduced by the down-regulation of K+ currents. In support of this hypothesis, we studied the effects of INaL inhibition by ranolazine (Antzelevitch et al., 2004) or GS-967 (Sicouri et al., 2013) (a potent and selective INaL blocker) in HCM cardiomyocytes (Coppini et al., 2013; Ferrantini et al., 2018): INaL inhibition significantly reduced APD by approximately 30% in all HCM cardiomyocytes (Figure 1). Of note, ranolazine (at the clinically relevant concentration of 10 μM) and GS-967 (at 0.5 μM) reduced INaL by 70% in HCM cardiomyocytes. Consistently, the likelyhood of EADs in HCM cardiomyocytes was nearly halved by ranolazine or GS-967. In agreement with the increased role of INaL as a determinant of APD in the presence of an impaired repolarization reserve, the efficacy of ranolazine in shortening APD was more pronounced at low pacing frequencies and in cells with a longer APD at baseline. Studies in a mathematical cardiomyocyte model (Passini et al., 2016) confirmed that inhibition of 70% of INaL in HCM myocytes is sufficient to reduce APD, abolish EADs and reduce APD dispersion, as experimentally observed with pharmacological blockers.
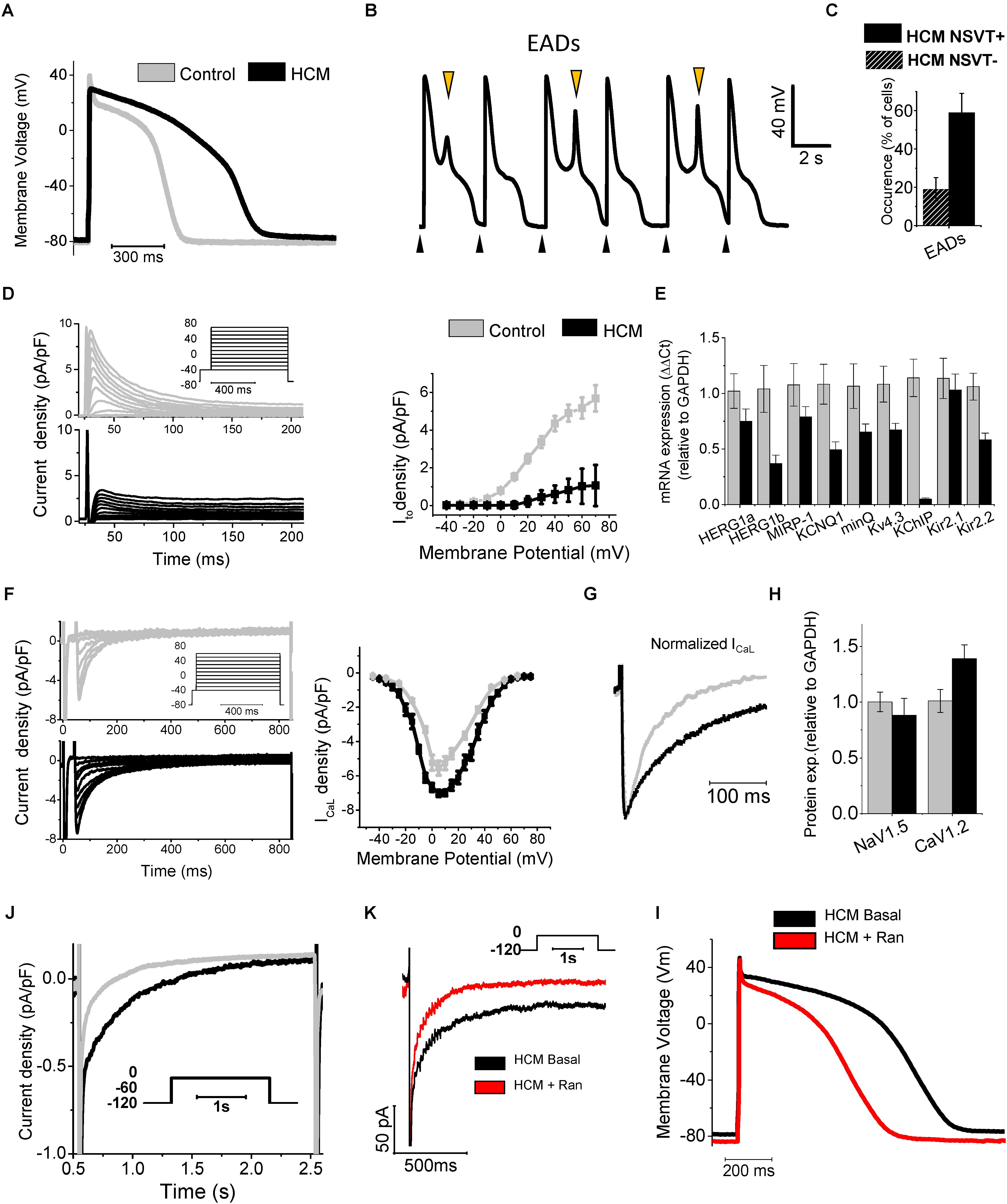
FIGURE 1. Remodeling of ion currents in HCM cardiomyocytes (A) Superimposed representative action potentials recorded during stimulation at 0.2 Hz from HCM and control cardiomyocytes. (B) Representative recording from an HCM cardiomyocyte paced at 0.2 Hz, showing EADs. Black arrows mark stimuli. Orange arrows mark EADs. (C) Occurrence of EADs in 23 cells from patients without NSVT (NSVT-) and 29 from patients with NSVT (NSVT+). (D) Representative traces (left, top panel control, bottom panel HCM) and average Ito activation curves from control and HCM cardiomyocytes (right panel). (E) RNA expression of potassium current genes, relative to GAPDH in control (N = 11, gray) and HCM (N = 15, black) samples. (F) Left: representative ICaL traces at different voltages. Right: ICaL activation curves. (G) Superimposed normalized ICaL recordings at 0 mV. (H) Protein expression of ICaL and I Na main channel subunits (Cav1.2 and Nav1.5). (J) Representative INaL traces from control and HCM cardiomyocytes. (K) Ranolazine inhibits INaL in HCM cardiomyocytes: representative INaL traces from an HCM cardiomyocyte during depolarization to -20 mV in the absence (Basal) or presence of Ran. (I) Action potentials at 0.2 Hz from an HCM cardiomyocyte before (Basal) and after exposure to 10 μmol/L ranolazine (Ran). Modified from Coppini et al., 2013 (ref. 19).
Remodeling of Ca2+ Handling in HCM Cardiomyocytes
Notwithstanding the large variabilities observed among different disease models, the common feature that is described in all transgenic animal models, human samples and cellular models of HCM is the sustained increase of diastolic intracellular calcium concentration [(Ca2+)]i within ventricular cardiomyocytes (Knollmann et al., 2003; Haim et al., 2007; Ashrafian et al., 2011; Fraysse et al., 2012; Lan et al., 2013). The increase of [Ca2+]i is likely to be a direct consequence of some of the sarcomere mutations that cause the disease and thus it may among the first pathological changes in the hearts of HCM-mutation carriers. Indeed, the majority of HCM-related mutations cause an increase of the Ca2+-sensitivity of the myofilaments and determine an increased ATP-consumption by the cardiac sarcomeres (Ashrafian et al., 2011), thus reducing the energetic efficiency of force production by the myocardium. Both these primary sarcomeric changes are associated with an increase of [Ca2+]i: the increased myofilament Ca2+- sensitivity determines a slower release of Ca2+ from Troponin-C that in turn prolongs the decay of Ca2+-transients and leads to elevated diastolic [Ca2+]i (Baudenbacher et al., 2008). ATP depletion caused by the reduced efficiency of mutated myofilaments can reduce the function of the sarcoplasmic reticulum Ca2+-ATPase (SERCA), thus reducing the rate of Ca2+ reuptake from the cytosol during relaxation (Ashrafian et al., 2003). Regardless of the cause that primarily increases [Ca2+]i, the complex remodeling of the Ca2+-handling apparatus in the cardiomyocytes of HCM hearts determines a global alteration of intracellular Ca2+ fluxes, ultimately contributing to aggravate Ca2+ overload in the cytosol and in the sarcoplasmic reticulum (SR) (Figure 2).
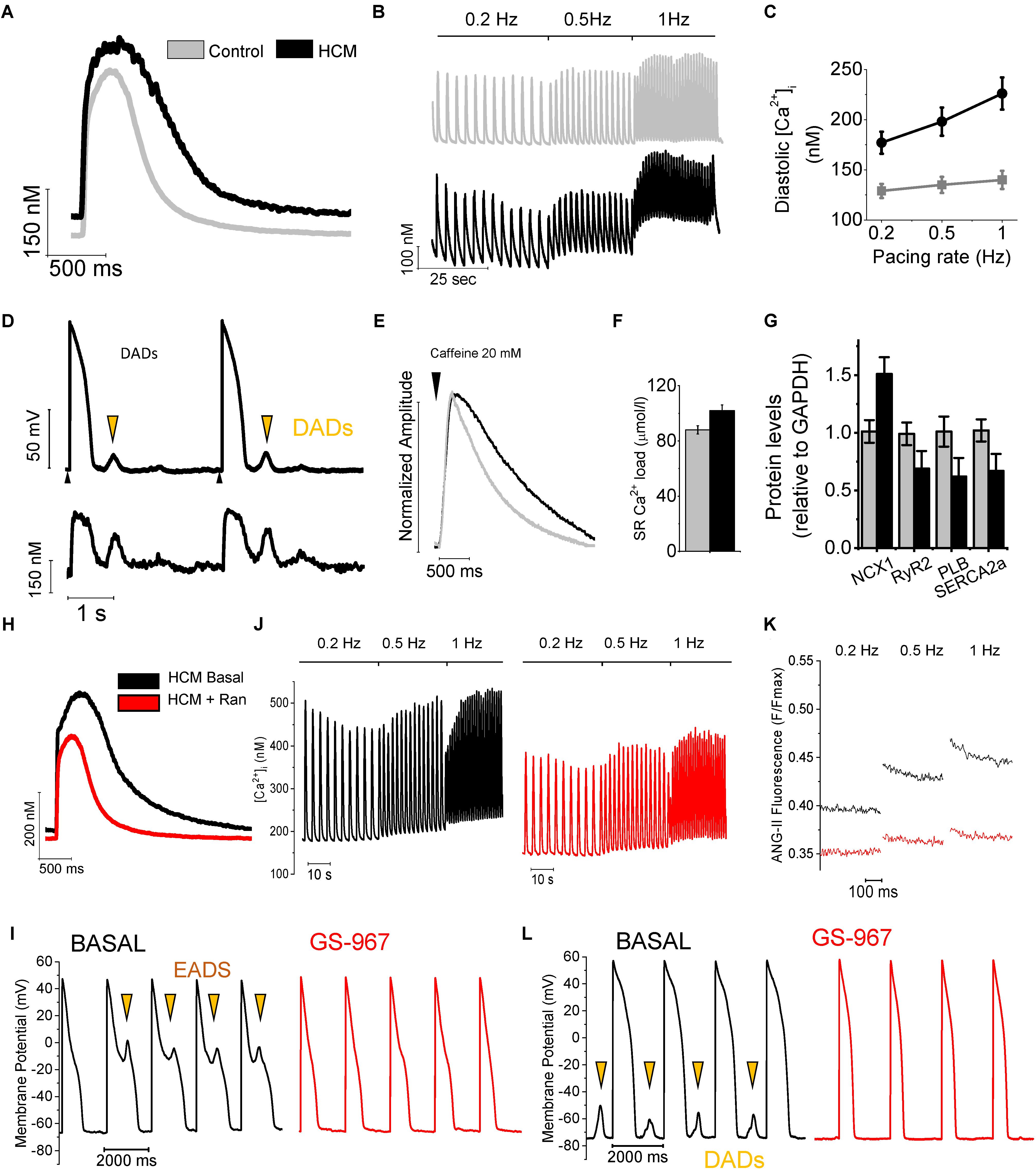
FIGURE 2. Remodeling of Ca2+ handling in HCM cardiomyocytes. (A) Superimposed representative Ca2+i transients recorded during stimulation at 0.2 Hz. from control and HCM cardiomyocytes. (B) Continuous recordings of Ca2+i transients elicited at 0.2, 0.5, and 1 Hz frequency of stimulation, from a control (above) and an HCM (below) cardiomyocyte. (C) Average diastolic Ca2+i fluorescence levels at increasing pacing frequencies. (D) Representative recording from an HCM cardiomyocyte, showing DADs, occurring in response to spontaneous Ca2+ release (calcium waves). Black arrows mark stimuli. Orange arrows mark DADs. (E) Ca2+ fluorescence traces during the protocol to measure SR Ca2+ content with caffeine: superimposed normalized caffeine-induced calcium transients from HCM and control cardiomyocytes. (F) SR Ca2+ content (left) in control and HCM cardiomyocytes. (G) Protein expression of NCX1, SERCA2a, RYR2 and PLB in HCM (N = 10) and control (N = 10) specimens. (H) Superimposed representative Ca2+i transients at 0.2 Hz from an HCM cardiomyocyte at baseline and with ranolazine. (J) Trains of Ca2+i transients elicited at 0.2, 0.5, and 1 Hz in an HCM myocyte before (left) and following (right) exposure to Ran. (K) Representative traces of ANG-II florescence (intracellular sodium) during diastole, recorded at steady state stimulation of 0.2, 0.5, and 1 Hz in the absence (black) and presence (red) of 10 μM Ran. (I) Representative trains of action potentials elicited at 0.5 Hz at baseline (black traces) and in the presence of GS-967 0.5 μM (red traces). Early after-depolarizations (EADs) are marked by arrows. (L) Representative trains of action potentials elicited at 0.5 Hz. Delayed after-depolarizations (DADs) are marked by arrows. Modified from Coppini et al. (2013) and from Ferrantini et al. (2018).
In human HCM cardiomyocytes, abnormalities of APD and ion current were paralleled by marked alterations of intracellular Ca2+ handling (Coppini et al., 2013; Ferrantini et al., 2018), as studied by virtue of fluorescence measurements using Ca2+-sensitive dyes. Ca2+ transient amplitude was similar in HCM and control myocytes. Conversely, Ca2+ transient kinetics was significantly slower and intracellular diastolic Ca2+ concentration ([Ca2+]i) was higher in HCM cells as compared to control cardiomyocytes, especially at higher rates of stimulation (Figures 2A–C). The elevated [Ca2+]i contributed to the abnormally high rate of spontaneous Ca2+ releases from the SR observed in HCM myocytes, resulting in diastolic Ca2+-waves and delayed after-depolarizations (DADs), thus contributing to cellular arrhythmogenesis (Figure 2D). Additionally, SR Ca2+ content was slightly increased in HCM cardiomyocytes (Figure 2F), at variance with human and animal models of heart failure with reduced systolic function, where decreased SR Ca2+ content and Ca2+ transients amplitude are common observations (Beuckelmann et al., 1992; Shan et al., 2010). The altered Ca2+ fluxes in HCM myocytes are the result of several concurrent alterations: (i)increased amplitude and slower inactivation kinetics of L-Type Ca2+-current (see above), (ii)reduced expression of SERCA and reduced SERCA/phospholamban ratio (Figure 2G), (iii)loss or disorganization of t-tubules (see below), (iv) increase leakage of Ca2+ from the SR, and (v)abnormal function of the Na+/Ca2+ exchanger (NCX). The latter is the consequence of the increased intracellular concentration of Na+ ([Na+]i). In agreement with these results, we calculated a negative shift of the NCX reversal potential (ENCX), suggesting increased [Na+]i. Indeed, we directly observed an increase of [Na+]i in HCM myocytes, as measured in human HCM myocytes using the Na+-selective fluorescent dye Asante Natrium Green II (Figure 2K). Based on this observation, we proposed that enhanced Na+ influx mediated by the larger INaL leads to a sustained increase in cytosolic [Na+]i, providing the driving force for an increased rate of Ca2+ entry trough NCX, as previously observed in secondary LV hypertrophy (Terracciano et al., 2001), albeitnot in heart failure. Combined with the increased expression of NCX and the prolongation of APs, this mechanism leads to an increased total Ca2+ entry during the plateau of the AP, thus helping HCM cardiomyocytes to maintain normal SR Ca2+ load, Ca2+ transients amplitude and contractility (Weisser-Thomas et al., 2003) despite SERCA down-regulation. This is at variance with reports on human failing cardiomyocytes (Beuckelmann et al., 1992), where SERCA down-regulation reduces SR Ca2+ load and Ca2+ release. However, the increased cytosolic [Na+]i in HCM myocytes also reduces forward-mode NCX activity, thus determining the observed decrease of Ca2+ extrusion rate during exposure to caffeine (Figure 2E) and likely contributing to prolong Ca2+ transient decay and increase diastolic [Ca2+]i, in combination with the reduced activity of SERCA. Notably, prolongation of APs likely contributes to reduce Ca2+ extrusion through the NCX, as the exchanger can effectively work in forward mode only at diastolic potentials. The central role of the increased INaL in determining Na+ and Ca2+ overload in HCM cardiomyocytes is highlighted by the effects of INaL inhibition by ranolazine or GS-967 (Coppini et al., 2013; Ferrantini et al., 2018). In human HCM cardiomyocytes, besides shortening APD, INaL inhibition reduced intracellular [Na+] (Figure 2K), as directly assessed using fluorescence measurements. The reduction of [Na+]i shifted ENCX back to positive levels; this in turn led to a potentiation of the forward-mode activity of the NCX (Ca2+ extrusion/Na+ entry), while it decreased the reverse-mode function (Ca2+ entry/Na+ extrusion). The enhanced Ca2+ extrusion via the NCX resulted in an acceleration of Ca2+-transient decay, determined a reduction of diastolic [Ca2+]i, and lessened diastolic [Ca2+]i rise in response to increases of pacing frequency (Figures 2H–J). In keeping with these observations, we observed that ranolazine hastened the decay of caffeine-induced Ca2+ transients and slightly reduced SR Ca2+-content in HCM cardiomyocytes (Coppini et al., 2013). Interestingly, the reduction of diastolic [Ca2+]i by ranolazine or GS-967 reduced the occurrence of spontaneous diastolic Ca2+ waves, DADs and triggered activity (Figures 2I–L).
Intracellular Ca2+ overload negatively affected diastolic function in HCM myocardium: kinetics of relaxation was slower in HCM vs. control trabeculae and diastolic tension was higher, especially at high stimulation frequencies. Ranolazine and GS-967, by lowering diastolic [Ca2+]i, accelerated relaxation of HCM myocardium (Coppini et al., 2013; Ferrantini et al., 2018). Of note, when used in control myocytes and trabeculae, we observed none of the effects shown by ranolazine and GS-967 on action potentials, Ca2+-handling and contraction in HCM myocardium (Coppini et al., 2013; Ferrantini et al., 2018); these results highlight the selectivity of these compounds for INaL over peak INa and support the idea that INaL augmentation plays a leading role in the remodeling of cardiac electro-mechanical function in HCM.
Most of the abnormalities of Ca2+ handling and contraction we observed in human cardiomyocytes and trabeculae were present also in cardiomyocytes isolated from the hearts of transgenic mice carrying the clinically relevant R92Q mutation of the Troponin T gene (Coppini et al., 2017; Ferrantini et al., 2017): these include slower Ca2+-transients, elevated [Ca2+]i, increased INaL and [Na+]i, as well as impaired relaxation and elevated diastolic tension. Interestingly, in cardiomyocytes from the R92Q mouse, ranolazine hastened Ca2+ transients, normalized [Ca2+]i and [Na+]i and reduced Ca2+-dependent arrhythmias. The observed antiarrhythmic effect of ranolazine may, at least in part, depend on the direct stabilization of ryanodine receptors by the drug (Parikh et al., 2012), which may have contributed to reduce spontaneous diastolic Ca2+ release and arrhythmogenic Ca2+ waves in human and mouse HCM cardiomyocytes.
Role of CaMKII in HCM Pathophysiology
Sustained activation of the signaling pathways driven by calcium-calmodulin dependent protein kinase II (CaMKII) appear to play a central role as a determinant of cardiomyocyte remodeling and dysfunction in HCM myocardium (Figure 3), as observed in several human and animal models of cardiac hypertrophy and heart failure (Ling et al., 2009; Toischer et al., 2010; Fischer et al., 2013). Sustained activation of CaMKII in disease conditions is driven by the increase of cytosolic [Ca2+]I within the cardiomyocyte, as Ca2+-bound calmodulin is the most important activator of this kinase (Hudmon and Schulman, 2002). Additionally, enhanced generation of reactive oxygen species in diseased myocytes, a likely consequence of myocardial energetic derangement, can be a strong contributor to CaMKII over-activation in HCM cardiac muscle (Erickson et al., 2011). Once activated, CaMKII then phosphorylates itself (at threonine 286 site), thereby prolonging and potentiating its activated state. In HCM vs. control specimens (Coppini et al., 2013), CaMKII auto-phosphorylation was increased 3.5-fold (Figure 3A), indicating increased activity (Lai et al., 1986). This in turn potentiates the phosphorylation of all the downstream targets of CaMKII (Toischer et al., 2010; Anderson et al., 2011; Fischer et al., 2013). CaMKII targets several proteins involved in the regulation of cardiomyocyte electrophysiology and calcium fluxes. The observed 2.5-fold higher phosphorylation of cardiac Na+ channel (NaV1.5) by CaMKII in HCM samples (Figure 3A; Coppini et al., 2013) may have significantly contributed to increase INaL in HCM cardiomyocytes (Wagner et al., 2006); this suggests that CaMKII activation is the most relevant cause of INaL augmentation in HCM cardiac myocytes, although additional mechanisms might be involved (e.g., oxidation of Na+channels) (Lu et al., 1999). The increased phosphorylation of L-Type Ca2+ channel (Figure 3A) may have contributed to slow down ICaL inactivation in HCM cells (Hudmon et al., 2005; Xu et al., 2010). The slower ICaL inactivation may also be a consequence of the loss of T-tubules observed in HCM cardiomyocytes (see below) (Brette et al., 2004). Moreover, the observed 1.5-fold higher CaMKII-dependent phosphorylation of RyR2 (Figure 3A) may have contributed to increase the rate of spontaneous releases during diastole (Shannon et al., 2003) and DADs (Curran et al., 2010) in HCM myocytes. Finally, the observed 3-fold higher phosphorylation of PLB at CaMKII site (Figure 3A), via reduced SERCA inhibition, may have partially counteracted the consequences of reduced SERCA expression in terms of SR Ca2+ reuptake, ultimately contributing to maintain SR Ca2+ content and steady state Ca2+-transient amplitude in HCM cardiomyocytes (Mattiazzi and Kranias, 2011). In support of the previous observations, we here tested the effects of the acute inhibition of CaMKII in HCM cardiomyocytes and trabeculae by using the cell-permeant version of Autocamtide-related inhibitory peptide II (AIP-II, see Figures 3B–C). In single human HCM cardiomyocytes, AIP-II reduced diastolic [Ca2+]i and limited the increase of diastolic [Ca2+]i at higher stimulation frequencies, without affecting the amplitude or the kinetics of Ca2+-transients (Figure 3B). In line with that, when used in intact human HCM while measuring twitch force, AIP-II reduced diastolic tension and limited the increase of diastolic tension upon increase of pacing rate (Figure 3C). Interestingly, AIP-II accelerated time-to-peak contraction in HCM trabeculae, suggesting that acute inhibition of CaMKII may directly affect the kinetics of force generation by the myofilaments.
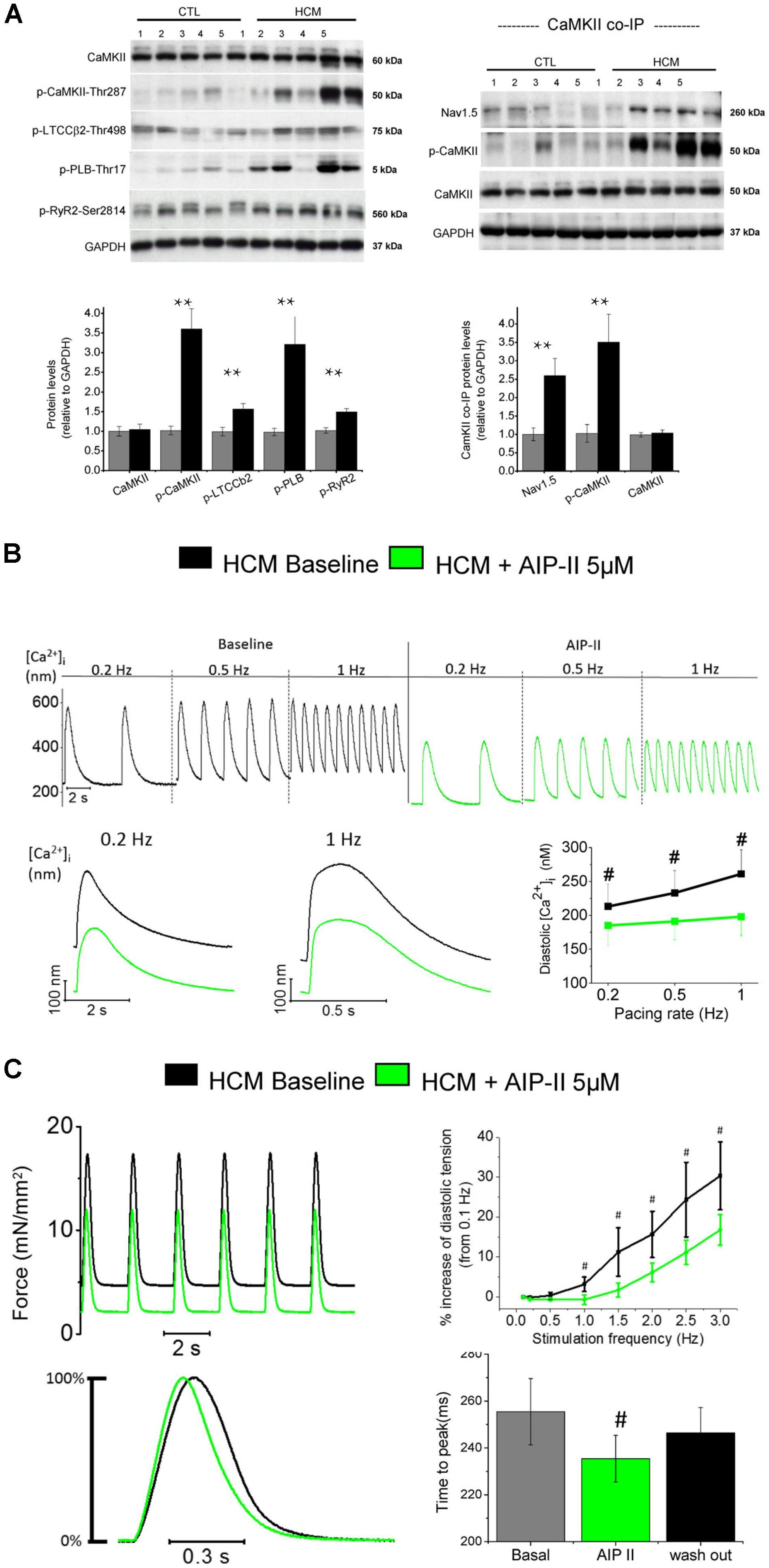
FIGURE 3. Increased CaMKII activity underlies electro-mechanical remodeling in HCM cardiomyocytes: acute effects of CaMKII block with Autocamtide 2-related inhibitory peptide II (AIP II) on Ca2+ transients and twitch force. (A) Right: Representative Western blots (above) for total CaMKII, (phosphorylated CaMKII at threonine 287 (p-CaMKII), phosphorylated L-type Ca2+ channel β2 subunit at threonine 498 (p-LTCCb2), phosphorylated phospholamban at threonine 17 (p-PLB) and phosphorylated ryanodine receptor 2 at serine 2814 (p-RyR2). Average values from septum of control (n = 10) and HCM patients (n = 10) are reported below. Left: Representative Western blots (above) and mean values (below) for Co-IP of Nav1.5 with CaMKII from control (n = 10) and HCM patients (n = 10), probed with antibodies for Nav1.5, p-CaMKII and total CaMKII. (A,B) For each protein, 5 blots representative of the 10 are shown. Relative intensity of individual bands was quantitated and normalized to GAPDH. The ratio for control was assigned a value of 1. ∗∗P < 0.01, t-test. Modified from Coppini et al., 2013. (B) Representative Ca2+ transients recorded from an HCM cardiomyocytes in the absence and in the presence of AIP II, showing a reduction of diastolic [Ca2+]i levels upon administration of AIP II. Data from 19 cells from 4 patients (C) Top: Representative force traces from an HCM trabecula in the absence and in the presence of AIPII, showing a reduction of diastolic tension after AIPII and mean diastolic tension at various frequency from 6 HCM trabeculae (5 patients), in the absence and in the presence of AIP II. Bottom: Normalized representative force traces highlighting the effects of AIPII on twitch kinetics and mean data for contraction peak time, at baseline, under AIP II and after washout of the drug (B,C). #P < 0.05 in paired t-test. Previously unpublished data.)
Besides the direct effects on cardiomyocyte function, CaMKII is able to alter the expression of genes involved in the hypertrophic remodeling process of cardiomyocytes, and to facilitate the production of collagen, the increase of extracellular matrix volume and the growth of cardiac fibroblasts (Kreusser and Backs, 2014). We verified that CaMKII participates in the development and progression of cardiac functional and structural phenotype in HCM by studying the transgenic R92Q-TnT mouse model. In the R92Q-TnT mouse, CaMKII activity, increased INaL and cardiomyocyte Ca2+ overload go hand in hand during disease development and are present since the earliest stages of disease development (Coppini et al., 2017; Ferrantini et al., 2017). In this mouse model, lifelong treatment with ranolazine prevented the development of all features of HCM-specific cardiac phenotype, including LV thickening, progression of LV diastolic dysfunction and intra-myocardial fibrosis and the establishment of an arrhythmogenic substrate (Coppini et al., 2017). The mechanism by which ranolazine prevents the hypertrophic HCM phenotype is related to the inhibition of the enhanced INaL, leading to decreased intracellular [Na+] and diastolic [Ca2+]i, eventually avoiding the pathological intensification of CaMKII function in treated mice (Coppini et al., 2017). The reduction of CaMKII-activity in treated mice prevented the progression of the hypertrophic remodeling in mutant hearts, thereby reducing the morphological and functional cardiac HCM-phenotype in mutation-carrier mice.
Loss of T-Tubules in HCM Cardiomyocytes
T-tubules (Ferrantini et al., 2013) play a fundamental role in myocardial function because they allow a quick propagation of APs within the inner portions of cardiac myocytes. The simultaneous electrical activation of the whole t-tubular system allows for a synchronous triggering of Ca2+release from the SR across the whole myocytes, even the central regions that are farther away from surface sarcolemma. This is essential to achieve an homogeneous activation of all myofilaments and thus a rapid simultaneous shortening of the entire cardiac cell. As a proof of concept, by acutely disrupting T-tubules trough osmotic-shock, Ca2+release was rendered asynchronous (Brette et al., 2005), resulting in a clear impairment of contractile function and a slower relaxation (Ferrantini et al., 2014). In cardiomyocytes from animal models of cardiac hypertrophy and heart failure, a delay of local Ca2+release was observed both in areas where t-tubules are disrupted (Song et al., 2006) and in regions adjacent to electrically uncoupled T-tubules (Sacconi et al., 2012; Crocini et al., 2014). Lyon et al. (2009) studied T-tubular structures in myocardial specimens from patients with HF caused by different diseases (i.e., post-ischemic HF, dilated cardiomyopathy and HCM) and observed a significant reduction of T-tubule density in all failing human hearts regardless of the underlying disease, including in end-stage HCM. In a mouse model of HCM we used Random Access Multi Photon (RAMP) microscopy to measure the local propagation of APs in the T-tubule and the correspondent release of Ca2+in the adjacent junctional area by simultaneously mapping multiple sites of an isolated cell. With this technique, we found that more than 20% of T-tubules are unable to propagate AP and the surrounding junctional regions display a significantly delayed local Ca2+release (Crocini et al., 2016). In this mouse model, asynchronous intracellular Ca2+ release due to altered T-tubules contribute to slow down Ca2+-transient kinetics and impair diastolic function.
In all cardiomyocytes subjected to patch-clamp measurements, we measured cell capacitance, an index of sarcolemma extension, and compared with cell volume, as estimated by cell surface (Figure 4A). The ratio between cell capacitance and volume (surface/volume ratio) was reduced in HCM vs. control human cardiomyocytes, suggesting reduction of T-tubules. In addition, we recently performed a preliminary assessment of the density of T-tubules in cardiomyocytes isolated from surgical septal samples of 10 HCM patient (Ferrantini et al., 2018; Figures 4B,C): in all the myocytes studied under the confocal microscope after membrane fluorescent labeling, we observed a very low density of T-tubules, much lower than what is expected in healthy human cardiomyocytes (Lyon et al., 2009). We previously demonstrated that the loss of t-tubules may directly contribute to slow down the kinetics of Ca2+ transients (Ferrantini et al., 2014), thus delaying relaxation, with possible detrimental effects on diastolic function. However, it is unclear whether and how loss of t-tubules affects the propensity toward arrhythmias of HCM cardiomyocytes. Loss of t-tubules could be protective against arrhythmias because orphan RyR channels (RyR uncoupled from t-tubules) have a reduced likelihood of diastolic spontaneous opening (Brette et al., 2005); however, the rate of Ca2+ waves is increased in human HCM. Loss of t-tubules leads to reduction of capacitance/volume ratio, thus increasing conduction velocity in detubulated myocardial tissue: this effect may prevent the formation of small re-entry circuits. On the contrary, loss of t-tubules may promote arrhythmias because it reduces synchronicity of Ca2+ release, thus raising the likelihood of APD and effective-refractory-period (ERP) temporal fluctuations (alternans): APD and ERP alternans facilitate the formation of dynamic reentry circuits (Heinzel et al., 2011). In support of this, we found that Ca2+ release is indeed asynchronous in human HCM cardiomyocytes (Figure 4D). Moreover, loss of t-tubules reduces Ca2+-dependent inactivation of ICaL, as subsarcolemmal systolic [Ca2+] is lower below surface sarcolemma than in proximity of T-tubules (Morotti et al., 2012): indeed, ICaL inactivation is slower in human HCM cells as compared with controls. Finally, the presence of dysfunctional residual t-tubules within the myocyte may facilitate spontaneous Ca2+-release events at SR sites adjacent to those tubules, as we previously showed in a model of heart failure (Crocini et al., 2014), increasing the overall risk of Ca2+ waves and DADs.
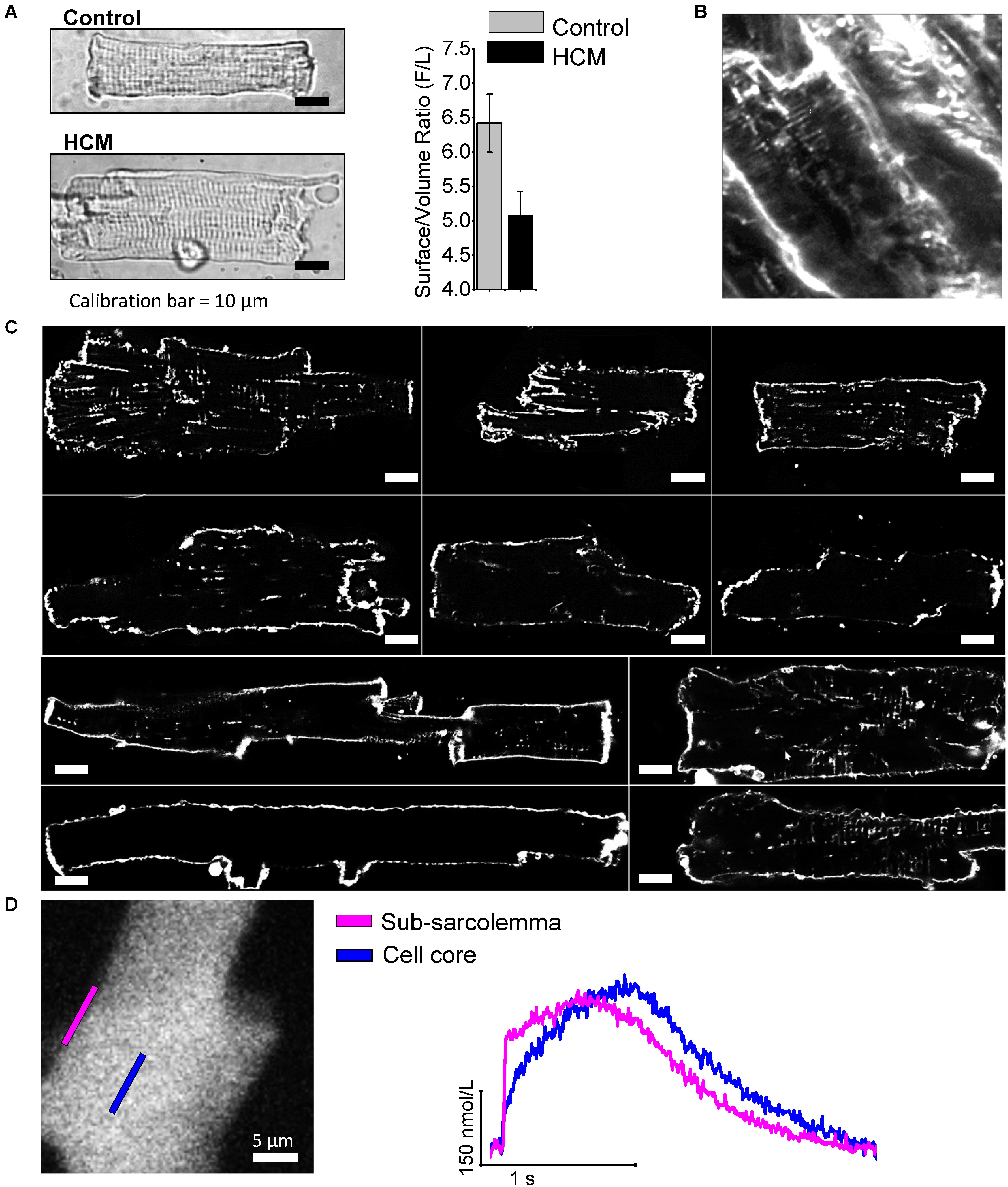
FIGURE 4. The density of T-tubules is markedly low in HCM cardiomyocytes. (A) Left: Representative images of a control (top) and an HCM(bottom) cardiomyocyte, showing cell hypertrophy in HCM. Right: surface/volume ratio in HCM and control cardiomyocytes;surface is derived from cell capacitance, volume estimated from cell area. Data from 64 cells (14 patients). Previously unpublished data. (B) 2-photon image recorded in HCM intact tissue after membrane labeling with anepp dyes, showing a severe reduction of T-tubules. (C)Each cell derives from a different patient sample (ID of the patient is indicated next to the cell in each respective image). Cells were stained with Di-3-ANEPPDHQ (Thermo-Fisher) and imaged with a Leica Confocal microscope using the 488 nm laser line. Sections were taken at mid cell. While the outer sarcolemma is well stained in all myocytes, T-tubules are barely visible in most of them and some cells are completely devoid of T-tubules. White bars equal 10 μm. (D) Recordings of intracellular calcium from an HCM myocytes using a fast camera; right: calcium variations during an elicited electrical activation in the subsarcolemma and in the cell core. In agreement with the loss of t-tubule, calcium rise in the core is significantly delayed. Previously unpublished data.
Abnormal Response of HCM Myocardium to Beta-Adrenergic Stimulation
We recently investigated the characteristics and anomalies of β-adrenergic signaling in the myocardium of HCM patients by comparing the response of control cardiac muscle to β-adrenergic stimulation with that observed in HCM cardiomyocytes or trabeculae (Ferrantini et al., 2018; Figure 5). In particular, we observed that the mechanical response to the β-adrenergic agonist isoproterenol (that is, augmentation of twitch force amplitude and acceleration of relaxation) was qualitatively similar in HCM and control trabeculae, albeit the kinetics of contraction and relaxation remained slower in HCM myocardium, even upon maximal β-adrenergic activation (Ferrantini et al., 2018; Figures 5A–D). These results suggest that the molecular mechanisms responsible for the acceleration of relaxation upon β-adrenergic activation [i.e., myofilament Ca2+-desensitization (Sequeira et al., 2013) and phospholamban phosphorylation (Coppini et al., 2013; Helms et al., 2016)] are preserved in HCM myocardium.
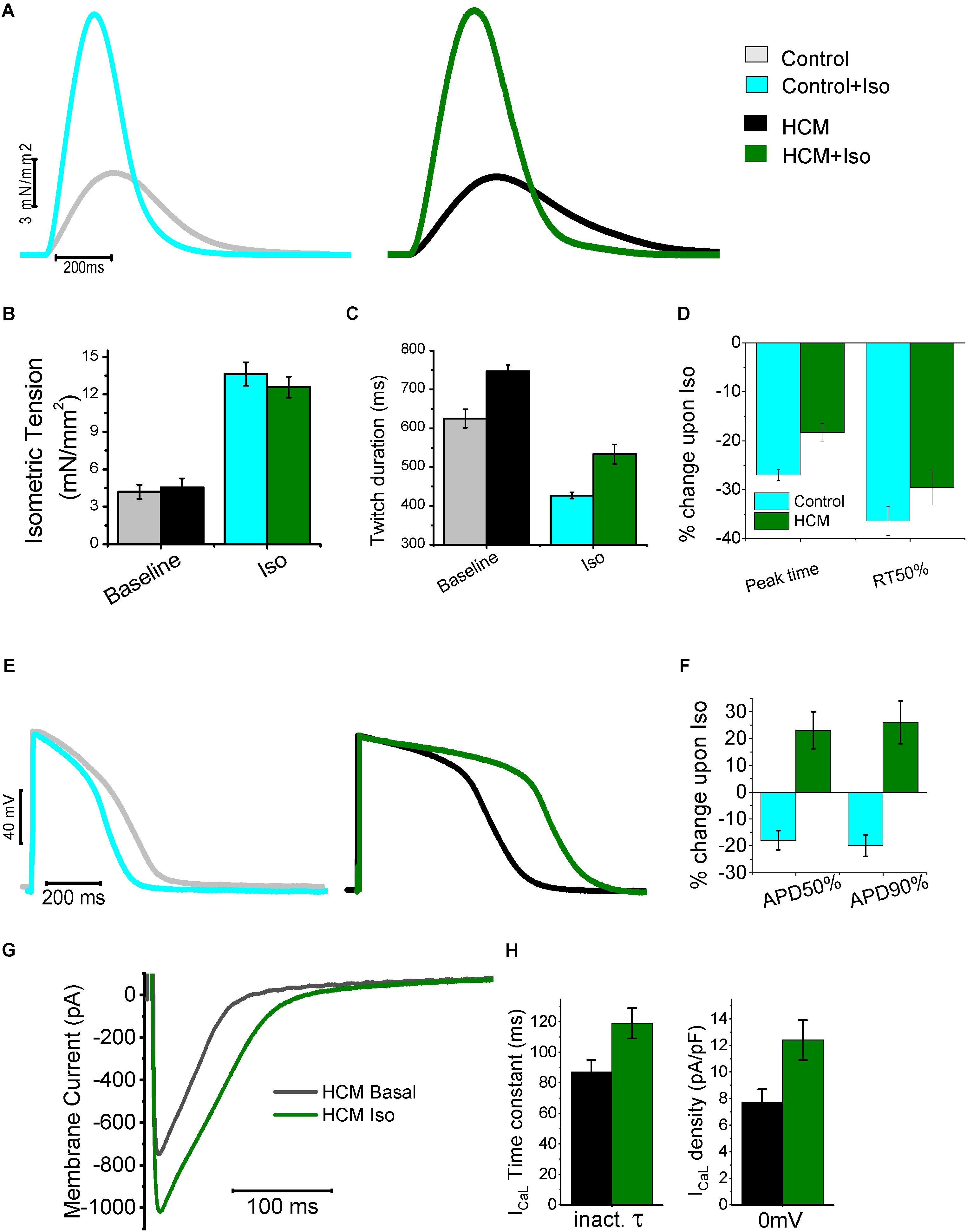
FIGURE 5. Mechanical and electrical response to β-adrenergic stimulation of HCM myocardium. (A) Representative superimposed force twitches elicited at 0.5 Hz in control (left) and HCM (right) trabeculae in the absence and presence of Isoproterenol 10-7M (Iso). (B) Isometric tension during steady state stimulation at 0.5 in the absence and presence of Iso in control and HCM trabeculae. (C) Duration of force twitches (from stimulus to 90% of relaxation) elicited at 0.5 Hz in the absence and presence of Isoproterenol 10-7M. (D) Percentages of Change in the parameters of twitch kinetics (0.5 Hz) upon exposure to Iso in Control (cyan) and HCM (green) trabeculae: time from stimulus to peak contraction (peak time) and time from peak to 50% of relaxation (RT50%). (E) Representative superimposed action potentials elicited at 0.5 Hz in control (left) and HCM (right) cardiomyocytes, in the absence and presence of Iso. (F) Percentages of Change in the parameters of action potential kinetics upon exposure to Iso in Control (cyan) and HCM (green) cardiomyocytes: time from stimulus to 50% repolarization (APD50%) and time from peak to 90% of repolarization (APD90%). (G) Representative superimposed L-Type Ca-current traces at baseline (black traces) and in the presence of Iso (green). (H) L-Type Ca-current inactivation time-constant(left) and density (right) at baseline (black) and with Iso (green) in HCM cells. Modified from Ferrantini et al. (2018).
On the contrary The electrical response to isoprenalin observed in single HCM cardiomyocytes was profoundly different as compared with control cardiac cells (Figures 5E–G). Activation of β-adrenergic receptors physiologically leads to the enhancement of both ICaL and the slow delayed-rectifier K+ current (IKs). In healthy human cardiomyocytes the increase of repolarizing K+ currents prevails over the augmentation of ICaL (Terrenoire et al., 2005), thus determining a net reduction of AP duration (Taggart et al., 2003; Figure 5E). In HCM cardiomyocytes, however, we observed unbalanced changes in the expression of Ca2+ and K+ currents (Coppini et al., 2013). As shown in Figure 1, we observed a reduction of the expression of all K+ channels (including IKs) while the expression of Ca2+ channels and the density of ICaL was slightly increased. Therefore, in response to β-stimulation, the potentiation of ICaL prevails over the increase of K+ currents, ultimately causing a net increase of inward currents during the plateau of the AP, thus determining a “paradoxical” prolongation of APD in HCM cardiomyocytes (Ferrantini et al., 2018; Figures 5E,F). Moreover, we observed that β-adrenergic stimulation in HCM cardiomyocytes not only increased peak ICaL amplitude, but also slowed down ICaL inactivation (Ferrantini et al., 2018; Figures 5G,H), further contribute to the prolongation of APs by β-adrenergic agonists. Additionally, recent work suggested that β-adrenergic stimulation is able to rapidly and transiently increase INaL in cardiac myocytes (Dybkova et al., 2014): a further augmentation of the already increased INaL in HCM myocytes may have played a relevant role in the paradoxical prolongation of APs with isoprenaline. As expected, AP prolongation by β-adrenergic agonists further increased the occurrence of arrhythmogenic early afterdepolarizations, triggered activity and spontaneous premature contractions in HCM cardiomyocytes and trabeculae (Ferrantini et al., 2018). This paradoxical electrical response may have severe consequences in HCM patients, that is, it may increase the risk of exercise/stress-induced arrhythmias in HCM patients.
In parallel, the response of HCM cardiomyocytes to β-adrenergic stimulation in terms of Ca2+-handling changes was also abnormal. In HCM cardiomyocytes the β-adrenergic-induced increase of Ca2+ release may primarily rely on the prolongation of Ca2+ entry via ICaL channels caused by the slower current inactivation. In addition to the increase of net Ca2+ influx through ICaL, the potentiation of Ca2+-entry via reverse-mode NCX may have contributed to the augmentation of Ca2+-transients β-receptor activation (Perchenet et al., 2000). The increase of NCX-mediated Ca2+-entry upon β-adrenergic stimulation is probably caused by the rise of intracellular [Na+] in response to the transient augmentation of INaL and by the further prolongation of AP plateau, as reverse-mode NCX is only active at positive membrane potentials (Coppini et al., 2013; Dybkova et al., 2014). The idea that the increase of contractile tension in response to β-adrenergic activation mainly depends on the larger Ca2+-entry through the sarcolemma apparently contrasts with the largely accepted idea that the inotropic response of β-stimulus mainly stems from the increase of SR Ca2+ content (Desantiago et al., 2008), mediated by the enhancement of SR Ca2+-uptake by SERCA via PKA-dependent phospholamban phosphorylation, ultimately causing an enhanced release of Ca2+ from the SR. The hastening of Ca-transient decay in response to isoprenaline in HCM cardiomyocytes (Ferrantini et al., 2018) suggests that the β-adrenergic-induced increase of SERCA function (via PkA-mediated phospholamban phosphorylation) is preserved in HCM myocardium, as Ca-transient decay is physiologically accelerated by isoproterenol. Despite the shortening of Ca2+ transient decay, their rise-time is so prolonged that the total duration of Ca2+-transients is not reduced upon β-stimulation. The rise of SR Ca2+ load upon exposure to isoprenaline in HCM myocytes may be limited by the increased phosphorylation of ryanodine receptors (due to the higher CaMKII activity), which in turn causes a larger rate of Ca2+ leakage from the SR during the diastolic period. This may render positive inotropic responses more dependent on the increase of Ca2+ entry from the sarcolemma, as the possibility to accumulate Ca2+ in the SR is limited. An additional contributor to this aberrant behavior is the lower density of t-tubules observed in HCM myocytes (see above) (Orchard and Brette, 2008). The physiological response to β-adrenergic activation may be radically different in disease-remodeled myocytes showing a sparse and disorganized the T-tubular system. In HCM cells, where T-tubules are nearly absent, we expect a large redistribution of ICaL channels to the surface sarcolemma (Coppini et al., 2013). Under such conditions, modulation of cellularinotropism essentially relies on the magnitude of sarcolemmal Ca2+ triggers (Ferrantini et al., 2014), that is, the amplitude and duration of ICaL plus the rate of NCX-mediated Ca2+ entry (reverse mode). The prolongation of APs causing increased sarcolemmal Ca2+-entry appears to be an essential requisite for the preservation of the positive-inotropic effect of β-adrenergic stimulation in HCM myocardium. In line with that hypothesis, the application of ranolazine or GS-967 on top of isoproterenol, which prevented the β-adrenergic-induced AP prolongation, also greatly reduced the positive inotropic response to β-stimulation in HCM myocardium (Ferrantini et al., 2018). The preservation of inotropic response in HCM myocardium comes at the expense of a further impairment of diastolic function (due to prolonged Ca2+ transient rise-time) and a further increase of the likelihood of cellular arrhythmias (due to increased cytosolic Ca2+ accumulation and AP prolongation).
Conclusion
Hypertrophic remodeling in the myocardium of patients with HCM features several pathological alterations of cardiomyocyte electrical function and intracellular Ca2+-handling (Figure 6), that contribute to increase the likelihood of EADs, DADs and premature contractions. Such arrhythmogenic changes occur since the early stages of HCM disease development and may explain the occurrence of ventricular arrhythmias and cardiac arrest in young HCM patients who lack advanced structural alterations of myocardial structure (e.g., diffuse fibrosis, myocardial scars, massive hypertrophy, severe microvascular abnormalities). Changes of the expression and function of ion channels and EC-coupling proteins in HCM myocardium concur to generate an “acquired channelopathy” phenotype in HCM patients that raises the risk of arrhythmias even before the establishment of a structural substrate for sustained arrhythmias. Among the several molecular determinants of cellular arrrhythmogenesis, increased INaL appear to play a leading role in HCM and may represent a selective target for pharmacological prevention of arrhythmias in this disease, with possible clinical implications. Although targeting INaL is proven to be effective antiarrhytmic approach, one cannot discount NCX (Passini et al., 2016), leaky RyR channels or cardiac CaMKII as potential targets to reduce Ca2+-dependent arrhythmias in HCM.
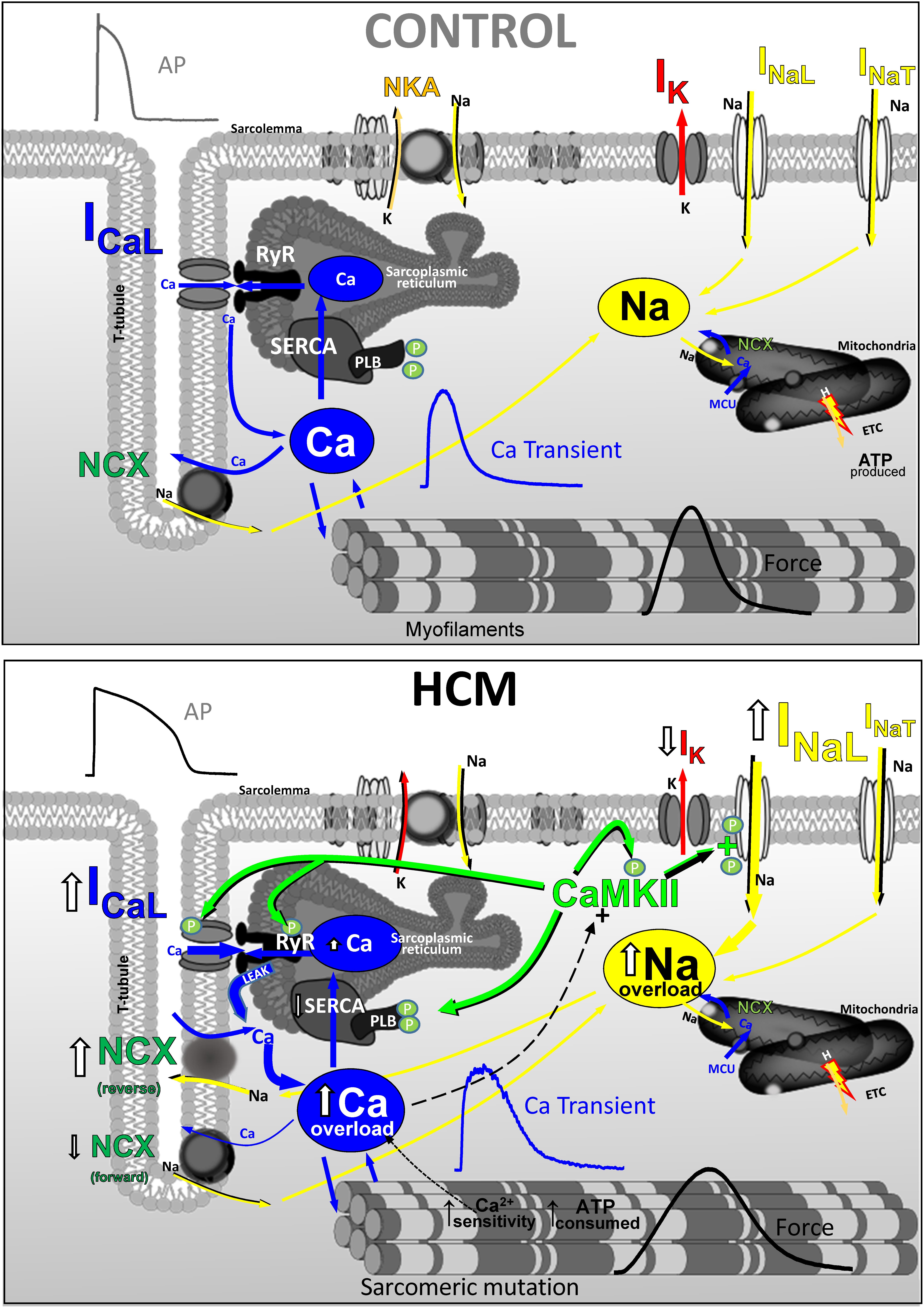
FIGURE 6. Functional changes of ion currernts and EC-coupling in human HCM vs. control myocardium (CARTOON).
Author Contributions
RC performed most of the original experiments described in this review and drafted this manuscript. CF contributed to the original data presented and discussed here, drafted the figures and copyedited the manuscript. AM, CP, and EC supervised the original projects that led to production of the results shown here; moreover, they critically reviewed and edited the manuscript. All authors concur with the current submitted version.
Funding
Original data described in the review are from research work supported by: the Regione Toscana (FAS-Salute 2014, ToRSADE project), Telethon Italy (GGP13162), the EU (STREP Project 241577 BIG HEART, 7th European Framework Program and project SILICOFCM, grant agreement N. 777204, Horizon 2020 research and innovation program) and the Italian Ministry of Health (RF 2010-2313451, RF-2013-02356787, and GR-2011-02350583).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adabag, A. S., Casey, S. A., Kuskowski, M. A., Zenovich, A. G., and Maron, B. J. (2005). Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 45, 697–704. doi: 10.1016/j.jacc.2004.11.043
Anderson, M. E., Brown, J. H., and Bers, D. M. (2011). CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell Cardiol. 51, 468–473. doi: 10.1016/j.yjmcc.2011.01.012
Antzelevitch, C., and Belardinelli, L. (2006). The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J. Cardiovasc. Electrophysiol. 17(Suppl. 1), S79–S85. doi: 10.1111/j.1540-8167.2006.00388.x
Antzelevitch, C., Belardinelli, L., Zygmunt, A. C., Burashnikov, A., Di Diego, J. M., Fish, J. M., et al. (2004). Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation 110, 904–910. doi: 10.1161/01.CIR.0000139333.83620.5D
Ashrafian, H., McKenna, W. J., and Watkins, H. (2011). Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ. Res. 109,86–96. doi: 10.1161/CIRCRESAHA.111.242974
Ashrafian, H., Redwood, C., Blair, E., and Watkins, H. (2003). Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 19, 263–268. doi: 10.1016/S0168-9525(03)00081-87
Authors/Task Force Members Elliott, P. M., Anastasakis, A., Borger, M. A., Borggrefe, M., Cecchi, F., et al. (2014). 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2733–2779. doi: 10.1093/eurheartj/ehu284
Avanesov, M., Munch, J., Weinrich, J., Well, L., Saring, D., Stehning, C., et al. (2017). Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 27, 5136–5145. doi: 10.1007/s00330-017-4869-x
Baudenbacher, F., Schober, T., Pinto, J. R., Sidorov, V. Y., Hilliard, F., Solaro, R. J., et al. (2008). Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J. Clin. Invest. 118, 3893–3903. doi: 10.1172/JCI36642
Beuckelmann, D. J., Nabauer, M., and Erdmann, E. (1992). Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85, 1046–1055. doi: 10.1161/01.CIR.85.3.1046
Brette, F., Despa, S., Bers, D. M., and Orchard, C. H. (2005). Spatiotemporal characteristics of SR Ca2+ uptake and release in detubulated rat ventricular myocytes. J. Mol. Cell Cardiol. 39, 804–812. doi: 10.1016/j.yjmcc.2005.08.005
Brette, F., Salle, L., and Orchard, C. H. (2004). Differential modulation of L-type Ca2 + current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ. Res. 95, e1–e7. doi: 10.1161/01.RES.0000135547.53927.F6
Cecchi, F., Olivotto, I., Gistri, R., Lorenzoni, R., Chiriatti, G., and Camici, P. G. (2003). Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 349, 1027–1035. doi: 10.1056/NEJMoa025050
Chan, R. H., Maron, B. J., Olivotto, I., Pencina, M. J., Assenza, G. E., Haas, T., et al. (2014). Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130, 484–495. doi: 10.1161/CIRCULATIONAHA.113.007094
Cho, J. H., Zhang, R., Kilfoil, P. J., Gallet, R., de Couto, G., Bresee, C., et al. (2017). Delayed repolarization underlies ventricular arrhythmias in rats with heart failure and preserved ejection fraction. Circulation 136, 2037–2050. doi: 10.1161/CIRCULATIONAHA.117.028202
Coppini, R., Ferrantini, C., Yao, L., Fan, P., Del Lungo, M., Stillitano, F., et al. (2013). Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127, 575–584. doi: 10.1161/CIRCULATIONAHA.112.134932
Coppini, R., Mazzoni, L., Ferrantini, C., Gentile, F., Pioner, J. M., Laurino, A., et al. (2017). Ranolazine prevents phenotype development in a mouse model of hypertrophic cardiomyopathy. Circ. Heart Fail. 10:e003565. doi: 10.1161/CIRCHEARTFAILURE.116.003565
Crocini, C., Coppini, R., Ferrantini, C., Yan, P., Loew, L. M., Tesi, C., et al. (2014). Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure. Proc. Natl. Acad. Sci. U.S.A. 111, 15196–15201. doi: 10.1073/pnas.1411557111
Crocini, C., Ferrantini, C., Scardigli, M., Coppini, R., Mazzoni, L., Lazzeri, E., et al. (2016). Novel insights on the relationship between T-tubular defects and contractile dysfunction in a mouse model of hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 91, 42–51. doi: 10.1016/j.yjmcc.2015.12.013
Curran, J., Brown, K. H., Santiago, D. J., Pogwizd, S., Bers, D. M., and Shannon, T. R. (2010). Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J. Mol. Cell Cardiol. 49, 25–32. doi: 10.1016/j.yjmcc.2010.03.013
Dass, S., Suttie, J. J., Piechnik, S. K., Ferreira, V. M., Holloway, C. J., Banerjee, R., et al. (2012). Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ. Cardiovasc. Imaging 5, 726–733. doi: 10.1161/CIRCIMAGING.112.976738
Desantiago, J., Ai, X., Islam, M., Acuna, G., Ziolo, M. T., Bers, D. M., et al. (2008). Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ. Res. 102, 1389–1397. doi: 10.1161/CIRCRESAHA.107.169011
Dybkova, N., Wagner, S., Backs, J., Hund, T. J., Mohler, P. J., Sowa, T., et al. (2014). Tubulin polymerization disrupts cardiac beta-adrenergic regulation of late INa. Cardiovasc. Res. 103, 168–177. doi: 10.1093/cvr/cvu120
Erickson, J. R., He, B. J., Grumbach, I. M., and Anderson, M. E. (2011). CaMKII in the cardiovascular system: sensing redox states. Physiol. Rev. 91, 889–915. doi: 10.1152/physrev.00018.2010
Ferrantini, C., Coppini, R., Pioner, J. M., Gentile, F., Tosi, B., Mazzoni, L., et al. (2017). Pathogenesis of hypertrophic cardiomyopathy is mutation rather than disease specific: a comparison of the cardiac troponin T E163R and R92Q mouse models. J. Am. Heart Assoc. 6, e005407. doi: 10.1161/JAHA.116.005407
Ferrantini, C., Coppini, R., Sacconi, L., Tosi, B., Zhang, M. L., Wang, G. L., et al. (2014). Impact of detubulation on force and kinetics of cardiac muscle contraction. J. Gen. Physiol. 143, 783–797. doi: 10.1085/jgp.201311125
Ferrantini, C., Crocini, C., Coppini, R., Vanzi, F., Tesi, C., Cerbai, E., et al. (2013). The transverse-axial tubular system of cardiomyocytes. Cell Mol. Life Sci. 70, 4695–4710. doi: 10.1007/s00018-013-1410-1415
Ferrantini, C., Pioner, J. M., Mazzoni, L., Gentile, F., Tosi, B., Rossi, A., et al. (2018). Late sodium current inhibitors to treat exercise-induced obstruction in hypertrophic cardiomyopathy: an in vitro study in human myocardium. Br. J. Pharmacol. 175, 2635–2652. doi: 10.1111/bph.14223
Fischer, T. H., Herting, J., Tirilomis, T., Renner, A., Neef, S., Toischer, K., et al. (2013). Ca2 + /calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2 + leak in human cardiac pathology. Circulation 128, 970–981. doi: 10.1161/CIRCULATIONAHA.113.001746
Fraysse, B., Weinberger, F., Bardswell, S. C., Cuello, F., Vignier, N., Geertz, B., et al. (2012). Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J. Mol. Cell Cardiol. 52, 1299–1307. doi: 10.1016/j.yjmcc.2012.03.009
Galati, G., Leone, O., Pasquale, F., Olivotto, I., Biagini, E., Grigioni, F., et al. (2016). Histological and histometric characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy: a clinical-pathological study of 30 explanted hearts. Circ. Heart Fail. 9:e003090. doi: 10.1161/CIRCHEARTFAILURE.116.003090
Gersh, B. J., Maron, B. J., Bonow, R. O., Dearani, J. A., Fifer, M. A., Link, M. S., et al. (2011). 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124, e783–e831. doi: 10.1161/CIR.0b013e318223e2bd
Grandi, E., Pasqualini, F. S., and Bers, D. M. (2010). A novel computational model of the human ventricular action potential and Ca transient. J. Mol. Cell Cardiol. 48, 112–121. doi: 10.1016/j.yjmcc.2009.09.019
Haim, T. E., Dowell, C., Diamanti, T., Scheuer, J., and Tardiff, J. C. (2007). Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J. Mol. Cell Cardiol. 42, 1098–1110. doi: 10.1016/j.yjmcc.2007.03.906
Heinzel, F. R., MacQuaide, N., Biesmans, L., and Sipido, K. (2011). Dyssynchrony of Ca2+ release from the sarcoplasmic reticulum as subcellular mechanism of cardiac contractile dysfunction. J. Mol. Cell Cardiol. 50, 390–400. doi: 10.1016/j.yjmcc.2010.11.008
Helms, A. S., Alvarado, F. J., Yob, J., Tang, V. T., Pagani, F., Russell, M. W., et al. (2016). Genotype-dependent and -independent calcium signaling dysregulation in human hypertrophic cardiomyopathy. Circulation 134, 1738–1748. doi: 10.1161/CIRCULATIONAHA.115.020086
Ho, C. Y., Abbasi, S. A., Neilan, T. G., Shah, R. V., Chen, Y., Heydari, B., et al. (2013). T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ. Cardiovasc. Imaging 6, 415–422. doi: 10.1161/CIRCIMAGING.112.000333
Hudmon, A., and Schulman, H. (2002). Structure-function of the multifunctional Ca2 + /calmodulin-dependent protein kinase II. Biochem. J. 364(Pt 3), 593–611. doi: 10.1042/BJ20020228
Hudmon, A., Schulman, H., Kim, J., Maltez, J. M., Tsien, R. W., and Pitt, G. S. (2005). CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J. Cell Biol. 171, 537–547. doi: 10.1083/jcb.200505155
Hurtado-de-Mendoza, D., Corona-Villalobos, C. P., Pozios, I., Gonzales, J., Soleimanifard, Y., Sivalokanathan, S., et al. (2017). Diffuse interstitial fibrosis assessed by cardiac magnetic resonance is associated with dispersion of ventricular repolarization in patients with hypertrophic cardiomyopathy. J. Arrhythm 33, 201–207. doi: 10.1016/j.joa.2016.10.005
Johnson, J. N., Grifoni, C., Bos, J. M., Saber-Ayad, M., Ommen, S. R., Nistri, S., et al. (2011). Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur. Heart J. 32, 1114–1120. doi: 10.1093/eurheartj/ehr021
Knollmann, B. C., Kirchhof, P., Sirenko, S. G., Degen, H., Greene, A. E., Schober, T., et al. (2003). Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ. Res. 92, 428–436. doi: 10.1161/01.RES.0000059562.91384.1A
Kreusser, M. M., and Backs, J. (2014). Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front. Pharmacol. 5:36. doi: 10.3389/fphar.2014.00036
Lai, Y., Nairn, A. C., and Greengard, P. (1986). Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. U.S.A. 83, 4253–4257. doi: 10.1073/pnas.83.12.4253
Lan, F., Lee, A. S., Liang, P., Sanchez-Freire, V., Nguyen, P. K., Wang, L., et al. (2013). Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12, 101–113. doi: 10.1016/j.stem.2012.10.010
Ling, H., Zhang, T., Pereira, L., Means, C. K., Cheng, H., Gu, Y., et al. (2009). Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J. Clin. Invest. 119, 1230–1240. doi: 10.1172/JCI38022
Lu, T., Lee, H. C., Kabat, J. A., and Shibata, E. F. (1999). Modulation of rat cardiac sodium channel by the stimulatory G protein alpha subunit. J. Physiol. 518( Pt 2), 371–384.
Lyon, A. R., MacLeod, K. T., Zhang, Y., Garcia, E., Kanda, G. K., Lab, M. J., et al. (2009). Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. U.S.A. 106, 6854–6859. doi: 10.1073/pnas.0809777106
Maltsev, V. A., Sabbah, H. N., Higgins, R. S., Silverman, N., Lesch, M., and Undrovinas, A. I. (1998). Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 98, 2545–2552. doi: 10.1161/01.CIR.98.23.2545
Maron, B. J., McKenna, W. J., Danielson, G. K., Kappenberger, L. J., Kuhn, H. J., Seidman, C. E., et al. (2003). American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J. Am. Coll. Cardiol. 42, 1687–1713. doi: 10.1016/S0735-1097(03)00941-0
Maron, B. J., Olivotto, I., Spirito, P., Casey, S. A., Bellone, P., Gohman, T. E., et al. (2000). Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102, 858–864. doi: 10.1161/01.CIR.102.8.858
Maron, M. S., Rowin, E. J., Olivotto, I., Casey, S. A., Arretini, A., Tomberli, B., et al. (2016). Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 67, 1399–1409. doi: 10.1016/j.jacc.2016.01.023
Mattiazzi, A., and Kranias, E. G. (2011). CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm 8, 784–787. doi: 10.1016/j.hrthm.2010.11.035
Maurizi, N., Passantino, S., Spaziani, G., Girolami, F., Arretini, A., Targetti, M., et al. (2018). Long-term outcomes of pediatric-onset hypertrophic cardiomyopathy and age-specific risk factors for lethal arrhythmic events. JAMA Cardiol. 3, 520–525. doi: 10.1001/jamacardio.2018.0789
Morotti, S., Grandi, E., Summa, A., Ginsburg, K. S., and Bers, D. M. (2012). Theoretical study of L-type Ca2+ current inactivation kinetics during action potential repolarization and early afterdepolarizations. J. Physiol. 590,4465–4481. doi: 10.1113/jphysiol.2012.231886
Olivotto, I., Cecchi, F., Poggesi, C., and Yacoub, M. H. (2012). Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ. Heart Fail. 5, 535–546. doi: 10.1161/CIRCHEARTFAILURE.112.967026
Olivotto, I., Girolami, F., Nistri, S., Rossi, A., Rega, L., Garbini, F., et al. (2009). The many faces of hypertrophic cardiomyopathy: from developmental biology to clinical practice. J. Cardiovasc. Transl. Res. 2, 349–367. doi: 10.1007/s12265-009-9137-9132
Orchard, C., and Brette, F. (2008). t-Tubules and sarcoplasmic reticulum function in cardiac ventricular myocytes. Cardiovasc. Res. 77, 237–244. doi: 10.1093/cvr/cvm002
Parikh, A., Mantravadi, R., Kozhevnikov, D., Roche, M. A., Ye, Y., Owen, L. J., et al. (2012). Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm 9, 953–960. doi: 10.1016/j.hrthm.2012.01.010
Passini, E., Minchole, A., Coppini, R., Cerbai, E., Rodriguez, B., Severi, S., et al. (2016). Mechanisms of pro-arrhythmic abnormalities in ventricular repolarisation and anti-arrhythmic therapies in human hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 96, 72–81. doi: 10.1016/j.yjmcc.2015.09.003
Perchenet, L., Hinde, A. K., Patel, K. C., Hancox, J. C., and Levi, A. J. (2000). Stimulation of Na/Ca exchange by the beta-adrenergic/protein kinase A pathway in guinea-pig ventricular myocytes at 37 degrees C. Pflugers Arch. 439, 822–828.
Pieske, B., and Houser, S. R. (2003). [Na + ]i handling in the failing human heart. Cardiovasc. Res. 57, 874–886. doi: 10.1016/S0008-6363(02)00841-6
Pogwizd, S. M., and Corr, P. B. (1987). Electrophysiologic mechanisms underlying arrhythmias due to reperfusion of ischemic myocardium. Circulation 76,404–426. doi: 10.1161/01.CIR.76.2.404
Pogwizd, S. M., Sipido, K. R., Verdonck, F., and Bers, D. M. (2003). Intracellular Na in animal models of hypertrophy and heart failure: contractile function and arrhythmogenesis. Cardiovasc. Res. 57, 887–896. doi: 10.1016/S0008-6363(02)00735-6
Priori, S. G., Blomstrom-Lundqvist, C., Mazzanti, A., Blom, N., Borggrefe, M., Camm, J., et al. (2015). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 36, 2793–2867. doi: 10.1093/eurheartj/ehv316
Ravens, U., and Cerbai, E. (2008). Role of potassium currents in cardiac arrhythmias. Europace 10, 1133–1137. doi: 10.1093/europace/eun193
Sacconi, L., Ferrantini, C., Lotti, J., Coppini, R., Yan, P., Loew, L. M., et al. (2012). Action potential propagation in transverse-axial tubular system is impaired in heart failure. Proc. Natl. Acad. Sci. U.S.A. 109, 5815–5819. doi: 10.1073/pnas.1120188109
Sato, D., Xie, L. H., Sovari, A. A., Tran, D. X., Morita, N., Xie, F., et al. (2009). Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc. Natl. Acad. Sci. U.S.A. 106, 2983–2988. doi: 10.1073/pnas.0809148106
Sequeira, V., Wijnker, P. J., Nijenkamp, L. L., Kuster, D. W., Najafi, A., Witjas-Paalberends, E. R., et al. (2013). Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ. Res. 112, 1491–1505. doi: 10.1161/CIRCRESAHA.111.300436
Shan, J., Betzenhauser, M. J., Kushnir, A., Reiken, S., Meli, A. C., Wronska, A., et al. (2010). Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J. Clin. Invest. 120, 4375–4387. doi: 10.1172/JCI37649
Shannon, T. R., Pogwizd, S. M., and Bers, D. M. (2003). Elevated sarcoplasmic reticulum Ca2 + leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 93, 592–594. doi: 10.1161/01.RES.0000093399.11734.B3
Sicouri, S., Belardinelli, L., and Antzelevitch, C. (2013). Antiarrhythmic effects of the highly selective late sodium channel current blocker GS-458967. Heart Rhythm 10, 1036–1043. doi: 10.1016/j.hrthm.2013.03.023
Song, L. S., Sobie, E. A., McCulle, S., Lederer, W. J., Balke, C. W., and Cheng, H. (2006). Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. U.S.A. 103, 4305–4310. doi: 10.1073/pnas.0509324103
Sotgia, B., Sciagra, R., Olivotto, I., Casolo, G., Rega, L., Betti, I., et al. (2008). Spatial relationship between coronary microvascular dysfunction and delayed contrast enhancement in patients with hypertrophic cardiomyopathy. J. Nucl. Med. 49, 1090–1096. doi: 10.2967/jnumed.107.050138
Taggart, P., Sutton, P., Chalabi, Z., Boyett, M. R., Simon, R., Elliott, D., et al. (2003). Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation 107, 285–289. doi: 10.1161/01.CIR.0000044941.13346.74
Terracciano, C. M., Philipson, K. D., and MacLeod, K. T. (2001). Overexpression of the Na+/Ca2+ exchanger and inhibition of the sarcoplasmic reticulum Ca2+-ATPase in ventricular myocytes from transgenic mice. Cardiovasc. Res. 49, 38–47. doi: 10.1016/S0008-6363(00)00205-4
Terrenoire, C., Clancy, C. E., Cormier, J. W., Sampson, K. J., and Kass, R. S. (2005). Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ. Res. 96, e25–e34. doi: 10.1161/01.RES.0000160555.58046.9a
Toischer, K., Rokita, A. G., Unsold, B., Zhu, W., Kararigas, G., Sossalla, S., et al. (2010). Differential cardiac remodeling in preload versus afterload. Circulation 122, 993–1003. doi: 10.1161/CIRCULATIONAHA.110.943431
Ulus, T., Kudaiberdieva, G., and Gorenek, B. (2013). The onset mechanisms of ventricular tachycardia. Int. J. Cardiol. 167, 619–623. doi: 10.1016/j.ijcard.2012.09.034
van der Velden, J., Ho, C. Y., Tardiff, J. C., Olivotto, I., Knollmann, B. C., and Carrier, L. (2015). Research priorities in sarcomeric cardiomyopathies. Cardiovasc. Res. 105, 449–456. doi: 10.1093/cvr/cvv019
Wagner, S., Dybkova, N., Rasenack, E. C., Jacobshagen, C., Fabritz, L., Kirchhof, P., et al. (2006). Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 116, 3127–3138. doi: 10.1172/JCI26620
Wagner, S., Hacker, E., Grandi, E., Weber, S. L., Dybkova, N., Sossalla, S., et al. (2009). Ca/calmodulin kinase II differentially modulates potassium currents. Circ. Arrhythm Electrophysiol. 2, 285–294. doi: 10.1161/CIRCEP.108.842799
Weisser-Thomas, J., Piacentino, V. III, Gaughan, J. P., Margulies, K., and Houser, S. R. (2003). Calcium entry via Na/Ca exchange during the action potential directly contributes to contraction of failing human ventricular myocytes. Cardiovasc. Res. 57, 974–985. doi: 10.1016/S0008-6363(02)00732-0
Xu, L., Lai, D., Cheng, J., Lim, H. J., Keskanokwong, T., Backs, J., et al. (2010). Alterations of L-type calcium current and cardiac function in CaMKII{delta} knockout mice. Circ. Res. 107, 398–407. doi: 10.1161/CIRCRESAHA.110.222562
Keywords: cardiac hypertrophy, ion channels, ranolazine, arrhythmias, afterdepolarization, calmodulin kinase (CaMKII), T-tubules, beta adrenergic
Citation: Coppini R, Ferrantini C, Mugelli A, Poggesi C and Cerbai E (2018) Altered Ca2+ and Na+ Homeostasis in Human Hypertrophic Cardiomyopathy: Implications for Arrhythmogenesis. Front. Physiol. 9:1391. doi: 10.3389/fphys.2018.01391
Received: 29 June 2018; Accepted: 13 September 2018;
Published: 16 October 2018.
Edited by:
Gaetano Santulli, Columbia University, United StatesReviewed by:
Dmitry Terentyev, Brown University, United StatesJordi Heijman, Maastricht University, Netherlands
Copyright © 2018 Coppini, Ferrantini, Mugelli, Poggesi and Cerbai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Coppini, cmFmZmFlbGUuY29wcGluaUB1bmlmaS5pdA==
 Raffaele Coppini
Raffaele Coppini Cecilia Ferrantini
Cecilia Ferrantini Alessandro Mugelli
Alessandro Mugelli Corrado Poggesi
Corrado Poggesi Elisabetta Cerbai
Elisabetta Cerbai