- Department of Biomedical Sciences, College of Veterinary Medicine, University of Missouri, Columbia, MO, United States
The prevalence of diabetes is rapidly increasing and closely associated with cardiovascular morbidity and mortality. While the major cardiovascular complication associated with diabetes is coronary artery disease, it is becoming increasingly apparent that diabetes impacts the electrical conduction system in the heart, resulting in atrial fibrillation, and ventricular arrhythmias. The relationship between diabetes and arrhythmias is complex and multifactorial including autonomic dysfunction, atrial and ventricular remodeling and molecular alterations. This review will provide a comprehensive overview of the link between diabetes and arrhythmias with insight into the common molecular mechanisms, structural alterations and therapeutic outcomes.
Introduction
Diabetes mellitus is a group of metabolic disorders where there are high blood sugar levels over time. Prolonged elevations in sugar levels lead to a number of health complications including cardiovascular disease and kidney disease (Forbes and Cooper, 2013). There are two main forms of diabetes mellitus including type 1, which has an unknown etiology and is characterized by a loss of insulin-producing β-cells in the pancreas resulting in the inability of the pancreas to produce enough insulin (van Belle et al., 2011). Type 1 diabetes is often juvenile in onset, insulin dependent, and comprises roughly 10% of the diabetic patient population. Type 2 diabetes results from insulin resistance and the body's inability to respond to insulin (Kahn et al., 2014). It is generally adult-onset and is a result of genetics and lifestyle choices including excessive body weight, lack of exercise and poor diet. Type 2 diabetes is rapidly increasing in incidence (Centers for Disease C and Prevention, 2008). As of 2015 there were an estimated 415 million people with diabetes worldwide (Federation, 2014).
Cardiac arrhythmia is a group of conditions where the heart beats too fast (tachycardia), too slow (bradycardia) or irregularly (Roberts-Thomson et al., 2011). While most arrhythmias are not serious acutely, prolonged arrhythmic episodes increase an individual's likelihood of stroke, heart failure and cardiac arrest (Nattel et al., 2014). Arrhythmias arise due to a problem in the electrical conduction of the heart however, the cause of these complications is not fully defined. Atrial fibrillation is the most common type of arrhythmia and is associated with significant morbidity and mortality (Kannel et al., 1983). It is becoming increasingly apparent that diabetes mellitus is a significant promoter of cardiac arrhythmias (Kannel et al., 1998).
While diabetes likely contributes to multiple types of cardiac arrhythmias, the connection between diabetes and atrial fibrillation has been the most extensively studied to date. Observational studies looking at the association between diabetes mellitus and atrial fibrillation have been inconclusive and inconsistent (Benjamin et al., 1994; Psaty et al., 1997; Wilhelmsen et al., 2001; Nichols et al., 2009; Pallisgaard et al., 2016; Dahlqvist et al., 2017). The incidence of diabetes is most commonly associated with coronary artery disease however, electrical conduction complications are also an important cardiovascular problem associated with both type 1 and type 2 diabetes (Huxley et al., 2011; Dahlqvist et al., 2017). In a 38 year follow up of Framingham heart study patients, diabetes mellitus was identified as an independent risk factor of atrial fibrillation (Benjamin et al., 1994). However, other studies failed to see a connection between atrial fibrillation and diabetes (Wilhelmsen et al., 2001). Discrepancies in these studies may be a result of the populations examined since differences in the association of diabetes and atrial fibrillation appear to be variable depending on age (Pallisgaard et al., 2016), gender (Nichols et al., 2009; Dahlqvist et al., 2017) and ethnicity (Lipworth et al., 2012; Dewland et al., 2013; Rodriguez et al., 2016; O'Neal et al., 2017). In a comprehensive meta-analysis, diabetic patients were found to have an ~40% greater risk for developing atrial fibrillation compared to non-diabetic patients (Huxley et al., 2011) and a more recent meta-analysis, identified a 20% increase in the risk of developing atrial fibrillation for prediabetic patients whereas in patients with diabetes, this number was elevated to 28% greater change of atrial fibrillation development (Aune et al., 2018). Furthermore, this meta-analysis identified a dose dependent relationship between increased blood glucose levels and atrial fibrillation suggesting that rises in glucose may be an important contributor to atrial fibrillation. In a large study, over 845,000 patients, diabetes was found to be a strong, independent risk factor for atrial fibrillation and other cardiovascular diseases mellitus (Movahed et al., 2005). However, obesity, which is common in patients with diabetes mellitus is independently associated with atrial fibrillation and might also be a contributing factor (Grundvold et al., 2015; Baek et al., 2017). Levels of pericardial fat have been linked to atrial fibrillation in humans (Al Chekakie et al., 2010). Though not as extensively characterized, diabetes likely also contributes to ventricular arrhythmias since there is electrocardiographic evidence of this in humans and the underlying mechanisms linking diabetes with atrial fibrillation would apply to other types of arrhythmias (Cardoso et al., 2003). Table 1 summarizes the clinical studies examining the correlation between diabetes and arrhythmias. While there is a clear link between diabetes and cardiac arrhythmias, the mechanisms underlying these changes are not fully elucidated. Potential causes include changes in glucose levels, the autonomic nervous system, structural and electrical remodeling, mitochondrial alterations and inflammation will be reviewed herein (Figure 1).
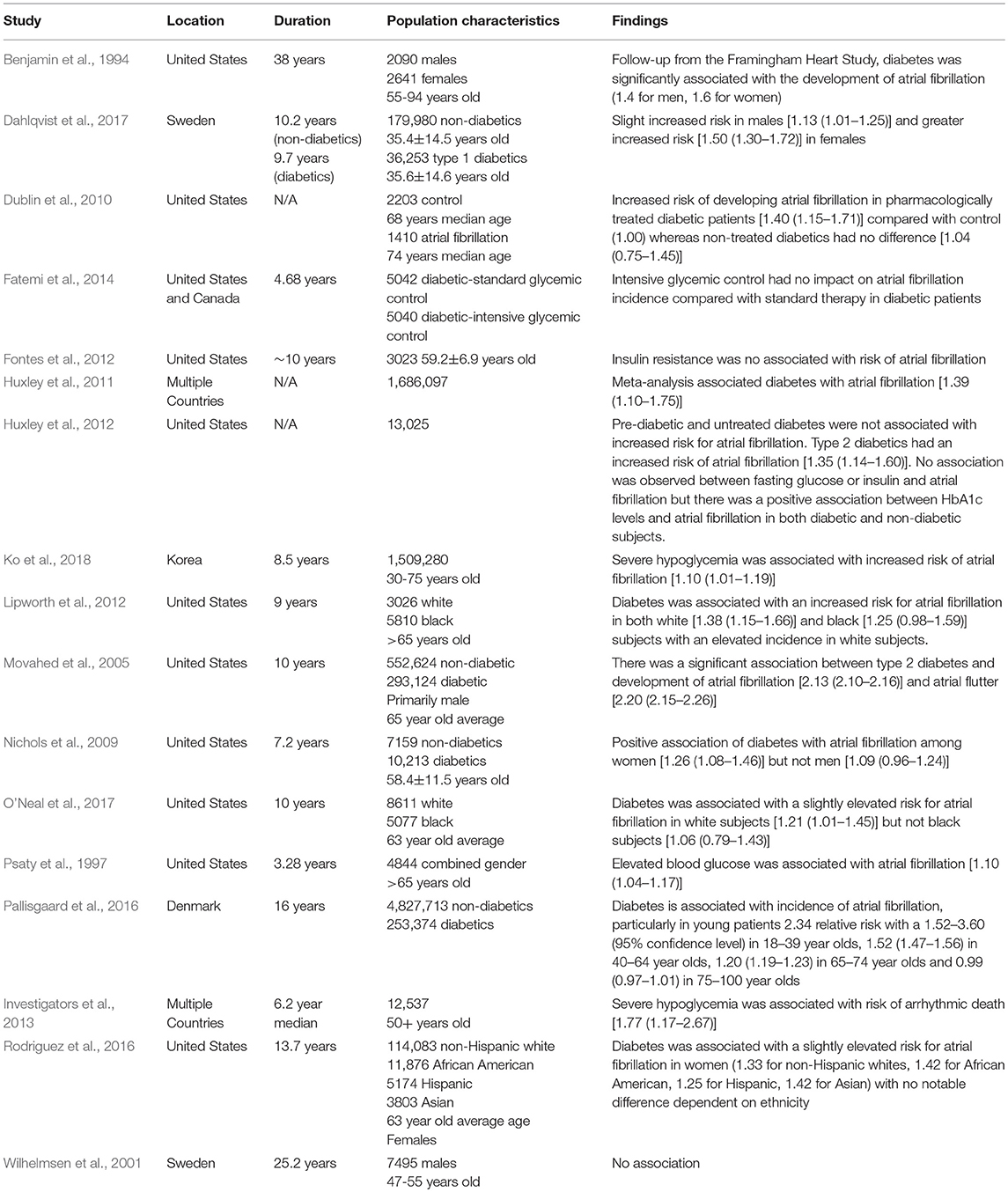
Table 1. Characterization of studies evaluating the correlation between diabetes and arrhythmias. Statistics are reported as [risk ratio (95% confidence interval)].
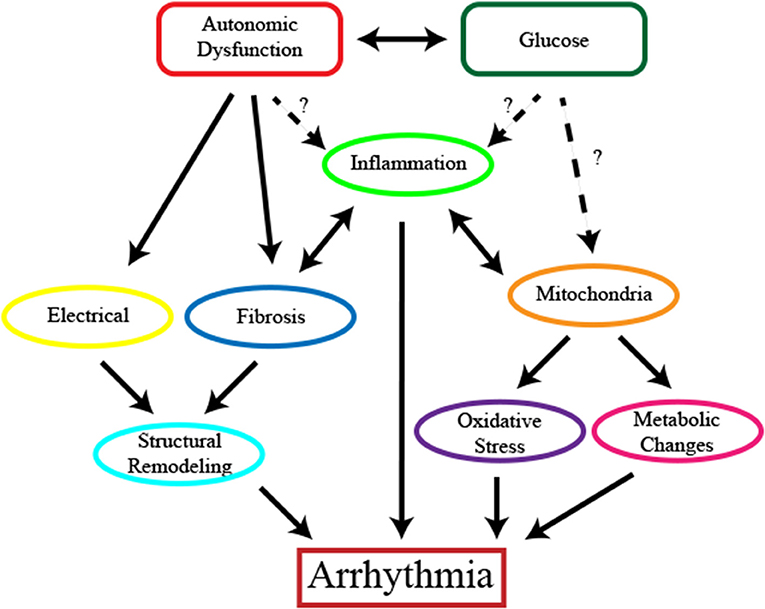
Figure 1. The complex relationship between diabetes and cardiac arrhythmias. Potential contributors to the induction of cardiac arrhythmias including hypoglycemia, hyperglycemia or glucose fluctuations and autonomic dysfunction activate multiple mechanisms to contribute to the development of cardiac arrhythmias. Structural remodeling including changes in the electrical conduction of the heart and fibrosis promote and potentiate the progression of the disease. Mitochondrial dysfunction leads to changes in cardiomyocyte function and metabolism and contributes to disease progression through oxidative stress. Inflammation is present and may arise as a result of oxidative stress and structural changes.
Blood Glucose Levels
Meta-analysis of clinical populations suggests a dose-dependent relationship between blood glucose levels and atrial fibrillation, implying that glucose levels may be an important contributor to atrial fibrillation onset (Aune et al., 2018). However, this may not be the case since intensive glucose control has not been shown to be beneficial in reducing death from cardiovascular causes or all-cause death in multiple large trials (Group et al., 2008; Duckworth et al., 2009) and has been associated with increased mortality in another (Action to Control Cardiovascular Risk in Diabetes Study et al., 2008). Duration of pharmacological treatment (Dublin et al., 2010) and poorly controlled diabetes have also been linked to increased incidence of atrial fibrillation (Huxley et al., 2012). However, in a prospective study of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial cohort of patients, intensive glycemic control did not impact the rate of new-onset atrial fibrillation (Fatemi et al., 2014). Animal studies investigating the involvement diabetes in atrial fibrillation suggest it may be due to glucose fluctuations rather than hyperglycemia (Saito et al., 2014). In a streptozotocin-induced rat model, glucose fluctuations increased incidence of atrial fibrillation, atrial fibrosis, and reactive oxygen species (Saito et al., 2014).
There is increasing evidence that hypoglycemic states may contribute to atrial fibrillation. Several reports of hypoglycemic triggered atrial fibrillations have been reported clinically (Odeh et al., 1990; Celebi et al., 2011; Ko et al., 2018) and information gathered from the Framingham Heart Study suggests insulin resistance does not play a role (Fontes et al., 2012). Severe hypoglycemia in type 2 diabetics was associated with a range of adverse clinical outcomes including death from cardiovascular cause (Chow et al., 2014) and in patients from the Outcomes Reduction with an Initial Glargine Intervention (ORIGIN) trial, severe hypoglycemia was associated with greater risk for all-cause mortality and arrhythmic death (Investigators et al., 2013). In a study using 30 patients with type 2 diabetes and known cardiovascular disease, glucose monitoring in conjunction with electrocardiograms showed patients taking insulin and/or sulfonylurea had a high incidence of severe (< 3.1 mmol/L) but asymptomatic hypoglycemia whereas patients taking metformin and/or dipeptidyl peptidase-4 inhibitors did not and patients with severe hypoglycemia had more ventricular arrhythmias (Stahn et al., 2014). In a separate but similar study, type 2 diabetic patients with a history or risk of cardiovascular disease were monitored for interstitial glucose and ambulatory electrocardiogram simultaneously (Chow et al., 2014). Bradycardia and atrial and ventricular ectopic counts were higher during episodes of nocturnal hypoglycemia further suggesting a role for hypoglycemia in arrhythmic events.
Autonomic Dysfunction
The autonomic nervous system is an important regulator of heart rhythm through innervation by sympathetic and parasympathetic nerves. Dysfunction of the autonomic nervous system is recognized as a risk for development of atrial fibrillation and a contributing factor to disease progression (Agarwal et al., 2017). A link between type 2 diabetes and autonomic dysfunction has also been well established and is recognized as a complication that damages multiple organs including the heart (Mäkimattila et al., 1997; Oberhauser et al., 2001). While the etiology of diabetic autonomic neuropathy is not fully understood, it is thought that metabolic insult, neurovascular insufficiency, autoimmune damage and deficiency in neurohormonal growth factors may be contributing factors to the damage or loss of nerves (Vinik et al., 2003). Despite the fact that cardiovascular autonomic neuropathy has been extensively studied, it remains largely overlooked and serious complication of diabetes (Vinik et al., 2003).
In a study of nearly 2,000 men and women from the Framingham Offspring Study, heart rate variability, an indicator of autonomic nervous system function, was associated with plasma glucose levels and reduced in diabetic and patients with impaired fasting glucose levels (Singh et al., 2000). In healthy, non-diabetic adults, impaired heart rate recovery, another measure of autonomic dysfunction, and a predictor of all cause death in diabetic patients (Wheeler et al., 2002; Cheng et al., 2003), was more common in participants with abnormal fasting plasma glucose levels, which was also true in diabetic patients (Panzer et al., 2002). A closer examination of the link between diabetes and autonomic neuropathy using heart rate recovery as measure of autonomic dysfunction also associated diabetes and autonomic dysfunction with new-onset atrial fibrillation and heart failure independent of other cardiovascular risk factors (Negishi et al., 2013). Interestingly, this study demonstrated an incremental and predictive association between diabetes, heart rate recovery and new-onset atrial fibrillation. There is some evidence that the presence of cardiac autonomic neuropathy in asymptomatic type 1 and type 2 diabetes patients could predict major cardiovascular events including arrhythmias (Valensi et al., 2001). The recurrence of atrial fibrillation is increased in diabetic patients with autonomic neuropathy, which was determined by Ewing's test. Electrocardiographic measurements from diabetic patients with autonomic neuropathy had a longer P-wave duration and dispersion compared to control patients or diabetic patients, suggesting that autonomic neuropathy is causing inhomogeneous atrial depolarization to trigger atrial fibrillation (Bissinger et al., 2011). In a study investigating the changes in autonomic function and repolarization that occurring during prolonged hypoglycemia in type 2 diabetic patients, twelve type 2 diabetic patients and eleven age and body mass index-matched control patients had their glucose levels maintained by hyperinsulinemic clamps at euglycemia (6 mmol/L) or hypoglycemia (2.5 mmol/L; Chow et al., 2017). Differences in autonomic regulation during periods of hypoglycemia, as indicated by heart rate, heart rate variability and blood pressure, occurred between patients with type 2 diabetes and controls during hypoglycemia, suggesting that changes in cardiac autonomic regulation in diabetic patients may occur during hypoglycemic episodes and may contribute to arrhythmias (Chow et al., 2017).
Animal models of diabetes show alterations in cardiac innervation (Gando et al., 1993; Otake et al., 2009; Švíglerová et al., 2011; Thaung et al., 2015). In a streptozotocin-induced diabetic rat model, diabetic rats had increased heterogeneity of sympathetic nerves as measure by immunohistochemistry for tyrosine hydroxylase (sympathetic nerves) and acetylcholinesterase (parasympathetic nerves; Otake et al., 2009). This study also found that sympathetic nerve stimulation increased incidence of atrial fibrillation in diabetic rats. Zucker diabetic fatty rats have elevated resting cardiac sympathetic nerve activity (Thaung et al., 2015). Signs of chronic β-adrenergic stimulation were observed in hearts from these rats including impaired responses to dobutamine stimulation, downregulation of β1-adrenergic receptors and increases in Gαi proteins. These studies demonstrate dysfunction of the autonomic nervous system in diabetes, confirming the findings from human studies.
Structural Remodeling
Structural remodeling likely plays a large role in which diabetes mellitus and obesity promote cardiac arrhythmias. Atrial hypertrophy, fibrosis and fat deposits are observed in the hearts of obese and type II diabetes patients (Tadic and Cuspidi, 2015). Extensive atrial fibrosis is a hallmark of atrial fibrillation and is thought to play a role in both initiating and perpetuation the arrhythmia. Fibrotic tissue in the myocardium disrupts the geometry of the heart and alters the mechanical, electrical and chemical composition (Figure 2). There is extensive evidence of cardiac fibrosis in both type 1 Sutherland et al., 1989 and type 2 diabetes (Regan et al., 1977; Fischer et al., 1984; Nunoda et al., 1985; van Hoeven and Factor, 1990; Shimizu et al., 1993; Kawaguchi et al., 1997) however, the contribution of fibrosis to atrial fibrillation is not fully understood in the context of diabetes. Structural remodeling is particularly relevant in diabetic cardiomyopathy where architectural changes including fibrosis and cardiomyocyte length changes as a result of cardiac dilation increased axial resistance in cardiomyocytes which exasperates conduction dysfunction (Aromolaran and Boutjdir, 2017).
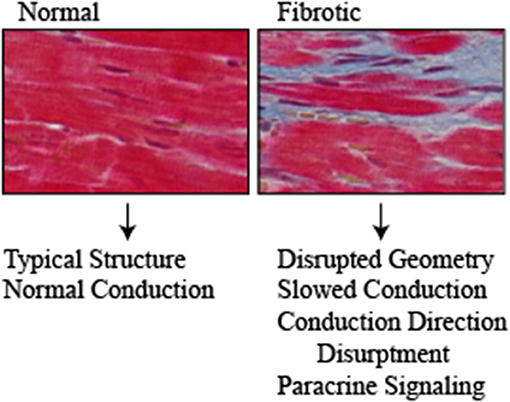
Figure 2. Normal and fibrotic cardiac tissue highlights the structural changes that occur with fibrosis (Red = cardiomyocytes, Blue = fibrosis). Structural changes that occur with diabetes contribute to the pathogenesis of arrhythmias through disrupting the normal architecture of the heart. Fibrosis and fat deposits slow the electrical conduction and disrupt the direction. Furthermore, they serve as a source of paracrine signaling molecules including cytokines/chemokines, adipokines and pro-fibrotic that exasperate the disease.
Animal models of diabetes also exhibit signs of increased cardiac fibrosis (Kato et al., 2006; Liu et al., 2012). In Goto-Kakizaki rats, a genetic non-overweight type 2 diabetes model with slight impairments of glucose tolerance, Goto-Kakizaki rats had significantly greater atrial arrhythmogenicity and increased atrial fibrosis compared with control rats (Kato et al., 2006). However, this study did not examine the progression of the disease making it difficult to determine if arrhythmias arose due to atrial fibrosis or if fibrosis is a contributing factor to atrial fibrillation. In a rabbit alloxan-induced diabetic model, diabetic rabbits had increased atrial interstitial fibrosis and electrophysiological changes including a prolonged inter-atrial conduction time and increased atrial effective refractory period which increased the inducibility of atrial fibrillation (Liu et al., 2012).
Advanced glycation end products (AGEs) are proteins or lipids that become glycated as a result of sugar exposure and have become recognized as a major contributor to complications from diabetes (Ramasamy et al., 2011). AGEs and AGE receptors (RAGEs) may contribute to the structural remodeling seen in the diabetic heart. AGEs and RAGEs are increased in streptozotocin-induced diabetic rat atrial and inhibition of AGE formation significantly reduced elevated levels of connective tissue growth factor and atrial fibrosis observed in the diabetic rats (Kato et al., 2008). Elevated RAGE has been associated with atrial fibrillation in humans, however the link between RAGE and diabetes was not observed in this study (Lancefield et al., 2016). However, other mechanisms likely also contribute to the fibrosis seen in the diabetic heart. Studies using fasudil, a Rho-kinase (ROCK) inhibitor, and a high-fat/low dose streptozotocin rat model of diabetes, implemented the RhoA/ROCK pathway in cardiac fibrosis through decreasing RhoA, ROCK and collagen expression (Chen et al., 2014).
While not as extensively investigated as fibrosis, increases in epicardial and pericardial fat is associated with type 2 diabetes (Rosito et al., 2008; Noyes et al., 2014; Levelt et al., 2016) and are correlated with left atrial enlargement, cardiac structural changes, and has been associated with increased atrial fibrillation risk (Al Chekakie et al., 2010; Batal et al., 2010; Wong et al., 2011). Increased epicardial fat is associated with adipocyte infiltration into the myocardium, which contributes to changes in the electrical conduction between cardiomyocytes due to physical disruption and slowing of the conduction time (Friedman et al., 2014; Mahajan et al., 2015; Haemers et al., 2017). Epicardial and pericardial fat is also an abundant source of adipokines and cytokines which have pro-fibrotic and pro-inflammatory effects on the heart. Studies have shown that the secretome from human epicardial fat, including TGF-β family members and matrix metalloproteinases, produces a pro-fibrotic response in rat atrial myocardium (Venteclef et al., 2015). Additionally, pericardial and epicardial fat have increased markers of inflammation including C-reactive protein, IL-6, IL-1β, and TNF-α, which are associated with increased incidence, severity and reoccurrence of atrial fibrillation (Abe et al., 2018). Furthermore, inflammatory mediator production is not reversed by standard therapies including angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (Mazurek et al., 2003).
Role of Electrical Conduction
Studies consistently demonstrate prolonged action potentials in diabetic patients and animal models. Type 1 (Sivieri et al., 1993) and type 2 (Jermendy et al., 1990; Veglio et al., 2002; Ramirez et al., 2011) diabetic patients have been identified as having slowed conduction velocity and an increased prevalence of prolonged QT interval. In some cases, this has been linked with autonomic neuropathy (Ewing and Neilson, 1990). This is also observed in numerous animal models of type 1 and type 2 diabetes (Xu et al., 2002; Lengyel et al., 2007; Huang et al., 2013). Studies in Goto-Kakizaki (Howarth et al., 2008) and Zucker Diabetic Fatty rats (VanHoose et al., 2010) also identified prolonged QT intervals with additional changes in the R wave amplitudes and signs of autonomic neuropathy. While prolongation of action potentials consistently occurs throughout studies, the mechanisms responsible are less clear. Potassium channels have been the most widely identified change leading to slowed action potentials in diabetic hearts. Prolongation of the QT interval is observed in diet-induced obese mice, which was attributed to a protein kinase D-dependent reduction in voltage gated potassium channel expression (Huang et al., 2013). Oxidative stress-induced alterations in GsH redox state, which will be discussed in more detail in subsequent sections, may regulate K+ channels, that have been shown to be decreased in myocytes isolated from diabetic rats and be reversed by insulin application (Xu et al., 2002). This has also been further supported in follow-up studies, corroborating the involvement of glucose metabolism in these changes (Xu et al., 1996). Changes in outward K+ currents are observed early after streptozotocin injection and corresponded with increases in glucose levels, which were prevented by blocking hyperglycemia (Shimoni et al., 1994). Periodic changes in K+ ion current have been linked to oscillations in energy metabolism in cardiomyocytes, which could be modulated by changing glucose metabolism (O'Rourke et al., 1994). Studies have shown that in cardiomyocytes, glycolysis is more effective than oxidative phosphorylation at regulating K+ channel opening (Weiss and Lamp, 1987). These changes in K+ channels observed in small animals appear to also hold true in larger, more human relevant animal species. In a type 1 diabetes model in dogs, while only slight lengthening in ventricular repolarization was observed, there were decreases in transient outward K+ and slow delayed rectifier potassium currents (Lengyel et al., 2007). In a streptozotocin-induce type 1 diabetes mouse model, prolongation of the QT interval were observed along with increased susceptibility to arrhythmia and decreased K+ currents (Meo et al., 2016). Many of these studies make it difficult to determine the mechanisms responsible for changes in electrical conduction since animal models often have a number of alterations in metabolism including hyperglycemia and hyperlipidemia. However, studies using a cardiac-specific insulin receptor knockout mouse model, several K+ channel components that are important for ventricle repolarization were identified as being decreased, in particular components of the transient outward K+ current fast component, which were also associated with a reduction in the current (Lopez-Izquierdo et al., 2014). Similar with what is seen in other diabetic models and human patients, cardiac-specific insulin receptor knockout mice also had a longer ventricular action potential duration due to a prolonged QT interval, substantiating the role of insulin signaling in diabetes-induced arrhythmias.
Contrarily, not all research implicates K+ currents in action potential changes with diabetes. In a fructose-fat fed rat model of pre-diabetes, QRS prolongation was present, slower conduction velocity, and increased propensity for ventricular fibrillation (Axelsen et al., 2015). There were no changes in Na+ or K+ currents, fibrosis or gap junctions, suggesting another mechanism for dysfunction. In an alloxan-induced diabetic rabbit model, no changes in action potential duration were observed, but there was a reduction in conduction velocity (Stables et al., 2014). A reduction in cell capacitance and Na+ channel density were present in diabetic hearts however, no changes in gap junctions or fibrosis were observed. In obesity-induced QT interval prolongation, there is extensive literature connecting abnormal calcium conduction with arrhythmias (Aromolaran and Boutjdir, 2017). However, this mechanism of arrhythmogenesis is not clear in diabetes and if Na+ or Ca+ channels play an important role in diabetes-induced arrthymias remains to be determined. Energy in the form of ATP is necessary for maintaining membrane potential and generating action potentials, which is mainly supplied by oxidative phosphorylation in the mitochondria, and oxidative phosphorylation to a lesser extent (Barth and Tomaselli, 2009). It is likely that metabolic activity and arrhythmias are interdependent since changes in the cellular energy promotes arrhythmias however arrhythmias also influence metabolic activity. Whole transcriptome analysis of human atrial tissue revealed an upregulation of metabolic process related genes with atrial fibrillation (Barth et al., 2005). This was confirmed in a study using human atrial appendages where metabolomics and proteomics were performed comparing patients with sinus rhythm compared to patients that developed persistent atrial fibrillation following cardiac surgery (Mayr et al., 2008). Patients with atrial fibrillation had an elevation in substrates and enzymes for ketogenic metabolism and other metabolic processes. Additionally, mutantion or knockout of important ion channel genes cause both prolonged ventricular repolarization as well as diabetes (Hu et al., 2014). Hypoglycaemia is also associated with hypokalemia, which could contribute to delayed repolarization (Petersen et al., 1982; Heller and Robinson, 2000; Christensen et al., 2009). As mentioned above, there is extensive clinical and experimental evidence to suggest that structural alterations contribute to the occurrence and persistence of atrial fibrillation (Nattel and Harada, 2014). Changes in gap junctions, which are important for the electrical impulse propagation and synchronization in the heart, are observed with fibrosis and hypertrophy and affect the electrical conduction of the heart (Spach et al., 1988; Saffitz and Kléber, 2004; Ten Tusscher and Panfilov, 2007; dos Santos et al., 2016). In the adult heart, connexin-43 is the main cardiac gap junction component and changes in expression, distribution or post-translational modifications contribute to heart rhythm disturbances (Boengler et al., 2006). Therapies to restore connexin levels are capable of improving conduction disturbances in atrial fibrillation models, further supporting the importance of connexins in the pathogenesis of atrial fibrillation (Igarashi et al., 2012).
There may be alterations in gap junctions in the heart with diabetes. Decreases in phosphorylated and overall connexin-43 levels, a major gap junction protein which has been linked with atrial fibrillation, have been shown in a streptozotocin-induced diabetes model (Mitasíková et al., 2009) which may occur through protein kinase Cε-dependent mechanisms (Lin et al., 2006). These changes were associated with a decreased in connexin-43 phosphorylation and ventricular conduction abnormalities. However, in a different study that also used a streptozotocin-induced diabetes model, connexin-43 levels were elevated and distribution changes were evident (Hage et al., 2017). Other studies using diabetic (db/db) mice show decreased connexin-43 expression that can be reversed with exercise (Veeranki et al., 2016). In this study, exercise resulted in improvements in mitochondrial oxygen consumption rate, tissue ATP levels and reduced cardiac fibrosis with diabetes. Further convoluting the involvement of cardiac connexin-43 in diabetes-induced arrhythmias, an obese diabetic (db/db) mouse model of diabetes showed was atrial hypertrophy and fibrosis without alterations in connexin-43 staining (Hanif et al., 2017). No alterations in connexin-43 levels were also observed in a Zucker Diabetic Fatty rat model of type 2 diabetes, where the conduction velocity was significantly slower in diabetic rats, but levels of connexin-43 were unchanged (Olsen et al., 2013). However, this study did observe distribution changes in connexin-43, which may contribute to functional changes.
Changes in other connexins may also contribute to the pathogenesis of cardiac arrhythmia with diabetes. In a streptozotocin-induced diabetic rat model where connexin-40, 43, and 45 mRNA expression was measured in the sinoatrial node, right ventricle and right atrium, connexin-45 expression was significantly elevated in the sinoatrial node with no changes seen in the atrial or ventricles (Howarth et al., 2007). Using a streptozotocin-induced diabetic rat model, the duration of atrial tachyarrhythmia induced by atrial stimulation was extended in diabetic rats while the conduction velocity was decreased (Watanabe et al., 2012). Increased atrial fibrosis was also observed in diabetic rats compared with controls and had decreased connexin 40 expression with no significant differences in connexin 43. A separate study examining mRNA changes in sinoatrial node of streptozotocin-induced diabetic rats failed to see differences in connexin 40 expression but identified increased transcript expression for connexin 45 among changes in numerous other ion channels including transient receptor potential channel (TRPC) 1, TRPC6, voltage gated calcium channel (Cav) 3.1, Cavβ3, ryanodine receptor 3 and Cavγ4 (Ferdous et al., 2016). This same group identified a unique profile of ion channel alterations in the atrioventricular node with no changes in connexins (Howarth et al., 2017).
Role of Mitochondria and Oxidative Stress
The contribution of oxidative stress to the pathogenesis of cardiac arrhythmias is becoming increasingly recognized (Yang and Dudley, 2013; Samman Tahhan et al., 2017). There are a number of signs of oxidative stress with atrial fibrillation including increased levels of superoxide and hydrogen peroxide (Dudley et al., 2005; Kim et al., 2005; Reilly et al., 2011; Zhang et al., 2012), decreased nitric oxide bioavailability (Cai et al., 2002; Bonilla et al., 2012), changes in the ratio of oxidized glutathione disulfide to reduced glutathione and differences in the ratio of oxidized cysteine to reduced cysteine (Neuman et al., 2007). There is also known to be increased oxidative stress in diabetes, which contributes to the damage of multiple tissue types throughout the body including the heart (Giacco and Brownlee, 2010; Rochette et al., 2014). In diabetes, there is a known increase in superoxide production, which contributes to a reduction in vascular nitric oxide bioactivity through increased NADPH oxidases and dysfunction endothelial nitric oxide synthase (Guzik et al., 2002) which is similar decreases in endothelial nitric oxide synthase and nitric oxide bioavailability are associated with atrial fibrillation (Cai et al., 2002).
Changes in oxidative stress that are present in the heart during diabetes are likely mitochondrial in origin since in diabetic human atrial tissue, where mitochrondrial changes in metabolism of multiple substrates are observed (Anderson et al., 2009). Hydrogen peroxide emissions are increased regardless of the substrate, suggesting alterations in the electron transport system or antioxidant capacity (Anderson et al., 2009). In permeabilized myofibers from right atrial appendages obtained from non-diabetic and type 2 diabetic patients, mitochondria from diabetic patients had a decreased capacity for glutamate and fatty acid-supported respiration, increased content of myocardial triglycerides and increased mitochondrial hydrogen peroxide emission during oxidation of carbohydrate and lipid based substrates (Anderson et al., 2009). There is some evidence that oxidative stress contributes to the atrial remodeling and inflammation seen with atrial fibrillation. In a rabbit alloxan-induced diabetes model, Langendorff perfused diabetic hearts had greater induction of atrial fibrillation following burst pacing, which was decreased with use of the antioxidant probucol (Fu et al., 2015). Antioxidant administration also attenuated atrial interstitial fibrosis and signs of decreased oxidative stress including reductions in serum and tissue malonaldehyde, NF-κB, TGF-β, and TNF-α. However, several studies in humans have shown little or no cardiovascular benefits from antioxidant supplementation demonstrating the need for better understanding of the mechanisms that oxidative stress contributes to atrial fibrillation in the context of diabetes (Sesso et al., 2008; Violi et al., 2014).
There has been limited investigation into the mechanisms linking oxidative stress, diabetes and arrhythmias. One of the most extensively studied mechanisms is Ca2+/calmodulin-dependent protein kinase II (CaMKII). CaMKII is a serine-threonine kinase that has emerged as an important nodal point to allow cardiomyocytes to respond to perturbances in calcium and reactive oxygen species through the activation of a diverse group of downstream targets to regulate membrane excitability and calcium cycling (Voigt et al., 2012; Mesubi and Anderson, 2016). CaMKII is increased in atria of atrial fibrillation patients and in mouse models of susceptible to atrial fibrillation (Purohit et al., 2013). CaMKII has been shown to influence calcium dynamics through several different mechanisms in the diabetic heart. In obese Zucker rats and high-fat-fed rodents, there is increased muscle mitochondrial content and CaMKII activation (Jain et al., 2014). Increases in mitochondrial reactive oxygen species and S-nitrosylation of the ryanodine receptor lead to increased SR calcium leak and activation of CAMKII. CaMKII may also contribute to connexin alterations and electrical conduction changes observed in diabetes (Zhong et al., 2017). In ApoE knockout mice fed a high fat diet, downregulation of the ion channels, connexin-43 upregulation and ventricular remodeling could be prevented by administration of a CaMKII antagonist.
Post-translational modifications of CamKII may contribute to its role in diabetes-induced arrhythmias. Animal studies show that mitochondria isolated from streptazocin treated rat hearts have increased total O-linked N-acetylglucosamine (O-GlcNAc) and O-GlcNAc transferase levels, with O-GlcNAc transferase being localized in the mitochondrial matrix as opposed to an inner membrane localization in control rats (Banerjee et al., 2015). Mislocalization of O-GlcNAc transferase results in decreased interactions with complex IV of the electron transport chain, resulting in impairments of its activity. Acute hyperglycemia in cardiomyocytes has been shown to result in covalent and persisting modifications of Ca2+/calmodulin-dependent protein kinase II (CaMKII) by O-GlcNAc, which can also be observed in the heart and brain of diabetic humans and rats (Erickson et al., 2013). O-GlcNAc modification of CaMKII leads to increased activation of spontaneous sarcoplasmic reticulum Ca2+ release resulting in arrhythmias (Erickson et al., 2013). In diabetic humans and mouse models of diabetes, there are increased levels of oxidized CaMKII (Luo et al., 2013). Oxidized CaMKII has been linked with ventricular arrhythmia (Wang et al., 2018) and atrial fibrillation (Purohit et al., 2013) however, how oxidized CaMKII contributes to atrial fibrillation with diabetes is not currently defined. In addition to changes in CaMKII, abnormal calcium handling has been observed in diabetic hearts however, how this relates to arrhythmogenesis has no yet been investigated (Belke and Dillmann, 2004; Lacombe et al., 2007).
Role of Inflammation
Inflammation has been identified as a risk factor for cardiac arrhythmias due to the increased frequency of incidence following cardiac surgery (Bruins et al., 1997), genetic studies (Gaudino et al., 2003) and increased occurrence during myocarditis (Spodick, 1976). In human patients, C-reactive protein (Aviles et al., 2003) has been associated with incidence of atrial fibrillation and was able to predict future development and polymorphisms in the interleukin-1 family affect risk for atrial fibrillation (Cauci et al., 2010; Gungor et al., 2013). Inflammation has also been suggested as an underlying pathogenic mediator for diabetes. Since it was identified that TNF-α secretion by adipocytes played a role in the body's update of glucose and response to insulin which contributes to the development of diabetes (Hotamisligil et al., 1993), extensive research has been done looking at the role of inflammation in diabetes (Wellen and Hotamisligil, 2005; Calle and Fernandez, 2012).
The connection between inflammation, arrhythmias and diabetes is not currently well characterized and an ongoing area of research however, hypoglycemia, which has been suggested to trigger atrial fibrillation (Odeh et al., 1990; Celebi et al., 2011; Ko et al., 2018), increases markers of inflammation (Investigators et al., 2013). In a recent study, toll-like receptor (TLR) 2 knockout mice have decreased incidence of arrhythmias compared to wild-type mice in a streptozotcin model of diabetes mellitus (Monnerat et al., 2016). This is thought to occur through IL-1β production by macrophages since macrophages from TLR2 knockout animals had lower levels of MCHIIhigh macrophages and NLRP3 inflammasome. IL-1β was decreased in the hearts of TLR2 knockout animals and IL-1β could decreased potassium current and increase calcium sparks in isolated cardiomyocytes. Furthermore, inhibition of the NLRP3 inflammasome or IL-1β reversed diabetes-induced arrhythmias.
The Impact of Diabetes Therapies on Arrhythmias
Current type 2 diabetes therapies aim to treat hyperglycemia to reduce and maintain glucose concentration to normal levels in an effort to prevent the development of complications (Kahn et al., 2014). Since the process through which diabetes causes arrhythmias is not currently know, the impact of current therapies is just beginning to be understood. Studies suggest that merely controlling glucose levels is not beneficial (Group et al., 2008; Duckworth et al., 2009) and potentially detrimental (Action to Control Cardiovascular Risk in Diabetes Study et al., 2008) in controlling cardiovascular complications. This lack of benefit from intense glycemic control includes the rate impact the rate of new-onset atrial fibrillation (Fatemi et al., 2014). However, poorly controlled diabetes has also been linked to increased incidence of atrial fibrillation showing that the role of glycemic control is not fully understood at this time (Huxley et al., 2012).
Metformin is currently the most widely used medication to treat type 2 diabetes and acts to suppress gluconeogenesis thus lowering glucose levels. Metformin has been associated with decreased atrial fibrillation risk compared with diabetic patients not taking medication (Chang et al., 2014). In vitro studies using an atrial cell line demonstrated that metformin decreased reactive oxygen species in response to pacing and prevented cardiomyocyte remodeling (Chang et al., 2014). In diabetic Goto-Kakizaki rats, metformin treatment decreased cardiac fibrosis and arrhythmias (Fu et al., 2018). Alterations in small conductance calcium-activated potassium channels were observed in the atria of these animals, which was corrected with metformin treatment, suggesting that metformin may restore the atrial electrophysiology. Metformin has also been shown to prevent high glucose induction of apoptosis, autophagy and connexin-43 downregulation in H9C2 cells, a ventricular myoblast cell line (Wang et al., 2017). However, there have been reported incidences of onset of atrial fibrillation with metformin use in diabetic patients (Boolani et al., 2011), which may be attributed to lactic acidosis, which occurs rarely with metformin treatment (Salpeter et al., 2010).
Thiazolinediones are peroxisome proliferator-activated receptor-γ agonists, which decrease glucose levels by increasing storage of fatty acids in adipocytes, also decrease incidences of atrial fibrillation onset, which might also be influenced by their anti-inflammatory actions (Chao et al., 2012; Pallisgaard et al., 2017; Zhang Z. et al., 2017). However, other studies have shown in patients with coronary disease, thiazolinediones have no improvements in atrial fibrillation compared with other diabetes treatments including metformin, insulin, sulfonylurea or meglitinides, suggesting that the anti-inflammatory effects of thiazolinediones does not further improve anti-arrhythmia effects of controlling glucose levels (Pallisgaard et al., 2018).
Dipeptidyl peptidase-4 (DPP-4) inhibitors, such as alogliptin, which increases incretin levels to inhibit glucagon release leading to increased insulin secretion are also a common treatment for type 2 diabetes. In an alloxan-induced rabbit model of diabetes mellitus, diabetic rabbits had increased left ventricular hypertrophy and left atrial dilation (Zhang X. et al., 2017). Diabetic hearts had a higher level of atrial fibrillation inducibility and treatment with alogliptin prevented morphological changes and increased propensity for atrial fibrillation. Additionally reactive oxygen species, mitochondrial membrane depolariazation and mitochondrial biogenesis were improved with alogliptin. In a Taiwanese population, DPP-4 inhibitors, in conjunction with metformin, decreased the onset of atrial fibrillation compared to diabetics taking metformin and other second-line therapies (Chang et al., 2017). Other standard diabetes therapies may also have beneficial effects on cardiac arrhythmias. Retrospective studies suggest that there may be differences in sudden cardiac arrest and ventricular arrhythmias between types of sulonylurea medications, where glyburide was found to have lower risk for sudden cardiac arrest and ventricular arrhythmias compared with glipizide (Leonard et al., 2018).
Arrhythmia Therapies in Diabetic Patients
Pharmacological therapies for arrhythmia include agents that control rate and rhythm. Currently, there has been no research examining the efficacy of anti-arrhythmic medications in patients with diabetes (Dobbin et al., 2018). Surgically, catheter ablation is an established therapeutic option for heart rhythm control in drug resistant patients. Catheter ablation has been shown in a large study composed of 1,464 patients to have the same efficacy and safety in diabetes patients as the general population (Anselmino et al., 2015). However, due to the presence of other atrial fibrillation recurrence predictors such as alterations in the electrical and anatomical composition of the atrial myocytes, metabolic alterations and other comorbidities (D'Ascenzo et al., 2013), the need to redo ablation is more common (Chao et al., 2010; Anselmino et al., 2015). However, smaller studies have shown that while ablation is equally effective in diabetic patients, there are increased numbers of thrombotic or hemorrhagic complications (Tang et al., 2006).
Thromboprophylaxis therapies are also commonly used in patients with atrial fibrillation to decrease risk of stroke and other complications. A number of clinical trials investigating the effectiveness of various theromboprophylaxis therapies have included diabetic subpopulations. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial, 40% of the patients had diabetes (Patel et al., 2011). The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial comparing warfarin and high dose dibagatran was composed of about 23% diabetic patients (Connolly et al., 2009). The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction (ENGAGE AF-TIMI) trial had ~36% diabetic participants (Giugliano et al., 2013). No differences were observed between diabetic and non-diabetic patients in any of these studies. The Apixaban for Reduction in Stroke and Other Thromoembolic Events in Atrial Fibrillation (ARISTOTLE) trail, composed of ~25% diabetic patients, found no difference in the primary outcome of decreased stroke and thromboembolic events between diabetic and non-diabetic patients (Granger et al., 2011). However, diabetic patients had an increased risk of bleeding compared with non-diabetic participants. Taken together, these studies suggest that anti-arrhythmic and anti-thromboprophylaxis therapies are effective in diabetic patients with few adverse effects.
The renin-angiotensin system is involved in the genesis of arrhythmias through its impact on structural and electrical remodeling (Iravanian and Dudley, 2008). Therapies targeting this pathway including angiotensin-converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARB) have been hypothesized to be beneficial in preventing atrial fibrillation occurrence and are currently the focus of numerous studies (Iravanian and Dudley, 2008). Activation of the renin-angiotensin system is often associated with diabetes where it is thought to impact the initiation and progression of the disease (Giacchetti et al., 2005). While there have not been direct studies linking the renin-angiotensin system and diabetes-induced arrhythmias, it is likely that they are intertwined since the renin-angiotensin system impacts nearly all contributing factors including cardiac remodeling, electrical remodeling and inflammation (Iravanian and Dudley, 2008). Inhibitors of the renin-angiotensin system have been shown to reduce cardiovascular events, decrease diabetic complications and can reduce incidence of new onset diabetes (Hansson et al., 1999; Heart Outcomes Prevention Evaluation Study, 2000; Brenner et al., 2001; Dahlof, 2002; Bangalore et al., 2011). This has been confirmed in a large clinical trial investigating the effects of the ARB valsartan on diabetes development in patients with impaired glucose tolerance where valsartan was found to reduce incidence of diabetes but did not reduce the rate of cardiovascular events (Group et al., 2010). However, in patients with impaired fasting glucose or impaired glucose tolerance, the ACE inhibitor Ramipril was not able to reduce new incidence of diabetes in patients with impaired fasting glucose but did promote normoglycemia (Investigators et al., 2006). While renin-angiotensin system targeted therapies show promise in reducing incidence of cardiac arrhythmias and diabetes, additional research is necessary to further understand the mechanisms involved and confirm studies performed in small patient populations.
Conclusions
The impact of diabetes on the electrical conduction of the heart and development of cardiac arrhythmias is becoming increasingly apparent. Due to the complex, multifactorial nature of diabetes, the relationship between diabetes and cardiac arrhythmias is not yet fully understood however, correlations between increased blood glucose levels, glucose fluctuation and hypoglycemia, and arrhythmias have been observed and are a likely initiator of the disease. Autonomic dysfunction, which is known to contribute to diabetic complications in other tissues potentiates disease progression through changes in the heart's energy needs, the production of paracrine signaling factors and alterations in receptors that influence ion channel activity in the heart. Alterations in the architecture of the heart including fibrosis, fat deposition and hypertrophy change the electrical conduction of the heart and disrupt the pattern of the electrical signal. They are also an important source of paracrine factors that enhance disease progression. Taken together, these alterations within the heart change the electrical conduction by regulating ion channels and gap junctions between cardiomyocytes changing the electrical signaling. While anti-arrhythmic therapies appear to be effective in diabetic patients, the effectiveness of diabetes therapeutics on prevention of cardiac arrhythmias is unclear. Due to the complex nature of diabetes and cardiac arrhythmias, further experimental and clinical research is necessary to fully elucidate the relationship between diabetes and arrhythmias in the hope of developing improved therapeutic strategies in the future.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by an American Heart Association Scientific Development Grant 17SDG33400114 (LG).
References
Abe, I., Teshima, Y., Kondo, H., Kaku, H., Kira, S., Ikebe, Y., et al. (2018). Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 15, 1717–27. doi: 10.1016/j.hrthm.2018.06.025
Action to Control Cardiovascular Risk in Diabetes Study, G., Gerstein, H. C., Miller, M. E., Byington, R. P., Goff, D. C., Bigger, J. T., et al. (2008). Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559. doi: 10.1056/NEJMoa0802743
Agarwal, S. K., Norby, F. L., Whitsel, E. A., Soliman, E. Z., Chen, L. Y., Loehr, L. R., et al. (2017). Cardiac autonomic dysfunction and incidence of atrial fibrillation: results from 20 years follow-up. J. Am. Coll. Cardiol. 69, 291–299. doi: 10.1016/j.jacc.2016.10.059
Al Chekakie, M. O., Welles, C. C., Metoyer, R., Ibrahim, A., Shapira, A. R., Cytron, J., et al. (2010). Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 56, 784–788. doi: 10.1016/j.jacc.2010.03.071
Anderson, E. J., Kypson, A. P., Rodriguez, E., Anderson, C. A., Lehr, E. J., and Neufer, P. D. (2009). Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 54, 1891–1898. doi: 10.1016/j.jacc.2009.07.031
Anselmino, M., Matta, M., D'ascenzo, F., Pappone, C., Santinelli, V., Bunch, T. J., et al. (2015). Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace 17, 1518–1525. doi: 10.1093/europace/euv214
Aromolaran, A. S., and Boutjdir, M. (2017). Cardiac ion channel regulation in obesity and the metabolic syndrome: relevance to long qt syndrome and atrial fibrillation. Front. Physiol. 8:431. doi: 10.3389/fphys.2017.00431
Aune, D., Feng, T., Schlesinger, S., Janszky, I., Norat, T., and Riboli, E. (2018). Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J. Diabetes Complicat. 32, 501–511. doi: 10.1016/j.jdiacomp.2018.02.004
Aviles, R. J., Martin, D. O., Apperson-Hansen, C., Houghtaling, P. L., Rautaharju, P., Kronmal, R. A., et al. (2003). Inflammation as a risk factor for atrial fibrillation. Circulation 108, 3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F
Axelsen, L. N., Calloe, K., Braunstein, T. H., Riemann, M., Hofgaard, J. P., Liang, B., et al. (2015). Diet-induced pre-diabetes slows cardiac conductance and promotes arrhythmogenesis. Cardiovasc. Diabetol. 14:87. doi: 10.1186/s12933-015-0246-8
Baek, Y. S., Yang, P. S., Kim, T. H., Uhm, J. S., Park, J., Pak, H. N., et al. (2017). Associations of Abdominal Obesity and New-Onset Atrial Fibrillation in the General Population. J. Am. Heart Assoc. 6:e004705. doi: 10.1161/JAHA.116.004705
Banerjee, P. S., Ma, J., and Hart, G. W. (2015). Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc. Natl. Acad. Sci. U.S.A. 112, 6050–6055. doi: 10.1073/pnas.1424017112
Bangalore, S., Kumar, S., Wetterslev, J., and Messerli, F. H. (2011). Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ 342:d2234. doi: 10.1136/bmj.d2234
Barth, A. S., Merk, S., Arnoldi, E., Zwermann, L., Kloos, P., Gebauer, M., et al. (2005). Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ. Res. 96, 1022–1029. doi: 10.1161/01.RES.0000165480.82737.33
Barth, A. S., and Tomaselli, G. F. (2009). Cardiac metabolism and arrhythmias. Circ. Arrhythm. Electrophysiol. 2, 327–335. doi: 10.1161/CIRCEP.108.817320
Batal, O., Schoenhagen, P., Shao, M., Ayyad, A. E., Van Wagoner, D. R., Halliburton, S. S., et al. (2010). Left atrial epicardial adiposity and atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3, 230–236. doi: 10.1161/CIRCEP.110.957241
Belke, D. D., and Dillmann, W. H. (2004). Altered cardiac calcium handling in diabetes. Curr. Hypertens. Rep. 6, 424–429. doi: 10.1007/s11906-004-0035-3
Benjamin, E. J., Levy, D., Vaziri, S. M., D'Agostino, R. B., Belanger, A. J., and Wolf, P. A. (1994). Independent risk factors for atrial fibrillation in a population-based cohort. Framingham Heart Study. JAMA 271, 840–844. doi: 10.1001/jama.1994.03510350050036
Bissinger, A., Grycewicz, T., Grabowicz, W., and Lubinski, A. (2011). The effect of diabetic autonomic neuropathy on P-wave duration, dispersion and atrial fibrillation. Arch. Med. Sci. 7, 806–812. doi: 10.5114/aoms.2011.25555
Boengler, K., Schulz, R., and Heusch, G. (2006). Connexin 43 signalling and cardioprotection. Heart 92, 1724–1727. doi: 10.1136/hrt.2005.066878
Bonilla, I. M., Sridhar, A., Gyorke, S., Cardounel, A. J., and Carnes, C. A. (2012). Nitric oxide synthases and atrial fibrillation. Front. Physiol. 3:105. doi: 10.3389/fphys.2012.00105
Boolani, H., Shanberg, D., Chikkam, V., and Lakkireddy, D. (2011). Metformin associated atrial fibrillation - a case report. J. Atr. Fibrillation 4, 411. doi: 10.4022/jafib.411
Brenner, B. M., Cooper, M. E., de Zeeuw, D., Keane, W. F., Mitch, W. E., Parving, H. H., et al. (2001). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869. doi: 10.1056/NEJMoa011161
Bruins, P., te Velthuis, H., Yazdanbakhsh, A. P., Jansen, P. G., van Hardevelt, F. W., de Beaumont, E. M., et al. (1997). Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 96, 3542–3548. doi: 10.1161/01.CIR.96.10.3542
Cai, H., Li, Z., Goette, A., Mera, F., Honeycutt, C., Feterik, K., et al. (2002). Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation 106, 2854–2858. doi: 10.1161/01.CIR.0000039327.11661.16
Calle, M. C., and Fernandez, M. L. (2012). Inflammation and type 2 diabetes. Diabetes Metab. 38, 183–191. doi: 10.1016/j.diabet.2011.11.006
Cardoso, C. R., Salles, G. F., and Deccache, W. (2003). Prognostic value of QT interval parameters in type 2 diabetes mellitus: results of a long-term follow-up prospective study. J. Diabetes Complicat. 17, 169–178. doi: 10.1016/S1056-8727(02)00206-4
Cauci, S., Di Santolo, M., Ryckman, K. K., Williams, S. M., and Banfi, G. (2010). Variable number of tandem repeat polymorphisms of the interleukin-1 receptor antagonist gene IL-1RN: a novel association with the athlete status. BMC Med. Genet. 11:29. doi: 10.1186/1471-2350-11-29
Celebi, S., Celebi, O. O., Aydogdu, S., and Diker, E. (2011). A peculiar medical cardioversion of atrial fibrillation with glucose infusion–a rare cause of atrial fibrillation: hypoglycemia. Am. J. Emerg. Med. 29, 134.e1–134.e3. doi: 10.1016/j.ajem.2010.02.012
Centers for Disease C and Prevention (2008). State-specific incidence of diabetes among adults–participating states, 1995-1997 and 2005-2007. MMWR Morb. Mortal. Wkly. Rep. 57, 1169–1173. Available online at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5743a2.htm
Chang, C. Y., Yeh, Y. H., Chan, Y. H., Liu, J. R., Chang, S. H., Lee, H. F., et al. (2017). Dipeptidyl peptidase-4 inhibitor decreases the risk of atrial fibrillation in patients with type 2 diabetes: a nationwide cohort study in Taiwan. Cardiovasc. Diabetol. 16, 159. doi: 10.1186/s12933-017-0640-5
Chang, S. H., Wu, L. S., Chiou, M. J., Liu, J. R., Yu, K. H., Kuo, C. F., et al. (2014). Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc. Diabetol. 13:123. doi: 10.1186/s12933-014-0123-x
Chao, T. F., Leu, H. B., Huang, C. C., Chen, J. W., Chan, W. L., Lin, S. J., et al. (2012). Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int. J. Cardiol. 156, 199–202. doi: 10.1016/j.ijcard.2011.08.081
Chao, T. F., Suenari, K., Chang, S. L., Lin, Y. J., Lo, L. W., Hu, Y. F., et al. (2010). Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am. J. Cardiol. 106, 1615–1620. doi: 10.1016/j.amjcard.2010.07.038
Chen, J., Li, Q., Dong, R., Gao, H., Peng, H., and Wu, Y. (2014). The effect of the Ras homolog gene family, (Rho), member A/Rho associated coiled-coil forming protein kinase pathway in atrial fibrosis of type 2 diabetes in rats. Exp. Ther. Med. 8, 836–840. doi: 10.3892/etm.2014.1843
Cheng, Y. J., Lauer, M. S., Earnest, C. P., Church, T. S., Kampert, J. B., Gibbons, L. W., et al. (2003). Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care 26, 2052–2057. doi: 10.2337/diacare.26.7.2052
Chow, E., Bernjak, A., Walkinshaw, E., Lubina-Solomon, A., Freeman, J., Macdonald, I. A., et al. (2017). Cardiac autonomic regulation and repolarization during acute experimental hypoglycemia in type 2 diabetes. Diabetes 66, 1322–1333. doi: 10.2337/db16-1310
Chow, E., Bernjak, A., Williams, S., Fawdry, R. A., Hibbert, S., Freeman, J., et al. (2014). Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 63, 1738–1747. doi: 10.2337/db13-0468
Christensen, T. F., Baekgaard, M., Dideriksen, J. L., Steimle, K. L., Mogensen, M. L., Kildegaard, J., et al. (2009). A physiological model of the effect of hypoglycemia on plasma potassium. J. Diabetes Sci. Technol. 3, 887–894. doi: 10.1177/193229680900300436
Connolly, S. J., Ezekowitz, M. D., Yusuf, S., Eikelboom, J., Oldgren, J., Parekh, A., et al. (2009). Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151. doi: 10.1056/NEJMoa0905561
Dahlof, B. (2002). Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study, (LIFE): a randomised trial against atenolol. Lancet 359, 995–1003. doi: 10.1016/S0140-6736(02)08089-3
Dahlqvist, S., Rosengren, A., Gudbjörnsdottir, S., Pivodic, A., Wedel, H., Kosiborod, M., et al. (2017). Risk of atrial fibrillation in people with type 1 diabetes compared with matched controls from the general population: a prospective case-control study. Lancet Diabetes Endocrinol. 5, 799–807. doi: 10.1016/S2213-8587(17)30262-0
D'Ascenzo, F., Corleto, A., Biondi-Zoccai, G., Anselmino, M., Ferraris, F., di Biase, L., et al. (2013). Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int. J. Cardiol. 167, 1984–1989. doi: 10.1016/j.ijcard.2012.05.008
Dewland, T. A., Olgin, J. E., Vittinghoff, E., and Marcus, G. M. (2013). Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 128, 2470–2477. doi: 10.1161/CIRCULATIONAHA.113.002449
Dobbin, S., Fisher, M., and McKay, G. (2018). Management of atrial fibrillation in diabetes. Pract Diabetes 35, 27–31. doi: 10.1002/pdi.2155
dos Santos, D. O., Blefari, V., Prado, F. P., Silva, C. A., Fazan, R., Salgado, H. C., et al. (2016). Reduced expression of adherens and gap junction proteins can have a fundamental role in the development of heart failure following cardiac hypertrophy in rats. Exp. Mol. Pathol. 100, 167–176. doi: 10.1016/j.yexmp.2015.12.009
Dublin, S., Glazer, N. L., Smith, N. L., Psaty, B. M., Lumley, T., Wiggins, K. L., et al. (2010). Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J. Gen. Intern. Med. 25, 853–858. doi: 10.1007/s11606-010-1340-y
Duckworth, W., Abraira, C., Moritz, T., Reda, D., Emanuele, N., Reaven, P. D., et al. (2009). Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139. doi: 10.1056/NEJMoa0808431
Dudley, S. C., Hoch, N. E., McCann, L. A., Honeycutt, C., Diamandopoulos, L., Fukai, T., et al. (2005). Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 112, 1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108
Erickson, J. R., Pereira, L., Wang, L., Han, G., Ferguson, A., Dao, K., et al. (2013). Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502, 372–376. doi: 10.1038/nature12537
Ewing, D. J., and Neilson, J. M. (1990). QT interval length and diabetic autonomic neuropathy. Diabet. Med. 7, 23–26. doi: 10.1111/j.1464-5491.1990.tb01301.x
Fatemi, O., Yuriditsky, E., Tsioufis, C., Tsachris, D., Morgan, T., Basile, J., et al. (2014). Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus, (from the Action to Control Cardiovascular Risk in Diabetes Study). Am. J. Cardiol. 114, 1217–1222. doi: 10.1016/j.amjcard.2014.07.045
Federation, I. D. (2014). IDF Diabetes Atlas. Epidemiology and Morbidity. International Diabetes Federation. Available online at: http://www.idf.org
Ferdous, Z., Qureshi, M. A., Jayaprakash, P., Parekh, K., John, A., Oz, M., et al. (2016). Different profile of mRNA Expression in sinoatrial node from streptozotocin-induced diabetic rat. PLoS ONE 11:e0153934. doi: 10.1371/journal.pone.0153934
Fischer, V. W., Barner, H. B., and Larose, L. S. (1984). Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum. Pathol. 15, 1127–1136. doi: 10.1016/S0046-8177(84)80307-X
Fontes, J. D., Lyass, A., Massaro, J. M., Rienstra, M., Dallmeier, D., Schnabel, R. B., et al. (2012). Insulin resistance and atrial fibrillation, (from the Framingham Heart Study). Am. J. Cardiol. 109, 87–90. doi: 10.1016/j.amjcard.2011.08.008
Forbes, J. M., and Cooper, M. E. (2013). Mechanisms of diabetic complications. Physiol. Rev. 93, 137–188. doi: 10.1152/physrev.00045.2011
Friedman, D. J., Wang, N., Meigs, J. B., Hoffmann, U., Massaro, J. M., Fox, C. S., et al. (2014). Pericardial fat is associated with atrial conduction: the Framingham Heart Study. J. Am. Heart Assoc. 3:e000477. doi: 10.1161/JAHA.113.000477
Fu, H., Li, G., Liu, C., Li, J., Wang, X., Cheng, L., et al. (2015). Probucol prevents atrial remodeling by inhibiting oxidative stress and TNF-alpha/NF-kappaB/TGF-beta signal transduction pathway in alloxan-induced diabetic rabbits. J. Cardiovasc. Electrophysiol. 26, 211–222. doi: 10.1111/jce.12540
Fu, X., Pan, Y., Cao, Q., Li, B., Wang, S., Du, H., et al. (2018). Metformin restores electrophysiology of small conductance calcium-activated potassium channels in the atrium of GK diabetic rats. BMC Cardiovasc. Disord. 18, 63. doi: 10.1186/s12872-018-0805-5
Gando, S., Hattori, Y., and Kanno, M. (1993). Altered cardiac adrenergic neurotransmission in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 109, 1276–1281. doi: 10.1111/j.1476-5381.1993.tb13761.x
Gaudino, M., Andreotti, F., Zamparelli, R., Di Castelnuovo, A., Nasso, G., Burzotta, F., et al. (2003). The−174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation 108 (Suppl. 1):II195–199. doi: 10.1161/01.cir.0000087441.48566.0d
Giacchetti, G., Sechi, L. A., Rilli, S., and Carey, R. M. (2005). The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol. Metab. 16, 120–126. doi: 10.1016/j.tem.2005.02.003
Giacco, F., and Brownlee, M. (2010). Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070. doi: 10.1161/CIRCRESAHA.110.223545
Giugliano, R. P., Ruff, C. T., Braunwald, E., Murphy, S. A., Wiviott, S. D., Halperin, J. L., et al. (2013). Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 369, 2093–2104. doi: 10.1056/NEJMoa1310907
Granger, C. B., Alexander, J. H., McMurray, J. J., Lopes, R. D., Hylek, E. M., Hanna, M., et al. (2011). Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365, 981–992. doi: 10.1056/NEJMoa1107039
Group, A. C., Patel, A., MacMahon, S., Chalmers, J., Neal, B., Billot, L., et al. (2008). Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572. doi: 10.1056/NEJMoa0802987
Group, N. S., McMurray, J. J., Holman, R. R., Haffner, S. M., Bethel, M. A., Holzhauer, B., et al. (2010). Effect of valsartan on the incidence of diabetes and cardiovascular events. N. Engl. J. Med. 362, 1477–1490. doi: 10.1056/NEJMoa1001121
Grundvold, I., Bodegard, J., Nilsson, P. M., Svennblad, B., Johansson, G., Östgren, C. J., et al. (2015). Body weight and risk of atrial fibrillation in 7,169 patients with newly diagnosed type 2 diabetes; an observational study. Cardiovasc. Diabetol. 14:5. doi: 10.1186/s12933-014-0170-3
Gungor, B., Ekmekci, A., Arman, A., Ozcan, K. S., Ucer, E., Alper, A. T., et al. (2013). Assessment of interleukin-1 gene cluster polymorphisms in lone atrial fibrillation: new insight into the role of inflammation in atrial fibrillation. Pacing Clin. Electrophysiol. 36, 1220–1227. doi: 10.1111/pace.12182
Guzik, T. J., Mussa, S., Gastaldi, D., Sadowski, J., Ratnatunga, C., Pillai, R., et al. (2002). Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105, 1656–1662. doi: 10.1161/01.CIR.0000012748.58444.08
Haemers, P., Hamdi, H., Guedj, K., Suffee, N., Farahmand, P., Popovic, N., et al. (2017). Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 38, 53–61. doi: 10.1093/eurheartj/ehv625
Hage, C., Michaëlsson, E., Linde, C., Donal, E., Daubert, J. C., Gan, L. M., et al. (2017). Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction: a holistic proteomic approach. Circ. Cardiovasc. Genet. 10:e001633. doi: 10.1161/CIRCGENETICS.116.001633
Hanif, W., Alex, L., Su, Y., Shinde, A. V., Russo, I., Li, N., et al. (2017). Left atrial remodeling, hypertrophy, and fibrosis in mouse models of heart failure. Cardiovasc. Pathol. 30:27–37. doi: 10.1016/j.carpath.2017.06.003
Hansson, L., Lindholm, L. H., Niskanen, L., Lanke, J., Hedner, T., Niklason, A., et al. (1999). Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project, (CAPPP) randomised trial. Lancet 353, 611–616. doi: 10.1016/S0140-6736(98)05012-0
Heart Outcomes Prevention Evaluation Study, I. (2000). Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 342, 145–153. doi: 10.1056/NEJM200001203420301
Heller, S. R., and Robinson, R. T. (2000). Hypoglycaemia and associated hypokalaemia in diabetes: mechanisms, clinical implications and prevention. Diabetes Obes. Metab. 2, 75–82. doi: 10.1046/j.1463-1326.2000.00050.x
Hotamisligil, G. S., Shargill, N. S., and Spiegelman, B. M. (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91. doi: 10.1126/science.7678183
Howarth, F. C., Jacobson, M., Shafiullah, M., and Adeghate, E. (2008). Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp. Physiol. 93, 362–369. doi: 10.1113/expphysiol.2007.040055
Howarth, F. C., Nowotny, N., Zilahi, E., El Haj, M. A., and Lei, M. (2007). Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Mol. Cell. Biochem. 305(1–2):145–151. doi: 10.1007/s11010-007-9537-z
Howarth, F. C., Parekh, K., Jayaprakash, P., Inbaraj, E. S., Oz, M., Dobrzynski, H., et al. (2017). Altered profile of mRNA expression in atrioventricular node of streptozotocininduced diabetic rats. Mol. Med. Rep. 16, 3720–3730. doi: 10.3892/mmr.2017.7038
Hu, Z., Kant, R., Anand, M., King, E. C., Krogh-Madsen, T., Christini, D. J., et al. (2014). Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ. Cardiovasc. Genet. 7, 33–42. doi: 10.1161/CIRCGENETICS.113.000315
Huang, H., Amin, V., Gurin, M., Wan, E., Thorp, E., Homma, S., et al. (2013). Diet-induced obesity causes long QT and reduces transcription of voltage-gated potassium channels. J. Mol. Cell. Cardiol. 59:151–158. doi: 10.1016/j.yjmcc.2013.03.007
Huxley, R. R., Alonso, A., Lopez, F. L., Filion, K. B., Agarwal, S. K., Loehr, L. R., et al. (2012). Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 98, 133–138. doi: 10.1136/heartjnl-2011-300503
Huxley, R. R., Filion, K. B., Konety, S., and Alonso, A. (2011). Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am. J. Cardiol. 108, 56–62. doi: 10.1016/j.amjcard.2011.03.004
Igarashi, T., Finet, J. E., Takeuchi, A., Fujino, Y., Strom, M., Greener, I. D., et al. (2012). Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation 125, 216–225. doi: 10.1161/CIRCULATIONAHA.111.053272
Investigators, D. T., Bosch, J., Yusuf, S., Gerstein, H. C., Pogue, J., Sheridan, P., et al. (2006). Effect of ramipril on the incidence of diabetes. N. Engl. J. Med. 355, 1551–1562. doi: 10.1056/NEJMoa065061
Investigators, O. T., Mellbin, L. G., Rydén, L., Riddle, M. C., Probstfield, J., Rosenstock, J., et al. (2013). Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur. Heart J. 34, 3137–3144. doi: 10.1093/eurheartj/eht332
Iravanian, S., and Dudley, S. C. Jr. (2008). The renin-angiotensin-aldosterone system, (RAAS) and cardiac arrhythmias. Heart Rhythm 5(Suppl. 6), S12–17. doi: 10.1016/j.hrthm.2008.02.025
Jain, S. S., Paglialunga, S., Vigna, C., Ludzki, A., Herbst, E. A., Lally, J. S., et al. (2014). High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes 63, 1907–1913. doi: 10.2337/db13-0816
Jermendy, G., Koltai, M. Z., and Pogatsa, G. (1990). QT interval prolongation in type 2, (non-insulin-dependent) diabetic patients with cardiac autonomic neuropathy. Acta Diabetol. Lat. 27, 295–301. doi: 10.1007/BF02580933
Kahn, S. E., Cooper, M. E., and Del Prato, S. (2014). Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083. doi: 10.1016/S0140-6736(13)62154-6
Kannel, W. B., Abbott, R. D., Savage, D. D., and McNamara, P. M. (1983). Coronary heart disease and atrial fibrillation: the Framingham Study. Am. Heart J. 106, 389–396. doi: 10.1016/0002-8703(83)90208-9
Kannel, W. B., Wolf, P. A., Benjamin, E. J., and Levy, D. (1998). Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am. J. Cardiol. 82, 2N−9N.
Kato, T., Yamashita, T., Sekiguchi, A., Sagara, K., Takamura, M., Takata, S., et al. (2006). What are arrhythmogenic substrates in diabetic rat atria? J. Cardiovasc. Electrophysiol. 17, 890–894. doi: 10.1111/j.1540-8167.2006.00528.x
Kato, T., Yamashita, T., Sekiguchi, A., Tsuneda, T., Sagara, K., Takamura, M., et al. (2008). AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J. Cardiovasc. Electrophysiol. 19, 415–420. doi: 10.1111/j.1540-8167.2007.01037.x
Kawaguchi, M., Techigawara, M., Ishihata, T., Asakura, T., Saito, F., Maehara, K., et al. (1997). A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels 12, 267–274. doi: 10.1007/BF02766802
Kim, Y. M., Guzik, T. J., Zhang, Y. H., Zhang, M. H., Kattach, H., Ratnatunga, C., et al. (2005). A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ. Res. 97, 629–636. doi: 10.1161/01.RES.0000183735.09871.61
Ko, S. H., Park, Y. M., Yun, J. S., Cha, S. A., Choi, E. K., Han, K., et al. (2018). Severe hypoglycemia is a risk factor for atrial fibrillation in type 2 diabetes mellitus: Nationwide population-based cohort study. J. Diabetes Complicat. 32, 157–163. doi: 10.1016/j.jdiacomp.2017.09.009
Lacombe, V. A., Viatchenko-Karpinski, S., Terentyev, D., Sridhar, A., Emani, S., Bonagura, J. D., et al. (2007). Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1787–1797. doi: 10.1152/ajpregu.00059.2007
Lancefield, T. F., Patel, S. K., Freeman, M., Velkoska, E., Wai, B., Srivastava, P. M., et al. (2016). The Receptor for Advanced Glycation End Products, (RAGE) Is Associated with Persistent Atrial Fibrillation. PLoS ONE 11:e0161715. doi: 10.1371/journal.pone.0161715
Lengyel, C., Virág, L., Bíró, T., Jost, N., Magyar, J., Biliczki, P., et al. (2007). Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc. Res. 73, 512–520. doi: 10.1016/j.cardiores.2006.11.010
Leonard, C. E., Brensinger, C. M., Aquilante, C. L., Bilker, W. B., Boudreau, D. M., Deo, R., et al. (2018). Comparative safety of sulfonylureas and the risk of sudden cardiac arrest and ventricular arrhythmia. Diabetes Care 41, 713–722. doi: 10.2337/dc17-0294
Levelt, E., Pavlides, M., Banerjee, R., Mahmod, M., Kelly, C., Sellwood, J., et al. (2016). Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J. Am. Coll. Cardiol. 68, 53–63. doi: 10.1016/j.jacc.2016.03.597
Lin, H., Ogawa, K., Imanaga, I., and Tribulova, N. (2006). Remodeling of connexin 43 in the diabetic rat heart. Mol. Cell. Biochem. 290, 69–78. doi: 10.1007/s11010-006-9166-y
Lipworth, L., Okafor, H., Mumma, M. T., Edwards, T. L., Roden, D. M., Blot, W. J., et al. (2012). Race-specific impact of atrial fibrillation risk factors in blacks and whites in the southern community cohort study. Am. J. Cardiol. 110, 1637–1642. doi: 10.1016/j.amjcard.2012.07.032
Liu, C., Fu, H., Li, J., Yang, W., Cheng, L., Liu, T., et al. (2012). Hyperglycemia aggravates atrial interstitial fibrosis, ionic remodeling and vulnerability to atrial fibrillation in diabetic rabbits. Anadolu Kardiyol. Derg. 12, 543–550. doi: 10.5152/akd.2012.188
Lopez-Izquierdo, A., Pereira, R. O., Wende, A. R., Punske, B. B., Abel, E. D., and Tristani-Firouzi, M. (2014). The absence of insulin signaling in the heart induces changes in potassium channel expression and ventricular repolarization. Am. J. Physiol. Heart Circulat. Physiol. 306, H747–754. doi: 10.1152/ajpheart.00849.2013
Luo, M., Guan, X., Luczak, E. D., Lang, D., Kutschke, W., Gao, Z., et al. (2013). Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J. Clin. Invest. 123, 1262–1274. doi: 10.1172/JCI65268
Mahajan, R., Lau, D. H., Brooks, A. G., Shipp, N. J., Manavis, J., Wood, J. P., et al. (2015). Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J. Am. Coll. Cardiol. 66, 1–11. doi: 10.1016/j.jacc.2015.04.058
Mäkimattila, S., Mäntysaari, M., Groop, P. H., Summanen, P., Virkamäki, A., Schlenzka, A., et al. (1997). Hyperreactivity to nitrovasodilators in forearm vasculature is related to autonomic dysfunction in insulin-dependent diabetes mellitus. Circulation 95, 618–625. doi: 10.1161/01.CIR.95.3.618
Mayr, M., Yusuf, S., Weir, G., Chung, Y. L., Mayr, U., Yin, X., et al. (2008). Combined metabolomic and proteomic analysis of human atrial fibrillation. J. Am. Coll. Cardiol. 51, 585–594. doi: 10.1016/j.jacc.2007.09.055
Mazurek, T., Zhang, L., Zalewski, A., Mannion, J. D., Diehl, J. T., Arafat, H., et al. (2003). Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5
Meo, M., Meste, O., Signore, S., Sorrentino, A., Cannata, A., Zhou, Y., et al. (2016). Reduction in Kv current enhances the temporal dispersion of the action potential in diabetic myocytes: insights from a novel repolarization algorithm. J. Am. Heart Assoc. 5:e003078. doi: 10.1161/JAHA.115.003078
Mesubi, O. O., and Anderson, M. E. (2016). Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal. Cardiovasc. Res. 109, 542–557. doi: 10.1093/cvr/cvw002
Mitasíková, M., Lin, H., Soukup, T., Imanaga, I., and Tribulova, N. (2009). Diabetes and thyroid hormones affect connexin-43 and PKC-epsilon expression in rat heart atria. Physiol. Res. 58, 211–217.
Monnerat, G., Alarcón, M. L., Vasconcellos, L. R., Hochman-Mendez, C., Brasil, G., Bassani, R. A., et al. (2016). Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 7:13344. doi: 10.1038/ncomms13344
Movahed, M. R., Hashemzadeh, M., and Jamal, M. M. (2005). Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int. J. Cardiol. 105, 315–318. doi: 10.1016/j.ijcard.2005.02.050
Nattel, S., Guasch, E., Savelieva, I., Cosio, F. G., Valverde, I., Halperin, J. L., et al. (2014). Early management of atrial fibrillation to prevent cardiovascular complications. Eur. Heart J. 35, 1448–1456. doi: 10.1093/eurheartj/ehu028
Nattel, S., and Harada, M. (2014). Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J. Am. Coll. Cardiol. 63, 2335–2345. doi: 10.1016/j.jacc.2014.02.555
Negishi, K., Seicean, S., Negishi, T., Yingchoncharoen, T., Aljaroudi, W., and Marwick, T. H. (2013). Relation of heart-rate recovery to new onset heart failure and atrial fibrillation in patients with diabetes mellitus and preserved ejection fraction. Am. J. Cardiol. 111, 748–753. doi: 10.1016/j.amjcard.2012.11.028
Neuman, R. B., Bloom, H. L., Shukrullah, I., Darrow, L. A., Kleinbaum, D., Jones, D. P., et al. (2007). Oxidative stress markers are associated with persistent atrial fibrillation. Clin. Chem. 53, 1652–1657. doi: 10.1373/clinchem.2006.083923
Nichols, G. A., Reinier, K., and Chugh, S. S. (2009). Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care 32, 1851–1856. doi: 10.2337/dc09-0939
Noyes, A. M., Dua, K., Devadoss, R., and Chhabra, L. (2014). Cardiac adipose tissue and its relationship to diabetes mellitus and cardiovascular disease. World J. Diabetes 5, 868–876. doi: 10.4239/wjd.v5.i6.868
Nunoda, S., Genda, A., Sugihara, N., Nakayama, A., Mizuno, S., and Takeda, R. (1985). Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels 1, 43–47. doi: 10.1007/BF02066486
Oberhauser, V., Schwertfeger, E., Rutz, T., Beyersdorf, F., and Rump, L. C. (2001). Acetylcholine release in human heart atrium: influence of muscarinic autoreceptors, diabetes, and age. Circulation 103, 1638–1643. doi: 10.1161/01.CIR.103.12.1638
Odeh, M., Oliven, A., and Bassan, H. (1990). Transient atrial fibrillation precipitated by hypoglycemia. Ann. Emerg. Med. 19, 565–567. doi: 10.1016/S0196-0644(05)82191-2
Olsen, K. B., Axelsen, L. N., Braunstein, T. H., Sørensen, C. M., Andersen, C. B., Ploug, T., et al. (2013). Myocardial impulse propagation is impaired in right ventricular tissue of Zucker diabetic fatty, (ZDF) rats. Cardiovasc. Diabetol. 12:19. doi: 10.1186/1475-2840-12-19
O'Neal, W. T., Judd, S. E., Limdi, N. A., McIntyre, W. F., Kleindorfer, D. O., Cushman, M., et al. (2017). Differential Impact of Risk Factors in Blacks and Whites in the Development of Atrial Fibrillation: the Reasons for Geographic And Racial Differences in Stroke, (REGARDS) Study. J. Racial Ethn. Health Disparit. 4, 718–724. doi: 10.1007/s40615-016-0275-3
O'Rourke, B., Ramza, B. M., and Marban, E. (1994). Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265, 962–966. doi: 10.1126/science.8052856
Otake, H., Suzuki, H., Honda, T., and Maruyama, Y. (2009). Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int. Heart J. 50, 627–641. doi: 10.1536/ihj.50.627
Pallisgaard, J. L., Brooks, M. M., Chaitman, B. R., Boothroyd, D. B., Perez, M., Hlatky, M. A., et al. (2018). Thiazolidinediones and risk of atrial fibrillation among patients with diabetes and coronary disease. Am. J. Med. 131, 805–812. doi: 10.1016/j.amjmed.2018.02.026
Pallisgaard, J. L., Lindhardt, T. B., Staerk, L., Olesen, J. B., Torp-Pedersen, C., Hansen, M. L., et al. (2017). Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. Eur. Heart J. Cardiovasc Pharmacother. 3, 140–146. doi: 10.1093/ehjcvp/pvw036
Pallisgaard, J. L., Schjerning, A. M., Lindhardt, T. B., Procida, K., Hansen, M. L., Torp-Pedersen, C., et al. (2016). Risk of atrial fibrillation in diabetes mellitus: A nationwide cohort study. Eur. J. Prev. Cardiol. 23, 621–627. doi: 10.1177/2047487315599892
Panzer, C., Lauer, M. S., Brieke, A., Blackstone, E., and Hoogwerf, B. (2002). Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes 51, 803–807. doi: 10.2337/diabetes.51.3.803
Patel, M. R., Mahaffey, K. W., Garg, J., Pan, G., Singer, D. E., Hacke, W., et al. (2011). Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891. doi: 10.1056/NEJMoa1009638
Petersen, K. G., Schluter, K. J., and Kerp, L. (1982). Regulation of serum potassium during insulin-induced hypoglycemia. Diabetes 31, 615–617. doi: 10.2337/diab.31.7.615
Psaty, B. M., Manolio, T. A., Kuller, L. H., Kronmal, R. A., Cushman, M., Fried, L. P., et al. (1997). Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96, 2455–2461. doi: 10.1161/01.CIR.96.7.2455
Purohit, A., Rokita, A. G., Guan, X., Chen, B., Koval, O. M., Voigt, N., et al. (2013). Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 128, 1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313
Ramasamy, R., Yan, S. F., and Schmidt, A. M. (2011). Receptor for AGE, (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x
Ramirez, A. H., Schildcrout, J. S., Blakemore, D. L., Masys, D. R., Pulley, J. M., Basford, M. A., et al. (2011). Modulators of normal electrocardiographic intervals identified in a large electronic medical record. Heart Rhythm 8, 271–277. doi: 10.1016/j.hrthm.2010.10.034
Regan, T. J., Lyons, M. M., Ahmed, S. S., Levinson, G. E., Oldewurtel, H. A., Ahmad, M. R., et al. (1977). Evidence for cardiomyopathy in familial diabetes mellitus. J. Clin. Invest. 60, 884–899. doi: 10.1172/JCI108843
Reilly, S. N., Jayaram, R., Nahar, K., Antoniades, C., Verheule, S., Channon, K. M., et al. (2011). Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation 124, 1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223
Roberts-Thomson, K. C., Lau, D. H., and Sanders, P. (2011). The diagnosis and management of ventricular arrhythmias. Nat. Rev. Cardiol. 8, 311–321. doi: 10.1038/nrcardio.2011.15
Rochette, L., Zeller, M., Cottin, Y., and Vergely, C. (2014). Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 1840, 2709–2729. doi: 10.1016/j.bbagen.2014.05.017
Rodriguez, F., Stefanick, M. L., Greenland, P., Soliman, E. Z., Manson, J. E., Parikh, N., et al. (2016). Racial and ethnic differences in atrial fibrillation risk factors and predictors in women: Findings from the Women's Health Initiative. Am. Heart J. 176:70–77. doi: 10.1016/j.ahj.2016.03.004
Rosito, G. A., Massaro, J. M., Hoffmann, U., Ruberg, F. L., Mahabadi, A. A., Vasan, R. S., et al. (2008). Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117, 605–613. doi: 10.1161/CIRCULATIONAHA.107.743062
Saffitz, J. E., and Kléber, A. G. (2004). Effects of mechanical forces and mediators of hypertrophy on remodeling of gap junctions in the heart. Circ. Res. 94, 585–591. doi: 10.1161/01.RES.0000121575.34653.50
Saito, S., Teshima, Y., Fukui, A., Kondo, H., Nishio, S., Nakagawa, M., et al. (2014). Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc. Res. 104, 5–14. doi: 10.1093/cvr/cvu176
Salpeter, S. R., Greyber, E., Pasternak, G. A., and Salpeter, E. E. (2010). Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst. Rev. 20:CD002967. doi: 10.1002/14651858.CD002967.pub3
Samman Tahhan, A., Sandesara, P. B., Hayek, S. S., Alkhoder, A., Chivukula, K., Hammadah, M., et al. (2017). Association between oxidative stress and atrial fibrillation. Heart Rhythm 14, 1849–1855. doi: 10.1016/j.hrthm.2017.07.028
Sesso, H. D., Buring, J. E., Christen, W. G., Kurth, T., Belanger, C., MacFadyen, J., et al. (2008). Vitamins, E., and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300, 2123–2133. doi: 10.1001/jama.2008.600
Shimizu, M., Umeda, K., Sugihara, N., Yoshio, H., Ino, H., Takeda, R., et al. (1993). Collagen remodelling in myocardia of patients with diabetes. J. Clin. Pathol. 46, 32–36. doi: 10.1136/jcp.46.1.32
Shimoni, Y., Firek, L., Severson, D., and Giles, W. (1994). Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ. Res. 74, 620–628. doi: 10.1161/01.RES.74.4.620
Singh, J. P., Larson, M. G., O'Donnell, C. J., Wilson, P. F., Tsuji, H., Lloyd-Jones, D. M., et al. (2000). Association of hyperglycemia with reduced heart rate variability, (The Framingham Heart Study). Am. J. Cardiol. 86, 309–312. doi: 10.1016/S0002-9149(00)00920-6
Sivieri, R., Veglio, M., Chinaglia, A., Scaglione, P., and Cavallo-Perin, P. (1993). Prevalence of QT prolongation in a type 1 diabetic population and its association with autonomic neuropathy. The neuropathy study group of the italian society for the study of diabetes. Diabet Med 10, 920–924. doi: 10.1111/j.1464-5491.1993.tb00007.x
Spach, M. S., Dolber, P. C., and Heidlage, J. F. (1988). Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res 62, 811–832. doi: 10.1161/01.RES.62.4.811
Spodick, D. H. (1976). Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 235, 39–41. doi: 10.1001/jama.1976.03260270025020
Stables, C. L., Musa, H., Mitra, A., Bhushal, S., Deo, M., Guerrero-Serna, G., et al. (2014). Reduced Na(+) current density underlies impaired propagation in the diabetic rabbit ventricle. J. Mol. Cell. Cardiol. 69:24–31. doi: 10.1016/j.yjmcc.2013.12.031
Stahn, A., Pistrosch, F., Ganz, X., Teige, M., Koehler, C., Bornstein, S., et al. (2014). Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care 37, 516–520. doi: 10.2337/dc13-0600
Sutherland, C. G., Fisher, B. M., Frier, B. M., Dargie, H. J., More, I. A., and Lindop, G. B. (1989). Endomyocardial biopsy pathology in insulin-dependent diabetic patients with abnormal ventricular function. Histopathology 14, 593–602. doi: 10.1111/j.1365-2559.1989.tb02200.x
Švíglerová, J., Mudra, J., Tonar, Z., Slavikova, J., and Kuncova, J. (2011). Alteration of the cardiac sympathetic innervation is modulated by duration of diabetes in female rats. Exp. Diabetes Res. 2011:835932. doi: 10.1155/2011/835932
Tadic, M., and Cuspidi, C. (2015). The influence of type 2 diabetes on left atrial remodeling. Clin. Cardiol. 38, 48–55. doi: 10.1002/clc.22334
Tang, R. B., Dong, J. Z., Liu, X. P., Fang, D. P., Long, D. Y., Liu, X. H., et al. (2006). Safety and efficacy of catheter ablation of atrial fibrillation in patients with diabetes mellitus–single center experience. J. Interv. Card. Electrophysiol. 17, 41–46. doi: 10.1007/s10840-006-9049-x
Ten Tusscher, K. H., and Panfilov, A. V. (2007). Influence of diffuse fibrosis on wave propagation in human ventricular tissue. Europace 9 Suppl 6:vi38–45. doi: 10.1093/europace/eum206
Thaung, H. P., Baldi, J. C., Wang, H. Y., Hughes, G., Cook, R. F., Bussey, C. T., et al. (2015). Increased efferent cardiac sympathetic nerve activity and defective intrinsic heart rate regulation in type 2 diabetes. Diabetes 64, 2944–2956. doi: 10.2337/db14-0955
Valensi, P., Sachs, R. N., Harfouche, B., Lormeau, B., Paries, J., Cosson, E., et al. (2001). Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care 24, 339–343. doi: 10.2337/diacare.24.2.339
van Belle, T. L., Coppieters, K. T., and von Herrath, M. G. (2011). Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol. Rev. 91, 79–118. doi: 10.1152/physrev.00003.2010
van Hoeven, K. H., and Factor, S. M. (1990). A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 82, 848–855. doi: 10.1161/01.CIR.82.3.848
VanHoose, L., Sawers, Y., Loganathan, R., Vacek, J. L., Stehno-Bittel, L., Novikova, L., et al. (2010). Electrocardiographic changes with the onset of diabetes and the impact of aerobic exercise training in the Zucker Diabetic Fatty, (ZDF) rat. Cardiovasc. Diabetol. 9:56. doi: 10.1186/1475-2840-9-56
Veeranki, S., Givvimani, S., Kundu, S., Metreveli, N., Pushpakumar, S., and Tyagi, S. C. (2016). Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell. Cardiol. 92:163–173. doi: 10.1016/j.yjmcc.2016.01.023
Veglio, M., Bruno, G., Borra, M., Macchia, G., Bargero, G., D'Errico, N., et al. (2002). Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J. Intern. Med. 251, 317–324. doi: 10.1046/j.1365-2796.2002.00955.x
Venteclef, N., Guglielmi, V., Balse, E., Gaborit, B., Cotillard, A., Atassi, F., et al. (2015). Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 36, 795–805a. doi: 10.1093/eurheartj/eht099
Vinik, A. I., Maser, R. E., Mitchell, B. D., and Freeman, R. (2003). Diabetic autonomic neuropathy. Diabetes Care 26, 1553–1579. doi: 10.2337/diacare.26.5.1553
Violi, F., Pastori, D., Pignatelli, P., and Loffredo, L. (2014). Antioxidants for prevention of atrial fibrillation: a potentially useful future therapeutic approach? A review of the literature and meta-analysis. Europace 16, 1107–1116. doi: 10.1093/europace/euu040
Voigt, N., Li, N., Wang, Q., Wang, W., Trafford, A. W., Abu-Taha, I., et al. (2012). Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125, 2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306
Wang, G. Y., Bi, Y. G., Liu, X. D., Zhao, Y., Han, J. F., Wei, M., et al. (2017). Autophagy was involved in the protective effect of metformin on hyperglycemia-induced cardiomyocyte apoptosis and Connexin43 downregulation in H9c2 cells. Int. J. Med. Sci. 14, 698–704. doi: 10.7150/ijms.19800
Wang, Q., Quick, A. P., Cao, S., Reynolds, J., Chiang, D. Y., Beavers, D., et al. (2018). Oxidized CaMKII, (Ca(2+)/calmodulin-dependent protein kinase II) is essential for ventricular arrhythmia in a mouse model of duchenne muscular dystrophy. Circ. Arrhythm. Electrophysiol. 11:e005682. doi: 10.1161/CIRCEP.117.005682
Watanabe, M., Yokoshiki, H., Mitsuyama, H., Mizukami, K., Ono, T., and Tsutsui, H. (2012). Conduction and refractory disorders in the diabetic atrium. Am. J Physiol. Heart Circ. Physiol. 303, H86–95. doi: 10.1152/ajpheart.00010.2012
Weiss, J. N., and Lamp, S. T. (1987). Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science 238, 67–69. doi: 10.1126/science.2443972
Wellen, K. E., and Hotamisligil, G. S. (2005). Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119. doi: 10.1172/JCI25102
Wheeler, S. G., Ahroni, J. H., and Boyko, E. J. (2002). Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res. Clin. Pract. 58, 131–138. doi: 10.1016/S0168-8227(02)00128-6
Wilhelmsen, L., Rosengren, A., and Lappas, G. (2001). Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J. Intern. Med. 250, 382–389. doi: 10.1046/j.1365-2796.2001.00902.x
Wong, C. X., Abed, H. S., Molaee, P., Nelson, A. J., Brooks, A. G., Sharma, G., et al. (2011). Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J. Am. Coll. Cardiol. 57, 1745–1751. doi: 10.1016/j.jacc.2010.11.045
Xu, Z., Patel, K. P., Lou, M. F., and Rozanski, G. J. (2002). Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc. Res. 53, 80–88. doi: 10.1016/S0008-6363(01)00446-1
Xu, Z., Patel, K. P., and Rozanski, G. J. (1996). Metabolic basis of decreased transient outward K+ current in ventricular myocytes from diabetic rats. Am. J. Physiol. 271(5 Pt 2):H2190–2196. doi: 10.1152/ajpheart.1996.271.5.H2190
Yang, K. C., and Dudley, S. C. Jr. (2013). Oxidative stress and atrial fibrillation: finding a missing piece to the puzzle. Circulation 128, 1724–1726. doi: 10.1161/CIRCULATIONAHA.113.005837
Zhang, J., Youn, J. Y., Kim, A. Y., Ramirez, R. J., Gao, L., Ngo, D., et al. (2012). NOX4-Dependent hydrogen peroxide overproduction in human atrial fibrillation and hl-1 atrial cells: relationship to hypertension. Front. Physiol. 3:140. doi: 10.3389/fphys.2012.00140
Zhang, X., Zhang, Z., Zhao, Y., Jiang, N., Qiu, J., Yang, Y., et al. (2017). Alogliptin, a dipeptidyl peptidase-4 inhibitor, alleviates atrial remodeling and improves mitochondrial function and biogenesis in diabetic rabbits. J. Am. Heart Assoc. 6:e005945. doi: 10.1161/JAHA.117.005945
Zhang, Z., Zhang, X., Korantzopoulos, P., Letsas, K. P., Tse, G., Gong, M., et al. (2017). Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc. Disord. 17:96. doi: 10.1186/s12872-017-0531-4
Keywords: diabetes mellitus, arrhythmia, atrial fibrillation, cardiac fibrosis, autonomic dysregulation
Citation: Grisanti LA (2018) Diabetes and Arrhythmias: Pathophysiology, Mechanisms and Therapeutic Outcomes. Front. Physiol. 9:1669. doi: 10.3389/fphys.2018.01669
Received: 19 June 2018; Accepted: 06 November 2018;
Published: 26 November 2018.
Edited by:
Laurent Metzinger, University of Picardie Jules Verne, FranceReviewed by:
Firdos Ahmad, University of Sharjah, United Arab EmiratesMartin Bishop, King's College London, United Kingdom
Copyright © 2018 Grisanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurel A. Grisanti, Z3Jpc2FudGlsQG1pc3NvdXJpLmVkdQ==
 Laurel A. Grisanti
Laurel A. Grisanti