- Department of Physiology and Cardiovascular Research Institute, Wayne State University School of Medicine, Detroit, MI, United States
During both static and dynamic exercise hypertensive subjects can experience robust increases in arterial pressure to such an extent that heavy exercise is often not recommended in these patients due to the dangerously high levels of blood pressure sometimes observed. Currently, the mechanisms mediating this cardiovascular dysfunction during exercise in hypertension are not fully understood. The major reflexes thought to mediate the cardiovascular responses to exercise in normotensive healthy subjects are central command, arterial baroreflex and responses to stimulation of skeletal muscle mechano-sensitive and metabo-sensitive afferents. This review will summarize our current understanding of the roles of these reflexes and their interactions in mediating the altered cardiovascular responses to exercise observed in hypertension. We conclude that much work is needed to fully understand the mechanisms mediating excessive pressor response to exercise often seen in hypertensive patients.
Introduction
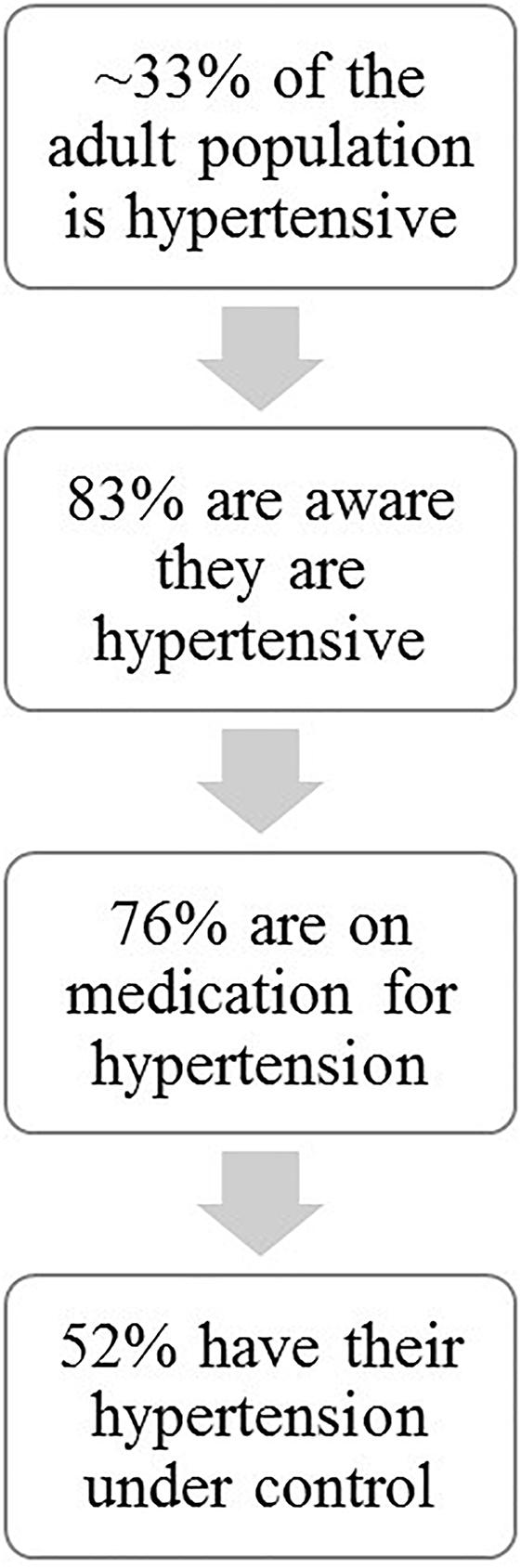
Hypertension is one of the major pathological diseases of our time affecting approximately 1/3 (29.1%) of the adult population. Only 83% of these individuals are aware that they are hypertensive and of these, only 76% are medicated and despite multiple available therapeutic interventions only about one-half of treated individuals have their hypertension under control (Nwankwo et al., 2013) (Figure 1). Current extrapolations predict that by 2030 an additional 27 million people will be hypertensive (Kirkland et al., 2018). HTN has a myriad of risk factors: obesity, sedentary lifestyle, high salt diet, high alcohol intake, insulin resistance, low potassium intake, low calcium intake, stress, sex, and age all can contribute in concert or alone in the disease development and pathology (Carretero and Oparil, 2000). Excessive activation of the sympathetic nervous system may play a role in hypertension (Head, 1995). Although, regular moderate exercise can reduce the subsequent resting sympathetic activity in hypertensive individuals, several studies have shown that during exercise sympathetic activity may increase excessively thereby increasing risk factors for adverse events including myocardial ischemia, arrhythmia, myocardial infarctions, and sudden cardiac death (Hoberg et al., 1990; Mittleman et al., 1993). Indeed The American College of Cardiology/American Heart Association 2002 guidelines for high blood pressure warns that individuals with uncontrolled hypertension (>200 mmHg systolic and/or >110 mmHg diastolic at rest) should not undergo exercise stress tests due to possible dangerously large increases in arterial blood pressure (Gibbons et al., 2002). Similarly, the Journal of Nuclear Cardiology and The American Family Physician guidelines considers systolic blood pressure above 230 mm Hg and diastolic above 115 mm Hg during exercise as contraindications to continue these tests (Darrow, 1999; Henzlova et al., 2016). Clearly, pathological increases in arterial pressure during these tests present potential risks for adverse events such as myocardial ischemia, arrhythmia, myocardial infarction, and stroke. The mechanisms mediating these exaggerated increases in sympathetic activity in hypertensive patients are not well-understood and are clinically important. This review summarizes current understanding of the roles of three major mechanisms thought to control autonomic outflow during exercise in hypertension: central command, arterial baroreflex, and skeletal muscle afferents.
Central Command
The initiation of exercise elicits immediate changes in autonomic outflow which have been in part ascribed to feed-forward reflex effects of the volition to exercise, termed central command. This reflex likely contributes importantly to the immediate partial reductions in parasympathetic activity to the heart causing a rapid tachycardia. Sympathetic activity can also increase with activation of central command. To our knowledge there have been no studies investigating whether neural control of cardiovascular function during exercise by central command is altered in patients with hypertension. However, Liang et al. (2016) showed in decerebrated rats that electrical stimulation of the mesencephalic locomotor region in spontaneous hypertensive rats (SHRs) had greater pressor and heart rate (HR) responses when compared to normotensive rats indicating that “central command” maybe exaggerated in hypertension.
Skeletal Muscle Afferents
Skeletal muscle contains afferents that are both mechanosensitive (predominantly group III afferents) and sensitive to the metabolic environment (predominantly group IV afferents) although some afferents are polymodal (Kaufman et al., 1983, 1984; Kaufman and Rybicki, 1987). Activation of these afferents can elicit a powerful pressor response. Previous studies have concluded that the pressor, HR, and renal sympathetic nerve activity (RSNA) responses to stimulation of both mechanosensitive and metabosenstive skeletal muscle afferents are exaggerated in SHR compared to the normotensive controls (Leal et al., 2008; Mizuno et al., 2011a; Liang et al., 2016). In addition, a recent study (Barbosa et al., 2016) showed in humans that the exaggerated pressor response to leg exercise could be normalized by blockade of leg afferents via intrathecal fentanyl. Although the drug also lowered resting arterial pressure, these results strongly suggest that the exaggerated pressor response to exercise in hypertension stems, in part, from activation of skeletal muscle afferents.
Muscle Mechanoreflex
In SHR, blockade of mechanoreceptors reduced the increases in mean arterial pressure (MAP), RSNA and HR when compared to control responses (Mizuno et al., 2011b) indicating that the muscle mechanoreflex is playing an important role in the exaggerated cardiovascular responses to exercise in hypertension. These studies (Mizuno et al., 2011a,b) provide some support for the role of the mechanoreflex, however, all of these studies were done either under anesthesia or in a decerebrated animal models. In a study using conscious humans Choi et al. (2013) observed that the pressor response to static forearm contraction was exaggerated in pre-hypertensive subjects whereas the pressor response to only mechanoreflex activation (via stretching of lower leg muscles) was only seen to be greater if expressed as absolute increases in pressure, % changes were not different.
Muscle Metaboreflex
Multiple studies have been conducted in order to elucidate whether hypertension affects the strength and mechanisms of the muscle metaboreflex although the conclusions have been varied. Delaney et al. (2010) showed that hypertensive individuals had accentuated increases in mean arterial blood pressure and muscle sympathetic nerve activity (MSNA) in response to hand grip exercise when compared to normotensive individuals, whereas the responses to the cold pressor test were not different indicating that the accentuated metaboreflex responses likely were not due to generalized increases in sympatho-excitatory reflexes. Furthermore, these exaggerated responses were maintained during post-exercise circulatory occlusion (PECO) – a setting that isolates any metaboreceptor activation during the recovery from exercise. These results indicate that the accentuated responses to hand grip exercise may be due to accentuated muscle metaboreflex activation. Furthermore, Chant et al. (2018) found that the enhanced pressor response to exercise and metaboreflex activation still occurred in medicated hypertensive patients whose pressure was deemed controlled at rest. Studies in decerebrated rats have shown that chemical activation of skeletal muscle afferents with capsaicin leads to exaggerated RSNA (Mizuno et al., 2011a) and pressor responses (Leal et al., 2008; Mizuno et al., 2011a) in SHR when compared to Wistar-Kyoto (WKY) normotensive controls. Blockade of purinergic receptors in hypertensive individuals during PECO reduced MSNA burst frequency more when compared to normotensive individuals, suggesting that the purinergic receptors are playing a role in the sympathoexcitation occurring during muscle metaboreflex activation (Greaney et al., 2015b). In addition, women with a positive family history of hypertension had greater pressor and MSNA to several stressors such as the cold pressor test, isometric hand grip, and PECO when compared to women with no family history of hypertension (Greaney et al., 2015a) suggesting there may be a genetic component contributing to these accentuated responses.
In contrast there are several studies that have shown that muscle metaboreflex induced cardiovascular responses are reduced in hypertension. Rondon et al. (2006) found that the increases in MSNA in response to moderate handgrip exercise were not sustained during PECO whereas in normal individuals this sympatho-activation is sustained in PECO. A study by Ranadive et al. (2017) showed that there was no differences in muscle metaboreflex responses between women with a history of hypertensive pregnancies when compared to women that had normotensive pregnancies. Studies in conscious dogs concluded that metaboreflex-induced increases in MAP, cardiac output (CO), stroke volume (SV) and HR were reduced in dogs after induction of hypertension (Sala-Mercado et al., 2013) and that these attenuated responses were less sustained during PECO (Spranger et al., 2015). Previous studies in canines and humans have shown that when cardiac function is impaired the mechanisms mediating the metaboreflex pressor response during submaximal dynamic exercise “switch” from primarily increases in CO to primarily peripheral vasoconstriction (Hammond et al., 2000; Kim et al., 2005a; Crisafulli et al., 2011; Sala-Mercado et al., 2013). Furthermore, a recent study in canines demonstrated that metaboreflex activation during dynamic exercise elicits beta receptor-mediated peripheral vasodilation likely via epinephrine release from the adrenal gland (Kaur et al., 2015b). However, in heart failure (HF) this response appears to be abolished (Kaur et al., 2015b). In the study by Spranger et al. (2015) the authors noted a hint of this shift in the mechanisms mediating the metaboreflex in the animals after induction of hypertension inasmuch as the small rise in peripheral vascular conductance during metaboreflex activation often noted in normal animals was abolished after induction of hypertension. This could indicate greater peripheral sympatho-activation during metaboreflex activation after induction of hypertension and/or reduced beta mediated vasodilation. In a subsequent study, Spranger et al. (2017) concluded that during metaboreflex activation in hypertensive canines there is enhanced vasoconstriction of the coronary vasculature which limits increases in ventricular function. When this restraint of coronary blood flow was blocked via prazosin, the increases in coronary blood flow and CO were restored toward normal. These data indicate that the primary reason for the attenuated metaboreflex responses seen in the canine studies was enhanced coronary vasoconstriction which limited increases in CO. Inasmuch as the rise in CO is the primary mechanism mediating the metaboreflex response when activated during exercise, when the cardiac component was decreased, the pressor response was likewise attenuated (Sala-Mercado et al., 2013; Spranger et al., 2015, 2017). Additionally, in HF previous studies have shown that muscle metaboreflex activation induces limitations in coronary blood flow resulting in a further reduced capacity to increase CO to match physiological demands (Kaur et al., 2015a). Inasmuch as the responses in hypertension coincide with those observed in HF, the mechanism ultimately resulting in the profound pressor response in hypertension may have the same functional origin. Whether the metaboreflex is increased or attenuated may be dependent on which response is used for analysis, e.g., when a shift in mechanisms occurs (from CO based pressor response to peripheral vasoconstriction as occurs in HF), the strength of the metaboreflex in the control of CO is reduced but the strength of this reflex in the ability to elicit vasoconstriction is enhanced.
Arterial Baroreflex
The arterial baroreceptor reflex is the primary reflex for beat-by-beat control of arterial pressure. In normotensive individuals during dynamic exercise many studies have shown that the operating point of the stimulus – response relationship for the arterial baroreflex is shifted to the right but the gain remains the same (Rowell and O’Leary, 1990; Papelier et al., 1994; O’Leary, 1996, 2006; Michelini et al., 2015). At rest, hypertensive individuals have reduced baroreflex control of HR (Judy and Farrell, 1979; Souza et al., 2008). Whether baroreflex control of sympathetic input to the peripheral vasculature is altered in hypertension is controversial (Sapru and Wang, 1976; Judy and Farrell, 1979; Thames et al., 1984; Matsukawa et al., 1991a,b; Dampney et al., 2005; Rossi et al., 2010). To our knowledge no study has systematically examined the effect of exercise on the strength and mechanisms of the arterial baroreflex during exercise in hypertension. Legramante et al. (1999) did observe the effect of changes in posture on the responses to handgrip in an older cohort of normotensive and hypertensive men and found that the effect of the static exercise on spontaneous baroreflex control of heart period was unaffected by the posture and was similar between normotensive and hypertensive groups. Inasmuch as posture changes unloads cardiopulmonary and arterial baroreceptors to variable extents, it is difficult to conclude whether the baroreflex is altered during exercise in hypertensive subjects. The authors attributed the lack of effect of hypertension on the baroreflex responses at rest to perhaps the age of the subjects. Some studies have investigated whether exercise training affects the baroreceptor reflex in hypertension but none have shown whether the arterial baroreflex strength and mechanisms are different between normotensive and hypertensive subjects during exercise. Burger et al. (1998) showed that in female exercise trained SHR, the baroreflex operating point of the systolic pressure – HR relationship was shifted to a higher point and the gain was reduced with increasing workloads, however, responses from normotensive animals were not investigated in that study. Moraes-Silva et al. (2010) demonstrated that treadmill training in during hypertension reduced absolute levels and the variability of blood pressure and HR when compared to sedentary hypertensive rats. Since a normotensive group was not utilized in the experiments previously mentioned, it is difficult to draw any conclusions about the arterial baroreflex function during exercise in hypertension. Furthermore the methods used to assess baroreceptor reflex activity is not consistent between studies, this provides a possible reason for the mismatch between the findings in the studies that have been done (O’Leary, 1996). Therefore further studies with appropriate controls are needed to fully understand impact of hypertension on arterial baroreflex control during exercise.
Baroreflex – Metaboreflex Interaction in Hypertension
The arterial baroreflex resets during exercise to a higher set point in normotensive subjects (Rowell and O’Leary, 1990; Papelier et al., 1994; O’Leary, 1996, 2006; Michelini et al., 2015). Even though the arterial baroreflex is at a higher set point it still restrains the rise in arterial pressure caused by the muscle metaboreflex during dynamic exercise under normotensive conditions (Sheriff et al., 1990; Kim et al., 2005b). Sheriff et al. (1990) observed that sino-aortic baroreceptor denervation (SAD) increased the rise in arterial pressure in response to muscle metaboreflex activation (induced via reductions in hindlimb blood flow) during mild treadmill exercise in dogs. These results indicate that the arterial baroreflex buffers the muscle metaboreflex by about 50%. Subsequently, Kim et al. (2005b) showed that this buffering is due to arterial baroreflex attenuation of metaboreflex-induced peripheral vasoconstriction inasmuch as after SAD pronounced vasoconstriction now occurred along with the substantial increases in CO during metaboreflex activation. Therefore, the baroreflex nearly completely prevents the metaboreflex from causing substantial peripheral vasoconstriction. As workload rises, the skeletal muscle vasculature progressively becomes the largest fraction of the total vascular conductance and therefore substantial pressor responses via peripheral vasoconstriction could only occur via constriction of the active muscle (O’Leary, 1991; Kaur et al., 2016, 2018). This could engender a positive feedback, vicious cycle where further metaboreflex activation causes further skeletal muscle vasoconstriction. Indeed, recent studies have shown that there is some vasoconstriction within the active skeletal muscle during metaboreflex activation and this serves as an amplifier of the original response (Kaur et al., 2016). After induction of HF, this vasoconstriction in skeletal muscle is substantially greater perhaps due to depressed ability of the baroreflex to buffer metaboreflex-induced peripheral vasoconstriction (Kim et al., 2005a; Kaur et al., 2018). During exercise, hypertensive individuals experience accentuated increases in arterial blood pressure. Whether this is due to attenuated baroreflex restraint of pressor responses to activation of the muscle metaboreflex is unknown. Further studies are need to determine whether there is diminished arterial baroreflex function in hypertension which may be a potential mechanism allowing for the exaggerated increases in blood pressure during dynamic exercise.
Conclusion
In summary, it is known that central command, skeletal muscle reflexes and the arterial baroreflex are important for regulating the cardiovascular system during exercise. To what extent hypertension alters these individual mechanisms and the interaction between these reflexes is not well-understood as some studies lack appropriate controls. Also since multiple models are utilized (i.e., humans, rats, and dogs), resting and locomotive postural position may be play a role in the sometimes disparate results. Therefore, more studies are needed to understand how hypertension alters neural control of cardiovascular function which allows for the often marked increases in arterial pressure observed in these patients during exercise (Gibbons et al., 2002). In particular how hypertension affects the central neural processes involved in integrative cardiovascular control during exercise in not understood and could be a target of therapeutic strategies.
Author Contributions
MD wrote the first draft of the manuscript. DO’L and JM wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by NIH HL-55473 and HL-126706.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barbosa, T. C., Vianna, L. C., Fernandes, I. A., Prodel, E., Rocha, H. N., Garcia, V. P., et al. (2016). Intrathecal fentanyl abolishes the exaggerated blood pressure response to cycling in hypertensive men. J. Physiol. 594, 715–725. doi: 10.1113/JP271335
Burger, H. R., Chandler, M. P., Rodenbaugh, D. W., and Dicarlo, S. E. (1998). Dynamic exercise shifts the operating point and reduces the gain of the arterial baroreflex in rats. Am. J. Physiol. 275, R2043–R2048. doi: 10.1152/ajpregu.1998.275.6.R2043
Carretero, O. A., and Oparil, S. (2000). Essential hypertension. Part I: definition and etiology. Circulation 101, 329–335. doi: 10.1161/01.CIR.101.3.329
Chant, B., Bakali, M., Hinton, T., Burchell, A. E., Nightingale, A. K., Paton, J. F. R., et al. (2018). Antihypertensive treatment fails to control blood pressure during exercise. Hypertension 72, 102–109. doi: 10.1161/HYPERTENSIONAHA.118.11076
Choi, H. M., Stebbins, C. L., Lee, O. T., Nho, H., Lee, J. H., Chun, J. M., et al. (2013). Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl. Physiol. Nutr. Metab. 38, 209–215. doi: 10.1139/apnm-2012-0143
Crisafulli, A., Piras, F., Filippi, M., Piredda, C., Chiappori, P., Melis, F., et al. (2011). Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J. Physiol. Sci. 61, 385–394. doi: 10.1007/s12576-011-0163-x
Dampney, R. A., Horiuchi, J., Killinger, S., Sheriff, M. J., Tan, P. S., and Mcdowall, L. M. (2005). Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin. Exp. Pharmacol. Physiol. 32, 419–425. doi: 10.1111/j.1440-1681.2005.04205.x
Darrow, M. D. (1999). Ordering and understanding the exercise stress test. Am. Fam. Phys. 59, 401–410.
Delaney, E. P., Greaney, J. L., Edwards, D. G., Rose, W. C., Fadel, P. J., and Farquhar, W. B. (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 299, H1318–H1327. doi: 10.1152/ajpheart.00556.2010
Gibbons, R. J., Balady, G. J., Timothy Bricker, J., Chaitman, B. R., Fletcher, G. F., Froelicher, V. F., et al. (2002). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American college of cardiology/American heart association task force on practice guidelines (committee to update the 1997 exercise testing guidelines). J. Am. Coll. Cardiol. 40, 1531–1540. doi: 10.1016/S0735-1097(02)02164-2
Greaney, J. L., Matthews, E. L., and Wenner, M. M. (2015a). Sympathetic reactivity in young women with a family history of hypertension. Am. J. Physiol. Heart Circ. Physiol. 308, H816–H822. doi: 10.1152/ajpheart.00867.2014
Greaney, J. L., Wenner, M. M., and Farquhar, W. B. (2015b). Exaggerated increases in blood pressure during isometric muscle contraction in hypertension: role for purinergic receptors. Auton. Neurosci. 188, 51–57. doi: 10.1016/j.autneu.2014.12.003
Hammond, R. L., Augustyniak, R. A., Rossi, N. F., Churchill, P. C., Lapanowski, K., and O’leary, D. S. (2000). Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 278, H818–H828. doi: 10.1152/ajpheart.2000.278.3.H818
Head, G. A. (1995). Baroreflexes and cardiovascular regulation in hypertension. J. Cardiovasc. Pharmacol. 26(Suppl. 2), S7–S16. doi: 10.1097/00005344-199512020-00002
Henzlova, M. J., Duvall, W. L., Einstein, A. J., Travin, M. I., and Verberne, H. J. (2016). ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J. Nucl. Cardiol. 23, 606–639. doi: 10.1007/s12350-015-0387-x
Hoberg, E., Schuler, G., Kunze, B., Obermoser, A.-L., Hauer, K., Mautner, H.-P., et al. (1990). Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am. J. Cardiol. 65, 583–589. doi: 10.1016/0002-9149(90)91034-4
Judy, W. V., and Farrell, S. K. (1979). Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension 1, 605–614. doi: 10.1161/01.HYP.1.6.605
Kaufman, M. P., Longhurst, J. C., Rybicki, K. J., Wallach, J. H., and Mitchell, J. H. (1983). Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J. Appl. Physiol. 55, 105–112. doi: 10.1152/jappl.1983.55.1.105
Kaufman, M. P., and Rybicki, K. J. (1987). Discharge properties of group-III and group-IV muscle afferents - their responses to mechanical and metabolic stimuli. Circ. Res. 61, 60–65.
Kaufman, M. P., Rybicki, K. J., Waldrop, T. G., and Ordway, G. A. (1984). Effect of ischemia on responses of group III and IV afferents to contraction. J. Appl. Physiol. 57, 644–650.
Kaur, J., Alvarez, A., Hanna, H. W., Krishnan, A. C., Senador, D., Machado, T. M., et al. (2016). Interaction between the muscle metaboreflex and the arterial baroreflex in control of arterial pressure and skeletal muscle blood flow. Am. J. Physiol. Heart Circ. Physiol. 311, H1268–H1276. doi: 10.1152/ajpheart.00501.2016
Kaur, J., Krishnan, A. C., Senador, D., Alvarez, A., Hanna, H. W., and O’leary, D. S. (2018). Altered arterial baroreflex - muscle metaboreflex interaction in heart failure. Am. J. Physiol. Heart Circ. Physiol. [Epub ahead of print].
Kaur, J., Machado, T. M., Alvarez, A., Krishnan, A. C., Hanna, H. W., Altamimi, Y. H., et al. (2015a). Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 309, H2145–H2151. doi: 10.1152/ajpheart.00679.2015
Kaur, J., Spranger, M. D., Hammond, R. L., Krishnan, A. C., Alvarez, A., Augustyniak, R. A., et al. (2015b). Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in beta2-mediated vasodilation. Am. J. Physiol. Heart Circ. Physiol. 308, H524–H529. doi: 10.1152/ajpheart.00648.2014
Kim, J. K., Sala-Mercado, J. A., Hammond, R. L., Rodriguez, J., Scislo, T. J., and O’leary, D. S. (2005a). Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am. J. Physiol. Heart Circ. Physiol. 289, H2416–H2423. doi: 10.1152/ajpheart.00648.2014
Kim, J.-K., Sala-Mercado, J. A., Rodriguez, J., Scislo, T. J., and O’leary, D. S. (2005b). Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am. J. Physiol. Heart Circ. Physiol. 288, H1374–H1380. doi: 10.1152/ajpheart.01040.2004
Kirkland, E. B., Heincelman, M., Bishu, K. G., Schumann, S. O., Schreiner, A., Axon, R. N., et al. (2018). Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003-2014. J. Am. Heart Assoc. 7:e008731. doi: 10.1161/JAHA.118.008731
Leal, A. K., Williams, M. A., Garry, M. G., Mitchell, J. H., and Smith, S. A. (2008). Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 295, H1429–H1438. doi: 10.1152/ajpheart.01365.2007
Legramante, J. M., Massaro, M., Raimondi, G., Castrucci, F., Cassarino, S., Peruzzi, G., et al. (1999). Effect of postural changes on cardiovascular responses to static exercise in hypertensive human beings. J. Hypertens. 17, 99–105. doi: 10.1097/00004872-199917010-00015
Liang, N., Mitchell, J. H., Smith, S. A., and Mizuno, M. (2016). Exaggerated sympathetic and cardiovascular responses to stimulation of the mesencephalic locomotor region in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 310, H123–H131. doi: 10.1152/ajpheart.00479.2015
Matsukawa, T., Gotoh, E., Hasegawa, O., Miyajima, E., Shionoiri, H., Tochikubo, O., et al. (1991a). Reduced arterial baroreflex control of muscle sympathetic nerve activity in young borderline hypertensives. Funct. Neurol. 6, 113–120.
Matsukawa, T., Gotoh, E., Hasegawa, O., Shionoiri, H., Tochikubo, O., and Ishii, M. (1991b). Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J. Hypertens. 9, 537–542.
Michelini, L. C., O’leary, D. S., Raven, P. B., and Nobrega, A. C. (2015). Neural control of circulation and exercise: a translational approach disclosing interacterions between central command, arterial baroreflex, and muscle metaboreflex. Am. J. Physiol. Heart Circ. Physiol. 309, H381–H392. doi: 10.1152/ajpheart.00077.2015
Mittleman, M. A., Maclure, M., Tofler, G. H., Sherwood, J. B., Goldberg, R. J., and Muller, J. E. (1993). Triggering of acute myocardial infarction by heavy physical exertion – protection against triggering by regular exertion. New Engl. J. Med. 329, 1677–1683. doi: 10.1056/NEJM199312023292301
Mizuno, M., Murphy, M. N., Mitchell, J. H., and Smith, S. A. (2011a). Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J. Physiol. 589, 6191–6204. doi: 10.1113/jphysiol.2011.214429
Mizuno, M., Murphy, M. N., Mitchell, J. H., and Smith, S. A. (2011b). Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 300, H968–H977. doi: 10.1152/ajpheart.01145.2010
Moraes-Silva, I. C., De La Fuente, R. N., Mostarda, C., Rosa, K., Flues, K., Damaceno-Rodrigues, N. R., et al. (2010). Baroreflex deficit blunts exercise training-induced cardiovascular and autonomic adaptations in hypertensive rats. Clin. Exp. Pharmacol. Physiol. 37, e114–e120. doi: 10.1111/j.1440-1681.2009.05333.x
Nwankwo, T., Yoon, S., Burt, V., and Gu, Q. (2013). Hypertension among adults in the United States: national health and nutrition examination survey, 2001-2012. NCHS Data Brief 133, 1–8.
O’Leary, D. S. (1991). Regional vascular resistance vs. conductance: which index for baroreflex responses? Am. J. Physiol. Heart Circ. Physiol. 260, H632–H637.
O’Leary, D. S. (1996). Heart rate control during exercise by baroreceptors and skeletal muscle afferents. Med. Sci. Sports Exerc. 28, 210–217. doi: 10.1097/00005768-199602000-00009
O’Leary, D. S. (2006). Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp. Physiol. 91, 73–77. doi: 10.1113/expphysiol.2005.031179
Papelier, Y., Escourrou, P., Gauthier, J. P., and Rowell, L. B. (1994). Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J. Appl. Physiol. 77, 502–506. doi: 10.1152/jappl.1994.77.2.502
Ranadive, S. M., Harvey, R. E., Lahr, B. D., Miller, V. M., Joyner, M. J., and Barnes, J. N. (2017). Sympathetic responsiveness is not increased in women with a history of hypertensive pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R49–R54. doi: 10.1152/ajpregu.00379.2016
Rondon, M. U., Laterza, M. C., De Matos, L. D., Trombetta, I. C., Braga, A. M., Roveda, F., et al. (2006). Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am. J. Hypertens. 19, 951–957. doi: 10.1016/j.amjhyper.2006.02.001
Rossi, N. F., Maliszewska-Scislo, M., Chen, H., Black, S. M., Sharma, S., Ravikov, R., et al. (2010). Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp. Physiol. 95, 845–857. doi: 10.1113/expphysiol.2009.051789
Rowell, L. B., and O’Leary, D. S. (1990). Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J. Appl. Physiol. 69, 407–418. doi: 10.1152/jappl.1990.69.2.407
Sala-Mercado, J. A., Spranger, M. D., Abu-Hamdah, R., Kaur, J., Coutsos, M., Stayer, D., et al. (2013). Attenuated muscle metaboreflex-induced increases in cardiac function in hypertension. Am. J. Physiol. Heart Circ. Physiol. 305, H1548–H1554. doi: 10.1152/ajpheart.00478.2013
Sapru, H., and Wang, S. (1976). Modification of aortic barorecptor resetting in the spontaneously hypertensive rat. Am. J. Physiol. Leg. Cont. 230, 664–674. doi: 10.1152/ajplegacy.1976.230.3.664
Sheriff, D. D., O’leary, D. S., Scher, A. M., and Rowell, L. B. (1990). Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am. J. Physiol. 258, H305–H310. doi: 10.1152/ajpheart.1990.258.2.H305
Souza, H. C. D., Martins-Pinge, M. C., Dias Da Silva, V. J., Borghi-Silva, A., Gastaldi, A. C., Blanco, J. H. D., et al. (2008). Heart rate and arterial pressure variability in the experimental renovascular hypertension model in rats. Auton. Neurosci. 139, 38–45. doi: 10.1016/j.autneu.2008.01.001
Spranger, M. D., Kaur, J., Sala-Mercado, J. A., Krishnan, A. C., Abu-Hamdah, R., Alvarez, A., et al. (2017). Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am. J. Physiol. Heart Circ. Physiol. 312, H68–H79. doi: 10.1152/ajpheart.00417.2016
Spranger, M. D., Kaur, J., Sala-Mercado, J. A., Machado, T. M., Krishnan, A. C., Alvarez, A., et al. (2015). Attenuated muscle metaboreflex-induced pressor response during postexercise muscle ischemia in renovascular hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R650–R658. doi: 10.1152/ajpregu.00464.2014
Keywords: hypertension, central command, arterial baroreflex, exercise pressor response, dynamic exercise
Citation: Dombrowski M, Mannozzi J and O’Leary DS (2018) Neural Control of Cardiovascular Function During Exercise in Hypertension. Front. Physiol. 9:1829. doi: 10.3389/fphys.2018.01829
Received: 27 September 2018; Accepted: 06 December 2018;
Published: 20 December 2018.
Edited by:
Antonio Crisafulli, Università degli Studi di Cagliari, ItalyReviewed by:
Ferdinando Iellamo, Università degli Studi di Roma Tor Vergata, ItalyLauro C. Vianna, Universidade de Brasília, Brazil
Konstantina Dipla, Aristotle University of Thessaloniki, Greece
Copyright © 2018 Dombrowski, Mannozzi and O’Leary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donal S. O’Leary, ZG9sZWFyeUBtZWQud2F5bmUuZWR1
 Maryetta Dombrowski
Maryetta Dombrowski Joseph Mannozzi
Joseph Mannozzi Donal S. O’Leary
Donal S. O’Leary