- 1Faculty of Medical Laboratory Sciences, Omdurman Islamic University, Omdurman, Sudan
- 2Faculty of Medical Laboratory Sciences, College of Applied Medical Sciences, Taibah University, Madinah, Saudi Arabia
- 3Africa City of Technology, Khartoum, Sudan
- 4Department of Obstetrics and Gynaecology, Unaizah College of Medicine and Medical Sciences, Qassim University, Unaizah, Saudi Arabia
- 5Department of Basic Medical Sciences, Unaizah College of Medicine and Medical Sciences, Qassim University, Unaizah, Saudi Arabia
- 6Faculty of Medicine, Al-Neelain University, Khartoum, Sudan
Introduction: Preeclampsia can lead to a number of adverse maternal and perinatal effects. The association between iron status [serum iron, ferritin and total iron-binding capacity (TIBC)], unsaturated iron-binding capacity, hepcidin, interleukin-6 (IL-6) levels and preeclampsia is not fully understood.
Objective: To assess the levels of iron status, hepcidin and interleukin-6 in women with preeclampsia compared with healthy pregnant women.
Method: A case-control study (60 women were recruited in each group) was conducted at Saad Abuelela Maternity Hospital in Khartoum, Sudan. Sociodemographic and clinical data were gathered through a questionnaire. The levels of iron status, hepcidin and IL-6 were measured using applicable methods.
Results: There was no significant difference in the median [interquartile range (IQR)] of age, parity or body mass index between the two groups. Moreover, the median (IQR) of the iron status, hepcidin and interleukin-6 did not differ between women with preeclampsia and healthy controls. There were no significant correlations between haemoglobin, hepcidin and IL-6. There were also no significant correlations between serum iron, serum ferritin, hepcidin and IL-6. However, there was a significant positive correlation between hepcidin and IL-6 (r = 0.393, p = 0.002).
Conclusion: In this study, women with preeclampsia had levels of iron status, hepcidin and IL-6 similar to those observed in healthy pregnant women. There was no significant correlation between iron status, hepcidin and IL-6.
Introduction
Preeclampsia is a multiorgan disease which occurs in the second half of pregnancy (i.e., after the 20th week of gestation), and it is common in pregnancy-related disorders (ACOG Committee on Obstetric Practice, 2002). It is a global health problem that affects 2%–8% of pregnant/parturient women (Abalos et al., 2013). Preeclampsia is the leading cause of maternal and perinatal adverse effects (Abalos et al., 2013). Several obstetrics, clinical, biochemical, nutritional and immunological factors have been identified as risk factors for preeclampsia (Meazaw et al., 2020).
Although the exact pathophysiology of preeclampsia remains to be understood, preeclampsia is characterised by poor placentation (shallow cytotrophoblast invasion), which results in defective remodelling of the maternal spiral arteries (Hoodbhoy and Payne, 2016). Poor placentation (under-perfused placenta) can lead to maternal angiogenic imbalance and results in activation of the inflammatory response (Hoodbhoy and Payne, 2016).
Hepcidin is a peptide hormone that plays an essential role in iron status to keep it tightly regulated for haemoglobin and erythropoiesis without allowing iron overload to occur in the body. It is an acute-phase reactant that can increase in response to inflammation (Chambers et al., 2021). It is expected that hepcidin is lower during pregnancy to enhance optimum iron bioavailability to the mother as well as for fetus. However, inflammatory states during pregnancy such as preeclampsia are reported to be associated with higher hepcidin levels during pregnancy (Koenig et al., 2014). The proposed elevated hepcidin level in women with preeclampsia could act as a protective mechanism to counteract iron overload (mediated cytotoxicity), oxidative stress and endothelial dysfunction that might occur in women with preeclampsia (Shaji Geetha et al., 2022).
The association between the levels of iron status, hepcidin and interleukin-6 (IL-6), and preeclampsia is not fully understood. Some studies have reported higher levels of iron status, hepcidin and IL-6 (Muhsin et al., 2016; Brunacci et al., 2018; Shaji Geetha et al., 2022; Ölmez et al., 2022) in healthy controls, while other studies have shown no significant difference in these elements in women with preeclampsia and controls (Duvan et al., 2015; Cardaropoli et al., 2018). Moreover, none of these studies were conducted in sub-Saharan Africa, including Sudan. In sub-Saharan Africa, there is a high prevalence of anaemia among pregnant women (Geta et al., 2022), which might have an effect on the association between iron status, hepcidin, IL-6 and preeclampsia. Likewise, the pathophysiology of preeclampsia might be different in Africa from other settings for a number of reasons, for example, communicable diseases such as malaria (Adam et al., 2011) and bacterial (Ahmed et al., 2020) and viral infections (Ahmed et al., 2018), which have been reported to be associated with preeclampsia in sub-Saharan Africa. Therefore, there is a need to assess the association between iron status, hepcidin, IL-6 and preeclampsia in sub-Saharan Africa. The current study was conducted to evaluate iron status, hepcidin and IL-6 levels in Sudanese women with preeclampsia.
Materials and methods
A case-control study was conducted at Saad Abuelela Maternity Hospital in Khartoum, Sudan, from June to December 2020. The cases were 60 pregnant women who presented with preeclampsia and have no history of pre-existing hypertension. Preeclampsia was defined as per the American College of Obstetricians and Gynaecologists criteria (ACOG Committee on Practice Bulletins—Obstetrics, 2020): pregnant women with onset of new hypertension (an average blood pressure reading of ≥140/90 mmHg taken on two occasions at least six hours apart) with proteinuria (≥ 300 mg/24 h) or features of end organ dysfunction in a previously normotensive woman. Preeclampsia was classified as severe in women with an average blood pressure reading of ≥ 160/110 mmHg on two occasions or HELLP syndrome, which includes haemolysis, elevated liver enzymes and low platelet count; otherwise, preeclampsia was considered mild (ACOG Committee on Practice Bulletins—Obstetrics, 2020). The condition was also categorised as early presentation or late-onset preeclampsia, before and after 34 weeks, respectively (Tranquilli et al., 2013). Sixty healthy pregnant women without any systemic disease, such as hypertension, diabetes mellitus, renal disease or thyroid disease, served as a control for each preeclampsia case. Women with multiple pregnancies, diabetic women, smokers and women with fetuses who had major anomalies or died were excluded from both groups in the study.
After signing informed consent, the women were asked about their sociodemographic, obstetrics and clinical data, including age, parity, educational level, residence of antenatal attendance and history of miscarriage and preeclampsia/hypertension. Body mass index (BMI) was computed from the measured weight and height.
Then, 5 mL of blood was collected from each subject at the diagnosis and separated into two equal aliquots for blood and serum analysis. Haemoglobin levels were measured using a modern haematology analyser (Sysmex KX21n, Japan) according to the manufacturer’s instructions. The blood was then centrifuged and stored at −20°C until the assay of these elements. Serum ferritin was determined using the ferritin chemiluminescent immunoassay sandwich method [TOSOH instrument (AIA360), Japan]. Serum iron and total iron-binding capacity (TIBC) were measured using a colorimetric assay (Roche Diagnostics, Germany Cobas 311). Serum hepcidin and IL-6 concentrations were measured using an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Euroimmun, Lubeck, Germany).
The sample included 60 women in each group (ratio of 1:1) and was calculated using mean difference of 5 in the iron levels between the women who had preeclampsia and the healthy controls as reported before (Duvan et al., 2015). The sample size was used to achieve 80% power and a precision of 5%. It was assumed that 10% of the women would not respond or would provide incomplete data.
Statistical analysis
The collected data were entered into SPSS version 22.0 for Windows for analysis. Continuous data were checked for normality using the Shapiro–Wilk test. The clinical data for the two groups (preeclampsia and controls) were compared using the Mann–Whitney U test for non-normally distributed data and Pearson’s chi-square (χ2) test for continuous and categorical data. Spearman correlations were performed between the continuous variables, and p-values of < 0.05 were considered significant.
Results
During the study period, 60 pregnant women were enrolled in the case (preeclampsia) and 60 control groups. Nine women (15.0%) and four women (6.6%) had severe and early presented preeclampsia, respectively. There was no significant difference in the median (IQR) of age, parity or BMI between the two groups. However, the median (IQR) of gestational age was significantly lower in women with preeclampsia (Table 1).
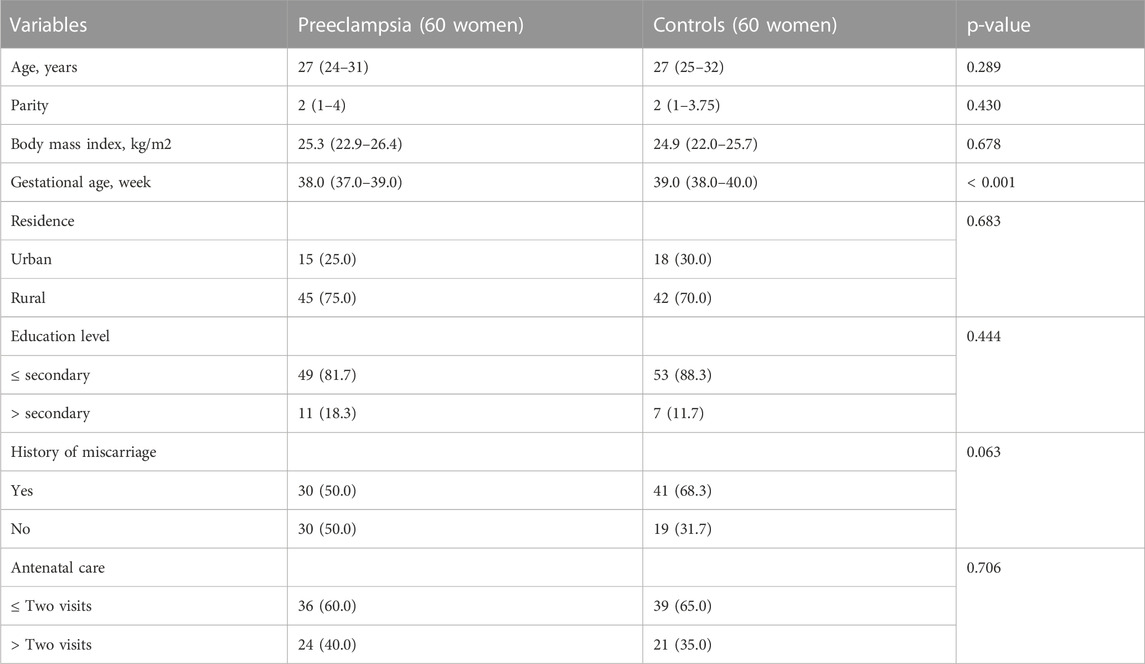
TABLE 1. Comparing frequency (proportions) or median (interquartile range) of the sociodemographic and clinical variables between women with preeclampsia and controls in Khartoum Sudan, 2020.
There was no significant difference in residence, education, antenatal care, history of miscarriage or blood group. The median (IQR) of the iron status, hepcidin and interleukin-6, did not differ between the women with preeclampsia and the healthy controls (Table 2).
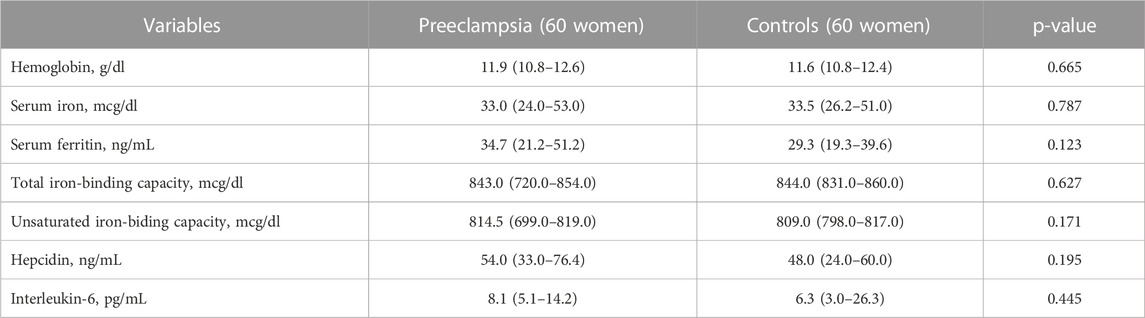
TABLE 2. Comparing the median (interquartile range) of the iron status indices, hepcidin, and interleukin-6 between women with preeclampsia and controls in Khartoum Sudan, 2020.
There was no significant correlation between haemoglobin, hepcidin and IL-6, nor was there a significant correlation between serum iron, serum ferritin, hepcidin and IL-6. However, there was a significant positive correlation between hepcidin and IL-6 (r = 0.393, p = 0.002) (Table 3).
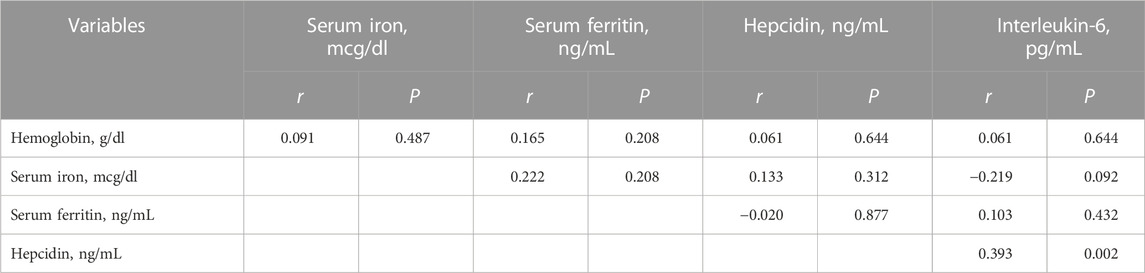
TABLE 3. Spearman correlation between the iron status, hepcidin, and interleukin-6 in pregnant women with preeclampsia in Khartoum Sudan, 2020.
Discussion
The main finding of the current study was that there was no difference in the levels of iron status, hepcidin and interleukin-6 between women with preeclampsia and healthy controls. This is in line with previous studies that showed no significant differences in maternal serum hepcidin levels in preeclampsia compared with age-matched controls (Cardaropoli et al., 2018). Duvan et al. (2015) reported no significant differences in pro-hepcidin, haemoglobin concentration, iron, ferritin or IL-6 in women with preeclampsia compared with controls.
On the other hand, several studies have shown higher serum iron and ferritin and serum hepcidin and pro-hepcidin levels in women with preeclampsia compared to healthy pregnant women (Muhsin et al., 2016; Brunacci et al., 2018; Shaji Geetha et al., 2022; Ölmez et al., 2022) It is worth mentioning that a recent meta-analysis (Bandyopadhyay et al., 2022), which included 760 individuals from seven studies, concluded that the pooled mean hepcidin levels were significantly higher in women with preeclampsia than in women without preeclampsia. Significantly lower iron-binding capacity, TIBC and transferrin levels have also been reported (Duvan et al., 2015).
Our results showed no significant difference in the levels of hepcidin and Il-6, and there was no significant correlation between the iron status, hepcidin and IL-6; however, we observed a significant positive correlation between hepcidin and IL-6. Some previous studies have reported higher levels of IL-6 in women with preeclampsia (Brunacci et al., 2018; Aggarwal et al., 2019). Our results (no significant correlation between iron status and hepcidin) were previously reported (Muhsin et al., 2016) among women with preeclampsia; however, in a later study, a positive correlation was found between iron status and hepcidin among women with normal pregnancies. Interestingly, Brunacci and colleagues reported significantly higher concentrations of serum iron, ferritin and transferrin saturation in women with preeclampsia; however, they reported lower hepcidin levels and no significant correlations between hepcidin concentration and iron status in these women (Brunacci et al., 2018).
It should be noted that our results should be compared to the results of other studies with caution. First, anaemia, which is highly prevalent (50%) among pregnant women, could have effects on the whole picture and the results of the iron status and hepcidin (Adam et al., 2018). Second, as previously mentioned, several communicable diseases (e.g., malaria and viral diseases) have been associated with preeclampsia in Sudan and these diseases could have influenced the iron status, hepcidin and IL6 (Adam et al., 2011; Ahmed et al., 2018, 2020). During states of acute or chronic inflammation, levels of hepcidin and other acute-phase reactants including ferritin increase in response to elevated IL-6 levels, leading to a decrease in serum iron levels as hepcidin levels rise. Increased hepcidin correlates with the pathophysiology of anaemia in chronic disease; the increase in inflammation causes a reduction in serum iron levels because the increase in hepcidin reduces iron transport out of cells (Chambers et al., 2021). In this study, ferritin levels were not correlated with IL-6, neither with hepcidin.
It has been reported that hepcidin is lower during pregnancy to ensure optimum iron bioavailability to the mother and fetus. However, inflammatory states, such as preeclampsia and parasitic (malaria) infection, have been associated with higher hepcidin levels during pregnancy (Koenig et al., 2014). The elevated hepcidin level in women with preeclampsia could be a protective mechanism to counteract iron overload (mediated cytotoxicity), oxidative stress and endothelial dysfunction that might occur in women with preeclampsia (Shaji Geetha et al., 2022).
For better interpretation of our findings, there are some limitations to be addressed. First; Sudan is endemic for many communicable diseases which is known to be associated with preeclampsia. However, we recruited apparently healthy pregnant women in both groups and did not screen for possible endemic diseases. Second; information regarding anaemia during entire course of pregnancy or before pregnancy is not available.
Conclusion
In this study, women with preeclampsia had levels of iron homeostasis parameters, hepcidin and IL-6 similar to those observed in healthy pregnant women. There was no significant correlation between iron homeostasis parameters, hepcidin and IL-6.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Department of Obstetrics and Gynecology, Faculty of Medicine, University of Khartoum, Sudan (#2020, 06). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, HY, MH, and IA; Methodology, YA and HH; Formal Analysis, YA, HY, and IA; Investigation, YA, HY, and IA; Data curation, HH, and IA; Writing—original draft preparation, YA, HY, MH, IA, and HH; Writing—review and editing, YA, HY, MH, IA, and HH; Supervision, MH and IA; Project administration, HY and HH.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University, Kingdom of Saudi Arabia for funding the publication of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abalos E., Cuesta C., Grosso A. L., Chou D., Say L. (2013). Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7. doi:10.1016/j.ejogrb.2013.05.005
ACOG Committee on Obstetric Practice (2002). ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. 77, 67–75. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12094777 (Accessed March 22, 2020).
ACOG Committee on Practice Bulletins—Obstetrics (2020). Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet. Gynecol. 135, e237–e260. doi:10.1097/AOG.0000000000003891
Adam I., Elhassan E. M., Mohmmed A. A., Salih M. M., Elbashir M. I. (2011). Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malar. J. 10, 258. doi:10.1186/1475-2875-10-258
Adam I., Ibrahim Y., Elhardello O. (2018). Prevalence, types and determinants of anemia among pregnant women in Sudan: A systematic review and meta-analysis. BMC Hematol. 18, 31. doi:10.1186/s12878-018-0124-1
Aggarwal R., Jain A. K., Mittal P., Kohli M., Jawanjal P., Rath G. (2019). Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 33, e22834. doi:10.1002/jcla.22834
Ahmed M. A., Hassan N. G., Omer M. E., Rostami A., Rayis D. A., Adam I. (2020). Helicobacter pylori and Chlamydia trachomatis in Sudanese women with preeclampsia. J. Matern. Neonatal Med. 33, 2023–2026. doi:10.1080/14767058.2018.1536738
Ahmed M. A., Sharif M. E., Rayis D. A., Nasr A. M., Adam I. (2018). Hepatitis B infection and preeclampsia among pregnant Sudanese women. Virol. J. 15, 20. doi:10.1186/s12985-018-0927-5
Bandyopadhyay A., Ahamed F., Palepu S., Ghosh T., Yadav V. (2022). Association of serum hepcidin with preeclampsia: A systematic review and meta-analysis. Cureus 14, e26699. doi:10.7759/CUREUS.26699
Brunacci F., Rocha V. S., De Carli E., Espósito B. P., Ruano R., Colli C. (2018). Increased serum iron in preeclamptic women is likely due to low hepcidin levels. Nutr. Res. 53, 32–39. doi:10.1016/j.nutres.2018.03.005
Cardaropoli S., Todros T., Nuzzo A. M., Rolfo A. (2018). Maternal serum levels and placental expression of hepcidin in preeclampsia. Pregnancy Hypertens. 11, 47–53. doi:10.1016/J.PREGHY.2017.12.008
Chambers K., Ashraf M. A., Sharma S. (2021). “Physiology, hepcidin - PubMed,” in StatPearls. Editor E. N. Veena Sangkhae (Treasure Island: StatPearls Publishing).
Duvan C. I., Simavli S., Keskin E. A., Onaran Y., Turhan N. O., Koca C. (2015). Is the level of maternal serum prohepcidin associated with preeclampsia? Hypertens. pregnancy 34, 145–152. doi:10.3109/10641955.2014.988350
Geta T. G., Gebremedhin S., Omigbodun A. O. (2022). Prevalence and predictors of anemia among pregnant women in Ethiopia: Systematic review and meta-analysis. PLoS One 17, e0267005. doi:10.1371/JOURNAL.PONE.0267005
Koenig M. D., Tussing-Humphreys L., Day J., Cadwell B., Nemeth E. (2014). Hepcidin and iron homeostasis during pregnancy. Nutrients 6, 3062–3083. doi:10.3390/NU6083062
Meazaw M. W., Chojenta C., Muluneh M. D., Loxton D. (2020). Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in sub-Saharan Africa. PLoS One 15, e0237600. doi:10.1371/JOURNAL.PONE.0237600
Muhsin N. A. A., Al-Mudalal S. S., Hameed B. M. (2016). Original article Evaluation of the changes in iron homeostasis and hepcidin concentration in. Iraqi J. Hematol. 5, 32–41.
Ölmez F., Oğlak S. C., Behram M., Özköse Z. G., Can E., Ünal Ö., et al. (2022). Serum prohepcidin concentrations in preeclamptic pregnant women: An analysis concerning serum iron status markers and compared to healthy pregnant women. East. J. Med. 27, 198–202. doi:10.5505/EJM.2022.32659
Shaji Geetha N., Bobby Z., Dorairajan G., Jacob S. E. (2022). Increased hepcidin levels in preeclampsia: A protective mechanism against iron overload mediated oxidative stress? J. Matern. Fetal. Neonatal Med. 35, 636–641. doi:10.1080/14767058.2020.1730322
Keywords: preeclampsia, hepcidin, iron homeostasis, interleukin-6, sub-Sahara Africa, pregnancy
Citation: Ahmed YIB, Yagoub HS, Hassan MA, Adam I and Hamdan HZ (2023) Maternal serum iron status, hepcidin and interleukin-6 levels in women with preeclampsia. Front. Physiol. 14:1049994. doi: 10.3389/fphys.2023.1049994
Received: 21 September 2022; Accepted: 16 February 2023;
Published: 24 February 2023.
Edited by:
Shyam Nandi, University of Nebraska Medical Center, United StatesCopyright © 2023 Ahmed, Yagoub, Hassan, Adam and Hamdan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamdan Z. Hamdan, aC5hYnVhbGJhc2hlckBxdS5lZHUuc2E=
 Yasir I. B. Ahmed
Yasir I. B. Ahmed Hind S. Yagoub
Hind S. Yagoub Mohamed A. Hassan3
Mohamed A. Hassan3 I. Adam
I. Adam Hamdan Z. Hamdan
Hamdan Z. Hamdan