Abstract
Lung transplantation remains the only effective treatment for end-stage lung disease, offering the potential to significantly prolong survival and enhance quality of life for recipients. However, primary graft dysfunction (PGD)-a severe form of lung injury occurring within the first 72 h post-transplantation-constitutes a major cause of early mortality and presents a substantial barrier to the broader clinical adoption of lung transplantation. Biomarkers, defined as specific molecules, cells, or other biological indicators detectable within or outside the body, can reflect physiological states, disease progression, or therapeutic responses. The identification of accurate and reliable biomarkers for the prediction and diagnosis of PGD is therefore critical for improving diagnostic precision and therapeutic outcomes. This review provides a comprehensive overview of recent advances in the discovery of PGD-related biomarkers, encompassing a wide range of candidates such as plasma proteins, hormones, cell-free DNA, and immunoreactive substances. The complex biomarker landscape associated with PGD involves multiple signaling pathways and cellular phenotypes. Despite ongoing research, no single biomarker has yet demonstrated sufficient predictive or diagnostic power to be used independently in clinical practice. Consequently, continued investigation is essential to validate existing biomarkers and develop optimized strategies for their integration into routine clinical application.
Introduction
Lung transplantation remains an effective treatment for end-stage lung diseases, although its success is often limited by primary graft dysfunction (PGD) (Shah and Diamond, 2018; Young and Dilling, 2019; Avtaar Singh et al., 2023). PGD presents as a severe form of lung injury within the first 72 h after transplantation and is characterized by hypoxemia, pulmonary edema, and decreased pulmonary compliance (Criner et al., 2021). With an incidence rate as high as 30%–50%, PGD is strongly associated with both early and long-term post-transplant mortality (Hunt and Cantu, 2023). Currently, there is a lack of reliable biomarkers and pharmacological treatments for PGD, making early diagnosis and intervention challenging. Therefore, identifying biomarkers that can predict, diagnose, and potentially guide treatment for PGD is a pressing need in the field of lung transplantation.
Biomarkers are substances that reflect physiological or pathological states and can be detected in bodily fluids such as blood, urine, bronchoalveolar lavage fluid (BALF), or organ perfusate to evaluate organ function or injury (Calfee and Ware, 2007; Chacon-Alberty et al., 2022; Hamilton et al., 2017; Nakata et al., 2023). In recent years, advances in molecular biology, proteomics, and metabolomics have led to some progress in identifying potential PGD biomarkers. However, challenges remain, including small sample sizes, inconsistent findings, and a lack of thorough validation.
This review aims to summarize current research on PGD biomarkers, including plasma proteins, hormones, cell-free DNA, and immunoreactive substances. It discusses their advantages, limitations, and potential clinical applications, while also highlighting the challenges and future directions in biomarker discovery for PGD.
Primary graft dysfunction and biomarkers
Biomarkers are substances that reflect physiological or pathological states, including genes, proteins, metabolites, and cytokines (Lozano-Edo et al., 2022; Lee and Christie, 2011; Suzuki et al., 2013). They can be detected in bodily fluids such as blood, urine, BALF, or organ perfusate, and are used to assess organ function or the extent of injury. PGD is one of the most common complications following lung transplantation, significantly affecting patient survival and quality of life (Wu et al., 2023). As such, identifying reliable biomarkers for the early prediction and accurate diagnosis of PGD remains a critical goal in the field. Despite encouraging progress, most studies on PGD biomarkers have been limited by relatively small sample sizes, and further research is needed to validate these findings for clinical use. Currently, PGD diagnosis primarily depends on the oxygenation index, chest radiographs, and clinical judgment (Sanchez-Gonzalez et al., 2022; Li et al., 2021; Neves et al., 2016). However, these methods often lack sensitivity and specificity, and may not effectively capture the onset or resolution of PGD. The discovery of biomarkers capable of predicting PGD risk, monitoring its progression, and guiding treatment strategies holds substantial clinical promise (Figure 1). Advancing biomarker research could ultimately transform the diagnosis, management, and outcomes of PGD in lung transplant recipients. Table 1 summarizes the detailed information of biomarkers for PGD following lung transplantation.
FIGURE 1
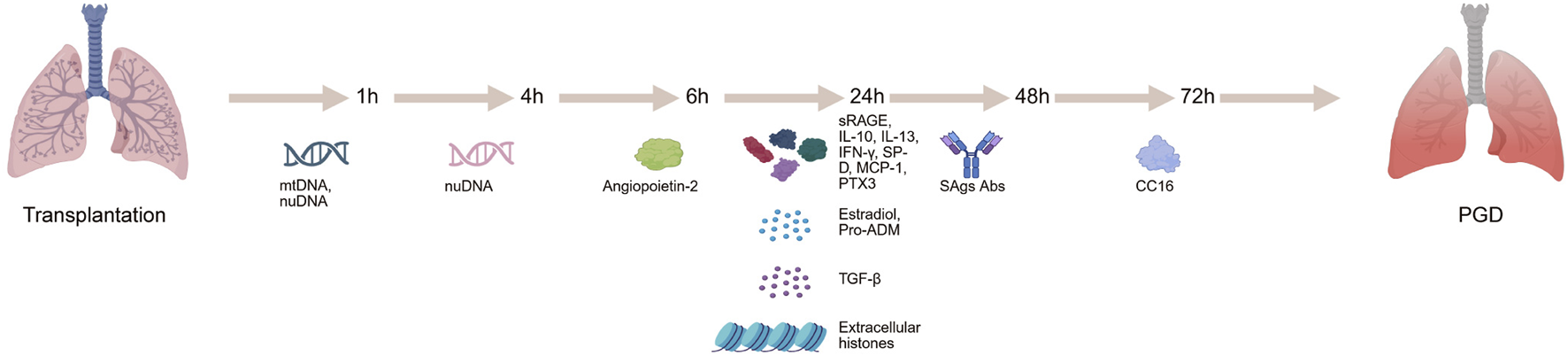
Identification of biomarkers in primary graft dysfunction can be evaluated at different time points in lung transplantation recipients.
TABLE 1
| Biomarker | Biological fucntion | Number of patients | Single/multi-center | Time points of biomarker detection after lung transplantation (/h) | With PGD | Without PGD | Detection method | References |
|---|---|---|---|---|---|---|---|---|
| Plasma Proteins | ||||||||
| sRAGE | Soluble receptor | 317 | multi-center | 24 | 4.3 ng/mL | 1.9 ng/mL | Sandwich ELISA | Christie et al. (2009) |
| IL-10 | Anti-inflammatory cytokine | 80 | single-center | 24 | 3(PGD1)/5.5(PGD2)/10.9(PGD3) pg/mL | 2 pg/mL | ELISA | Frick et al. (2020) |
| IL-13 | Inflammatory cytokine | 80 | single-center | 24 | 0.2(PGD1)/1(PGD2)/0.9(PGD3) pg/mL | 0.7 pg/mL | ELISA | Frick et al. (2020) |
| IFN-γ | Immune response regulator | 80 | single-center | 24 | 5.7(PGD1)/8.4(PGD2)/11.5(PGD3) pg/mL | 4.8 pg/mL | ELISA | Frick et al. (2020) |
| SP-D | Pulmonary surfactant | 80 | single-center | 24 | 3841(PGD1)/4247(PGD2)/3263(PGD3) pg/mL | 6001 pg/mL | ELISA | Frick et al. (2020) |
| CC16 | Secretory protein | 104 | multi-center | 72 | 13.8 ng/mL | 8.2 ng/mL | Sandwich ELISA | Diamond et al. (2011a) |
| MCP-1 | Chemotactic factor | 108 | multi-center | 24 | 167.95 pg/mL | 103.5 pg/mL | ELISA | Shah et al. (2012) |
| Angiopoietin-2 | Vascular growth stimulator | 119 | multi-center | 6 | 4578 pg/mL | 3218 pg/mL | ELISA | Diamond et al. (2017) |
| VEGF | Angiogenesis factor | 150 | single-center | 72 | 584(PGD1)/829(PGD2)/1191(PGD3)/pg/mL | 428 pg/mL | ELISA | Krenn et al. (2007) |
| PTX3 | Complement system regulator | 119 | multi-center | 24 | 88.9 ng/mL | 22.7 ng/mL | Sandwich ELISA | Diamond et al. (2011b) |
| Hormones | ||||||||
| Estradiol | Estrogen | 111 | multi-center | 24 | 77.4 pg/mL | 59.6 pg/mL | ELISA | Bastarache et al. (2012) |
| Pro-ADM | Precursor of adrenomedullin | 100 | single-center | 24 | 3.25 nmol/L | 1.61 nmol/L | An immune time-resolved amplified cryptate emission technology assay | Riera et al. (2016) |
| Cell-free DNA | ||||||||
| cfDNA | Fragmented DNA | 186 | multi-center | NA | 23.7 ng/mL | 12.9 ng/mL | Quantitative PCR | Balasubramanian et al. (2024) |
| mtDNA | Mitochondrial DNA | 62 | single-center | 1 | 1874 copies/μL | 1259 copies/μL | Quantitative PCR | Kanou et al. (2021) |
| nuDNA | Nuclear DNA | 62 | single-center | 1 | 1895 copies/μL | 675 copies/μL | Quantitative PCR | Kanou et al. (2021) |
| nuDNA | Nuclear DNA | 62 | single-center | 4 | 4521 copies/μL | 1764 copies/μL | Quantitative PCR | Kanou et al. (2021) |
| %ddcfDNA | Percentage donor-derived cell-free DNA | 99 | multi-center | 72 | 12.2% | 8.5% | An automated shotgun sequencing method | Keller et al. (2021) |
| Immunoreactive Substances | ||||||||
| TGF-β | Cytokine | 279 | single-center | 24 | - | - | ELISA | DerHovanessian et al. (2016) |
| SAgs Abs | lung-associated self-antigen antibodies | 317 | multi-center | 48 | - | - | ELISA | Tiriveedhi et al. (2013) |
| Others | ||||||||
| Extracellular histones | Cytotoxic proteins | 65 | single-center | 24 | 5.882 μg/ml | 2.478 μg/ml | Sandwich ELISA | Jin et al. (2020) |
Overview of biomarkers for primary graft dysfunction after lung transplantation.
Plasma proteins
Plasma proteins are essential for maintaining homeostasis, facilitating substance transport, supporting immune defense, and regulating coagulation (Christie et al., 2009; Covarrubias et al., 2007; Leon et al., 2009). A retrospective analysis examined the relationship between plasma cytokine levels before and after lung transplantation and the severity of PGD (Frick et al., 2020). Of the 30 proteins tested, eight showed significant differences between patients with mild and severe PGD: IL-6, IL-10, IL-13, eotaxin, G-CSF, IFN-γ, MIP-1α, and SP-D. Notably, IL-10 and IL-13 were associated with prolonged extubation times, extended ICU stays, and longer overall hospitalizations, independent of donor and recipient characteristics. Plasma IL-10 and IFN-γ levels in both donors and recipients correlated positively with PGD incidence and severity, whereas SP-D levels were inversely correlated with PGD severity.
Patients with PGD typically exhibit elevated levels of inflammatory mediators in early post-transplant serum, such as MCP-1, IP-10, IL-1β, IL-2, IFN-γ, and IL-12 (21). Further investigations revealed that PGD-induced inflammation may enhance donor HLA class II antigen expression on the graft, increase antigen presentation, and stimulate donor-specific immune responses (Bharat et al., 2008).
In a multicenter cohort study, protein C and plasminogen activator inhibitor-1 (PAI-1) levels were measured in the plasma of lung transplant recipients (Christie et al., 2007). Those who developed PGD had significantly lower post-transplant protein C levels and higher PAI-1 levels compared to those without PGD. Pre-transplant pulmonary artery systolic pressure was positively correlated with post-transplant PAI-1 levels, potentially linking pulmonary hypertension to PGD development.
Another study collected blood samples from lung transplant recipients before surgery and at 6, 24, 48, and 72 h post-transplant to evaluate plasma ICAM-1 and von Willebrand factor (vWF) levels (Covarrubias et al., 2007). ICAM-1 is a cell adhesion molecule predominantly expressed on the surface of endothelial and immune cells (Singh et al., 2023; Bui et al., 2020; van de Stolpe and van der Saag, 1996). It plays a critical role in the inflammatory response by interacting with integrins on leukocytes, thereby facilitating their adhesion and migration across the endothelium. This process promotes the infiltration of inflammatory cells into injured tissues. vWF is a large glycoprotein synthesized and secreted by endothelial cells (Zanetta et al., 2000; Xiang and Hwa, 2016; Nakhaei-Nejad et al., 2019). It is primarily involved in hemostasis, contributing to blood coagulation and platelet aggregation. ICAM-1 levels were significantly higher in patients with PGD and positively correlated with PGD occurrence. Although vWF levels tended to rise postoperatively, they were not significantly associated with PGD. ICAM-1 levels also correlated with pre-transplant pulmonary artery pressure and recipient diagnosis (Covarrubias et al., 2007).
CC16, a protein secreted by airway epithelial cells, has been significantly associated with PGD, particularly in recipients without idiopathic pulmonary fibrosis (non-IPF) (Diamond et al., 2011a). Produced by non-ciliated pulmonary epithelial cells, CC16 may serve as a biomarker for epithelial injury (Shah et al., 2014). In a prospective cohort, CC16 levels at 6 h post-transplant were notably higher in PGD patients, and a 15 ng/mL increase in CC16 was linked to a 1.6-fold higher PGD risk. Additionally, MCP-1, a chemotactic protein released by pulmonary epithelial cells, plays a key role in recruiting inflammatory cells and mediating ischemia-reperfusion injury (IRI) (Yoshimura, 2018; Singh et al., 2021; Ferreira et al., 2005). Elevated MCP-1 levels at 24 h post-transplant were positively correlated with PGD risk and severity within 72 h (Shah et al., 2012).
Angiopoietin-2 (Ang2), a vascular growth stimulator involved in angiogenesis, binds to the TIE2 receptor and acts as a negative regulator of the ANG1/TIE2 signaling pathway, modulating endothelial responses to cytokines (Akwii et al., 2019; Scholz et al., 2015; Song et al., 2012; Eklund et al., 2017; Nicolini et al., 2019). In PGD patients, Ang2 plasma levels changed significantly over time, particularly in those with idiopathic pulmonary fibrosis (IPF), but showed no significant association in chronic obstructive pulmonary disease (COPD) patients (Diamond et al., 2017).
Vascular endothelial growth factor (VEGF), a key regulator of vascular permeability, was evaluated preoperatively in lung transplant patients (Guzmán et al., 2023; Shi et al., 2022). VEGF serum levels were significantly higher in patients who developed grade 3 PGD compared to those with lower-grade PGD or healthy controls. Elevated VEGF levels were predictive of more severe PGD outcomes (Krenn et al., 2007).
Long pentraxin-3 (PTX3), a protein involved in complement activation and regulation, has also been linked to PGD (Cieślik and Hrycek, 2012; Chiari et al., 2023). In IPF recipients, PTX3 levels at 6 and 24 h post-transplant correlated positively with PGD risk (Diamond et al., 2011b). Genetic analysis in lung transplant recipients identified two PTX3 gene polymorphisms associated with increased PGD risk (Diamond et al., 2012).
The receptor for advanced glycation end-products (RAGE), a transmembrane protein of the immunoglobulin superfamily, has a soluble form (sRAGE) that includes its extracellular domain (Eva et al., 2022; Yue et al., 2022; Bongarzone et al., 2017). sRAGE levels measured at 6 and 24 h post-transplant were higher in PGD patients. These levels were influenced by right heart pressure and cardiopulmonary bypass and were associated with red blood cell transfusion and bypass usage (Kim T. et al., 2023).
Hormones
Prostaglandin E2 (PGE2) is a hormone-like lipid compound that plays a key role in numerous physiological processes, including smooth muscle contraction and relaxation, vasodilation and vasoconstriction, blood pressure regulation, and the modulation of inflammation (Képes et al., 2023; Cheng et al., 2021; Finetti et al., 2020; Finetti et al., 2023). In a large-scale gene association study, 17 genetic variants were significantly linked to PGD, four of which were located within genes related to the PGE2 pathway (Diamond et al., 2014). One notable variant involved a coding change in the PTGES2 gene, resulting in the substitution of arginine with histidine at position 298, which was associated with an increased risk of PGD. The other three variants were found in the promoter region and first intron of the PTGER4 gene and were associated with a decreased risk of PGD. Functional analysis showed that the rs4434423A variant in PTGER4 influenced the inhibitory function of regulatory T cells.
In another study, plasma estradiol levels were measured before transplantation and at 6 and 24 h post-transplantation to assess their relationship with PGD severity within 72 h after surgery. While no significant differences were found between male and female recipients overall, a positive correlation between estradiol levels at 24 h and PGD severity was observed in male recipients. This association was not present in female recipients (Bastarache et al., 2012).
Pro-adrenomedullin (pro-ADM), a precursor of adrenomedullin (ADM), has been identified as a potential biomarker in various acute conditions such as sepsis, acute heart failure, cardiac arrest, and stroke (Liang et al., 2023; Hagag et al., 2011; Spoto et al., 2023; Zelniker et al., 2023; Ishiyama et al., 2023). It reflects the rapid breakdown of ADM in circulation. In a prospective study of lung transplant recipients, pro-ADM levels were measured at 24, 48, and 72 h following ICU admission (Riera et al., 2016). Findings indicated that pro-ADM levels were strongly correlated with PGD severity and positively associated with ICU mortality. Patients with PGD grade 3 exhibited significantly higher pro-ADM levels at 72 h. Furthermore, pro-ADM levels measured at 24 h could predict the development of PGD grade 3 by 72 h. The predictive value of pro-ADM for ICU mortality surpassed that of PGD grading alone, and combining both enhanced prognostic accuracy. Elevated pro-ADM levels were strongly linked to early graft dysfunction and post-transplant mortality.
Endothelin-1 (ET-1) is a potent peptide hormone known for its vasoconstrictive and proliferative effects and plays a critical role in the pathogenesis of various pulmonary diseases (Banecki and Dora, 2023; Salama et al., 2010). In a study analyzing lung tissue and serum samples from lung transplant donors and recipients, both ET-1 mRNA expression in lung tissue and serum ET-1 concentrations were positively correlated with the severity of PGD(65).
Follistatin-like protein 1 (FSTL1) is a secretory glycoprotein involved in multiple biological functions, including the regulation of myocardial ischemia-reperfusion injury, airway remodeling, and inflammatory responses (Veraar et al., 2022; Kim DK. et al., 2023; Chiou et al., 2019). In a prospective cohort of bilateral lung transplant recipients with end-stage lung disease, post-transplant elevations in plasma FSTL1 levels were identified, showing significant associations with the incidence and clinical severity of PGD(66).
Cell-free DNA
Cell-free DNA (cfDNA) refers to the free DNA fragments existing in the extracellular environment (Valpione et al., 2018; Mattox et al., 2023). It mainly originates from apoptosis, necrosis, inflammatory responses, and tumor cells. Present in bodily fluids such as blood, saliva, and urine in the form of short fragments, cfDNA is characterized by its diverse sources, fragmentation, and short half-life (Bruhm et al., 2025; Hu et al., 2021; Sherwood and Weimer, 2018). Under normal physiological conditions, the concentration of cfDNA in the bloodstream of healthy individuals remains relatively low. However, in conditions that accelerate cell turnover, such as acute or chronic inflammation, cfDNA levels can rise significantly. Given its short half-life-typically less than one to two hours-cfDNA can serve as a real-time biomarker for disease. By distinguishing between donor- and recipient-specific single nucleotide polymorphisms (SNPs), the origin of circulating cfDNA can be determined, enabling the detection of donor-derived cfDNA. This has potential applications in identifying graft injury following transplantation (Magnusson et al., 2022; Li and Liang, 2023; Zou et al., 2017; Keller and Agbor-Enoh, 2021; Balasubramanian et al., 2024; Ju et al., 2023; Keller et al., 2022a; Keller et al., 2024; Kim et al., 2024; Keller et al., 2022b; Zhang et al., 2024; Keller MB. et al., 2022).
In lung transplant recipients, cfDNA levels are almost twice as high as those observed in healthy individuals, primarily originating from innate immune cells involved in inflammatory responses (Keller and Agbor-Enoh, 2022). Elevated cfDNA levels prior to transplantation are associated with a heightened risk of post-transplant pulmonary edema (such as PGD) and mortality. These levels also show potential in predicting both early and long-term complications, such as PGD, chronic lung allograft dysfunction (CLAD), and death, making cfDNA a promising molecular tool for risk stratification in transplant recipients (Keller et al., 2024; Keller and Agbor-Enoh, 2022).
Analysis of perfusate from donor lungs at one and 4 hours of perfusion revealed significantly higher levels of cfDNA-including mitochondrial DNA (mtDNA) and nuclear DNA (nuDNA)-in lungs that later developed severe PGD (grade 3) within 72 h after transplantation, particularly those from donation after circulatory death (DCD) donors. While cfDNA shows promise as a predictive marker for PGD, its diagnostic accuracy still requires further refinement (Kanou et al., 2021).
In a prospective study involving lung transplant recipients, plasma samples collected on days 1, 3, and 7 post-transplantation revealed that patients who developed PGD had elevated levels of percentage donor-derived cell-free DNA (%ddcfDNA). These levels correlated positively with the severity of PGD. Furthermore, PGD patients with higher %ddcfDNA levels were at increased risk for developing CLAD. Notably, %ddcfDNA levels in PGD patients who progressed to CLAD were approximately double those in PGD patients who did not develop CLAD (Keller et al., 2021).
Immunoreactive substances
Surfactant protein A (SP-A) is a key pulmonary surfactant involved in immune defense and the regulation of inflammation in the lungs (D'Ovidio et al., 2013; Depicolzuane et al., 2021; King and Chen, 2020). Low expression of SP-A mRNA in donor lungs has been significantly associated with reduced post-transplant survival. After transplantation, recipients with low SP-A mRNA levels show decreased SP-A concentrations in BALF, elevated levels of IL-2 and IL-12, and an increased incidence of rejection episodes (D'Ovidio et al., 2013).
Elevated levels of CCR5 and its ligands have been observed in both mouse and human models of ischemia-reperfusion injury (IRI). CCR5-positive natural killer (NK) cells accumulate in the lungs and airways, exhibiting markers of maturity and tissue residency. The CCR5 antagonist maraviroc has been shown to reduce NK cell migration to the airways, decrease pulmonary vascular permeability, improve oxygenation, and lower the incidence and severity of PGD (Santos et al., 2023).
Dectin-1, a C-type lectin receptor, plays a role in recognizing and activating a variety of ligands, including β-glucans, endogenous damage-associated molecular patterns (DAMPs), and fungal pathogen-associated molecular patterns (PAMPs) (Ochoa et al., 2023; Drummond et al., 2022; Yang et al., 2023). It is involved in modulating inflammatory responses and immune tolerance. A specific Dectin-1 mutation (Y238X) has been linked to acute rejection after lung transplantation, increased lymphocyte proportions in BALF, the development of bronchiolitis obliterans syndrome (BOS) (Calabrese et al., 2019). Additionally, levels of transforming growth factor-beta (TGF-β) increase during PGD and are associated with BOS development. Immunohistochemistry has revealed TGF-β expression in epithelial cells, interstitial cells, and macrophages in transplanted lungs, suggesting that TGF-β may serve as a critical mediator linking PGD and BOS, and could potentially function as a biomarker for both conditions (DerHovanessian et al., 2016).
In a large cohort of lung transplant recipients, patients were grouped by underlying conditions-such as COPD, IPF, and cystic fibrosis (CF)-to investigate the relationship between antibodies against lung-associated self-antigens (SAgs) and PGD. The highest pre-transplant positivity rates for SAg antibodies were observed in the IPF and CF groups. Recipients with pre-existing SAg antibodies exhibited higher rates of PGD and elevated serum levels of inflammatory cytokines (Tiriveedhi et al., 2013).
Type V collagen (col(V)), primarily located at the apex of lung epithelial cells, has been shown to induce complement-dependent cytotoxicity (Mak et al., 2016; Iwata et al., 2008; Zaffiri et al., 2019). In lung transplant recipients, high pre-transplant plasma levels of anti-col(V) antibodies have been significantly associated with severe PGD following surgery. Lung-restricted autoantibodies (LRAs) are recognized as important pathogenic contributors to PGD, with mechanisms involving IL-1β-mediated increases in pulmonary vascular endothelial permeability and activation of the complement cascade. These findings offer promising targets for preventive and therapeutic strategies (Yang et al., 2022).
Genetic studies have identified a variant (rs3168046) in the Toll-interacting protein (TOLLIP) gene that is significantly associated with PGD, as well as with plasma levels of plasminogen activator inhibitor-1 (PAI-1) (Cantu et al., 2016). Additionally, two IL-17 receptor (IL-17R) gene variants (rs882643 and rs2241049) have been linked to increased risk of PGD, with carriers of the risk genotypes more likely to experience higher PGD grades within the first 48 h post-transplantation (Somers et al., 2015).
Others
Extracellular histones are a novel class of highly tissue-damaging molecules released during cell death and the formation of neutrophil extracellular traps (NETs). These molecules exhibit diverse biological activities, including cytotoxic effects, promotion of inflammation, and enhancement of platelet aggregation (Jin et al., 2020; de Vries et al., 2023; Zhong et al., 2023). Following lung transplantation, extracellular histone levels increase significantly, particularly in patients who develop PGD. In vitro studies have shown that serum collected within 24 h post-transplantation from patients with high extracellular histone levels is markedly toxic to human pulmonary artery endothelial cells (HPAECs) and stimulates cytokine production in human monocytic cell lines (THP1). These effects are largely mitigated by heparin or anti-histone antibodies (Jin et al., 2020).
Telomere length in airway epithelial cells has also been associated with PGD severity (Greenland et al., 2023). In one analysis of lung transplant recipients, a negative correlation was observed between telomere length in airway epithelial cells and the severity of PGD within the first few weeks after transplantation. Further evidence suggests that PGD may contribute to telomere dysfunction, thereby enhancing immune activation. Telomere impairment in airway epithelial cells may represent a mechanistic link between PGD and the later development of CLAD (Spahn et al., 2015).
The transient receptor potential vanilloid 4 (TRPV4) channel in endothelial cells has emerged as a key mediator of lung IRI. Inhibition or genetic deletion of TRPV4 significantly improves pulmonary function, reduces pulmonary edema and inflammatory cell infiltration, and lowers levels of inflammatory cytokines. These findings suggest that TRPV4 channels may serve as promising therapeutic targets for preventing PGD after lung transplantation (Kuppusamy et al., 2023; Weber et al., 2020).
Limitations and future prospects
Although numerous biomarkers associated with PGD have been identified, most current studies are limited by small sample sizes and a lack of multicenter validation. Therefore, there is an urgent need for further research to identify biomarkers with high sensitivity and specificity, and to develop standardized detection methods and diagnostic criteria.
PGD is a dynamic and evolving condition, with biomarker levels fluctuating over time to reflect different pathophysiological mechanisms and prognostic implications. Thus, determining the optimal timing and frequency for biomarker collection, as well as defining clinically relevant thresholds, is crucial for early prediction and real-time monitoring. However, threshold values for biomarkers vary across studies, which may be due to differences in sample sizes, study designs, detection methods, and patient populations. The biomarkers highlighted in this review offer advantages such as higher sensitivity, stronger specificity, and the ability to guide therapeutic adjustments. For instance, cytokines like IL-10 and IL-13 show significantly elevated levels in early post-transplant serum and are closely associated with PGD severity. These changes often precede the appearance of clinical symptoms, enabling physicians to identify high-risk patients before PGD fully develops. Additionally, certain biomarkers are closely linked to the pathogenesis of PGD and demonstrate high specificity. For example, SP-A, which plays a key role in pulmonary immune defense and inflammation regulation, has been shown to correlate with reduced post-transplant survival when expressed at low levels in donor lungs. Such biomarkers, directly involved in the pathogenesis of PGD, more accurately reflect post-transplant pathological states and reduce the risk of misdiagnosis.
Furthermore, monitoring biomarker fluctuations allows clinicians to more precisely assess PGD severity and progression, facilitating timely therapeutic adjustments. For example, elevated levels of circulating cfDNA are strongly associated with both the occurrence and severity of PGD, as well as an increased risk of CLAD. By measuring cfDNA levels, physicians can identify patients at risk of developing CLAD in advance and implement appropriate preventive or therapeutic strategies.
Given the complexity and heterogeneity of PGD, a single biomarker may be insufficient for accurate diagnosis or prognosis. Therefore, combining and integrating multiple biomarkers may improve diagnostic precision. Advanced analytical approaches, such as multivariate statistical analyses and machine learning, can support the development of composite scoring systems or predictive models. Currently, most biomarker detection methods rely on ELISA and quantitative PCR, which are cost-effective and easily implemented. However, detection methods for some emerging biomarkers are still under development.
Biomarkers not only serve diagnostic and prognostic roles but may also act as therapeutic targets. Future research should focus on elucidating the functional roles, regulatory mechanisms, and detection strategies of these biomarkers, paving the way for effective prevention and treatment strategies for PGD.
Statements
Author contributions
LF: Investigation, Supervision, Writing – original draft. KL: Supervision, Investigation, Writing – original draft. YQ: Investigation, Supervision, Writing – original draft. RL: Methodology, Software, Supervision, Writing – original draft. CX: Investigation, Supervision, Writing – review and editing. SX: Investigation, Supervision, Writing – original draft. JL: Software, Writing – original draft, Writing – review and editing. YP: Software, Writing – original draft, Writing – review and editing. CM: Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AkwiiR. G.SajibM. S.ZahraF. T.MikelisC. M. (2019). Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells8 (5), 471. 10.3390/cells8050471
2
Avtaar SinghS. S.Das DeS.Al-AdhamiA.SinghR.HopkinsP. M.CurryP. A. (2023). Primary graft dysfunction following lung transplantation: from pathogenesis to future frontiers. World J. Transpl.13 (3), 58–85. 10.5500/wjt.v13.i3.58
3
BalasubramanianS.RichertM. E.KongH.FuS.JangM. K.AndargieT. E.et al (2024). Cell-free DNA maps tissue injury and correlates with disease severity in lung transplant candidates. Am. J. Respir. Crit. Care Med.209 (6), 727–737. Epub 2023/12/20. 10.1164/rccm.202306-1064OC
4
BaneckiKMRMDoraK. A. (2023). Endothelin-1 in Health and disease. Int. J. Mol. Sci.24 (14), 11295. 10.3390/ijms241411295
5
BastaracheJ. A.DiamondJ. M.KawutS. M.LedererD. J.WareL. B.ChristieJ. D. (2012). Postoperative estradiol levels associate with development of primary graft dysfunction in lung transplantation patients. Gend. Med.9 (3), 154–165. 10.1016/j.genm.2012.01.009
6
BharatA.KuoE.StewardN.AloushA.HachemR.TrulockE. P.et al (2008). Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg.86 (1), 189–195. ; discussion 96-97. 10.1016/j.athoracsur.2008.03.073
7
BongarzoneS.SavickasV.LuziF.GeeA. D. (2017). Targeting the receptor for advanced glycation endproducts (rage): a medicinal chemistry perspective. J. Med. Chem.60 (17), 7213–7232. 10.1021/acs.jmedchem.7b00058
8
BruhmD. C.VulpescuN. A.FodaZ. H.PhallenJ.ScharpfR. B.VelculescuV. E. (2025). Genomic and fragmentomic landscapes of cell-free DNA for early cancer detection. Nat. Rev. Cancer25, 341–358. 10.1038/s41568-025-00795-x
9
BuiT. M.WiesolekH. L.SumaginR. (2020). Icam-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol.108 (3), 787–799. Epub 2020/03/18. 10.1002/jlb.2mr0220-549r
10
CalabreseD. R.WangP.ChongT.HooverJ.SingerJ. P.TorgersonD.et al (2019). Dectin-1 genetic deficiency predicts chronic lung allograft dysfunction and death. JCI Insight4 (22), e133083. 10.1172/jci.insight.133083
11
CalfeeC. S.WareL. B. (2007). Biomarkers of lung injury in primary graft dysfunction following lung transplantation. Biomark. Med.1 (2), 285–291. 10.2217/17520363.1.2.285
12
CantuE.SuzukiY.DiamondJ. M.EllisJ.TiwariJ.BeduhnB.et al (2016). Protein quantitative trait loci analysis identifies genetic variation in the innate immune regulator tollip in post-lung transplant primary graft dysfunction risk. Am. J. Transpl.16 (3), 833–840. 10.1111/ajt.13525
13
Chacon-AlbertyL.KanchiR. S.YeS.Hochman-MendezC.DaoudD.CoarfaC.et al (2022). Plasma protein biomarkers for primary graft dysfunction after lung transplantation: a single-center cohort analysis. Sci. Rep.12 (1), 16137. 10.1038/s41598-022-20085-y
14
ChengH.HuangH.GuoZ.ChangY.LiZ. (2021). Role of prostaglandin E2 in tissue repair and regeneration. Theranostics11 (18), 8836–8854. 10.7150/thno.63396
15
ChiariD.PiraliB.PeranoV.LeoneR.MantovaniA.BottazziB. (2023). The crossroad between autoimmune disorder, tissue remodeling and cancer of the thyroid: the long pentraxin 3 (Ptx3). Front. Endocrinol. (Lausanne)14, 1146017. 10.3389/fendo.2023.1146017
16
ChiouJ.ChangY.-C.TsaiH.-F.LinY.-F.HuangM.-S.YangC.-J.et al (2019). Follistatin-like protein 1 inhibits lung cancer metastasis by preventing proteolytic activation of osteopontin. Cancer Res.79 (24), 6113–6125. 10.1158/0008-5472.CAN-19-0842
17
ChristieJ. D.RobinsonN.WareL. B.PlotnickM.De AndradeJ.LamaV.et al (2007). Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am. J. Respir. Crit. Care Med.175 (1), 69–74. 10.1164/rccm.200606-827OC
18
ChristieJ. D.ShahC. V.KawutS. M.MangalmurtiN.LedererD. J.SonettJ. R.et al (2009). Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am. J. Respir. Crit. Care Med.180 (10), 1010–1015. 10.1164/rccm.200901-0118OC
19
CieślikP.HrycekA. (2012). Long pentraxin 3 (Ptx3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity45 (2), 119–128. 10.3109/08916934.2011.611549
20
CovarrubiasM.WareL. B.KawutS. M.De AndradeJ.MilstoneA.WeinackerA.et al (2007). Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am. J. Transpl.7 (11), 2573–2578. 10.1111/j.1600-6143.2007.01981.x
21
CrinerR. N.ClausenE.CantuE. (2021). Primary graft dysfunction. Curr. Opin. Organ Transpl.26 (3), 321–327. 10.1097/MOT.0000000000000876
22
DepicolzuaneL.PhelpsD. S.FlorosJ. (2021). Surfactant protein-a function: knowledge gained from sp-a knockout mice. Front. Pediatr.9, 799693. 10.3389/fped.2021.799693
23
DerHovanessianA.WeigtS. S.PalchevskiyV.ShinoM. Y.SayahD. M.GregsonA. L.et al (2016). The role of tgf-Β in the association between primary graft dysfunction and bronchiolitis obliterans syndrome. Am. J. Transpl.16 (2), 640–649. 10.1111/ajt.13475
24
de VriesF.HuckriedeJ.WichapongK.ReutelingspergerC.NicolaesG. A. F. (2023). The role of extracellular histones in covid-19. J. Intern Med.293 (3), 275–292. 10.1111/joim.13585
25
DiamondJ. M.AkimovaT.KaziA.ShahR. J.CantuE.FengR.et al (2014). Genetic variation in the prostaglandin E2 pathway is associated with primary graft dysfunction. Am. J. Respir. Crit. Care Med.189 (5), 567–575. 10.1164/rccm.201307-1283OC
26
DiamondJ. M.CantuE.PorteousM. K.SuzukiY.MeyerK. C.LedererD. J.et al (2017). Peripheral blood gene expression changes associated with primary graft dysfunction after lung transplantation. Am. J. Transpl.17 (7), 1770–1777. 10.1111/ajt.14209
27
DiamondJ. M.KawutS. M.LedererD. J.AhyaV. N.KohlB.SonettJ.et al (2011a). Elevated plasma clara cell secretory protein concentration is associated with high-grade primary graft dysfunction. Am. J. Transpl.11 (3), 561–567. 10.1111/j.1600-6143.2010.03431.x
28
DiamondJ. M.LedererD. J.KawutS. M.LeeJ.AhyaV. N.BellamyS.et al (2011b). Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am. J. Transpl.11 (11), 2517–2522. 10.1111/j.1600-6143.2011.03702.x
29
DiamondJ. M.MeyerN. J.FengR.RushefskiM.LedererD. J.KawutS. M.et al (2012). Variation in Ptx3 is associated with primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med.186 (6), 546–552. 10.1164/rccm.201204-0692OC
30
D'OvidioF.KanedaH.ChaparroC.MuraM.LedererD.Di AngeloS.et al (2013). Pilot study exploring lung allograft surfactant protein a (Sp-a) expression in association with lung transplant outcome. Am. J. Transpl.13 (10), 2722–2729. 10.1111/ajt.12407
31
DrummondR. A.DesaiJ. V.HsuA. P.OikonomouV.VinhD. C.AcklinJ. A.et al (2022). Human dectin-1 deficiency impairs macrophage-mediated defense against phaeohyphomycosis. J. Clin. Invest132 (22), e159348. 10.1172/JCI159348
32
EklundL.KangasJ.SaharinenP. (2017). Angiopoietin-tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. (Lond)131 (1), 87–103. 10.1042/CS20160129
33
EvaT. A.BaruaN.ChowdhuryM. M.YeasminS.RakibA.IslamM. R.et al (2022). Perspectives on signaling for biological- and processed food-related advanced glycation end-products and its role in cancer progression. Crit. Rev. Food Sci. Nutr.62 (10), 2655–2672. 10.1080/10408398.2020.1856771
34
FerreiraA. M.RollinsB. J.FaunceD. E.BurnsA. L.ZhuX.DipietroL. A. (2005). The effect of mcp-1 depletion on chemokine and chemokine-related gene expression: evidence for a complex network in acute inflammation. Cytokine30 (2), 64–71. 10.1016/j.cyto.2004.12.006
35
FinettiF.ParadisiL.BernardiC.PanniniM.TrabalziniL. (2023). Cooperation between prostaglandin E2 and epidermal growth factor receptor in cancer progression: a dual target for cancer therapy. Cancers (Basel)15 (8), 2374. 10.3390/cancers15082374
36
FinettiF.TravelliC.ErcoliJ.ColomboG.BuosoE.TrabalziniL. (2020). Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biol. (Basel)9 (12), 434. 10.3390/biology9120434
37
FrickA. E.VerledenS. E.OrdiesS.SacreasA.VosR.VerledenG. M.et al (2020). Early protein expression profile in bronchoalveolar lavage fluid and clinical outcomes in primary graft dysfunction after lung transplantation. Eur. J. Cardiothorac. Surg.58 (2), 379–388. 10.1093/ejcts/ezaa043
38
GreenlandJ. R.GuoR.LeeS.TranL.KapseB.KukrejaJ.et al (2023). Short airway telomeres are associated with primary graft dysfunction and chronic lung allograft dysfunction. J. Heart Lung Transpl.42 (12), 1700–1709. 10.1016/j.healun.2023.08.018
39
GuzmánA.Hernández-CoronadoC. G.GutiérrezC. G.Rosales-TorresA. M. (2023). The vascular endothelial growth factor (vegf) system as a key regulator of ovarian follicle angiogenesis and growth. Mol. Reprod. Dev.90 (4), 201–217. 10.1002/mrd.23683
40
HagagA. A.ElmahdyH. S.EzzatA. A. (2011). Prognostic value of plasma pro-adrenomedullin and antithrombin levels in neonatal sepsis. Indian Pediatr.48 (6), 471–473. 10.1007/s13312-011-0074-1
41
HamiltonB. C. S.KukrejaJ.WareL. B.MatthayM. A. (2017). Protein biomarkers associated with primary graft dysfunction following lung transplantation. Am. J. Physiol. Lung Cell Mol. Physiol.312 (4), L531-L541–L41. 10.1152/ajplung.00454.2016
42
HuZ.ChenH.LongY.LiP.GuY. (2021). The main sources of circulating cell-free DNA: apoptosis, necrosis and active secretion. Crit. Rev. oncology/hematology157, 103166. Epub 2020/12/01. 10.1016/j.critrevonc.2020.103166
43
HuntM. L.CantuE. (2023). Primary graft dysfunction after lung transplantation. Curr. Opin. Organ Transpl.28 (3), 180–186. 10.1097/MOT.0000000000001065
44
IshiyamaH.TanakaT.SaitoS.KoyamaT.KitamuraA.InoueM.et al (2023). Plasma mid-regional pro-adrenomedullin: a biomarker of the ischemic penumbra in hyperacute stroke. Brain Pathol.33 (2), e13110. 10.1111/bpa.13110
45
IwataT.PhilipovskiyA.FisherA. J.PressonR. G.ChiyoM.LeeJ.et al (2008). Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J. Immunol.181 (8), 5738–5747. 10.4049/jimmunol.181.8.5738
46
JinY.SunM.LvX.WangX.JiangG.ChenC.et al (2020). Extracellular histones play a pathogenic role in primary graft dysfunction after human lung transplantation. RSC Adv.10 (21), 12485–12491. 10.1039/d0ra00127a
47
JuC.XuX.ZhangJ.ChenA.LianQ.LiuF.et al (2023). Application of plasma donor-derived cell free DNA for lung allograft rejection diagnosis in lung transplant recipients. BMC Pulm. Med.23 (1), 37. 10.1186/s12890-022-02229-y
48
KanouT.NakahiraK.ChoiA. M.YeungJ. C.CypelM.LiuM.et al (2021). Cell-free DNA in human ex vivo lung perfusate as a potential biomarker to predict the risk of primary graft dysfunction in lung transplantation. J. Thorac. Cardiovasc Surg.162 (2), 490–499.e2. 10.1016/j.jtcvs.2020.08.008
49
KellerM.Agbor-EnohS. (2021). Donor-derived cell-free DNA for acute rejection monitoring in heart and lung transplantation. Curr. Transplant. Rep.8 (4), 351–358. 10.1007/s40472-021-00349-8
50
KellerM.Agbor-EnohS. (2022). Cell-free DNA in lung transplantation: research tool or clinical workhorse?Curr. Opin. Organ Transpl.27 (3), 177–183. Epub 2022/06/02. 10.1097/mot.0000000000000979
51
KellerM.BushE.DiamondJ. M.ShahP.MatthewJ.BrownA. W.et al (2021). Use of donor-derived-cell-free DNA as a marker of early allograft injury in primary graft dysfunction (pgd) to predict the risk of chronic lung allograft dysfunction (clad). J. Heart Lung Transpl.40 (6), 488–493. 10.1016/j.healun.2021.02.008
52
KellerM.MutebiC.ShahP.LevineD.AryalS.IaconoA.et al (2022b). Biological variation of donor-derived cell-free DNA in stable lung transplant recipients. J. Appl. laboratory Med.7 (4), 901–909. Epub 2022/01/14. 10.1093/jalm/jfab171
53
KellerM.SunJ.MutebiC.ShahP.LevineD.AryalS.et al (2022a). Donor-derived cell-free DNA as a composite marker of acute lung allograft dysfunction in clinical care. J. Heart Lung Transpl.41 (4), 458–466. Epub 2022/01/23. 10.1016/j.healun.2021.12.009
54
KellerM. B.MedaR.FuS.YuK.JangM. K.CharyaA.et al (2022c). Comparison of donor-derived cell-free DNA between single versus double lung transplant recipients. Am. J. Transpl.22 (10), 2451–2457. Epub 2022/03/25. 10.1111/ajt.17039
55
KellerM. B.NewmanD.AlnababtehM.PonorL.ShahP.MathewJ.et al (2024). Extreme elevations of donor-derived cell-free DNA increases the risk of chronic lung allograft dysfunction and death, even without clinical manifestations of disease. J. Heart Lung Transpl.43 (9), 1374–1382. Epub 2024/05/06. 10.1016/j.healun.2024.04.064
56
KépesZ.DénesN.KertészI.HajduI.TrencsényiG. (2023). Overview of prostaglandin E2 (Pge2)-Targeting radiolabelled imaging probes from preclinical perspective: lessons learned and road ahead. Int. J. Mol. Sci.24 (8), 6942. 10.3390/ijms24086942
57
KimD. K.KangS. H.KimJ. S.KimY. G.LeeY. H.LeeD.-Y.et al (2023b). Clinical implications of circulating follistatin-like protein-1 in hemodialysis patients. Sci. Rep.13 (1), 6637. 10.1038/s41598-023-33545-w
58
KimH. D.BaeH.KangH.LeeH.EumS. H.YangC. W.et al (2024). Donor-derived cell-free DNA predicted allograft rejection and severe microvascular inflammation in kidney transplant recipients. Front. Immunol.15, 1433918. Epub 2024/07/24. 10.3389/fimmu.2024.1433918
59
KimT.KimS. J.ChoiH.ShinT. R.SimY. S. (2023a). Diagnostic utility and tendency of bronchial and serum soluble receptor for advanced glycation endproducts (srage) in lung cancer. Cancers (Basel)15 (10), 2819. 10.3390/cancers15102819
60
KingS. D.ChenS.-Y. (2020). Recent progress on surfactant protein A: cellular function in lung and kidney disease development. Am. J. Physiol. Cell Physiol.319 (2), C316-C320–C20. 10.1152/ajpcell.00195.2020
61
KrennK.KlepetkoW.TaghaviS.LangG.SchneiderB.AharinejadS. (2007). Recipient vascular endothelial growth factor serum levels predict primary lung graft dysfunction. Am. J. Transpl.7 (3), 700–706. 10.1111/j.1600-6143.2006.01673.x
62
KuppusamyM.TaH. Q.DavenportH. N.BazazA.KulshresthaA.DanevaZ.et al (2023). Purinergic P2y2 receptor-induced activation of endothelial Trpv4 channels mediates lung ischemia-reperfusion injury. Sci. Signal16 (808), eadg1553. 10.1126/scisignal.adg1553
63
LeeJ. C.ChristieJ. D. (2011). Primary graft dysfunction. Clin. Chest Med.32 (2), 279–293. 10.1016/j.ccm.2011.02.007
64
LeonI.VicenteR.MorenoI.RamosF.SoléA.MoralesP.et al (2009). Plasma levels of N terminal pro-brain natriuretic peptide as a prognostic value in primary graft dysfunction and a predictor of mortality in the immediate postoperative period of lung transplantation. Transpl. Proc.41 (6), 2216–2217. 10.1016/j.transproceed.2009.05.016
65
LiD.WeinkaufJ.HirjiA.NagendranJ.KapasiA.LienD.et al (2021). Chest X-ray sizing for lung transplants reflects pulmonary diagnosis and body composition and is associated with primary graft dysfunction risk. Transplantation105 (2), 382–389. 10.1097/TP.0000000000003238
66
LiY.LiangB. (2023). Circulating donor-derived cell-free DNA as a marker for rejection after lung transplantation. Front. Immunol.14, 1263389. Epub 2023/10/27. 10.3389/fimmu.2023.1263389
67
LiangJ.CaiY.ShaoY. (2023). Comparison of presepsin and mid-regional pro-adrenomedullin in the diagnosis of sepsis or septic shock: a systematic review and meta-analysis. BMC Infect. Dis.23 (1), 288. 10.1186/s12879-023-08262-4
68
Lozano-EdoS.Sánchez-LázaroI.PortolésM.Roselló-LletíE.TarazónE.Arnau-VivesM. A.et al (2022). Plasma levels of Serca2a as a noninvasive biomarker of primary graft dysfunction after heart transplantation. Transplantation106 (4), 887–893. 10.1097/TP.0000000000003798
69
MagnussonJ. M.RickstenA.DellgrenG.WasslavikC.NordénR.WestinJ.et al (2022). Cell-free DNA as a biomarker after lung transplantation: a proof-of-concept study. Immun. Inflamm. Dis.10 (5), e620. Epub 2022/04/29. 10.1002/iid3.620
70
MakK. M.PngC. Y. M.LeeD. J. (2016). Type V collagen in Health, disease, and fibrosis. Anat. Rec. Hob.299 (5), 613–629. 10.1002/ar.23330
71
MattoxA. K.DouvilleC.WangY.PopoliM.PtakJ.SillimanN.et al (2023). The origin of highly elevated cell-free DNA in healthy individuals and patients with pancreatic, colorectal, lung, or ovarian cancer. Cancer Discov.13 (10), 2166–2179. Epub 2023/08/11. 10.1158/2159-8290.Cd-21-1252
72
NakataK.OkazakiM.KawanaS.KuboY.ShimizuD.TanakaS.et al (2023). S100a8/A9 as a prognostic biomarker in lung transplantation. Clin. Transpl.37 (9), e15006. 10.1111/ctr.15006
73
Nakhaei-NejadM.FarhanM.MojiriA.JabbariH.MurrayA. G.JahroudiN. (2019). Regulation of von Willebrand factor gene in endothelial cells that are programmed to pluripotency and differentiated back to endothelial cells. Stem cells Dayt. Ohio37 (4), 542–554. Epub 2019/01/27. 10.1002/stem.2978
74
NevesD. B.RusiM. B.DiazL. G. G.SalvalaggioP. (2016). Primary graft dysfunction of the liver: definitions, diagnostic criteria and risk factors. Einstein (Sao Paulo)14 (4), 567–572. 10.1590/S1679-45082016RW3585
75
NicoliniG.ForiniF.KusmicC.IervasiG.BalzanS. (2019). Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci.239, 117080. 10.1016/j.lfs.2019.117080
76
OchoaA. E.CongelJ. H.CorleyJ. M.JanssenW. J.NickJ. A.MalcolmK. C.et al (2023). Dectin-1-Independent macrophage phagocytosis of Mycobacterium abscessus. Int. J. Mol. Sci.24 (13), 11062. 10.3390/ijms241311062
77
RieraJ.SennaA.CuberoM.RomanA.RelloJ.Vall d'Hebron Lung Transplant Study Group I (2016). Primary graft dysfunction and mortality following lung transplantation: a role for proadrenomedullin plasma levels. Am. J. Transpl.16 (2), 634–639. 10.1111/ajt.13478
78
SalamaM.AndrukhovaO.HodaM. A.TaghaviS.JakschP.HeinzeG.et al (2010). Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am. J. Transpl.10 (3), 628–636. 10.1111/j.1600-6143.2009.02957.x
79
Sanchez-GonzalezC.Fernández AguilarJ. L.Sánchez PérezB.MuñozM. Á. S.DagaJ. A. P.ReyesM. P.et al (2022). Primary graft dysfunction: factor V's value for its early diagnosis. Transpl. Proc.54 (9), 2531–2534. 10.1016/j.transproceed.2022.09.017
80
SantosJ.WangP.ShemeshA.LiuF.TsaoT.AguilarO. A.et al (2023). Ccr5 drives nk cell-associated airway damage in pulmonary ischemia-reperfusion injury. JCI Insight8 (21), e173716. 10.1172/jci.insight.173716
81
ScholzA.PlateK. H.ReissY. (2015). Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann. N. Y. Acad. Sci.1347, 45–51. 10.1111/nyas.12726
82
ShahR. J.DiamondJ. M. (2018). Primary graft dysfunction (pgd) following lung transplantation. Semin. Respir. Crit. Care Med.39 (2), 148–154. 10.1055/s-0037-1615797
83
ShahR. J.DiamondJ. M.LedererD. J.ArcasoyS. M.CantuE. M.DemissieE. J.et al (2012). Plasma monocyte chemotactic protein-1 levels at 24 hours are a biomarker of primary graft dysfunction after lung transplantation. Transl. Res.160 (6), 435–442. 10.1016/j.trsl.2012.08.003
84
ShahR. J.WickershamN.LedererD. J.PalmerS. M.CantuE.DiamondJ. M.et al (2014). Preoperative plasma club (clara) cell secretory protein levels are associated with primary graft dysfunction after lung transplantation. Am. J. Transpl.14 (2), 446–452. 10.1111/ajt.12541
85
SherwoodK.WeimerE. T. (2018). Characteristics, properties, and potential applications of circulating cell-free dna in clinical diagnostics: a focus on transplantation. J. Immunol. methods463, 27–38. Epub 2018/09/30. 10.1016/j.jim.2018.09.011
86
ShiY.HuY.CuiB.ZhuangS.LiuN. (2022). Vascular endothelial growth factor-mediated peritoneal neoangiogenesis in peritoneal dialysis. Perit. Dial. Int.42 (1), 25–38. 10.1177/08968608211004683
87
SinghS.AnshitaD.RavichandiranV. (2021). Mcp-1: function, regulation, and involvement in disease. Int. Immunopharmacol.101 (Pt B), 107598. 10.1016/j.intimp.2021.107598
88
SinghV.KaurR.KumariP.PasrichaC.SinghR. (2023). Icam-1 and vcam-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin. chimica acta; Int. J. Clin. Chem.548, 117487. Epub 2023/07/14. 10.1016/j.cca.2023.117487
89
SomersJ.RuttensD.VerledenS. E.VandermeulenE.PiloniD.WautersE.et al (2015). Interleukin-17 receptor polymorphism predisposes to primary graft dysfunction after lung transplantation. J. Heart Lung Transpl.34 (7), 941–949. 10.1016/j.healun.2015.03.009
90
SongS.-H.KimK. L.LeeK.-A.SuhW. (2012). Tie1 regulates the Tie2 agonistic role of angiopoietin-2 in human lymphatic endothelial cells. Biochem. Biophysical Res. Commun.419 (2), 281–286. 10.1016/j.bbrc.2012.02.009
91
SpahnJ. H.LiW.BribriescoA. C.LiuJ.ShenH.IbricevicA.et al (2015). Dap12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J. Immunol.194 (8), 4039–4048. 10.4049/jimmunol.1401415
92
SpotoS.ArgemiJ.Di CostanzoR.Gavira GomezJ. J.Salterain GonzalesN.BasiliS.et al (2023). Mid-regional pro-adrenomedullin and N-terminal pro-B-type natriuretic peptide measurement: a multimarker approach to diagnosis and prognosis in acute heart failure. J. Pers. Med.13 (7), 1155. 10.3390/jpm13071155
93
SuzukiY.CantuE.ChristieJ. D. (2013). Primary graft dysfunction. Semin. Respir. Crit. Care Med.34 (3), 305–319. 10.1055/s-0033-1348474
94
TiriveedhiV.GautamB.SarmaN. J.AskarM.BudevM.AloushA.et al (2013). Pre-transplant antibodies to Kα1 tubulin and collagen-V in lung transplantation: clinical correlations. J. Heart Lung Transpl.32 (8), 807–814. 10.1016/j.healun.2013.06.003
95
ValpioneS.GremelG.MundraP.MiddlehurstP.GalvaniE.GirottiM. R.et al (2018). Plasma total cell-free DNA (cfdna) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur. J. cancer (Oxford, Engl. 1990)88 (88), 1–9. Epub 2017/11/28. 10.1016/j.ejca.2017.10.029
96
van de StolpeA.van der SaagP. T. (1996). Intercellular adhesion molecule-1. J. Mol. Med. Berlin, Ger.74 (1), 13–33. Epub 1996/01/01. 10.1007/bf00202069
97
VeraarC.KirschnerE.SchwarzS.JakschP.HoetzeneckerK.TschernkoE.et al (2022). Follistatin-like 1 and biomarkers of neutrophil activation are associated with poor short-term outcome after lung transplantation on va-ecmo. Biol. (Basel)11 (10), 1475. 10.3390/biology11101475
98
WeberJ.RajanS.SchremmerC.ChaoY.-K.Krasteva-ChristG.KannlerM.et al (2020). Trpv4 channels are essential for alveolar epithelial barrier function as protection from lung edema. JCI Insight5 (20), e134464. 10.1172/jci.insight.134464
99
WuY.HuangL.LiM.CuiX.ZhanQ.WangC. (2023). The role of lung microbiota in primary graft dysfunction in lung transplant recipients. Clin. Transpl.37 (12), e15152. 10.1111/ctr.15152
100
XiangY.HwaJ. (2016). Regulation of vwf expression, and secretion in Health and disease. Curr. Opin. Hematol.23 (3), 288–293. Epub 2016/01/16. 10.1097/moh.0000000000000230
101
YangN.WangM.LinK.WangM.XuD.HanX.et al (2023). Dectin-1 deficiency alleviates diabetic cardiomyopathy by attenuating macrophage-mediated inflammatory response. Biochim. Biophys. Acta Mol. Basis Dis.1869 (6), 166710. 10.1016/j.bbadis.2023.166710
102
YangW.CerierE. J.Núñez-SantanaF. L.WuQ.YanY.KuriharaC.et al (2022). Il-1β-Dependent extravasation of preexisting lung-restricted autoantibodies during lung transplantation activates complement and mediates primary graft dysfunction. J. Clin. Invest132 (20), e157975. 10.1172/JCI157975
103
YoshimuraT. (2018). The chemokine mcp-1 (Ccl2) in the host interaction with cancer: a foe or ally?Cell Mol. Immunol.15 (4), 335–345. 10.1038/cmi.2017.135
104
YoungK. A.DillingD. F. (2019). The future of lung transplantation. Chest155 (3), 465–473. 10.1016/j.chest.2018.08.1036
105
YueQ.SongY.LiuZ.ZhangL.YangL.LiJ. (2022). Receptor for advanced glycation end products (rage): a pivotal hub in immune diseases. Molecules27 (15), 4922. 10.3390/molecules27154922
106
ZaffiriL.ShahR. J.StearmanR. S.RothhaarK.EmtiazjooA. M.YoshimotoM.et al (2019). Collagen type-V is a danger signal associated with primary graft dysfunction in lung transplantation. Transpl. Immunol.56, 101224. 10.1016/j.trim.2019.101224
107
ZanettaL.MarcusS. G.VasileJ.DobryanskyM.CohenH.EngK.et al (2000). Expression of von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int. J. cancer85 (2), 281–288. Epub 2000/01/11. 10.1002/(sici)1097-0215(20000115)85:2<281::aid-ijc21>3.0.co;2-3
108
ZelnikerT. A.SchwallD.HamidiF.SteinbachS.SchellerP.SpaichS.et al (2023). Mid-regional pro-adrenomedullin and lactate levels for risk stratification in patients with out-of-Hospital cardiac arrest. Eur. Heart J. Acute Cardiovasc Care12 (6), 364–371. 10.1093/ehjacc/zuad029
109
ZhangW.LiuB.JiaD.WangR.CaoH.WuH.et al (2024). Application of graft-derived cell-free DNA for solid organ transplantation. Front. Immunol.15, 1461480. Epub 2024/10/08. 10.3389/fimmu.2024.1461480
110
ZhongW.WangQ.ShenX.DuJ. (2023). The emerging role of neutrophil extracellular traps in cancer: from lab to ward. Front. Oncol.13, 1163802. 10.3389/fonc.2023.1163802
111
ZouJ.DuffyB.SladeM.YoungA. L.StewardN.HachemR.et al (2017). Rapid detection of donor cell free DNA in lung transplant recipients with rejections using donor-recipient hla mismatch. Hum. Immunol.78 (4), 342–349. Epub 2017/03/08. 10.1016/j.humimm.2017.03.002
Summary
Keywords
primary graft dysfunction, lung transplantation, biomarker, plasma proteins, cell-free DNA
Citation
Feng L, Luo K, Qiu Y, Li R, Xue C, Xi S, Liu J, Pei Y and Ma C (2025) Biomarkers for primary graft dysfunction after lung transplantation: a review of current evidence and future prospects. Front. Physiol. 16:1557182. doi: 10.3389/fphys.2025.1557182
Received
08 January 2025
Accepted
28 April 2025
Published
22 May 2025
Volume
16 - 2025
Edited by
Christina Maria Pabelick, Mayo Clinic, United States
Reviewed by
Sarah Julia Reiling, McGill University Health Centre, Canada
Sheng Li, Central Hospital Affiliated to Shenyang Medical College, China
Updates
Copyright
© 2025 Feng, Luo, Qiu, Li, Xue, Xi, Liu, Pei and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jixian Liu, 13923831537@139.com; Yuanmin Pei, peiym_1014@sina.com; Chao Ma, wfmc05@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.