- 1College of Traditional Chinese Medicine, Shanghai University of Traditional Chinese medicine, Shanghai, China
- 2Shanghai Baoshan Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai, China
Introduction: Coronary artery disease (CAD) diagnosis currently relies on invasive coronary angiography for stenosis severity assessment, carrying inherent procedural risks. This study develops a transformer-based multimodal prediction model to provide a clinically reliable non-invasive alternative. By integrating heterogeneous biomarkers including facial morphometrics, cardiovascular waveforms and biochemical indicators, we aim to establish an interpretable framework for precision risk stratification.
Methods: The study utilized a transformer-based architecture integrated with residual modules and adaptive weighting mechanisms. Multimodal data, including facial features, lip and tongue images, pulse and pressure wave amplitudes, and laboratory indicators, were collected from 488 CAD patients. These data were processed and analyzed to predict the severity of coronary artery stenosis. The model’s performance was evaluated using both internal and external validation datasets.
Results: The proposed model demonstrated high predictive accuracy, achieving over 90% accuracy in assessing coronary artery stenosis risk on the training dataset. External validation on real-world data further confirmed the model’s robustness, with an accuracy of 85% on the validation set. The integration of multimodal data and advanced architectural components significantly enhanced the model’s performance.
Conclusion: This study developed a non-invasive, transformer-based multimodal prediction model for assessing coronary artery stenosis severity. By combining diverse data sources and advanced machine learning techniques, the model offers a clinically viable alternative to invasive diagnostic methods. The results highlight the potential of multimodal data integration in improving CAD diagnosis and patient care.
1 Introduction
Coronary artery disease (CAD) is a prevalent chronic cardiovascular condition worldwide, characterized by consistently high incidence and mortality rates. Epidemiological studies across different countries have identified CAD as a high-risk disease (Roth et al., 2020; Duan et al., 2024a; SU Wei, 2024; Tian et al., 2024). In recent years, advancements in coronary computed tomography angiography (CTA) have established it as a critical diagnostic tool for assessing coronary artery stenosis, becoming one of the key diagnostic standards for CAD. However, due to the invasive nature of CTA, it is not suitable for all patients. In clinical practice, patients diagnosed with significant stenosis via CTA often undergo percutaneous coronary intervention (PCI) to restore blood flow. However, complications such as calcification, bifurcation issues, and multivessel disease can arise postoperatively, causing additional strain on the patient’s health (Iftikhar et al., 2024). Therefore, there is a pressing need for a non-invasive diagnostic method for coronary artery stenosis. Such a method could serve as an alternative to CTA for patients who are unsuitable candidates and provide auxiliary recommendations for stenosis severity. Furthermore, it could reduce the need for exploratory PCI procedures, thereby minimizing unnecessary vascular damage.
In recent years, advancements in deep learning and artificial intelligence have accelerated the adoption of non-invasive diagnostic techniques (Le et al., 2024; Liu et al., 2024a). In the domain of CAD, AI has been successfully applied to areas such as depression in CAD patients (Hou et al., 2024), atrial fibrillation prediction (Jian et al., 2024), genetic risk estimation, and hemodynamic modeling (Mishra et al., 2024; Rasmussen et al., 2024). Studies incorporating imaging data for CAD prediction have also shown promise. For instance, research has indicated that facial features of patients are associated with an increased risk of coronary artery disease. A deep learning algorithm developed for CAD prediction based on facial photographs achieved a sensitivity of 0.80, specificity of 0.54, and an area under the curve (AUC) of 0.730 (Lin et al., 2020). This suggests that facial photographs can, to some extent, predict CAD.
Additionally, other studies have demonstrated the predictive value of pulse pressure wave velocity (PWV) for CAD, particularly showing stronger associations in males compared to females (Chiha et al., 2016; Park et al., 2019; Hametner et al., 2021). Tongue features have also been employed in CAD diagnostics, improving model performance when included (accuracy = 0.760, precision = 0.773, AUC = 0.786), indicating the feasibility of using tongue characteristics for CAD detection (Duan et al., 2024b). In the context of non-invasive diagnostic techniques incorporating facial, tongue, and pulse features, prediction models for pulmonary diseases have also achieved promising results (AUC = 0.825 for the best-performing comprehensive syndrome diagnosis model) (Zhou et al., 2023).
Meanwhile, in recent years, there have been studies focusing on the prognostic prediction of coronary heart disease (CHD) using multimodal data, including the use of different types of clinical data to predict the prognosis of coronary artery disease (Xu et al., 2024), the combination of cardiovascular imaging techniques and biomarkers for the diagnosis of elderly patients with CHD (Liu et al., 2024b), and the use of multimodal laboratory indicators for the early prediction of cardiovascular and cerebrovascular diseases (Wang et al., 2024). Although current research demonstrates that multimodal data fusion is superior to single data in the diagnosis of CHD, there is still room for improvement in the research methods of multimodal data fusion.
Motivated by these findings, we aim to integrate clinical indicators, CAD risk factors, and multimodal data—including facial images, tongue images, and pulse data—into a unified multimodal representation learning framework (Zhang et al., 2019). This approach allows us to construct a multimodal disease risk prediction model (Zhou et al., 2023; Mishra et al., 2024), which combines image features, numerical data, and pressure wave values to enable non-invasive prediction of CAD risk. The model incorporates well-established CAD risk factors, such as age, smoking status, systolic blood pressure, diabetes history, and total cholesterol levels (Khera et al., 2016; Di Angelantonio et al., 2019). By training a machine learning model with these risk factors and clinical indicators, we aim to develop a binary classification model that predicts whether coronary artery stenosis exceeds 75%, Adaptive Weighted Cardiovascular Occlusion Prediction Model (AWCOP_Model). Patients with stenosis greater than 75% are generally considered candidates for PCI(Boden et al., 2007; Amsterdam et al., 2014). This model would thus assist in assessing high-risk cases requiring surgical intervention. Through the construction of multimodal data models, new avenues can be provided for the non-invasive prediction of coronary artery occlusion in coronary heart disease, offering additional value to clinicians when CTA diagnosis is unavailable.
2 Materials and methods
2.1 Case collection
2.1.1 Study subjects
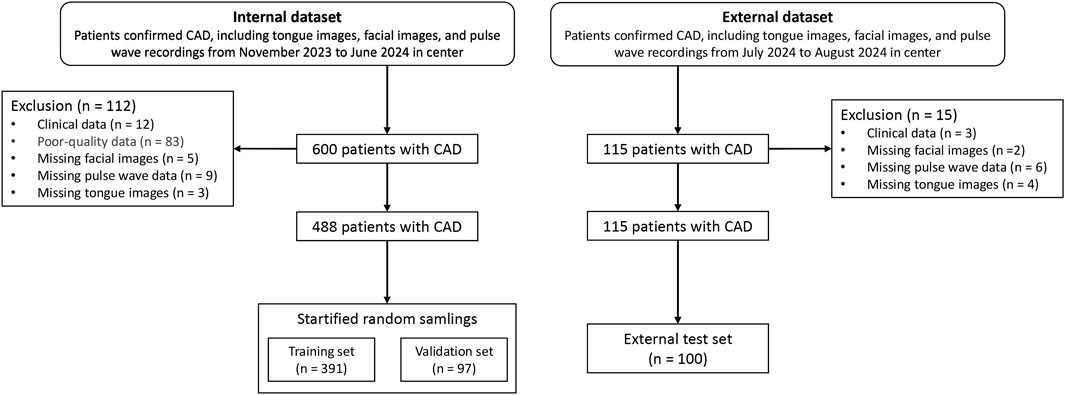
The study cohort consisted of patients hospitalized in the Cardiology Department of Shanghai Baoshan District Integrated Traditional Chinese and Western Medicine Hospital between October 2023 and July 2024. Among the participants, 243 patients were diagnosed with coronary artery stenosis ≥75% via coronary CTA, while 214 patients had stenosis <75%. External validation data were collected in August 2024 from additional patients hospitalized in the same department. Clinical data for the patients are summarized in Table 1. The case screening process is shown in Figure 1.
The study used the validation dataset (n = 97) to train the parameters, which served as the model’s Internal Data results, while the external test set (n = 100) was used as the results of the external dataset.
This study has been reviewed and approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, with Registration Number 2020-916-125. It complies with the Helsinki Declaration of the World Medical Association guidelines. All participants in this study provided written informed consent prior to their inclusion. The objectives, procedures, potential risks, and anticipated benefits of the study were comprehensively communicated to each participant. The research was conducted in full compliance with the principles of the Declaration of Helsinki, ensuring that the rights, safety, and welfare of all participants were safeguarded throughout the study. At the same time, the obtained patient pulse pressure waveform data, facial images, and tongue images were used with the patient’s consent, and efforts were made to protect any privacy that may be involved.
2.1.2 Diagnostic criteria
The diagnostic criteria for CAD were based on the ninth edition of Internal Medicine, published by People’s Medical Publishing House, which defines acute and chronic CAD. The classification includes chronic CAD—encompassing stable angina, ischemic cardiomyopathy, and latent CAD—and acute coronary syndrome (ACS), which includes unstable angina, non-ST-segment elevation myocardial infarction, and ST-segment elevation myocardial infarction (Ge et al., 2018).
2.1.3 Inclusion criteria
Patients were included in the study if they met the following criteria:
1. Diagnosed with CAD according to the established diagnostic criteria.
2. Exhibited typical chest pain symptoms, such as paroxysmal angina or compressive pain.
3. Showed diminished heart sounds on auscultation.
4. Had electrocardiogram (ECG) findings of ST-segment abnormalities.
5. Met the diagnostic criteria outlined in the 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes published by the European Society of Cardiology (Knuuti et al., 2020).
2.1.4 Exclusion criteria
Patients were excluded if they met any of the following criteria:
1. Did not meet the CAD diagnostic criteria.
2. Were younger than 20 years or older than 85 years.
3. Suffered from malignant tumors or critical illnesses.
4. Were pregnant or breastfeeding women.
5. Had incomplete clinical or imaging data.
2.2 Data collection equipment
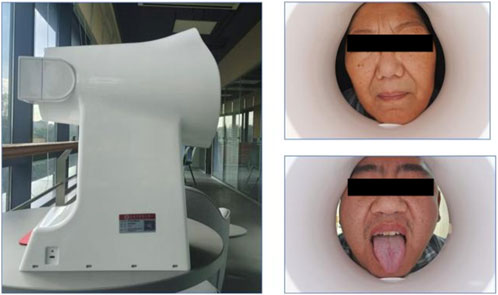
Tongue and facial images were collected using the TFDA-1 digital tongue and facial diagnostic instrument, developed by the Shanghai University of Traditional Chinese Medicine. Data analysis for tongue images was performed using the university’s proprietary Traditional Chinese Medicine Tongue Diagnosis Analysis System (TDAS) V2.0. Recent studies have summarized the classification and typology of tongue features, demonstrating the reliability of such diagnostic tools (Jiatuo et al., 2024). This device was specifically used for stable and standardized collection of tongue and facial images.
The imaging parameters were set as follows: Shutter Speed: 1/125, seconds Aperture: F6.3, ISO Sensitivity: ISO 200, Standardized tongue image analysis was conducted using the TDAS-3.0 software, which quantifies tongue features based on predefined parameters. This system ensures consistency and objectivity in the analysis of tongue and facial images (Figure 2).
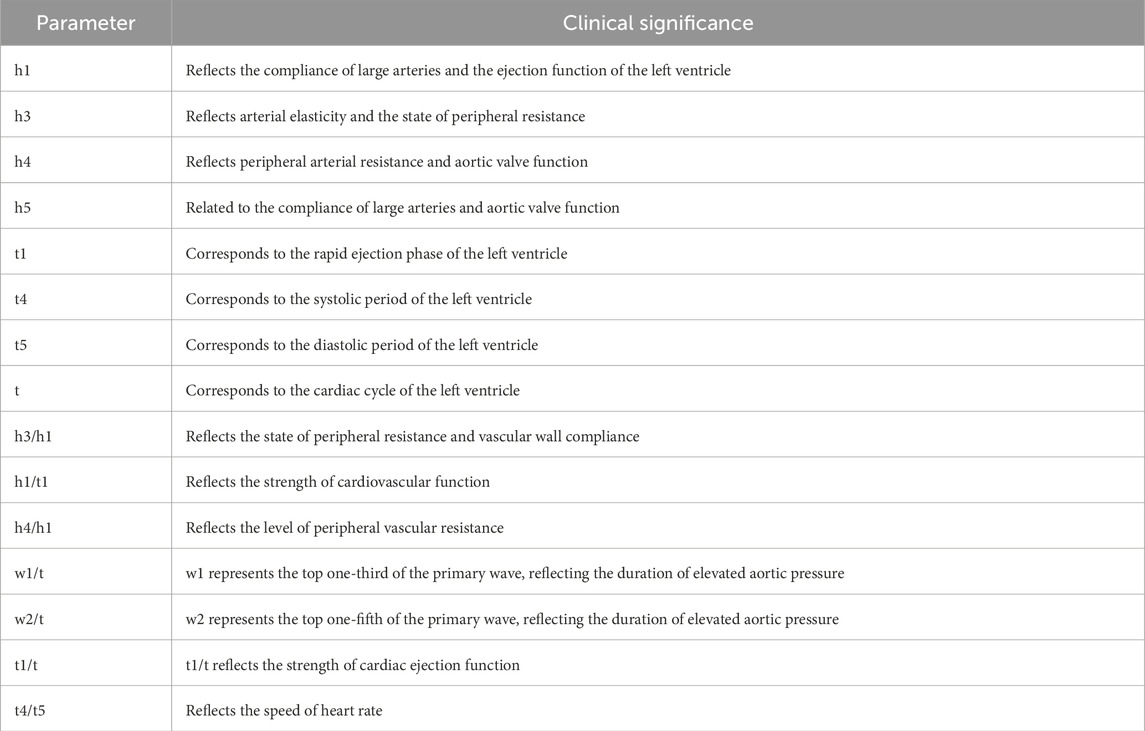
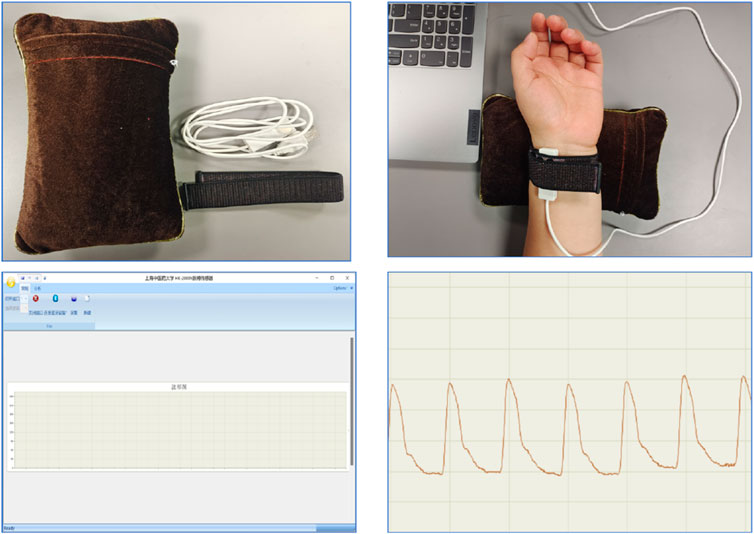
Pulse diagnostic indicators: including pulse intensity indicators, time indicators, ratio indicators (h1, h3, h4, h5, t, t1, t4, t5, h3/h1, h1/t1, h4/h1, t1/t, t4/t5, w1/t, w2/t). See Table 2 for the significance of the actual parameters of the instrument. The equipment parameters and acquisition process are shown in Figure 3 and Table 1.
3 Model construction
3.1 Model framework
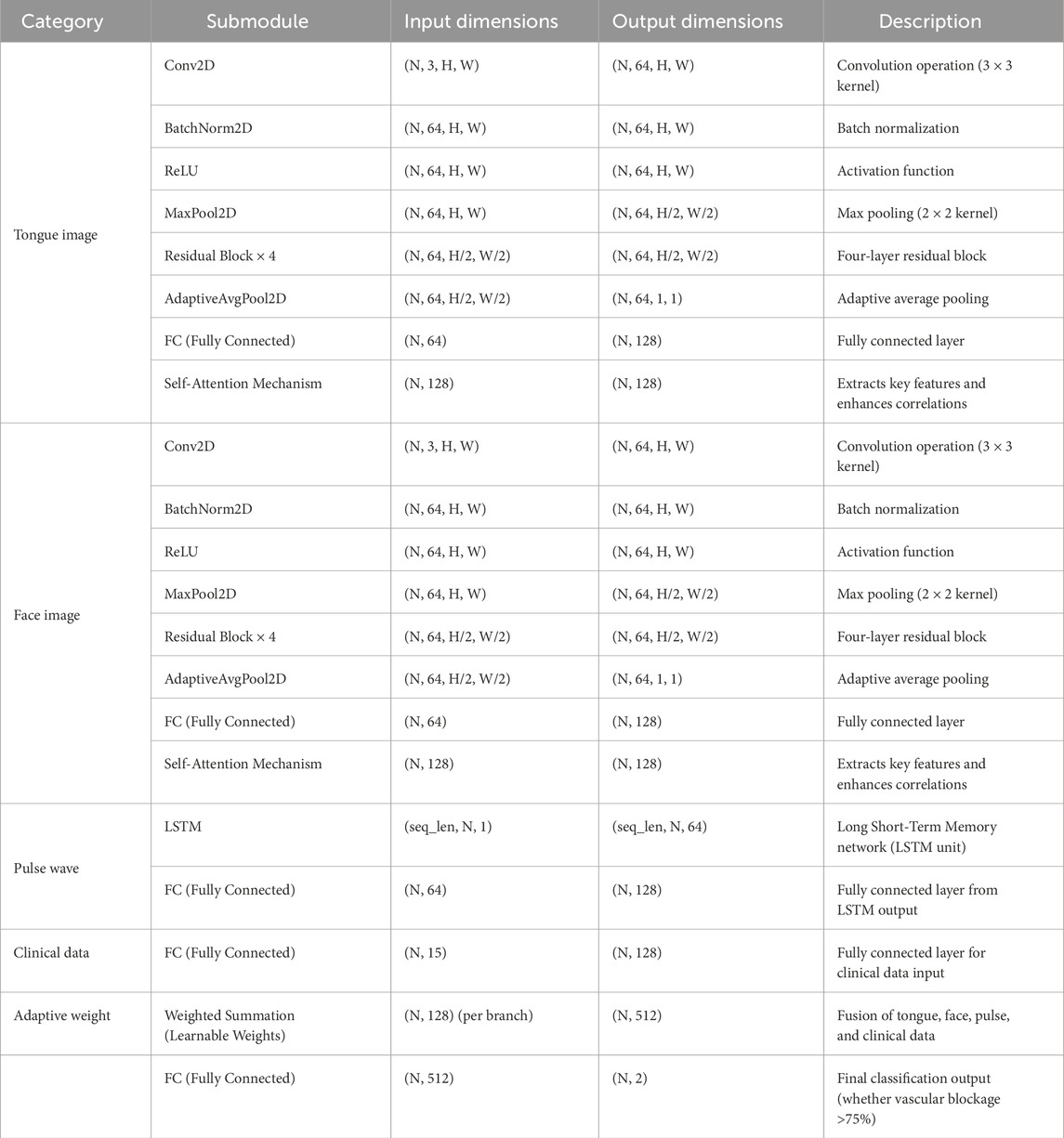
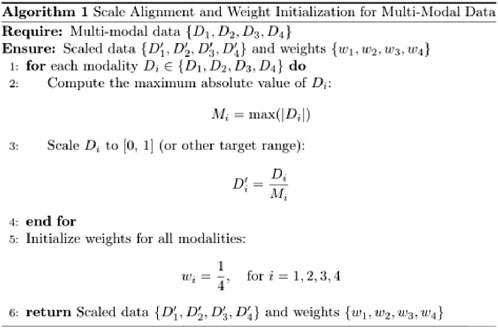
A fusion decision network model was constructed based on clinical data, facial images, tongue images, and radial pulse wave data (as illustrated in Figure 4, Table 2). Facial and tongue images were processed using 3 × 3 convolutional kernels followed by max-pooling operations before being passed through four residual modules. Residual modules, a well-established component in deep learning, have been proven to significantly enhance image classification performance in neural networks, achieving optimal results on public datasets such as ImageNet (He et al., 2016). Recent studies have also highlighted the broad application prospects of residual modules in various domains (Shafiq and Gu, 2022).
In the proposed model, we incorporated a transformer-based self-attention mechanism after the residual modules to further improve performance. The self-attention mechanism has been demonstrated to effectively capture multi-angle information and combine semantic features, thereby enhancing the model’s representational power and accuracy. Additionally, the residual modules improve gradient propagation when combined with the self-attention mechanism, mitigating issues such as vanishing or exploding gradients during training (Vaswani et al., 2017; Dosovitskiy et al., 2020).
Radial artery pulse wave data were collected using a PDA-1 pressure sensor-based pulse diagnosis instrument. During data acquisition, participants maintained a sitting or supine position, with their wrist resting on a pulse pillow. The sensor probe was placed on the radial artery of either the left or right hand. Once a stable pulse waveform was observed, data were collected for 30 s and saved. The raw data underwent preprocessing steps, including smoothing and noise removal, to eliminate artifacts and data drift. The pressure waveform data points were sampled at a frequency of 1/50 s, and a stable waveform segment of 6 s in duration was extracted.
For processing the pulse wave signal, we employed the long short-term memory (LSTM) mechanism (Hochreiter and Schmidhuber, 1997), which is particularly effective for sequential data processing. LSTM has been validated for use in disease diagnosis applications, demonstrating its robustness and reliability in similar tasks (Saif et al., 2024).
By integrating these components—convolutional layers, residual modules, self-attention mechanisms, and LSTM processing—we constructed a multimodal fusion model capable of leveraging diverse data sources to predict the severity of coronary artery stenosis.
3.2 Adaptive weight algorithm
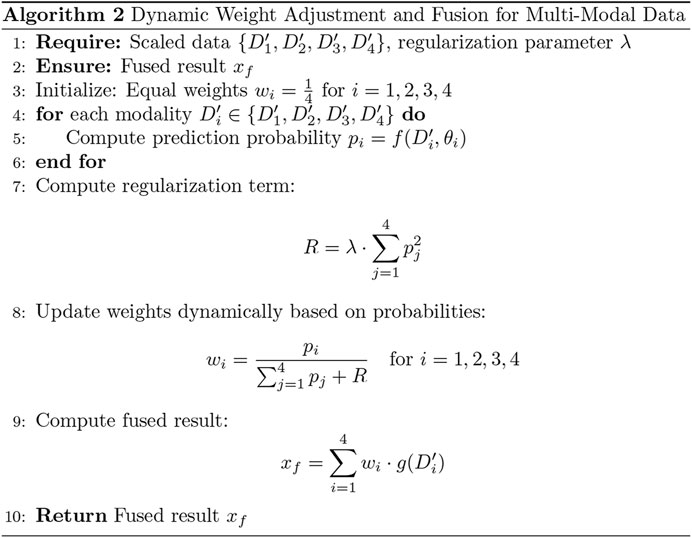
To enhance the model’s performance, we introduced an adaptive weight (adaptive_weight) mechanism into the final output layer. This algorithm dynamically adjusts the contribution of each modality (tongue, face, pulse, and laboratory data) to the final prediction by automatically allocating weights based on their individual output significance. The concept of fusing multimodal data at the backend through adaptive weighting has been explored in recent studies (Huq and Pervin, 2022; Wang et al., 2023). Additionally, we compared the performance of our adaptive weight module with other weight allocation methods to evaluate its effectiveness.
The adaptive weight algorithm addresses discrepancies in evaluation caused by differences in data dimensions during multimodal fusion at the backend. Through weight initialization and spatial alignment mapping, the algorithm ensures consistent weighting across modalities, mitigating biases resulting from dimensional variations. Specifically, the process aligns data from multiple modalities onto a shared temporal or feature space, which is critical when combining or comparing data from heterogeneous sources. This alignment step enhances classification consistency and improves the accuracy of the model’s predictions.
By leveraging this adaptive weight mechanism, the model can autonomously learn the relative importance of each modality and assign appropriate weights during the final decision-making process. This approach not only improves multimodal integration but also optimizes the model’s ability to utilize complementary information across modalities (as shown in Figures 5, 6). The R in Figure 6 represents the regularization term.
3.3 Heatmap algorithm
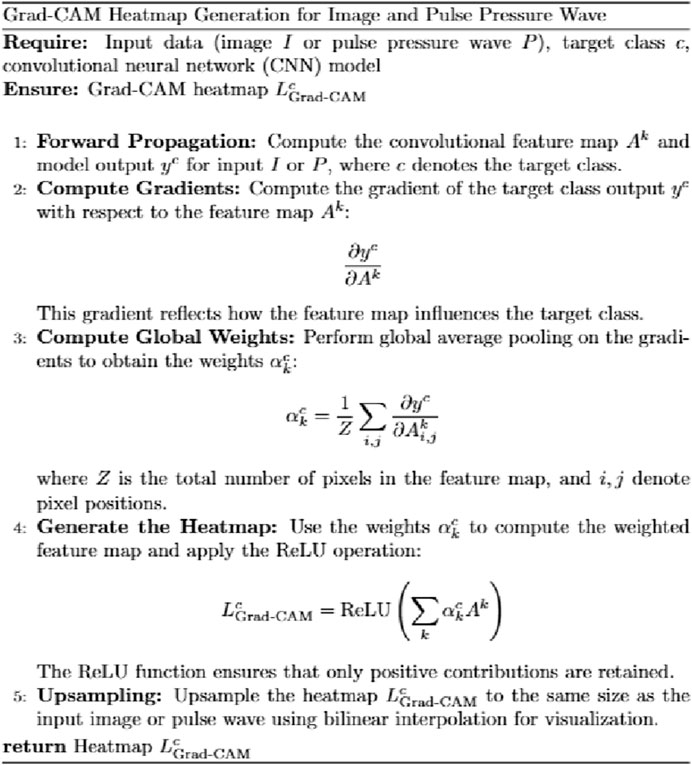
The heatmap visualization in our model utilizes the Grad-CAM (Gradient-weighted Class Activation Mapping) approach (Selvaraju et al., 2020; Li et al., 2024). Grad-CAM is well-suited for multimodal tasks and does not require retraining of the model, making it an efficient tool for understanding the learning process of convolutional neural networks (CNNs) in image classification tasks. By combining gradient information with feature maps, Grad-CAM highlights the key regions of input data that contribute most significantly to the model’s predictions, thereby providing interpretability for the internal mechanisms of the deep learning model (Figure 7).
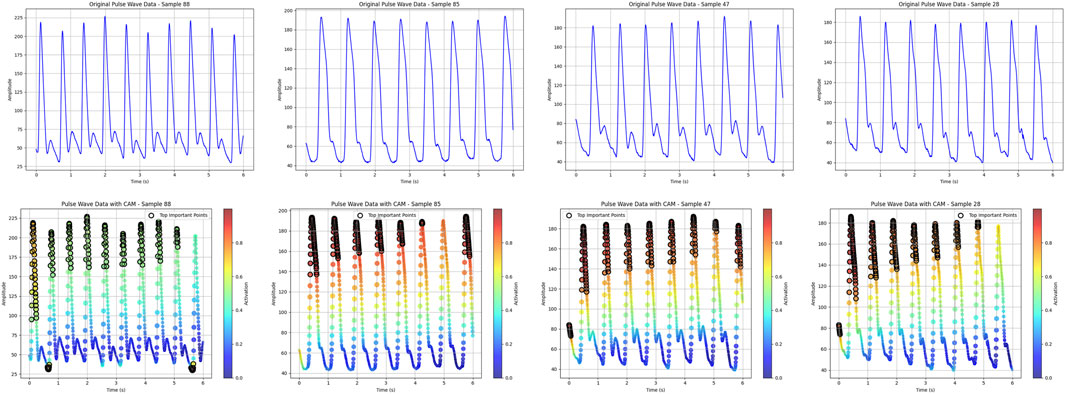
For the visualization of pulse wave data heatmaps, we marked the top 20% of points contributing to the prediction using black dots. This approach helps identify the primary regions of focus within the pulse waveform that the model considers critical for its decision-making process.
By employing Grad-CAM across the different modalities (e.g., tongue and facial images, pulse wave data), the algorithm enables a clearer understanding of how the model integrates and prioritizes information from each input source. This interpretability is crucial for validating the model’s behavior and gaining insights into its decision-making logic, particularly in clinical applications where trust in AI predictions is paramount.
3.4 Clinical feature importance screening
The clinical dataset in this study consisted of 50 dimensions, including patients’ basic physiological indicators, thromboelastography results, and coagulation parameters. To prevent overfitting and reduce the complexity of clinical data dimensions, random forest importance analysis was employed to evaluate the significance of each feature. The top 15 most important clinical features were selected and incorporated into the model training process to optimize its performance.
3.5 Machine learning
To compare the performance of the proposed model with traditional machine learning algorithms, we tested five classical methods: Logistic Regression (LR), Random Forest (RF), Support Vector Machine (SVM), K-Nearest Neighbors (KNN), and Extreme Gradient Boosting (XGBoost). Radial pulse wave parameters were extracted using the PDA-1 pulse pressure wave analysis system, while tongue and facial image parameters were collected using the TFDA-1 tongue and facial diagnostic instrument. These extracted parameters, combined with clinical data, formed four input dimensions—pulse, tongue, face, and clinical data—which were used for training and testing the machine learning models.
The performance of the models was evaluated using four standard metrics: AUC (Area Under the Curve): Reflects the overall performance of the model across different classification thresholds. ACC (Accuracy): Indicates the overall classification accuracy. F1-Score: Balances Precision and Recall to provide a comprehensive performance metric. Recall: Measures the ability of the model to correctly identify positive cases. This evaluation framework allowed for a systematic comparison of the proposed model with traditional machine learning approaches, ensuring the robustness and reliability of the multimodal prediction framework for clinical applications.
4 Results
4.1 Laboratory index screening
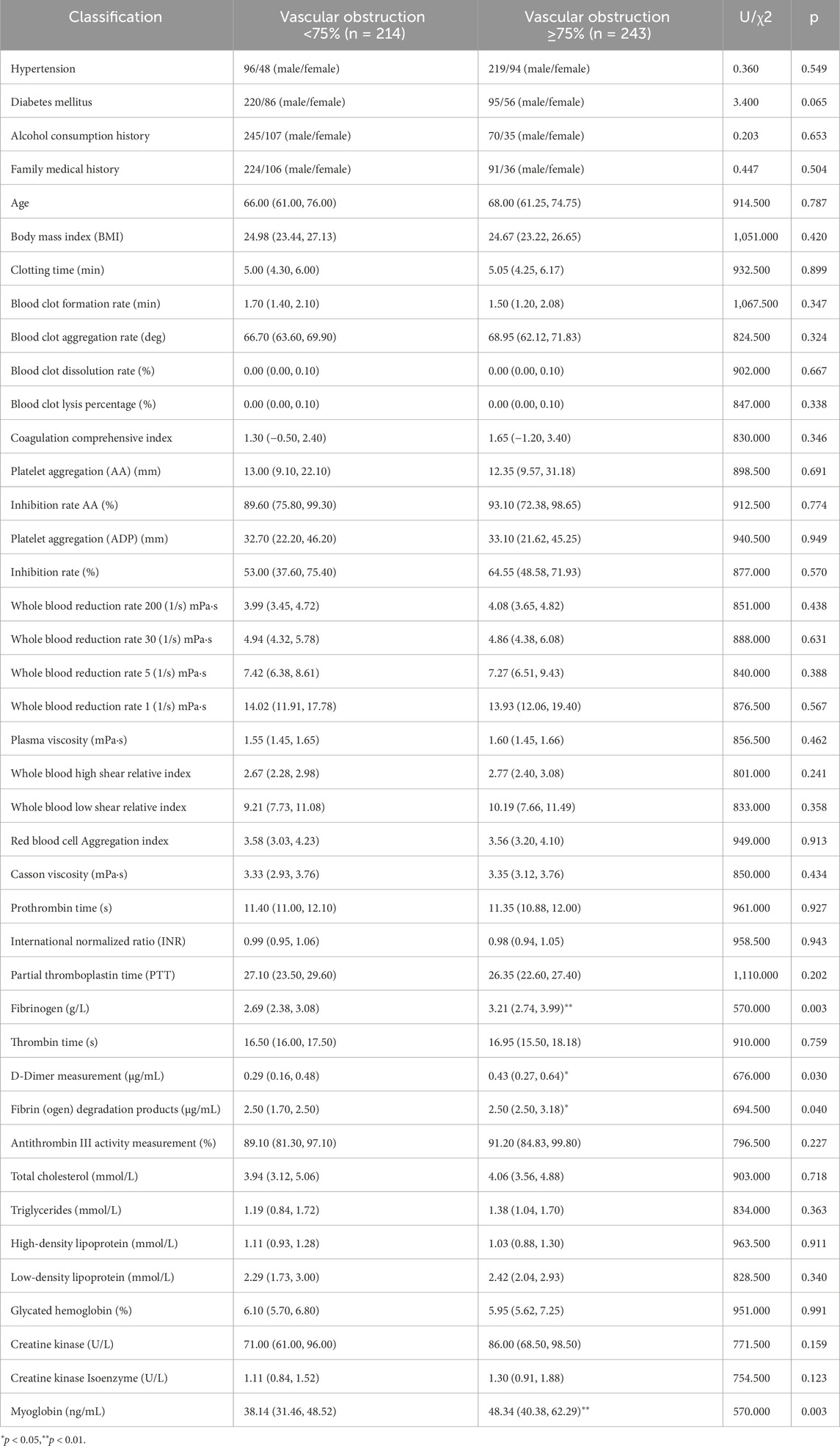
As shown in Table 3, the significantly different indicators between the two groups include Fibrinogen, D-Dimer, Fibrin (ogen) Degradation Products, and Myoglobin, suggesting that vascular obstruction is associated with increased fibrinogen levels, which may impact the vasculature.
4.2 Laboratory index screening based on machine learning
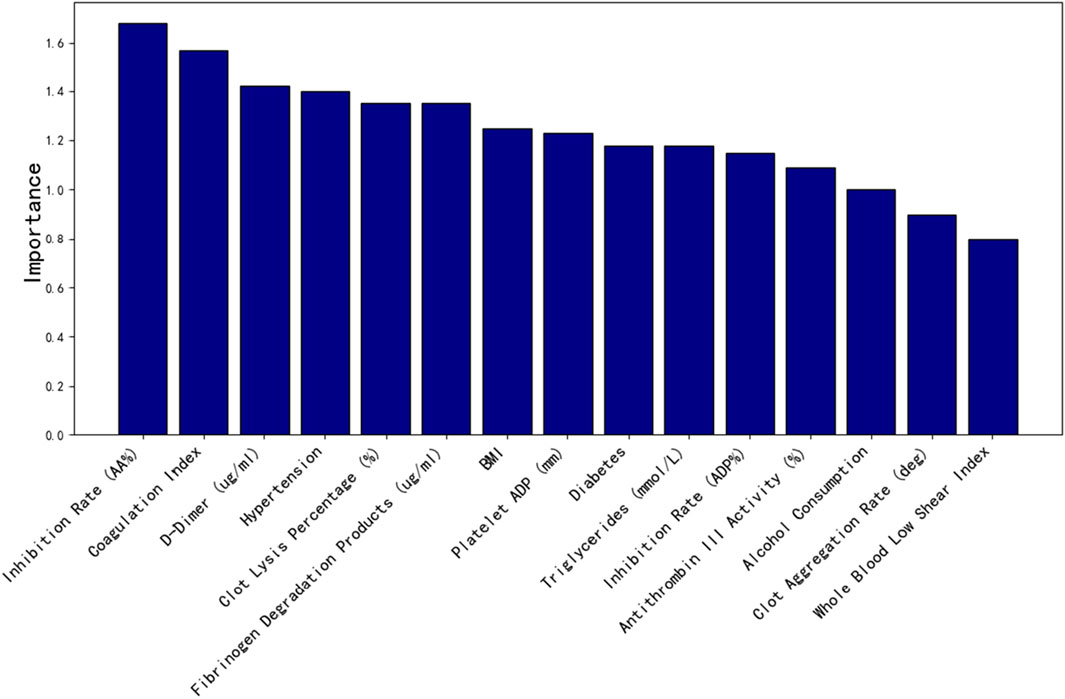
As shown in Figure 8, the top 15 important features screened by random forest factors are: Inhibition rate (AA%), Coagulation index, D-Dimer (ug/mL), Hypertension, Clot lysis percentage (%), Fibrinogen degradation products (ug/mL), BMI, Platelet ADP (mm), Diabetes, Triglycerides (mmol/L), Inhibition rate (ADP%), Antithrombin III activity (%),Alcohol consumption, Clot acceleration time (deg), Whole blood low shear index.
4.3 AWCOP and machine learning assessment
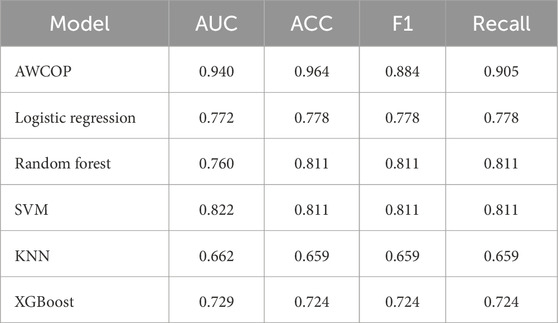
As shown in Table 4 and Figure 9, the evaluation performance of AWCOP is better than that of machine learning models. The AWCOP model performed best (AUC = 0.940, ACC = 0.964, F1 = 0.884, recall = 0.905). In the machine learning model, SVM performs best (AUC = 0.822, ACC = 0.811, F1 = 0.811, recall = 0.811).
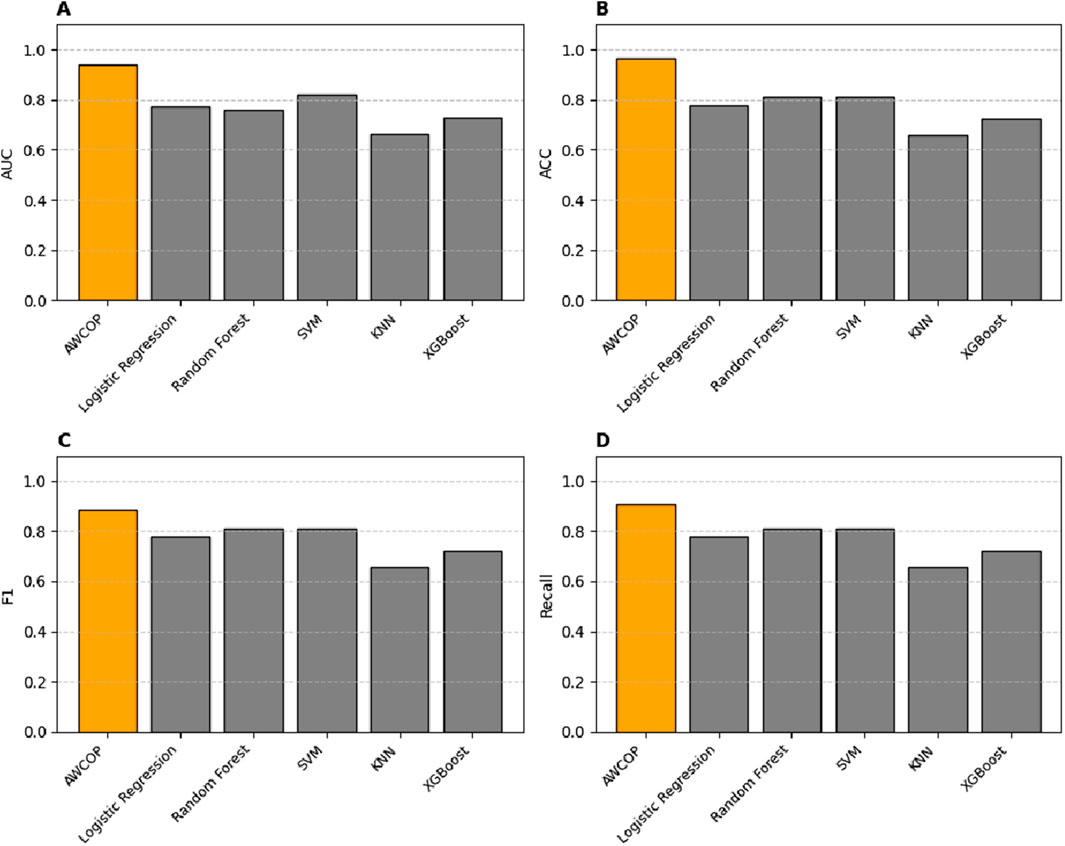
Figure 9. (A) Different models of AUC; (B) Different models of ACC; (C) Different models of F1; (D) Different models of Recall.
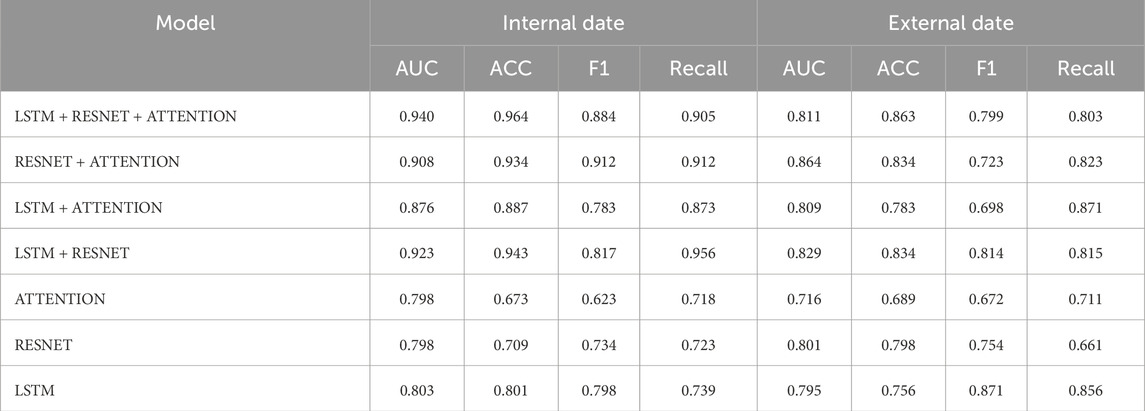
4.4 Ablation experiment of model module
Table 5 compares the performance of various models on both the internal dataset and the external dataset, evaluated using five metrics: AUC, ACC, F1, Recall, and Accuracy. Among the models, the LSTM + RESNET + ATTENTION model performed better on the internal dataset, achieving an AUC of 0.940, ACC of 0.964, F1 of 0.884, Recall of 0.905, and Accuracy of 0.863. On the external dataset, this model also demonstrated superior performance, with an AUC of 0.811, F1 of 0.799, and Recall of 0.803. In comparison, the RESNET + ATTENTION model ranked second in performance, achieving an AUC of 0.908, ACC of 0.934, and F1 and Recall values of 0.912 on the internal dataset. On the external dataset, it achieved an AUC of 0.864, F1 of 0.723, and Recall of 0.823. Single-component models, such as Attention, RESNET, and LSTM, showed relatively lower performance, with AUC values of 0.798, 0.798, and 0.803, respectively. This indicates that the multimodal integration of LSTM, RESNET, and Attention mechanisms effectively enhances the model’s performance, particularly in handling both internal and external datasets.
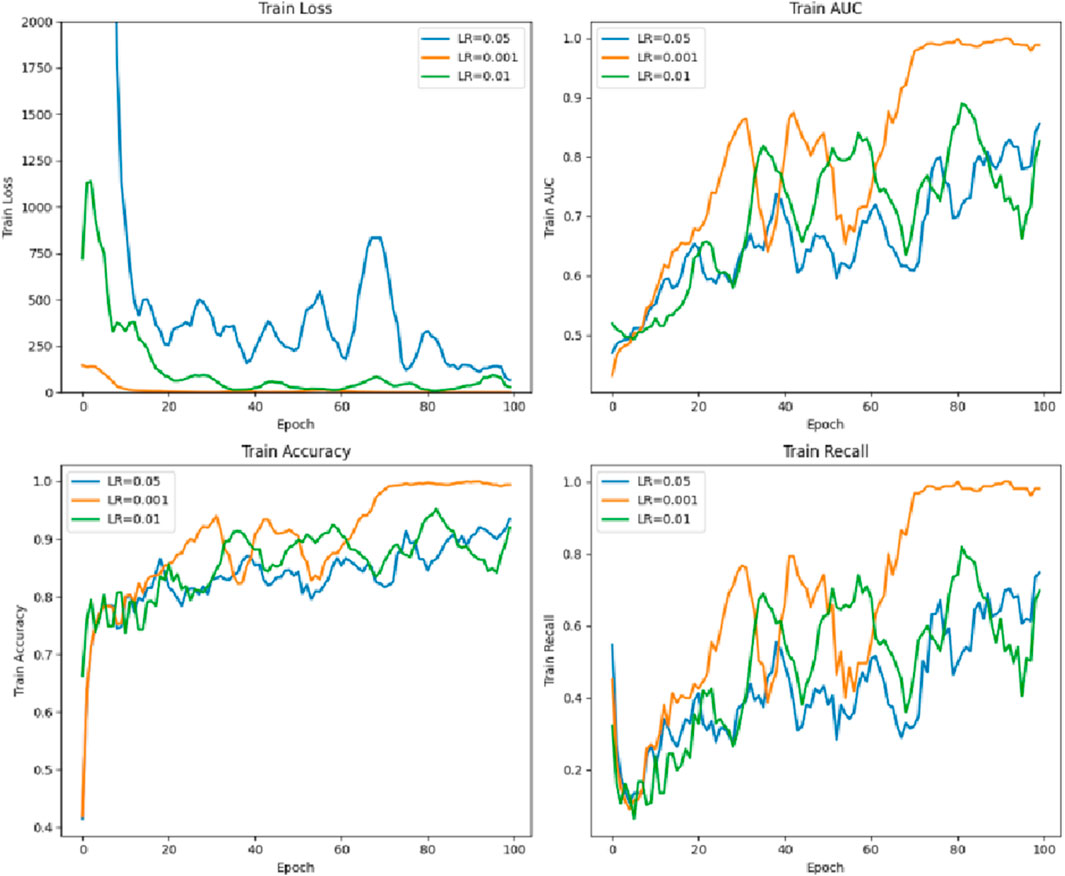
4.5 Parameter comparison of the different learning rates of the model
As shown in Figure 10, the learning rate LR = 0.001 is the best, while LR = 0.05 is poor.
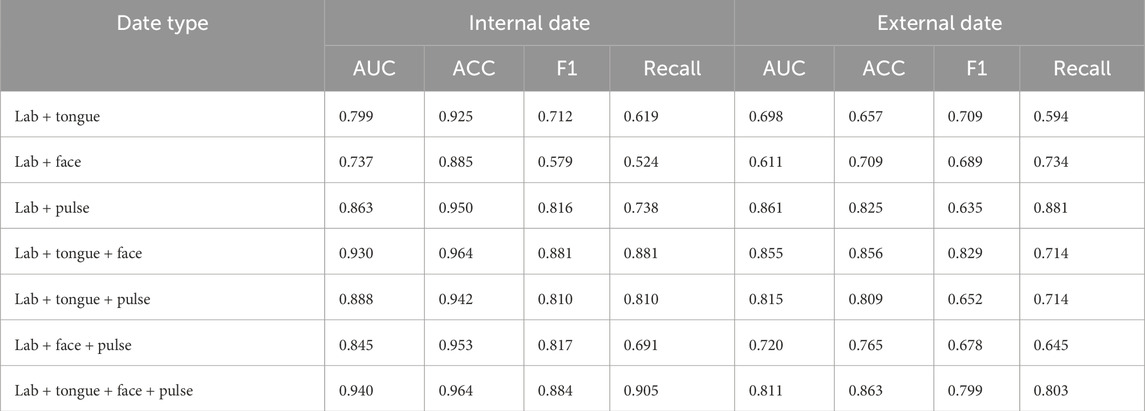
4.6 Data ablation experiment
As shown in Table 6, in the model data ablation analysis, the AUC, ACC, F1, and Recall values for the internal data outperform those of the external data. The training accuracy of laboratory and tongue images is the lowest, and the laboratory performance of tongue, surface and pulse data is the best. Internal training data (ACC = 0.964, AUC = 0.940, F1 = 0.884, Recall = 0.905) and external data training results were (ACC = 0.863, AUC = 0.811, F1 = 0.799, Recall = 0.803).
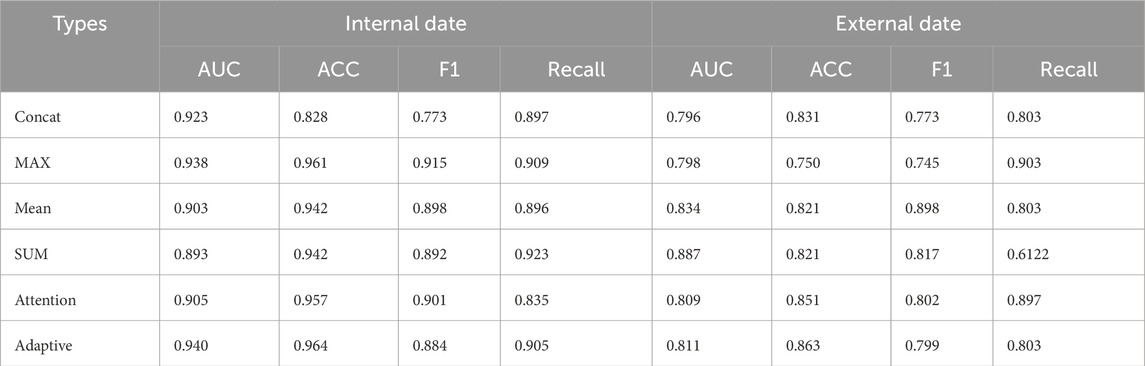
4.7 Fusion results of different decision-making layers
As shown in Table 7, the Adaptive method performed the best on the internal dataset with AUC of 0.940, ACC of 0.964, F1 0.884, and Recall 0.905; on the external dataset, AUC of 0.811, ACC 0.863, F1 0.799 and Recall 0.803 performed relatively stable. In contrast, the MAX method achieved an AUC of 0.938 on the internal dataset, ACC of 0.961 and F1 of 0.915, but its AUC and F1 values decreased to 0.798 and 0.745, respectively. The AUC of the method was 0.905 and ACC 0.957, while the AUC and F1 values on the external dataset were 0.809 and 0.802. The Concat method has an AUC of 0.923 in the internal dataset, but the AUC of its external dataset is 0.796. Adaptive method showed good comprehensive performance on both internal and external data sets.
4.8 Model heat map is presented
4.8.1 Pulse thermal map
As shown in Figure 11, the top 20% regions of the model focus are marked with black dots. In the data extraction of pulse pressure waves, the model focuses on the region of the main wave h1, focusing on the peak region of the h1 main wave.
4.8.2 Face image heat map
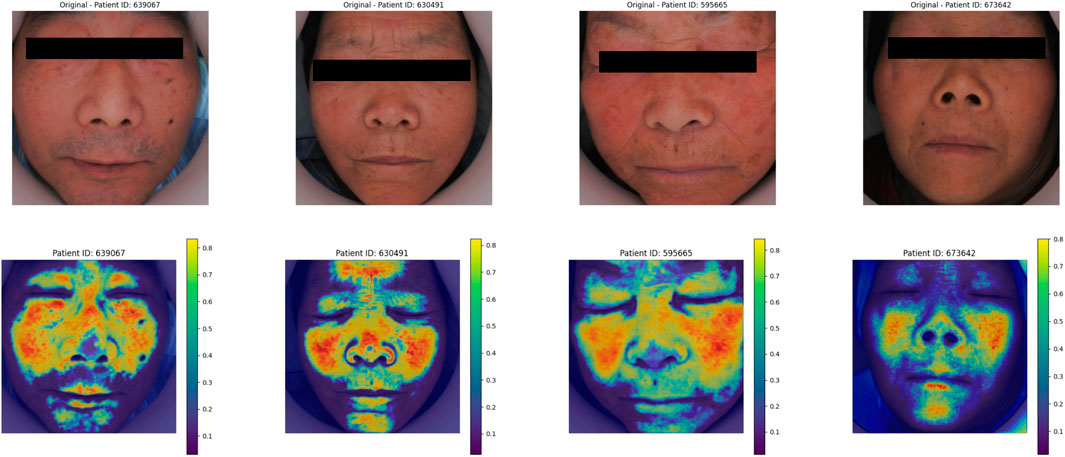
As shown in Figure 12, in the deep learning face image Grad_CAM thermal map, the model focus area was focused on the frontal, nasal and zygomatic regions of the patient.
4.8.3 Thermal map of the tongue image
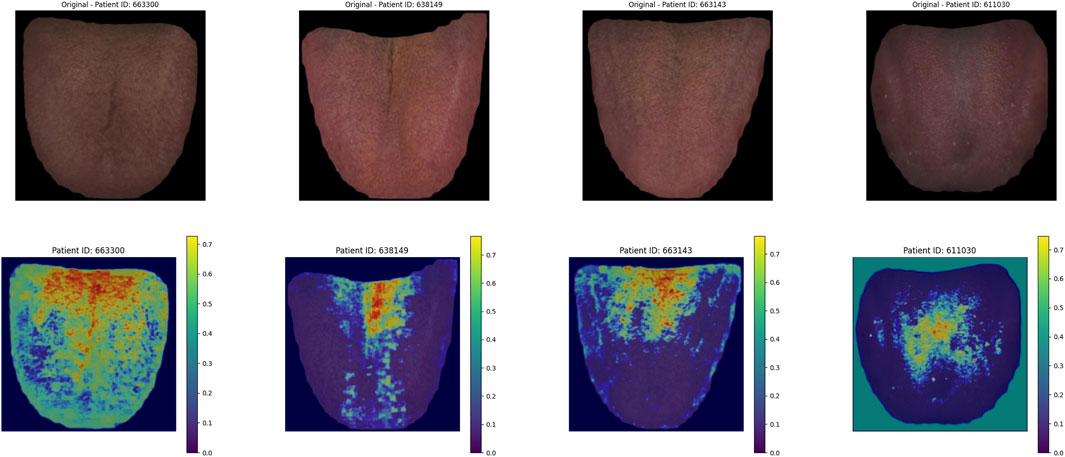
As shown in Figure 13, in the deep learning tongue Grad_CAM thermal map, the model focuses on the tongue coating area of the tongue.
5 Discussion
An artificial intelligence-based non-invasive predictive approach can serve as an alternative to angiographic results in the diagnosis of coronary artery disease and assist in determining the degree of vascular blockage. This not only reduces the risks associated with exploratory PCI procedures but also minimizes vascular damage to patients, enhancing the safety of diagnosis and treatment. This study predicted the severity of coronary artery stenosis through the fusion of multimodal data. Compared to previous machine learning studies conducted by the team—which used parameter extraction from tongue and facial images in different color spaces and time-domain and value-domain data extraction from pulse wave signals-this model demonstrated superior training accuracy. This indicates that deeper convolutional neural networks can extract richer features from image data (Ling et al., 2019). Additionally, the use of the LSTM model based on time series proved effective in fully capturing the characteristics of pulse wave signals. Unlike existing approaches that focus on time-domain or value-domain features, LSTM allows for a more comprehensive learning of the pulse wave signal, enabling the model to focus on finer details of the waveform over a complete cardiac cycle. Compared to existing non-invasive coronary artery disease risk prediction models, the prediction accuracy of this model showed significant improvement (Park et al., 2019; Lin et al., 2020).
In the data ablation and module ablation experiments, the combination of tongue, face, pulse, and laboratory data yielded the best performance. When analyzing individual data inputs, the combination of laboratory data and pulse wave signals showed the highest predictive capability. Pulse wave data, which reflect the vascular pressure of the radial artery, provide a direct indication of cardiovascular function. The model’s heatmap for pulse wave data revealed that it primarily focused on the peak region of the primary wave, which corresponds to the endpoint of cardiac contraction and the beginning of relaxation. This region may be highly correlated with the degree of vascular stenosis. In the data ablation analysis, the importance of pulse wave data was greater than that of facial features, which in turn was greater than tongue features.
In the module ablation experiments, standalone residual modules or self-attention modules performed suboptimally; however, their combination significantly improved model accuracy. Residual modules enhance the extraction of fine details from deep layers of image data, and the extracted features can then be amplified by the self-attention mechanism (Liu et al., 2020a).
The model’s heatmaps highlight its focus on different modalities. For pulse wave data, the model emphasized arterial compliance and left ventricular ejection function—functions that are directly affected by coronary artery stenosis. The model’s attention to these key features demonstrates its ability to capture the physiological impact of vascular obstruction. For tongue features, the focus was mainly on the tongue coating. Previous studies have suggested a relationship between tongue coating and gut microbiota, with different gut microbiota compositions leading to variations in tongue coating. Gut microbiota is also a major risk factor for coronary artery disease (Liu et al., 2020b; Guo et al., 2022). For facial image data, the model primarily focused on the forehead, cheekbones, and nose areas. These regions are richly supplied with blood, and coronary artery stenosis can impair the microcirculation in capillary networks. The rich capillary supply in these regions may explain why the model focuses on them (Sanchez-Garcia et al., 2018). Studies have also shown a certain correlation between facial microcirculation and coronary heart disease (Khedkar et al., 2024). The model in this study specifically focused on learning the facial microcirculation region. This suggests that the multimodal transformer model can effectively distinguish different modalities of data through visualization, providing valuable insights into the obstruction of coronary vessels.
This study also addresses the issue of unbalanced patient group sizes. In the experiment, we controlled the sample size of Vascular Obstruction ≥75% to be the same as that of Vascular Obstruction <75% and found that the results were similar to existing findings. Based on considerations regarding sample size, we decided to proceed with the experiment using the current sample size. However, this study has certain limitations. The sample size was relatively small, and larger datasets from multi-center and multi-regional studies would improve the model’s robustness and bring its predictive accuracy closer to real-world performance. Additionally, although uniform equipment was used to collect data in this study—minimizing variability caused by different devices—developing more portable data collection devices would facilitate the clinical application of this model as a diagnostic aid.
6 Conclusion
This study develops a multimodal AWCOP model integrating clinical data, tongue images, facial images, and pulse wave data, using adaptive weights for decision-making. The model effectively distinguishes coronary artery blockage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2020-916-125). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – original draft. JaX: Writing – review and editing. LT: Writing – review and editing. TJ: Writing – review and editing. YW: Writing – review and editing. JjX: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82104738), the High-Level Key Discipline Construction Project in Traditional Chinese Medicine Diagnostics (Grant No. ZYYZDXK-2023069) by the National Administration of Traditional Chinese Medicine, and the Clinical Research Project in the Health Industry (Grant No. 20244Y0129) by the Shanghai Municipal Health Commission, China. This study was supported by the School of Traditional Chinese Medicine at Shanghai University of Chinese Medicine and the Center for Traditional Chinese Medicine Information Science and Technology at Shanghai University of Chinese Medicine.
Acknowledgments
Thank you for the clinical data support from the Department of Cardiology at Baoshan District Integrated Traditional Chinese and Western Medicine Hospital in Shanghai.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amsterdam E. A., Wenger N. K., Brindis R. G., Casey D. E., Ganiats T. G., Holmes D. R., et al. (2014). 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130 (25), 2354–2394. doi:10.1161/CIR.0000000000000133
Boden W. E., O'Rourke R. A., Teo K. K., Hartigan P. M., Maron D. J., Kostuk W. J., et al. (2007). Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356 (15), 1503–1516. doi:10.1056/NEJMoa070829
Chiha J., Mitchell P., Gopinath B., Burlutsky G., Plant A., Kovoor P., et al. (2016). Prediction of coronary artery disease extent and severity using pulse wave velocity. PLoS One 11 (12), e0168598. doi:10.1371/journal.pone.0168598
Di Angelantonio E., Kaptoge S., Pennells L., De Bacquer D., Cooney M. T., Kavousi M., et al. (2019). World health organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob. Health 7 (10), E1332–E1345. doi:10.1016/S2214-109X(19)30318-3
Dosovitskiy A., Beyer L., Kolesnikov A., Weissenborn D., Zhai X., Unterthiner T., et al. (2020). An image is worth 16x16 words: transformers for image recognition at scale. arXiv preprint. doi:10.48550/arXiv.2010.11929
Duan M., Mao B., Li Z., Wang C., Hu Z., Guan J., et al. (2024a). Feasibility of tongue image detection for coronary artery disease: based on deep learning. Front. Cardiovasc. Med. 11, 1384977. doi:10.3389/fcvm.2024.1384977
Duan M., Zhang Y., Liu Y., Mao B., Li G., Han D., et al. (2024b). Machine learning aided non-invasive diagnosis of coronary heart disease based on tongue features fusion. Technol. Health Care 32 (1), 441–457. doi:10.3233/THC-230590
Ge J. B., Xu Y. J., Wang C., Tang C. W., Zhou J., Xiao H. P., et al. (2018). Internal medicine. 9th ed. Beijing, China: People's Medical Publishing House.
Guo X., Jiang T., Ma X., Hu X., Huang J., Cui L., et al. (2022). Relationships between diurnal changes of tongue coating microbiota and intestinal microbiota. Front. Cell. Infect. Microbiol. 12, 813790. doi:10.3389/fcimb.2022.813790
Hametner B., Wassertheurer S., Mayer C. C., Danninger K., Binder R. K., Weber T. (2021). Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography: a comparison of invasive measurements and noninvasive estimates. Hypertension 77 (2), 571–581. doi:10.1161/HYPERTENSIONAHA.120.15336
He K., Zhang X., Ren S., Sun J. (2016). Deep residual learning for image recognition. in: 2016 IEEE Conference on computer Vision and pattern recognition (CVPR). 2016 June 27–30; Las Vegas, NV, USA. IEEE. p. 770–778. doi:10.1109/CVPR.2016.90
Hochreiter S., Schmidhuber J. U. R. (1997). Long short-term memory. Neural comput. 9 (8), 1735–1780. doi:10.1162/neco.1997.9.8.1735
Hou X. Z., Wu Q., Lv Q. Y., Yang Y. T., Li L. L., Ye X. J., et al. (2024). Development and external validation of a risk prediction model for depression in patients with coronary heart disease. J. Affect. Disord. 367, 137–147. doi:10.1016/j.jad.2024.08.218
Huq A., Pervin M. T. (2022). Adaptive weight assignment scheme for multi-task learning. IAES Int. J. Artif. Intell. 11 (1), 173. doi:10.11591/ijai.v11.i1.pp173-178
Iftikhar S. F., Bishop M. A., Hu P. (2024). “Complex coronary artery lesions,” in StatPearls. St. Petersburg, Florida: StatPearls Publishing.
Jian J., Zhang L., He S., Wu W., Zhang Y., Jian C., et al. (2024). The identification and prediction of atrial fibrillation in coronary artery disease patients: a multicentre retrospective study based on bayesian network. Ann. Med. 56 (1), 2423789. doi:10.1080/07853890.2024.2423789
Jiatuo X. U., Tao J. I. A. N., Shi L. I. U. (2024). Research status and prospect of tongue image diagnosis analysis based on machine learning. Digit. Chin. Med. 7 (1), 3–12. doi:10.1016/j.dcmed.2024.04.002
Khedkar R., Jagtap M. A., Bhoje N. V., Patil V. N. (2024). Coronary artery disease prediction using facial features. in: 2024 OPJU International Technology Conference (OTCON) on Smart Computing for Innovation and Advancement in Industry 4.0. 2024 June 05-07; Raigarh, India. IEEE. p. 1–8. doi:10.1109/OTCON60325.2024.10687745
Khera A. V., Emdin C. A., Drake I., Natarajan P., Bick A. G., Cook N. R., et al. (2016). Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med. 375 (24), 2349–2358. doi:10.1056/NEJMoa1605086
Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., et al. (2020). 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41 (3), 407–477. doi:10.1093/eurheartj/ehz425
Le E. P. V., Wong M., Rundo L., Tarkin J. M., Evans N. R., Weir-McCall J. R., et al. (2024). Using machine learning to predict carotid artery symptoms from CT angiography: a radiomics and deep learning approach. Eur. J. Radiol. Open 13, 100594. doi:10.1016/j.ejro.2024.100594
Li J., Xiong D., Hong L., Lim J., Xu X., Xiao X., et al. (2024). Tongue color parameters in predicting the degree of coronary stenosis: a retrospective cohort study of 282 patients with coronary angiography. Front. Cardiovasc. Med. 11, 1436278. doi:10.3389/fcvm.2024.1436278
Lin S., Li Z., Fu B., Chen S., Li X., Wang Y., et al. (2020). Feasibility of using deep learning to detect coronary artery disease based on facial photo. Eur. Heart J. 41 (46), 4400–4411. doi:10.1093/eurheartj/ehaa640
Ling H., Wu J., Wu L., Huang J., Chen J., Li P. (2019). Self residual attention network for deep face recognition. IEEE Access 7, 55159–55168. doi:10.1109/ACCESS.2019.2913205
Liu H., Zhuang J., Tang P., Li J., Xiong X., Deng H. (2020a). The role of the gut microbiota in coronary heart disease. Curr. Atheroscler. Rep. 22 (12), 77. doi:10.1007/s11883-020-00892-2
Liu J., He H., Su H., Hou J., Luo Y., Chen Q., et al. (2024a). The predictive value of the ARC-HBR criteria for in-hospital bleeding risk following percutaneous coronary intervention in patients with acute coronary syndrome. IJC. Heart. Vasc. 55, 101527. doi:10.1016/j.ijcha.2024.101527
Liu Q., Jia R., Zhao C., Liu X., Sun H., Zhang X. (2020b). Face super-resolution reconstruction based on self-attention residual network. IEEE Access 8, 4110–4121. doi:10.1109/ACCESS.2019.2962790
Liu X., Li Y., Li W., Zhang Y., Zhang S., Ma Y., et al. (2024b). Diagnostic value of multimodal cardiovascular imaging technology coupled with biomarker detection in elderly patients with coronary heart disease. Br. J. Hosp. Med. 85 (6), 1–10. doi:10.12968/hmed.2024.0123
Mishra P. P., Mishra B. H., Lyytikainen L. P., Goebeler S., Martiskainen M., Hakamaa E., et al. (2024). Genetic risk score for coronary artery calcification and its predictive ability for coronary artery disease. Am. J. Prev. Cardiol. 20, 100884. doi:10.1016/j.ajpc.2024.100884
Park K. H., Park W. J., Han S. J., Kim H. S., Jo S. H., Kim S. A., et al. (2019). Association between intra-arterial invasive central and peripheral blood pressure and endothelial function (assessed by flow-mediated dilatation) in stable coronary artery disease. Am. J. Hypertens. 32 (10), 953–959. doi:10.1093/ajh/hpz100
Rasmussen L. D., Karim S. R., Westra J., Nissen L., Dahl J. N., Brix G. S., et al. (2024). Clinical likelihood prediction of hemodynamically obstructive coronary artery disease in patients with stable chest pain. JACC Cardiovasc. Imaging 17 (10), 1199–1210. doi:10.1016/j.jcmg.2024.04.015
Roth G. A., Mensah G. A., Johnson C. O., Addolorato G., Ammirati E., Baddour L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76 (25), 2982–3021. doi:10.1016/j.jacc.2020.11.010
Saif D., Sarhan A. M., Elshennawy N. M. (2024). Deep-kidney: an effective deep learning framework for chronic kidney disease prediction. Health Inf. Sci. Syst. 12 (1), 3. doi:10.1007/s13755-023-00261-8
Sanchez-Garcia M. E., Ramirez-Lara I., Gomez-Delgado F., Yubero-Serrano E. M., Leon-Acuna A., Marin C., et al. (2018). Quantitative evaluation of capillaroscopic microvascular changes in patients with established coronary heart disease. Med. Clin. Barc. 150 (4), 131–137. doi:10.1016/j.medcli.2017.06.068
Selvaraju R., Cogswell M., Das A., Vedantam R., Parikh D., Batra D. (2020). Grad-CAM: visual explanations from deep networks via gradient-based localization. Int. J. Comput. Vis. 128 (2), 336–359. doi:10.1007/s11263-019-01228-7
Shafiq M., Gu Z. (2022). Deep residual learning for image recognition: a survey. Appl. Sci.-Basel 12 (18), 8972. doi:10.3390/app12188972
Su Wei Z. Y. M. S. (2024). Trend and prediction analysis of the changing disease burden of ischemic heart disease in China and worldwide from 1990 to 2019. Zhongguo quanke yixue 27 (19), 2375–2381. doi:10.12114/j.issn.1007-9572.2023.0498
Tian Y., Li D., Cui H., Zhang X., Fan X., Lu F. (2024). Epidemiology of multimorbidity associated with atherosclerotic cardiovascular disease in the United States, 1999–2018. BMC Public Health 24 (1), 267. doi:10.1186/s12889-023-17619-y
Vaswani A., Shazeer N., Parmar N., Uszkoreit J., Jones L., Gomez A. N., et al. (2017). Attention is all you need. arXiv preprint. doi:10.48550/arXiv.1706.03762
Wang J., Xu Y., Zhu J., Wu B., Wang Y., Tan L., et al. (2024). Multimodal data-driven, vertical visualization prediction model for early prediction of atherosclerotic cardiovascular disease in patients with new-onset hypertension. J. Hypertens. 42 (10), 1757–1768. doi:10.1097/HJH.0000000000003798
Wang Y., Chen H., Heng Q., Hou W., Fan Y., Wu Z., et al. (2023). FreeMatch: self-adaptive thresholding for semi-supervised learning. arXiv preprint. doi:10.48550/arXiv.2205.07246
Xu Y., Wang J., Zhou Z., Yang Y., Tang L. (2024). Multimodal prognostic model for predicting chronic coronary artery disease in patients without obstructive sleep apnea syndrome. Arch. Med. Res. 55 (1), 102926. doi:10.1016/j.arcmed.2023.102926
Zhang C., Yang Z., He X., Deng L. (2019). Multimodal intelligence: representation learning, information fusion, and applications. IEEE J. Sel. Top. Signal Process 14, 478–493. doi:10.1109/JSTSP.2020.2987728
Keywords: coronary artery disease, multimodal prediction, deep learning approaches, cardiovascular risk assessment, machine learning for disease risk stratification
Citation: Zhang J, Xu J, Tu L, Jiang T, Wang Y and Xu J (2025) A non-invasive prediction model for coronary artery stenosis severity based on multimodal data. Front. Physiol. 16:1592593. doi: 10.3389/fphys.2025.1592593
Received: 12 March 2025; Accepted: 23 May 2025;
Published: 02 June 2025.
Edited by:
Dominik Obrist, University of Bern, SwitzerlandReviewed by:
Jafar A. Alzubi, Al-Balqa Applied University, JordanMichael Guckert, Technische Hochschule Mittelhessen, Germany
Copyright © 2025 Zhang, Xu, Tu, Jiang, Wang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiatuo Xu, eGp0QGZ1ZGFuLmVkdS5jbg==
 Jiyu Zhang
Jiyu Zhang Jiatuo Xu
Jiatuo Xu Liping Tu1
Liping Tu1 Tao Jiang
Tao Jiang Yu Wang
Yu Wang