Abstract
Lactate, traditionally seen as a byproduct of anaerobic metabolism, has gained attention for its dual role in human health. While it is associated with muscle fatigue, lactate also plays a crucial role in various physiological and pathological processes. This review explores lactate’s dual nature as both beneficial and detrimental. Under normal physiological conditions, lactate is an essential energy substrate, involved in the Cori cycle, where it is converted back to glucose in the liver. However, excessive lactate accumulation is linked to health issues, including cancer, metabolic disorders, and neurological diseases. The Warburg effect in cancer, characterized by increased lactate production even in oxygen-rich environments, promotes tumor progression and therapy resistance. In diseases like malaria and ischemic stroke, high lactate levels contribute to tissue damage and metabolic disturbances. Recent research also highlights lactate’s beneficial roles, including regulation of immune responses, enhanced exercise performance, and neuronal signaling. Furthermore, gut microbiota significantly impacts lactate metabolism, where beneficial bacteria use lactate to maintain gut health, while some pathogenic bacteria exacerbate disease through excess lactate production. Emerging therapeutic potential of lactate, including lactate dehydrogenase inhibitors, offers promising treatment avenues. This review provides a comprehensive overview of lactate’s complex role in health and disease, emphasizing the need for targeted strategies to harness its benefits while mitigating its harmful effects.
1 Introduction
Lactate, first discovered in 1780, was long considered a metabolic waste product generated under hypoxic conditions. However, the lactate shuttle hypothesis redefined its role, highlighting its function in oxidative metabolism, gluconeogenesis, and cellular signaling. Contrary to early misconceptions, lactate is actively produced and utilized under aerobic conditions, serving as a key modulator of systemic metabolism. It is transported via monocarboxylate transporters (MCTs) and signals through G protein-coupled receptor 81 (GPR81) (Lee, 2021).
In exercise physiology, lactate accumulation has traditionally been linked to muscle fatigue, though it is now recognized as an important energy source and metabolic regulator. In oncology, Otto Warburg’s 1920s discovery of aerobic glycolysis, where tumors ferment glucose into lactate even in oxygen-rich conditions, revealed lactate’s role in tumor metabolism. This Warburg effect is also observed in various non-cancerous conditions, including inflammatory diseases and metabolic disorders. Beyond cancer, lactate accumulation contributes to pathophysiological processes in pulmonary hypertension, pulmonary fibrosis, heart failure, atherosclerosis, and polycystic kidney disease (Figure 1). It is involved in stress responses during trauma, infection, and myocardial infarction. Moreover, lactate plays a crucial role in epigenetic regulation through lactylation, a posttranslational modification of histones that influences gene expression, particularly in inflammation and tumor progression (Mandadzhiev, 2025).
FIGURE 1
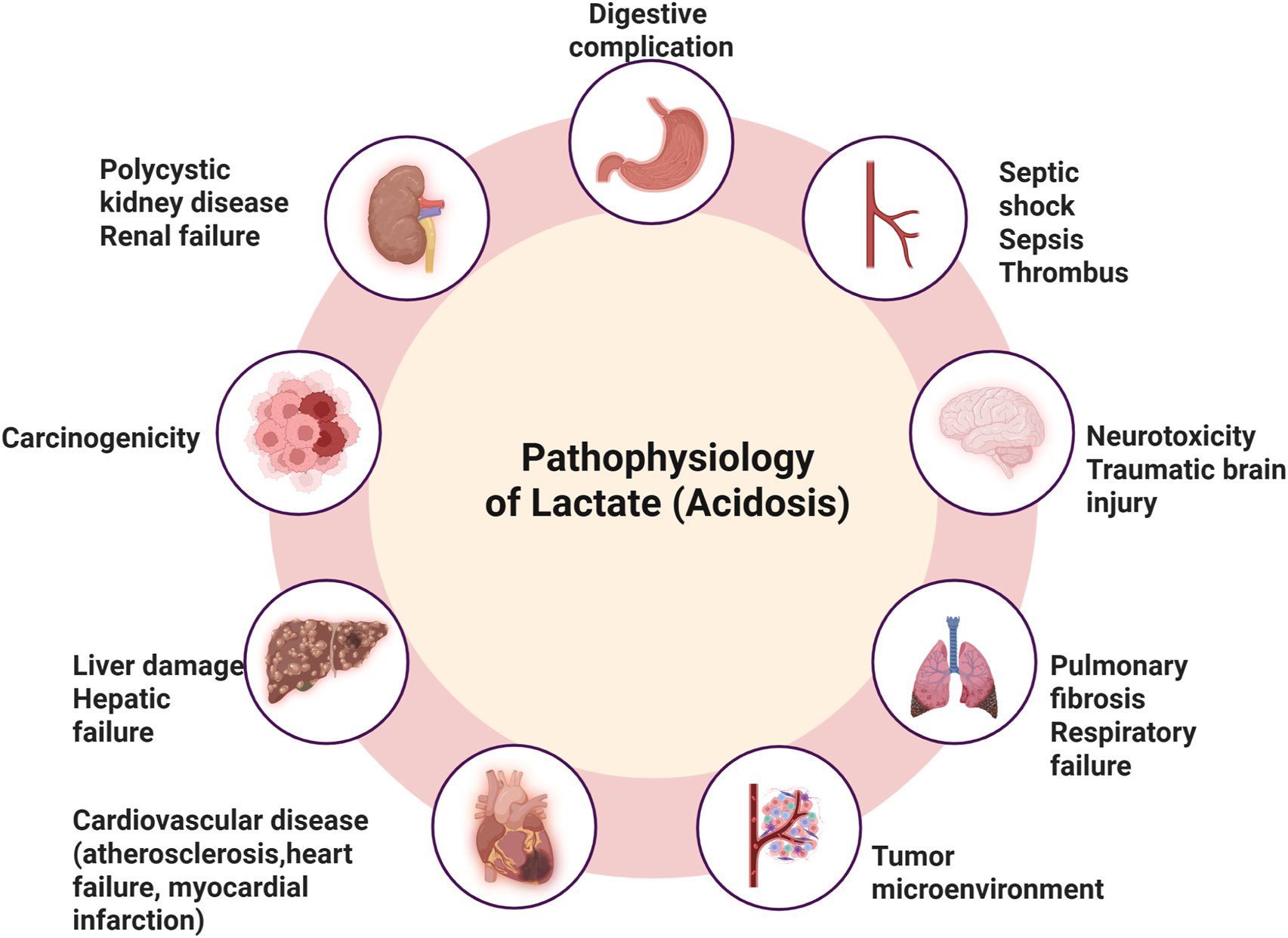
Pathophysiological impacts of lactate accumulation and acidosisElevated lactate levels leading to acidosis are implicated in various systemic complications, including digestive complications, septic shock, sepsis, and thrombus formation. Neurological effects include neurotoxicity and traumatic brain injury, while pulmonary consequences involve pulmonary fibrosis and respiratory failure. Lactate-driven acidosis also contributes to tumor microenvironment remodeling, promoting carcinogenicity. Furthermore, it is associated with cardiovascular diseases (such as atherosclerosis, heart failure, and myocardial infarction), liver damage leading to hepatic failure, and renal impairments, including polycystic kidney disease and renal failure.
This review examines lactate’s diverse functions in health and disease, emphasizing its roles in metabolic reprogramming, immune modulation, and disease progression, underscoring the need for further research on its broader physiological and pathological implications.
2 Lactate production and metabolism
Lactate synthesis occurs in the cytoplasm via lactate dehydrogenase (LDH), which catalyzes the conversion of pyruvate to lactate while regenerating NAD+ from NADH. This reaction is crucial for sustaining glycolysis under anaerobic or hypoxic conditions, ensuring ATP production when oxidative phosphorylation is impaired. The direction of this reversible reaction depends on oxygen availability, intracellular NADH/NAD+ ratios, and metabolic demands (Lee, 2021).
Under normoxic conditions, pyruvate enters the mitochondria for oxidation via the tricarboxylic acid (TCA) cycle, producing ATP efficiently. However, during hypoxia, intense exercise, or pathological conditions like ischemia and cancer, oxidative metabolism is restricted, leading to NADH accumulation. To maintain glycolytic flux, LDH reduces pyruvate to lactate, restoring NAD+ for continued glycolysis (Liu et al., 2025).
Lactate synthesis is regulated by enzyme isoforms, substrate availability, and signaling pathways. LDH exists as isoenzymes composed of LDHA and LDHB subunits, with tissue-specific functions. LDHA, predominant in glycolytic tissues like skeletal muscle, favors lactate production, while LDHB, abundant in oxidative tissues like the heart, facilitates lactate oxidation. The NADH/NAD+ ratio is a key determinant of LDH activity, with high NADH levels promoting lactate formation (Wang et al., 2018).
Cellular signaling also modulates lactate metabolism. Hypoxia-inducible factor 1-alpha (HIF-1α) upregulates glycolytic enzymes and LDHA under low oxygen conditions, enhancing lactate production. Adrenergic stimulation during exercise accelerates glycolytic flux, increasing lactate accumulation, whereas insulin and other metabolic regulators influence lactate utilization. Lactate is not merely a result of metabolism but a key intermediary in energy production. It is transported via MCTs and serves as a metabolic substrate in various tissues, supporting gluconeogenesis in the liver and oxidative metabolism in the heart, highlighting its role in systemic metabolic flexibility (Zhang T. et al., 2024).
2.1 Physiological processes
Once regarded merely as a metabolic waste product generated during anaerobic glycolysis, lactate has emerged as a pivotal metabolic and signaling molecule integral to diverse physiological and pathological processes. Traditionally associated with muscle fatigue during intense exercise, lactate’s role extends far beyond a simple byproduct; it serves as an essential energy substrate, a regulator of cellular metabolism, and a mediator of intercellular communication (Vavřička et al., 2024).
In cancer biology, lactate’s significance is profound. Tumors commonly exhibit a metabolic shift known as aerobic glycolysis or the “Warburg effect,” where glucose is preferentially converted to lactate despite sufficient oxygen availability (Zhong et al., 2022). This metabolic reprogramming leads to lactate accumulation within the tumor microenvironment, which promotes cancer cell proliferation, angiogenesis, and metastasis (de la Cruz-López et al., 2019). Elevated lactate also contributes to immune evasion by creating an acidic microenvironment that suppresses cytotoxic T cell and natural killer cell activity, facilitating tumor progression (Gu et al., 2025). Research has demonstrated that lactate acts through signaling pathways such as HIF-1α stabilization and GPR81 activation, further reinforcing cancer cell survival and immune modulation (Chen et al., 2025). Beyond oncology, lactate is implicated in several chronic inflammatory and metabolic diseases (Lee, 2021). For instance, in pulmonary hypertension and pulmonary fibrosis, elevated lactate levels correlate with vascular remodeling and fibrotic processes (Peng et al., 2025). Studies indicate that lactate stimulates fibroblast activation and extracellular matrix deposition, contributing to disease progression (Caslin et al., 2021). In heart failure and atherosclerosis, abnormal lactate metabolism is associated with impaired mitochondrial function and chronic inflammation. Elevated circulating lactate in these conditions reflects underlying tissue hypoxia and metabolic stress, exacerbating cardiac dysfunction and vascular pathology (Zymliński et al., 2018; Zhu et al., 2024). Polycystic kidney disease (PKD) also features altered lactate metabolism. Experimental models show that lactate accumulation in cystic epithelial cells supports their proliferation and survival, contributing to cyst growth (Ghazi et al., 2019; Pagliarini and Podrini, 2021). Similarly, metabolic disorders such as type 2 diabetes exhibit dysregulated lactate production, linking it to insulin resistance and chronic low-grade inflammation (Nareika et al., 2005; Wu et al., 2016). Lactate involvement extends to acute stress responses, including trauma, infection, myocardial infarction, and ischemia-reperfusion injury (Wang et al., 2025). Here, lactate serves as both a metabolic fuel for damaged tissues and a signaling molecule triggering adaptive responses. Elevated lactate levels in sepsis, for example, are a prognostic marker reflecting tissue hypoperfusion and metabolic derangement (Liu et al., 2024).
A groundbreaking discovery expanding lactate role in disease is its function in epigenetic regulation through histone lactylation. This recently identified posttranslational modification involves the addition of lactate-derived groups to histone lysine residues, altering chromatin structure and gene expression. Histone lactylation has been shown to modulate inflammatory gene expression in macrophages, promoting resolution of inflammation, while also contributing to oncogenic gene programs in cancer cells. This epigenetic mechanism underscores lactate’s capacity to link metabolic states with long-term changes in cellular phenotype and disease outcomes (Zhang et al., 2019).
Collectively, lactate acts as a critical mediator of metabolic adaptation, immune regulation, and pathophysiological remodeling across a spectrum of diseases. Its dual roles as an energy substrate and signaling molecule highlight the intricate connections between metabolism and cellular function. Ongoing research aims to eKMCTlucidate therapeutic strategies targeting lactate metabolism and signaling, offering promising avenues for treating cancer, inflammatory disorders, cardiovascular diseases, and metabolic syndromes. Understanding lactate’s diverse physiological and pathological functions is essential for developing novel interventions to modulate disease progression and improve patient outcomes (Li et al., 2022).
2.2 Key sites of lactate production
During vigorous exercise, skeletal muscles rely on anaerobic glycolysis, resulting in substantial lactate accumulation. Once thought to be simply a product of glycolysis, lactate is now recognized as a vital signaling molecule and energy source. It can be shuttled to the liver for gluconeogenesis or oxidized in oxidative muscle fibers and the heart. Lactate also modulates gene expression related to mitochondrial biogenesis and angiogenesis, supporting muscle adaptation during endurance training (Mandadzhiev, 2025).
Erythrocytes, which lack mitochondria, depend entirely on anaerobic glycolysis for ATP production and continuously produce lactate. Recent studies highlight the role of MCTs in lactate transport, essential for maintaining acid-base balance. Erythrocyte-derived lactate significantly influences systemic metabolism, particularly in the heart and brain, and emerging evidence suggests its levels may serve as biomarkers for metabolic disorders such as diabetes and sepsis (Zhang T. et al., 2024).
In the brain, lactate plays a vital role through the astrocyte-neuron lactate shuttle. Astrocytes convert glucose into lactate, which neurons utilize during heightened activity. Lactate supports synaptic plasticity, learning, and memory by enhancing brain-derived neurotrophic factor (BDNF) signaling. Impaired lactate metabolism is linked to neurodegenerative diseases like Alzheimer’s and Parkinson’s, highlighting its therapeutic potential in cognitive disorders (Beard et al., 2022).
Cancer cells exhibit the Warburg effect, favoring glycolysis even in oxygen-rich environments, leading to lactate accumulation. This promotes immune evasion, angiogenesis, metastasis, and epigenetic changes that drive tumor aggressiveness. Lactate transporters such as MCT1 and MCT4 are emerging targets, with their inhibition shown to reduce tumor progression in breast cancer models. Modulating lactate metabolism offers a promising strategy for cancer therapy (Zhong et al., 2022).
2.3 Lactate metabolism
Lactate is transported to the liver, heart, and oxidative muscle fibers, where it converts to pyruvate and enters the TCA cycle for ATP production (Wang et al., 2018). The lactate shuttle hypothesis, first introduced by Brooks (1985), describes lactate’s movement between glycolytic and oxidative tissues as a fuel source. The heart preferentially utilizes lactate as an energy substrate, with lactate oxidation playing a substantial role in myocardial energy production during both resting and stressed states (Brooks, 2021; Zhang H. et al., 2024) (Table 1). Through the Cori cycle, lactate is transported to the liver for gluconeogenesis (Figure 2), forming glucose that is released into the bloodstream (Melkonian et al., 2023). Lactate homeostasis is maintained through MCTs 1–4, enabling rapid cellular exchange (Figure 2). Elevated lactate levels (lactic acidosis) signal metabolic dysregulation. Lactate’s clearance mechanisms include oxidation via the TCA cycle, renal excretion through sodium/lactate transporters (Slc5a12 and Slc5a8), and microbiome incorporation (Lee, 2021). In a study, it has been demonstrated that hepatic gluconeogenesis is essential for glucose homeostasis during exercise and fasting (Meyer et al., 2002). The kidneys also contribute significantly, accounting for approximately 40% of systemic glucose production during prolonged fasting. Recent research challenges the traditional view of lactate as a glycolytic byproduct, showing that it directly fuels mitochondrial oxidative phosphorylation. Lactate enters mitochondria via the mitochondrial lactate oxidation complex (mLOC) and converts into pyruvate for ATP generation (Brooks et al., 2022). Hashimoto et al. (2008) provided compelling evidence that lactate oxidation occurs within mitochondria, highlighting its role in both normal and pathological states such as cancer and neurodegeneration (Hashimoto et al., 2008).
TABLE 1
| Beneficial role | Mechanism | Relevant system/Organ | References |
|---|---|---|---|
| Energy substrate | Provides an efficient fuel source for muscles, heart, and brain | Muscle, Heart, Brain | Hashimoto et al. (2008) |
| Muscle adaptation | Enhances mitochondrial biogenesis and oxidative capacity | Skeletal muscle | Nalbandian et al. (2017) |
| Neuroprotection | Supports neuronal metabolism, prevents excitotoxicity, and enhances synaptic plasticity | Brain | Suzuki et al. (2011) |
| Immune modulation | Regulates macrophage polarization, suppresses inflammation, and supports Treg function | Immune system | Zhang et al. (2024b) |
| Angiogenesis and wound healing | Stimulates vascular endothelial growth factor (VEGF) production | Endothelial cells, Skin | Hunt et al. (2007) |
| Gluconeogenesis | Serves as a precursor for glucose production via the Cori cycle | Liver | Rogatzki et al. (2015) |
| Lipid metabolism regulation | Modulates free fatty acid (FFA) oxidation and storage | Adipose tissue | Bergman et al. (1999) |
| Exercise recovery | Reduces muscle fatigue, maintains pH balance, and promotes lactate shuttling | Muscle | Gladden (2004) |
| Cancer metabolism | Supports tumor cell survival and immune evasion in the tumor microenvironment | Tumor microenvironment | Fischer et al. (2007) |
| Cardioprotection | Preferred substrate over glucose in ischemic conditions, reducing oxidative stress | Heart | Chatham (2002) |
Beneficial role of lactate in human health and underlying mechanisms.
FIGURE 2
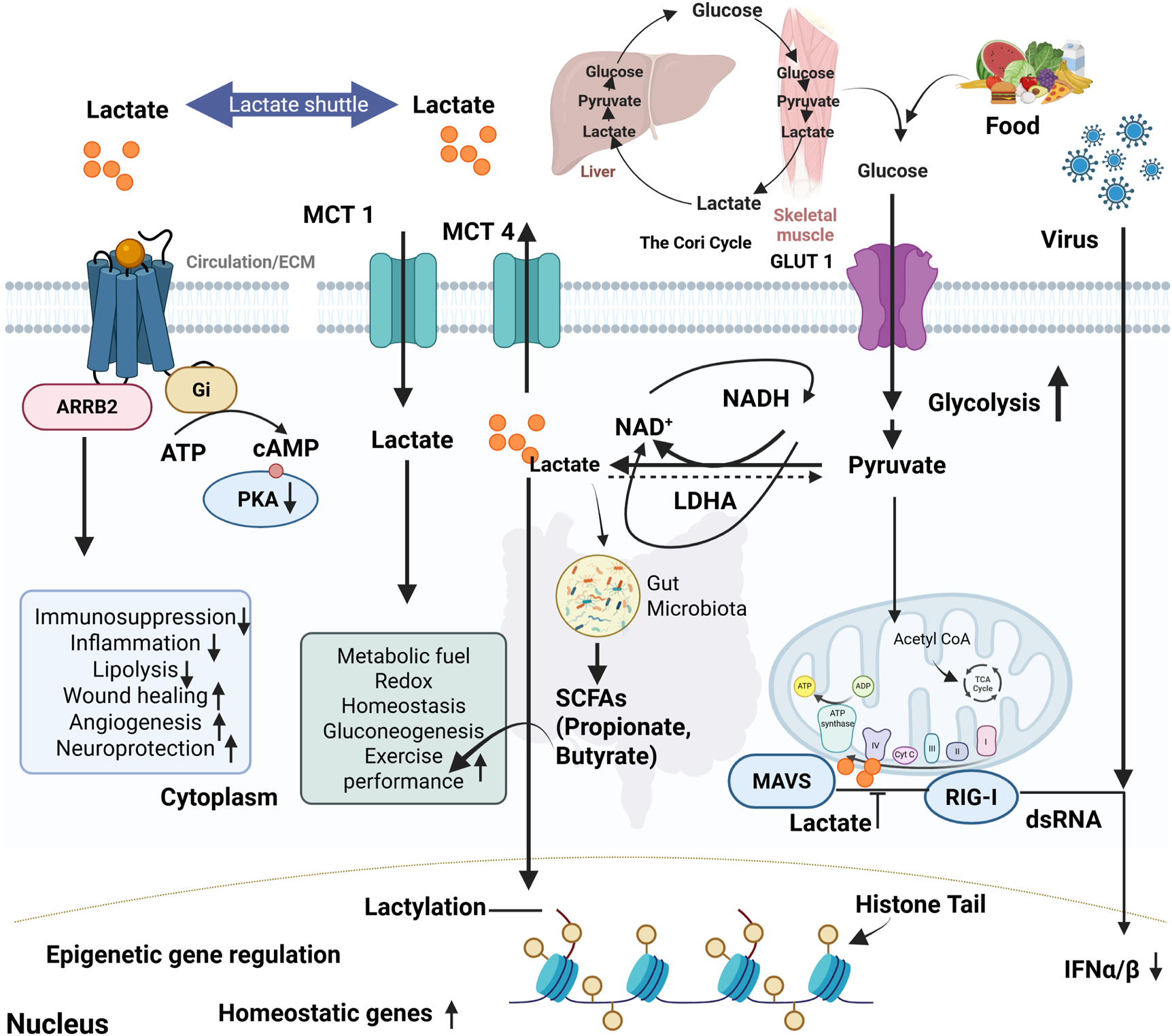
Lactate metabolism, signaling pathways, and its systemic impact on homeostasis and immunity. Food intake and viral infections drive glycolysis, leading to pyruvate and lactate production. Lactate is transported across membranes by MCT1& MCT4 and shuttled between tissues such as skeletal muscle and liver via the Cori cycle. Intracellularly, lactate modulates signaling through ARRB2-Gi protein-coupled pathways, reducing cAMP/PKA activity and influencing immunosuppression, inflammation, lipolysis, wound healing, angiogenesis, and neuroprotection. Lactate also serves as a metabolic fuel, supporting redox balance, gluconeogenesis, and exercise performance. In the cytoplasm, lactate-derived short-chain fatty acids (SCFAs) from gut microbiota contribute to systemic homeostasis. Lactate can inhibit mitochondrial antiviral signaling (MAVS) and RIG-I-mediated antiviral responses, reducing IFN-α/β production. Additionally, lactate enters the nucleus and promotes histone lactylation, driving epigenetic regulation of homeostatic gene expression.
2.4 Physiological roles of lactate
Lactate serves as an efficient energy source, particularly in oxidative tissues like the heart and brain. The heart preferentially oxidizes lactate over glucose, emphasizing its role in cardiac metabolism (Lee, 2021). Schurr et al. (1999) demonstrated that lactate is a primary energy source for neurons, reinforcing its significance beyond glycolysis (Schurr et al., 1999). Lactate regulates gene expression, angiogenesis, and immune responses (Figure 2). Lactate promotes the stabilization of HIF-1α by inhibiting prolyl hydroxylase activity. This stabilization enhances HIF-1-mediated transcription of glycolytic enzymes and VEGF, supporting metabolic adaptation and angiogenesis under hypoxic or pseudohypoxic conditions (Li et al., 2022). A study demonstrated that lactate accumulation under hypoxic conditions enhances VEGF expression, facilitating angiogenesis and tissue survival. Lactate accumulation during high-intensity exercise leads to metabolic acidosis but is rapidly cleared and utilized as an energy source. It aids recovery by replenishing glycogen stores and supporting mitochondrial respiration (Mandadzhiev, 2025). Gladden and Hogan (2006) showed that lactate metabolism during and after exercise is crucial for sustaining muscle function and preventing fatigue (Gladden and Hogan, 2006). Lactate is essential for brain metabolism. The astrocyte-neuron lactate shuttle theory suggests that astrocytes produce lactate, which neurons use as an energy source. Lactate enhances synaptic plasticity and memory formation (Hu et al., 2021). Pellerin et al. (1994) identified lactate as a key neuronal energy substrate, while another study showed that lactate enhances long-term memory formation in animal models (Pellerin et al., 1994). Lactate influences immune cell metabolism and function, regulating macrophage polarization. It promotes anti-inflammatory M2 macrophages while inhibiting pro-inflammatory M1 macrophages. This is particularly relevant in cancer metabolism and immune evasion (Zhang T. et al., 2024). In research study showed that lactate accumulation in tumors promotes M2 macrophage polarization, contributing to immune suppression and tumor progression (Zhong et al., 2022). Cancer cells convert glucose to lactate even in oxygen-rich conditions, a phenomenon known as the Warburg effect. Lactate accumulation in the tumor microenvironment promotes angiogenesis, immune evasion, and metastasis. Warburg first described this metabolic shift, and more recent studies highlighted how lactate metabolism supports tumor growth and resistance to therapy. Lactate is a vital metabolic intermediate with diverse roles beyond glycolysis. Its involvement in energy production, signaling, neuroprotection, and immune modulation underscores its physiological significance. The traditional perception of lactate as a waste product has evolved into its recognition as a key player in metabolic homeostasis (Nath and Balling, 2024).
In summary (Table 1), Lactate, an energy substrate, efficiently fuels muscles, the heart, and the brain, especially under high-demand conditions (Hashimoto et al., 2008). In skeletal muscle, lactate promotes adaptation by enhancing mitochondrial biogenesis and oxidative capacity, thereby improving endurance and performance (Nalbandian et al., 2017). In the brain, it supports neuronal metabolism, prevents excitotoxicity, and enhances synaptic plasticity, contributing to neuroprotection (Suzuki et al., 2011). Lactate also plays a crucial role in immune modulation by regulating macrophage polarization, suppressing inflammation, and supporting regulatory T cell function (Zhang T. et al., 2024). Furthermore, it stimulates angiogenesis and wound healing by promoting vascular endothelial growth factor (VEGF) production in endothelial cells and skin (Hunt et al., 2007). In the liver, lactate serves as a precursor for gluconeogenesis via the Cori cycle, helping to maintain blood glucose levels (Rogatzki et al., 2015). Its influence extends to lipid metabolism, where it modulates free fatty acid oxidation and storage in adipose tissue (Bergman et al., 1999). During exercise recovery, lactate helps reduce muscle fatigue, maintain pH balance, and facilitate lactate shuttling between tissues (Gladden, 2004). Interestingly, in the tumor microenvironment, lactate supports cancer cell survival and immune evasion (Fischer et al., 2007). Lastly, in the heart, lactate serves as a preferred substrate over glucose during ischemic conditions, offering cardioprotection by reducing oxidative stress (Chatham, 2002). Collectively, these findings highlight lactate’s multifaceted and beneficial roles across various organs and systems, challenging its outdated reputation as merely a metabolic waste product. In the following section, we provide a detailed explanation of key aspects highlighted in (Table 1).
3 The boon: beneficial role of lactate
3.1 Lactate as an energy source
Glycolysis, a key metabolic pathway, processes glucose to generate ATP and essential precursors for cellular functions. While glycolysis contributes only ∼6% of cellular ATP via substrate-level phosphorylation, its regulation by enzymes such as hexokinase (HEX), phosphofructokinase (PFK), and pyruvate kinase (PK) is crucial. Pyruvate dehydrogenase (PDH) and pyruvate carboxylase (PC) further link glycolysis to mitochondrial metabolism. Traditionally considered a waste product of anaerobic metabolism, lactate is now recognized as a critical energy source. It is converted to pyruvate by lactate dehydrogenase (LDH) and utilized by muscles, the heart, and the brain for oxidative phosphorylation, particularly during exercise or metabolic stress (Chaudhry and Varacallo, 2023).
Lactate, first identified in sour milk through microbial fermentation, becomes the predominant metabolite in mammals when oxygen and ATP demands surpass supply. Contrary to its reputation as a metabolic waste product, lactate is now understood as a key circulating carbohydrate fuel. By balancing the NADH/NAD+ ratio, lactate serves as both an energy substrate and a redox buffer, facilitating metabolic flexibility. This shift in perspective redefines lactate’s role in energy metabolism (Li et al., 2022).
3.2 Lactate as a preferred fuel
During physical exertion, skeletal muscles and the heart preferentially utilize lactate over glucose and fatty acids. In the brain, the Astrocyte-Neuron Lactate Shuttle (ANLS) facilitates lactate transfer from astrocytes to neurons, promoting energy metabolism and neuroprotection (Cauli et al., 2023). Recent findings challenge the notion that lactate is merely converted to pyruvate in the cytosol; instead, it is processed in mitochondria via the Mitochondrial Lactate Oxidation Complex (mLOC), particularly in high-energy-demand tissues (Chen et al., 2016).
In resting humans, the lactate-to-pyruvate (L/P) ratio is ∼10, increasing to ∼500 during moderate exercise, underscoring lactate’s role in metabolic adaptation. Major lactate utilization pathways include intramuscular oxidation, cardiac uptake, and hepatic gluconeogenesis (Liu et al., 2025).
3.3 Lactate and lipid metabolism
An inverse relationship exists between lactate and plasma free fatty acids (FFAs) during exercise. Lactate inhibits lipolysis in adipose tissue via Hydroxycarboxylic Acid Receptor 1 (HCAR1), modulating cAMP and CREB signaling pathways. Additionally, lactate impacts mitochondrial fatty acid oxidation by increasing acetyl-CoA and malonyl-CoA levels, inhibiting β-oxidation (Liu et al., 2009).
Lactate-induced secretion of Transforming Growth Factor Beta 2 (TGF-β2) from adipose tissue enhances glucose tolerance, highlighting its role in interorgan metabolic communication. These findings emphasize lactate’s dual role in acute metabolic regulation and long-term adaptation (Liu et al., 2025).
3.4 Lactate in brain energy metabolism and neuroprotection
Neurons rely on astrocytes for metabolic support. While glucose remains the primary fuel, lactate serves as an essential alternative, particularly during hypoglycemia or ischemic stress. Lactate supplementation enhances recovery from traumatic brain injury (TBI) and supports memory formation by modulating epigenetic mechanisms and brain-derived neurotrophic factor (BDNF) expression. Lactate metabolism is altered in neurodegenerative diseases like Alzheimer’s and Parkinson’s, with reduced lactate transport linked to cognitive decline. Enhancing lactate availability may offer therapeutic benefits in restoring metabolic balance and neuroprotection (Beard et al., 2022).
3.5 Lactate as a cellular signal and epigenetic modulator
Beyond metabolism, lactate functions as a signaling molecule influencing angiogenesis, tissue repair, and gene expression. It activates G-protein coupled receptors such as HCAR1, regulating neuroprotection and lipid metabolism. A novel post-translational modification, histone lactylation, links lactate to gene regulation (Figure 2). Increased lactate production under hypoxia or bacterial infections drives histone lactylation, influencing macrophage polarization, tumor progression, and inflammation resolution. These positions lactate as a crucial metabolic and epigenetic regulator with broad physiological and pathological implications (Zhang et al., 2019).
3.6 Lactate in hypoxia and cancer metabolism
Lactate stabilizes HIF-1α, promoting angiogenesis and metabolic adaptation under low-oxygen conditions. Lactate signaling plays a central role in tumor metabolism and immune evasion; thus, its targeting represents an emerging avenue for cancer therapeutics (Gu et al., 2025).
3.7 Lactate in exercise adaptation and immune modulation
Lactate modulates mitochondrial biogenesis and endurance adaptations through AMP-activated protein kinase (AMPK) and PGC-1α signaling. It also influences immune responses, shaping macrophage polarization and cytokine production, with implications for inflammation, metabolism, and disease progression (Takeda et al., 2022).
4 Gut microbiota: fuel or metabolize excess lactate
4.1 Lactate production by gut bacteria
Lactic Acid Bacteria (LAB) have adapted to survive in different environments, including the human gut, by relying primarily on fermentation for energy production. Unlike many other bacteria, LAB cannot perform respiration because they lack functional cytochromes, which are essential for oxidative metabolism. Instead, they obtain energy by breaking down sugars into lactic acid, which acidifies their surroundings and helps them outcompete harmful bacteria (Wang et al., 2020).
LAB have evolved unique metabolic pathways that enable their survival in diverse environments, particularly within the human gut. Among these pathways, amino acid deamination serves as a crucial mechanism for energy production, allowing LAB to thrive in nutrient-poor conditions by converting amino acids into usable energy. Additionally, acid decarboxylation plays a fundamental role in maintaining pH balance, which is essential for LAB survival in acidic environments such as the gastrointestinal tract (Feng and Wang, 2020).
Beyond their metabolic adaptability, LAB engage in intricate interactions with host cells, significantly influencing gut health and immune function. Through cross-talk with the host, LAB can modulate intestinal gene expression, thereby impacting digestive processes and immune responses. Furthermore, LAB contributes to gut homeostasis by producing bioactive compounds, such as gamma-aminobutyric acid (GABA), which not only relaxes gut smooth muscles but also has broader implications for mood regulation. Another essential feature of LAB is their antimicrobial effect, primarily mediated by lactic acid production. It acidifies the gut environment and inhibits the growth of pathogenic bacteria, thereby fostering a balanced microbiome (Yu et al., 2024).
Lactate plays a dual role in gut health. On the one hand, it serves as an important metabolic intermediate, supporting the growth of lactate-consuming bacteria, which convert it into beneficial SCFAs (Table 4). On the other hand, excessive lactate accumulation can lead to acidosis, which disrupts microbial stability. This phenomenon is well-documented in ruminants, where diets rich in fermentable carbohydrates can promote the overgrowth of lactate-producing bacteria, causing a dangerous drop in pH and leading to metabolic disorders such as lactic acidosis. In the human gut, lactate metabolism is tightly regulated through cross-feeding interactions between different bacterial species (Yu et al., 2024).
4.2 Lactate utilization by gut microbes
4.2.1 Butyrate production
Certain members of the phylum Firmicutes, particularly species belonging to the genera Anaerobutyricum and Anaerostipes, are capable of converting lactate into butyrate, a SCFA with well-established health benefits. This conversion typically occurs via a cross-feeding mechanism that requires acetate as a co-substrate. Butyrate is a critical energy source for colonocytes, where it enhances intestinal barrier function, regulates inflammatory pathways, and modulates the gut immune response. Additionally, butyrate has been associated with anti-inflammatory effects through its role in the inhibition of histone deacetylases (HDACs), contributing to immune homeostasis within the gut mucosa (Figure 3) (Louis et al., 2022).
FIGURE 3
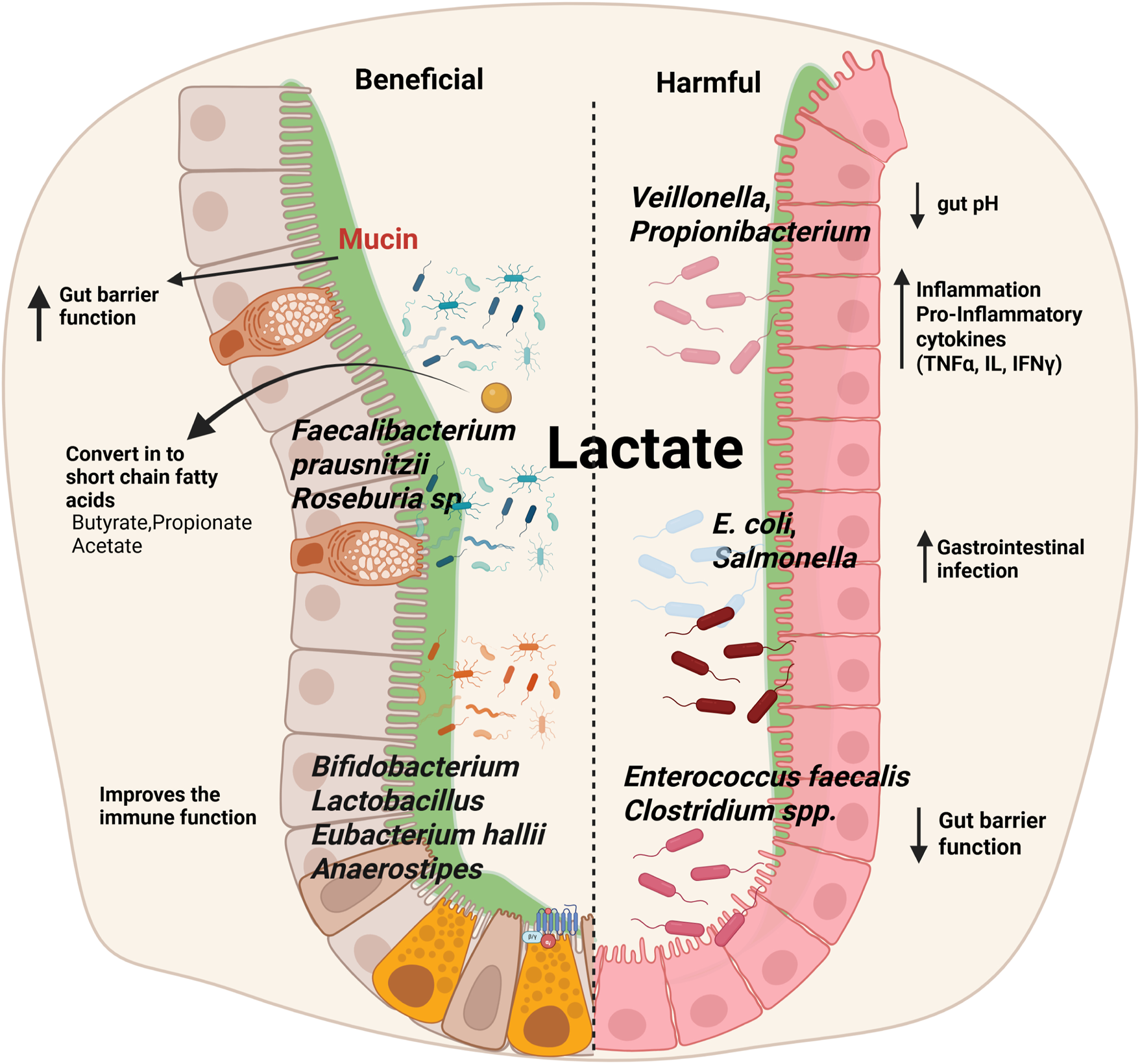
Impact of lactate on gut microbiota: balancing beneficial and harmful effects. Lactate influences gut microbiota composition, supporting beneficial bacteria such as Faecalibacterium prausnitzii, Roseburia spp., Bifidobacterium, Lactobacillus, Eubacterium hallii, and Anaerostipes, which enhance gut barrier integrity, improve immune function, and produce SCFAs like butyrate, propionate, and acetate. In contrast, elevated lactate levels can promote the growth of harmful bacteria, including Veillonella, Propionibacterium, E. coli, Salmonella, Enterococcus faecalis, and Clostridium spp., leading to reduced gut pH, increased inflammation via pro-inflammatory cytokines (TNF-α, ILs, IFN-γ), impaired gut barrier function, and a higher risk of gastrointestinal infections.
4.2.2 Propionate formation
Lactate can also be metabolized into propionate via multiple biochemical pathways, with distinct microbial taxa utilizing different metabolic route.
4.2.3 Acrylate pathway
This pathway involves the direct conversion of lactate into propionate and is employed by bacterial species such as Coprococcus catus (Reichardt et al., 2014) and Megasphaera elsdenii (Hino and Kuroda, 1993).
4.2.4 Succinate pathway
In this pathway, lactate is first converted to succinate, which is subsequently metabolized into propionate. This process is utilized by species within the genus Veillonella (Reichardt et al., 2014).
4.2.5 1,2-Propanediol pathway
An alternative route involves the conversion of lactate into 1,2-propanediol, which can then be further metabolized into propionate by species such as Limosilactobacillus reuteri and Anaerobutyricum hallii (Niu et al., 2019).
Propionate serves multiple physiological functions in the host, including modulating gluconeogenesis in the liver, influencing appetite regulation, and exerting anti-inflammatory properties through interactions with gut epithelial and immune cells (Louis et al., 2022).
4.2.6 Acetate production and sulfate reduction
Certain Proteobacteria, including species within the genus Desulfovibrio, are capable of metabolizing lactate into acetate through dissimilatory sulfate reduction. This process is coupled with the production of hydrogen sulfide (H2S), a metabolite that, at excessive levels, has been implicated in gut epithelial damage and inflammatory conditions such as inflammatory bowel disease (IBD). While low concentrations of H2S may have physiological roles in cell signaling, its accumulation can lead to mucosal toxicity and alterations in gut microbiota composition (Rey et al., 2013).
The microbial conversion of lactate into butyrate, propionate, and acetate plays a pivotal role in gut ecosystem stability and host physiology. Cross-feeding interactions between lactate-producing and lactate-utilizing bacteria regulate metabolite flux, influencing gut barrier integrity, immune function, and metabolic homeostasis (Dordević et al., 2020).
4.2.7 Cross-feeding among microbes
Bacterial cross-feeding plays a crucial role in shaping SCFA production and optimizing substrate utilization in the gut. These interactions enhance microbial diversity, prevent metabolic imbalances, and contribute to gut health by maintaining a stable environment. Bifidobacterium produces acetate when fermenting dietary fibers such as oligofructose, which is then utilized by butyrate-producing bacteria like Faecalibacterium prausnitzii and Roseburia sp. to produce butyrate. Lactate-producing bacteria like Bifidobacterium and Lactobacillus produce lactate during carbohydrate fermentation, which can be converted into butyrate by Eubacterium hallii and Anaerostipes to prevent acid build-up and contribute to gut health. Propionate-producing bacteria (Veillonella, Propionibacterium) convert lactate into propionate, playing a role in glucose metabolism and satiety (Figure 3) (Culp and Goodman, 2023).
4.3 Implications for intestinal homeostasis
Lactate plays a significant role in gut health, immunity, and disease prevention. If lactate-consuming bacteria are insufficient, lactate can accumulate, leading to a drop in pH and potential gut dysbiosis. Excess lactate has been linked to inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS), and metabolic disorders (Wang et al., 2020). Colonocytes (intestinal cells) utilize lactate to produce butyrate through cross-feeding by bacteria such as Eubacterium and Anaerostipes, which strengthen the gut lining and support overall intestinal health. Lactate lowers the pH, inhibiting harmful bacteria (Escherichia coli, Salmonella) while promoting beneficial microbes (Bifidobacteria, Akkermansia) (Zhao et al., 2024). Additionally, lactate helps regulate immune responses by modulating immune cells and reducing inflammatory cytokines (TNF-α, IL-6), preventing excessive inflammation (Llibre et al., 2025).
Recent studies highlight the pivotal role of lactate and its receptor, GPR81, in regulating intestinal homeostasis and immune responses, particularly in inflammatory bowel diseases (IBD) (Ranganathan et al., 2018). Understanding these metabolic pathways and their microbial contributors provides valuable insight into potential probiotic interventions aimed at promoting gut health and preventing dysbiosis-associated diseases (Li et al., 2022).
5 Lactate dehydrogenase (LDH) inhibitors: plant-based and synthetic
Lactate dehydrogenase (LDH) plays a crucial role in anaerobic glycolysis by catalyzing the reversible conversion of pyruvate to lactate, coupled with the regeneration of NAD+. In cancer, this pathway is hijacked even under aerobic conditions, a phenomenon known as the Warburg effect, whereby cancer cells preferentially convert glucose to lactate despite sufficient oxygen. This metabolic reprogramming supports rapid cell proliferation by sustaining glycolytic flux, maintaining redox balance, and promoting survival in the tumor microenvironment. LDH, particularly the LDH-A isoform, is thus considered a key metabolic enzyme and a promising therapeutic target in cancers (Valvona et al., 2016; Kim et al., 2019) and infectious diseases like malaria (Possemiers et al., 2021). LDH belongs to the 2-hydroxyacid oxidoreductase family and is widely found in animals, microorganisms, yeasts, and plants. The Table 2 summarizes various plant-based LDH inhibitors, highlighting their mechanisms of action and implications for human health (Table 2). Natural compounds such as pentagalloyl glucose from Rhus chinensis, polyphenols (including quercetin, EGCG, curcumin, and resveratrol), and berberine target LDH through different mechanisms, such as non-competitive inhibition, binding to the enzyme’s active or coenzyme sites, and suppression of LDH expression. These inhibitors show potential in cancer therapy by disrupting tumor metabolism, overcoming drug resistance, and modulating lactate production. Additionally, some compounds like those from Polygala tenuifolia demonstrate neuroprotective effects in ischemic stroke, while others, such as rutin and amentoflavone from Selaginella doederleinii, offer potential treatments for metabolic disorders. Overall, plant-based LDH inhibitors present promising therapeutic avenues for cancer, metabolic diseases, and neuroprotection (Table 2).
TABLE 2
| Category | LDH inhibitor | Role/Mechanism | Implications for human health | References |
|---|---|---|---|---|
| Plant-Based Inhibitors | Rhus chinensis | Pentagalloyl glucose (PGG) inhibits LDH non-competitively, reducing lactate in cancer cells | Potential cancer therapy by disrupting metabolic processes in tumors | Mendonca et al. (2021) |
| Polygala tenuifolia | Identified five LDH inhibitors, a potential treatment for ischemic stroke | Neuroprotective effects in ischemic stroke | Shen et al. (2025) | |
| Rutin | Binds to the coenzyme site of LDH, inhibiting its activity in a dose-dependent manner | Modulates lactate production, useful in various metabolic disorders | Ding et al. (2024) | |
| Selaginella doederleinii | Amentoflavone and robustaflavone inhibit LDH | Potential treatment for metabolic disorders and cancer | Zhang et al. (2022) | |
| Polyphenols (Quercetin, EGCG, Curcumin, Resveratrol) | Binds to the LDH active site, reduces its activity, and suppresses LDH expression | Anti-cancer and anti-inflammatory effects, metabolic modulation | Han et al. (2023) | |
| Catechin | Inhibits lactate production and LDHA activity, overcomes 5FU resistance in cancer | Potential adjuvant therapy for drug resistance in cancer | Han et al. (2023) | |
| Berberine | Targets LDH-A, suppressing pancreatic cancer progression via AMPK/mTOR pathway | Potential cancer therapy, metabolic disorder treatment | Davoodvandi et al. (2024) | |
| Betulinic Acid | Binds strongly to LDH, reducing its activity, and exerting antitumor effects | Anti-cancer properties, modulates lactate production in tumors | Davoodvandi et al. (2024) | |
| Synthetic Inhibitors | Pyrazole-based inhibitors | Block LDH enzymatic activity, developed through high-throughput screening | Potential cancer and metabolic disorder therapy | Rai et al. (2020) |
| Galloflavin | Inhibits LDH-A and LDH-B, induces apoptosis in tumor cells | Selective anticancer agent, blocks aerobic glycolysis in tumors | Yao et al. (2022) | |
| Oxamate | Inhibits LDH-A in NSCLC cells, reduces ATP levels and induces apoptosis | Targeting LDH-A for cancer therapy, particularly in lung cancer | Li et al. (2020) | |
| GSK2837808A | Specific LDH-A inhibitor, reduces lactate secretion in TMJOA synovial fibroblasts | Potential treatment for joint disorders and metabolic dysfunctions | Alobaidi et al. (2023) | |
| FX-11 and AR-C155858 | Combination therapy targeting LDHA and MCT1, reducing tumor cell proliferation | Effective in breast and colorectal cancer treatment | Alobaidi et al. (2023) | |
| N-hydroxyindole-based inhibitor (NHI-Glc-2) | Inhibits glycolysis and cell proliferation in cancer | Potential anticancer agent with antiglycolytic effects | Hassouni et al. (2020) | |
| Itraconazole, Atorvastatin, Posaconazole | Potential inhibitors of Plasmodium LDH, with posaconazole most effective | Treatment for malaria, particularly against Plasmodium LDH | Comandatore et al. (2022) | |
| Stiripentol | Antiepileptic drug, inhibits LDH, reducing seizures and epileptiform activity | Potential treatment for epilepsy, reducing lactate-related neuronal activity | Wheless and Weatherspoon (2025) |
Summarizes the plant-based and synthetic LDH inhibitors.
Beyond its metabolic role, LDH is an important diagnostic marker, as its sudden increase in serum levels often indicates acute disease conditions. High LDH levels are commonly observed in malignancies, megaloblastic anemia, myocardial infarction, liver disorders, hematological diseases, and skeletal muscle conditions. Due to its strong clinical significance, LDH measurement is widely used for disease diagnosis and monitoring (Gupta, 2022).
5.1 LDH in malaria and cancer
In Plasmodium falciparum (the parasite responsible for malaria), the enzyme pfLDH is essential for energy production, as the parasite relies on anaerobic glycolysis due to the absence of a citric acid cycle. Inhibitors of pfLDH could potentially lead to the death of the malaria parasite (Penna-Coutinho et al., 2011). Similarly, in cancer, human LDH isoform-5 (hLDH-5 or LDH-A) is upregulated in tumor cells, supporting the Warburg effect, where tumors rely more on anaerobic glycolysis than oxidative phosphorylation for energy. Targeting hLDH-5 could disrupt tumor growth and invasiveness (Alam et al., 2014).
6 The curse: potential detrimental effects of lactate
6.1 Lactic acidosis and metabolic dysregulation
While lactate serves as a critical metabolic intermediate, excessive accumulation can lead to lactic acidosis, a pathological condition characterized by a decrease in blood pH. This occurs in cases of severe hypoxia, sepsis, or mitochondrial dysfunction, where lactate clearance is impaired. Studies have shown that elevated lactate levels in critically ill patients are associated with poor outcomes due to systemic metabolic disturbances (Table 3) (Li et al., 2022). Recently, research highlighted the role of excessive lactate in disrupting cellular homeostasis, leading to impaired enzyme function and metabolic stress (Cai et al., 2024).
TABLE 3
| Effect | Mechanism | Relevant organs | References |
|---|---|---|---|
| Cancer progression | Lactate supports tumor growth by driving enhanced glycolysis and suppressing immune responses | Tumors (Various organs) | de la Cruz-López et al. (2019) |
| Increased inflammation | Lactate accumulation can activate inflammatory pathways and cytokine release | Immune system, tissues | Llibre et al. (2025) |
| Metabolic disorders | High lactate levels contribute to metabolic dysfunction, affecting glucose and lipid metabolism | Liver, muscles, adipose tissue | Ishitobi et al. (2019) |
| Muscle fatigue | Accumulation of lactate in muscles leads to acidosis and impairs muscle contraction | Skeletal muscles | Chen et al. (2025) |
| Brain dysfunction | Lactate buildup in the brain may contribute to neurological diseases like epilepsy and Alzheimer’s | Brain (CNS) | Cai et al. (2022) |
| Cardiac stress | Elevated lactate in the heart can cause reduced oxygen availability, increasing the risk of ischemia | Heart | Fang et al. (2024) |
Summary of the impact of excess lactate on human health and the mechanisms involved in disease progression.
Excessive lactate accumulation, as detailed in Table 3, has significant implications for human health by contributing to the progression of various diseases. In cancer, elevated lactate levels promote tumor growth through enhanced glycolysis and suppression of immune responses, affecting multiple organs (de la Cruz-López et al., 2019) (Table 3). Lactate buildup can also activate inflammatory pathways and cytokine release, leading to increased inflammation in the immune system and other tissues (Llibre et al., 2025) (Table 3). Metabolic disorders arise when high lactate concentrations disrupt glucose and lipid metabolism, particularly impacting the liver, muscles, and adipose tissue (Ishitobi et al., 2019). In skeletal muscles, lactate accumulation results in acidosis and impairs muscle contraction, contributing to muscle fatigue (Chen et al., 2025) (Table 3). The brain is also vulnerable, as lactate buildup may play a role in neurological diseases such as epilepsy and Alzheimer’s (Cai et al., 2022) (Table 3). Lastly, in the heart, elevated lactate can reduce oxygen availability and increase the risk of ischemia (Fang et al., 2024) (Table 3). This summary underscores the multifaceted and detrimental effects of excess lactate on various organ systems.
6.2 Tumor microenvironment and cancer progression
The Warburg effect, a hallmark of cancer metabolism, leads to high lactate production, which significantly alters the tumor microenvironment. Accumulated lactate promotes immune evasion, angiogenesis, and metastasis by modulating tumor-associated macrophages and regulatory T cells. Studies have shown that lactate enhances HIF-1α stabilization, upregulating VEGF expression and supporting tumor growth. In study showed that targeting lactate transporters, such as MCT1 and MCT4, can disrupt tumor metabolism and enhance the efficacy of cancer therapies (Zhong et al., 2022).
6.3 Cardiovascular disease and impaired cardiac function
Elevated lactate levels are frequently observed in cardiovascular diseases, especially in conditions like heart failure and ischemic injury, where tissue hypoxia impairs oxidative metabolism. The heart preferentially oxidizes lactate as an energy source under normal conditions; however, in pathological states, excessive lactate accumulation can contribute to acidosis and impaired myocardial function (Ouyang et al., 2023). A study illustrated that heart failure patients exhibit altered lactate metabolism, leading to reduced cardiac efficiency (Revelly et al., 2005). Moreover, lactate-driven inflammation in vascular endothelial cells has been implicated in atherosclerosis progression, highlighting the need for therapeutic strategies targeting lactate balance in cardiovascular health (Dong et al., 2021).
6.4 Neurological disorders and cognitive dysfunction
Dysregulated lactate metabolism has been implicated in neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and multiple sclerosis. While lactate plays a neuroprotective role under physiological conditions, impaired lactate transport and utilization can contribute to neuronal energy deficits and oxidative stress (Yang et al., 2024). A research study demonstrated that disruptions in the astrocyte-neuron lactate shuttle lead to cognitive impairment (Suzuki et al., 2011). Furthermore, excessive lactate accumulation in the brain has been associated with neuroinflammation and excitotoxicity, exacerbating disease progression in conditions such as epilepsy and traumatic brain injury (Roh et al., 2023).
6.5 Muscular dystrophy and exercise intolerance
In individuals with muscular dystrophy and metabolic myopathies, lactate metabolism is often impaired, leading to exercise intolerance and muscle fatigue. Mutations affecting glycolytic enzymes or lactate transporters result in an inability to efficiently clear lactate, leading to muscle damage and weakness (Jeppesen et al., 2013). A study highlighted the role of lactate accumulation in muscle dysfunction, emphasizing its contribution to oxidative stress and mitochondrial impairment. Therapeutic approaches aimed at optimizing lactate metabolism are being explored to improve muscle function in dystrophic conditions (Tarnopolsky, 2018; Wen et al., 2025).
6.6 Lactate-producing bacteria harmful to human health
While many lactate-producing bacteria play beneficial roles in maintaining gut homeostasis, certain species can contribute to dysbiosis and inflammation when their growth is dysregulated (Louis et al., 2022). For instance, an overgrowth of Lactobacillus or Enterococcus species can lead to excessive lactate accumulation, which may exacerbate gastrointestinal conditions such as IBS, IBD, and even metabolic diseases like obesity. In these cases, the excess lactate can lower gut pH, leading to an imbalance in the microbiota and promoting the growth of pathogenic bacteria (Figure 3; Table 4) (Portincasa et al., 2024).
TABLE 4
| Bacteria | Category | Mechanism of lactate production | Impact on human health | Potential health effects | References |
|---|---|---|---|---|---|
| Lactobacillus spp. (Lactobacillus acidophilus; Lactobacillus casei) | Beneficial | Ferments carbohydrates to produce lactate | Helps maintain gut acidity, beneficial for digestion, and supports gut health by preventing pathogenic overgrowth | Prevents pathogen colonization, supports immune function, and promotes gut barrier integrity | Dempsey and Corr (2022) |
| Bifidobacterium spp. | Beneficial | Produces lactate from dietary fibers and oligosaccharides | Modulates gut microbiota, improves gut health, and has anti-inflammatory effects | Supports healthy digestion, reduces inflammation, and promotes the production of beneficial short-chain fatty acids | Victoria Obayomi et al. (2024) |
| Faecalibacterium prausnitzii | Beneficial | Converts carbohydrates into lactate and butyrate | Supports gut health and maintains a balanced microbiome | Enhances anti-inflammatory responses and strengthens gut barrier function | Martín et al. (2023) |
| Lactobacillus plantarum | Beneficial | Produces lactic acid by fermenting sugars in anaerobic conditions | Promotes gut barrier integrity and reduces inflammation | May help in managing irritable bowel syndrome (IBS) and other inflammatory conditions | Liu et al. (2021) |
| Lactobacillus rhamnosus | Beneficial | Ferments glucose to lactic acid, regenerating NAD+ for glycolysis | Strengthens gut microbiota and supports immune function | Reduces risk of respiratory infections and improves skin health | Rastogi and Singh (2022) |
| Lactobacillus reuteri | Beneficial | Convert sugars to lactic acid via the lactate dehydrogenase enzyme | Produces antimicrobial substances that inhibit pathogens | May reduce colic in infants and improve oral health | Singh et al. (2022) |
| Streptococcus thermophilus | Beneficial | Converts lactose to lactic acid during fermentation | Aids in lactose digestion and supports gut health | Reduces symptoms of lactose intolerance and improves gut microbiota | Saleem et al. (2024) |
| Enterococcus faecium | Beneficial | Produces lactic acid as a byproduct of carbohydrate metabolism | Competes with harmful bacteria in the gut | May reduce the risk of gastrointestinal infections | Vieco-Saiz et al. (2019) |
| Pediococcus acidilactici | Beneficial | Ferments sugars to lactic acid, lowering pH and inhibiting spoilage organisms | Used in food preservation and supports gut health | Enhances food safety and promotes gut microbiota balance | Todorov et al. (2022) |
| Leuconostoc mesenteroides | Beneficial | Produces lactic acid and other metabolites during carbohydrate fermentation | Contributes to food fermentation and supports gut health | Improves digestion and enhances the flavor of fermented foods | Kim et al. (2025) |
| Weissella confusa | Beneficial | Ferments sugars to lactic acid, producing antimicrobial compounds | Inhibits harmful bacteria and supports gut health | May reduce the risk of infections and improve gut microbiota | Petrariu et al. (2023) |
| Oenococcus oeni | Beneficial | Converts malic acid to lactic acid during malolactic fermentation | Used in winemaking and supports gut health | Enhances the flavor of wine and promotes gut microbiota balance | James et al. (2023) |
| Carnobacterium maltaromaticum | Beneficial | Produces lactic acid and bacteriocins during fermentation | Inhibits spoilage organisms in food and supports gut health | Enhances food safety and promotes gut microbiota balance | Bisht et al. (2024) |
| Propionibacterium freudenreichii | Beneficial | Produces lactic acid and propionic acid during carbohydrate fermentation | Supports gut health and produces vitamin B12 | Enhances nutrient absorption and reduces gut inflammation | Tripathi et al. (2024) |
| Streptococcus mutans | Harmful | Produces lactate from sugars, especially sucrose | Key contributor to dental cavities and oral infections | Causes tooth decay and promotes plaque formation | Luo et al. (2024) |
| Clostridium spp. | Harmful | Ferments carbohydrates to produce lactate, often in excess | Overproduction of lactate in gut can lower pH, cause metabolic disturbances, and exacerbate gut inflammation | Contributes to conditions like colorectal cancer, IBS, and IBD. | Hou et al. (2022) |
| Enterococcus faecalis | Harmful | Produces lactate from sugars under anaerobic conditions | Associated with gut dysbiosis, promotes inflammation and infection, and is pathogenic in immunocompromised individuals. | Can lead to sepsis, urinary tract infections, and gut-related issues like IBS and IBD. | Chancharoenthana et al. (2023) |
| Staphylococcus aureus | Harmful | Anaerobic fermentation of glucose | Produces lactate, which can contribute to tissue damage and inflammation | Causes skin infections, pneumonia, and toxic shock syndrome | Taylor and Unakal (2023) |
| Escherichia coli (E. coli) | Harmful | Glycolysis followed by fermentation under oxygen-limited conditions | Lactate production can exacerbate inflammation in the gut | Causes food poisoning, urinary tract infections, and septicemia | Milani et al. (2017) |
| Streptococcus pyogenes | Harmful | Lactic acid fermentation via the Embden-Meyerhof pathway | Lactate can fuel bacterial growth and worsen tissue damage | Causes strep throat, scarlet fever, and necrotizing fasciitis | Stevens and Bryant (2022) |
| Clostridium perfringens | Harmful | Fermentation of pyruvate in anaerobic conditions | Lactate production contributes to the acidic environment, aiding bacterial survival | Causes gas gangrene and food poisoning | Juneja et al. (2023) |
| Lactobacillus acidophilus | Harmful | Homolactic fermentation, where glucose is converted entirely into lactate | Overproduction of lactate can lead to acidosis in certain conditions | Normally beneficial but can cause infections in immunocompromised individuals | Gao et al. (2022) |
| Propionibacterium acnes | Harmful | Fermentation of carbohydrates under anaerobic or oxygen-restricted conditions | Lactate production can contribute to inflammation in sebaceous glands | Associated with acne and other skin conditions | Gannesen et al. (2019) |
| Helicobacter pylori | Harmful | Adaptive metabolic pathways for survival in acidic stomach conditions | Lactate production can alter the stomach’s microenvironment, aiding bacterial colonization | Causes stomach ulcers and is linked to gastric cancer | Somiah et al. (2022) |
| Klebsiella pneumoniae | Harmful | Facultative anaerobic fermentation of sugars | Lactate production can worsen lung inflammation and tissue damage | Causes pneumonia, urinary tract infections, and septicemia | Liang et al. (2022) |
| Pseudomonas aeruginosa | Harmful | Lactate synthesis during anaerobic respiration | Lactate production supports biofilm formation, making infections harder to treat | Causes infections in wounds, lungs, and urinary tract | Moser et al. (2021) |
| Enterococcus faecalis | Harmful | Fermentation via the glycolytic pathway | Lactate production can contribute to biofilm formation and antibiotic resistance | Causes urinary tract infections, endocarditis, and bacteremia | Said et al. (2024) |
| Bacteroides fragilis | Harmful | Anaerobic glycolysis | Lactate production can promote bacterial survival in anaerobic environments | Causes abdominal infections and abscesses | Tufail and Schmitz (2024) |
| Clostridium difficile | Harmful | Lactic acid fermentation in low-oxygen environments | Lactate production can disrupt gut microbiota balance | Causes severe diarrhea and colitis | Sehgal and Khanna (2021) |
| Mycobacterium tuberculosis | Harmful | Modulation of metabolic pathways under hypoxic conditions | Lactate production can contribute to the acidic environment in infected tissues | Causes tuberculosis | Kiran and Basaraba (2021) |
| Neisseria gonorrhoeae | Harmful | Oxygen-limited glycolysis followed by fermentation | Lactate production can enhance bacterial survival in mucosal tissues | Causes gonorrhea | Llibre et al. (2021) |
| Salmonella enterica | Harmful | Facultative fermentation of carbohydrates | Lactate production can exacerbate gut inflammation and bacterial invasion | Causes foodborne illnesses and typhoid fever | Llibre et al. (2021) |
Summarized table of microbiota showing their beneficial and harmful impacts on human health in the presence of lactate.
The Table 4 highlights the beneficial roles of various gut bacteria in human health through their mechanisms of lactate production. Lactobacillus spp. (including L. acidophilus and L. casei) ferment carbohydrates to produce lactate, which helps maintain gut acidity, supports digestion, and prevents pathogenic overgrowth by enhancing immune function and barrier integrity. Bifidobacterium spp. generate lactate from dietary fibers and oligosaccharides, modulating the gut microbiota, improving gut health, and exerting anti-inflammatory effects by promoting the production of beneficial short-chain fatty acids. Faecalibacterium prausnitzii converts carbohydrates into lactate and butyrate, supporting a balanced microbiome and enhancing anti-inflammatory responses, thereby strengthening gut barrier function. Lactobacillus plantarum produces lactic acid under anaerobic conditions, promoting gut barrier integrity and potentially aiding in the management of irritable bowel syndrome (IBS) and other inflammatory conditions. Together, these bacteria contribute significantly to gut health by modulating inflammation, supporting digestion, and maintaining a balanced microbiome (Table 4).
7 Conclusion and future directions
Lactate, once primarily associated with muscle fatigue and anaerobic metabolism, is increasingly recognized for its complex and dual role in human health. Under normal physiological conditions, lactate serves as a critical energy substrate and plays a pivotal role in metabolic processes like the Cori cycle. However, its excessive accumulation, particularly in diseases like cancer, ischemic stroke, and metabolic disorders, can be detrimental, contributing to disease progression and poor prognosis.
Recent studies have highlighted lactate’s potential as both a boon and a curse. While its role in tumor metabolism, immune regulation, and neuronal signaling shows promise, the harmful effects of chronic lactate accumulation cannot be overlooked. The emerging therapeutic strategies targeting lactate, such as lactate dehydrogenase inhibitors, offer new avenues for disease treatment.
Future research should focus on developing precise methods to regulate lactate metabolism, exploring the role of gut microbiota in lactate production, and understanding the complex interactions between lactate and immune responses. Additionally, investigating the potential of lactate in enhancing exercise performance and as a therapeutic agent in neurological and metabolic diseases warrants attention. Ultimately, advancing our understanding of lactate’s multifaceted role will enable the development of targeted interventions to harness its benefits while mitigating its harmful effects.
Statements
Author contributions
SK: Methodology, Resources, Writing – original draft, Writing – review and editing, Software. NS: Writing – original draft, Writing – review and editing. TJ: Writing – review and editing. HJ: Writing – review and editing. PU: Conceptualization, Methodology, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AlamA.NeyazM. K.HasanS. I. (2014). Exploiting unique structural and functional properties of malarial glycolytic enzymes for antimalarial drug development. Malar. Res. Treat.2014, 451065. 10.1155/2014/451065
2
AlobaidiB.HashimiS. M.AlqosaibiA. I.AlQurashiN.AlhazmiS. (2023). Targeting the monocarboxylate transporter MCT2 and lactate dehydrogenase A LDHA in cancer cells with FX-11 and AR-C155858 inhibitors. Eur. Rev. Med. Pharmacol. Sci.27, 6605–6617. 10.26355/EURREV_202307_33131
3
BeardE.LengacherS.DiasS.MagistrettiP. J.FinsterwaldC. (2022). Astrocytes as key regulators of brain energy metabolism: new therapeutic perspectives. Front. Physiol.12, 825816. 10.3389/FPHYS.2021.825816
4
BergmanB. C.WolfelE. E.ButterfieldG. E.LopaschukG. D.CasazzaG. A.HorningM. A.et al (1999). Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol.87, 1684–1696. 10.1152/JAPPL.1999.87.5.1684
5
BishtV.DasB.HussainA.KumarV.NavaniN. K. (2024). Understanding of probiotic origin antimicrobial peptides: a sustainable approach ensuring food safety. npj Sci. Food8 (18), 67–16. 10.1038/s41538-024-00304-8
6
BrooksG. A. (2021). Role of the heart in lactate shuttling. Front. Nutr.8, 663560. 10.3389/FNUT.2021.663560
7
BrooksG. A.CurlC. C.LeijaR. G.OsmondA. D.DuongJ. J.ArevaloJ. A. (2022). Tracing the lactate shuttle to the mitochondrial reticulum. Exp. & Mol. Med.54 (54), 1332–1347. 10.1038/s12276-022-00802-3
8
CaiM.LiS.CaiK.DuX.HanJ.HuJ. (2024). Empowering mitochondrial metabolism: exploring L-lactate supplementation as a promising therapeutic approach for metabolic syndrome. Metabolism152, 155787. 10.1016/J.METABOL.2024.155787
9
CaiM.WangH.SongH.YangR.WangL.XueX.et al (2022). Lactate is answerable for brain function and treating brain diseases: energy substrates and signal molecule. Front. Nutr.9, 800901. 10.3389/FNUT.2022.800901
10
CaslinH. L.AbebayehuD.PinetteJ. A.RyanJ. J. (2021). Lactate is a metabolic mediator that shapes immune cell fate and function. Front. Physiol.12, 688485. 10.3389/fphys.2021.688485
11
CauliB.DusartI.LiD. (2023). Lactate as a determinant of neuronal excitability, neuroenergetics and beyond. Neurobiol. Dis.184, 106207. 10.1016/J.NBD.2023.106207
12
ChancharoenthanaW.KamolratanakulS.SchultzM. J.LeelahavanichkulA. (2023). The leaky gut and the gut microbiome in sepsis – targets in research and treatment. Clin. Sci. (Lond)137, 645–662. 10.1042/CS20220777
13
ChathamJ. C. (2002). Lactate - the forgotten fuel. J. Physiology542, 333. 10.1113/jphysiol.2002.020974
14
ChaudhryR.VaracalloM. A. (2023). Biochemistry, glycolysis. Treasure Island, FL: StatPearls Publishing. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK482303/(Accessed April 25, 2025).
15
ChenJ.HuangZ.ChenY.TianH.ChaiP.ShenY.et al (2025). Lactate and lactylation in cancer. Signal Transduct. Target. Ther.10 (1), 38–26. 10.1038/s41392-024-02082-x
16
ChenY. J.MahieuN. G.HuangX.SinghM.CrawfordP. A.JohnsonS. L.et al (2016). Lactate metabolism is associated with Mammalian mitochondria. Nat. Chem. Biol.12, 937–943. 10.1038/NCHEMBIO.2172
17
ComandatoreA.FranczakM.SmolenskiR. T.MorelliL.PetersG. J.GiovannettiE. (2022). Lactate dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin. Cancer Biol.86, 93–100. 10.1016/J.SEMCANCER.2022.09.001
18
CulpE. J.GoodmanA. L. (2023). Cross-feeding in the gut microbiome: ecology and mechanisms. Cell Host Microbe31, 485–499. 10.1016/J.CHOM.2023.03.016
19
DavoodvandiA.SadeghiS.AlaviS. M. A.AlaviS. S.JafariA.KhanH.et al (2024). The therapeutic effects of berberine for gastrointestinal cancers. Asia Pac J. Clin. Oncol.20, 152–167. 10.1111/AJCO.13941
20
de la Cruz-LópezK. G.Castro-MuñozL. J.Reyes-HernándezD. O.García-CarrancáA.Manzo-MerinoJ. (2019). Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol.9, 1143. 10.3389/FONC.2019.01143
21
DempseyE.CorrS. C. (2022). Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front. Immunol.13, 840245. 10.3389/FIMMU.2022.840245
22
DingP.YangK.WangH.KuangL.GaoL.LuoJ.et al (2024). Exploring the therapeutic potential of rutin through investigating its inhibitory mechanism on lactate dehydrogenase: multi-Spectral methods and computer simulation. Bioorg Chem.149, 107503. 10.1016/J.BIOORG.2024.107503
23
DongS.QianL.ChengZ.ChenC.WangK.HuS.et al (2021). Lactate and myocardiac energy metabolism. Front. Physiol.12, 715081. 10.3389/FPHYS.2021.715081
24
DordevićD.JančíkováS.VítězováM.KushkevychI. (2020). Hydrogen sulfide toxicity in the gut environment: meta-Analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res.27, 55–69. 10.1016/J.JARE.2020.03.003
25
FangY.LiZ.YangL.LiW.WangY.KongZ.et al (2024). Emerging roles of lactate in acute and chronic inflammation. Cell Commun. Signal.22 (22), 276–22. 10.1186/S12964-024-01624-8
26
FengT.WangJ. (2020). Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes12, 1801944. 10.1080/19490976.2020.1801944
27
FischerK.HoffmannP.VoelklS.MeidenbauerN.AmmerJ.EdingerM.et al (2007). Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood109, 3812–3819. 10.1182/BLOOD-2006-07-035972
28
GannesenA. V.ZdorovenkoE. L.BotchkovaE. A.HardouinJ.MassierS.KopitsynD. S.et al (2019). Composition of the biofilm matrix of Cutibacterium acnes acneic strain RT5. Front. Microbiol.10, 1284. 10.3389/fmicb.2019.01284
29
GaoH.LiX.ChenX.HaiD.WeiC.ZhangL.et al (2022). The functional roles of Lactobacillus acidophilus in different physiological and pathological processes. J. Microbiol. Biotechnol.32, 1226–1233. 10.4014/JMB.2205.05041
30
GhaziS.PoleselM.HallA. M. (2019). Targeting glycolysis in proliferative kidney diseases. Am. J. Physiol. Ren. Physiol.317, F1531-F1535–F1535. 10.1152/ajprenal.00460.2019
31
GladdenL. B. (2004). Lactate metabolism: a new paradigm for the third millennium. J. Physiology558, 5–30. 10.1113/JPHYSIOL.2003.058701
32
GladdenL. B.HoganM. C. (2006). Lactic acid accumulation is an advantage/disadvantage during muscle activity. J. Appl. Physiol.100, 2100–2101. 10.1152/japplphysiol.00213.2006
33
GuX. Y.YangJ. L.LaiR.ZhouZ. J.TangD.HuL.et al (2025). Impact of lactate on immune cell function in the tumor microenvironment: mechanisms and therapeutic perspectives. Front. Immunol.16, 1563303. 10.3389/FIMMU.2025.1563303
34
GuptaG. S. (2022). The lactate and the lactate dehydrogenase in inflammatory diseases and major risk factors in COVID-19 patients. Inflamm. 202245 (6), 2091–2123. 10.1007/S10753-022-01680-7
35
HanJ. H.LeeE. J.ParkW.HaK. T.ChungH. S. (2023). Natural compounds as lactate dehydrogenase inhibitors: potential therapeutics for lactate dehydrogenase inhibitors-related diseases. Front. Pharmacol.14, 1275000. 10.3389/FPHAR.2023.1275000
36
HashimotoT.BrooksG. A. (2008). Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med. Sci. Sports Exerc40, 486–494. 10.1249/MSS.0B013E31815FCB04
37
HassouniB.FranczakM.CapulaM.VonkC. M.GomezV. M.SmolenskiR. T.et al (2020). Lactate dehydrogenase A inhibition by small molecular entities: steps in the right direction. Oncoscience7, 76–80. 10.18632/ONCOSCIENCE.519
38
HinoT.KurodaS. (1993). Presence of lactate dehydrogenase and lactate racemase in Megasphaera elsdenii grown on glucose or lactate. Appl. Environ. Microbiol.59, 255–259. 10.1128/AEM.59.1.255-259.1993
39
HouK.WuZ. X.ChenX. Y.WangJ. Q.ZhangD.XiaoC.et al (2022). Microbiota in health and diseases. Signal Transduct. Target. Ther.7 (1), 135–28. 10.1038/s41392-022-00974-4
40
HuJ.CaiM.ShangQ.LiZ.FengY.LiuB.et al (2021). Elevated lactate by high-intensity interval training regulates the hippocampal BDNF expression and the mitochondrial quality control system. Front. Physiol.12, 629914. 10.3389/FPHYS.2021.629914
41
HuntT.BergstenJ.LevkanicovaZ.PapadopoulouA.St. JohnO.WildR.et al (2007). A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science1979 (318), 1913–1916. 10.1126/SCIENCE.1146954
42
IshitobiM.HosakaT.MoritaN.KondoK.MurashimaT.KitaharaA.et al (2019). Serum lactate levels are associated with serum alanine aminotransferase and total bilirubin levels in patients with type 2 diabetes mellitus: a cross-sectional study. Diabetes Res. Clin. Pract.149, 1–8. 10.1016/J.DIABRES.2019.01.028
43
JamesA.YaoT.KeH.WangY. (2023). Microbiota for production of wine with enhanced functional components. Food Sci. Hum. Wellness12, 1481–1492. 10.1016/J.FSHW.2023.02.008
44
JeppesenT. D.OrngreenM. C.Van HallG.VissingJ. (2013). Lactate metabolism during exercise in patients with mitochondrial myopathy. Neuromuscul. Disord.23, 629–636. 10.1016/J.NMD.2013.05.007
45
JunejaV. K.TanejaN. K.ThakurS. (2023). Clostridium perfringens infection. Encycl. Food Saf. (1–4), V2–V128. 10.1016/B978-0-12-822521-9.00089-7
46
KimE. Y.ChungT. W.HanC. W.ParkS. Y.ParkK. H.JangS. B.et al (2019). A novel lactate dehydrogenase inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci. Rep. 20199 (1), 3969–12. 10.1038/s41598-019-40617-3
47
KimM. J.JeongJ. Y.HwangI. M.LeeJ. H. (2025). Modulation of fermentation dynamics in kimchi using Leuconostoc mesenteroides starter. Food Biosci.66, 106317. 10.1016/J.FBIO.2025.106317
48
KiranD.BasarabaR. J. (2021). Lactate metabolism and signaling in tuberculosis and cancer: a comparative review. Front. Cell Infect. Microbiol.11, 624607. 10.3389/FCIMB.2021.624607
49
LeeT.-Y. (2021). Lactate: a multifunctional signaling molecule. Yeungnam Univ. J. Med.38, 183–193. 10.12701/YUJM.2020.00892
50
LiH. M.GuoH. L.XuC.LiuL.HuS. Y.HuZ. H.et al (2020). Inhibition of glycolysis by targeting lactate dehydrogenase A facilitates hyaluronan synthase 2 synthesis in synovial fibroblasts of temporomandibular joint osteoarthritis. Bone141, 115584. 10.1016/j.bone.2020.115584
51
LiX.YangY.ZhangB.LinX.FuX.AnY.et al (2022). Lactate metabolism in human health and disease. Signal Transduct. Target. Ther.7 (1), 305–322. 10.1038/s41392-022-01151-3
52
LiangZ.WangY.LaiY.ZhangJ.YinL.YuX.et al (2022). Host defense against the infection of klebsiella pneumoniae: new strategy to kill the bacterium in the era of antibiotics?Front. Cell Infect. Microbiol.12, 1050396. 10.3389/fcimb.2022.1050396
53
LiuC.WuJ.ZhuJ.KueiC.YuJ.SheltonJ.et al (2009). Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem.284, 2811–2822. 10.1074/jbc.M806409200
54
LiuH.WangS.WangJ.GuoX.SongY.FuK.et al (2025). Energy metabolism in health and diseases. Signal Transduct. Target Ther.10, 69. 10.1038/S41392-025-02141-X
55
LiuS.YangT.JiangQ.ZhangL.ShiX.LiuX.et al (2024). Lactate and lactylation in sepsis: a comprehensive review. J. Inflamm. Res.17, 4405–4417. 10.2147/JIR.S459185
56
LiuY.YuX.YuL.TianF.ZhaoJ.ZhangH.et al (2021). Lactobacillus plantarum CCFM8610 alleviates irritable bowel syndrome and prevents gut microbiota dysbiosis: a randomized, double-blind, placebo-controlled, pilot clinical trial. Engineering7, 376–385. 10.1016/J.ENG.2020.06.026
57
LlibreA.GrudzinskaF. S.O’SheaM. K.DuffyD.ThickettD. R.MauroC.et al (2021). Lactate cross-talk in host–pathogen interactions. Biochem. J.478, 3157–3178. 10.1042/BCJ20210263
58
LlibreA.KucukS.GopeA.CertoM.MauroC. (2025). Lactate: a key regulator of the immune response. Immunity58, 535–554. 10.1016/J.IMMUNI.2025.02.008
59
LouisP.DuncanS. H.SheridanP. O.WalkerA. W.FlintH. J. (2022). Microbial lactate utilisation and the stability of the gut microbiome. Gut Microbiome3, e3. 10.1017/GMB.2022.3
60
LuoS. C.WeiS. M.LuoX. T.YangQ. Q.WongK. H.CheungP. C. K.et al (2024). How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: an oral microbiota perspective. Biofilms Microbiomes10 (1), 14–15. 10.1038/s41522-024-00488-7
61
MandadzhievN. (2025). The contemporary role of lactate in exercise physiology and exercise prescription – a review of the literature. Folia Medica67 (1), e144693. 10.3897/FOLMED.67.E144693
62
MartínR.Rios-CovianD.HuilletE.AugerS.KhazaalS.Bermúdez-HumaránL. G.et al (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev.47, fuad039. 10.1093/FEMSRE/FUAD039
63
MelkonianE. A.AsukaE.SchuryM. P. (2023). Physiology, Gluconeogenesis. Treasure Island, FL: StatPearls Publishing. . Available online at: https://www.ncbi.nlm.nih.gov/books/NBK541119/.
64
MendoncaP.AlghamdiS.MessehaS.SolimanK. F. A. (2021). Pentagalloyl glucose inhibits TNF‐α‐activated CXCL1/GRO-α expression and induces apoptosis‐related genes in triple-negative breast cancer cells. Sci. Rep.11, 5649. 10.1038/S41598-021-85090-Z
65
MeyerC.StumvollM.DostouJ.WelleS.HaymondM.GerichJ. (2002). Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am. J. Physiol. Endocrinol. Metab.282, 428–434. 10.1152/AJPENDO.00116.2001
66
MilaniC.DurantiS.BottaciniF.CaseyE.TurroniF.MahonyJ.et al (2017). The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev.81, e00036-17. 10.1128/MMBR.00036-17
67
MoserC.JensenP. Ø.ThomsenK.KolpenM.RybtkeM.LaulandA. S.et al (2021). Immune responses to Pseudomonas aeruginosa biofilm infections. Front. Immunol.12, 625597. 10.3389/FIMMU.2021.625597
68
NalbandianH. M.RadakZ.TakedaM. (2017). Active recovery between interval bouts reduces blood lactate while improving subsequent exercise performance in trained men. Sports5, 40. 10.3390/SPORTS5020040
69
NareikaA.HeL.GameB. A.SlateE. H.SandersJ. J.LondonS. D.et al (2005). Sodium lactate increases LPS-Stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am. J. Physiol. Endocrinol. Metab.289, 534–542. 10.1152/ajpendo.00462.2004
70
NathS.BallingR. (2024). The warburg effect reinterpreted 100 yr on: a first-principles stoichiometric analysis and interpretation from the perspective of ATP metabolism in cancer cells. Function5, zqae008. 10.1093/FUNCTION/ZQAE008
71
NiuW.KramerL.MuellerJ.LiuK.GuoJ. (2019). Metabolic engineering of Escherichia coli for the de novo stereospecific biosynthesis of 1,2-propanediol through lactic acid. Metab. Eng. Commun.8, e00082. 10.1016/J.MEC.2018.E00082
72
OuyangJ.WangH.HuangJ. (2023). The role of lactate in cardiovascular diseases. Cell Commun. Signal21, 317. 10.1186/S12964-023-01350-7
73
PagliariniR.PodriniC. (2021). Metabolic reprogramming and reconstruction: integration of experimental and computational studies to set the path forward in ADPKD. Front. Med. (Lausanne)8, 740087. 10.3389/fmed.2021.740087
74
PellerinL.MagistrettiP. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U S A91, 10625–10629. 10.1073/PNAS.91.22.10625
75
PengT. Y.LuJ. M.ZhengX. L.ZengC.HeY. H. (2025). The role of lactate metabolism and lactylation in pulmonary arterial hypertension. Respir. Res.26, 99. 10.1186/S12931-025-03163-3
76
Penna-CoutinhoJ.CortopassiW. A.OliveiraA. A.FrançaT. C. C.KrettliA. U. (2011). Antimalarial activity of potential inhibitors of Plasmodium falciparum lactate dehydrogenase enzyme selected by docking studies. PLoS One6, e21237. 10.1371/JOURNAL.PONE.0021237
77
PetrariuO. A.BarbuI. C.NiculescuA. G.ConstantinM.GrigoreG. A.CristianR. E.et al (2023). Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol.14, 1296447. 10.3389/fmicb.2023.1296447
78
PortincasaP.KhalilM.GrazianiA.FrühbeckG.BaffyG.GarrutiG.et al (2024). Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations?Eur. J. Intern Med.119, 13–30. 10.1016/J.EJIM.2023.10.002
79
PossemiersH.VandermostenL.Van Den SteenP. E. (2021). Etiology of lactic acidosis in malaria. PLoS Pathog.17, e1009122. 10.1371/JOURNAL.PPAT.1009122
80
RaiG.UrbanD. J.MottB. T.HuX.YangS. M.BenavidesG. A.et al (2020). Pyrazole-based lactate dehydrogenase inhibitors with optimized cell activity and pharmacokinetic properties. J. Med. Chem.63, 10984–11011. 10.1021/ACS.JMEDCHEM.0C00916
81
RanganathanP.ShanmugamA.SwaffordD.SuryawanshiA.BhattacharjeeP.HusseinM. S.et al (2018). GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol.200, 1781–1789. 10.4049/JIMMUNOL.1700604
82
RastogiS.SinghA. (2022). Gut microbiome and human health: exploring how the probiotic genus lactobacillus modulate immune responses. Front. Pharmacol.13, 1042189. 10.3389/fphar.2022.1042189
83
ReichardtN.DuncanS. H.YoungP.BelenguerA.McWilliam LeitchC.ScottK. P.et al (2014). Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J.8, 1323–1335. 10.1038/ISMEJ.2014.14
84
RevellyJ. P.TappyL.MartinezA.BollmannM.CayeuxM. C.BergerM. M.et al (2005). Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit. Care Med.33, 2235–2240. 10.1097/01.CCM.0000181525.99295.8F
85
ReyF. E.GonzalezM. D.ChengJ.WuM.AhernP. P.GordonJ. I. (2013). Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. U S A110, 13582–13587. 10.1073/pnas.1312524110
86
RogatzkiM. J.FergusonB. S.GoodwinM. L.GladdenL. B. (2015). Lactate is always the end product of glycolysis. Front. Neurosci.9, 22. 10.3389/FNINS.2015.00022
87
RohY.LeeS. B.KimM.KimM. H.KimH. J.ChoK. O. (2023). Alleviation of hippocampal necroptosis and neuroinflammation by NecroX-7 treatment after acute seizures. Front. Pharmacol.14, 1187819. 10.3389/FPHAR.2023.1187819
88
SaidM. S.TirthaniE.LeshoE. (2024). Enterococcus infections. 1–15. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK567759/(Accessed April 25, 2025).
89
SaleemG. N.GuR.QuH.Bahar KhaskheliG.Rashid RajputI.QasimM.et al (2024). Therapeutic potential of popular fermented dairy products and its benefits on human health. Front. Nutr.11, 1328620. 10.3389/fnut.2024.1328620
90
SchurrA.MillerJ. J.PayneR. S.RigorB. M. (1999). An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J. Neurosci.19, 34–39. 10.1523/JNEUROSCI.19-01-00034.1999
91
SehgalK.KhannaS. (2021). Gut microbiome and Clostridioides difficile infection: a closer look at the microscopic interface. Ther. Adv. Gastroenterol.14, 1756284821994736. 10.1177/1756284821994736
92
ShenP.ZhangL.JiangX.RajR.YuB.ZhangJ. (2025). Polygala tenuifolia root extract attenuates ischemic stroke by inhibiting HMGB1 trigger neuroinflammation. Fitoterapia180, 106280. 10.1016/J.FITOTE.2024.106280
93
SinghR. P.ShadanA.MaY. (2022). Biotechnological applications of probiotics: a multifarious weapon to disease and metabolic abnormality. Probiotics Antimicrob. Proteins14 (6), 1184–1210. 10.1007/S12602-022-09992-8
94
SomiahT.GebremariamH. G.ZuoF.SmirnovaK.JonssonA. B. (2022). Lactate causes downregulation of Helicobacter pylori adhesin genes sabA and labA while dampening the production of proinflammatory cytokines. Sci. Rep.12, 20064–13. 10.1038/S41598-022-24311-5
95
StevensD. L.BryantA. E. (2022) “Streptococcus pyogenes impetigo, erysipelas, and cellulitis,” in Streptococcus pyogenes: basic biology to clinical manifestations. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK587091/(Accessed April 25, 2025).
96
SuzukiA.SternS. A.BozdagiO.HuntleyG. W.WalkerR. H.MagistrettiP. J.et al (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell144, 810–823. 10.1016/j.cell.2011.02.018
97
TakedaR.NonakaY.KakinokiK.MiuraS.KanoY.HoshinoD. (2022). Effect of endurance training and PGC-1α overexpression on calculated lactate production volume during exercise based on blood lactate concentration. Sci. Rep.12, 1635. 10.1038/S41598-022-05593-1
98
TarnopolskyM. A. (2018). Myopathies related to glycogen metabolism disorders. Neurotherapeutics15, 915–927. 10.1007/S13311-018-00684-2
99
TaylorT. A.UnakalC. G. (2023). Staphylococcus aureus infection. Encycl. Food Saf. (1–4), V2–V310. 10.1016/B978-0-12-822521-9.00048-4
100
TodorovS. D.DiosoC. M.LiongM. T.NeroL. A.Khosravi-DaraniK.IvanovaI. V. (2022). Beneficial features of pediococcus: from starter cultures and inhibitory activities to probiotic benefits. World J. Microbiol. Biotechnol.39, 4. 10.1007/S11274-022-03419-W
101
TripathiA.PandeyV. K.PanesarP. S.TaufeeqA.MishraH.RustagiS.et al (2024). Fermentative production of vitamin B12 by propionibacterium shermanii and Pseudomonas denitrificans and its promising health benefits: a review. Food Sci. Nutr.12, 8675–8691. 10.1002/FSN3.4428
102
TufailM. A.SchmitzR. A. (2024). Exploring the probiotic potential of bacteroides spp. within one health paradigm. Probiotics Antimicrob. Proteins17 (2), 681–704. 10.1007/S12602-024-10370-9
103
ValvonaC. J.FillmoreH. L.NunnP. B.PilkingtonG. J. (2016). The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol.26, 3–17. 10.1111/BPA.12299
104
VavřičkaJ.BrožP.FollprechtD.NovákJ.KroužeckýA. (2024). Modern perspective of lactate metabolism. Physiol. Res.73, 499–514. 10.33549/PHYSIOLRES.935331
105
Victoria ObayomiO.Folakemi OlaniranA.Olugbemiga OwaS. (2024). Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: a review. J. Funct. Foods119, 106337. 10.1016/J.JFF.2024.106337
106
Vieco-SaizN.BelguesmiaY.RaspoetR.AuclairE.GancelF.KempfI.et al (2019). Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol.10, 57. 10.3389/fmicb.2019.00057
107
WangF. X.MuG.YuZ. H.ShiZ. A.LiX. X.FanX.et al (2025). Lactylation: a promising therapeutic target in ischemia-reperfusion injury management. Cell Death Discov.11, 100. 10.1038/S41420-025-02381-4
108
WangS. P.RubioL. A.DuncanS. H.DonachieG. E.HoltropG.LoG.et al (2020). Pivotal roles for pH, lactate, and lactate-utilizing bacteria in the stability of a human colonic microbial ecosystem. mSystems5, e00645-20–20. 10.1128/MSYSTEMS.00645-20
109
WangY.HuangY.YangJ.ZhouF. Q.ZhaoL.ZhouH. (2018). Pyruvate is a prospective alkalizer to correct hypoxic lactic acidosis. Mil. Med. Res.5, 13. 10.1186/S40779-018-0160-Y
110
WenH.DengH.LiB.ChenJ.ZhuJ.ZhangX.et al (2025). Mitochondrial diseases: from molecular mechanisms to therapeutic advances. Signal Transduct. Target. Ther.10 (1), 9–54. 10.1038/s41392-024-02044-3
111
WhelessJ.WeatherspoonS. (2025). Use of stiripentol in Dravet syndrome: a guide for clinicians. Pediatr. Neurol.162, 76–86. 10.1016/J.PEDIATRNEUROL.2024.10.015
112
WuY.DongY.AtefiM.LiuY.ElshimaliY.VadgamaJ. V. (2016). Lactate, a neglected factor for diabetes and cancer interaction. Mediat. Inflamm.2016, 6456018. 10.1155/2016/6456018
113
YangC.PanR.-Y.GuanF.YuanZ. (2024). Lactate metabolism in neurodegenerative diseases. Neural Regen. Res.19, 69–74. 10.4103/1673-5374.374142
114
YaoH.YangF.LiY. (2022). Natural products targeting human lactate dehydrogenases for cancer therapy: a mini review. Front. Chem.10, 1013670. 10.3389/fchem.2022.1013670
115
YuJ.LiL.TaoX.ChenY.DongD. (2024). Metabolic interactions of host-gut microbiota: new possibilities for the precise diagnosis and therapeutic discovery of gastrointestinal cancer in the future—A review. Crit. Rev. Oncol. Hematol.203, 104480. 10.1016/J.CRITREVONC.2024.104480
116
ZhangD.TangZ.HuangH.ZhouG.CuiC.WengY.et al (2019). Metabolic regulation of gene expression by histone lactylation. Nature574, 575–580. 10.1038/s41586-019-1678-1
117
ZhangF.LiH.LiuC.FangK.JiangY.WuM.et al (2022). Lactate dehydrogenase-inhibitors isolated from ethyl acetate extract of Selaginella doederleinii by using a rapid screening method with enzyme-immobilized magnetic nanoparticles. Front. Biosci. - Landmark27, 229. 10.31083/j.fbl2708229
118
ZhangH.ZhaoJ.YuJ.ZhangX.RanS.WangS.et al (2024a). Lactate metabolism and lactylation in cardiovascular disease: novel mechanisms and therapeutic targets. Front. Cardiovasc Med.11, 1489438. 10.3389/fcvm.2024.1489438
119
ZhangT.ChenL.KuethG.ShaoE.WangX.HaT.et al (2024b). Lactate’s impact on immune cells in sepsis: unraveling the complex interplay. Front. Immunol.15, 1483400. 10.3389/FIMMU.2024.1483400
120
ZhaoS.LauR.ZhongY.ChenM. H. (2024). Lactate cross-feeding between bifidobacterium species and Megasphaera indica contributes to butyrate formation in the human colonic environment. Appl. Environ. Microbiol.90, e0101923. 10.1128/AEM.01019-23
121
ZhongX.HeX.WangY.HuZ.HuangH.ZhaoS.et al (2022). Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. & Oncol.15 (15), 160–29. 10.1186/S13045-022-01358-5
122
ZhuW.GuoS.SunJ.ZhaoY.LiuC. (2024). Lactate and lactylation in cardiovascular diseases: current progress and future perspectives. Metabolism158, 155957. 10.1016/J.METABOL.2024.155957
123
ZymlińskiR.BiegusJ.SokolskiM.SiwołowskiP.Nawrocka-MillwardS.ToddJ.et al (2018). Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail20, 1011–1018. 10.1002/EJHF.1156
Summary
Keywords
lactate, lactate dehydrogenase, cancer, metabolic disorders, gut microbiota
Citation
Kumar S, Sahu N, Jawaid T, Jayasingh Chellammal HS and Upadhyay P (2025) Dual role of lactate in human health and disease. Front. Physiol. 16:1621358. doi: 10.3389/fphys.2025.1621358
Received
02 May 2025
Accepted
18 July 2025
Published
01 August 2025
Volume
16 - 2025
Edited by
Charles Mobbs, Icahn School of Medicine at Mount Sinai, United States
Reviewed by
Elen H. Miyabara, University of São Paulo, Brazil
John Andrés Quiroga Ardiles, Universidad Austral de Chile, Chile
Updates
Copyright
© 2025 Kumar, Sahu, Jawaid, Jayasingh Chellammal and Upadhyay.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prabhat Upadhyay, pupadhyay@mgh.harvard.edu, upadhyayprabhat.89@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.