- 1Reproductive Genetics Department, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 2Department of Reproductive Medicine, Guangzhou Women and Children’s Medical Center Liuzhou Hospital, Liuzhou, Guangxi, China
- 3Department of Gynecology, The First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Objective: To systematically evaluate whether the melatonin supplementation could improve the embryo development and pregnancy outcomes of infertile women undergoing assisted reproductive technologies (ART).
Methods: This systematic review and meta-analysis followed the PRISMA guidelines and was prospectively registered in PROSPERO (CRD420251003042). The randomized controlled trials (RCTs) published before March 5, 2025 are included to evaluate the efficacy of melatonin on infertile women undergoing ART. Eligible studies reported at least one embryo development or pregnancy-related outcome. Primary outcome was clinical pregnancy rate; secondary outcomes including oocyte yield, fertilization rate, MII oocyte number, and high-quality embryo formation. Subgroup analyses were conducted based on stimulation protocols, melatonin dosage, and population characteristics. Risk of bias was assessed using the Cochrane Risk of Bias tool, and pooled effect sizes were calculated using fixed- or random-effects models depending on heterogeneity. Totally, eleven RCTs with a total of 1,481 participants were analyzed here.
Data sources: PubMed/MEDLINE, Embase, and Cochrane Library.
Results: Melatonin supplementation significantly improved clinical pregnancy rate (OR = 1.59, 95% CI: 1.22–2.07). Regarding embryo development, melatonin significantly increased the number of high-quality embryos (MD = 0.43, 95% CI: 0.07–0.79), MII oocyte (SMD=0.99, 95% CI: 0.29–1.69), and fertilization rates (OR = 1.32, 95% CI: 1.01–1.73). No significant difference was observed in oocyte yield (SMD = 0.45, 95% CI: −0.04 to 0.94). Subgroup analysis revealed enhanced clinical pregnancy outcomes with ≤3 mg/day melatonin and under GnRH-a long protocols. Moderate to high heterogeneity was observed in some secondary outcomes, with publication bias suggested for the MII oocyte outcome.
Conclusions: Melatonin supplementation may improve intermediate outcomes such as fertilization, embryo quality, and clinical pregnancy rates in women undergoing ART. With a favorable safety profile, it could be a low-cost adjunct for selected patients, though standardized guidelines are lacking and large-scale RCTs are needed to clarify long-term effects.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD420251003042, PROSPERO CRD420251003042.
Introduction
Infertility has become an increasingly prevalent global public health concern in recent years. According to the data of World Health Organization (WHO), approximately 10% to 15% of couples with reproductive age worldwide are affected by infertility (1). Beyond its physiological implications for women, infertility also imposes significant psychological, social, and familial burdens (2, 3). With ongoing advancements in assisted reproductive technologies (ART), in vitro fertilization (IVF) has become the cornerstone of infertility treatment. Current strategies primarily aim to improve embryo quality and optimize endometrial receptivity (4, 5). However due to the delayed childbearing, high levels of psychological stress, mental health issues including anxiety, insomnia, and depression, which could cause the the poor oocyte quality with the higher level of reactive oxygen species (ROS) and might cause a plausible poor oocyte quality, lower fertilization and pregnancy rates, and failed ART (6). Further enhancement of embryo viability and pregnancy outcomes remains a major challenge in reproductive medicine. As such, the development of safe and effective adjunctive interventions has emerged as a critical research priority.
Melatonin, an indoleamine hormone secreted by the pineal gland, is well known for regulating circadian rhythms and exerting potent antioxidant effects (7, 8). As an antioxidant, melatonin has been proposed as a promising therapeutic agent for improving oocyte quality and reproductive outcomes. It is thought to mitigate oxidative stress in the ovaries, thereby enhancing the quality of oocytes (9). In ovarian granulosa cells, melatonin has also been shown to inhibit autophagy via modulating of signaling pathways such as PI3K–Akt–mTOR, offer further cytoprotective effects (10).
Although several randomized controlled trials (RCTs) have demonstrated that melatonin supplementation might increase the proportion of metaphase II (MII) oocytes of ART (11), enhance endometrial thickness and clinical pregnancy rates (12), and reduce oxidative stress in follicular fluid (13), however, other study failed to observe significant improvements in clinical pregnancy or live birth rates (11). These inconsistencies may be due to melatonin dosage, treatment duration, or the concomitant use of adjunctive agents such as myo-inositol, thus which limited cross-study comparability and generalizability (14–16). Despite some encouraging findings, current evidences on the efficacy of melatonin in ART are remain inconsistent. Yet, several meta-analyses also have yielded conflicting results, while one analysis suggested improvements in clinical pregnancy rate and oocyte quality without significant benefit for live birth rates (17), another work highlighted considerable uncertainty across key outcomes (18), Mejlhede et al. (6) observed the increased mature oocyte counts with melatonin, however, the clinical pregnancy rates did not significantly improve.
These discrepancies underscore the necessary for an updated and methodologically rigorous meta-analysis. Previous reviews have been restricted by the limited available articles, incomplete outcome reporting, and insufficient heterogeneity assessment. Therefore, the present study aims to provide a comprehensive synthesis of the evidence through a systematic review and meta-analysis, evaluating the impact of melatonin on embryo development and pregnancy outcomes in women undergoing ART. Additionally, this study seeks to investigate potential effect modifiers, such as melatonin dosage, patient characteristics, and COS protocols, thereby offering more clinically actionable insights.
Materials and methods
This review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was prospectively registered in the PROSPERO database (registration number: CRD420251003042). We included all randomized controlled trials (RCTs) that evaluated the effects of melatonin on oocyte quality and pregnancy outcomes in women with infertility, and reported at least one embryo- or pregnancy-related outcome. Eligible participants were women of any age undergoing assisted reproductive treatment and receiving melatonin supplementation. The primary outcome was clinical pregnancy rate. Secondary outcomes included the number of oocytes retrieved, fertilization rate or fertilized oocytes, and the rate or number of high-quality embryos or blastocysts. Studies were excluded if they were quasi-randomized trials, cohort studies, case-control studies, case reports, animal or in vitro experiments, or if they lacked sufficient statistical data for the outcomes of interest. In some instances, investigators discussed outcomes in their conclusions without providing extractable data; in such cases, we cited the original interpretation but did not include it in the pooled analysis. A comprehensive search was performed in PubMed/MEDLINE, Cochrane Library, and Embase for studies published in English up to March 5, 2025. The search strategy included combinations of the following keywords: “melatonin”, “subfertility”, “fertilization in vitro”, “premature ovarian failure”, and “ovarian reserve”. The complete search terms are provided in Supplementary Table S1.
Study selection and data collection process
Two reviewers (W.Y. and H.W.) independently screened the titles and abstracts of all retrieved articles. Studies deemed irrelevant by both reviewers were excluded. The screening process were blinded to author names, institutional affiliations, journal titles, and study outcomes. Any disagreements were resolved through discussion with a third reviewer. Subsequently, four reviewers (W.Y., H.W., C.H., and W.H.) independently assessed the full texts of potentially eligible articles according to the predefined inclusion criteria and evaluated their methodological validity. All extracted data were cross-checked for accuracy, and discrepancies were resolved by consensus.
The extracted data included: first author, year of publication, sample size, number of participants in each group, stimulation protocols, intervention details, control measures, and reported outcomes. These characteristics were summarized in a comparative table (Table 1) for ease of cross-study comparison.
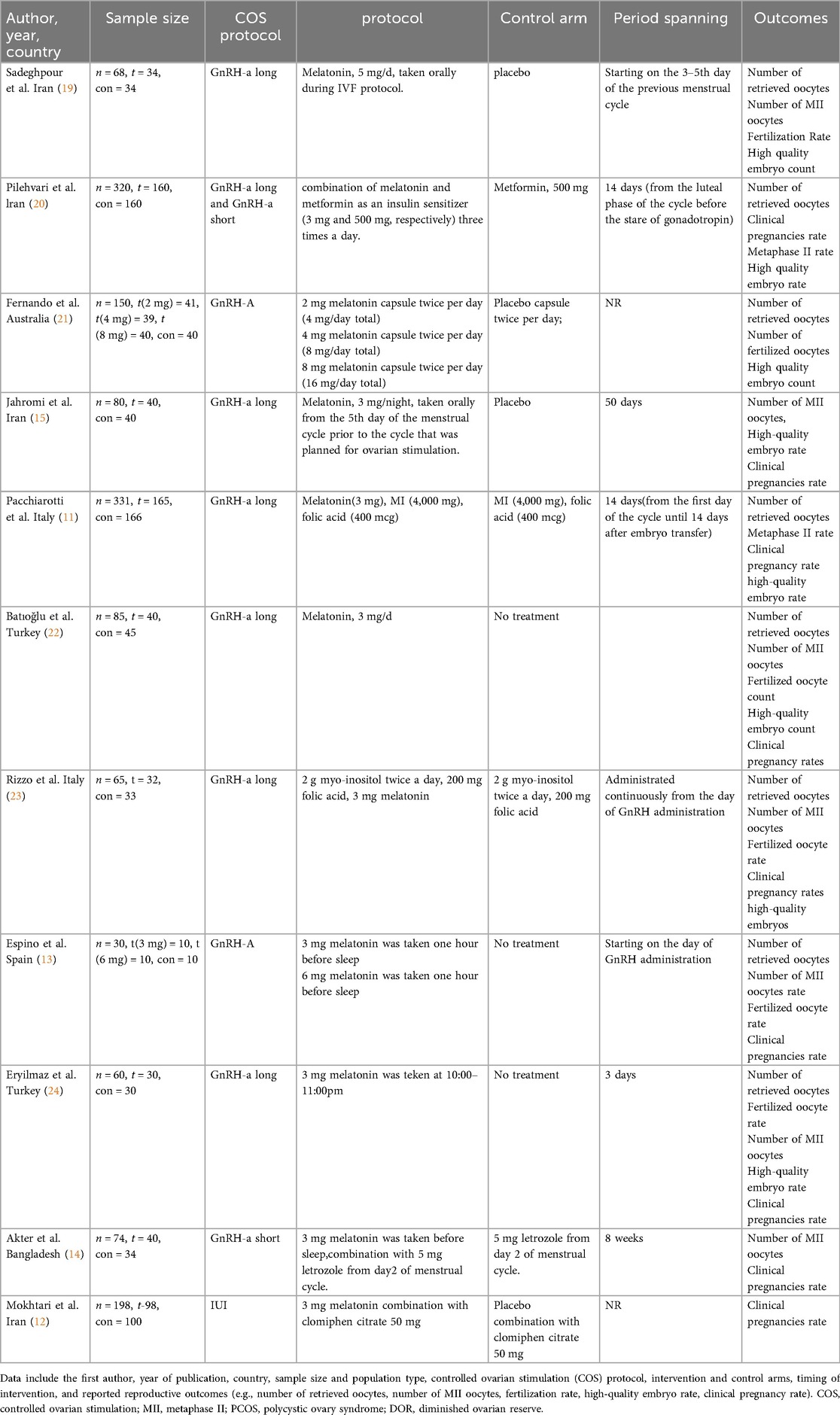
Table 1. Characteristics of the included studies on melatonin supplementation in assisted reproduction.
Risk of bias and quality assessment
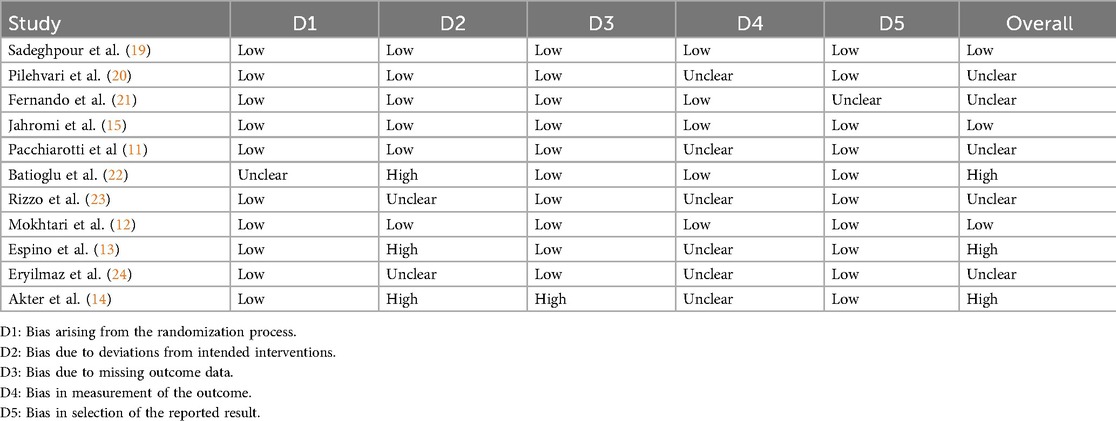
The risk of bias was independently assessed by four reviewers (W.Y., H.W., C.H., and W.H.) using the Cochrane Handbook methodology and the Cochrane Risk of Bias Tool. The assessment covered the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other potential sources of bias. Each domain was rated as having a “low”, “high”, or “unclear” risk of bias. Based on the overall assessment across these domains, studies were categorized as having low, unclear, or high overall risk of bias.
Statistical analysis
Statistical analyses were performed using the meta package in R (version 4.4.2). Heterogeneity was assessed using Cochran's Q test and the I² statistic. An I2 > 50% or a p-value < 0.05 was considered indicative of substantial heterogeneity. When heterogeneity was present, subgroup analyses and meta-regression were conducted to explore potential sources, such as variation in stimulation protocols or participant characteristics. Funnel plots were used to qualitatively evaluate publication bias. Sensitivity analyses were performed to assess the robustness of the pooled results. Both random-effects and fixed-effects models were applied as appropriate, with the random-effects model used for the primary analysis in the presence of heterogeneity.
Subgroup analyses were conducted based on stimulation protocols, melatonin dosage, and participant characteristics, including diminished ovarian reserve (DOR), polycystic ovary syndrome (PCOS), and infertility type. For each subgroup, we reported effect estimates (odds ratios [OR], mean differences [MD], or standardized mean differences [SMD]), along with 95% confidence intervals and I² statistics. Sensitivity analyses were conducted by excluding studies with a high risk of bias or those that had a disproportionate influence on the overall estimates.
Results
Study selection
A total of 178 records were retrieved from PubMed with the process described in Figure 1, Embase, and the Cochrane Library. After automatic deduplication using Endnote X9, 32 additional duplicate records were manually removed. Title and abstract screening excluded 99 clearly irrelevant studies. Full texts of 47 potentially eligible articles were assessed. Among them, 3 literatures belonged to meta-analyses works, 1 literature was assigned to narrative review, 28 papers were excluded due to non-RCT designs, and 11 works were removed for lacking relevant outcome data. Ultimately, 8 studies met the predefined inclusion criteria were analyzed here. Additionally, 3 eligible studies were identified through citation tracking of existing reviews and meta-analyses, resulting in a final total of 11 studies included in the quantitative synthesis (11–15, 19–24).
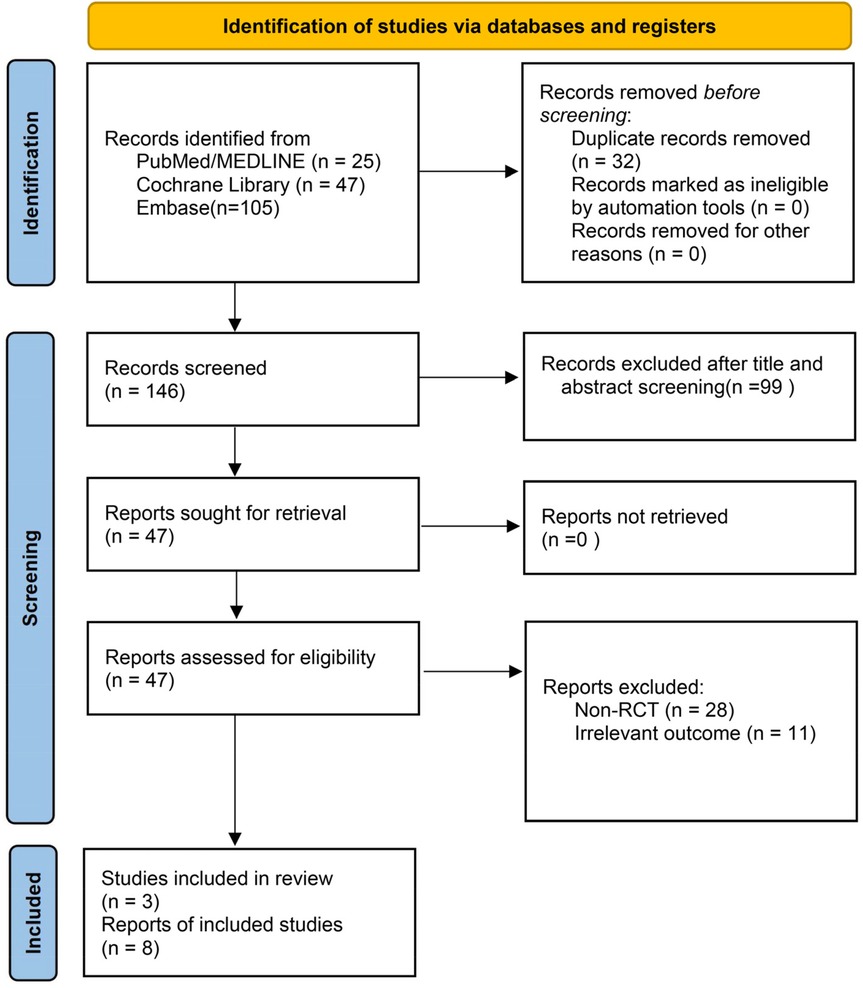
Figure 1. PRISMA flow chart for a systematic review and meta-analysis of clinical pregnancy rate following melatonin treatment in women undergoing assisted reproduction.
The risk of bias assessment is presented in Supplementary Figure S1 and Table 2. All included studies were evaluated using the Cochrane Risk of Bias Tool. Most studies demonstrated low risk in random sequence generation and allocation concealment. However, three studies exhibited potential bias in the blinding of participants, personnel, and outcome assessment. As a result, the overall risk of bias across studies was judged to be moderate. Notably, three studies were categorized as high risk, primarily due to inadequate blinding procedures and insufficient outcome assessment measures. Table 1 summarizes the key characteristics of the included studies, including sample size, controlled ovarian stimulation (COS) protocols, study design, control group details, and treatment duration.
Clinical pregnancy rate
Figure 2 presents the meta-analysis of clinical pregnancy rate. Nine studies involving a total of 1,235 participants (613 in the melatonin group and 622 in the control group) were included. The pooled analysis showed that melatonin supplementation significantly improved clinical pregnancy rates by comparing that with control group (OR = 1.59, 95% CI: 1.22–2.07), reflecting a statistically significant 59% increase in the odds of clinical pregnancy. Heterogeneity was negligible (I2 = 0.0%, p = 0.8307), supporting the use of a fixed-effects model. Visual inspection of the funnel plot did not suggest substantial publication bias (Supplementary Figure S3E).
![Forest plot of a meta-analysis showing odds ratios and confidence intervals for nine studies comparing experimental and control groups. Studies listed include Pilehvari 2023 to Akter 2022. Diamonds represent common and random effects models with odds ratio of 1.59, 95% CI [1.22; 2.07], and weights. Heterogeneity is low with \\(I^2 = 0.0\\%\\) and \\(p = 0.8307\\).](https://www.frontiersin.org/files/Articles/1680984/frph-07-1680984-HTML/image_m/frph-07-1680984-g002.jpg)
Figure 2. Forest plot of odds ratios (OR) for clinical pregnancy rate following melatonin treatment in assisted reproduction. The plot summarizes the results of individual studies and the overall effect estimate using both common and random effects models.
Number of retrieved oocytes
Eight studies (n = 983) reported on the number of oocytes retrieved. Pooled analysis showed no significant difference between the melatonin and control groups (SMD = 0.45, 95% CI: −0.04 to 0.94; random-effects model). Substantial heterogeneity was observed (I2 = 86.1%, p < 0.0001) (Figure 3A). Sensitivity analysis identified the study by Sadeghpour et al. (19) as a major source of heterogeneity. Excluding this study reduced the effect size to SMD = 0.17 (95% CI: −0.04 to 0.38) and decreased heterogeneity (I2 = 38.0%, p = 0.1051) (Supplementary Figure S2A). Visual inspection of the funnel plot did not suggest substantial publication bias (t = 1.57, p = 0.1515) (Supplementary Figures S3A, S4A).
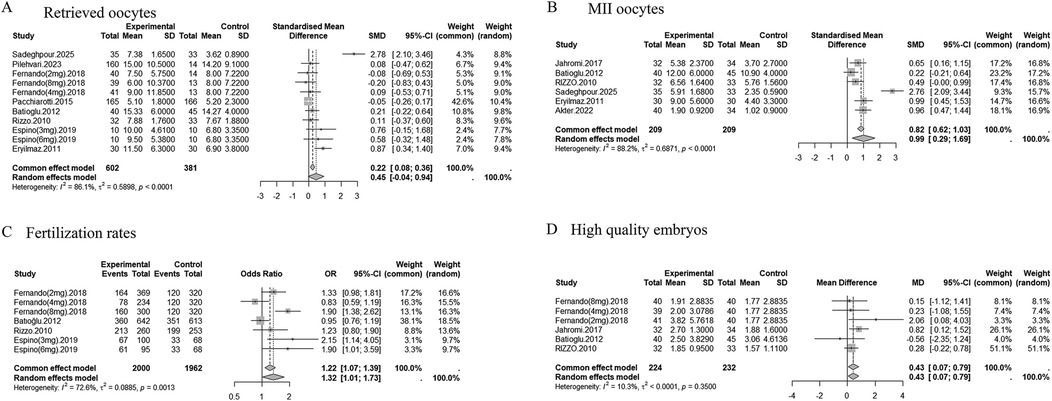
Figure 3. Forest plots of the effects of melatonin treatment on oocyte and embryo outcomes in assisted reproduction. (A) Number of retrieved oocytes (SMD). (B) Number of MII (metaphase II) oocytes (SMD). (C) Number of fertilized oocytes (OR). (D) Number of high-quality embryos (MD). The results are presented with 95% confidence intervals (CI) under a random effects model.
Number of MII oocytes
Six studies (n = 418) reported MII oocyte counts. Melatonin supplementation significantly increased the number of MII oocytes (SMD = 0.99, 95% CI: 0.29 to 1.69; random-effects model), though heterogeneity was high (I2 = 88.2%, p < 0.0001) (Figure 3B). When high risk-of-bias studies were excluded, the effect remained significant (SMD = 1.21, 95% CI: 0.20–2.21), but heterogeneity persisted (I2 = 90.7%) (Supplementary Table S5). Excluding the Sadeghpour study reduced the effect to SMD = 0.63 (95% CI: 0.41 to 0.84) and heterogeneity to I2 = 45.5% (p = 0.1188) (Supplementary Figure S2B). Visual inspection of the funnel plot suggested potential publication bias, particularly before exclusion of the Sadeghpour study (Supplementary Figure S3B).
Fertilization rate
Four studies (n = 275) evaluated fertilization rate. Meta-analysis showed that melatonin significantly improved fertilization outcomes (OR = 1.32, 95% CI: 1.01 to 1.73; random-effects model). Moderate heterogeneity was detected (I2 = 72.6%, p = 0.0013) (Figure 3C). After excluding high risk-of-bias studies, the effect was attenuated and became non-significant (OR = 1.28, 95% CI: 0.91–1.80), with heterogeneity remaining moderate (I2 = 74.2%) (Supplementary Table S5). Stratified analyses suggested that COS protocols contributed to heterogeneity, with I2 reduced to 3.9% in specific subgroups. Visual inspection of the funnel plot did not indicate substantial publication bias (Supplementary Figure S3C).
Number of high-quality embryos
Four studies (n = 456) reported high-quality embryo counts. Melatonin significantly increased the number of high-quality embryos (MD = 0.43, 95% CI: 0.07 to 0.79; fixed-effects model). Heterogeneity was low (I2 = 10.3%, p = 0.3500) (Figure 3D). Visual inspection of the funnel plot did not suggest substantial publication bias (Supplementary Figure S3D).
Subgroup analyses of supplement melatonin additionally
Multidimensional subgroup analyses were performed to explore sources of heterogeneity (Supplementary Tables S2–S4). Stratification by COS protocol showed that melatonin significantly improved clinical pregnancy rates in patients receiving long GnRH agonist protocols (OR = 1.41, 95% CI: 1.04–1.90) and in IUI populations (OR = 2.17, 95% CI: 1.06–4.11). However, oocyte-related outcomes remained highly heterogeneous (I2 > 90%) within the long GnRH-a subgroup. Dose-based analysis revealed that ≤3 mg melatonin significantly improved clinical pregnancy (OR = 1.59, 95% CI: 1.22–2.07) and high-quality embryo formation (MD = 0.48, 95% CI: 0.09–0.87), with low heterogeneity. By contrast, studies using doses >3 mg showed considerable variability (I2 > 80%) and yielded inconsistent results, though the limited number of such trials and differences in study design may partly explain these findings. Additionally, analysis stratified by patient population showed that melatonin had the most consistent benefits in women with PCOS and diminished ovarian reserve (DOR), particularly for clinical pregnancy, oocyte quality, and embryo outcomes.
Discussion
This systematic review included 11 randomized controlled trials (RCTs) encompassing a total of 1,481 infertile women and evaluated the efficacy of melatonin supplementation in assisted reproductive technology (ART). Our meta-analysis demonstrated that melatonin significantly improves clinical pregnancy rates (OR = 1.59, 95% CI: 1.22–2.07) and the number of high-quality embryos (MD = 0.43, 95% CI: 0.07–0.79). These findings support its potential clinical utility in enhancing ART outcomes. While an upward trend was observed in the number of retrieved oocytes, the difference did not reach statistical significance. This discrepancy may be attributed to methodological heterogeneity, including variations in trial design, patient demographics, and melatonin dosing regimens.
Melatonin, a neuroendocrine hormone, is well known for its roles in circadian rhythm regulation, antioxidant defense, and anti-aging mechanisms (25, 26). These physiological functions provide a compelling rationale for its use in ART. Notably, prior studies have highlighted the associations between sleep quality, oxidative stress, and reproductive outcomes. Improved sleep has been linked to increased clinical pregnancy rates (27), while antioxidant supplementation has been shown to enhance oocyte yield and embryo quality, and reduce gonadotropin requirements—ultimately improving ART efficacy (28).
Building upon this mechanistic foundation, our meta-analysis confirms and extends previous evidence supporting melatonin's reproductive benefits in ART contexts (6, 17). Specifically, we observed a pooled odds ratio for clinical pregnancy (OR = 1.59) that exceeds that reported by Hu et al. (OR = 1.43) (17), likely reflecting the inclusion of more recent trials and stringent methodological criteria. In contrast to prior reviews that reported significant increases in oocyte yield (e.g., MD = 0.98, 95% CI: 0.52–1.44) (17, 28), our analysis did not find a statistically significant effect of melatonin on this outcome. This divergence may be due to the inclusion of multi-ingredient antioxidant supplements in earlier studies (e.g., combinations with myo-inositol, folic acid, or selenium), which confounded the specific contribution of melatonin (29). To address this, we included only trials evaluating melatonin as a standalone intervention, thereby improving the precision of our effect estimates. Subgroup analyses suggested that lower doses (≤3 mg/day) of melatonin consistently improved clinical pregnancy rates and embryo quality. However, the apparent inferiority of higher doses should be interpreted cautiously, as it may reflect differences in study design or patient populations rather than a true biological threshold. Evidence from other fields reinforces this caution: while animal models show broad protective effects (30, 31), clinical data in humans are less consistent. At higher doses, melatonin remains generally safe but has been associated with increased adverse events such as drowsiness, headache, and dizziness (32), and in some contexts even retinal damage (33). These observations suggest that variability at higher doses may relate not only to study design, but also to non-linear pharmacodynamics and tolerability issues in humans.
Melatonin exerts multiple physiological actions that may underlie its reproductive benefits. As a potent endogenous antioxidant, it mitigates mitochondrial reactive oxygen species (ROS) accumulation, which is critical for maintaining oocyte and embryo quality (34). Melatonin activates the Nrf2 signaling pathway, upregulating antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), thereby reducing oxidative damage and apoptosis, particularly in porcine and murine aging oocytes (35, 36). Endocrinologically, melatonin may enhance luteal phase support by modulating progesterone production and delaying luteolysis, thereby improving sheep embryo implantation and survival (37). Indeed, recent evidence indicates that pharmacological interventions and luteal support strategies during transfer cycles can significantly affect outcomes, further underscoring the potential endocrine relevance of melatonin (38, 39).
Beyond clinical data, several mechanisms have been demonstrated only in preclinical models. In animal models, it has also been shown to influence early embryonic development via regulation of IGF-1 and pineal–gonadal axis signaling (40). From a cellular perspective, melatonin promotes cytoplasmic maturation by optimizing the spatial arrangement of intracellular organelles such as the Golgi apparatus and endoplasmic reticulum, ultimately facilitating fertilization and blastocyst development (41). At the molecular level, melatonin modulates key signaling cascades such as the PI3K–AKT–FOXO3 pathway, delaying follicular activation and ovarian aging (42). In PCOS models, it has also been shown to improve uterine redox balance and energy metabolism, further supporting fertility (43).
This review offers several methodological strengths. We conducted a comprehensive literature search, included only RCTs, and applied rigorous criteria for data extraction and synthesis. Outcomes assessed encompassed both clinical endpoints (e.g., pregnancy rates) and laboratory markers (e.g., MII oocyte count, embryo quality). Random-effects models were employed to account for between-study heterogeneity, and sensitivity analyses confirmed the robustness of our findings. Publication bias appeared minimal, based on visual inspection of funnel plots.
Nonetheless, limitations should be acknowledged. Several included trials had small sample sizes, and outcomes such as fertilization rate and high-quality embryo count were reported in a limited subset of studies, increasing the risk of imprecision. Moreover, residual confounding from factors such as age, ovarian reserve, endocrine status, and lifestyle cannot be ruled out. Moreover, residual confounding from factors such as age, ovarian reserve, endocrine status, and lifestyle cannot be ruled out (44, 45). Beyond these factors, baseline uterine characteristics—such as endometrial receptivity and structural factors—can also materially influence implantation and ART success, as highlighted in recent studies (46, 47). In addition, evidence on the long-term or high-dose use of melatonin in reproductive-age women is limited, and potential cumulative effects remain uncertain. Importantly, live birth and ongoing pregnancy outcomes, which are more patient-relevant than clinical pregnancy, were rarely reported across the included trials, thereby limiting the clinical interpretability of our findings. Publication bias assessment was also limited, as several endpoints included fewer than 10 studies and thus relied mainly on visual inspection of funnel plots. Additionally, sensitivity analyses revealed that the pooled results for some endpoints were influenced by individual studies, such as the trial by Sadeghpour et al. (19) and by high risk-of-bias trials, particularly for MII oocytes and fertilization, underscoring the need for further large-scale, high-quality RCTs.
Conclusion
This meta-analysis consolidates evidence on melatonin's potential to improve intermediate reproductive outcomes, including fertilization, embryo quality, and clinical pregnancy rates, among women undergoing ART. However, evidence regarding definitive outcomes such as live birth and miscarriage remains insufficient. With a favorable safety profile, melatonin could be a low-cost adjunct, particularly for patients with diminished ovarian reserve or oxidative stress-related infertility, though standardized guidelines are lacking. Large-scale RCTs are needed to define optimal regimens, identify responsive subgroups, and clarify its impact on long-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
YW: Writing – original draft, Writing – review & editing, Formal analysis. WH: Conceptualization, Writing – review & editing, Data curation, LT: Writing – review & editing. YF: Writing – review & editing. HC: Writing – review & editing. MP: Writing – review & editing. JP: Writing – review & editing. CL: Writing – review & editing. HW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Applied Basic Research Joint Project of Yunnan Province Science and Technology Department and Kunming Medical University (No. 202301AY070001-087); the First-Class Discipline Team of Kunming Medical University (2024XKTDTS02); the Medical Youth Reserve Talent Training Program of the Health Commission of Guangxi Zhuang Autonomous Region (Gui Wei Ren Fa (2025) No. 5); and the Fund of Kunming Medical University First Affiliated Hospital Textbook Construction Cultivation Project (Grant Number: 2025-JC-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2025.1680984/full#supplementary-material
References
1. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. (2012) 9(12):e1001356. doi: 10.1371/journal.pmed.1001356
2. Hyun JY, Jung HS, Park JY. Herbal therapeutics for female infertility: a systematic review and meta-analysis. J Ethnopharmacol. (2024) 319(Pt 2):117258. doi: 10.1016/j.jep.2023.117258
3. Addisu D, Mekuriaw BY, Berihun Erega B, Yazie Ferede W, Mihretie GN, Dagnew E, et al. Factors associated with female infertility in Ethiopia: a systematic review and meta-analysis. PLoS One. (2025) 20(5):e0323181. doi: 10.1371/journal.pone.0323181
4. Jones BP, Bracewell-Milnes T, Kasaven L, L'Heveder A, Spearman M, Marcus D, et al. Pre-implantation genetic testing for aneuploidy: motivations, concerns, and perceptions in a UK population. J Assist Reprod Genet. (2021) 38(8):1987–96. doi: 10.1007/s10815-021-02130-3
5. Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, González-Foruria I, et al. Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod. (2021) 36(6):1552–60. doi: 10.1093/humrep/deab031
6. Mejlhede MAB, Jepsen JB, Knudsen UB. Oral melatonin supplementation during in vitro fertilization treatment: a systematic PRISMA review and meta-analysis of randomized controlled trials. Gynecol Endocrinol. (2021) 37(12):1079–85. doi: 10.1080/09513590.2021.1974378
7. Sun SY, Chen GH. Treatment of circadian rhythm sleep-wake disorders. Curr Neuropharmacol. (2022) 20(6):1022–34. doi: 10.2174/1570159X19666210907122933
8. Alruhaimi RS, Hassanein EHM, Bin-Jumah MN, Mahmoud AM. Cadmium-induced lung injury is associated with oxidative stress, apoptosis, and altered SIRT1 and Nrf2/HO-1 signaling; protective role of the melatonin agonist agomelatine. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397(4):2335–45. doi: 10.1007/s00210-023-02754-5
9. Park HJ, Park JY, Kim JW, Yang SG, Jung JM, Kim MJ, et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J Pineal Res. (2018) 64(2):e12458. doi: 10.1111/jpi.12458
10. Wu D, Zhao W, Xu C, Zhou X, Leng X, Li Y. Melatonin suppresses serum starvation-induced autophagy of ovarian granulosa cells in premature ovarian insufficiency. BMC Womens Health. (2022) 22(1):474. doi: 10.1186/s12905-022-02056-7
11. Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. (2016) 32(1):69–73. doi: 10.3109/09513590.2015.1101444
12. Mokhtari F, Akbari Asbagh F, Azmoodeh O, Bakhtiyari M, Almasi-Hashiani A. Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int J Fertil Steril. (2019) 13(3):225–9. doi: 10.22074/ijfs.2019.5717
13. Espino J, Macedo M, Lozano G, Ortiz Á, Rodríguez C, Rodríguez AB, et al. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants (Basel). (2019) 8(9):338. doi: 10.3390/antiox8090338
14. Akter S, Banu J, Ishrat S, Rani C, Jahan S, Nazneen S, et al. Melatonin enhances ovarian response in infertile women with polycystic ovary syndrome. Bangladesh J Med Sci. (2023) 22(4):850–8. doi: 10.3329/bjms.v22i4.67124
15. Jahromi BN, Sadeghi S, Alipour S, Parsanezhad ME, Alamdarloo SM. Effect of melatonin on the outcome of assisted reproductive technique cycles in women with diminished ovarian reserve: a double-blinded randomized clinical trial. Iran J Med Sci. (2017) 42(1):73–8.28293053
16. Tagliaferri V, Romualdi D, Scarinci E, Cicco S, Florio CD, Immediata V, et al. Melatonin treatment may be able to restore menstrual cyclicity in women with PCOS: a pilot study. Reprod Sci. (2018) 25(2):269–75. doi: 10.1177/1933719117711262
17. Hu KL, Ye X, Wang S, Zhang D. Melatonin application in assisted reproductive technology: a systematic review and meta-analysis of randomized trials. Front Endocrinol. (2020) 11:160. doi: 10.3389/fendo.2020.00160
18. Seko LM, Moroni RM, Leitao VM, Teixeira DM, Nastri CO, Martins WP. Melatonin supplementation during controlled ovarian stimulation for women undergoing assisted reproductive technology: systematic review and meta-analysis of randomized controlled trials. Fertil Steril. (2014) 101(1):154–61.e4. doi: 10.1016/j.fertnstert.2013.09.036
19. Sadeghpour S, Ghasemnejad-Berenji M, Maleki F, Behroozi-Lak T, Bahadori R, Ghasemnejad-Berenji H. The effects of melatonin on follicular oxidative stress and art outcomes in women with diminished ovarian reserve: a randomized controlled trial. J Ovarian Res. (2025) 18(1):5. doi: 10.1186/s13048-024-01584-0
20. Pilehvari S, Yavangui M, Paknahad E, Cheraghi Z, Ghorbani M. The boosting effects of melatonin on the in vitro fertilization (IVF) of women with polycystic ovary syndrome. Chonnam Med J. (2023) 59(3):188–93. doi: 10.4068/cmj.2023.59.3.188
21. Fernando S, Wallace EM, Vollenhoven B, Lolatgis N, Hope N, Wong M, et al. Melatonin in assisted reproductive technology: a pilot double-blind randomized placebo-controlled clinical trial. Front Endocrinol. (2018) 9:545. doi: 10.3389/fendo.2018.00545
22. Batıoğlu AS, Sahin U, Gürlek B, Oztürk N, Unsal E. The efficacy of melatonin administration on oocyte quality. Gynecol Endocrinol. (2012) 28(2):91–3. doi: 10.3109/09513590.2011.589925
23. Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur Rev Med Pharmacol Sci. (2010) 14(6):555–61.20712264
24. Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. (2011) 28(9):815–20. doi: 10.1007/s10815-011-9604-y
25. Poza JJ, Pujol M, Ortega-Albás JJ, Romero O. Melatonin in sleep disorders. Neurologia. (2022) 37(7):575–85. doi: 10.1016/j.nrl.2018.08.002
26. Liu Z, Gan L, Luo D, Sun C. Melatonin promotes circadian rhythm-induced proliferation through clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J Pineal Res. (2017) 62(4):e12383.
27. Habibi F, Nikbakht R, Jahanfar S, Ahmadi M, Eslami M, Azizi M, et al. Relationship between sleep disturbances and in vitro fertilization outcomes in infertile women: a systematic review and meta-analysis. Brain Behav. (2025) 15(2):e70293. doi: 10.1002/brb3.70293
28. Shang Y, Song N, He R, Wu M. Antioxidants and fertility in women with ovarian aging: a systematic review and meta-analysis. Adv Nutr. (2024) 15(8):100273. doi: 10.1016/j.advnut.2024.100273
29. Jiménez Tuñón JM, Trilles PP, Molina MG, Duvison MH, Pastor BM, Martín PS, et al. A double-blind, randomized prospective study to evaluate the efficacy of previous therapy with melatonin, myo-inositol, folic acid, and selenium in improving the results of an assisted reproductive treatment. Clin Med Insights Ther. (2017) 9:1179559X17742902.
30. Hrenak J, Paulis L, Repova K, Aziriova S, Nagtegaal EJ, Reiter RJ, et al. Melatonin and renal protection: novel perspectives from animal experiments and human studies (review). Curr Pharm Des. (2015) 21(7):936–49. doi: 10.2174/1381612820666140929092929
31. Lin HW, Lee EJ. Effects of melatonin in experimental stroke models in acute, sub-acute, and chronic stages. Neuropsychiatr Dis Treat. (2009) 5:157–62. doi: 10.2147/NDT.S4815
32. Menczel Schrire Z, Phillips CL, Chapman JL, Duffy SL, Wong G, D'Rozario AL, et al. Safety of higher doses of melatonin in adults: a systematic review and meta-analysis. J Pineal Res. (2022) 72(2):e12782. doi: 10.1111/jpi.12782
33. Tao Y, Hu B, Ma Z, Li H, Du E, Wang G, et al. Intravitreous delivery of melatonin affects the retinal neuron survival and visual signal transmission: in vivo and ex vivo study. Drug Deliv. (2020) 27(1):1386–96. doi: 10.1080/10717544.2020.1818882
34. Rodríguez-Varela C, Labarta E. Clinical application of antioxidants to improve human oocyte mitochondrial function: a review. Antioxidants. (2020) 9(12):1197. doi: 10.3390/antiox9121197
35. Kim EH, Ridlo MR, Lee BC, Kim GA. Melatonin-Nrf2 signaling activates peroxisomal activities in porcine cumulus cell-oocyte complexes. Antioxidants. (2020) 9(11):1080. doi: 10.3390/antiox9111080
36. Qu J, Hu H, Niu H, Sun X, Li Y. Melatonin restores the declining maturation quality and early embryonic development of oocytes in aged mice. Theriogenology. (2023) 210:110–8. doi: 10.1016/j.theriogenology.2023.07.021
37. Abecia JA, Forcada F, Vázquez MI, Muiño-Blanco T, Cebrián-Pérez JA, Pérez-Pe R, et al. Role of melatonin on embryo viability in sheep. Reprod Fertil Dev. (2018) 31(1):82–92. doi: 10.1071/RD18308
38. Etrusco A, Ata B, Agrifoglio V, D'Amato A, Wyns C, Vitagliano A, et al. Reproductive outcome after frozen embryo transfer with hormone replacement therapy according to luteal-phase support protocol: systematic review and network meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. (2025). doi: 10.1002/uog.29302
39. Salang L, Teixeira DM, Solà I, Sothornwit J, Martins WP, Bofill Rodriguez M, et al. Luteal phase support for women trying to conceive by intrauterine insemination or sexual intercourse. Cochrane Database Syst Rev. (2022) 8(8):Cd012396. doi: 10.1002/14651858.CD012396.pub2
40. Wang P, Sun Y, Li Y, Fan J, Zong Y, Isa AM, et al. Monochromatic green light stimulation during incubation shortened the hatching time via pineal function in White Leghorn eggs. J Anim Sci Biotechnol. (2021) 12(1):17. doi: 10.1186/s40104-020-00539-x
41. Sun JT, Liu JH, Zhao L, Chen HY, Wang RF, Li YJ, et al. Melatonin decreases excessive polyspermy for single oocyte in pigs through the MT2 receptor. Sci Rep. (2024) 14(1):23153. doi: 10.1038/s41598-024-74969-2
42. Yang C, Liu Q, Chen Y, Wang X, Ran Z, Fang F, et al. Melatonin delays ovarian aging in mice by slowing down the exhaustion of ovarian reserve. Commun Biol. (2021) 4(1):534. doi: 10.1038/s42003-021-02042-z
43. Hansda SR, Haldar C. Uterine anomalies in cell proliferation, energy homeostasis and oxidative stress in PCOS hamsters, M. auratus: therapeutic potentials of melatonin. Life Sci. (2021) 281:119755. doi: 10.1016/j.lfs.2021.119755
44. Riemma G, De Franciscis P, La Verde M, Ravo M, Fumiento P, Fasulo DD, et al. Impact of the hemostatic approach after laparoscopic endometrioma excision on ovarian reserve: systematic review and network meta-analysis of randomized controlled trials. Int J Gynaecol Obstet. (2023) 162(1):222–32. doi: 10.1002/ijgo.14621
45. Li YL, Yan EQ, Zhao GN, Jin L, Ma BX. Effect of body mass index on ovarian reserve and ART outcomes in infertile women: a large retrospective study. J Ovarian Res. (2024) 17(1):195. doi: 10.1186/s13048-024-01521-1
46. Riemma G, De Franciscis P, Torella M, Narciso G, La Verde M, Morlando M, et al. Reproductive and pregnancy outcomes following embryo transfer in women with previous cesarean section: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2021) 100(11):1949–60. doi: 10.1111/aogs.14239
47. Friedenthal J, Alkon-Meadows T, Hernandez-Nieto C, Gounko D, Lee JA, Copperman A, et al. The association between prior cesarean delivery and subsequent in vitro fertilization outcomes in women undergoing autologous, frozen-thawed single euploid embryo transfer. Am J Obstet Gynecol. (2021) 225(3):287.e1–e8. doi: 10.1016/j.ajog.2021.03.026
Keywords: melatonin, clinical pregnancy rate, assisted reproductive technologies (ART), RCT, embryo quality, systematic review and meta-analysis
Citation: Wu Y, Huang W, Tang L, Feng Y, Chen H, Pan M, Peng J, Li C and Wang H (2025) Melatonin improved the outcomes of women with ART: a systematic review and meta-analysis of randomized trials. Front. Reprod. Health 7:1680984. doi: 10.3389/frph.2025.1680984
Received: 6 August 2025; Accepted: 9 September 2025;
Published: 23 September 2025.
Edited by:
Andrea Etrusco, University of Palermo, ItalyReviewed by:
Gaetano Riemma, University of Campania Luigi Vanvitelli, ItalyMarco La Verde, Università degli Studi della Campania "Luigi Vanvitelli", Italy
Copyright: © 2025 Wu, Huang, Tang, Feng, Chen, Pan, Peng, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huawei Wang, d2FuZ2h1YXdlaTk5QDE2My5jb20=; Chen Li, MTI1MjU2NDkyQHFxLmNvbQ==
†These authors have contributed equally to this work
 Yilin Wu
Yilin Wu Wenjie Huang
Wenjie Huang Li Tang1
Li Tang1 Huawei Wang
Huawei Wang