- 1Goergen Institute for Data Science, University of Rochester, Rochester, NY, United States
- 2Department of Physiology College of Medicine Tucson, University of Arizona, Tucson, AZ, United States
- 3School of Nursing, University of Rochester, Rochester, NY, United States
- 4School of Nursing, University of Pittsburgh, Pittsburgh, PA, United States
- 5Statistics Consulting Lab, BIO5 Institute, University of Arizona, Tucson, AZ, United States
- 6Department of Pharmacy Practice and Science, The University of Arizona R. Ken Coit College of Pharmacy, Tucson, AZ, United States
- 7Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Rochester Medical Center, Rochester, NY, United States
Hormonal contraceptives (HCs) are commonly prescribed medications that have had immeasurable impacts on quality of life and health of women and families globally. However, usage of exogenous hormones is not without risks, and patients often report a variety of side effects, ranging from burdensome to life-threatening. For some patients, side effects of HCs are severe enough to cause medication discontinuation or switching to alternative forms of contraception. Variability in side effect profiles may indicate heritable risk factors for some side effects. Understanding these patterns or risk profiles may help clinicians anticipate severe adverse events, match patients with suitable medications more rapidly, and improve patient outcomes and adherence. To support further research in this field, this narative review summarizes what is currently known about pharmacogenetic interactions with respect to HCs and specific polymorphisms suspected to contribute to adverse side effects and outcomes.
Introduction
Hormonal contraceptives (HCs) offer major benefits for many individuals, including control over family planning and pregnancy, regulation of menses, and improvement in numerous health conditions (e.g., endometriosis, dysmenorrhea, abnormal uterine bleeding, acne). HCs, medications intended to decrease the risk of pregnancy following sexual intercourse, contain a progestin with or without an estrogen (1). By introducing exogenous versions of these hormones at levels significantly higher than baseline, HCs can affect multiple body systems and produce numerous side effects in addition to pregnancy prevention (1–3). Patient response to HCs is highly variable, ranging from positive side effects (such as improved health and mood) to severe negative side effects such as depression, hypertension, stroke, and venous thromboembolism (4–6). Due to dissatisfaction with their current HC method, many women discontinue HCs despite wanting to prevent pregnancy (7). The majority of women cite side effects such as breakthrough bleeding, weight gain, or mood changes as the cause for discontinuation (5, 8, 9). Switching or discontinuing these medications can lead to unwanted pregnancies, as many women who discontinue HCs take up less reliable methods, use barrier methods inconsistently, or report no contraceptive methods at all (5, 9).
Emerging evidence suggests that genetic variation may play a role in individual susceptibility to certain adverse effects of HC, although this area remains underexplored (10). Due to the variability of responses to HC exposure and the familial tendencies of some adverse effects, it has been postulated that genetics may influence HC effects (10). No studies to date have systematically evaluated whether genetic factors (e.g., single nucleotide polymorphisms, epigenetic changes) can be used as a predictive tool for hormonal contraceptive side effects, and limited research has been focused on the effects of genotype on HC response. The purpose of this narrative review is to identify side effect profiles associated with HCs and single nucleotide polymorphisms (SNPs) that are known, or suspected, to alter these phenotypic responses. We summarized side effects that have been reported as common reasons for discontinuation of HCs and have been studied in conjunction with genetic risk factors (Table 1). We aim to identify trends in genetic susceptibility to these side effects, which will aid in prediction of adverse reactions, offer a pathway toward more individualized, risk-informed contraceptive counseling, and highlight gaps remaining in research.
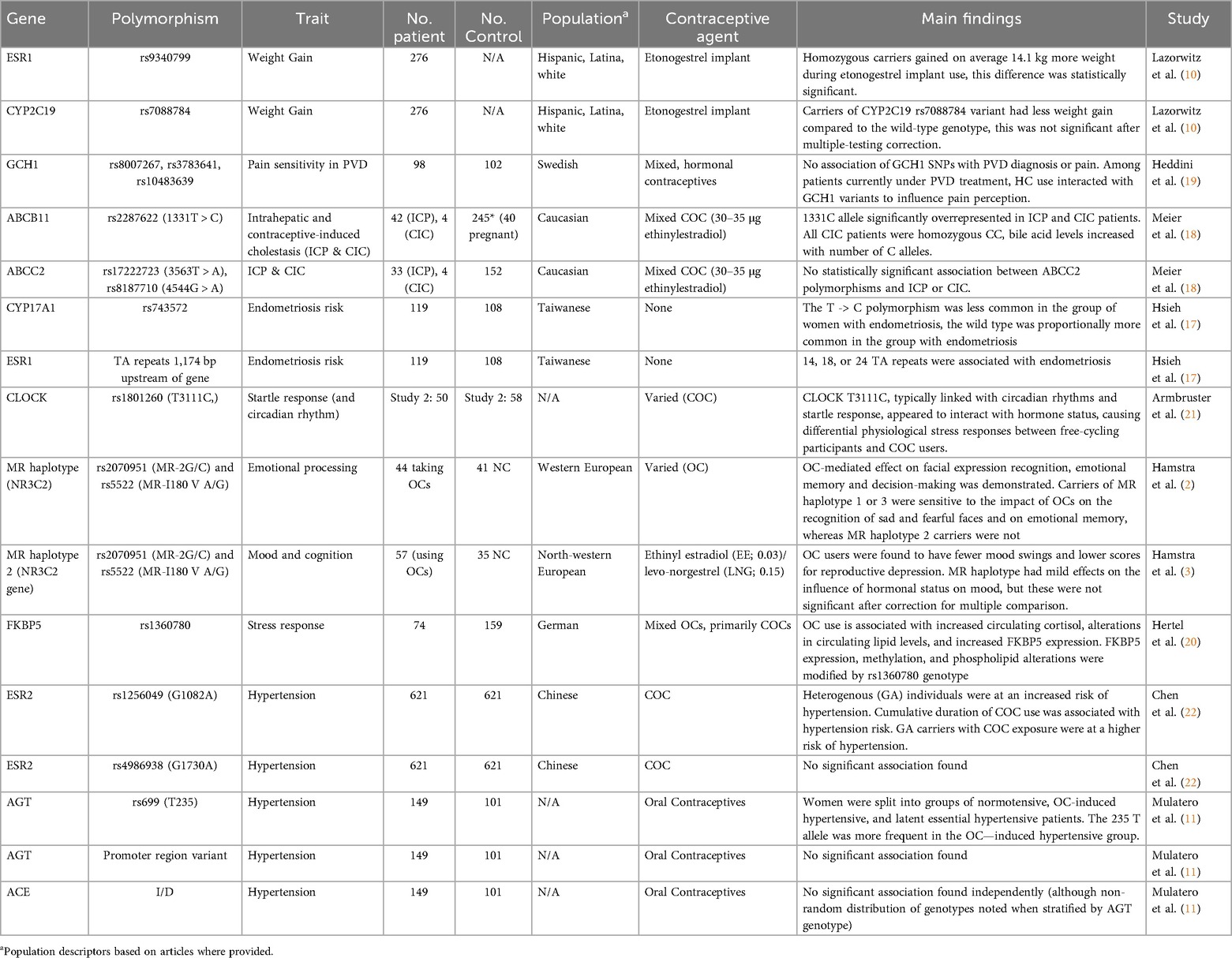
Table 1. A summary of polymorphisms studied in relation to altered hormonal contraceptive response. Polymorphisms with evidence of positive interaction effects are highlighted in green.
Weight gain
One side effect most associated with HCs, particularly DepoProvera or medroxyprogesterone acetate (12), is weight gain. Providers are recommended to counsel women about the possibility of weight change prior to new HC medication and weight changes are frequently cited as a concern or reason for discontinuation (5, 6, 10, 13–15). Despite anecdotal data, the risk of weight gain remains heavily debated in the literature, with many researchers questioning the link between HCs and weight gain given the lack of randomized controlled trials and the tendency for weight to fluctuate naturally over time (6, 13, 14). Multiple studies with patient-control groups have found no statistically significant differences between weight outcomes, however, these studies also show that outcomes at the individual level vary widely (13, 15). The variation may suggest a multifactorial source of weight change including possible genetic predispositions which make some patients more likely to gain weight once introduced to exogenous hormones (10, 15). In one retrospective analysis, Lazorwitz et al. considered 99 genetic variants among reproductive-aged women with an etonogestrel contraceptive implant placed 12–36 months prior (Table 2) (10). Current weight was compared to pre-insertion weight using data from medical records. One SNP on the estrogen receptor 1 (ESR1) gene (rs9340799) was found to have a statistically significant association with weight change, with participants homozygous for the variant (genotype GG) gaining 14.1 kg more weight on average than those with at least 1 wild-type (A) allele (10). Although etonogestrel is not known to bind to estrogen receptors, progesterone receptor activation is suspected of altering ESR1 transcription, and the authors posit exogenous progesterone sources may affect estrogen receptor protein production or action (10).
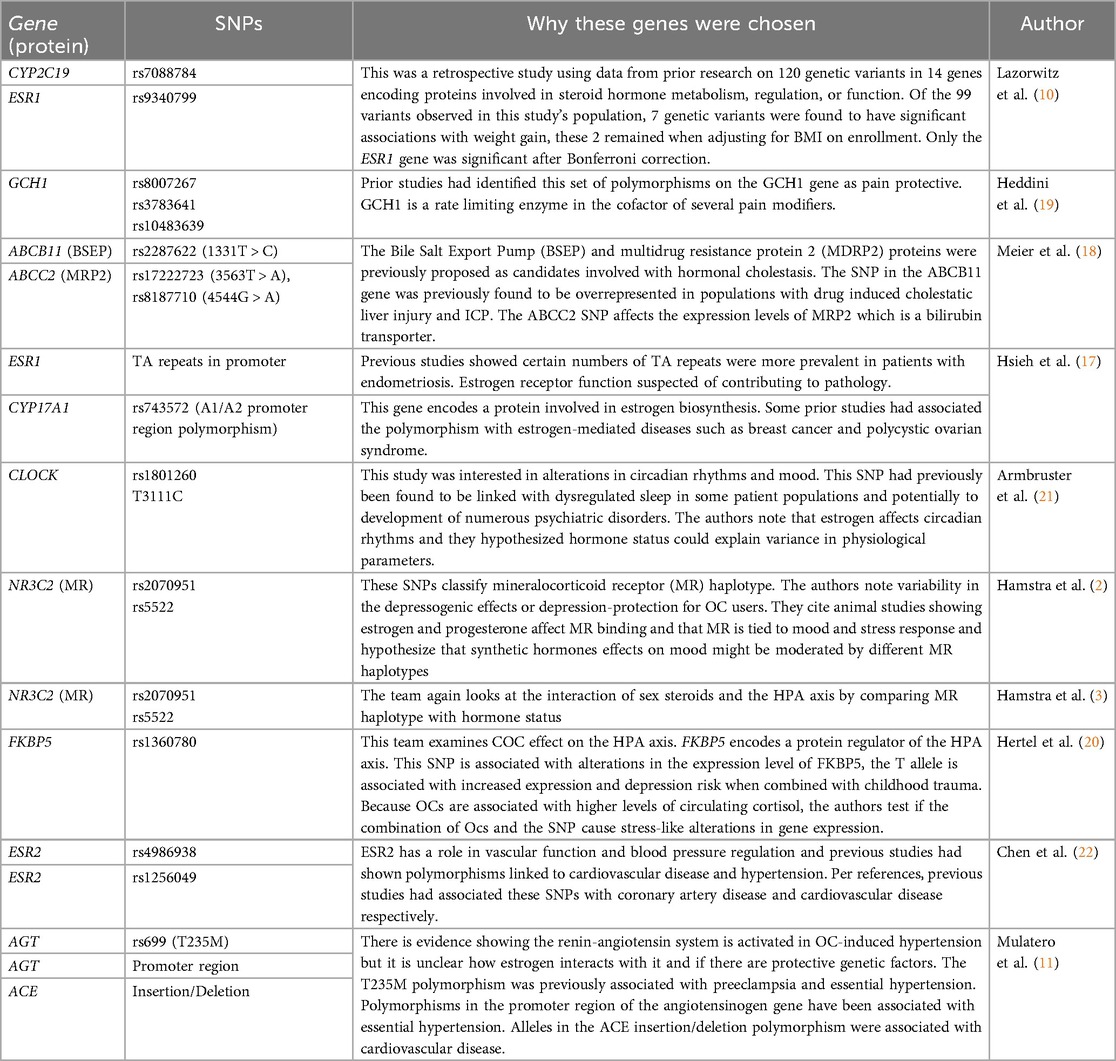
Table 2. A summary of rationale given in referenced articles as to why selected polymorphisms were studied .
Including genomic considerations into analyzing weight gain in the setting of contraceptive use appeared to explain a substantial amount of variance compared with studies focused on all HC users. A genetic predisposition to weight change could markedly impact a patient's outcome, and their choice to take or continue a medication. While these results are significant, we found this field to be understudied. Additional research would be warranted to confirm these results and examine relevance to alternative sources of exogenous hormones.
Abdominopelvic symptoms
Abdominal and pelvic pain are common complaints with HC use, particularly with oral contraceptive pills (5, 9). Much of the literature describing abdominal pain with HC use focuses on specific patient groups with, or at risk for, pre-existing conditions such as endometriosis. Consequently, the study of abdominal pain, HCs, and genotype interactions is broad and tends to be condition specific.
Estrogen itself may play a primary role in the effect of HCs on abdominal and GI symptoms as it has been implicated in multiple “estrogen-dependent” diseases. Endometriosis, as an example, is an estrogen-dependent disease with strong familial tendencies which is associated with severe pelvic pain and dysmenorrhea (16, 17). Estrogen-dependent conditions may be particularly affected by combined hormonal contraceptives, as some clinicians have concerns that high doses of estrogen may cause progression in these conditions, making them more severe or difficult to treat (16). Preliminary genomics studies have found associations between endometriosis and polymorphisms in the ESR1 and CYP17A1 genes. However, the interaction between these polymorphisms and contraceptives has not yet been examined and may benefit from further research.
A more direct connection can be found between HCs and the development of oral contraceptive-induced cholestasis (CIC), an acquired form of cholestasis characterized by impaired bile flow from the liver to the duodenum. CIC is known to resolve after discontinuation of HCs and has been suspected of genetic origins due to regional clustering and similarity to intrahepatic cholestasis of pregnancy, which has known familial tendencies (18). In a study of CIC and intrahepatic cholestasis of pregnancy, Meier et al. found higher frequency of the ABCB11 1331T > C polymorphism (rs2287622) in patients with ICP or CIC than in pregnant controls. Although this study was limited due to the small population of patients (with only four CIC patients included), it may offer preliminary evidence of the role of the ABCB11 bile salt export pump gene in the development of CIC. Further work in these conditions could support to the theory that certain individuals could be at elevated risk when starting exogenous steroid hormones, although the etiology of CIC is likely multifactorial and joint risk factors would need to be considered to establish a full risk profile.
While estrogen-dependent disease progression may be mediated by HCs due to the introduction of exogenous hormones, there is additional evidence that HC use can alter pain sensation overall or modify pre-existing conditions through interactions with other genotypes. Research on provoked vestibulodynia, a chronic pain condition characterized by pain localized to the area surrounding the vaginal opening, found an interaction between hormone status, pain, and SNPs on the GCH1 gene. This gene codes for a rate limiting enzyme for B4, a cofactor in pain modulator synthesis. A 15 SNP combination on the GCH1 gene has been identified as protective and can be identified by genotyping as few as 3 SNPs: rs8007267, rs3783641, and rs10483639 (19). When studied in women currently receiving treatment for vestibulodynia, a treatment-hormonal interaction emerged. Carriers who did not take HCs reported lower coital pain and higher pressure pain thresholds on the arm while patients using HCs noted the reverse, with carriers reporting more pain and lower pain thresholds than non-carriers (19). The reversal may indicate that some aspect of exogenous hormonal medication blocked protective pathways of inherited polymorphisms, making these participants more likely to develop or notice worsening symptoms of pre-existing conditions after HC use was initiated. The authors speculated that this could explain the tendency for some patients to do better when taken off HCs, supporting the concern for frequent discontinuations or medication switching in this group (19).
While numerous diseases may depend on gene-environment interactions resulting in alterations in abdominal, pelvic, or vulvar pain attributed to HC use, there is very limited coverage in the literature, with conclusions muddled by contradictory results. While it is difficult to determine how patients of a given genotype may respond to specific hormonal medications given the paucity of research, there is evidence that multiple genes, perhaps most notably those responsible for mediating effects of sex steroids, may mitigate or worsen disease and symptom progression. Additional research may be able to further compare the risks or benefits of different HCs for these patients or investigate contraceptive users as a whole rather than specific patient groups.
Mood, energy, and emotional effects
Along with physiological changes, HCs have been studied as having numerous psychiatric side effects, ranging from depressogenic to depression-protective in different patients (2). Depression and mood-related side effects are frequently reported following new HC prescriptions, and are commonly cited causes for discontinuation (2, 4, 5).
One of the most studied interactions is that of estrogen and progesterone with regulators of the HPA-axis. Contraceptives containing these hormones (in the form of progestins and ethinyl estradiol) are thought to alter mineralcorticoid receptor (MR) action in regulating stress responses, thereby affecting emotion information processing, and to suppress or increase circulating cortisol levels through different phases of the menstrual cycle (2, 3, 20). In researching this relationship, MR haplotype (defined by SNPs rs2070951 and rs5522) was found to moderate the effect of combined oral contraceptives on emotional recognition and memory (2). Carriers of MR haplotypes 1 or 3 were found to be more sensitive to the effects of HCs on negative emotional memory and perception-bias (2). Participants with these haplotypes taking HCs were more aware of sad or fearful faces, a finding previously recognized in patients with depression (2). However, follow-up studies showed that haplotype was not significant in moderating HC effect on affect itself (3).
Another HPA regulator, the FKBP5 protein (which inhibits the role of glucocorticoid receptors in stress response to the HPA cycle), is known to have upregulated expression in high-cortisol environments (20). Hertel et al. found that while circulating cortisol levels were higher in oral contraceptive users (a known effect of combined HCs), FKBP5 transcription was only upregulated in a subset of individuals. They postulated that an FKBP5 SNP (rs1360780) could modulate the effect of oral contraceptives and high cortisol on expression. Although they were not able to show a direct effect of this SNP on depressive outcomes, they proposed that specific FKBP5 genotypes may help identify patients at high risk for HC-related mood disorders (20).
Beyond the HPA axis, pharmacogenomic associations have been found between HCs, startle response, and circadian rhythm, potentially influencing perceived energy and stress. The CLOCK gene, which plays a central role in circadian rhythm and is implicated in numerous psychiatric disorders, was studied by Armbruster et al. and found to have an interaction effect with hormone status. SNP rs1801260 is linked with evening wakefulness and sleep dysregulation, as well as possible affective changes (21). In one subset of their study, Armbruster et al. specifically tested reaction to emotional stimuli and circadian rhythms in patients taking combined oral contraceptives compared with free-cycling participants separated by CLOCK genotype. It was found that while certain measures of startle response (increased skin conductance and decreased corrugator activity) was stronger in homozygous carriers of the T allele in naturally-cycling users, these trends reversed in HC users (21). HC users also exhibited less differential diurnal preferences based on genotype than non-users (21). The differential physiological stress response and reported sleep preferences may indicate that in some users, HCs counteract natural energy fluctuations and stress response mechanisms. New users adjusting to these changes might notice these side effects as altered mood, affect changes, or fatigue.
Overall, there is compelling, yet limited, evidence that genotype can compound the effect of hormone status on mood and energy levels. Certain combinations of alleles might make some women more likely to experience unpleasant mood alterations, depression, or sleep disturbances while taking HCs. Identifying the link between specific HCs and varied emotional lability is difficult in experimental research, however. One limitation is that contraceptives were generally not compared within these studies, with the majority of participants taking combined oral contraceptives.
Hypertension
While HCs are generally associated with an increased risk of hypertension, the mechanism is not well understood, and a complex interplay between steroid hormones, the renin-angiotensin system (RAS), and blood pressure is inferred (11). The angiotensinogen (AGT) gene and ESR genes may all be involved in the etiology of HC-induced hypertension (11, 22). Angiotensinogen levels are found to be higher in women using HCs, as estrogens induce production in the liver, likely driving increased vasoconstrictor production (11, 22). Two polymorphisms on the angiotensinogen gene (rs699 and promoter region variants) and one on the angiotensin I converting enzyme gene (an insertion/deletion polymorphism) have been researched as possible links to the physiological pathways behind HC-induced hypertension (11). In women with HC-induced hypertension compared with women with essential hypertension, the frequency of the rs699T allele was higher, a difference which increased when stratified by the insertion/deletion polymorphism on the ACE gene (11). This suggests not only genetic predisposition to angiotensinogen dysregulation following exogenous estrogen introduction, but also that numerous SNPs interact to produce this predisposition, implying a complex, multifactorial genetic risk among the RAS alone.
Further complicating the system, ESR1 and ESR2 may also contribute to the pathology of this HC side effect. Estrogen receptors present on smooth muscle cells are known to regulate vasodilatory and vasoconstrictive proteins in these tissues (22). At least one ESR2 polymorphism (rs1256049) has been shown to have an interaction effect with combined oral contraceptive use, as the heterozygote variant genotype (G/A) was found to be significantly associated with hypertension, a trend amplified in the population of subjects using HCs (22). Furthermore, cumulative combined oral contraceptive use of 15 years or more was found to have an increased effect on hypertension risk, meaning that not only did combined oral contraceptive use affect hypertension, but risk was dependent on duration of exposure. The recessive genotype (A/A) was only slightly associated with hypertension and was not a significant risk factor, nor was a second ESR2 SNP (rs4986938) (22).
The multifactorial risk profile demonstrates the complexity inherent to pharmacogenomic interactions. While evidence suggests certain polymorphisms have a role in the development of hypertension, additional SNPs on related genes, the interaction effect between these genotypes, and drug-exposure length could all contribute to the complex picture of hormone-induced hypertension, making it extremely difficult to get a comprehensive picture of the syndrome. Blood pressure is easily monitored in most clinical settings, which could allow for early detection of HC-induced hypertension and medication discontinuation, although without additional research, it may be difficult to pinpoint which medications would be the safest alternatives, or which patients are at the highest risk prior to initiation.
Additional side effects linked to HC usage
In addition to the side effects reviewed above, HCs have been implicated in many serious disease processes, including thrombus formation, stroke risk, and breast cancer development (4, 6). Some work has been done on gene/hormone-status interactions in relation to breast cancer and deep vein thrombosis, implicating variants on the BRCA1 and the F5 genes as risk factors (23, 24). While increased risk in any one of these conditions may be of serious medical consequence, these variants were not covered in depth by this review as our focus was common, burdensome side-effects. These more serious, life-threatening conditions may not produce symptoms until significant disease progression occurs and patients are generally counselled against certain HCs prior to initiating based on personal and family history (4, 6, 25). However, the potential for a joint effect of genetic and environmental (contraceptive use) risk factors in influencing these significant health risks bears further study and clinical consideration.
In contrast, additional common side effects, such as migraines, fatigue, and irregular vaginal bleeding are major sources of medication discontinuation (5, 26). Unfortunately, the scarcity of research into genetic risk factors for these symptomologies limited their contribution to this review. Recent work in transcriptomics does indicate differential expression of CXCL9 and TIMP1 plays a role in timing and severity of abnormal uterine bleeding in conjunction with HC use (26). Expanding the current body of work into genomics may help explain additional variance between side effect profiles. The contribution of these side effects to HC discontinuation should not be overlooked and holds potential as future avenues of study.
Conclusion
This narrative review synthesizes the current evidence on genetic variation associated with hormonal contraceptive side effects and identifies key gaps for future research. Although there is a significant body of work supporting the role of pharmacogenetic contributions to side effects in HC use, this remains a broad and under-researched topic, limiting our ability to understand the intricacies of individual drug response. The diversity of contraceptive options and the large number of genes that may contribute to side effects are obstacles to efforts to build a more comprehensive systematic review. The strength of this narrative review is our ability to integrate heterogeneous early-stage findings from pharmacogenetics studies that are not yet suited to systematic review methods. The primary limitation is the restricted scope of available literature, which limits the strength of our conclusions.
Despite these challenges, it is important to predict side-effect risks to the best of our ability, as trial and error with contraceptive prescriptions carries risks of discontinuation, unwanted pregnancies, patient discomfort, and even severe health risks. This narrative review offers a glimpse at the current state of research and highlights the importance of further study in examining the complex relationship between hormonal birth control and genetics, supporting the notion that their interactions alter side effect profiles in patients. Future directions may include large-scale, genome-wide association studies and considerations of hormone status in other groups, such as post-menopausal women or pregnant women.
Author contributions
MN: Methodology, Writing – original draft, Investigation, Data curation. EE: Investigation, Writing – review & editing, Resources, Methodology, Supervision. SG: Resources, Writing – review & editing, Supervision. YY: Methodology, Conceptualization, Writing – review & editing. TK: Methodology, Conceptualization, Writing – review & editing. HL: Writing – review & editing, Methodology. KM: Methodology, Writing – review & editing. AZ: Methodology, Writing – review & editing, Data curation. CD: Funding acquisition, Project administration, Resources, Methodology, Supervision, Writing – original draft, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the University of Rochester School of Nursing Research Support Grant and the National Institutes of Health (K01NR020504, R25HG012324).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2025.1720994/full#supplementary-material
References
1. Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: a review. JAMA. (2021) 326(24):2507–18. doi: 10.1001/jama.2021.21392
2. Hamstra DA, de Kloet ER, van Hemert AM, de Rijk RH, Van der Does AJW. Mineralocorticoid receptor haplotype, oral contraceptives and emotional information processing. Neuroscience. (2015) 286:412–22. doi: 10.1016/j.neuroscience.2014.12.004
3. Hamstra DA, de Kloet ER, de Rover M, Van der Does W. Oral contraceptives positively affect mood in healthy PMS-free women: a longitudinal study. J Psychosom Res. (2017) 103:119–26. doi: 10.1016/j.jpsychores.2017.10.011
4. Britton LE, Alspaugh A, Greene MZ, McLemore MR. An evidence-based update on contraception. Am J Nurs. (2020) 120(2):22–33. doi: 10.1097/01.NAJ.0000654304.29632.a7
5. Al-Ghashri F, Al-Harthi H, Al Shukri M, Al Shidhani A. Discontinuation of hormonal contraception in Oman: prevalence and reasons. East Mediterr Health J. (2021) 27(10):993–1000. doi: 10.26719/emhj.21.031
6. Pitts SAB, Emans SJ. Controversies in contraception. Curr Opin Pediatr. (2008) 20(4):383–9. doi: 10.1097/MOP.0b013e328305e13f
7. Le Guen M, Schantz C, Régnier-Loilier A, de La Rochebrochard E. Reasons for rejecting hormonal contraception in western countries: a systematic review. Soc Sci Med. (2021) 284:114247. doi: 10.1016/j.socscimed.2021.114247
8. Daniels K, Abma JC. Contraceptive methods women have ever used: United States, 2015–2019. Natl Health Stat Rep. (2023) (195):1–18.PMID: 38170816
9. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. (1998) 179(3 Pt 1):577–82. doi: 10.1016/S0002-9378(98)70047-X
10. Lazorwitz A, Dindinger E, Harrison M, Aquilante CL, Sheeder J, Teal S. An exploratory analysis on the influence of genetic variants on weight gain among etonogestrel contraceptive implant users. Contraception. (2020) 102(3):180–5. doi: 10.1016/j.contraception.2020.05.002
11. Mulatero P, Rabbia F, di Cella SM, Schiavone D, Plazzotta C, Pascoe L, et al. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms are non-randomly distributed in oral contraceptive-induced hypertension. J Hypertens. (2001) 19(4):713–9. doi: 10.1097/00004872-200104000-00008
12. Sims J, Lutz E, Wallace K, Kassahun-Yimer W, Ngwudike C, Shwayder J. Depo-medroxyprogesterone acetate, weight gain and amenorrhea among obese women adolescent and adult women. Eur J Contracept Reprod Health Care. (2020) 25(1):54–9. doi: 10.1080/13625187.2019.1709963
13. Gallo MF, Legardy-Williams J, Hylton-Kong T, Rattray C, Kourtis AP, Jamieson DJ, et al. Association of progestin contraceptive implant and weight gain. Obstet Gynecol. (2016) 127(3):573–6. doi: 10.1097/AOG.0000000000001289
14. Lopez LM, Ramesh S, Chen M, Edelman A, Otterness C, Trussell J, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev. (2016) 2016(8):CD008815. doi: 10.1002/14651858.CD008815.pub4
15. Vickery Z, Madden T, Zhao Q, Secura G, Allsworth JE, Peipert JF. Weight change at 12 months in users of three progestin-only contraceptive methods. Contraception. (2013) 88(4):503–8. doi: 10.1016/j.contraception.2013.03.004
16. Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. (2017) 107(3):533–6. doi: 10.1016/j.fertnstert.2017.01.003
17. Hsieh YY, Chang CC, Tsai FJ, Lin CC, Tsai CH. Estrogen receptor alpha dinucleotide repeat and cytochrome P450c17alpha gene polymorphisms are associated with susceptibility to endometriosis. Fertil Steril. (2005) 83(3):567–72. doi: 10.1016/j.fertnstert.2004.07.977
18. Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T > C polymorphism in the bile salt export pump. World J Gastroenterol. (2008) 14(1):38–45. doi: 10.3748/wjg.14.38
19. Heddini U, Bohm-Starke N, Grönbladh A, Nyberg F, Nilsson KW, Johannesson U. GCH1-polymorphism And pain sensitivity among women with provoked vestibulodynia. Mol Pain. (2012) 8:68. doi: 10.1186/1744-8069-8-68
20. Hertel J, König J, Homuth G, Van der Auwera S, Wittfeld K, Pietzner M, et al. Evidence for stress-like alterations in the HPA-axis in women taking oral contraceptives. Sci Rep. (2017) 7:14111. doi: 10.1038/s41598-017-13927-7
21. Armbruster D, Brocke B, Kirschbaum C, Witt SH, Lesch KP, Strobel A. Rhythm and blues: influence of CLOCK T3111C on peripheral electrophysiological indicators of negative affective processing. Physiol Behav. (2020) 219:112831. doi: 10.1016/j.physbeh.2020.112831
22. Chen Y, Holzman C, Chung H, Senagore P, Talge NM, Siler-Khodr T. Levels of maternal serum corticotropin-releasing hormone (CRH) at midpregnancy in relation to maternal characteristics. Psychoneuroendocrinology. (2010) 35(6):820–32. doi: 10.1016/j.psyneuen.2009.11.007
23. Phillips KA, Kotsopoulos J, Domchek SM, Terry MB, Chamberlain JA, Bassett JK, et al. Hormonal contraception and breast cancer risk for carriers of germline mutations in BRCA1 and BRCA2. J Clin Oncol. (2025) 43(4):422–31. doi: 10.1200/JCO.24.00176
24. Bergendal A, Persson I, Odeberg J, Sundström A, Holmström M, Schulman S, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol. (2014) 124(3):600–9. doi: 10.1097/AOG.0000000000000411
25. Project RHA. Medical Eligibility for Initiating Contraception. Atlanta, GA: Reproductive Health Access Project. (2025). Available online at: https://www.reproductiveaccess.org/resource/medical-eligibility-initiating-contraception/ (Accessed October 8, 2025).
Keywords: pharmacogenomics, contraception, symptoms, side effects, single nucleotid polymorphism (SNP)
Citation: Nuzzo M, Erickson EN, Groth SW, Yu Y, Koleck T, Li H, Martinez K, Zaman A and Dreisbach C (2025) Genetic variation associated with side effects of hormonal contraception exposure: a narrative review. Front. Reprod. Health 7:1720994. doi: 10.3389/frph.2025.1720994
Received: 8 October 2025; Accepted: 30 October 2025;
Published: 14 November 2025.
Edited by:
Jan Tesarik, MARGen Clinic, SpainReviewed by:
Marie Grace Sandra Musabwasoni, University of Rwanda, RwandaCopyright: © 2025 Nuzzo, Erickson, Groth, Yu, Koleck, Li, Martinez, Zaman and Dreisbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caitlin Dreisbach, Y2FpdGxpbl9kcmVpc2JhY2hAdXJtYy5yb2NoZXN0ZXIuZWR1
 Mariah Nuzzo1
Mariah Nuzzo1 Elise N. Erickson
Elise N. Erickson Yang Yu
Yang Yu Adnin Zaman
Adnin Zaman Caitlin Dreisbach
Caitlin Dreisbach