- 1College of Medicine, University of Central Florida, Orlando, FL, United States
- 2Division of Urology, Nemours Children's Health System/Nemours Children's Hospital, Orlando, FL, United States
Introduction: Management of urachal anomalies in pediatric patients has historically lacked a clear consensus between conservative and surgical management. We aimed to review and summarize the literature on the diagnosis, symptoms, and evolution in the management of urachal anomalies in pediatric patients.
Methods: We performed a scoping literature review of PubMed/Medline and WebOfScience from January 2000 to February 2022.
Results: 32 publications were selected for inclusion in this analysis. 1,438 unique studies were identified with 32 studies meeting inclusion criteria. 15/32 studies discussed both conservative and surgical management, 14/32 studies discussed only surgical management outcomes, and 3/32 studies discussed diagnostic methods. The studies discussing conservative management supported the treatment of urachal anomalies with an initial conservative approach, which includes watchful waiting, repeated ultrasounds, lesion measurement, and antibiotic use. 5/32 of the included studies identified patients that were converted from conservative to surgical management with conversion rates ranging from 12.5% to 43.5% per study. 14/20 converted patients were identified to have a urachal cyst and 13/20 had a persistent infection.
Conclusions: Strong evidence exists that supports initial conservative management over surgical management of pediatric urachal anomalies. However, predictive factors for determining which patients will require surgical management remain elusive. Treatment algorithms can potentially be developed once carefully developed prospective studies delineate statistically significant patient factors which necessitate surgical management over observation.
Introduction
The urachus is a connection between the early fetal bladder and the allantois that aids in the removal of nitrogenous waste through the umbilical cord and placenta during gestation. This connection typically obliterates during fetal development or early in the neonatal course, forming the median umbilical ligament (1, 2). This lumen may fail to obliterate, thus leading to the abnormal persistence of an embryonic urachal remnant. Urachal remnants can be subtyped into different morphological anomalies including a patent urachus which has a fully patent lumen between the bladder and umbilicus, a vesicourachal diverticulum which contains a blind pouch attached to the bladder, a urachal cyst which contains a small patent lumen with two closed ends, and an umbilical-urachal sinus which contains a patent lumen at the umbilicus but does not extend to the bladder (Figure 1) (3). These four subtypes are classically reported in the literature, however, they may be further subdivided into anatomical variants based on length and connection with the lateral umbilical ligaments (4).
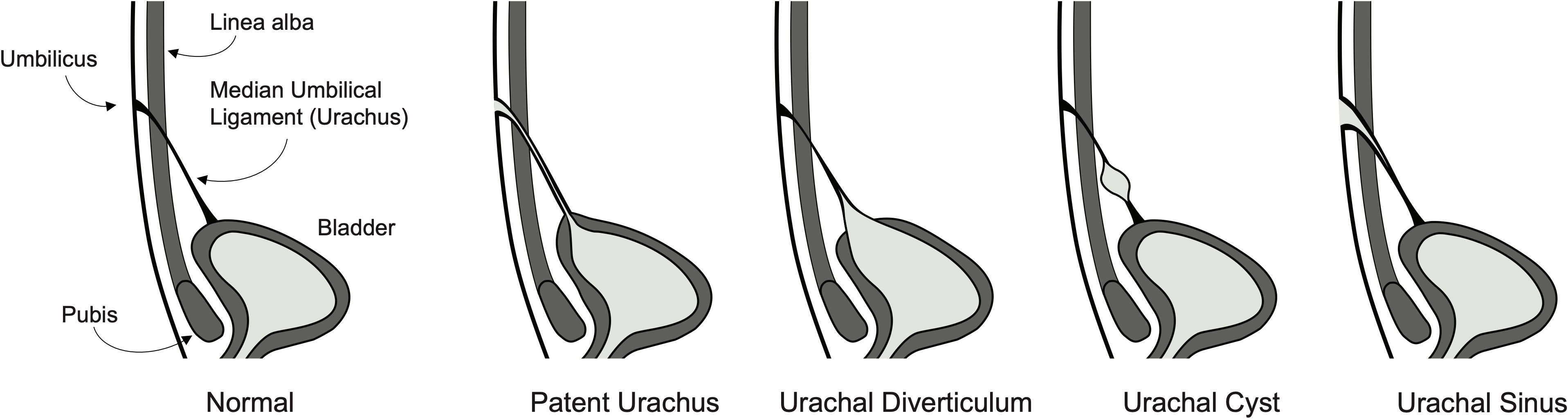
Figure 1 Illustration of a normal fully developed bladder with urachus and four of the most common urachal anomaly subtypes including patent urachus, vesicourachal diverticulum, urachal cyst, and umbilical-urachal sinus.
Urachal anomalies (UAs) were historically thought to be a rare occurrence, but modern increased utilization of imaging studies has revealed that UAs are more common than previously recorded. In 2015, Gleason et al. reported a 1.03% prevalence in the general pediatric population (5). Urachal malformations have varied clinical presentations, from asymptomatic (as the most common) to presenting with multiple non-specific symptoms including umbilical leakage, fever, abdominal pain, or acute/recurrent infection of the structure (6). Current management strategies for UAs include non-operative, conservative management in which the patient is followed to ensure symptoms do not worsen or recur, as well as surgical management in which the structure is removed, either in an open, laparoscopic, or robotically-assisted laparoscopic approach. The lack of a clear consensus on preferred management continues to persist due to the rarity of these defects and a paucity of existing data. Therefore, both approaches continue to be utilized. In this study, we aim to review the literature and identify current management strategies for pediatric patients with UAs.
Materials and methods
Search strategy
The following keywords were used to search the entire PubMed database on our search date of February 29, 2022, as follows: (urachal remnant) OR (urachal anomaly) OR (urachal abnormality) OR (patent urachus) OR (urachal diverticulum) OR (urachal sinus) OR (urachal cyst); these search terms were adjusted for use in Web of Science. Duplicates, articles published before 2000, and studies published in non-English languages were then removed using automated PubMed and WebOfScience filters.
Inclusion and exclusion criteria
Published original research was analyzed to evaluate the diagnosis and management of UAs in pediatric patients. Studies were included if they contained a group of pediatric patients with a diagnosed UA and data available on patient age, diagnostic techniques, and management. To focus on more recent management strategies, only articles published during or after the year 2000 were included. The abstract and title of the remaining studies were screened by two medical student independent reviewers (YG and DG) and the decision to include and exclude studies was taken in consensus with pediatric urology faculty. Figure 2 summarizes the article identification process. Studies were excluded if they fell into the following categories: animal studies, case reports, adult population included (>18 years of age), reviews, lack of abstract and full text, non-primary research (i.e., systematic review, literature reviews, etc.), and articles not primarily focused on UAs and their management. All studies that met the inclusion criteria were utilized to minimize study selection bias. Articles that remained were read in their entirety.
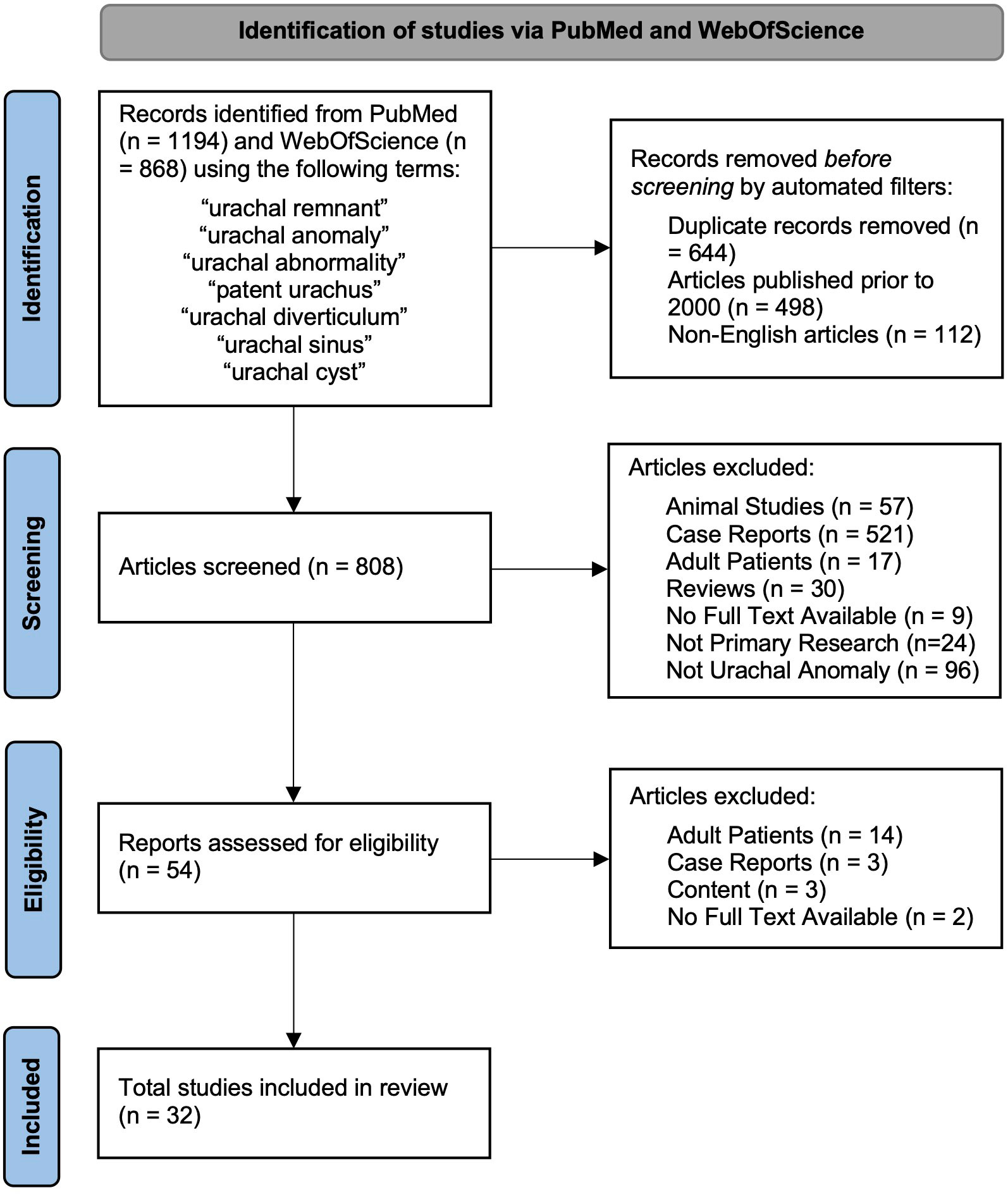
Figure 2 Flowchart of identification, screening, eligibility, and inclusion of studies in final analysis.
Study identification and characteristics
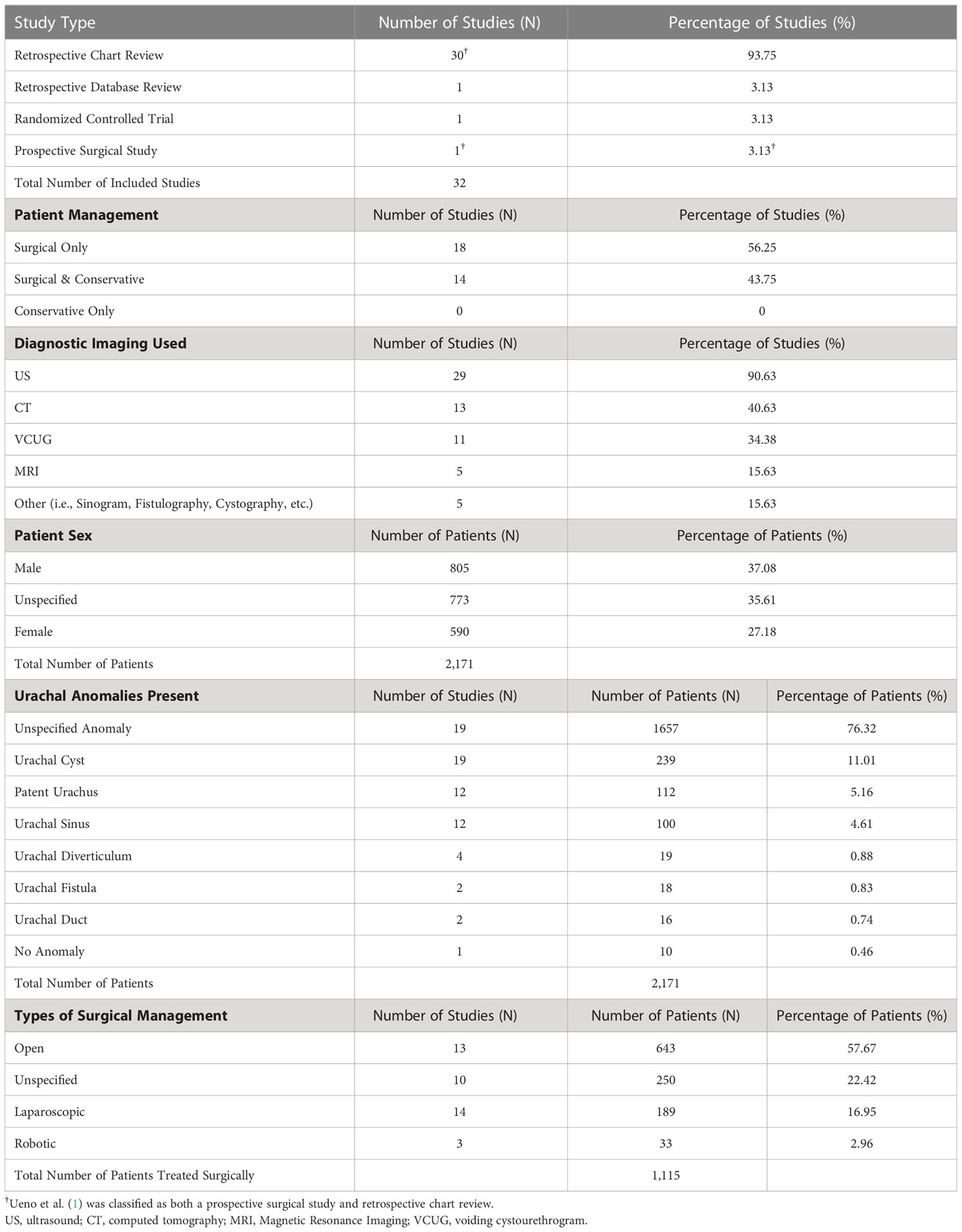
Of the 1,418 unique studies identified through the initial literature search, 32 were ultimately included in this review as described in Figure 2 along with reasons for exclusion. The characteristics of each individual study are listed in Appendix 1 including the study type, number of patients, diagnostic imaging, type of UA present (if specified), and management strategy. These characteristics are summarized in Table 1.
Results
Characteristics of the included studies, including study type, are noted in Table 1 (1, 5, 7–36). One of the retrospective chart reviews also contained elements of a prospective surgical study where 8 patients were randomly assigned to receive surgical treatment (1). Three of the studies (9.4%) classified as retrospective chart reviews discussed diagnostic methods and reviewed imaging studies used in diagnosis without primarily focusing on the treatment of UAs (8, 25, 31).
Of the 32 included studies, 18 of them (56.3%) reviewed patients who were only treated surgically and discussed variations in surgical technique and management, while 14 of them (43.8%) reviewed patients who received either conservative, surgical, or combined management. None of the studies reviewed solely contained patients that were treated conservatively. Every study included at least one patient requiring surgical intervention. All the studies used at least one form of imaging to confirm the diagnosis of urachal remnants, with ultrasound (US) being used most (in 90.6% of studies), followed by CT (40.6%) and VCUG (34.4%).
There was a total of 2,171 patients across all 32 studies which includes 805 male patients (37.1%), 590 female patients (27.2%), and 773 patients (35.6%) of unspecified sex. Many studies did not list specific UA subtypes for patients, however, where specified, urachal cysts were the most common anomaly - found in 19 studies, comprising 239 total patients (11%). A patent urachus and urachal sinus were the next most common anomalies, found in 19 and 12 studies, comprising 112 (5.2%) and 100 (4.6%) patients, respectively. Every study had at least one patient with a surgically managed urachal remnant, making up a total of 1,115 patients who were treated surgically. Though many studies did not list the specific surgical approach, open surgery was the most common (in 643 patients) and made up 57.7% of all surgical cases. Unspecified surgical approaches made up 22.4% of cases, and laparoscopic and robotic-assisted laparoscopic approaches made up 17% and 3% of all surgical cases, respectively. Laparoscopic techniques mentioned in the literature began in 2007, and robotic techniques began in 2013.
When specified, the subtype of the UA was listed in descending order of prevalence for each study within Appendix 1. Most studies also included a distinction between symptomatic and asymptomatic (incidental finding) UAs, and this is listed in Appendix 1 along with presenting symptoms in descending order of prevalence. Recommendations gathered from each included article are listed in Appendix 1 and recommendations regarding imaging or surgical techniques are listed in Appendix 1
Conversion from conservative to surgical management
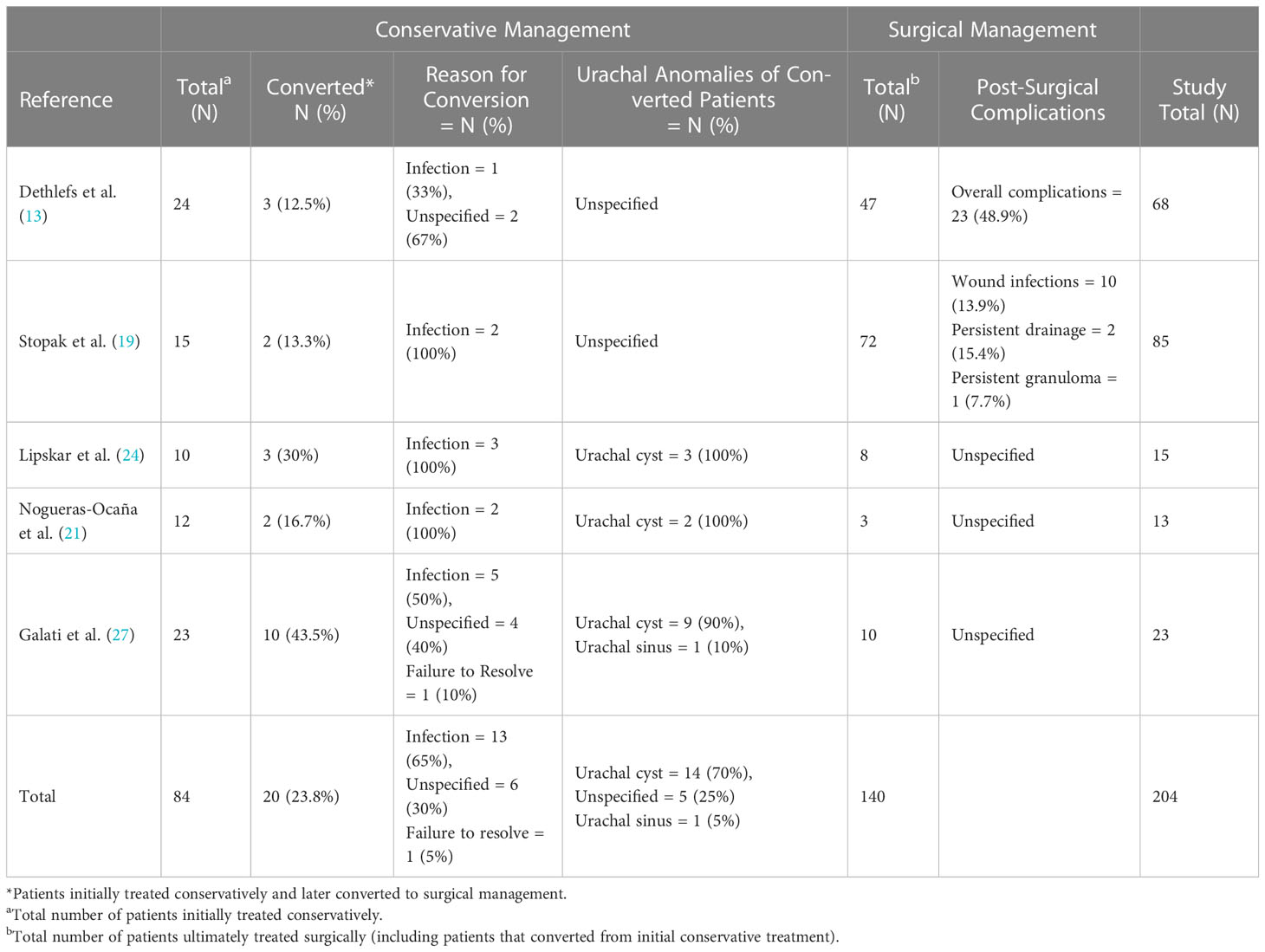
Out of the 32 total studies, our review identified five studies in the past two decades that included patients who were initially tried on conservative therapy that failed, leading to surgical intervention. Within these studies, we identified a total of 20 patients that were converted to surgical management with conversion rates ranging from 13% to 44% per study (13, 19, 21, 24, 27). The conversion percentage, the reason for the conversion, specific UA involved, and surgical complications are listed for each study in Table 2. The conservative management tab lists the total number of patients who were initially tried on conservative management and the number of patients who were ultimately converted to surgical correction. In the surgical management tab, the total number of surgical patients listed includes all surgically managed patients in the study, including those who were converted from initial conservative management. Out of these patients converted to surgical treatment, 14 of them had a urachal cyst (70%), 1 patient had a urachal sinus (5%), and 5 patients had unspecified anomalies (25%). The most common reason for conversion was an infection, found in 13 patients (65%). Of the total 20 patients who converted from conservative to surgical management, all were recurrently symptomatic. 5 (25%) initially presented symptomatically and 15 (75%) were unspecified. 2 (10%) were males and 18 (90%) were of unspecified gender.
Discussion
Originally, obliteration of the urachus was believed to be a strictly prenatal phenomenon, and thus persistence of any part of the urachus was thought to be pathological. In some reports from the early 1970s, it was recommended to remove all urachal remnants when discovered, even those discovered incidentally (3). This sentiment continued through the early 2000s, from 2000-2006, the recommended management of urachal anomalies was surgical excision regardless of symptom presentation, citing the potential for later infection or evolution into cancer. Even in 2006, Choi et al. supported surgical resection regardless of symptomatic status citing a lack of spontaneous involution and the potential for infection or evolution into cancer (30). In the past few decades, the more frequent use of advanced imaging studies (such as US) has increased the number of UAs diagnosed incidentally and provided insight regarding the presentation of these abnormalities, with the majority of UAs lacking any noticeable symptoms and containing a minimal risk of malignancy (1). With increased imaging data, studies have shown that involution of the urachus may even occur postnatally (1, 2). In 1998, Ziegler et al. demonstrated that a group of random, asymptomatic newborns all had a urachal remnant on US which spontaneously involuted on a second US exam in 3-5 months (2). In 2003, Ueno et al. corroborated spontaneous involutions in children up to 1 year of age, and was the earliest study to recommend conservative management, recommending non-invasive management for both symptomatic and asymptomatic presentations (1). In 2008 and 2009, the literature showed opposing recommendations for management. Three studies were published, with each giving a different suggestion for management: Yapo et al. continued to support surgical management regardless of symptom presentation (notably, the basis for excision of asymptomatic patients was due to a stated 51% chance of malignancy), Galati et al. supported conservative management for all asymptomatic patients and all symptomatic patients under 6 months of age, and Copp et al. stated an inability to provide recommendations based on inconclusive evidence (26–28).
Starting in 2010, the literature appears to address the inconsistency in management recommendations and sought to investigate the best course of management. While these most recent studies consistently support the use of conservative management for asymptomatic presentation. From this time point, it is debated which patients require surgery based on age and symptom presentation. In 2010, Lipskar et al. offered the first proposed treatment algorithm for distinguishing between surgical and conservative management for symptomatic patients, and based the algorithm on symptom presentation, type of UA, and whether UA resolution was achieved after antibiotic treatment (24).
Further studies found that the associated pathological findings are typically benign, and the calculated number needed to treat to prevent the risk of adenocarcinoma formation is large, at 5,721 persons (5). Thus, removal of asymptomatic remnants during the first year of life is likely unnecessary. Most remnants may be observed conservatively, with infected remnants initially receiving antibiotic treatment. If a patient fails conservative management and/or presents with severe symptoms, then surgical management should be considered. Surgical excision by an open approach has typically been the gold standard, but over the last two decades, laparoscopic and robotic approaches have shown to be safe and effective alternatives to open surgery with increased cosmetic advantages (9, 10, 12, 14–17, 22, 35). The current landscape of UA management consistently juggles conservative and surgical therapies, with no clear predictive signs or guidance on when patients who fail conservative treatment should be switched to definitive surgical management.
Effectiveness of conservative treatment
In 2008, Galati et al. performed a retrospective chart review and discovered 23 patients with UAs (including 12 urachal cysts, 9 urachal sinuses, and 2 patent urachus) who were all initially managed conservatively with silver nitrate and/or unspecified length of observation (27). Urachal sinuses were more common in boys (8 out of 9) and urachal cysts were more common in girls (9 out of 12), while patent urachi occurred equally in both sexes. About half of the patients required surgical intervention and they recommended surgery for recurrent symptoms or for failure of involution after 6 months of follow-up. The length of time from the initial diagnosis to involution was not noted. Of the 10 patients who converted to surgical management, 9 had urachal cysts and 1 had a urachal sinus. In 2010, Lipskar et al. performed a retrospective chart review of 15 initially symptomatic patients of unspecified gender (24). The group was composed of 10 urachal cysts and 5 patent urachal sinuses. 10 patients were initially managed conservatively with infected urachal cysts receiving percutaneous drainage and antibiotics. 3 out of the 10 patients had recurrent infections and were converted to surgical management.
In a retrospective case series performed by Nogueras-Ocana et al. in 2014, 13 patients with UAs were identified, 12 of whom were initially managed conservatively with US imaging every 6 months for the first two years and annually thereafter (21). Two patients (both male, initially presenting symptomatically, and with urachal cysts) were converted to surgical treatment due to reinfection after an initial course of antibiotics. In patients who achieved spontaneous resolution, the time between diagnosis and resolution ranged from 2 to 24 months. In 2015, Stopak et al. performed a retrospective review of 85 patients with UAs which was followed up by a 68-patient retrospective review by Dethlefs et al. in 2019 to monitor the evolution of treatment within the same health system (13, 19). In the initial study, it was recommended that UAs be treated conservatively in the first 6 to 12 months of life, and surgically thereafter. Only 15 patients were managed conservatively, with 2 of them requiring surgical management. In the follow-up study, it was noted that care was better shifted to nonoperative management. 24 patients were managed conservatively, with only 3 patients ultimately requiring surgery.
Though we were able to identify five articles that contained patients who were converted from conservative to surgical management (13, 19, 21, 24, 27), the lack of consistency between these studies makes it difficult to ascertain salient features of patient demographics or presentations that are predictive of conversion to surgical therapy. The included studies did not have enough patients convert from conservative to surgical management to determine if there are predictive variables for those necessitating surgery such as: certain urachal remnant subtypes, presenting symptoms, age, or gender. In addition, although there were a few patients with noted symptomatic patent urachi who studies claimed to be treated with conservative management (27), their degree of patency was unknown, and further investigation into this specific anomaly subtype is required to determine the proper management of these patients. Surgical correction may be needed for treatment if this anomaly is persistently symptomatic (e.g., drainage of urine directly from the umbilicus) does not close spontaneously within the first few months of life.
To our knowledge, there has been no comprehensive review to date on UAs. This review identifies recent shifts in preference towards avoiding surgical management for patients under one year of age, with evidence absent for whether this suggestion in management remains true regardless of UA type, presenting symptoms, gender, premature birth status, or specific age. We are currently unable to identify patterns of patients that would be more likely to require a conversion to surgical management. Given the lack of information, we are unable to algorithmically decipher which patients are ideal candidates for surgery and which patients should be observed. Also, due to the lack of randomized controlled trials and prospective studies in the literature, we were also unable to determine an ideal conservative management strategy with included follow-up timelines. Thus it would be necessary to perform a large-scale, multi-site prospective study analyzing demographic information, UA subtypes, and symptomatology regarding different protocol strategies to determine the most efficacious treatment strategy for this pathology. Though, this is not feasible due to the relative rarity of complications and potential for conversion to adenocarcinoma (1).
Conclusions
The studies included in this review support initial conservative management over prophylactic surgical excision of pediatric UAs, even in symptomatic cases. In the past few decades, a conservative approach has been increasingly favored, especially for the treatment of symptomatic remnants in children 6 months to 1 year of age, where spontaneous resolution and involution were likely, even if initially infected. In older patients, conservative treatment with antibiotics in infected cases has been shown to effectively treat symptoms – thereby eliminating the need for surgical intervention except for rare cases presenting with recurrent infections. There remains a question regarding which associated patient demographics, UA subtypes, or presenting symptoms predict the need for eventual surgical intervention. Currently available studies lack large enough sample sizes to draw any apt correlations. Without sufficient data from prospective studies, the authors are unable to provide a worthwhile management algorithm.
Author contributions
YG, DG, KI, PE, and AS made substantial contributions to conception and design. YG, DG and KI performed acquisition and interpretation of data. YG, DG, KI drafted the article. YG, DG, KI, PE and AS revised the paper critically for important intellectual content. YG, DG, KI, PE, and AS approved of the final version to be published. All authors contributed to the article and approved the submitted version.
Acknowledgments
Publication made possible in part by support from the Nemours Grants for Open Access from Library Services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2023.1159439/full#supplementary-material
Abbreviations
UA, urachal anomaly; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; VCUG, voiding cystourethrogram.
References
1. Ueno T, Hashimoto H, Yokoyama H, Ito M, Kouda K, Kanamaru H. Urachal anomalies: Ultrasonography and management. J Pediatr Surg (2003) 38(8):1203–7. doi: 10.1016/S0022-3468(03)00268-9
2. Zieger B, Sokol B, Rohrschneider WK, Darge K, Tröger J. Sonomorphology and involution of the normal urachus in asymptomatic newborns. Pediatr Radiol (1998) 28(3):156–61. doi: 10.1007/s002470050318
3. Blichert-Toft M, Nielsen OV. Congenital patient urachus and acquired variants. diagnosis and treatment. Review of the literature and report of five cases. Acta Chir Scand (1971) 137(8):807–14.
4. Zenitani M, Nose S, Oue T. Prevalence of urachal remnants in children according to age and their anatomic variants. Pediatr Surg Int (2022) 38(10):1495–500. doi: 10.1007/s00383-022-05183-2
5. Gleason JM, Bowlin PR, Bagli DJ, Lorenzo AJ, Hassouna T, Koyle MA, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. (2015) 193(2):632–6. doi: 10.1016/j.juro.2014.09.004
6. Snyder CL. Current management of umbilical abnormalities and related anomalies. Semin Pediatr Surg (2007) 16(1):41–9. doi: 10.1053/j.sempedsurg.2006.10.006
7. Nissen M, Rogge P, Sander V, Alrefai M, Romanova A, Tröbs RB. Pediatric urachal anomalies: Monocentric experience and mini-review of literature. Children (2022) 9(1):72. doi: 10.3390/children9010072
8. Olthof DC, Reemst S, Sleeboom C, Kuijper CF, van Schuppen J, Derikx JPM, et al. Diagnostic accuracy of abdominal ultrasound to detect pathology that needs surgical exploration in children with umbilical discharge. J Pediatr Surg (2021) 56(8):1436–40. doi: 10.1016/j.jpedsurg.2020.07.036
9. Osumah TS, Granberg CF, Butaney M, Gearman DJ, Ahmed M, Gargollo PC. Robot-assisted laparoscopic urachal excision using hidden incision endoscopic surgery technique in pediatric patients. J Endourol. (2021) 35(6):937–43. doi: 10.1089/end.2019.0525
10. Hashizume N, Ohtaki M, Nihei K, Sakamoto K, Shirahata Y, Shimada T, et al. Laparoscopic surgery for urachal remnants in pubescent children: a case series. Surg Case Rep (2020) 6(1):120. doi: 10.1186/s40792-020-00884-z
11. Orbatu D, Alaygut D. Evaluation and management of urachal remnants in children. Pediatr Int (2020) 62(10):1158–61. doi: 10.1111/ped.14272
12. Basuguy E, Okur MH, Zeytun H, Arslan S, Aydogdu B, Otcu S, et al. Management of symptomatic urachal cysts in children. Niger J Clin Pract (2019) 22(1):113–6. doi: 10.4103/njcp.njcp_228_18
13. Dethlefs CR, Abdessalam SF, Raynor SC, Perry DA, Allbery SM, Lyden ER, et al. Conservative management of urachal anomalies. J Pediatr Surg (2019) 54(5):1054–8. doi: 10.1016/j.jpedsurg.2019.01.039
14. Tanaka K, Misawa T, Baba Y, Ohashi S, Suwa K, Ashizuka S, et al. Surgical management of urachal remnants in children: Open versus laparoscopic approach. Med (Baltimore). (2019) 98(40):e17480. doi: 10.1097/MD.0000000000017480
15. Ahmed H, Howe AS, Dyer LL, Fine RG, Gitlin JS, Schlussel RN, et al. Robot-assisted laparoscopic urachal excision in children. Urology (2017) 106:103–6. doi: 10.1016/j.urology.2017.03.044
16. Bertozzi M, Riccioni S, Nardi N, Appignani A. The role of laparoscopy in the management of urachal anomalies in children. Ann Pediatr Surg (2017) 13(2):85–90. doi: 10.4314/aps.v13i2
17. Sukhotnik I, Aranovich I, Mansur B, Matter I, Kandelis Y, Halachmi S. Laparoscopic surgery of urachal anomalies: A single-center experience. Isr Med Assoc J (2016) 18(11):673–6.
18. Sato H, Furuta S, Tsuji S, Kawase H, Kitagawa H. The current strategy for urachal remnants. Pediatr Surg Int (2015) 31(6):581–7. doi: 10.1007/s00383-015-3712-1
19. Stopak JK, Azarow KS, Abdessalam SF, Raynor SC, Perry DA, Cusick RA. Trends in surgical management of urachal anomalies. J Pediatr Surg (2015) 50(8):1334–7. doi: 10.1016/j.jpedsurg.2015.04.020
20. Bertozzi M, Riccioni S, Appignani A. Laparoscopic treatment of symptomatic urachal remnants in children. J Endourol. (2014) 28(9):1091–6. doi: 10.1089/end.2014.0203
21. Nogueras-Ocaña M, Rodríguez-Belmonte R, Uberos-Fernández J, Jiménez-Pacheco A, Merino-Salas S, Zuluaga-Gómez A. Urachal anomalies in children: Surgical or conservative treatment? J Pediatr Urol. (2014) 10(3):522–6. doi: 10.1016/j.jpurol.2013.11.010
22. Masuko T, Uchida H, Kawashima H, Tanaka Y, Deie K, Iwanaka T. Laparoscopic excision of urachal remnants is a safe and effective alternative to open surgery in children. J Laparoendosc Adv Surg Tech. (2013) 23(12):1016–9. doi: 10.1089/lap.2013.0127
23. Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg (2013) 48(10):2148–52. doi: 10.1016/j.jpedsurg.2013.02.069
24. Lipskar AM, Glick RD, Rosen NG, Layliev J, Hong AR, Dolgin SE, et al. Nonoperative management of symptomatic urachal anomalies. J Pediatr Surg (2010) 45(5):1016–9. doi: 10.1016/j.jpedsurg.2010.02.031
25. Widni EE, Höllwarth ME, Haxhija EQ. The impact of preoperative ultrasound on correct diagnosis of urachal remnants in children. J Pediatr Surg (2010) 45(7):1433–7. doi: 10.1016/j.jpedsurg.2010.01.001
26. Copp HL, Wong IY, Krishnan C, Malhotra S, Kennedy WA. Clinical presentation and urachal remnant pathology: Implications for treatment. J Urol. (2009) 182(4S):1921–4. doi: 10.1016/j.juro.2009.03.026
27. Galati V, Donovan B, Ramji F, Campbell J, Kropp BP, Frimberger D. Management of urachal remnants in early childhood. J Urol. (2008) 180(4S):1824–7. doi: 10.1016/j.juro.2008.03.105
28. Yapo BR, Gerges B, Holland AJA. Investigation and management of suspected urachal anomalies in children. Pediatr Surg Int (2008) 24(5):589–92. doi: 10.1007/s00383-008-2136-6
29. Turial S, Hueckstaedt T, Schier F, Fahlenkamp D. Laparoscopic treatment of urachal remnants in children. J Urol. (2007) 177(5):1864–6. doi: 10.1016/j.juro.2007.01.049
30. Choi YJ, Kim JM, Ahn SY, Oh JT, Han SW, Lee JS. Urachal anomalies in children: A single center experience. Yonsei Med J (2006) 47(6):782–6. doi: 10.3349/ymj.2006.47.6.782
31. Little DC, Shah SR, St Peter SD, Calkins CM, Murphy JP, Gatti JM, et al. Urachal anomalies in children: the vanishing relevance of the preoperative voiding cystourethrogram. J Pediatr Surg (2005) 40(12):1874–6. doi: 10.1016/j.jpedsurg.2005.08.029
32. Huang CS, Luo CC, Chao HC, Chen HM, Chu SM. Urachal anomalies in children: Experience at one institution. Chang Gung Med J (2003) 26(6):412–6.
33. McCollum MO, MacNeily AE, Blair GK. Surgical implications of urachal remnants: Presentation and management. J Pediatr Surg (2003) 38(5):798–803. doi: 10.1016/jpsu.2003.50170
34. Pesce C, Costa L, Musi L, Campobasso P, Zimbardo L. Relevance of infection in children with urachal cysts. Eur Urol. (2000) 38(4):457–60. doi: 10.1159/000020324
35. Sun J, Zhu YJ, Shi CR, Zhao HT, He R, Liu GH. Laparoscopic radical excision of urachal remnants with recurrent infection in infants. J Endourol. (2010) 24(8):1329–32. doi: 10.1089/end.2009.0141
Keywords: pediatrics - children, urachal abnormalities, management, literature review, urachus, infection
Citation: Ghattas YS, Gelikman DG, Ibanez KR, Ellsworth P and Seth A (2023) Current management strategies of urachal anomalies in pediatric patients: A scoping review. Front. Urol. 3:1159439. doi: 10.3389/fruro.2023.1159439
Received: 05 February 2023; Accepted: 02 March 2023;
Published: 17 March 2023.
Edited by:
Seth A. Alpert, Nationwide Children’s Hospital, United StatesReviewed by:
Alessandro Boscarelli, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyJessica Hannick, Rainbow Babies & Children’s Hospital, United States
Copyright © 2023 Ghattas, Gelikman, Ibanez, Ellsworth and Seth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhishek Seth, YWJoaXNoZWsuc2V0aEBuZW1vdXJzLm9yZw==
 Yasmine S. Ghattas
Yasmine S. Ghattas David G. Gelikman
David G. Gelikman Kristen R. Ibanez
Kristen R. Ibanez Pamela Ellsworth1,2
Pamela Ellsworth1,2 Abhishek Seth
Abhishek Seth