- 1UroPartners/SolarisHealth Partners, Chicago, IL, United States
- 2Convergent Genomics, South San Francisco, CA, United States
Introduction and aim of study: Metachronous upper tract urothelial carcinoma (UTUC) is a rare yet aggressive malignancy that is often multifocal and invasive at the time of diagnosis. Unfortunately, the rarity of metachronous UTUC results in a paucity of targeted data, as current literature and clinical management of this tumor is largely extrapolated from that of bladder cancer. Urinary comprehensive genomic profiling with the UroAmp assay identifies six general classes of tumor-mutations present in the urine and thus, may aid in detecting UTUC when the limitations of current tools impede definitive diagnosis. We describe the utility of urinary comprehensive genomic profiling in confirming the provider’s suspicion for metachronous UTUC and recommending radical nephroureterectomy.
Patient case: A 68-year-old male with a history of recurrent carcinoma in situ (CIS) of the bladder presented to the urology clinic in 2022 for continued surveillance. Abnormal soft tissue thickening surrounding the proximal right ureter, revealed on computerized tomography urography, prompted further evaluation. Selective right upper tract cytology was indeterminate, and urinary comprehensive genomic profiling was ordered to adjudicate. No tumor was visualized on ureteroscopy however the cytologic brush biopsy of the renal pelvis and proximal ureter were positive for urothelial carcinoma (UC) and/or CIS. UroAmp testing identified genomic features associated with high-grade UC, risk of invasion, and a high genomic disease burden.
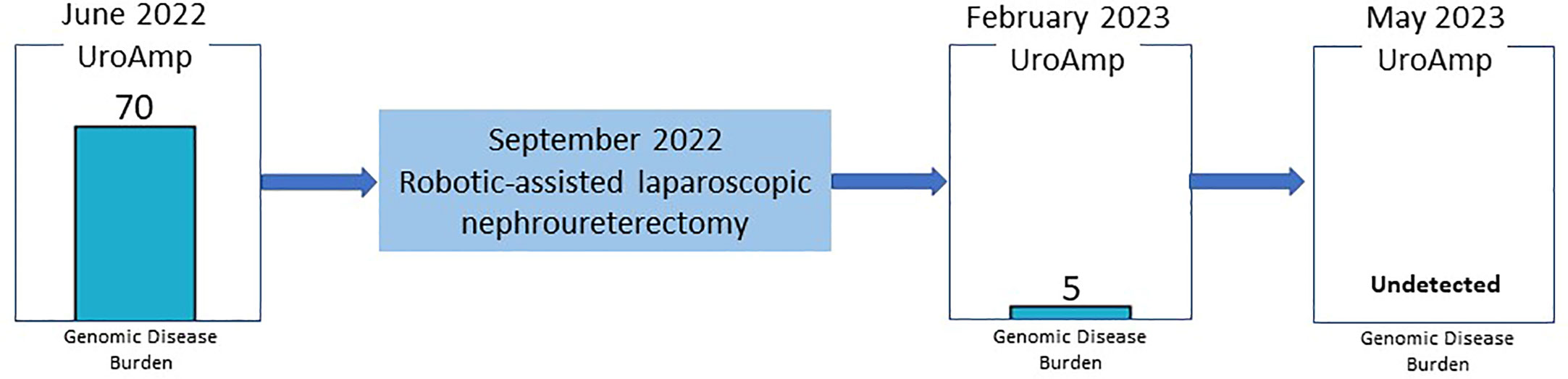
Results: The patient underwent a right kidney and ureter nephroureterectomy in September 2022. Surgical pathology confirmed non-invasive multifocal urothelial CIS. A postoperative urinary comprehensive genomic profiling in February and May of 2023 detected no evidence of residual disease, consistent with complete resection of the tumor. The provider will continue intensive urinary comprehensive genomic profile monitoring coupled with conventional surveillance.
Conclusion: Urinary measurement of mutated UC genes correlate with disease burden, pathologic grade, and invasion risk and provide clinical utility when reliance on visual confirmation and cytology were not definitive or feasible.
Introduction
Upper tract urothelial carcinoma (UTUC) accounts for 5-10% of cancers derived from the urothelium (1–3). The incidence of primary UTUC with concomitant bladder cancer is 17%; however, metachronous UTUC following a primary bladder cancer diagnosis occurs in 0.7-5% of patients (2–4). Although rare, UTUC is an aggressive malignancy, which is often multifocal and invasive at time of diagnosis; thus, early, and accurate recognition is critical (1, 5). Current diagnostic tools, such as cytology and cytologic brush biopsy, have documented sensitivity, specificity, and discordance limitations that reduce confidence when recommending guideline indicated interventions, such as radical nephroureterectomy. The Paris System for Reporting Urinary Cytology (6) has created standardized cytologic criteria for diagnosis and improved urologic cytology accuracy, but nephrolithiasis, artifact, and inflammation still make it difficult to obtain a definitive diagnosis, and atypical or suspicious findings are common. The limitations of traditional UTUC evaluation highlight the need for new tools. A noninvasive diagnostic that quantitatively identifies the presence of UTUC could help confidently risk stratify patients, enable guideline adherence, and improve outcomes. Urinary comprehensive genomic profiling uses next-generation sequencing to identify tumor-mutations present in the urine. The UroAmp™ assay (Convergent Genomics, South San Francisco, CA) performs urinary comprehensive genomic profiling to identify six classes of tumor mutations: single-nucleotide variants, gene-level copy-number variants, insertion-deletions, copy-neutral loss of heterozygosity, microsatellite instability, and whole-genome aneuploidy. It was built to identify mutations associated with UC as well as predict molecular grade, disease progression, and recurrence risk (7, 8). Here, we describe the use of urinary comprehensive genomic profiling to confirm metachronous UTUC and reassure the provider’s recommendation for a patient to proceed with a radical nephroureterectomy.
Case presentation
A 68-year-old non-smoking male with a history of recurrent carcinoma in situ (CIS) of the bladder since 2016, hypertension, hyperlipidemia, diabetes mellitus, and coronary artery disease presented to the urology clinic in 2022 for continued surveillance. The patient’s most recent recurrence was 2017, when a surveillance cystoscopy showed a cobblestone appearance of the right trigone and imaging revealed new right hydronephrosis. A transurethral resection and retrograde ureteropyelogram were performed and demonstrated mild hydroureteronephrosis with no filling defect and no specific pathology identified in the upper tract. Pathology from the bladder demonstrated recurrent CIS which was treated with repeat resection followed by induction and a full course of maintenance Bacillus Calmette-Guérin (BCG) therapy. No recurrences had been detected since, and the mild hydronephrosis was stable on imaging.
In April 2022, surveillance computerized tomography (CT) urography showed new abnormal imaging changes in the right ureter suggesting recent passage of kidney stone(s). Repeat CT urography in May demonstrated abnormal soft tissue thickening surrounding the right proximal ureter with intact lumen and drainage. The patient returned to clinic later that month for follow-up cystoscopy, which was negative. Office-based cytology, selective cytology, and retrograde pyelogram were performed, with the pyelogram showing normal upper tract contours and drainage with no filling defect. Cytology of the lower tract was negative for dysplastic cells; however, the right upper tract cytology was “suspicious” for malignancy and dysplastic urothelial cells were suggestive of high-grade UC/CIS. In early June, the physician ordered urinary comprehensive genomic profiling using UroAmp (results described below) to help adjudicate the abnormal cytology. Nine days later, the patient underwent a diagnostic ureteroscopy of the right renal pelvis and proximal ureter with brush biopsies. No visual lesions, either papillary or sessile, were seen in the upper tract; however, selective cytology and cytologic brush biopsy of the renal pelvis and proximal ureter were positive for high-grade UC and/or CIS.
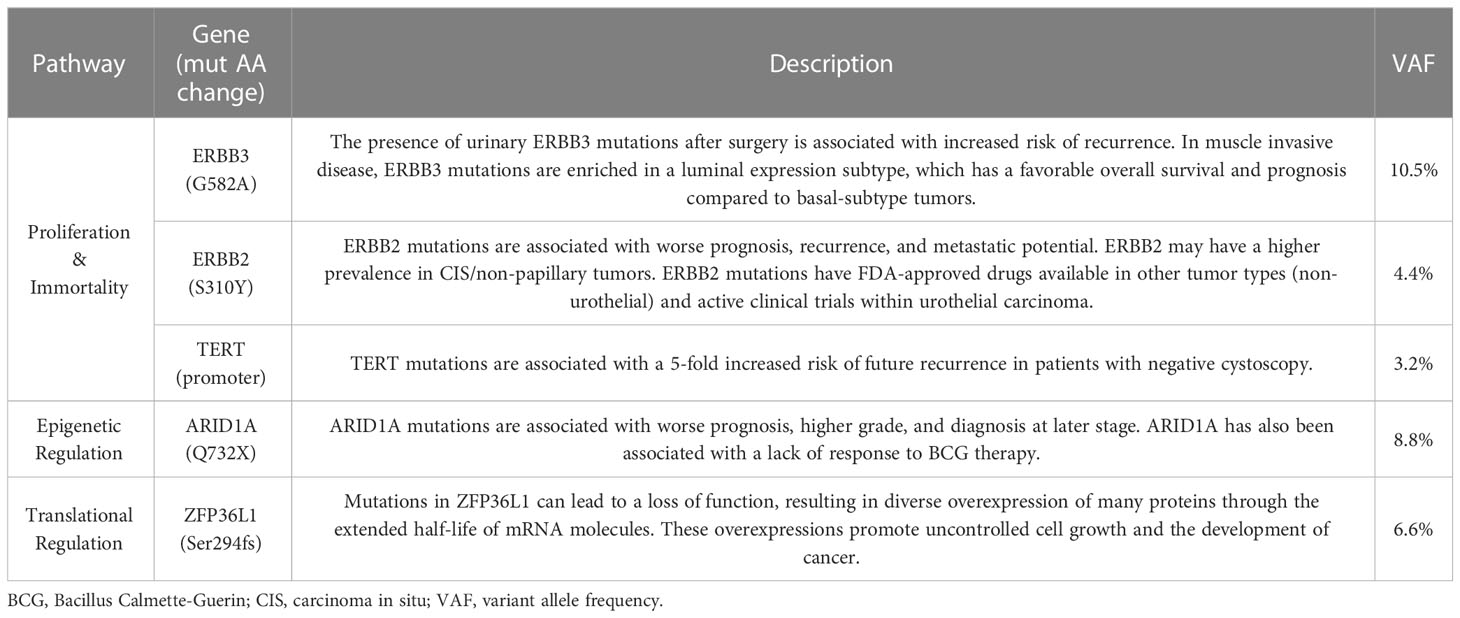
In late June, the patient was seen in clinic, post-operatively, to discuss UTUC diagnosis and recommendations for treatment. Notably, the UroAmp surveillance algorithm reported a high-risk for cancer recurrence and identified genomic features associated with high-grade UC. Urinary comprehensive genomic profiling identified five somatic mutations at variant allele frequencies ranging from 3.2%-10.5%. The mutational profile consisted of single nucleotide variants in ARID1A (premature stop codon), ERBB2 (TCGA hotspot), ERBB3, and TERT (TCGA hotspot), and a multi-base deletion causing a frameshift in ZFP36L1. The following prognostic insight was summarized from available literature: ARID1A mutation has been associated with BCG-resistance (9), ERBB2 and ERBB3 mutations are more prevalent in non-papillary/CIS tumors (consistent with negative ureteroscopy) (10), and the presence of insertion-deletions are also enriched in high-grade tumors (8). UroAmp further revealed a high genomic disease burden of 70 which alerted the provider that this patient’s mutational intensity is higher than 70% of UC patients previously evaluated.
Based on the combination of cytologic brush biopsy, selective cytology, and urinary comprehensive genomic profiling results, the patient was referred to an academic medical center, and subsequently underwent a right kidney and ureter robotic-assisted laparoscopic nephroureterectomy in September 2022. Surgical pathology confirmed multifocal urothelial high-grade CIS of the right renal pelvis and ureter. No invasive carcinoma was identified, and the renal parenchyma was uninvolved but with mild interstitial inflammation. Surgical margins were free of tumor (Figure 1).
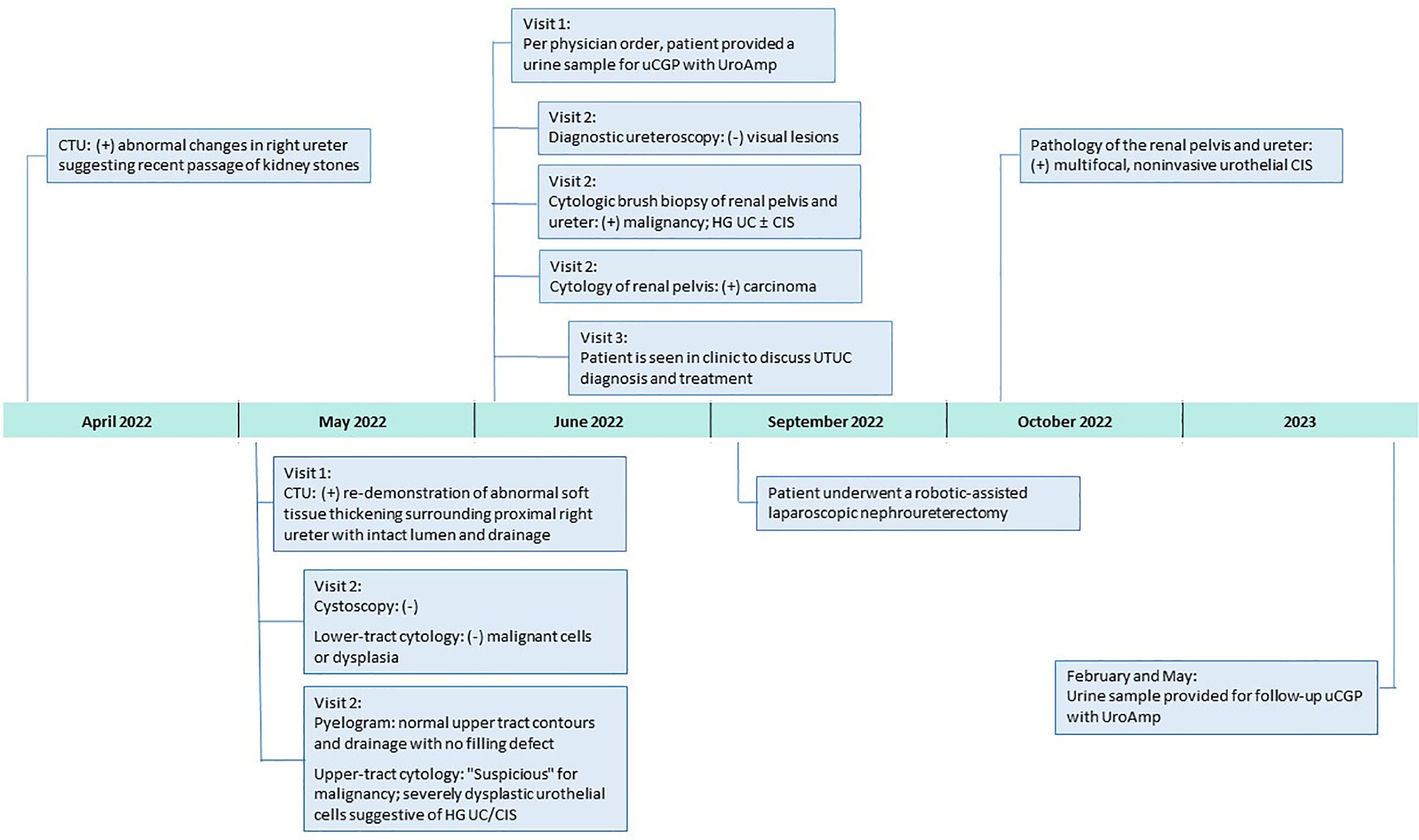
Figure 1 Clinical Course After 2022 Surveillance Visit. CIS, carcinoma in situ; CTU, computerized tomography urography; HG, high-grade; uCGP, urinary comprehensive genomic profiling; UC, urothelial carcinoma.
Since the nephroureterectomy, the patient has provided two urine samples for urinary comprehensive genomic profiling during follow-up visits. The first test, in February 2023, reported a genomic disease burden of 5 and found none of the original mutations present. No genomic disease burden was detected in the second test obtained in May 2023 (Figure 2). At this time, the provider plans to continue intensive monitoring with urinary comprehensive genomic profile testing coupled with conventional surveillance.
Discussion
Metachronous UTUC following primary bladder cancer is rare and difficult to confidently identify given quandaries that arise from canonical diagnostic tools. Here, the initial workup showed abnormal soft tissue thickening on CT scan, indeterminate cytology in the presence of negative cystoscopy, negative ureteroscopy, and normal retrograde pyelogram. Follow-up diagnostic ureteroscopy was visually negative and only found positive findings via selective cytology and cytologic brush biopsy. These cytologic findings were used to diagnose this patient with UTUC and recommend surgical intervention as per standard of care. There were, however, significant concerns from both the patient and surgeon about choosing radical nephroureterectomy based solely on cytology given its limitations (1, 6, 11, 12). Upper tract urine cytology has grade-dependent specificity for the diagnosis of carcinoma (67%-96%) and poor sensitivity (29%-76%, with most studies around 50%) (1, 6, 7, 13–20). Along with inter-observer variability and a high rate of indeterminate findings, urologists are unable to confidently diagnose UTUC when contemplating radical nephroureterectomy (1, 6, 7, 13–20). Here, the patient’s initial cytology was “suspicious” for malignancy, thus prompting the provider to order urinary comprehensive genomic profiling to adjudicate the indeterminate result and complement any additional findings from the planned ureteroscopy. Notably, urinary comprehensive genomic profiling results encouraged the provider to continue his investigation for potential malignancy.
Given the challenges of staging UTUC, the decision to recommend radical nephroureterectomy is largely based on the diagnosis of high-grade tumor(s) (1). Because histologic evaluation may be impacted by insufficient tissue volume, artifacts, and technique/instrumentation, the use of cytologic brush biopsy is common (1). In a recent study, concordance between brush biopsy and radical nephroureterectomy tissue pathology was 41.1% (grading) and 34.5% (staging), creating insufficient clarity for preoperative planning (21) and highlighting the need for definitive preoperative diagnostic tools to affirm radical nephroureterectomy recommendations.
UroAmp’s previously validated diagnostic algorithm has been shown to identify UC with 96% sensitivity and 90% specificity for de novo tumors, and a molecular grading algorithm identifies high pathologic grade with 88% positive predictive value and 95% specificity (8). In a cohort of previously analyzed UTUC urine compared to bladder cancer, UroAmp correctly identified 100% of UTUC specimens as disease positive (Figure 3; Supplemental Material) (8). This patient’s mutation profile included ERBB2 and ERBB3 mutations, which promote cell proliferation (22). They were also positive for ARID1A mutation, which is associated with high-grade UC and resistance to BCG (9). Found to occur in up to 84% of UC cases, the presence of TERT promoter mutations is associated with a higher risk of recurrence (23). ERBB2/ERBB3 are also associated with non-papillary/CIS tumors and predict responsiveness to ERBB pathway inhibitors (trastuzumab, lapatinib) (9, 22) (Table 1). After reviewing the mutations and genomic disease burden provided by UroAmp, the physician was able to confidently recommend radical nephroureterectomy. Notably, when the patient returned to clinic for post-surgical follow-up, UroAmp surveillance testing revealed no evidence of residual disease as none of the tumor’s defining mutations could be detected. Continued surveillance will look for re-emergence of these mutations as evidence of recurrence.
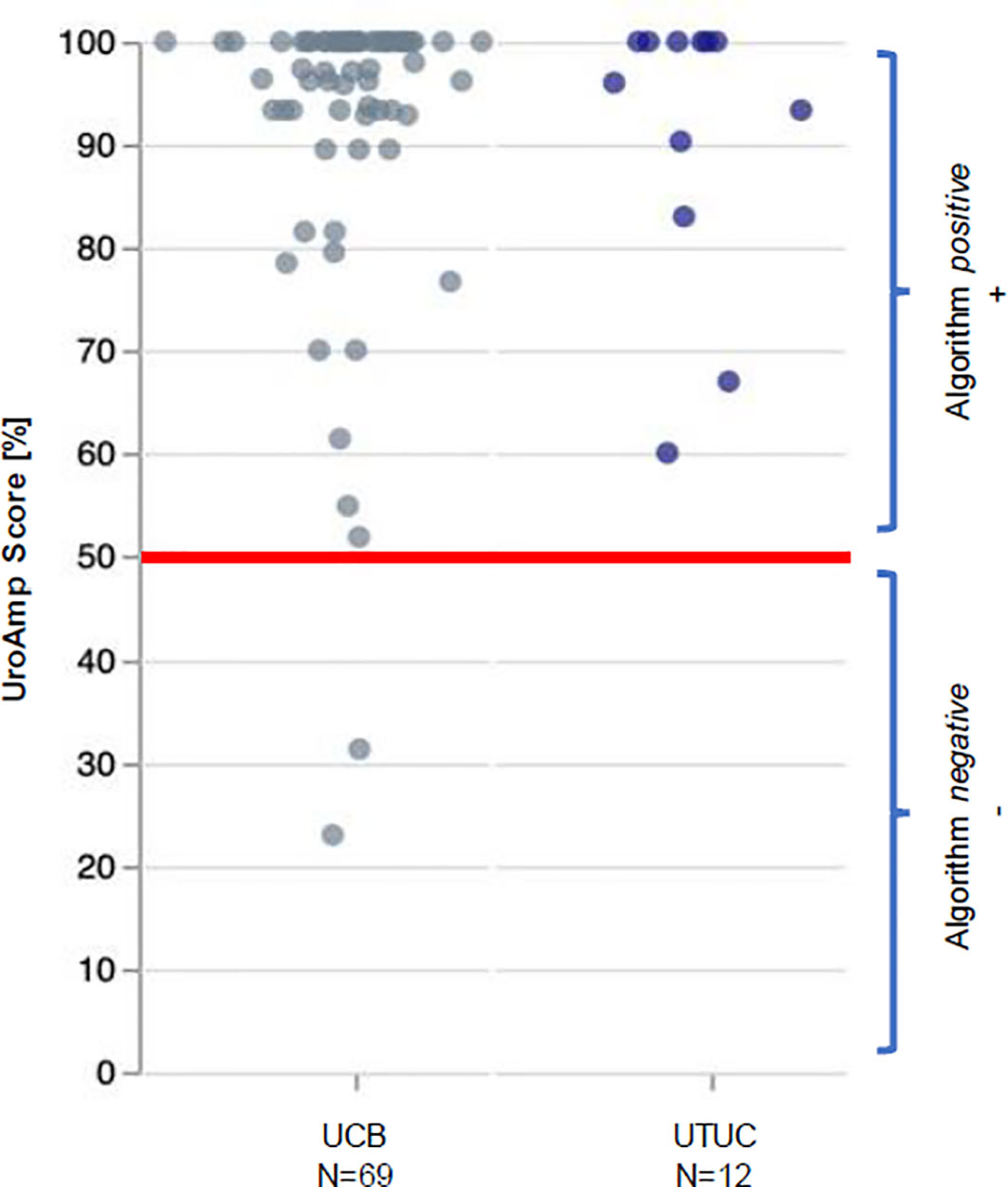
Figure 3 UroAmp Disease Classification of Urothelial Carcinoma. UCB, urothelial carcinoma of the bladder; UTUC, upper tract urothelial carcinoma.
In patients with non-muscle invasive UC, the projected 2030 annual costs of $19 billion in the United States will be largely driven by disease recurrence and progression as they necessitate the need for continuous treatment and intensive surveillance (24). Compared to other cancers, management of UC yields the highest economic burden, as rates of recurrence and disease progression remain high (>45%) (25–28). The high cost of care has not translated into improved care, as annual mortality has declined only 2% since 2015 (24). With genomic information, physicians are equipped to make decisions about de-intensifying surveillance, de-escalating therapy in non-responsive patients and/or hastening the time to recommend surgical intervention. These modifications in care have been proven to mitigate high costs (24). Although the financial impact of urinary comprehensive genomic profiling has yet to be determined, access to a genomic profile may allow for cost mitigation strategies as described by Joyce and provide actionable, patient specific data. For our patient, the availability of a urinary comprehensive genomic profile reassured the physician’s decision to proceed with a radical nephroureterectomy which averages $11,793 to $23,235 per patient (29). Alternatively, delaying surgical intervention in a patient with high-grade disease may have led to additional costs related to management of persistent and/or progressive disease.
For patients with non-metastatic high-grade UTUC, radical nephroureterectomy is recommended; however, this procedure has significant perioperative risks, especially in older patients with comorbidities (1, 5, 30). After radical nephroureterectomy, the risk of serious complications is between 11.3-18.2% (5). The rate of perioperative complications secondary to radical nephroureterectomy is limited; however, the most frequently reported include infection (surgical site, sepsis), blood loss requiring transfusion, and renal failure (5, 30, 31). In another alternative clinical scenario, the risk of performing an radical nephroureterectomy where surgical pathology is ultimately negative for malignancy and does not confirm the initial brush biopsy also presents a significant potential healthcare expense, risk for future renal insufficiency to the patient without clinical benefit, and medical liability risk to the treating physicians. Given this patient’s age and comorbidities, an accurate diagnosis of UTUC is prudent when contemplating risks associated with radical nephroureterectomy.
Conclusion
The diagnosis of metachronous UTUC and recommendation for radical nephroureterectomy were reassured with urinary comprehensive genomic profiling, a new noninvasive diagnostic validated to detect UC with high sensitivity and positive predictive value. Urinary measurement of prognostic genes correlating with high pathologic grade, invasion risk, and genomic disease burden provided clinical utility in this case when reliance on visual confirmation and cytologic brush biopsy were not definitive or feasible. Urinary comprehensive genomic profiling may also prove beneficial in adjudicating indeterminate cytology, detecting UTUC, and providing assessment of grade and invasion risk in scenarios where cytologic brush biopsy is unavailable or insufficient in size for definitive diagnosis, grading, and staging. A prospective study to corroborate findings from this case and the case-controlled cohort is underway.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
All the procedures involving human subjects described in the study were performed in accordance with the Declaration of Helsinki and were approved by WCG IRB (IRB00000533) under IRB protocol number 120160486. The patients/participants provided their written informed consent to participate in this study. Written and verbal informed consent was obtained from the participant(s)/patient(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PY and CW contributed equally to this work and share first authorship. PY was responsible for conception and design of the study. PY and KP were responsible for the acquisition of data. CW and BM were responsible for drafting the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Source of Funding: Research support by Convergent Genomics
Conflict of interest
PY is a research investigator for Convergent Genomics. CW, BM, KP, BJ, VB, and TL report being employees and shareholders of Convergent Genomics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2023.1229709/full#supplementary-material
References
1. Baard J, de Bruin DM, Zondervan PJ, Kamphuis G, de la Rosette J, Laguna P, et al. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol (2017) 14:181–91. doi: 10.1038/nrurol.2016.252
2. Petros FG. Epidemiology, clinical presentation, and evaluation of upper-tract urothelial carcinoma. Transl Androl Urol (2020) 9:1794–8. doi: 10.21037/tau.2019.11.22
3. van Doeveren T, van de Werken HJG, van Riet J, Aben KKH, van Leeuwen PJ, Zwarthoff EC, et al. Synchronous and metachronous urothelial carcinoma of the upper urinary tract and the bladder: are they clonally related? A systematic review. Urol Oncol (2020) 38:590–8. doi: 10.1016/j.urolonc.2020.01.008
4. Audenet F, Isharwal S, Cha EK, Donoghue MTA, Drill EN, Ostrovnaya I, et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res (2018) 25:967–76. doi: 10.1158/1078-0432.CCR-18-2039
5. Levy A, Canes D. Perioperative complications and adverse sequelae of radical nephroureterectomy. Transl Androl Urol (2020) 9:1853–9. doi: 10.21037/tau.2019.12.25
6. VandenBussche CJ, Hang J, McIntire PJ, Miki Y, Peyton S, Vohra P, et al. “Cytopathology of the upper urinary tract”. In: Wojcik EM, Kurtycz DFI, Rosenthal DL, editors. The Paris System for Reporting Urinary Cytology, 2nd ed., vol. 115-141 . Cham, Switzerland: Springer Nature Switzerland AG (2022). p. 243–5.
7. Bicocca VT, Phillips KG, Fischer DS, Caruso VM, Goudarzi M, Garcia-Ransom M, et al. Urinary comprehensive genomic profiling correlates with urothelial carcinoma mutations with clinical risk and efficacy of intervention. J Clin Med (2022) 11:5827. doi: 10.3390/jcm11195827
8. Salari K, Sundi D, Lee JJ, Wu S, Wu C, DiFiore G, et al. Development and multicenter case-control validation of urinary comprehensive genomic profiling for urothelial carcinoma diagnosis, surveillance, and risk prediction. Clin Cancer Res (2023). doi: 10.1158/1078-0432.CCR-23-0570
9. Pietzak EJ, Bagrodia A, Cha EK, Drill EN, Iyer G, Isharwal S, et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol (2017) 72:952–9. doi: 10.1016/j.eururo.2017.05.032
10. Garczyk S, Ortiz-Brüchle N, Schneider U, Lurje I, Guricova K, Gaisa NT, et al. Next-generation sequencing reveals potential predictive biomarkers and targets of therapy for urothelial carcinoma in situ of the urinary bladder. Am J Pathol (2020) 190:323–32. doi: 10.1016/j.ajpath.2019.10.0004
11. Straub J, Strittmatter F, Karl A, Stief CG Tritschler S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. Urol Oncol (2013) 31:1166–70. doi: 10.1016/j.urolonc.2011.12.021
12. Wang JK, Tollefson MK, Krambeck AE, Trost LW, Thompson RH. High rate of pathologic upgrading at nephroureterectomy for upper tract urothelial carcinoma. Oncology (2012) 79:615–9. doi: 10.1016/j.urology.2011.11.049
13. Zhang ML, Rosenthal DL, VandenBussche CJ. Upper urinary tract washings outperform voided urine specimens to detect upper tract high-grade urothelial carcinoma. Diagn Cytopathol (2017) 45:700–4. doi: 10.1002/dc.23746
14. Dev HS, Poo S, Armitage J, Wiseman O, Shah N, Al-Hayek S. Investigating upper urinary tract urothelial carcinomas: a single-centre 10-year experience. World J Urol (2017) 35:131–8. doi: 10.1007/s00345-016-1820-8
15. Simon CT, Skala SL, Weizer AZ, Ambani SN, Chinnaiyan AM, Palapattu G, et al. Clinical utility and concordance of upper urinary tract cytology and biopsy in predicting clinicopathological features of upper urinary tract urothelial carcinoma. Hum Pathol (2019) 86:76–84. doi: 10.1016/j.humpath.2018.11.021
16. Tanaka T, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: A multi-institutional study. Urol Oncol (2014) 32:48e19–26. doi: 10.1016/j.urolonc.2013.07.003
17. Sverrisson EF, Kim T, Espiritu PN, Sexton WJ, Pow-Sang JM, Dhillon J, et al. The merits of cytology in the workup for upper tract urothelial carcinoma – a contemporary review of a perplexing issue. Int Braz J Urol (2014) 40:493–8. doi: 10.1590/S1677-5538.IBJU.2014.04.07
18. Dodd LG, Johnston WW, Robertson CN, Layfield LJ. Endoscopic brush cytology of the upper urinary tract. Evaluation of its efficacy and potential limitations in diagnosis. Acta Cytol (1997) 41:377–84. doi: 10.1159/000332528
19. Low RK, Moran ME, Anderson KR. Ureteroscopic cytologic diagnosis of upper tract lesions. J Endourol (1993) 7:311–5. doi: 10.1089/end.1993.7.311
20. Gill WB, Lu C, Bibbo M. Retrograde brush biopsy of the ureter and renal pelvis. Urol Clin North Am (1979) 6(3):573–86. doi: 10.1016/S0094-0143(21)01214-3
21. Mori K, Katayama S, Laukhtina E, Schuettfort VM, Pradere B, Quhal F, et al. Discordance between clinical and pathological staging and grading in upper tract urothelial carcinoma. Clin Genitourin Cancer (2021) 20:95–100. doi: 10.1016/j.clgc.2021.10.002
22. Zangouei AS, Barjasteh AH, Rahimi HR, Mojarrad M, Moghbeli M. Role of tyrosine kinases in bladder cancer progression: an overview. Cell Commun Signal (2020) 18:127. doi: 10.1186/s12964-020-00625-7
23. Wan S, Liu X, Hua W, Xi M, Zhou Y, Wan Y. The role of telomerase reverse transcriptase (TERT) promoter mutations in prognosis in bladder cancer. Bioengineered (2021) 12:1495–504. doi: 10.1080/21655979.2021.1915725
24. Joyce DD, Sharma V, Williams SB. Cost-effectiveness and economic impact of bladder cancer management: an updated review of the literature. Pharmacoeconomics (2023) 41:751–69. doi: 10.1007/s40273-023-01273-8
25. Tobert CM, Nepple KG, McDowell BD, Charlton ME, Mott SL, Gruca TS, et al. Compliance with American Urological Association guidelines for non-muscle invasive bladder cancer remains poor: assessing factors associated with noncompliance and survival in a rural state. Urology (2019) 132:150–5. doi: 10.1016/j.urology.2019.06.021
26. Cumberbatch MGK, Foerster B, Catto JWF, Kamat AM, Kassouf W, Jubber I, et al. Repeat transurethral resection in non-muscle invasive bladder cancer: a systematic review. Eur Urol (2018) 73:925–33. doi: 10.1016/j.eururo.2018.02.014
27. Kamat AM, Georgieva MV, Song J, Bocharova I, Qian K, Guo A, et al. Disease progression among patients who receive available bladder preservation therapies after failure of BCG therapy in the SEER-Medicare data. J Clin Oncol (2020) 38:453. doi: 10.1200/JCO.2020.38.6_suppl.453
28. Aly A, Johnson C, Doleh Y, Chirikov V, Botterman M, Shenolikar R, et al. The real-world lifetime economic burden of urothelial carcinoma by stage at diagnosis. J Clin Pathways (2020) 6:51–60. doi: 10.25270/jcp.2020.5.00001
29. Thacker K, Raman JD, McLean T, Said J, Oliver L, Gore JL. Understanding the economic burden of treating low-grade upper tract urothelial cancer in the United States. Urol Pract (2021) 8:1–7. doi: 10.1097/UPJ.0000000000000161
30. Raman JD, Jafri SM. Complications following radical nephroureterectomy. Curr Urol Rep (2016) 17:36. doi: 10.1007/s11934-016-0595-1
Keywords: metachronous neoplasm, bladder cancer, diagnostic test, DNA mutational analysis, precision medicine
Citation: Yonover PM, Ward CT, Mazzarella BC, Phillips KG, Jensen BW, Bicocca VT, Duffy K, Yonover J, Cherry A and Levin TG (2023) Clinical utility of urinary comprehensive genomic profiling in diagnosing metachronous upper tract urothelial carcinoma: a case report. Front. Urol. 3:1229709. doi: 10.3389/fruro.2023.1229709
Received: 26 May 2023; Accepted: 19 July 2023;
Published: 09 August 2023.
Edited by:
Mohammed Shahait, King Hussein Medical Center, JordanReviewed by:
Liang Qu, University of Melbourne, AustraliaRamiz AbuHijlih, King Hussein Cancer Center, Jordan
Copyright © 2023 Yonover, Ward, Mazzarella, Phillips, Jensen, Bicocca, Duffy, Yonover, Cherry and Levin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul M. Yonover, UFlvbm92ZXJAdXJvcGFydG5lcnMuY29t; Ceressa T. Ward, Y3dhcmRAY29udmVyZ2VudGdlbm9taWNzLmNvbQ==
 Paul M. Yonover1*
Paul M. Yonover1* Ceressa T. Ward
Ceressa T. Ward Brad W. Jensen
Brad W. Jensen Trevor G. Levin
Trevor G. Levin