- 1CTU Bern, University of Bern, Bern, Switzerland
- 2HIV/AIDS Unit, Department of Infectious Diseases, Geneva University Hospitals, Geneva, Switzerland
- 3Private Practice Office, Geneva, Switzerland
- 4Department of Infectious Diseases, Bern University Hospital, University of Bern, Bern, Switzerland
- 5Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland
- 6Laboratory of Virology, Geneva University Hospitals, Geneva, Switzerland
- 7Division of Infectious Diseases and Hospital Epidemiology, University Hospital of Basel, University of Basel, Basel, Switzerland
- 8Division of Infectious Diseases, Regional Hospital of Lugano, Lugano, Switzerland
- 9Department of Infectious Diseases and Hospital Epidemiology, University Hospital of Zurich, University of Zurich, Zurich, Switzerland
- 10Institute of Medical Virology, University of Zurich, Zurich, Switzerland
- 11Division of Infectious Diseases and Hospital Epidemiology, Kantonspital St. Gallen, St. Gallen, Switzerland
- 12Department of Infectious Diseases, Lausanne University Hospital, Lausanne, Switzerland
- 13Pharmacology Laboratory, Clinical Pharmacology Department, University of Lausanne, Lausanne, Switzerland
HIV-1 reservoir size and dynamics are promising parameters to ensure the safe prescription of simplified maintenance antiretroviral therapy in chronically HIV-1 infected patients. In the SIMPL’HIV trial, HIV-1 DNA was quantified in peripheral blood mononuclear cells obtained at baseline and week 48 to investigate changes over time and evidence of a predictive relationship to maintain HIV-1 RNA <20 copies/ml. Measurements were available for 175 patients, with no differences observed between treatment strategies. Findings showed that baseline HIV-1 DNA was lower in those with durable HIV-1 RNA <20 copies/ml compared with patients with incomplete viral suppression over 48 weeks.
Introduction
Currently available standard combination antiretroviral therapy (cART) regimens effectively suppress HIV-1 replication. However, the virus persists in infected cells and discontinuation of treatment allows it to re-emerge from the latent reservoir (1). This HIV-1 reservoir is particularly maintained in latently-infected CD4 T-cells circulating in the peripheral blood or disseminated in lymphoid organs and associated tissues and can be measured as the total cell-associated HIV-1 DNA per 1 million peripheral blood mononuclear cells (PBMCs) (2). Although only a small part of the latent reservoir is attributable to peripheral PBMCs, the latter is a well validated marker and relatively easy to measure.
The HIV-1 reservoir establishes itself very early in the course of HIV-1 infection and is a strong predictor of disease progression and virological rebound during ART interruptions (3). Although the HIV-1 DNA level decreases in most patients on cART, the decline varies among individuals and is associated with pre-therapeutic HIV-1 DNA and HIV-1 RNA levels, as well as the baseline CD4 cell count (4, 5). Total HIV-1 DNA levels could be prognostic of the response to cART and have been found to be predictive of the success of boosted protease-inhibitor–based simplification strategies, including recent dolutegravir (DTG) monotherapy-based simplification trials (2, 6–8). Although the threshold of HIV-1 DNA is debatable, recent French guidelines state that HIV-1 DNA <3 log copies/106 PBMC is associated with the success of maintenance strategies (9).
Randomized controlled trials have already demonstrated the efficacy of dual therapy both either in treatment-naïve and experienced patients (10–12). The current study is embedded in the SIMPL’HIV trial, a randomized controlled trial aimed at investigating maintenance therapy using a dual therapy consisting of dolutegravir + emtricitabine (DTG+FTC) compared to standard of care (cART). In this study, we assessed the applicability of the baseline HIV-1 DNA level as a potential predictor to determine the success of a reductive antiretroviral strategy, thus sparing patients the risk of HIV-1 RNA detection.
Materials and Methods
SIMPL’HIV Trial
The SIMPL’HIV trial was a multicenter, non-inferiority, open-label, randomized, factorial design trial conducted within the Swiss HIV Cohort Study from May 2017 to May 2018 (NCT03160105). The study showed the non-inferiority of DTG+FTC in terms of maintaining viral suppression, defined as HIV-RNA <100 copies/ml through 48 weeks of follow-up compared to standard triple therapy. All measurements and collections of clinical data used here are reported in the main publication of the study, as well as the details concerning the inclusion and exclusion criteria and a flowchart of the study (12). Written informed consent was obtained from each participant before the initiation of study procedures.
HIV-1 DNA Quantification
HIV-1 DNA quantification in PBMCs was performed using the GENERIC HIV DNA Cell kit (Biocentric). Briefly, DNA was isolated from 3 to 5 million cryopreserved PBMCs and HIV-1 DNA values determined as the absolute HIV-1 DNA copy number per 1 million genomic equivalents. All the undetectable values (9 out of 352 measurements) that were below the individually calculated detection limit, were censored at this maximal value. In one case, the maximal value was set to 35.1.
Statistical Analysis
Descriptive analyses were performed for baseline characteristics using frequencies and percentages for categorical variables and medians (interquartile range [IQR]) for continuous variables. A comparison of HIV-1 DNA level changes in the two groups (DTG+FTC dual therapy intervention vs. standard care) were analyzed using the observed values from baseline to 48 weeks of treatment using a paired Wilcoxon signed rank test. In order to compare the values between the two groups, the Wilcoxon rank sum test was applied. The same comparison was repeated to compare patients who presented a value of HIV-1 RNA >20 copies/ml and those who did not. To account for the skewed distribution, the log of the HIV-1 DNA was used to compare the different time points and different groups.
Predictive factors of the virological response were assessed using univariable and multivariable logistic models, including HIV-1 DNA at the time of randomization, time of treatment and the CD4 nadir. We used the receiver operating characteristics (ROC) curve methodology to identify a cut-off value of the HIV-1 DNA measurements to successfully predict the therapeutic response based on the Youden Index. This allowed to discriminate between patients who presented a value of HIV-1 RNA >20 copies/ml plasma during the 48-week study period and those who successfully maintained their virological suppression upon DTG+FTC dual therapy and cART therapy. All statistical analyses were performed using R software (version 3.6.1 or higher) through the RStudio interface.
Results
A total of 175 patients (of 188 randomized in the study) had available HIV-1 DNA reservoir data at baseline and at week 48 (87 in the DTG+FTC group and 88 in the cART group). Demographic and baseline characteristics are shown in Supplementary Table 1. The median CD4 nadir was 240 cells/mm3 for DTG+FTC and 242 cells/mm3 for cART, while the median CD4 at baseline was 669 cells/mm3 and 677 cells/mm3, respectively. The median change of the log10-scale HIV-1 DNA between baseline and week 48 was not statistically significant in either treatment group. It was 0.06 (95% confidence interval [CI], -0.06-0.19, P = 0.352) for the DTG+FTC group and 0.07 (95% CI, -0.06-0.21, P = 0.302) for the cART group. Moreover, the difference in medians between the two groups was not statistically significant either; it was 0.01 (95% CI, -0.11-0.14; P = .838) at baseline and 0.00 (95% CI, -0.15-0.14; P = .992) at week 48 (Table 1). The graphical representation can be found in Figure 1, where the distributions of the different groups and time points show comparable results.
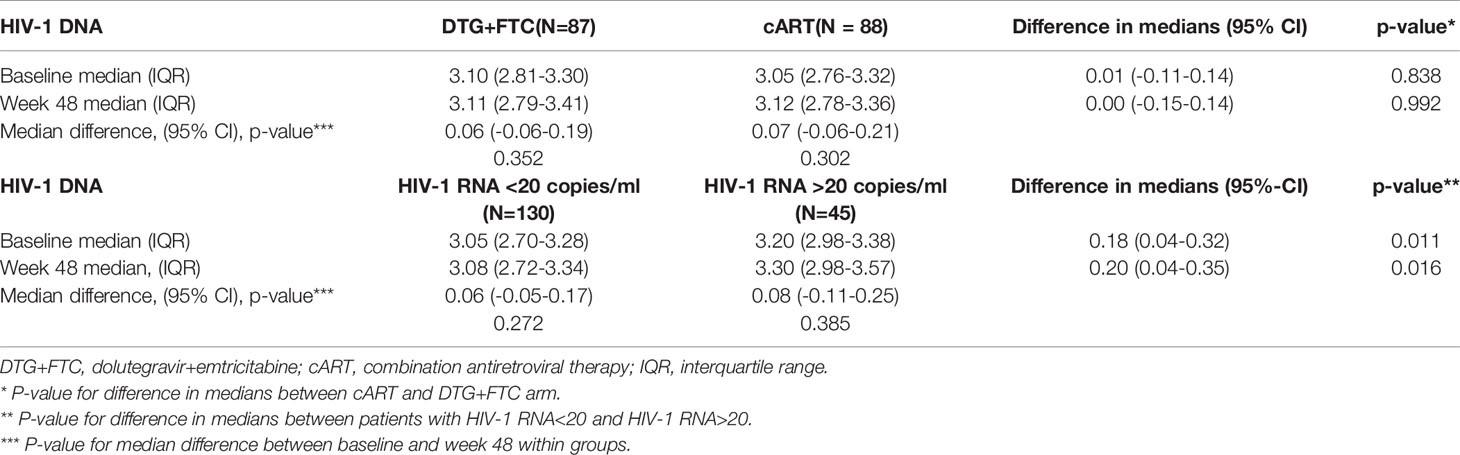
Table 1 Comparison of the median values of the HIV-1 DNA reservoir between treatment groups on a log10-scale and between patients with and without values of HIV-1 RNA >20 copies/ml throughout the 48-week study.
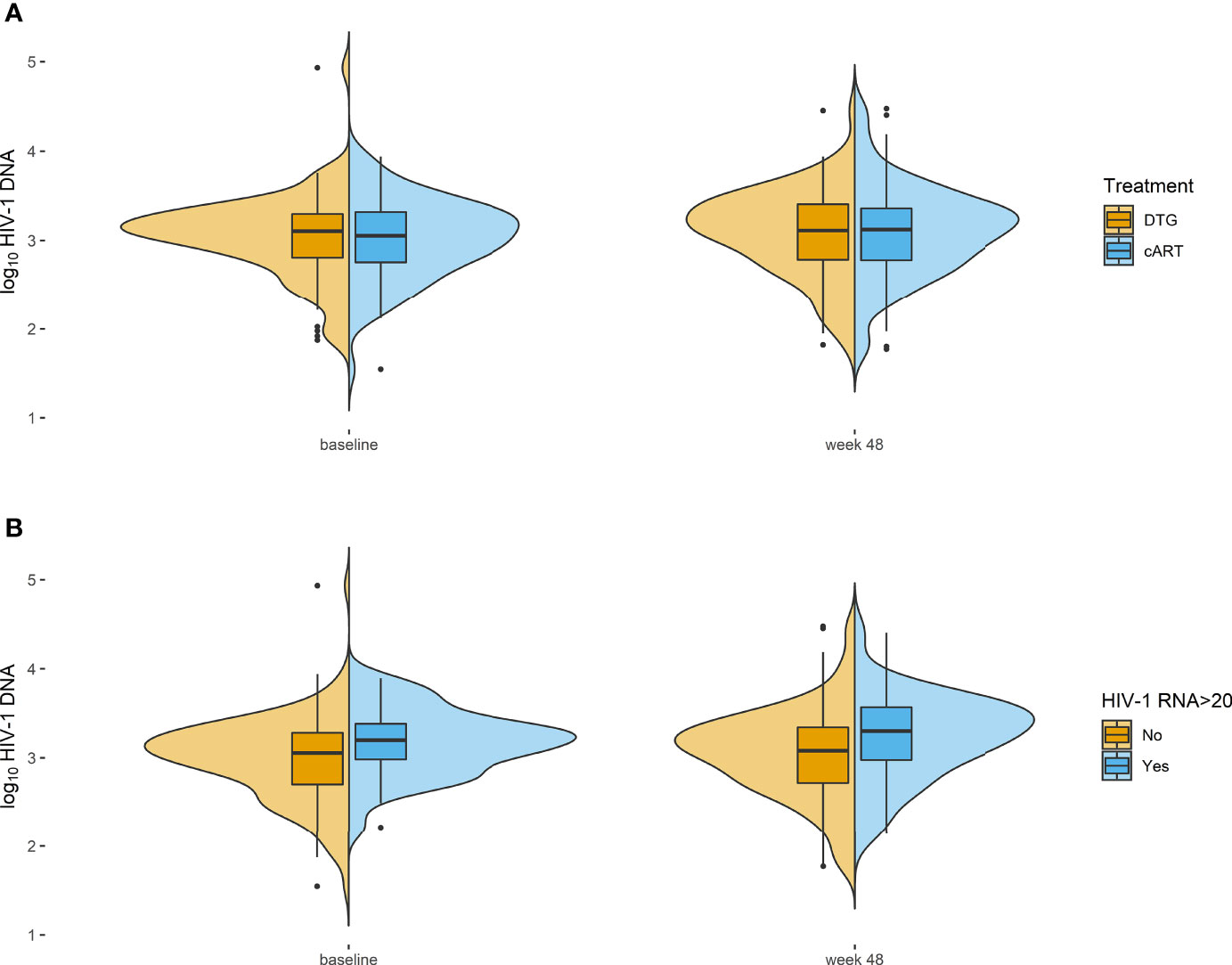
Figure 1 Violin plots representing the HIV-1 DNA reservoir with boxplots showing median with interquartile range (IQR) and whiskers with maximum length of 1.5 IQR together with the density distribution. (A) Treatment groups on a log10-scale. (B) Patients with and without values of HIV-1 RNA >20 copies/ml throughout the 48-week study. DTG, dolutegravir; cART, combination antiretroviral therapy.
Forty-five patients had at least a single value of HIV-1 RNA >20 copies/ml among the 7 or more measurements obtained during the 48 weeks (22/87 and 23/88 in the DTG+FTC cART groups, respectively), corresponding to 42 and 36 single events, respectively. Among these patients, 15 had >50 HIV-1 RNA copies/ml (7 values in 5 patients in the DTG+FTC group and 8 values in 7 patients in the cART group). There was a significant difference in the baseline value of HIV-1 DNA between patients with at least a single value of HIV-1 RNA >20 copies/ml compared to those who remained suppressed throughout the study (difference in medians, 0.18 [95% CI, 0.04-0.32]; P = .011; odds ratio [OR], 2.90 [95% CI, 1.25-6.73]; P = .013) (Table 2). A multivariable logistic model including HIV-1 DNA levels at baseline, CD4 nadir and time since ART initiation showed that HIV-1 DNA remained the only significant parameter (OR, 2.67 [95% CI, 1.13-6.32]; P = 0.025) to predict values of HIV-1 RNA >20 copies/ml. A significant difference was also observed at the time of week 48 measurements between patients with at least a single value of HIV-1 RNA >20 copies/ml throughout the 48 weeks and those without an event (difference in medians in log10-scale, 0.20 [95% CI, 0.04-0.35]; P = .016). No significant difference was observed within the groups comparing baseline and week 48 results; the median change was 0.06 (95% CI, -0.05-0.17, P = 0.272) for HIV-1 RNA <20 copies/ml and 0.08 (95% CI, -0.11-0.25, P = 0.385) for HIV-1 RNA >20 copies/ml.
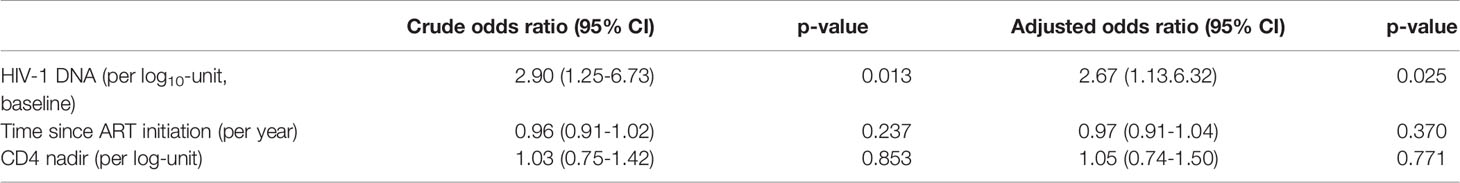
Table 2 Odds ratio estimates with 95% confidence intervals based on simple and multivariable models for potential predictive factors of patients with HIV-1 RNA values >20 copies/ml.
ROC curve analysis of HIV-1 DNA for the discrimination of patients with and without HIV-1 RNA >20 copies/ml yielded an area under the curve (AUC) of 0.63 (95%-CI 0.54 to 0.72) and a cut-off point based on the Youden index of 2.98 (log10-transformed baseline value). The sensitivity at the cut-off point is 75.6%, i.e. the power to rule out elevated HIV-1 RNA is rather good if the baseline DNA level is below the cut point. The specificity is 45.4%, i.e. the power to rule in elevated HIV-1 RNA is rather low if the baseline DNA level is above the cut point (Table 3).

Table 3 Receiver operating characteristic curve analysis for HIV-1 DNA at baseline to predict virological values of HIV-1 RNA >20 copies/ml throughout the 48-week study.
Discussion
In this study of HIV-suppressed patients included in a nationwide randomized trial, there was no significant difference in the HIV-1 DNA reservoir size between study groups over 48 weeks, thus showing that the HIV-1 DNA level remained stable in patients under dual or standard therapy. It was also observed that higher levels of HIV-1 DNA at baseline in both treatment groups could predict values of HIV-1 RNA above the threshold value of 20 copies/ml during the following 48 weeks. In the EARLY-SIMPLIFIED study (8), it was observed that the reservoir was relatively stable over time in most patients who started ART during primary HIV infection. Of note, the observation period in the SIMPL’HIV trial started more than 7 years (median value) after initiation of ART therapy. Thus, we were able to investigate a period where the stability of the HIV-1 reservoir was even higher compared to the first years of ART.
The SIMPL’HIV study compares favorably with many other previous trials comparing HIV-1 DNA measurements in patients under various ART regimens, including simplified regimens of mono- or dual therapies. The AtLaS study (13), which included patients switching from triple to dual therapy, observed a decrease of HIV-1 DNA levels in leucocytes from baseline to week 48, but no significant difference between dual and triple therapy. Another small study conducted in 30 patients with plasma HIV-1 RNA suppression <50 copies/ml (14) observed that HIV-1 DNA decay in patients occurred in the first years and then remained stable after the fourth year of standard ART treatment. In a Swiss cohort study (15), the authors observed differences in HIV-1 DNA levels in early vs late treatment initiation and in non-treated patients. In a Greek cohort study (6), the level of HIV-1 DNA was the only parameter significantly associated with viral rebound, as observed in the SIMPL’HIV analysis. Furthermore, low baseline values of HIV-1 DNA load levels were able to predict a non-virologic rebound throughout the study period (6). Moreover, adverse events and side effects of the tested therapies never showed a difference among them and reported similar events as the most frequent ones, e.g. upper respiratory infections, headaches, diarrhea and urinary tract (12, 13).
In the SIMPL’HIV Trial, the HIV-1 RNA events of >20 copies/ml throughout the 48-week measurements occurred in approximately one out of four patients in both treatment strategies. Results observed in the SIMPL’HIV study on the maintenance of HIV-1 suppression were comparable with other reports. For example, another study (6) showed that patients with a low level of HIV-1 DNA at baseline did not show an event of HIV-1 RNA >20 copies/ml during the following weeks of treatment. Other parameters of interest, such as CD4 nadir and time since ART initiation, There was no significant difference among other parameters of interest (such as CD4 nadir and time since ART initiation) between patients that did not have a HIV-1 RNA event >20 copies/ml and those that did experience such an event. The finding that HIV-1 DNA could be used as a possible suppression predictor is particularly interesting in the context of widely-used dual therapy, and as also previously observed (2, 6, 7). Importantly, the assessment of HIV-1 DNA level could translate into a promising prognostic marker of long-term HIV-1 viral suppression on a simplified reduced regimen. The estimated optimal cut-off point regarding the maintenance of a successful strategy of HIV-1 RNA suppression throughout the study is comparable to the value given in the recent French guidelines (9), i.e., <3 log copies/106 PBMC. This confirms that our threshold value is in line with current guidelines and provides a further confirmation of the potentiality of HIV-1 DNA as a predictor for the maintenance of low levels of HIV-1 RNA.
Nevertheless, a limitation of this study concerns the fact that the estimated optimal cut-off could be assessed only for single values of HIV-1 RNA over the minimum value of 20 copies/ml. Due to the low number of non-virological suppressed patients observed, it is not possible to determine an estimation of the cut-off for virological suppression as defined in the main analysis of the SIMPL’HIV study. Additionally, the results of the SIMPL’HIV study suggest that dual therapy is safe when also taking into consideration the fact that HIV-1 DNA levels remained low throughout the study. The limitation of this finding is the short duration of the study measurements, both at baseline and after 48 weeks. Of further note is the fact that measuring total HIV-1 DNA is not only quantifying replication-competent viruses, however, has been shown to have predictive values (2).
In conclusion, in the context of the SIMPL’HIV study, no difference in HIV-1 DNA was observed among patients, whether on cART or on a simplified regimen of DTG+FTC. Our findings also tend to suggest that baseline HIV-1 DNA could be potentially used as a predictive factor to maintain the level of HIV-1 RNA below the threshold of 20 copies/ml, showing a threshold that was observed in previous studies and in the guidelines.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Commission cantonale d’éthique de la recherche scientifique de Genève (CCER). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DS, GW, SY, MS, EB, DB, PV, MC, MBu, LD, HG, PS, AL, KM, and AC contributed to conception and design of the SIMPL’HIV study. Data curation and validation was done by AM, KN, AC, KM, and MBr. MBr and AL performed the statistical analysis. MBr and AM wrote the first draft of the manuscript. KM and AC wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was financed within the framework of the Swiss National Science Foundation (grant # 166819 and 17481) and the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant #177499), by SHCS project #826 and by the Swiss HIV Cohort Study research foundation. Data were collected by the five Swiss university hospitals, two cantonal hospitals, 15 affiliated hospitals and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers).
Conflict of Interest
DB received honoraria for advisory boards from the companies Gilead, MSD and Merck outside the submitted work. GW received honoraria for advisory boards from Gilead Sciences, ViiV and research grants from Gilead Sciences, all paid to his institution. KM received travel grants and honoraria from Gilead Sciences, Roche Diagnostics, GlaxoSmithKline, Merck Sharp & Dohme, Bristol-Myers Squibb, ViiV and Abbott; the University of Zurich received research grants from Gilead Science, Novartis, Roche, and Merck Sharp & Dohme for studies where KM served as principal investigator, and advisory board honoraria from Gilead Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the study participants for their cooperation in this study. They also thank the following persons for their contributions: Rosemary Sudan (review and editing); Charlotte Barbieux (investigation, data curation, validation); and the Clinical Research Center, Geneva University Hospitals and the University of Geneva Faculty of Medicine (validation). Membership of the Swiss HIV Cohort Study (SHCS): Aebi-Popp K, Anagnostopoulos A, Battegay M, EB, Böni J, DB, Bucher HC, AC, MC, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Haerry D (Deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (Chairman of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, KM, Müller N, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (Chairman of the Scientific Board), Rudin C, Scherrer AU (Head of Data Centre), PS, Speck R, MS (Chairman of the Clinical and Laboratory Committee), Tarr P, Trkola A, PV, GW, Weber R, and SY.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2022.855437/full#supplementary-material.
References
1. Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The Challenge of Finding a Cure for HIV Infection. Science (2009) 323(5919):1304–7. doi: 10.1126/science.1165706
2. Rouzioux C, Avettand-Fenoel V. Total HIV DNA: A Global Marker of HIV Persistence. Retrovirology (2018) 15(1):30. doi: 10.1186/s12977-018-0412-7
3. Tsiara CG, Nikolopoulos GK, Bagos PG, Goujard C, Katzenstein TL, Minga AK, et al. Impact of HIV Type 1 DNA Levels on Spontaneous Disease Progression: A Meta-Analysis. AIDS Res Hum Retroviruses (2012) 28(4):366–73. doi: 10.1089/aid.2011.0032
4. Yerly S, Perneger TV, Vora S, Hirschel B, Perrin L. Decay of Cell-Associated HIV-1 DNA Correlates With Residual Replication in Patients Treated During Acute HIV-1 Infection. AIDS (2000) 14(18):2805–12. doi: 10.1097/00002030-200012220-00001
5. Bachmann N, von Siebenthal C, Vongrad V, Turk T, Neumann K, Beerenwinkel N, et al. Determinants of HIV-1 Reservoir Size and Long-Term Dynamics During Suppressive ART. Nat Comm (2019) 10(1):1–11. doi: 10.1038/s41467-019-10884-9
6. Hatzakis AE, Touloumi G, Pantazis N, Anastassopoulou CG, Katsarou O, Karafoulidou A, et al. Cellular HIV-1 DNA Load Predicts HIV-RNA Rebound and the Outcome of Highly Active Antiretroviral Therapy. AIDS (2004) 18(17):2261–7. doi: 10.1097/00002030-200411190-00006
7. Lambert-Niclot S, Flandre P, Valantin MA, Peytavin G, Duvivier C, Haim-Boukobza S, et al. Factors Associated With Virological Failure in HIV-1-Infected Patients Receiving Darunavir/Ritonavir Monotherapy. J Infect Dis (2011) 204(8):1211–6. doi: 10.1093/infdis/jir518
8. Braun DL, Turk T, Tschumi F, Grube C, Hampel B, Depmeier C, et al. Noninferiority of Simplified Dolutegravir Monotherapy Compared to Continued Combination Antiretroviral Therapy That was Initiated During Primary Human Immunodeficiency Virus Infection: A Randomized, Controlled, Multisite, Open-Label, Noninferiority Trial. Clin Infect Dis (2019) 69(9):1489–97. doi: 10.1093/cid/ciy1131
9. Morlat P. Prise En Charge Médicale Des Personnes Vivant Avec Le VIH: Recommandations Du Groupe D'experts: Rapport 2017. Paris, France: Direction de l'information légale et administrative (2017). Available at: https://cns.sante.fr/actualites/prise-en-charge-du-vih-recommandations-du-groupe-dexperts/.
10. Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, et al. Dolutegravir Plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection. N Engl J Med (2013) 369(19):1807–18. doi: 10.1056/NEJMoa1215541
11. Clotet B, Feinberg J, Van Lunzen J, Khuong-Josses M-A, Antinori A, Dumitru I, et al. Once-Daily Dolutegravir Versus Darunavir Plus Ritonavir in Antiretroviral-Naive Adults With HIV-1 Infection (FLAMINGO): 48 Week Results From the Randomised Open-Label Phase 3b Study. Lancet (2014) 383(9936):2222–31. doi: 10.1016/S0140-6736(14)60084-2
12. Sculier D, Wandeler G, Yerly S, Marinosci A, Stoeckle M, Bernasconi E, et al. Efficacy and Safety of Dolutegravir Plus Emtricitabine Versus Standard ART for the Maintenance of HIV-1 Suppression: 48-Week Results of the Factorial, Randomized, Non-Inferiority SIMPL’HIV Trial. PloS Med (2020) 17(11):e1003421. doi: 10.1371/journal.pmed.1003421
13. Lombardi F, Belmonti S, Quiros-Roldan E, Latini A, Castagna A, D'Ettorre G, et al. Evolution of Blood-Associated HIV-1 DNA Levels After 48 Weeks of Switching to Atazanavir/Ritonavir+Lamivudine Dual Therapy Versus Continuing Triple Therapy in the Randomized AtLaS-M Trial. J Antimicrob Chemother (2017) 72(7):2055–9. doi: 10.1093/jac/dkx068
14. Besson GJ, Lalama CM, Bosch RJ, Gandhi RT, Bedison MA, Aga E, et al. HIV-1 DNA Decay Dynamics in Blood During More Than a Decade of Suppressive Antiretroviral Therapy. Clin Infect Dis (2014) 59(9):1312–21. doi: 10.1093/cid/ciu585
Keywords: HIV-1 reservoir, dolutegravir+emtricitabine, dual therapy, cART, HIV-1 RNA
Citation: Branca M, Marinosci A, Sculier D, Wandeler G, Yerly S, Stoeckle M, Bernasconi E, Braun DL, Neumann K, Vernazza P, Cavassini M, Buzzi M, Decosterd LA, Schmid P, Limacher A, Günthard HF, Metzner KJ and Calmy A (2022) Role of the HIV-1 Reservoir to Maintain Viral Suppression in a Simplified Strategy for the Long-Term Management of HIV-1 Infection (The SIMPL’HIV Trial). Front.Virol. 2:855437. doi: 10.3389/fviro.2022.855437
Received: 15 January 2022; Accepted: 20 April 2022;
Published: 19 May 2022.
Edited by:
Judd Hultquist, Northwestern University, United StatesReviewed by:
Jan Weber, Academy of Sciences of the Czech Republic (ASCR), CzechiaAmit Kumar, Laval University, Canada
Copyright © 2022 Branca, Marinosci, Sculier, Wandeler, Yerly, Stoeckle, Bernasconi, Braun, Neumann, Vernazza, Cavassini, Buzzi, Decosterd, Schmid, Limacher, Günthard, Metzner and Calmy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra Calmy, QWxleGFuZHJhLkNhbG15QGhjdWdlLmNo
†These authors have contributed equally to this work and share last authorship
 Mattia Branca
Mattia Branca Annalisa Marinosci2
Annalisa Marinosci2 Pietro Vernazza
Pietro Vernazza Laurent A. Decosterd
Laurent A. Decosterd Huldrych F. Günthard
Huldrych F. Günthard