- 1Department of Oncology and Metabolism, University of Sheffield, Sheffield, United Kingdom
- 2Biosciences Department, Sheffield Hallam University, Sheffield, United Kingdom
- 3CeraVe, L’Oréal, New York, NY, United States
- 4Department of Infection and Immunity, University of Sheffield, Sheffield, United Kingdom
Background: Washing hands with soap and lukewarm water for 20 s is a fundamental measure advocated especially within the UK to help control the spread of viral disease. However, these practices can induce irritant contact dermatitis, particularly in healthcare professionals (HCPs). HCPs typically manage their condition by replacing soap or alcohol-based hand sanitizers with cleansers containing mild surfactants and/or emollient ingredients [skin-friendly cleansers (SFCs)] to mitigate skin damage and/or using topical emollients after washing for repair. Despite this widespread practice, there is very limited evidence supporting the efficacy of these interventions in the prevention of viral propagation.
Methodology: Within this study a range of viruses comprising human coronavirus (HCoV), herpes simplex virus (HSV)-1, influenza (IVA), adenovirus (Ad), and murine norovirus (MNV) were tested against multiple hand wash products, including SFCs. In vitro analysis using plaque assays and tissue culture infectious dose 50 (TCID50) were used to assess virus infectability after incubation with the test products (soaps and SFCs) over a range of concentrations and time points. Transmission electron microscopy (TEM) was used to determine virus architecture and size, while viral replication genes were measured by reverse transcription-polymerase chain reaction (RT-PCR).
Results/conclusions: Enveloped viruses demonstrated greater susceptibility over a range of test products, suggesting some SFCs are a suitable alternative to soap (depending on the presence of a viral envelope). However, no virucidal activity was observed for non-enveloped viruses. Water type (i.e., soft/hard) and pre-exposed hand hygiene conditions (i.e., clean/dirty) made little difference to the effectiveness of both soaps and SFCs. Therefore, new hand hygiene regimens should be implemented based on trying to encompass all viruses with varying structures, with specific emphasis on the absence of a viral envelope.
1 Introduction
Viral outbreaks are threats to human health. Washing hands thoroughly with soap and lukewarm water more often and for at least 20 s is a fundamental measure advocated worldwide to help control the spread of infectious viruses, including SARS-CoV-2. However, these practices are causing unintended adverse effects on skin integrity, which particularly affect healthcare professionals (HCPs), where the incidence and severity of irritant contact dermatitis (ICD) of the hands increased from 20% to over 80% during the COVID-19 pandemic (1). This can have a significant impact on workplace productivity. Contact dermatitis of the hands is a common occupational skin disease characterized by red and swollen skin with a dry, damaged surface. Frequent hand washing is a contributing factor to ICD of the hands (1, 2) and accounts for 70%–90% of all occupational skin diseases in Europe and the USA (3–5). HCPs are at an increased risk of ICD because of the need for regular hand washing (2), which can have a multitude of negative effects including decreased compliance with proper personal protective equipment, inadequate hand washing, and, consequently, increased carriage of bacteria and viruses on the skin.
Typically, individuals with ICD are advised to avoid exposure to potential triggers and to use soap substitutes [i.e., skin-friendly cleansers (SFCs) that contain milder surfactant systems, such as emollients] and leave-on emollients during and after work. However, more recently and contrary to established guidelines for the management of occupational hand dermatitis, individuals in countries such as India (which experience a higher incidence of viral outbreaks due to a dense population) are being instructed to follow government advice and use traditional soap and water for hand hygiene during epidemics, and to wash their skin a second time using an alternative cleanser formulation containing emollients to remove harmful surfactant residues and protect the skin barrier (6). Although necessary to protect against the spread of SARS-CoV-2, this is expected to increase the incidence and severity of hand dermatitis (2). To protect HCPs and the wider public from ICD, there is a need to identify hand hygiene products and practices that are gentler on the skin and also help prevent viral transmission.
Therefore, the aim of this study is to investigate the efficacy of a range of hand wash product types, including SFC (named for their formulae content), and washing parameters against a range of enveloped [human coronavirus (HCoV), herpes simplex virus (HSV)-1, and influenza (IVA)] and non-enveloped [adenovirus (Ad) and murine norovirus (MNV)] viruses using a simulated hand washing model.
2 Materials and methods
2.1 Materials
All culture media were supplemented with 10% fetal bovine serum (FBS) (Biosera), 4 mM L-glutamine (200 mm in 0.85% sodium chloride solution), 1% penicillin–streptomycin, and Fungizone®, phosphate-buffered saline (PBS), trypsin/EDTA (Lonza, BioWhittaker Ltd), High-Capacity cDNA Reverse Transcription Kit, RNaseZap (Thermo Fisher Scientific), trypan blue (0.4%), 4% agarose, 0.5% crystal violet, sucrose (Sigma-Aldrich), paraformaldehyde 4% (Merck Millipore), and ReliaPrep RNA Cell Miniprep System (Promega) × 2 qPCR Master Mix (primer design). The hand wash products used in this study are listed in Table 1.
2.2 Propagation of cells and viruses
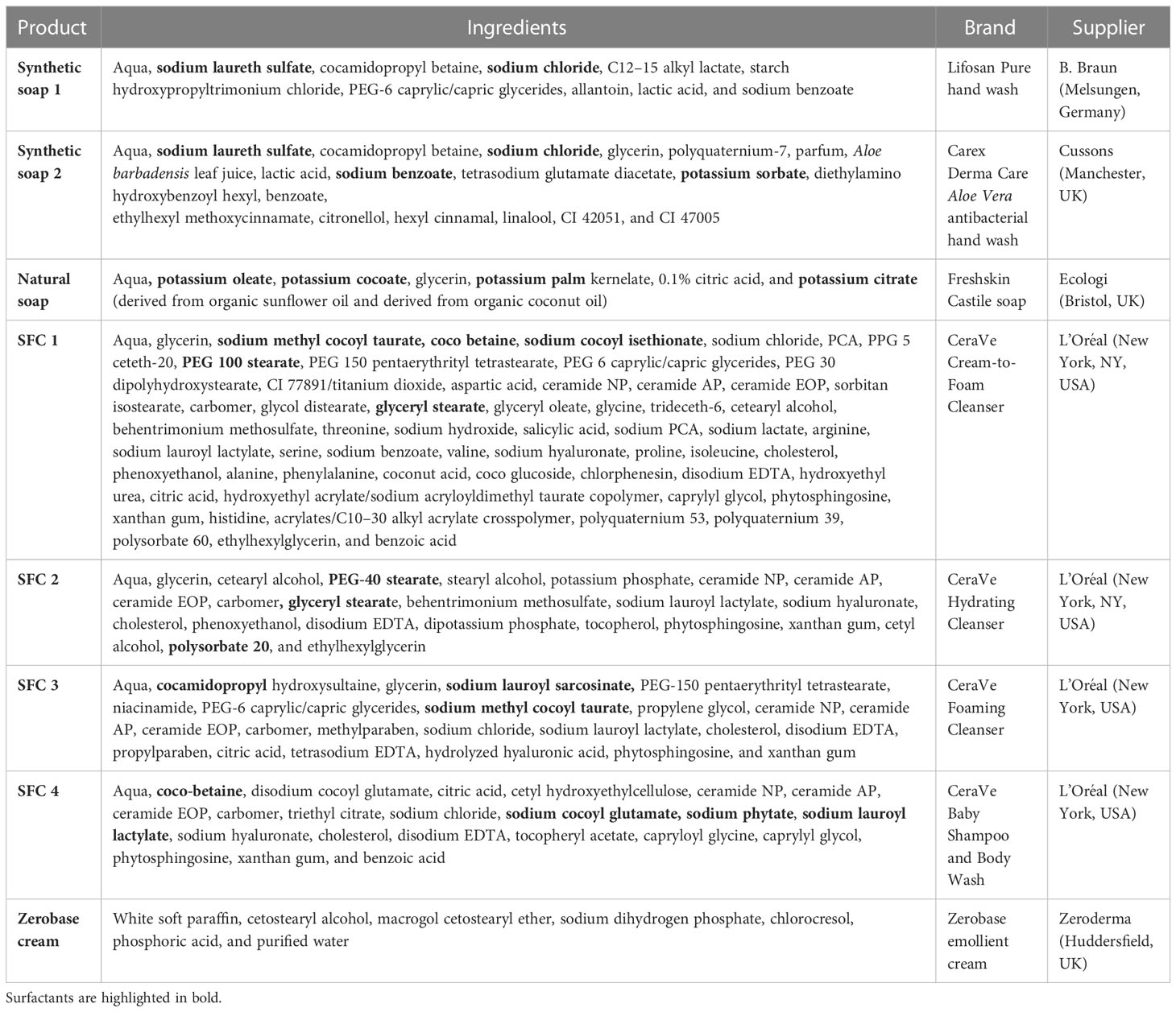
Viruses and the host cell lines required for propagation and titration are described in Table 2. Viruses were diluted in PBS to make working stocks of 1 × 108 pfu/mL. Cell lines were maintained in a culture medium of Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12) (Vero cells), RPMI Roswell Park Memorial Institute (RPMI) (A549 and Raw 264.7 cells), DMEM (HuH-7 cells), or Eagle's minimum essential medium (EMEM) [Madin-Darby canine kidney (MDCK) cells]. EMEM required the addition of 1% non-essential amino acids and DMEM required the addition of 1% sodium pyruvate. Cells were seeded at 2.5 × 105 cells/mL 24 h prior to inoculation in 12-well plates.
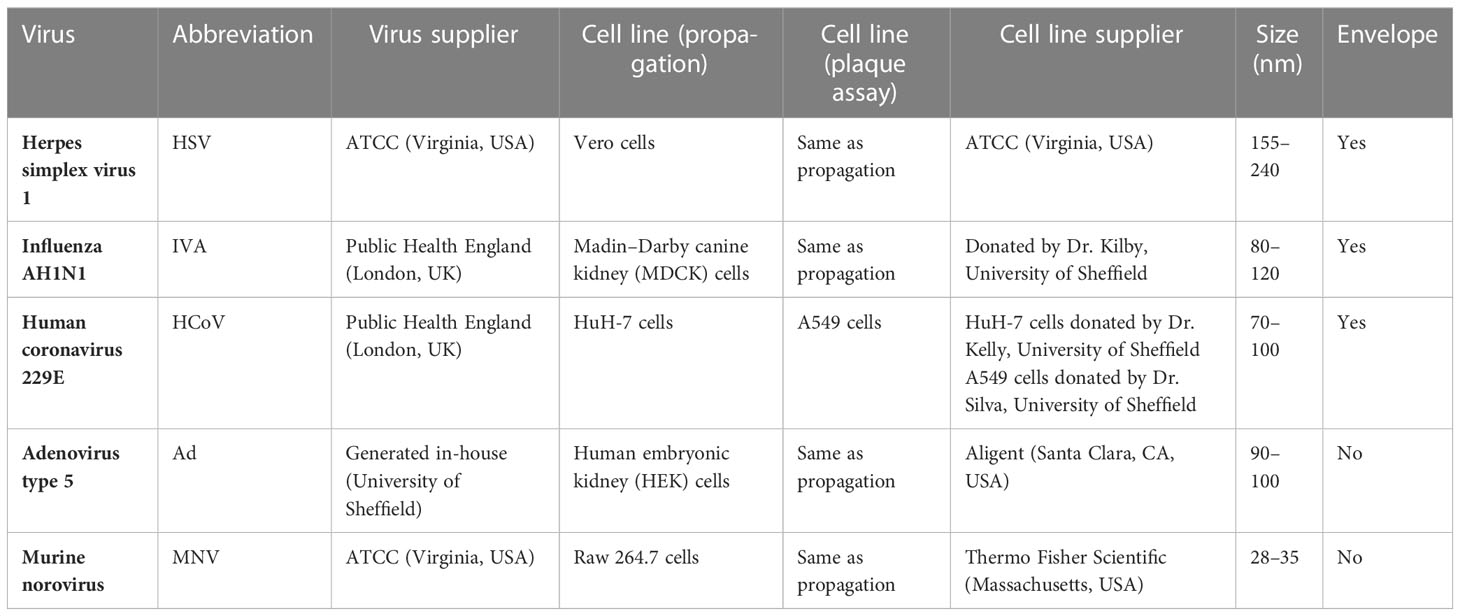
Table 2 List of viruses, their abbreviations, place of origin, and cell lines used for their analysis. .
2.3 Assessing the antiviral properties of hand wash products
The antiviral activity of the test products was determined according to British and European Standards BS EN 14476 (7). Each virus (1 × 108 pfu/mL) was exposed to the wash products at a final concentration of 20% or 97% in soft water (with a low calcium and magnesium content) containing 0.3% BSA for 20–60 s to simulate the conditions of hand washing; all products were heated to 20°C in a water bath prior to the experiment. The assay was repeated with the following variations: in hard water (with a concentrated calcium and magnesium content); under simulated dirty conditions, wherein the final BSA concentration was increased to 3%; and with 20% emollient cream (Zerobase cream) included (simulating the residues left from the use of topical leave-on emollients). Bleach (1% final concentration) and formaldehyde (1.4%, not shown) were included as a positive control. No product was included as the negative control; however, the viruses and an interfering substance were used alternately to ensure that the infectability of the virus was sustained. The effect of the products on virus activity was determined by plaque assay (MNV-1, IVA, HSV-1, and HCoV) or tissue culture infectious dose (TCID) 50 (Ad). For each wash condition a “suppression” control was also performed to establish infectability for ≥ 30 min (the maximum time prior to plating in plaque assay or TCID50) and to rule out cytotoxicity of the test products and control treatments to the cells used (data not shown). The experiment was repeated three times for each experimental condition to fulfill the British and European Standard EN 14476 (7).
2.4 Plaque assay (MNV-1, IVA, HSV-1, and HCoV)
The plaque assays were used to determine viral infectivity. The appropriate cells (Table 2) were inoculated with the viral suspensions following a serial log10 dilution. After 1 h, inocula were removed and the cell monolayers were overlaid with a 1 in 10 agarose (4%) solution in the appropriate medium and allowed to solidify before being transferred to a humidified incubator at 37°C for 48 (IVA and MNV), 72 (HSV), or 168 h (HCoV). Formaldehyde (4%) was applied to the solidified medium for 1 h to fix the cell monolayers before their removal. The cell monolayers were washed with PBS, stained with 1 mL of crystal violet (0.5%) for 5 min, and rinsed with tap water. Once dried, the pfu/mL was calculated.
2.5 TCID50 (Ad)
The HEK 293 cells were seeded at 1 × 104 cells per well in a 96-well plate, using standard DMEM growth medium. Immediately after seeding, the cells were inoculated with a serial log10 dilution of virus test solutions and controls. Cells were cultured for 10 days at 37°C, with medium replaced every 2–3 days. After 10 days, cytopathic effect (CPE) was observed. The cell monolayers were fixed with formaldehyde (4%) for 20 min at room temperature. Cells were then washed with PBS and stained with crystal violet (0.5%) for 5 min, before being rinsed with tap water. Once dried, TCID50 was calculated using a ratio scoring system and converted into pfu/mL, in order to aid comparison with results generated from the plaque assays. TCID50-to-pfu/mL conversion was accomplished using the method outlined in Pourianfar and Javadi (2012) (7).
2.6 Transmission electron microscopy
Virus architecture and size was determined using TEM after incubation with the test products, as viruses will begin to bleb and become smaller when they are no longer able to infect and replicate within a host effectively. TEM images were taken under set conditions. In brief, 10 μL of virus and 10 μL of clean interference substance were mixed before the addition of 80 μL of test product. The reaction took place for 20 s before termination through fixation (4% formaldehyde). The virus was fixed for 20 min on ice and 5 μL was transferred onto a carbon grid. The virus was negatively stained with 2% phosphotungstic acid before visualization on an FEI Tecnai T20 (Thermo Fisher Scientific). The TEM was operated at 80 kV with an embedded charge-coupled device camera. TEM images were analyzed using ImageJ software.
2.7 RT-PCR of viral replication genes
Viral RNA was extracted from cells after 4 and 24 h. The virus was allowed to interact with wash products for 20 s (as per the UK government guidelines) before the inoculation of cells. For the negative control (cells alone), positive control (virus alone), and test (virus combined with test products) RNA was extracted using the ReliaPrep™ RNA Cell Miniprep System. DNA was generated from an RNA template, via reverse transcription, resulting in complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription Kit. cDNA construction was carried out by using a thermal cycler (Bio-Rad) for 10 minutes at 25°C, 2 h at 37°C, then 5 min at 85°C. Viral gene amplification was achieved using × 2 qPCR Master Mix with R and SY primers (Sigma). Genes used to determine viral replication for Ad were as follows: E4F: 5’-ATGGGCAGTCGGTGATAGAGT-3’ and E4R: 5’-CTCAGGCTCAGGTTCAGAC-3’. As for MNV, genes used were as follows: NS3F: 5’-GATATCACCACCATGGGACCCTTCGACCTT-3’ and NS3R: 5’-ACTAGTTCAATGATGATGATGATGATGCTGGAGGCCGAAATC-3. The amplification of genes was achieved using QuantStudio™ 7 (Thermo Fisher Scientific) at ×40 cycles of 2 min at 50°C, 10 min and 15 s at 95°C, followed by 1 min at 60°C.
2.8 Statistics
All data were analyzed using GraphPad Prism 9 (Dotmatics, San Diego, CA, USA) and presented as mean ± standard deviation (SD). Products were considered antiviral if a log4 or greater reduction in viral titer was observed compared with the virus negative control. All PCR data were analyzed using CFX Maestro Software (Bio-Rad, CA, USA).
3 Results
3.1 Non-enveloped viruses displayed greater resistance to all wash conditions than enveloped viruses
To assess the antiviral properties of each product (Table 1), viruses were mixed with individual wash products. At both concentrations, a significant reduction in viral titer (log4 or greater) was observed in HSV, HCoV, and IVA (Figures 1A–F), across all products after 20 s of incubation, except for SFC 2, which had no effect at either concentration. In contrast, non-enveloped viruses including Ad and MNV demonstrated greater resistance, and largely showed little to no change in viral titer after incubation with all products (Figures 1G–J). The natural soap did show antiviral properties against Ad at the highest concentration, but not against MNV (Figure 1G). Increasing the incubation time did not alter these findings (Figures S1, S2). Emollient creams are recommended by physicians to treat ICD and are also frequently recommended for use as soap substitutes, despite not being designed for this purpose. Results showed a similar trend, whereby HSV, HCoV, and IVA demonstrated susceptibility to all products except SFC2 (Figures 1B, D and F). Similarly, both Ad and MNV appear to remain resistant across all products at 20 s (Figures 1H, J). Importantly, the virus negative control, containing the virus and 20% emollient cream (Table 1), demonstrated no effect on viral titer which is in agreement with clean conditions; this indicates that 20% emollient cream has no antiviral action against any of the viruses tested. Increasing the incubation time (to 40 and 60 s) did not alter the findings (Figures S1, S2). Further studies were performed using a more extensive panel of cleansers including synthetic soap 2 and SFCs 3 and 4 (Table 1); these yielded similar results as above, in that they demonstrated antiviral activity against enveloped viruses but not non-enveloped viruses (S3 Figure).
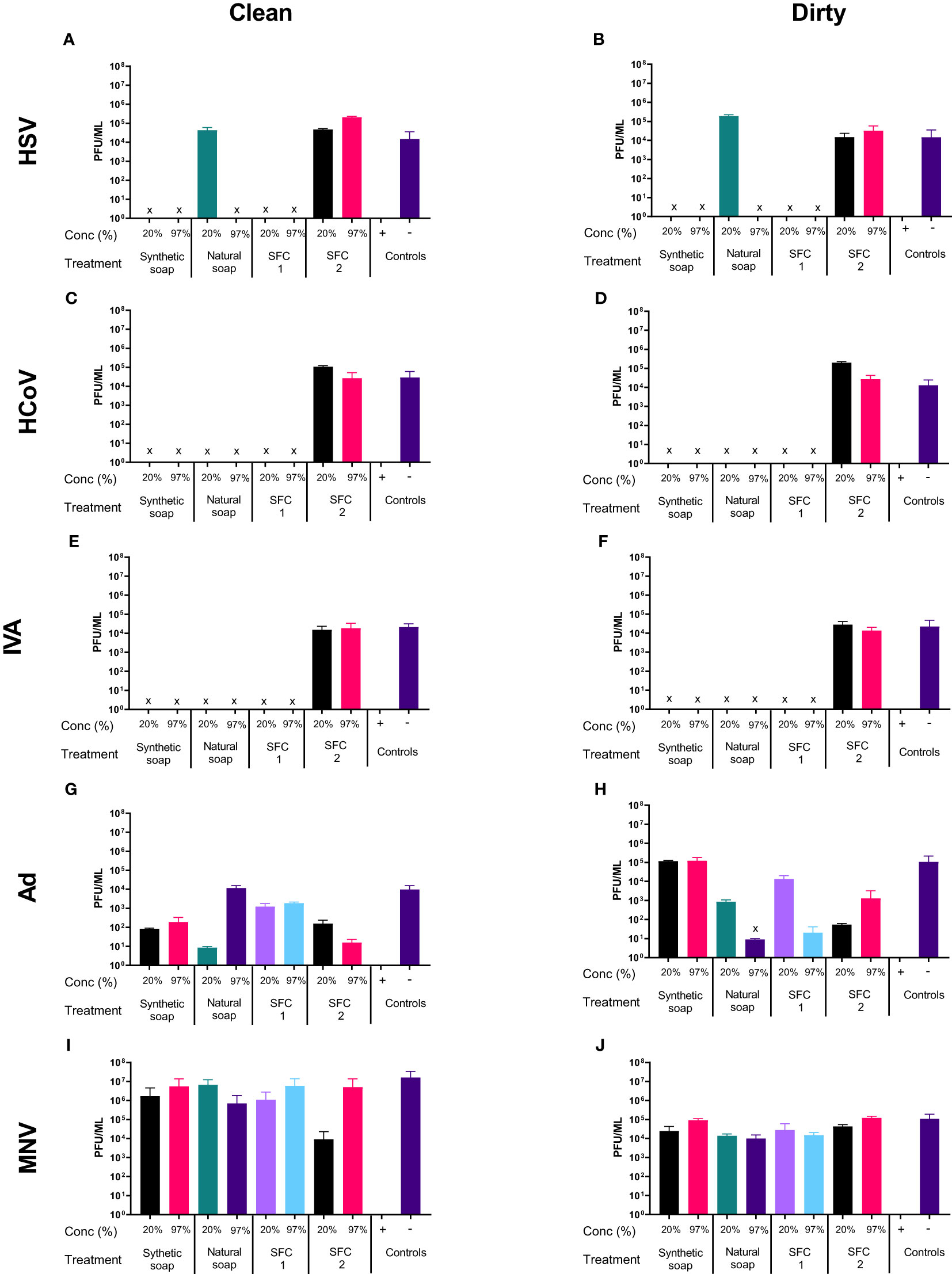
Figure 1 Non-enveloped viruses displayed resistance against soaps and skin-friendly cleansers, but enveloped viruses did not. The antiviral efficacy of a range of wash products (at 20% and 97% concentrations) was determined under simulated clean and dirty hand washing conditions (clean: 0.3% BSA; dirty: 3% BSA with 20% emollient cream) with soft water for 20 s. The wash products were incubated with HSV (A, B), HCoV (C, D), IVA (E, F), Ad (G, H), and MNV (I, J). Viral counts were obtained by plaque assay or TCID50 and expressed as pfu/mL (mean ± SD for n = 3). The wash products were considered antiviral if a log4 reduction or greater was observed (indicated by X). The positive control consisted of 1% bleach instead of a wash product and the negative control consisted of the virus and an interference substance (no wash product).
3.2 Water hardness affects the antiviral activity of wash products
As in the soft water conditions Figures 1A–F, most products showed antiviral properties when incubated with HSV, except natural soap, which lost its antiviral properties when diluted to a 20% concentration in soft water (Figures 1A, B). However, the antiviral effects of the natural soaps were observed in HSV1716, when the wash products was diluted in hard water (Figures 2A, B). In contrast, Ad and MNV, again, showed resistance to most wash products regardless of water type (Figures 2G–J). Tests using “dirty” conditions (Figures 2B, D, F, H, J) showed a similar trend, whereby HSV, HCoV, and IVA displayed susceptibility to all products except SFC 2, whereas both Ad and MNV remained resistant across most products at 20 s. Once more, similar trends were observed when increasing the incubation time (Figures S7, S8) and when using synthetic soap 2 and SFCs 3 and 4 as alternative wash products (Figure S3).
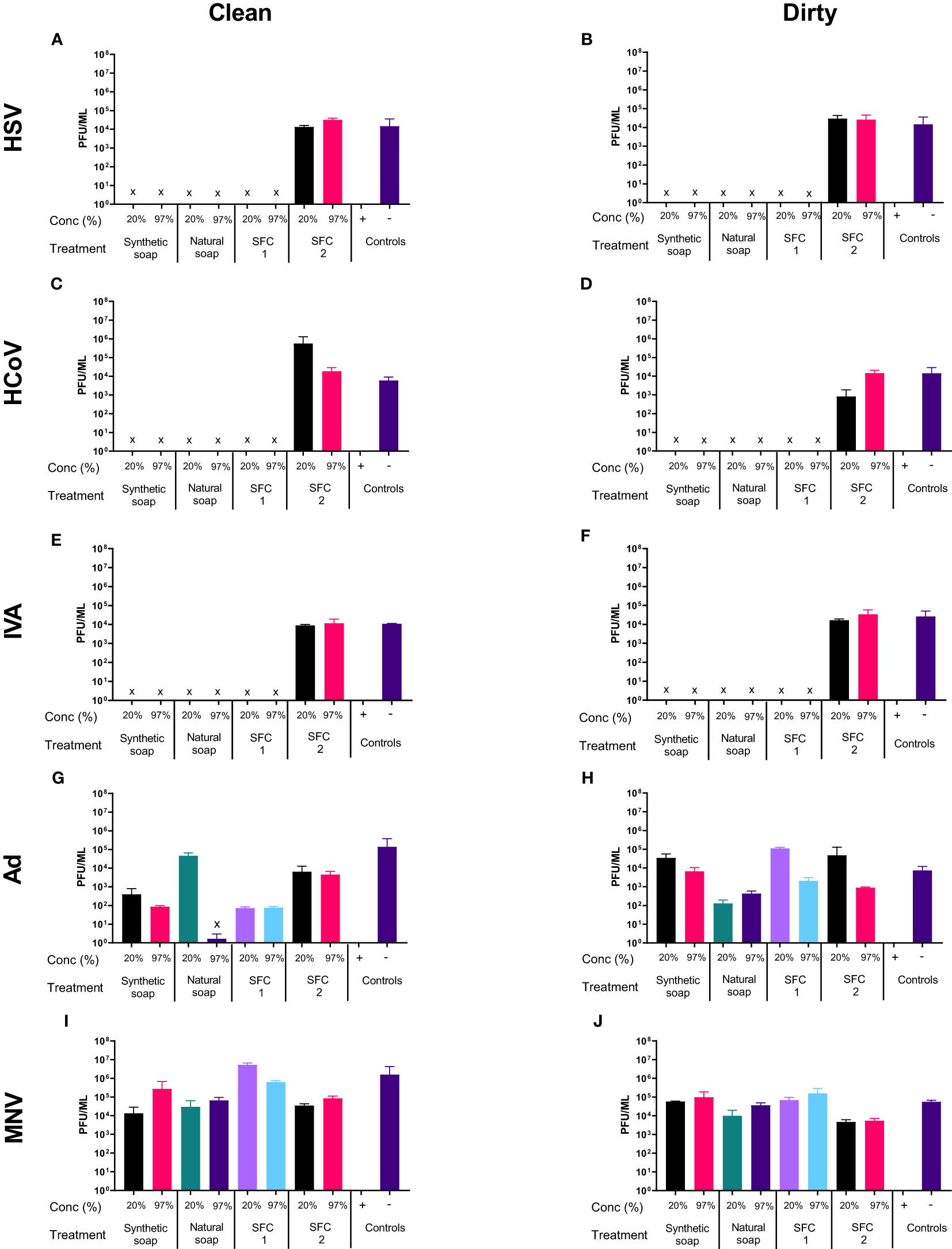
Figure 2 Hard water modifies the antiviral activity of traditional soaps. The antiviral efficacy of a range of wash products (at 20% and 97% concentrations) was determined under simulated clean and dirty hand washing conditions (clean: 0.3% BSA; dirty: 3% BSA with 20% emollient cream) with hard water for 20 s. The wash products were incubated with HSV (A, B), HCoV (C, D), IVA (E, F), Ad (G, H), and MNV (I, J). Viral counts were obtained by plaque assay or TCID50 and expressed as pfu/mL (mean ± SD for n = 3). The wash products were considered antiviral if a log4 reduction or greater was observed (indicated by X). The positive control consisted of 1% bleach instead of a wash product and the negative control consisted of the virus and an interference substance (no wash product).
3.3 TEM confirms that non-enveloped viruses are more resistant to routine hand hygiene practices
TEM was used to determine changes to the normal shape, size, and architecture of the viruses after interaction with the wash products, in order to further support viral titer assays and examine if the viruses had potentially lost their infectibility. The enveloped viruses HSV, HCoV, and IVA exhibited signs of morphological changes after incubation with synthetic and natural soaps, and with SFC 1 (Figures 3A–C). These morphological changes can be attributed to slight alterations in viral shape and structure (highlighted by red arrows), along with a reduction in the normal size for an enveloped virus. SFC 2 appears to be the only product tested that had no visible effect on enveloped virus morphology, supporting data seen within Figures 1A–F. Moreover, all non-enveloped viruses showed no distinct changes to shape, size, and architecture, again supporting data from Figures 1G–J and 3D, E. This indicates that these products are not capable of inducing morphological changes, which may potentially contribute to the non-enveloped viruses’ sustained infectivity.
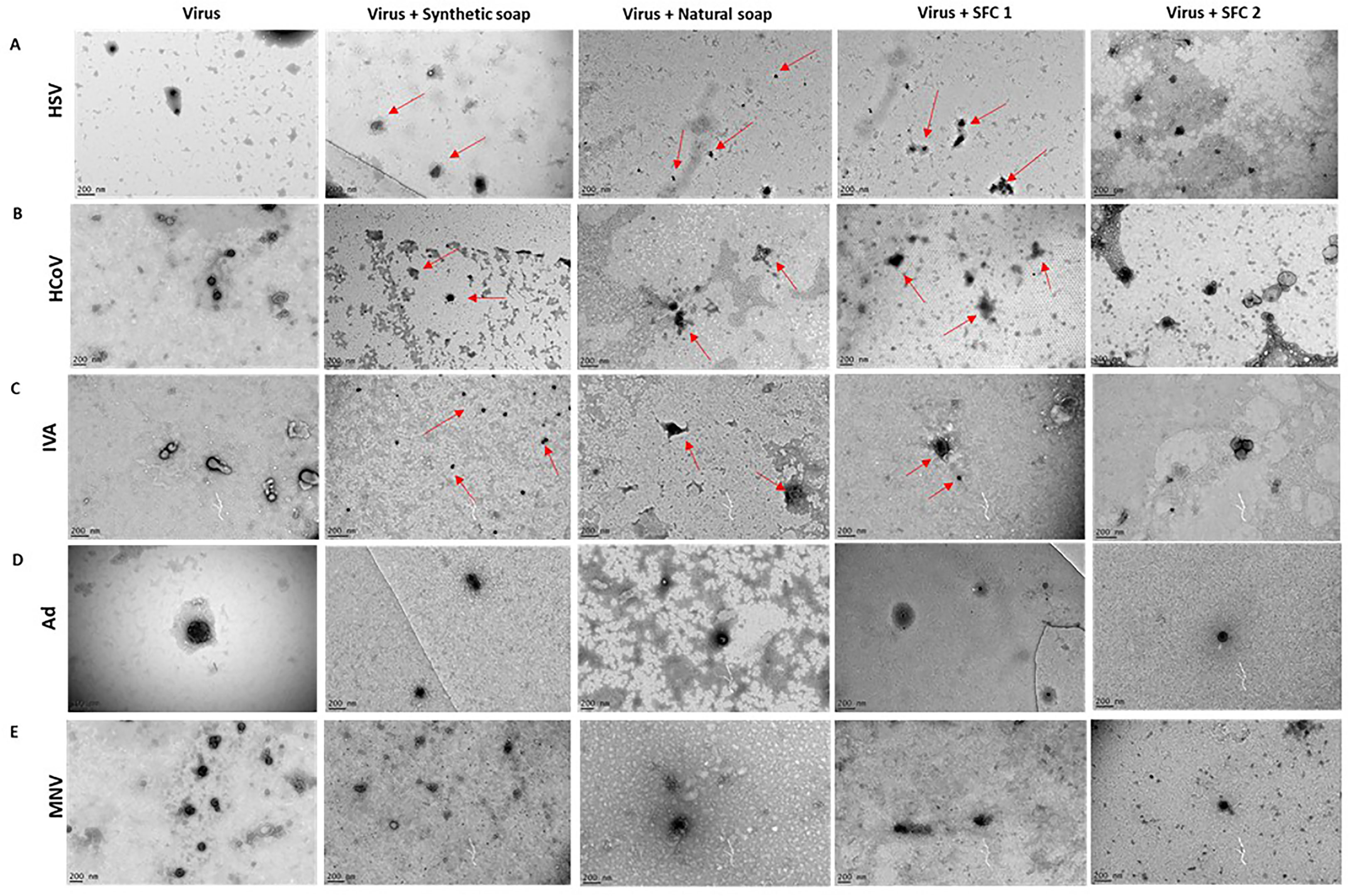
Figure 3 Non-enveloped viral structure remains unchanged after incubation with wash products. Representative TEM images of (A) HSV, (B) HCoV, (C) IVA, (D) Ad, and (E) MNV, alone or after incubation with wash products for 20 s. Red arrows depict changes to normal virus morphology. The TEM images were taken in order to support viral titer data, which can be seen in the above figures. Images were taken on a FEI Tecnai T20 and analyzed using ImageJ software. A scale bar can be seen in the lower left-hand corner.
3.4 Synthetic soap upregulates Ad viral replication genes
Results showed that synthetic soap upregulated the viral replication gene E4, as soon as 4 h after interaction (Figures 4A, B). Moreover, E4 was still upregulated, compared with the positive control, 24 h after exposure. However, interaction with SFC 1 showed no significant upregulation (Figure 4B). MNV showed no significant upregulation of the viral replication gene NS3 with any product or at any time point (Figures 4C, D).
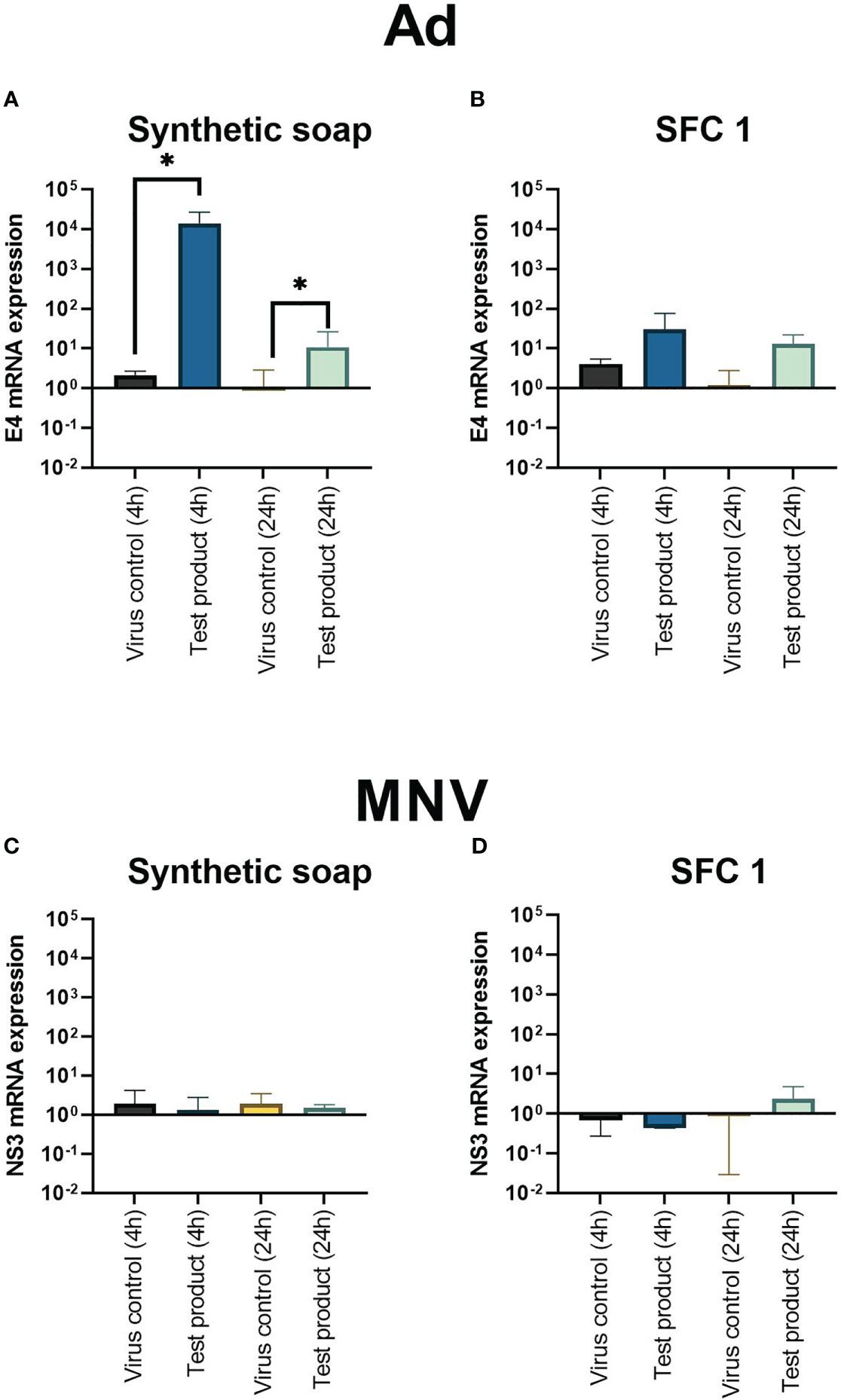
Figure 4 Adenoviral replication gene E4 is upregulated after incubation with synthetic soap and skin-friendly cleanser 1. The expression of viral replication genes E4 on Ad (A, B) and NS3 on MNV (C, D) after incubation with synthetic soap and SFC 1 (97% concentrations) was determined under simulated clean hand washing conditions (0.3% BSA) with soft water for 20 s. Synthetic soap (A) demonstrated a significant increase in viral replication gene expression when compared with the viral control, whereas SFC 1 (B) showed no statistically significant difference. MNV showed that no viral replication genes were upregulated or downregulated after incubation with either wash product. Data are presented as mean ± SD for n = 3 independent experiments. Statistical analysis comprised a ratio-paired t-test, with significance determined by a p-value = * > 0.05.
4 Discussion
In this study, hand washing conditions were replicated in vitro according to the British and European Standard to test a range of commonly used wash product types under the stringent conditions recommended by the British and European Standard (BS EN 14476:2013 + A2:2019). The results reveal that non-enveloped viruses were resistant to all types of wash products tested, including synthetic soaps, which are commonly used within hospitals. In contrast, enveloped viruses showed broad susceptibility to the different wash products. Increasing the exposure time from 20 (current worldwide guidance) to 60 s did not alter the findings and supports the current UK guidelines on hand washing duration (8).
Viral structure, specifically the viral envelope (lipid bilayer), is known to be destabilized in the presence of harsh surfactants (9). This is consistent with the results of the present study, which found that all enveloped viruses (HSV, HCoV, and IVA) were susceptible to both synthetic and natural soaps that contain anionic surfactants, such as sodium laureth sulfate, potassium cocoate, and sodium dodecyl sulfate, commonly found in household detergents. These data are supported by previous publications, which have shown the effectiveness of hand wash products, especially on enveloped viruses (such as IVA), demonstrating that hand washing is an effective intervention in the prevention of viral outbreaks generated from enveloped viruses (10–12). However, these surfactants exert the same effects on natural oils found in the skin, which are essential for maintaining skin health, leading to epidermal damage (13); therefore, a balance needs to be found between effective antimicrobial protection and mitigation of skin damage. The SFCs used within our study contain milder surfactant mixtures, designed to reduce skin damage. Most SFCs tested demonstrated antiviral effects. SFCs 1, 3, and 4 (Supplementary Figures) all showed antiviral properties across all enveloped viruses, due to their formulas containing some more effective surfactants.
It should be noted that the nature of these surfactant changes depend on the water type in which they interact. The high mineral content found in hard water makes soluble anionic surfactants insoluble. The effects of this are seen here, with the modulation of antimicrobial efficacy in natural soaps. In addition to reducing antiviral efficacy, hard water facilitates a build-up of insoluble surfactants on the skin surface, which is known to induce skin irritation and could potentially interfere with the destabilization of viruses (14). Therefore, individuals living in areas with hard water are at greater risk of developing atopic dermatitis (atopic eczema) and, based on our findings, may also be at increased risk of increased viral transmission if using natural soaps as opposed to synthetic cleansers.
Non-enveloped viruses (MNV and Ad) demonstrated greater resistance across all test products used within this study, which aligns with the current literature, as such viruses lack a phospholipid layer that can be easily destabilized by detergents and surfactants (15–17). The lack of a phospholipid membrane around the virus provides greater structural stability, thus enabling non-enveloped viruses to remain infectious outside of a host (or in more “hostile” environments) making it incredibly hard to neutralize them in a micro-community, such as a hospital (18). Our results demonstrated that the synthetic soaps (taken from a hospital setting) were incapable of sufficiently reducing the infectiousness of MNV and Ad, which may highlight why NV outbreaks within hospitals are difficult to control. In addition, TEM analysis showed that these hand wash products did not substantially change the morphology of the non-enveloped viruses (Figure 3). These findings suggest that good hand hygiene practices, especially those imposed within a healthcare setting (primarily regular hand washing with soap and water), may be insufficient at controlling the spread of norovirus (NV) within the population, as results from this study demonstrated an insufficient effect on MNV (a surrogate for human norovirus) (17, 19). Current guidelines given during norovirus outbreaks within a community health and social care setting advise regular disinfection with bleach for all surfaces (16, 17, 20); however, bleach-based hand wash products are not a feasible option due to the high level of sodium hypochlorite, which is a corrosive agent (21). However, one option that we did not explore within this series of studies was alcohol-based hand sanitizers. Another study looked at the antimicrobial and antiviral effects of hand wash products, including hand sanitizers; however, only alcohol-based hand sanitizers (> 70% ethanol) demonstrated antiviral effects (based on British and European standards) against MNV (22). This is not the only publication to report such findings, as Park et al. (23) supports these conclusions, with hand sanitizers being a potential candidate to replace current UK hand hygiene regimes and wash products found within hospitals, owing to more resilient viruses such as MNV and NV (23). However, consideration is required when picking the correct hand sanitizers that will provide protection from all viruses, including non-enveloped viruses, as Park et al. (23) demonstrated that out of seven potential hand sanitizer candidates, only two—a 72% ethanol, pH 2.9 sanitizer and 0.1% triclosan, pH 3.0 triclosan-based sanitizer—were effective at reducing the infectivity of MNV. Moreover, it should be noted from this study that, although MNV viral particles were reduced, some MNV still remained after incubation with the hand sanitizers (23). With data such as these generating encouraging results for the reduction of NV infectivity, and hence the potential prevention of Norovirus outbreaks, individuals suffering with ICD could consider an alcohol-based hand sanitizer as part of their hand hygiene routine. This could be followed by an emollient cleanser to help restore moisture within the skin’s epidermis and remove any excess alcohol that may lead to unwanted irritation. Furthermore, data has suggested that some alcohol-based hand sanitizers do not show significant signs of skin barrier irritation, especially if they contain emollients, providing an appropriate replacement for the hand wash products currently provided to HCPs within healthcare settings (24, 25).
An alternative to dedicated wash products advocated by HCPs is the use of emollient lotions and creams for hand washing. We show that at a 20% concentration of an emollient lotion, diluted by water to imitate an in vitro “real-life” washing scenario, did not exhibit any antiviral efficacy. Given that the removal of potentially harmful microbes is a key requirement for any cleansing routine, this highlights the fundamental ineffectiveness of this practice. However, Styles et al. (26) demonstrated that an antimicrobial emollient lotion containing benzalkonium chloride and chlorohexidine dihydrochloride, used in the management of ICD, exhibited antiviral properties against IVA strain H1N1 and SARS-CoV-19. The viruses were exposed to the diluted lotion (25% concentration) for a total of 60 min for SARS-CoV-19 and 120 min for IVA, and both viruses were neutralized as effectively as they were with common hand sanitizers (26). However, this result could be seen as somewhat ambiguous, as the log4 or greater reduction in viral titer required to substantiate an antiviral claim according to the British and European standard (BS EN 14476:2013 + A2:2019) was only observed for IVA after 120 min (26). This means that while this antimicrobial lotion may be appropriate for a topical leave-on emollient that also provides protection, it is not an effective alternative to hand wash products in a “real-life” scenario, as washing hands for 120 minutes is an unattainable expectation. Therefore, data such as these shows the importance of our findings that SFCs 1, 3, and 4 are capable of meeting UK government guidelines by deactivating enveloped viruses (HSV, HCoV, and IVA) within the desired hand washing time (20 s).
The emollient content of washing products is considered an important factor in formula design for maintaining a healthy skin barrier and providing antimicrobial protection. The application of topical emollients after washing has also been shown to reduce the damaging effects of washing on the skin (27). With the addition of emollient cream to our dirty test conditions, we showed that significant emollient residues do not interfere with the antiviral efficacy of wash products and that the inclusion of emollients in wash products should not necessarily impact on their antiviral efficacy.
Although the data generated from this study have demonstrated a potential and promising substitute for hand wash products (SFCs), it is difficult to determine which individual surfactant or combination of surfactants within the SFCs delivered the antiviral properties observed against enveloped viruses. Moreover, the concentration of surfactants is as important as the type and combination. In addition, the in vitro nature of these experiments does not reflect the “real-life” aspects of hand washing, such as the physical action of hand washing and drying, which has been shown to contribute to debris and microbial removal (28). In this way, this study provides a stepping stone for further in-depth pre-clinical in vivo research.
In conclusion, our results demonstrate a need for virus-dependent hand hygiene procedures, specifically in relation to viral structure, or better hand hygiene practices that account for all viruses. Enveloped viruses demonstrated susceptibility to a range of hand wash products, including both soaps and SFCs, which is encouraging in light of the current COVID-19 pandemic. Therefore, substituting harsh soaps containing anionic surfactants with milder wash products with proven antiviral activity against enveloped viruses has the potential to reduce the burden of ICD and should be considered in the future. Further studies will be required to demonstrate that these interventions can help reduce the prevalence and severity of ICD. Moreover, non-enveloped viruses demonstrated greater resistance across all product types, including both harsh anionic and milder non-ionic surfactants, with MNV being the most resilient virus. Consequently, greater future emphasis on developing hand wash products to eliminate non-enveloped viruses is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Conceptualization: MC, SD, and MM. Data curation: NW, SG, and ZA. Formal analysis: NW. funding acquisition: SD, MC, and MM. Investigation: NW, SG, and ZA. Methodology: NW. Validation: NW, SG, and ZA. Supervision: NW, SD, and MM. Visualization: NW, NB, MC, SD, and MM. contributed to writing (draft and reviewed): NW, SD, NB, and MM. Proofreading: NW, SD, NB, and MM. Formatting: NW. Editing: NW, SG, and MM. Supervision: NW and MM. Visualization of the review: NW and SG. All authors contributed to the article and approved the submitted version.
Funding
Financial support was provided by CeraVe (L’Oréal grant number R/167369-11-1) and the University of Sheffield Institutional Open Access Fund.
Acknowledgments
I would also like to acknowledge Kylie Stark (Sheffield Hallam University), who provided support throughout the project in their technical lab role.
Conflict of interest
NB was employed by company L’Oréal.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this this study received funding from L'Oréal. The funder had the following involvement in the study: Contribution to reviewing and proofreading of the manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1180815/full#supplementary-material
References
1. Babino G, Argenziano G, Balato A. Impact in contact dermatitis during and after SARS-CoV2 pandemic. Published in current treatment options in allergy. (2022) 9(1):19. doi: 10.1007/s40521-022-00298-2
2. The Health and Work Development Unit. Diagnosis, management and prevention of occupational contact dermatitis. RCP London: Royal College of Physicians (2011). Available at: https://www.rcplondon.ac.uk/guidelines-policy/diagnosis-management-and-prevention-occupational-contact-dermatitis-0.
3. Brasch J, Becker D, Aberer W, Bircher A, Kränke B, Jung K, et al. Guideline contact dermatitis: S1-guidelines of the German contact allergy group (DKG) of the German dermatology society (DDG), the information network of dermatological clinics (IVDK), the German society for allergology and clinical immunology (DGAKI), the working group for occupational and environmental dermatology (ABD) of the DDG, the medical association of German allergologists (AeDA), the professional association of German dermatologists (BVDD) and the DDG. Allergo J Int (2014) 23(4):126. doi: 10.1007/s40629-014-0013-5
4. Cashman MW, Reutemann PA, Ehrlich A. Contact dermatitis in the united states: epidemiology, economic impact, and workplace prevention. Dermatologic Clinics. (2012) 30(1):87–98. doi: 10.1016/j.det.2011.08.004
5. Johnston GA, Exton LS, Mohd Mustapa MF, Slack JA, Coulson IH, English JSC, et al. British Association of dermatologists’ guidelines for the management of contact dermatitis 2017. Br J Dermatol (2017) 176(2):317–29. doi: 10.1111/bjd.15239
6. Kar D, Das A, Sil A. An upsurge of hand dermatitis cases amidst COVID-19 pandemic. Indian J Dermatol (2021) 66(2):218. doi: 10.4103/ijd.IJD_631_20
7. Pourianfar HR, Javadi A, Grollo L. A colorimetric-based accurate method for the determination of enterovirus 71 titer. Indian J Virol (2012) 23(3):303–10. doi: 10.1007/s13337-012-0105-0
8. Department of Health and Social Care. Public information campaign focuses on handwashing (2020). Available at: https://www.gov.uk/government/news/public-information-campaign-focuses-on-handwashing.
9. Nazari M, Kurdi M, Heerklotz H. Classifying surfactants with respect to their effect on lipid membrane order. Published in Biophysical Journal (2012) 102(3):498–506. doi: 10.1016/j.bpj.2011.12.029
10. Grayson ML, Melvani S, Druce J, Barr IG, Ballard SA, Johnson PDR, et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis (2009) 48(3):285–91. doi: 10.1086/595845
11. Li JZ, Mack EC, Levy JA. Virucidal efficacy of soap and water against human immunodeficiency virus in genital secretions. Antimicrobial Agents Chemotherapy (2003) 47(10):3321. doi: 10.1128/AAC.47.10.3321-3322.2003
12. Lavelle GC, Gubbe SL, Neveaux JL, Bowden BJ. Evaluation of an antimicrobial soap formula for virucidal efficacy in vitro against human immunodeficiency virus in a blood-virus mixture. Antimicrobial Agents Chemotherapy (1989) 33(12):2034. doi: 10.1128/aac.33.12.2034
13. Walters KA, Bialik W, Brain KR. The effects of surfactants on penetration across the skin*. Int J Cosmetic Sci (1993) 15(6):260–71. doi: 10.1111/j.1467-2494.1993.tb00572.x
14. Baviere M, Bazin B, Aude R. Calcium effect on the solubility of sodium dodecyl sulfate in sodium chloride solutions. J Colloid And Interface Sci (1983) 92(2):580–3. doi: 10.1016/0021-9797(83)90179-0
15. Lichtenberg D, Ahyayauch H, Goñi FM. The mechanism of detergent solubilization of lipid bilayers. Biophys J (2013) 105(2):289. doi: 10.1016/j.bpj.2013.06.007
16. Escudero-Abarca BI, Goulter RM, Manuel CS, Leslie RA, Green K, Arbogast JW, et al. Comparative assessment of the efficacy of commercial hand sanitizers against human norovirus evaluated by an in vivo fingerpad method. Front Microbiol (2022) 13:869087(1). doi: 10.3389/fmicb.2022.869087
17. Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol (2010) 76(2):394. doi: 10.1128/AEM.01729-09
18. Firquet S, Beaujard S, Lobert PE, Sané F, Caloone D, Izard D, et al. Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environments (2015) 30(2):140. doi: 10.1264/jsme2.ME14145
19. Barclay L, Park GW, Vega E, Hall A, Parashar U, Vinjé J, et al. Infection control for norovirus. Clin Microbiol infection (2014) 20(8):731. doi: 10.1111/1469-0691.12674
20. CDC. Preventing norovirus. Center for Disease Control (2021). Available at: https://www.cdc.gov/norovirus/about/prevention.html.
21. Goffin V, Piérard GE, Henry F, Letawe C, Maibach HI. Sodium hypochlorite, bleaching agents, and the stratum corneum. Ecotoxicology Environ Saf (1997) 37(3):199–202. doi: 10.1006/eesa.1997.1537
22. Edmonds SL, Mccormack RR, Zhou SS, Macinga DR, Fricker CM. Hand hygiene regimens for the reduction of risk in food service environments. J Food Prot (2012) 75(7):1303–9. doi: 10.4315/0362-028X.JFP-11-449
23. Park GW, Barclay L, MacInga D, Charbonneau D, Pettigrew CA, Vinjé J. Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII.4 norovirus. J Food Prot (2010) 73(12):2232–8. doi: 10.4315/0362-028x-73.12.2232
24. Löffler H, Kampf G. Hand disinfection: how irritant are alcohols? J Hosp Infection (2008) 70(SUPPL. 1):44–8. doi: 10.1016/S0195-6701(08)60010-9
25. Rundle CW, Presley CL, Militello M, Barber C, Powell DL, Jacob SE, et al. Hand hygiene during COVID-19: recommendations from the American contact dermatitis society. J Am Acad Dermatol (2020) 83(6):1730. doi: 10.1016/j.jaad.2020.07.057
26. Styles CT, Vanden Oever M, Brown J, Rai S, Walsh S, Ryan FM, et al. Treatment of irritant contact dermatitis in healthcare settings during the COVID19 pandemic: 1 the emollient dermol 500 exhibits virucidal activity against influenza a virus and SARS-CoV-2. 2 3. Cold Spring Harbor Laboratory (2021). doi: 10.1101/2021.02.12.21251419
27. Williams C, Wilkinson SM, McShane P, Lewis J, Pennington D, Pierce S, et al. A double-blind, randomized study to assess the effectiveness of different moisturizers in preventing dermatitis induced by hand washing to simulate healthcare use. Br J Dermatol (2010) 162(5):1088–92. doi: 10.1111/j.1365-2133.2010.09643.x
Keywords: coronaviruses, norovirus, hand hygiene, dermatitis, skin-friendly cleanser
Citation: Winder N, Ashraf Z, Gohar S, Baalbaki N, Cork M, Danby S and Muthana M (2023) Are mild cleansers appropriate for hand hygiene in the COVID era? An in vitro investigation of the antiviral efficacy of different hand hygiene products. Front. Virol. 3:1180815. doi: 10.3389/fviro.2023.1180815
Received: 06 March 2023; Accepted: 02 May 2023;
Published: 06 June 2023.
Edited by:
Gregory Q. Del Prete, National Cancer Institute at Frederick (NIH), United StatesReviewed by:
Raymond Whiting Nims, RMC Pharmaceutical Solutions, Inc., United StatesDonald W. Schaffner, Rutgers, The State University of New Jersey, United States
Copyright © 2023 Winder, Ashraf, Gohar, Baalbaki, Cork, Danby and Muthana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalie Winder, bmp3aW5kZXIxQHNoZWZmaWVsZC5hYy51aw==; Munitta Muthana, bS5tdXRoYW5hQHNoZWZmaWVsZC5hYy51aw==
 Natalie Winder
Natalie Winder Zahra Ashraf2
Zahra Ashraf2 Sara Gohar
Sara Gohar Munitta Muthana
Munitta Muthana