- 1Department of Microbiology and Immunology, Georgetown University School of Medicine, Washington, DC, United States
- 2Division of Infectious Diseases and Travel Medicine, Georgetown University School of Medicine, Washington, DC, United States
Background: In people with HIV (PWH) and suppressed viral replication by antiretroviral therapy persistent T cell activation and inflammation are important contributors of the increased risk of morbidity and mortality. CD8 T cells express checkpoint receptors and are dysfunctional. IL-27, a member of the IL-6/IL-12 family has shown anti-viral properties against various human viruses, including HIV. The role of IL-27 on HIV-specific T cells remains unclear. We hypothesized that IL-27 will enhance the function of HIV-specific T cells.
Methods: IL-27 effects on T cell function was evaluated by measuring cytokine secretion, proliferation, and cytotoxicity.
Results: Our findings show that IL-27 upregulates cytokine secretion and cytotoxic potential, and trafficking of proliferating HIV-specific CD8 T cells expressing checkpoint receptors TIGIT and PD-1. Unbiased clustering analysis showed that IL-27 may have differential effects on distinct populations of HIV-specific T cells.
Conclusion: Altogether these results suggest that IL-27 may enhance T cell function in the setting of chronic HIV infection.
Introduction
While antiretroviral therapy (ART) successfully suppresses human immunodeficiency virus (HIV) replication, immune activation and inflammation persist, although at lower levels, and have been identified as an important contributor of morbidity and mortality in people with HIV (PWH) (1–4). Particularly, chronic T cell immune activation is associated with an “activated/exhausted phenotype”, expression of several checkpoint receptors including programmed cell death protein-1 (PD-1), lymphocyte activation gene-3 (LAG-3), T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain (TIGIT), CD160, and T cell immunoglobulin and mucin domain containing-3 (TIM-3), and diminished effector function (5–13). CD8 T cells are central players in controlling HIV/SIV replication during acute and chronic infection (14–20). In contrast, CD4 T cells are the main target of infection and harbor the reservoirs that compromise their role in T-cell-mediated immunity (9, 21–26). Immune modulators such as cytokines and checkpoint receptors have been considered to complement treatment strategies to improve T-cell-mediated immunity against HIV (27–35).
The observations that interleukin-27 (IL-27), a member of the IL-12 family of cytokines, has anti-viral properties against human viruses including zika virus, dengue, chikungunya, HIV, influenza, Hepatitis B virus (HBV), and Hepatitis C virus (HCV) has highlighted the therapeutic potential of this cytokine during viral infection (36–43).
IL-27 is mainly produced by antigen presenting cells. IL-27 is a heterodimeric cytokine composed of IL-27p28 and Epstein-Barr virus induced gene 3 (EBI3) subunits. In addition, EBI3 chain is a common element for other members of this family, IL-35 and IL-39 (44–47). IL-27 signals through a heterodimeric receptor formed by IL-27Rα (also known as WSX-1 or TCCR) and gp130 (IL6ST) (46, 48). Binding of IL-27 to its receptor induces activation of the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT1 and STAT3), as well as the mitogen activated protein kinase (MAPK) signaling pathway (48–56).
The role of IL-27 in the setting of viral infections and CD8 T-cell-mediated immunity is not well understood. In naïve CD8 T cells, studies have shown that IL-27 promotes effector differentiation. In vitro, TCR stimulation of naïve CD8 T cells in the presence of IL-27 enhanced expression of T-bet and effector molecules including granzyme B and perforin (57). In the setting of influenza infection, IL-27R signaling promoted upregulation of T-bet-dependent IFNγ secretion by effector CD8 T cells (58). Moreover, IL-27 promotes macrophages activation, increases antigen processing and presentation, and expression of costimulatory molecules boosting T cell-mediated responses against viruses (37).
In the setting of chronic HIV infection, the regulation of IL-27 is not well understood. Studies on plasma levels of IL-27 at distinct stages of the infection including untreated and successfully suppressed viremia by ART showed no changes (59). In contrast, in a cohort of individuals with HIV and cytomegalovirus (CMV) coinfection, IL-27 plasma levels were negatively associated with viral load and positively associated with CD4 T cell counts suggesting a beneficial effect of immunity against HIV (60). Further, another study described a positive association between IL-27 plasma levels and HIV proviral DNA in PBMCs (61).
The effects of IL-27 signaling in HIV-specific CD8 T cells is largely unknown. We previously showed that T cells from PWH express functional IL27Rα and IL-27 stimulation induces transcriptional changes associated with IFN/STAT1-pathways leading to upregulation of T-bet and cytokine secretion by TIGIT+ HIV-specific CD8 T cells (62). In the present study, we further investigated the effects of IL-27 in proliferation and cytotoxic function of HIV-specific CD8 T cells.
Materials and methods
Patient volunteers
The human study was conducted according to the principles expressed in the Declaration of Helsinki. Participants were studied under an Institutional Review Board at MedStar Georgetown University Hospital (ID CR00004576). PWH provided written informed consent for the collection of samples and subsequent analysis. The characteristics of PWH used in this study are described in Supplementary Table S1.
Flow cytometry
Proliferation and cytokine secretion assay
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA collected whole blood by Ficoll-Paque gradient centrifugation (Cytiva, MA). After centrifugation, cells were collected and washed twice with sterile phosphate buffered saline (Gibco, MA) containing 2% fetal bovine serum (GeminiBio, CA). PBMCs were counted and viability was assessed using a cellometer (Nexcelome, MA). Cells were cryopreserved in Recovery™ Cell Culture Freezing Medium (Gibco, MA). PBMCs were stored in nitrogen liquid vapor. In some experiments, PBMCs were used fresh after isolation.
For proliferation assay, frozen PBMCs were thawed with X-Vivo media (Lonza, MD) containing Benzonase nuclease (Millipore Sigma). After 1 hour of resting, cells were labeled with Cell Trace Violet (CTV; Invitrogen, MA) and plated 1x106 per well. Cells were cultured in the presence of HIVGag peptide pool (2 μg/mL; National Institutes of Health [NIH] HIV Reagent Program) or dimethyl sulfoxide (DMSO) as control in the presence or absence of recombinant human (rh) IL-27–100 ng/mL (PeproTech, NJ). After 5 days of culture, cells were restimulated with HIVGag peptides or DMSO. After 2 hours of stimulation, brefeldin A (Calbiochem, CA) was added, and cells were cultured for additional 4 hours. Cells were harvested and stained with LIVE/DEAD staining (Invitrogen, CA). Cells were washed and incubated with human IgG (10 µg/mL; Sigma, MO) for 10 minutes to block Fc receptors followed by staining with a cocktail of monoclonal antibodies (mAbs) specific for CD3, CD4, CD8, CD69, CD107a, TIGIT, PD-1 and PAR1 (Supplementary Table S2). After 30 minutes incubation, cells were washed, fixed and permeabilized with FoxP3/Transcription Factor Fixation/Permeabilization (eBiosceince, CA) for staining of intracellular markers including perforin, granzyme B, TNF-α, IRF4, IFN-γ, T-bet and Eomes (Supplementary Table S2). Cells were acquired at low speed using BD Symphony A5 flow cytometer and analyzed using FlowJo 10 software. High dimensional flow cytometry analysis was performed using Phenograph and tSNE. Phenograph and tSNE is an algorithm that builds a self-organizing map and computes a meta-clustering result. In this analysis, manual gates were applied to exclude dead cells and doublets to gate CD3+ cells. Live CD3+CTVlow cells (1500 from each HIV-infected patient) were concatenated for culture condition: HIVGag peptides stimulation in the presence or absence of rhIL-27.
Isolation and culture of HIV-specific CD8 T cells
Fresh PBMCs were stimulated overnight with HIVGag peptide pool (2 µg/mL; NIH HIV Reagent Program, Catalog HRP-12425) at 3x106 cells/mL and 1x106 with DMSO control. The following day, the cells were harvested, washed and assessed for viability. The PBMCs stimulated with HIVGag peptide pool in the absence of IL-27 had a median viability of 94.4% (interquartile range [IQR]: 89.0-96.3); while the PBMCs stimulated in the presence of IL-27 had a median viability of 93.5% (IQR: 88.9-97.6). PBMCs were centrifuged and resuspended in staining buffer (Hanks’ Balanced Salt Solution [HBSS] containing 0.01% of bovine serum albumin and 0.01% NaN3) and incubated with human IgG (10 µg/mL; Sigma, MO) for 10 minutes to block Fc receptors. The cells were then stained with a cocktail of mAbs specific for CD3, CD4, CD8, CD137, CD45RA, and CD27 for 30 minutes. CD137+ CD8 T cells were sorted using a BD FACSAria™ at the Georgetown University Shared Resource. The sorted CD137+ CD8 T cells were centrifuged at 1000 rpm for 30 minutes. Due to the low frequencies of HIVGag specific CD8 T cells in the blood, we used irradiated (30 Gy) autologous feeder cells at a 1:10 T cell-to-feeder cells ratio to promote optimal expansion (63). Cells were cultured in a 96-well round-bottom plate either with rhIL-27 at a concentration of 100 ng/mL or media alone. For expansion rhIL-2 and rhIL-15 at concentration of 50 U/mL and 20 ng/mL respectively was included in the culture media. Autologous CD4 T cells were cultured separately with rhIL-2 (50 U/mL) and recombinant human IL-7 (20 ng/mL) and used as target cells. After 15 to 20 days of culture, cells were tested for cytotoxicity and degranulation.
Cytotoxicity assays
This flow cytometry-based assay measures the delivery of granzyme B inside the target cells which is detected by a fluorogenic substrate (PanToxiLux™, OncoImmunin, Inc, MD) that detects granzyme B and downstream caspase activities inside the target cells (64, 65).
Autologous CD4 T cells were labeled with Cell Trace Far Red (CTFR) dye to differentiate them from effector cells. Target cells were pulsed with either DMSO or HIVGag peptide pool for 1 hour at 37°C with 5% CO2. NFL-1 probe (OncoImmunin, Inc, MD), a Live/Dead reagent, was added during the last 15 minutes of incubation.
Effector cells were washed and cultured with the targets at 15:1 effector-to-target (E:T) ratio and the fluorogenic substrate was added to the wells. Cells were incubated at 37°C with 5% CO2 for 1 hour. In the last 15 minutes of incubation, the cell mixtures were stained with a cocktail of mAbs specific for CD3, CD8, CD4, PD-1, TIGIT, and CD107a. After staining, cells were washed twice and immediately acquired on a BD Symphony A5 flow cytometer. Data was analyzed with FlowJo 10 software (BD Biosciences, CA).
Redirected killing assay was used to assess the full cytotoxic capacity of in vitro stimulated CD8 T cells. TIGIT+ and TIGIT- CD8 T cells were sorted from PBMCs from PWH and polyclonally T Cell Receptor (TCR) activated using TransACT beads (anti-CD3, -CD2, -CD28 mAbs) at a dilution of 1:100 (Miltenyi Biotech, MD). Cells were cultured either with rhIL-27 at a concentration 100 ng/mL or media alone. For expansion the media was supplemented with rhIL-2 at concentration of 50 U/mL. Cells were tested in the assay after 10 to 15 days of culture.
Redirected killing assay was performed as described (64, 65) In brief, FAS−L1210 (lymphocytic leukemia cell line) were used as targets to evaluate the granule exocytosis pathway. FAS−L1210 were stained with 0.2 mM CTFR (ThermoFisher/Invitrogen, MA). Cells were biotinylated with 0.2 mM biotin (EMD, MO) and incubated with 0.02 mg/mL streptavidin (MilliporeSigma, MO).
FAS−L1210 and activated TIGIT+ and TIGIT- CD8 T cells were labeled with NFL1 (OncoImmunin, MD) to exclude dead cells. TIGIT+ and TIGIT- CD8 T cells were mixed with FAS− L1210 at 27:1 effector-to-target ratio and cultured in media alone or adding biotin labeled anti-CD3 mAb (eBioscience, CA) and incubated for one hour. In the last 15 minutes of incubation, the cell mixtures were stained with a cocktail of antibodies targeting CD3, CD8, CD4, PD-1, TIGIT, and CD107a to evaluate degranulation of the effector cells. Cells were washed twice and immediately acquired on a BD Symphony A5 flow cytometer, with data analysis performed using FlowJo 10 software.
Statistical analysis
A comparison between conditions in the flow cytometry experiments was performed using a non-parametric Wilcoxon test to compare culture conditions. P value < 0.05 was considered significant. Correlations were performed using non-parametric Spearman correlation and p value ≤ 0.01 was considered significant.
Results
IL-27 enhances cytokine secretion and cytotoxic potential (CD107a) of TIGIT+PD-1+ proliferating HIV-specific CD8 T cells
We investigated the effects of IL-27 in proliferation and cytokine secretion by HIV-specific T cells. Study participants had chronic HIV infection and suppressed viral replication by antiretroviral therapy (Supplementary Table S1). Peripheral blood mononuclear cells (PBMCs) from PWH (n= 20, Supplementary Table S1) were thawed and labeled with cell trace violet (CTV). Cells were stimulated with HIVGag peptide pool in the absence or presence of rhIL-27 (100 ng/ml). DMSO was used as negative control. After 5 days of culture, cells were re-stimulated with HIVGag peptide or DMSO as control to further evaluate the cytokine production by proliferating HIV-specific T cells (Figure 1).
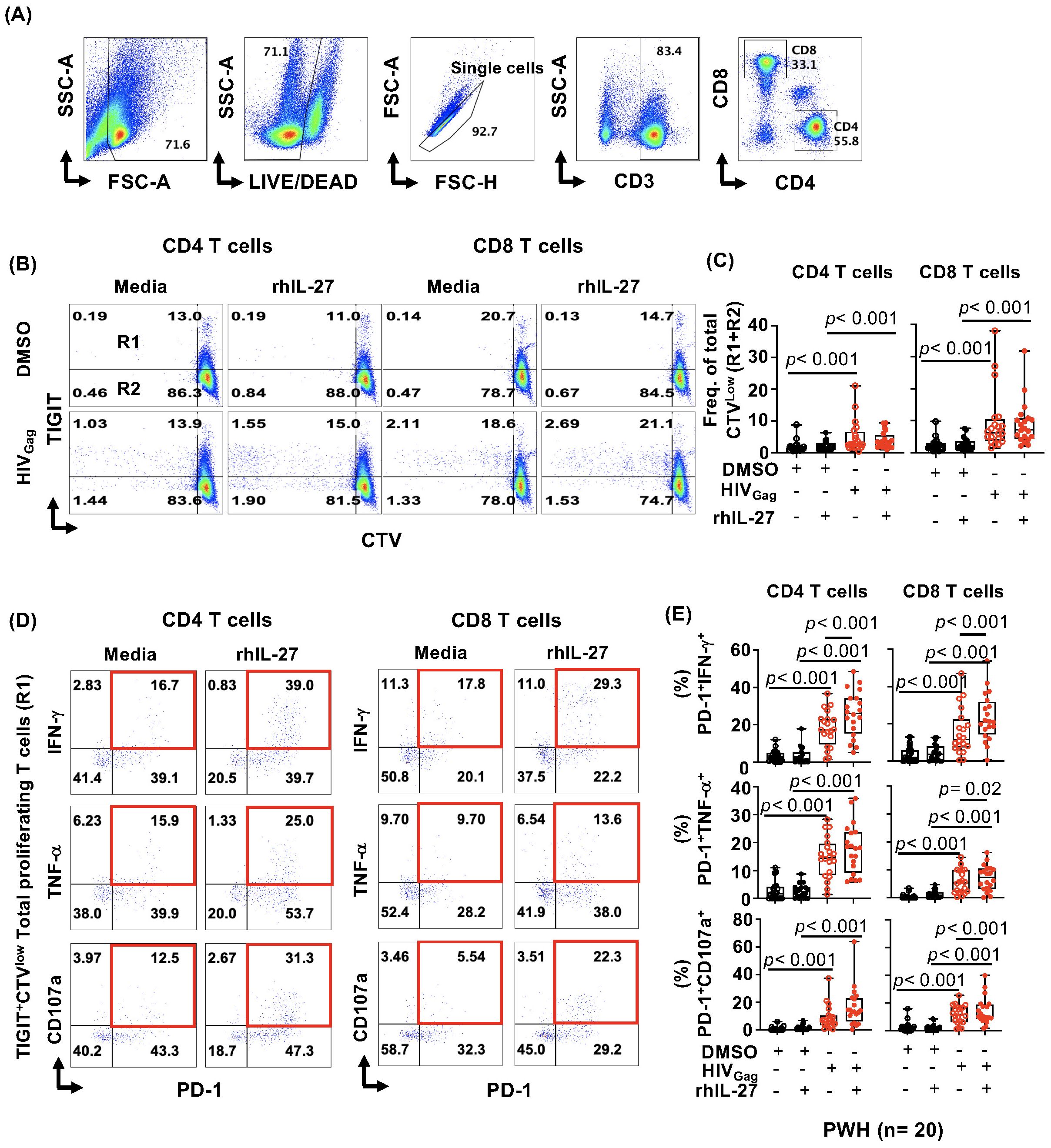
Figure 1. IL-27 enhances cytokine secretion, and degranulation of proliferating TIGIT+PD-1+HIV-specific CD8 T cells. Peripheral blood mononuclear cells (PBMCs) from PWH (n= 20) were thawed, rested for one hour, labeled with Cell Trace Violet (CTV), and stimulated with 2 μg/mL HIVGag peptide pool and 100 ng/mL recombinant human IL-27 (rhIL-27) for five days. DMSO was used as control. (A) Flow cytometry analysis gating strategy. (B) Representative example of gating total (R1+R2) proliferating (CTVlow) CD4 and CD8 HIVGag-specific T cells and TIGIT expression (R1= TIGIT+, R2=TIGIT-). (C) Frequency of total CTVlow (R1+R2) proliferating cells within CD4 and CD8 T cell populations. (D) Representative example dot plots of cytokine secretion by TIGIT+PD-1+CTVlowHIVGag-specific CD4 and CD8 T cells. (E) Frequency of proliferating TIGIT+ expressing PD-1+IFN-γ+, PD-1+TNF-α+, PD-1+CD107a+ within CD4 and CD8 T cells. Data are presented as box-and-whisker plots, showing the median, first and third quartiles, and whiskers extending to the minimum and maximum values. Comparisons between study conditions were performed using the non-parametric Wilcoxon test, with p-values < 0.05 considered statistically significant.
In vitro, IL-27 did not induce a significant increase in the frequency of total (R1+R2) proliferating CTVlow HIV-specific CD4 and CD8 T cells (Figures 1A–C). In addition, IL-27 did not show a differential effect in the proliferation of TIGIT+ (R1) and TIGIT- (R2) HIV-specific CD4 and CD8 T cells (Supplementary Figures S1A, B).
In the setting of HIV infection, chronic immune activation leads to the co-expression of checkpoint receptors and reduced functional ability to secrete cytokines by HIV-specific T cells (5–13, 19, 20). We next evaluated whether IL-27 has an effect on HIV-specific T cells co-expressing TIGIT and PD-1 (Figure 1D). Total proliferating (CTVlow) TIGIT+HIV-specific T cells were assessed for expression of PD-1 and their functional capacity to secrete cytokines. In addition, we assessed their cytotoxic potential by measuring surface expression of CD107a (Figures 1D, E).
We observed that IL-27 significantly increased secretion of IFNγ (p< 0.001) and TNF-α (p= 0.02) in proliferating CTVlowTIGIT+PD-1+HIV-specific CD8 T cells (Figures 1D, E). In addition, IL-27 also enhanced the degranulation of PD-1+CD107a+ (p< 0.001) (Figures 1D, E).
In CTVlowTIGIT+PD-1+HIV-specific CD4 T cells, IL-27 significantly increased the secretion of IFN-γ (p< 0.001) and an increased trend was observed in TNF-α secretion and CD107a, but it did not reach statistical significance (Figure 1E). Similar observations were noted in proliferating TIGIT-PD-1+HIV-specific T cells (Supplementary Figures S1C, D).
These data show that in vitro, IL-27 does not have an effect on the proliferation in HIV-specific T cells, however increased T cell cytokine secretion. IL-27 enhanced cytokine secretion and degranulation (CD107a) by proliferating HIV-specific CD8 T cells, and IFN-γ secretion by HIV-specific CD4 T cells. Particularly, these effects were observed in HIV-specific T cells expressing checkpoint receptors (TIGIT and PD-1).
HIV-specific T cells are heterogenous in their functionality and expression of checkpoint receptors. To better understand IL-27 effects on the function of distinct subsets of HIV-specific T cells, we performed an unbiased cluster analysis using t-distributed stochastic neighbor embedding (t-SNE) analysis of total proliferating (CD3+CTVlow) T cells (Figures 2A, B).
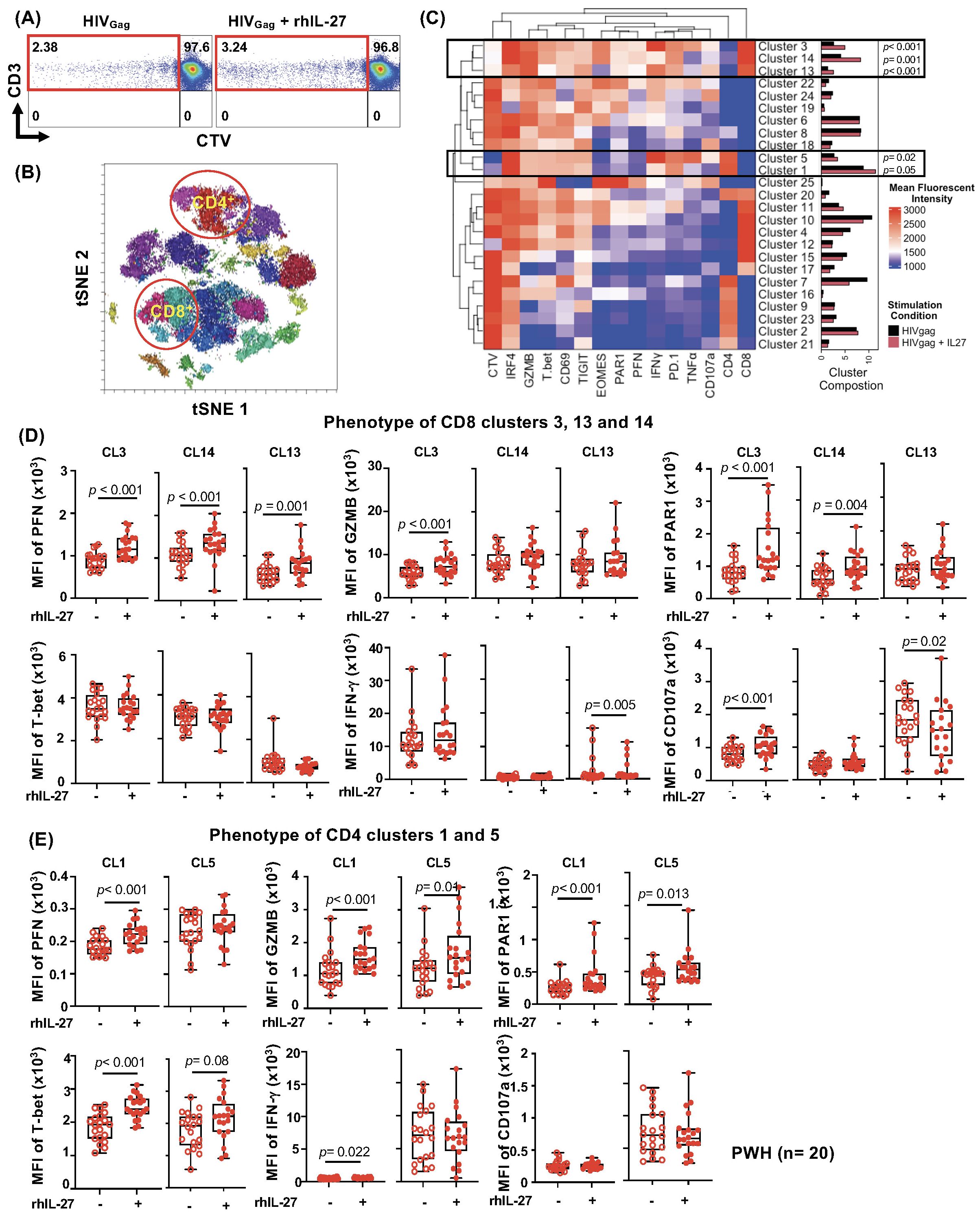
Figure 2. Unbiased clustering analysis of IL-27 effects on HIV-specific T Cells in PWH. (A) Gating strategy depicting the 1500 proliferating (CTVlow) vs CD3 T cells in HIVGag and HIVGag+IL-27 T cells. (B) A high-dimensional t-SNE plot showing the multiple clusters of CD4 and CD8 T cells is highlighted in red circles. (C) Unsupervised heatmap analysis of CD3+CTVlow cells showing 25 total clusters. The frequencies of the clusters are represented by bars, black (culture in absence of IL-27) and red (culture in presence of IL-27). The frequencies of the clusters expanded by IL-27 for both CD8 T cells (CL3, CL14, and CL13) and CD4 T cell clusters (CL5 and CL1) are represented by black box and p-values are indicated. (D) Median fluorescence intensity (MFI) of Perforin, Granzyme B, PAR1, T-bet, IFN-γ, and CD107a in CD8 T cell clusters (CL3, CL14, CL13. (E) MFI of Perforin, Granzyme B, PAR1, T-bet, IFN-γ, CD107a in CD4 T cell clusters (CL5 and CL1). Data are presented as box-and-whisker plots, showing the median, interquartile range, and whiskers representing the full data range. Statistical comparisons were performed using the non-parametric Wilcoxon test, with p-values <0.05 considered significant.
The clustering analysis revealed that IL-27 significantly increased the frequency of some clusters of proliferating CTVlowHIV-specific T cells. CTVlowHIV-specific CD8 T cells showed significant expansion of CL3, CL14, and a discrete CL13 (Figure 2C, bar graphs). In addition, in CTVlowHIV-specific CD4 T cells, IL-27 promoted expansion of cluster CL5 and a trend although not significant was observed for CL1 (Figure 2C).
The most prominent clusters of CTVlowHIV-specific CD8 T cells (CL3 and CL14) expressed high levels of T-bet, and IL-27 led to upregulation of perforin (PFN), granzyme B (only in CL3), and the Protease Activated Receptor 1 (PAR1), a receptor involved in the trafficking and cytotoxic function of CD8 T cells (65, 66). CL3 and CL14 expressed high levels of T-bet, and IL-27 increased expression of Eomes (Figure 2D; Supplementary Figure S2A). In addition, the presence of IL-27 led to an increased degranulation and a trend on IFN-γ production although it was not statistically significant (Figure 2D). Moreover, IL-27 increased PD-1 expression in CL3 and CL14 and had no effects on expression of TIGIT (Supplementary Figure S2A). CL13, a small cluster, expressed low levels of T-bet and higher levels of TIGIT and in the presence of IL-27 showed decreased degranulation (Figure 2D; Supplementary Figure S2A).
In proliferating (CTVlow) CD4 T cells, IL-27 induced proliferation of cluster CL5 and a trend that did not reach statistical significance was observed in CL1. IL-27 promoted a cytotoxic phenotype with increased perforin (but not in CL5), GZMB, and PAR1, although at lower levels than those observed in the CD8 T cell clusters (Figure 2E). In addition, IL-27 significantly increased expression of PD-1 in CL1 but not CL5.
We next evaluated the associations between proliferating TIGIT+PD-1+ HIV-specific T cells and CD4 and CD8 frequencies, absolute counts, and the CD4/CD8 ratio (Supplementary Tables S4–S6). We observed a trend of negative association although not significant between proliferating TIGIT+PD-1+TNFα+ HIV-specific CD4 T cells and CD8 counts, both in the presence of IL-27 (R= -0.697, p= 0.025) and in its absence (R = -0.677, p = 0.035).
Altogether these data suggest that IL-27 may induce differential effects on HIV-specific T cells by promoting proliferation and enhancing T cell function.
GZMB delivery by HIV-specific CD8 T cells is not modulated by IL-27
The results above suggest that IL-27 upregulation of effector molecules (perforin and GZMB) and PAR1 may enhance the cytotoxic function of HIV-specific CD8 T cells. Particularly, PAR1 is involved in polarized secretion of the granule content upon TCR signaling in CD8 T cells (48, 49). We then investigated if HIV-specific CD8 T cells expanded in vitro in the presence of IL-27 has enhanced cytotoxic activity.
PBMCs from PWH (n= 9) were thawed and stimulated with HIVGag peptides in the presence and absence of IL-27 (100 ng/mL) (Figures 3A, B). After overnight stimulation, HIV-specific CD8 T cells were sorted based on surface expression of CD137 (4-1BB) (67, 68). Sorted CD137+HIV-specific CD8 T cells were expanded in vitro in the presence or absence of IL-27 as described in the Material and Methods section. Autologous CD4 T cells were cultured and used as targets.
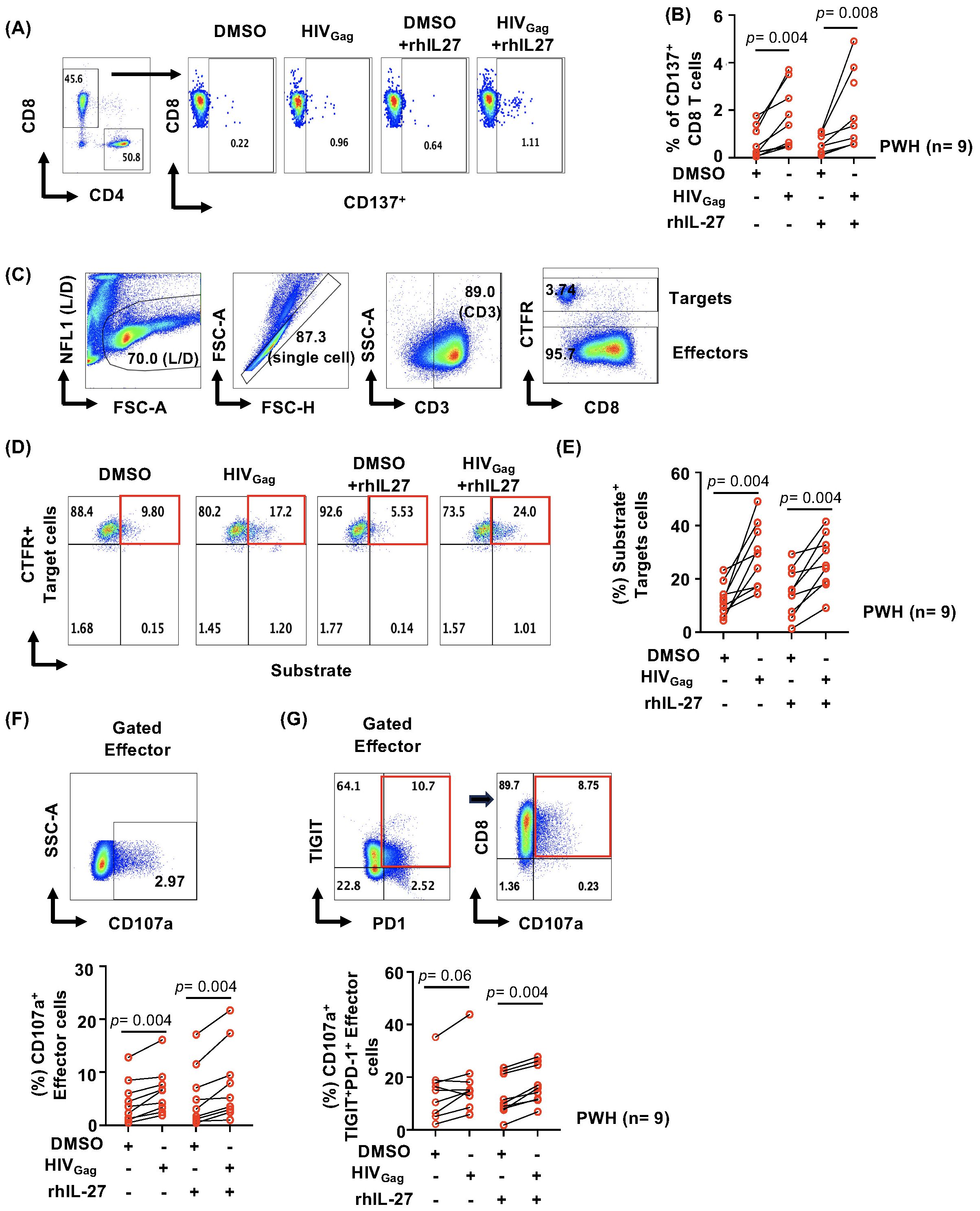
Figure 3. IL-27 effects on GZMB delivery by HIV-specific CD8 T cells. PBMCs from PWH (n= 9) were thawed and stimulated overnight with 2 μg/mL HIVGag peptide pool and 100 ng/mL rhIL-27. After culture, CD137+HIV-specific CD8 T cells were sorted using FACS and in vitro expanded with autologous feeder cells in the presence or absence of IL-27 (100ng/mL) and rhIL-2 (50 U/mL), rhIL-15 (20 ng/mL) for 15–20 days. Autologous CD4 T cells were also sorted and cultured separately with rhIL-2 (50 U/mL) and rhIL-7 (20 ng/mL) and used as target cells. (A) Gating strategy for isolating HIV-specific CD8 T cells based on CD137+ expression. (B) Frequency of the sorted HIV-specific CD8 T cells (CD137+) across the donors. DMSO was used as control. (C) Gating strategy of the cytotoxic assay. Autologous CD4 T cells we labeled with CTFR (targets) to differentiate from the effector cells. (D) Representative example of gating substrate fluorescent signal in target cells (CTFR+substrate+ Target cells). (E) Comparison of the cytotoxic activity of HIV-specific CD8 T cells expanded in the absence or presence of IL-27. DMSO was used as control. (F) Representative graph of the gating strategy to evaluate degranulation of the effector CD8 T cells (upper panel) and frequency of CD107a effector CD8 T cells (lower panel). (G) Representative graph of the gating strategy to evaluate degranulation of the effector TIGIT+PD-1+CD8 T cells (upper panel) and frequency of CD107a effector CD8 T cells (lower panel). Data are presented as line graphs between DMSO control and HIVGag peptides for CD8 T cells expanded in the presence and absence of IL-27. Statistical comparisons between study conditions were performed using the non-parametric Wilcoxon test, with p-values <0.05 considered statistically significant.
To determine the effects of IL-27 on the cytotoxic function, we used an assay that evaluates an early event of the delivery of granzymes inside the target cells. Granzyme B (GZMB) and caspase activities are detected using a substrate which emits a fluorescent signal when cleaved. In this assay, we also measured degranulation of effector cells by detecting surface expression of CD107a (64, 65).
To facilitate the gating of the target cells, autologous CD4 T cells were stained with Cell Trace Far Red (CTFR), (Figure 3C, gating strategy). Target cells were pulsed with HIVGag peptides and DMSO and loaded with the substrate (64, 65). In vitro expanded HIV-specific CD8 T cells were added to the target cells at 15:1 E:T ratio and incubated for one hour. The last fifteen minutes of the culture, a cocktail of mAb specific to CD8, TIGIT, PD-1 and CD107a was added to stain effectors T cells.
CTFR+ target cells were gated and analyzed for substrate activity (Figures 3C, D, gating strategy). Effector degranulation (CD107a surface expression) was analyzed in total CD8 T cells and TIGIT+PD-1+ CD8 T cells (Figures 3F, G).
The analysis showed that HIVGag-specific CD8 T cells whether expanded in the presence or absence of IL-27 have similar ability to deliver granule content inside the target cells (Figure 3E). The frequencies of substrate+ CTFR+ target cells ranged between 9.18 to 41.5% and 14.4 to 49.1% for effectors cultured in the presence or absence of IL-27 respectively (Figure 3E). Similar fold change relative to DMSO control was observed in both conditions (data not shown).
HIVGag-specific CD8 T cells expanded in the presence of IL-27 showed similar degranulation compared to those cultured in the absence of IL-27 (Figure 3F). In addition, similar degranulation was also observed in TIGIT+PD-1+HIVGag-specific CD8 T cells (Figure 3G). These results suggest that IL-27 in vitro neither enhanced nor inhibited degranulation and granule content delivery despite expression of checkpoint receptors of activated HIV-specific CD8 T cells (Supplementary Figure S3).
The in vitro expansion of HIVGag-specific CD8 T cells may result in the selection of clones with proliferative capacity. To better understand the effects of IL-27 in cytotoxic function, we evaluated cytotoxic function of TCR stimulated TIGIT+ and TIGIT- CD8 T cells and address their overall cytotoxic potential in a redirected killing assay.
We sorted TIGIT+ and TIGIT- memory (CD45RA-CD27+, CD45RA-CD27- and CD45RA+CD27-) CD8 T cells from PBMCs from PWH (n= 6). Cells were stimulated with anti-CD3 and anti-CD28 mAbs and expanded in the presence or absence of rhIL-27 (Figure 4A).
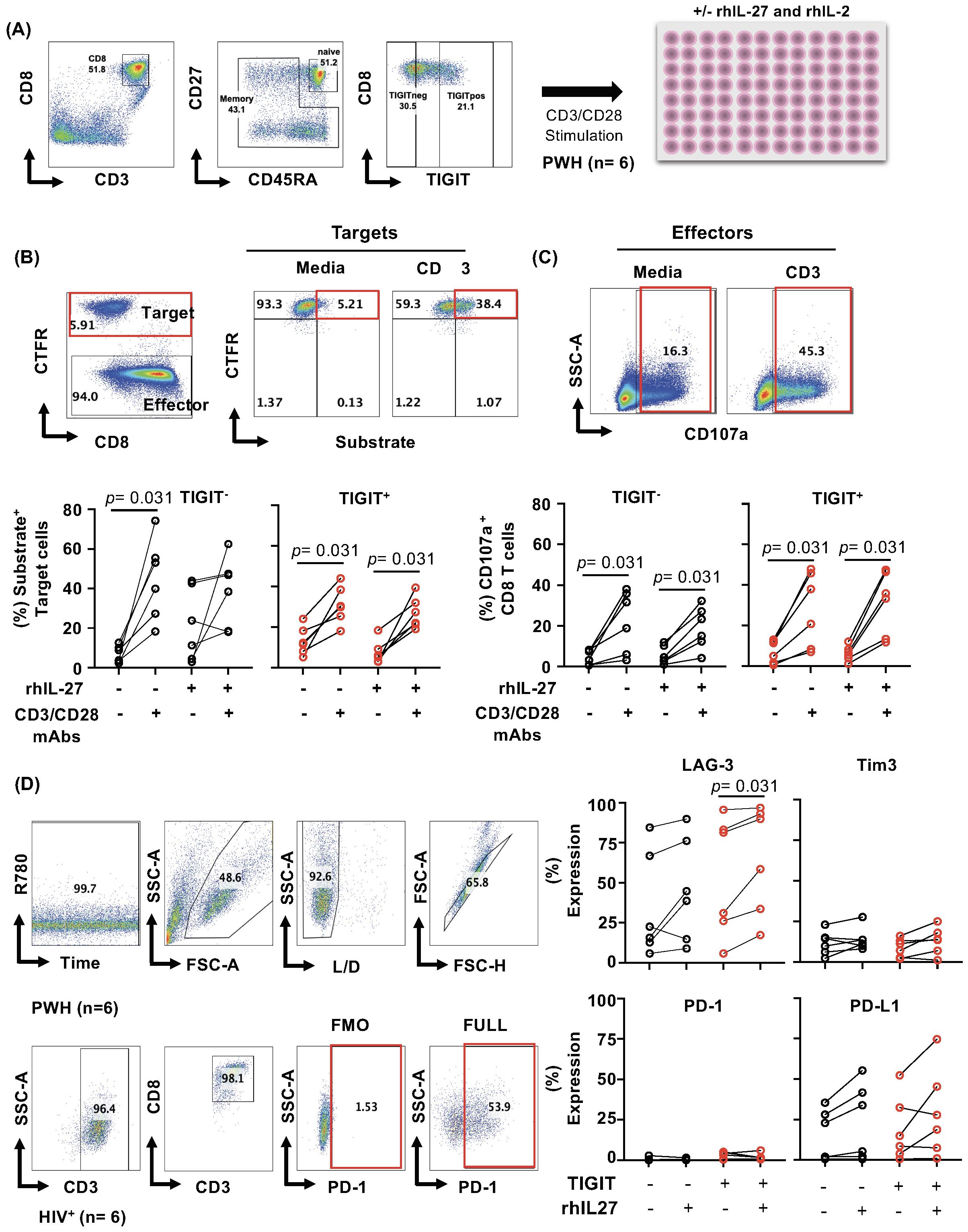
Figure 4. IL-27 does not modulate GZMB delivery by TIGIT+ CD8 T cells. PBMCs from PWH, (n= 6) were thawed, and FACS sorted CD8 T cells based on TIGIT expression, and Fas-L1210 cells were used as targets in redirected killing assay. (A) Gating strategy showing sorting and TIGIT+ and TIGIT- CD8 T memory phenotypes (CD45RA-CD27+, CD45RA-CD27- and CD45RA+CD27-) CD8 T cells from PBMCs from PWH (n= 6). (B) Gating strategy to identify Cell Trace Far Red positive target cells and effector cells. Representative example of the gating of target cells and substrate activity in media and TCR activation with CD3 mAb (Upper panel). Frequency of substrate-positive cells, representing granzyme B delivery into target cells, in both TIGIT+ and TIGIT− CD8 T cell subsets (lower panel). (C) Representative example of the gating of the effector CD8 T cells to assess degranulation in media and TCR activation with CD3 mAb (upper panel). Frequency of CD107a expressing effectors in both TIGIT+ and TIGIT- effector. (D) Phenotype of the effector cells for expression of checkpoint receptors on the day of the assay. Statistical comparisons between conditions were performed using the non-parametric Wilcoxon test, with p-values <0.05 considered significant.
We tested the delivery of granule content using a redirected antigen-independent assay. Effector degranulation is triggered by TCR signaling using cross-linked anti-CD3 mAb on the surface of the target cells (as described in the Material and Methods section). A FAS-deficient L1210 cell line (mouse lymphocytic leukemia) was used as a target to measure the granule exocytosis pathway (64, 65).
Similar to HIV-specific CD8 T cells, IL-27 did not significantly increase the delivery of granzyme by TIGIT+CD8 T cells compared to TIGIT- (Figures 4B, C). The frequency of CD107a+ CD8 T cells tended to be higher than TIGIT-CD107+, however, did not reach statistical significance. In addition, IL-27 did not induce a robust expression of checkpoint receptors cells (LAG-3, Tim-3, PD-1, and PD-L1) in TIGIT+ compared to TIGIT- CD8 T (Figure 4D). The data suggest that IL-27 does not modulate cytotoxic function.
Discussion
In the setting of chronic HIV infection, immune activation drives expression of checkpoint receptors and dysfunction of HIV-specific T cells (7–13, 22, 26, 69, 70). Therefore, enhancing HIV-specific T-cell-mediated immunity is part of the strategies to improve clinical outcomes in PWH (71). In the present study, we investigated the effects of IL-27 on HIV-specific CD8 T cell responses. We found that in vitro while IL-27 did not enhance HIV-specific T cell proliferation, but promoted cytokine secretion and enhanced degranulation of proliferating TIGIT+PD-1+ HIV-specific T cells.
IL-27 has been widely studied in CD4 T cells and showed immunomodulatory functions (72–74). In the setting of viral infections IL-27 effects on T cell immunity is not well understood and both beneficial and modulatory functions have been described (43, 75–77).
IL-27 is a member of the IL-6/IL-12 superfamily of cytokines, and in vitro, has anti-viral properties against HIV in CD4 T cells, monocyte-derived macrophages (MDMs) and dendritic cells (39–42). However, the expression regulation of IL-27 during stages of HIV infection is not well understood. In PWH, plasma levels of IL-27 did not show modulation at distinct stages of disease (59). Other studies of PWH and CMV coinfection showed that plasma levels of IL-27 were negatively associated with viral load and showed a positive association with CD4 T cell reconstitution in coinfected individuals, suggesting beneficial effects of IL-27 (60). The differences observed in these and other studies may be due to the non-disulfide-linked nature of IL-27 (IL-27p28 and EBI3) that makes the detection of the biological active heterodimeric molecule difficult (44, 78–81). The studies in non-human primates infected with simian immunodeficiency virus (SIV) showed that IL-27p28 mRNA expression is induced during acute infection and remain high in the chronic phase (82).
In antigen presenting cells, the transcription of the IL-27 subunits is differentially regulated. Upon activation of pattern recognition receptor, IL27p28 subunit expression is induced by IRF1 and/or IFN-dependent signaling pathways, while EBI3 expression is mainly driven by NF-kB activation (83). Whether HIV infection alters the transcription of IL-27 subunits is not well understood. In mesenteric lymph nodes from non-human primates infected with SIV, mRNA expression of IL27p28 and EBI3 showed no significant changes compared to uninfected controls (84).
The effects of IL-27 in modulating CD8 T-cell-mediated immunity against HIV remains to be defined. We previously showed that T cells from PWH express functional IL-27R and stimulation with IL-27 drives transcriptional changes associated with IFN/STAT1-dependent pathways in T cells. More importantly, IL-27 enhanced T-bet expression and promoted IFN-γ secretion by TIGIT+HIVGag-specific T cells. In addition, IL-27 enhanced degranulation of TIGIT+HIVGag-specific CD8 T cells (62). In the present study we further assessed IL-27 roles in proliferation and cytotoxicity. IL-27 did not enhance the proliferation of total CD4 and CD8 T cells HIV-specific T cells. However, IL-27 promoted cytokine secretion and degranulation in HIV-specific T cells even in those that expressed TIGIT and PD-1.
Previous reports shown that in vitro, IL-27 and TCR signaling induce expression of checkpoint receptors in human T cells suggesting a regulatory effect (85, 86). In this study, we found that IL-27 increased secretion of cytokines of virus-specific T cells expressing checkpoint receptors. Our observations are consistent with a recent report in which IL-27 enhanced antitumor activity of PD-1 and CD39 expressing T cells, and expression of checkpoint receptors was associated with higher levels of T cell activation rather than dysfunction (87–90). Moreover, the potential beneficial effects of IL-27 in T cell immunity were also observed in the setting of the murine model of chronic lymphocytic choriomeningitis virus (LCMV) infection. This infection model recapitulates some features of CD8 T cell exhaustion similar to that observed during HIV infection. In these studies, IL-27 showed a beneficial effect and IL-27 treatment led to increased virus-specific CXCR5+CD8 T cells with stem-cell-like properties (76, 77). In addition, IL-27 also enhanced the function of virus-specific CD4 T cells, TFH cells and humoral immunity controlling the virus at a later phase of the infection (76, 91).
In the present study, a more in-depth examination of the proliferating T cells showed that IL-27 exerts distinct effects on HIV specific T cells. IL-27 promoted proliferation of some clusters of HIV-specific CD4 and CD8 T cells with increased expression of cytotoxic molecules and PAR1, a receptor involved in cytotoxicity and trafficking of effector cells (65). It is possible that IL-27 promotes proliferation and function in certain clones of HIV-specific T cells but not others. In some HIV-specific T cells, IL-27 increased IFNγ secretion. In contrast, there was a very small cluster (CL13), expressing high levels of TIGIT that showed a modest reduction of CD107a suggesting that IL-27 exerts a differential effect on distinct T cell clones. What drives these heterogenous responses to IL-27 in chronically activated HIV-specific T cells may be linked to clonal differences and requires further investigation.
IL-27 is involved in effector differentiation by upregulating transcription factors including T-bet and Eomes (92, 93). In vitro expansion of HIV-specific CD8 T cells did not lead to either an enhanced delivery of granule content inside the target cell nor an inhibitory effect. One limitation of this assay is that while it allows us to investigate early steps in the delivery of the granule content inside the target cells and measure active granzyme and caspase activity, we are not detecting target cell death. In addition, the in vitro expansion of T cells was performed in the presence of IL-2 and IL-15 which may mask the effects of IL-27.
IL-27 promotes antigen processing and presentation, and expression of costimulatory molecules T cell responses (37). In this study, we used autologous CD4 T cells as targets, future studies should address whether IL-27 treatment sensitize CD4 T cells for killing, and whether it enhances HIV presentation in CD4 HIV infected T cells.
IL-27 role in the setting of chronic infection remains to be defined. A recent study in cancer reported beneficial effects of IL-27 in boosting of CD8 T-cell-mediated immunity against tumor (87). Moreover, human T cells engineered to express B-cell maturation antigen (BCMA)-specific T-cell antigen coupler and secreted single chain IL-27 showed that while reducing secretion of GMCSF and TNF-α maintain cytotoxic activity against myeloma cells (94). Therefore, studying the role of IL-27R/IL-27 signaling pathways in the setting of chronic HIV infection and other human chronic infection warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board at MedStar Georgetown University Hospital (ID CR00004576). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MA: Writing – original draft. GK: Writing – review & editing. JC: Writing – review & editing. CM: Writing – review & editing. FM: Writing – review & editing. DM: Writing – review & editing. PK: Writing – review & editing. MC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Leidos Biomedical Research, Inc. has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under Contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. MC is also supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number NIH R01AI145549-02 and by the District of Columbia Center for AIDS Research, an NIH funded program (P30AI117970) which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, 21 NHLBI, NIDA, NIMH, NIA, NIDDK, NIMHD, NIDCR, NINR, FIC and OAR. TGM is supported by the Intramural Research Program of the NIH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2025.1600802/full#supplementary-material
References
1. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PloS Med. (2008) 5:e203. doi: 10.1371/journal.pmed.0050203
2. Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS One. (2012) 7:e44454. doi: 10.1371/journal.pone.0044454
3. Deeks SG, Overbaugh J, Phillips A, and Buchbinder S. HIV infection. Nat Rev Dis Primers. (2015) 1:15035. doi: 10.1038/nrdp.2015.35
4. Brown TT and Guaraldi G. Multimorbidity and burden of disease. Interdiscip Top Gerontol Geriatr. (2017) 42:59–73.
5. Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol. (2004) 173:2410–8. doi: 10.4049/jimmunol.173.4.2410
6. Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci United States America. (2008) 105:19851–6. doi: 10.1073/pnas.0810032105
7. Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. (2006) 203:2281–92. doi: 10.1084/jem.20061496
8. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. (2006) 12:1198–202. doi: 10.1038/nm1482
9. Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. (2007) 8:1246–54. doi: 10.1038/ni1515
10. Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PloS Pathog. (2012) 8:e1002840. doi: 10.1371/journal.ppat.1002840
11. Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PloS Pathog. (2016) 12:e1005349. doi: 10.1371/journal.ppat.1005349
12. Wykes MN and Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. (2018) 18:91–104. doi: 10.1038/nri.2017.112
13. Chen H, Moussa M, and Catalfamo M. The role of immunomodulatory receptors in the pathogenesis of HIV infection: A therapeutic opportunity for HIV cure? Front Immunol. (2020) 11:1223. doi: 10.3389/fimmu.2020.01223
14. Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. (1994) 68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994
15. Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Sci (New York NY). (1999) 283:857–60. doi: 10.1126/science.283.5403.857
16. Lifson JD, Rossio JL, Piatak M, Parks T, Li L, Kiser R, et al. Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol. (2001) 75:10187–99. doi: 10.1128/JVI.75.21.10187-10199.2001
17. Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. (1999) 189:991–8. doi: 10.1084/jem.189.6.991
18. McDermott AB and Koup RA. CD8(+) T cells in preventing HIV infection and disease. AIDS (London England). (2012) 26:1281–92. doi: 10.1097/QAD.0b013e328353bcaf
19. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. (2006) 107:4781–9. doi: 10.1182/blood-2005-12-4818
20. Hersperger AR, Migueles SA, Betts MR, and Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS. (2011) 6:169–73. doi: 10.1097/COH.0b013e3283454c39
21. Soghoian DZ and Streeck H. Cytolytic CD4(+) T cells in viral immunity. Expert Rev Vaccines. (2010) 9:1453–63.
22. Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. (2013) 208:50–6. doi: 10.1093/infdis/jis630
23. Morou A, Palmer BE, and Kaufmann DE. Distinctive features of CD4+ T cell dysfunction in chronic viral infections. Curr Opin HIV AIDS. (2014) 9:446–51. doi: 10.1097/COH.0000000000000094
24. Buggert M, Frederiksen J, Lund O, Betts MR, Biague A, Nielsen M, et al. CD4+ T cells with an activated and exhausted phenotype distinguish immunodeficiency during aviremic HIV-2 infection. AIDS (London England). (2016) 30:2415–26. doi: 10.1097/QAD.0000000000001223
25. Morou A, Brunet-Ratnasingham E, Dube M, Charlebois R, Mercier E, Darko S, et al. Altered differentiation is central to HIV-specific CD4(+) T cell dysfunction in progressive disease. Nat Immunol. (2019) 20:1059–70. doi: 10.1038/s41590-019-0418-x
26. Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PloS Pathog. (2016) 12:e1005761. doi: 10.1371/journal.ppat.1005761
27. Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. (2011) 117:4787–95. doi: 10.1182/blood-2010-10-311456
28. Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, et al. Transient and persistent effects of IL-15 on lymphocyte homeostasis in nonhuman primates. Blood. (2010) 116:3238–48. doi: 10.1182/blood-2010-03-275438
29. Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, et al. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. (2004) 22:3510–21. doi: 10.1016/j.vaccine.2003.07.022
30. Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, et al. IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood. (2011) 118:6845–8. doi: 10.1182/blood-2011-09-377804
31. Miller JS, Davis ZB, Helgeson E, Reilly C, Thorkelson A, Anderson J, et al. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat Med. (2022) 28:392–400. doi: 10.1038/s41591-021-01651-9
32. Goshu BA, Chen H, Moussa M, Cheng J, and Catalfamo M. Combination rhIL-15 and anti-PD-L1 (Avelumab) enhances HIVGag-specific CD8 T-cell function. J Infect Dis. (2020) 222:1540–9. doi: 10.1093/infdis/jiaa269
33. Chen P, Chen H, Moussa M, Cheng J, Li T, Qin J, et al. Recombinant human interleukin-15 and anti-PD-L1 combination therapy expands a CXCR3+PD1-/low CD8 T-cell subset in simian immunodeficiency virus-infected rhesus macaques. J Infect Dis. (2020) 221:523–33. doi: 10.1093/infdis/jiz485
34. Harwood O and O’Connor S. Therapeutic potential of IL-15 and N-803 in HIV/SIV infection. Viruses. (2021) 13. doi: 10.3390/v13091750
35. King HAD and Lewin SR. Immune checkpoint inhibitors in infectious disease. Immunol Rev. (2024) 328:350–71. doi: 10.1111/imr.v328.1
36. Kwock JT, Handfield C, Suwanpradid J, Hoang P, McFadden MJ, Labagnara KF, et al. IL-27 signaling activates skin cells to induce innate antiviral proteins and protects against Zika virus infection. Sci Adv. (2020) 6:eaay3245. doi: 10.1126/sciadv.aay3245
37. Valdes-Lopez JF, Hernandez-Sarmiento LJ, Tamayo-Molina YS, Velilla-Hernandez PA, Rodenhuis-Zybert IA, and Urcuqui-Inchima S. Interleukin 27, like interferons, activates JAK-STAT signaling and promotes pro-inflammatory and antiviral states that interfere with dengue and chikungunya viruses replication in human macrophages. Front Immunol. (2024) 15:1385473. doi: 10.3389/fimmu.2024.1385473
38. Valdes-Lopez JF, Fernandez GJ, and Urcuqui-Inchima S. Interleukin 27 as an inducer of antiviral response against chikungunya virus infection in human macrophages. Cell Immunol. (2021) 367:104411. doi: 10.1016/j.cellimm.2021.104411
39. Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, et al. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PloS One. (2013) 8:e59194. doi: 10.1371/journal.pone.0059194
40. Dai L, Lidie KB, Chen Q, Adelsberger JW, Zheng X, Huang D, et al. IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J Exp Med. (2013) 210:517–34. doi: 10.1084/jem.20120572
41. Poudyal D, Yang J, Chen Q, Goswami S, Adelsberger JW, Das S, et al. IL-27 posttranslationally regulates Y-box binding protein-1 to inhibit HIV-1 replication in human CD4+ T cells. AIDS (London England). (2019) 33:1819–30. doi: 10.1097/QAD.0000000000002288
42. Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, et al. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS (London England). (2008) 22:39–45. doi: 10.1097/QAD.0b013e3282f3356c
43. Amsden H, Kourko O, Roth M, and Gee K. Antiviral activities of interleukin-27: A partner for interferons? Front Immunol. (2022) 13:902853.
44. Jones LL and Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. (2011) 51:5–14. doi: 10.1007/s12026-011-8209-y
45. Collison LW and Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. (2008) 226:248–62.
46. Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. (2002) 16:779–90. doi: 10.1016/S1074-7613(02)00324-2
47. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. (2007) 450:566–9. doi: 10.1038/nature06306
48. Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. (2004) 172:2225–31. doi: 10.4049/jimmunol.172.4.2225
49. Lucas S, Ghilardi N, Li J, and de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci United States America. (2003) 100:15047–52. doi: 10.1073/pnas.2536517100
50. Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. (2003) 170:4886–90. doi: 10.4049/jimmunol.170.10.4886
51. Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. (2003) 19:645–55. doi: 10.1016/S1074-7613(03)00300-5
52. Kastelein RA, Hunter CA, and Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. (2007) 25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758
53. Hibbert L, Pflanz S, De Waal Malefyt R, and Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. (2003) 23:513–22. doi: 10.1089/10799900360708632
54. Owaki T, Asakawa M, Morishima N, Mizoguchi I, Fukai F, Takeda K, et al. STAT3 is indispensable to IL-27-mediated cell proliferation but not to IL-27-induced Th1 differentiation and suppression of proinflammatory cytokine production. J Immunol. (2008) 180:2903–11. doi: 10.4049/jimmunol.180.5.2903
55. Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, and Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. (2004) 173:3871–7. doi: 10.4049/jimmunol.173.6.3871
56. Hirahara K, Onodera A, Villarino AV, Bonelli M, Sciume G, Laurence A, et al. Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity. (2015) 42:877–89. doi: 10.1016/j.immuni.2015.04.014
57. Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, and Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. (2005) 175:1686–93. doi: 10.4049/jimmunol.175.3.1686
58. Mayer KD, Mohrs K, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. (2008) 180:693–7. doi: 10.4049/jimmunol.180.2.693
59. Swaminathan S, Hu Z, Rupert AW, Higgins JM, Dewar RL, Stevens R, et al. Plasma interleukin-27 (IL-27) levels are not modulated in patients with chronic HIV-1 infection. PloS One. (2014) 9:e98989. doi: 10.1371/journal.pone.0098989
60. Garg A, Trout R, and Spector SA. Human immunodeficiency virus type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through interleukin-27 and B7-H4. Sci Rep. (2017) 7:44485. doi: 10.1038/srep44485
61. Ruiz-Riol M, Berdnik D, Llano A, Mothe B, Galvez C, Perez-Alvarez S, et al. Identification of interleukin-27 (IL-27)/IL-27 receptor subunit alpha as a critical immune axis for In Vivo HIV Control. J Virol. (2017) 91. doi: 10.1128/JVI.00441-17
62. Cheng J, Myers TG, Levinger C, Kumar P, Kumar J, Goshu BA, et al. IL-27 induces IFN/STAT1-dependent genes and enhances function of TIGIT(+) HIVGag-specific T cells. iScience. (2022) 25:103588. doi: 10.1016/j.isci.2021.103588
63. Catalfamo M, Roura-Mir C, Sospedra M, Aparicio P, Costagliola S, Ludgate M, et al. Self-reactive cytotoxic gamma delta T lymphocytes in Graves’ disease specifically recognize thyroid epithelial cells. J Immunol. (1996) 156:804–11.
64. Li T, Smith M, Abdussamad M, Katz G, and Catalfamo M. A flow-cytometry-based assay to assess granule exocytosis and GZB delivery by human CD8 T cells and NK cells. STAR Protoc. (2023) 4:101939. doi: 10.1016/j.xpro.2022.101939
65. Chen H, Smith M, Herz J, Li T, Hasley R, Le Saout C, et al. The role of protease-activated receptor 1 signaling in CD8 T cell effector functions. iScience. (2021) 24:103387. doi: 10.1016/j.isci.2021.103387
66. Hurley A, Smith M, Karpova T, Hasley RB, Belkina N, Shaw S, et al. Enhanced effector function of CD8(+) T cells from healthy controls and HIV-infected patients occurs through thrombin activation of protease-activated receptor 1. J Infect Dis. (2013) 207:638–50. doi: 10.1093/infdis/jis730
67. Reiss S, Baxter AE, Cirelli KM, Dan JM, Morou A, Daigneault A, et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PloS One. (2017) 12:e0186998. doi: 10.1371/journal.pone.0186998
68. Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, et al. Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol. (2016) 197:994–1002. doi: 10.4049/jimmunol.1600320
69. Tian X, Zhang A, Qiu C, Wang W, Yang Y, Qiu C, et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J Immunol. (2015) 194:3873–82. doi: 10.4049/jimmunol.1402176
70. Pombo C, Wherry EJ, Gostick E, Price DA, and Betts MR. Elevated expression of CD160 and 2B4 defines a cytolytic HIV-specific CD8+ T-cell population in elite controllers. J Infect Dis. (2015) 212:1376–86. doi: 10.1093/infdis/jiv226
71. Collins DR, Gaiha GD, and Walker BD. CD8(+) T cells in HIV control, cure and prevention. Nat Rev. (2020) 20:471–82. doi: 10.1038/s41577-020-0274-9
72. Hunter CA and Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. (2012) 37:960–9. doi: 10.1016/j.immuni.2012.11.003
73. Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. (2012) 36:1017–30. doi: 10.1016/j.immuni.2012.03.024
74. Villarino AV, Larkin J, Saris CJ, Caton AJ, Lucas S, Wong T, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. (2005) 174:7684–91. doi: 10.4049/jimmunol.174.12.7684
75. Andres-Martin F, James C, and Catalfamo M. IL-27 expression regulation and its effects on adaptive immunity against viruses. Front Immunol. (2024) 15:1395921. doi: 10.3389/fimmu.2024.1395921
76. Harker JA, Wong KA, Dallari S, Bao P, Dolgoter A, Jo Y, et al. Interleukin-27R signaling mediates early viral containment and impacts innate and adaptive immunity after chronic lymphocytic choriomeningitis virus infection. J Virol. (2018) 92. doi: 10.1128/JVI.02196-17
77. Huang Z, Zak J, Pratumchai I, Shaabani N, Vartabedian VF, Nguyen N, et al. IL-27 promotes the expansion of self-renewing CD8(+) T cells in persistent viral infection. J Exp Med. (2019) 216:1791–808. doi: 10.1084/jem.20190173
78. Tormo AJ, Meliani Y, Beaupre LA, Sharma M, Fritz JH, Elson G, et al. The composite cytokine p28/cytokine-like factor 1 sustains B cell proliferation and promotes plasma cell differentiation. J Immunol. (2013) 191:1657–65. doi: 10.4049/jimmunol.1201595
79. Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, et al. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. (2009) 128:e816–825. doi: 10.1111/j.1365-2567.2009.03088.x
80. Stumhofer JS, Tait ED, Quinn WJ, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. (2010) 11:1119–26. doi: 10.1038/ni.1957
81. Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. (2009) 183:7692–702. doi: 10.4049/jimmunol.0901464
82. Williams DW, Engle EL, Shirk EN, Queen SE, Gama L, Mankowski JL, et al. Splenic damage during SIV infection: role of T-cell depletion and macrophage polarization and infection. Am J Pathol. (2016) 186:2068–87. doi: 10.1016/j.ajpath.2016.03.019
83. Valdes-Lopez JF, Fernandez GJ, and Urcuqui-Inchima S. Synergistic effects of toll-like receptor 1/2 and toll-like receptor 3 signaling triggering interleukin 27 gene expression in chikungunya virus-infected macrophages. Front Cell Dev Biol. (2022) 10:812110. doi: 10.3389/fcell.2022.812110
84. Moukambi F, Rabezanahary H, Fortier Y, Rodrigues V, Clain J, Benmadid-Laktout G, et al. Mucosal T follicular helper cells in SIV-infected rhesus macaques: contributing role of IL-27. Mucosal Immunol. (2019) 12:1038–54. doi: 10.1038/s41385-019-0174-0
85. DeLong JH, O'Hara Hall A, Rausch M, Moodley D, Perry J, Park J, et al. IL-27 and TCR stimulation promote T cell expression of multiple inhibitory receptors. Immunohorizons. (2019) 3:13–25. doi: 10.4049/immunohorizons.1800083
86. Chihara N, Madi A, Kondo T, Zhang H, Acharya N, Singer M, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. (2018) 558:454–9. doi: 10.1038/s41586-018-0206-z
87. Breart B, Williams K, Krimm S, Wong T, Kayser BD, Wang L, et al. IL-27 elicits a cytotoxic CD8(+) T cell program to enforce tumor control. Nature. (2025) 639:746–53.
88. Liu Z, Liu JQ, Talebian F, Wu LC, Li S, and Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol. (2013) 43:468–79. doi: 10.1002/eji.201242930
89. Gerhardt L, Hong MMY, Yousefi Y, Figueredo R, and Maleki Vareki S. IL-12 and IL-27 promote CD39 expression on CD8+ T cells and differentially regulate the CD39+CD8+ T cell phenotype. J Immunol. (2023) 210:1598–606. doi: 10.4049/jimmunol.2200897
90. Xiong H, Mittman S, Rodriguez R, Pacheco-Sanchez P, Moskalenko M, Yang Y, et al. Coexpression of inhibitory receptors enriches for activated and functional CD8(+) T cells in murine syngeneic tumor models. Cancer Immunol Res. (2019) 7:963–76. doi: 10.1158/2326-6066.CIR-18-0750
91. Pratumchai I, Zak J, Huang Z, Min B, Oldstone MBA, and Teijaro JR. B cell-derived IL-27 promotes control of persistent LCMV infection. Proc Natl Acad Sci United States America. (2022) 119. doi: 10.1073/pnas.2116741119
92. Schneider R, Yaneva T, Beauseigle D, El-Khoury L, and Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. (2011) 41:47–59. doi: 10.1002/eji.201040804
93. Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, et al. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol. (2010) 2010:605483. doi: 10.1155/2010/605483
Keywords: IL27, HIV, T cell immune activation, HIV-specific T cells, IL-27 effects on T cells
Citation: Abdussamad M, Katz G, Cheng J, Mehta C, Andres-Martin F, Mahmood D, Kumar P and Catalfamo M (2025) IL-27 effects on HIVGag-specific CD4 and CD8 T cell function. Front. Virol. 5:1600802. doi: 10.3389/fviro.2025.1600802
Received: 26 March 2025; Accepted: 30 April 2025;
Published: 02 June 2025.
Edited by:
Masaaki Miyazawa, Kindai University, JapanReviewed by:
Chihiro Motozono, Kumamoto University, JapanJuan Felipe Valdés-López, University of Antioquia, Colombia
Copyright © 2025 Abdussamad, Katz, Cheng, Mehta, Andres-Martin, Mahmood, Kumar and Catalfamo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Catalfamo, bWMyMTUxQGdlb3JnZXRvd24uZWR1
 Maryam Abdussamad1
Maryam Abdussamad1 Grace Katz
Grace Katz Fernando Andres-Martin
Fernando Andres-Martin Marta Catalfamo
Marta Catalfamo