Abstract
Background:
This study analyzed the nongenetic risk factors that contributed to colorectal cancer (CRC) incidence in the South Sulawesi population through a case-control study.
Methods:
The sample consisted of 89 cases and 84 controls, aged between 19–86, with 99 males and 74 females from different ethnic groups. Univariate analysis was carried out using chi-square, Fisher’s exact test, -test, and Mann-Whitney U test. Significant nongenetic risk factors were selected through the logit model L1 regularization, adjusted for age, gender, and ethnicity. The analyzed risk factors were the patient’s weight, height, body mass index (BMI), defecation location, exercise habit, smoking habit, marital status, occupation, education level, and distance to the nearest health center. The estimated odds ratio from the logit model was used to analyze the significance of the selected risk factors.
Results:
The significant risk factors from the logit model were smoking habit, education level, marital status, distance to the nearest health center, and weight. CRC cases were more likely to have lower education (OR = 1.819, 95% CI 1.354–2.443), residing in remote areas (OR = 1.44, 95% CI 1.17–1.772), experiencing decreasing weight (OR = 1.03, 95% CI 1.013–1.048). Controls were more likely to be non-smokers (OR = 0.325, 95% CI 0.149–0.707) and unmarried (OR = 0.161, 95% CI 0.036–0.716).
Conclusion:
The study determined that other nongenetic risk factors, including education level, distance to the nearest health center, weight, smoking habit, and marital status, contributed to the CRC incidence within the South Sulawesi population. The study emphasized the importance in accounting for these risk factors for further, targeted CRC preventions.
1 Introduction
Colorectal cancer (CRC) is a type of epithelial, solid cancer that leads to both mortality and morbidity. Adenocarcinoma is the most common type, accounting for more than 90% of all colorectal carcinoma cases (1). Anatomically, CRC includes both colon and rectal cancers. Its origin remains a subject of debate, centered on the stochastic versus cancer stem cells paradigms and hypotheses (2). Considering many resemblances between carcinogenesis and colorectal ontogenesis, remnant fetal stem cells or normal mature colonic cells are thought to possess the potential to develop into cancer stem cells (3, 4). Molecular regulators on gut ontogenesis, such as Nuclear β-catenin, Krüppel-like factor (KLF) proteins, Sonic Hedgehog, or Notch-1 receptor, are observed to be over-expressed in CRC (5, 6, 7, 8). Recent genome-wide association studies (GWAS) have also revealed the role of acetaldehyde as a prognostic risk factor through alcohol consumption habits in light and moderate drinkers (9), highlighting the strong dynamic between environmental risk factors and the molecular mechanisms of onco-pathogenesis.
In 2022, there are 1,926,118 new colorectal cancer (CRC) cases, which places CRC third among other types of cancer in terms of incidence; CRC is also the second most lethal cancer with 903,859 deaths for 2022 (10). Although it is not the most lethal cancer, as of yet, the rates of incidences and mortality are rapidly increasing compared to 2020 (11). Recently, early-onset CRC has also emerged more frequently (12, 13, 14, 15). Some studies locate the tumor of this early-onset cases in the distal (14) and rectal subsites (13). Typically, those with early-onset CRCs are diagnosed at more advanced stages than those with later-onset (16), with dietary and lifestyle changes during the 1950s–1980s hypothesized as the leading cause (17).
Some studies report a regional decline in CRC incidence (12, 13, 14, 15, 18, 19), but this is not the case for many regions in Asia and Africa (20, 21), in which many low- and middle-income countries (LMICs) are situated. Even though high-income countries (HICs) have more CRC incidence (10), the mortality rate is lower compared to LMICs due to healthier lifestyles and public awareness of cancer screening, such as fecal immunochemical test (FIT) and fecal occult blood tests (FOBT) (22). Meanwhile, the inadequate health infrastructure and personnels in LMICs hinders those CRC screening, resulting in failure of incidence and mortality rate reduction (22). Indonesia, to date, remains part of LMICs, and is still struggling with CRC. One study reports that CRC cases in Indonesia are more prevalent in males than females (23). Other studies regarding CRC in Indonesia are divided either geographically (24, 25, 26) or ethnically (27) to accommodate the diverse Indonesian population. The heterogeneous nature requires a comprehensive approach in identifying the unique risk factors for each population.
Guided by the known interplay between environmental and genetic risk factors, this study aimed to connect and decipher the interplay among nongenetic variables linked to CRC occurrence through a case-control study, particularly in the South Sulawesi population. Previously, a GWAS of the same population was conducted (28). Nongenetic risk factors were briefly studied, but limited to the scope of dietary intake variables (29). This study analyzed other nongenetic variables related to patient health, socio-economy, and daily-life routines/behaviors to determine the nongenetic risk factors that were significantly associated with CRC incidence. Through this experiment, this study contributed to the development of targeted CRC prevention for the South Sulawesi population.
2 Materials and methods
2.1 Patient data collection
This study utilized the same set of observations as in (28, 29). Cases are patients diagnosed with CRC, with the group’s eligibility confirmed by physician examinations. The case samples were collected from seven hospitals: Grestelina Hospital, Wahidin Sudirohusodo Hospital, Akademis Hospital, Hasanuddin University Hospital, Stella Maris Hospital, Hikmah Hospital, and Ibnu Sina Hospital. The seven hospitals are based in Makassar City. Controls are individuals not diagnosed with CRC. This control group was frequently matched to the case group by age, ethnicity, and gender. Through an in-person interview, all patients completed a detailed questionnaire related to demography, family history, smoking behavior, alcohol consumption, and dietary intake summary. Information on clinical and histopathology observations was also collected for the case group. In total, there are 382 questions for the case group and 319 questions for the controls.
Although the samples were collected from hospitals across the city of Makassar, the patients comprised diverse ethnicities in South Sulawesi and across Indonesia. Initially, 162 cases and 193 controls were recruited. However, some patients were unwilling to answer some follow-up questions, which resulted in many missing values for those patients' variables. These observations were not used in this study. The sample used in this study consisted of 173 patients, with 89 cases and 84 controls.
2.2 Statistical analysis
Risk factors related to the subject’s health, behavior, and socio-economy were selected from the list of risk factors collected in (27, 28). The risk factors related to the patient’s health were smoking habits, body mass index (BMI), weight, and height. Risk factors related to the participant’s socioeconomic status and daily-life routines/behaviors were distance to the nearest health center, marital status, profession, location of defecation, and education level. The distance to the nearest health center variable contained missing values, which were imputed through linear regression using other variables, with the exclusion of CRC incidence to prevent circularity. While preserving the sample size, this imputation neglects the variance of the missing values and the cause of its randomness, which introduces bias. However, since the percentage of missing samples is relatively small (∼7%), the overall impact of the bias is minimal.
For univariate analysis, nominal risk factors were analyzed using the chi-square test (30). In conditions where the chi-square test assumptions were not met, Fisher’s exact test was utilized (30). Continuous risk factors were analyzed primarily using t-test (31). When the risk factor distribution was ordinal, or the assumptions of the t-test were not met, the Mann-Whitney U test (32) was utilized instead. Multivariate analysis was conducted afterwards using the logit model with L1 regularization (33) to obtain the significant nongenetic risk factors from the non-shrinking logit coefficients. Age, sex, and ethnicity were included in the logit model as the baseline covariate. The L1 regularization coefficient () candidates ranged between 0 1 with inversed regularization strength. A value of closer to 0 yielded a stricter logit coefficient shrinkage, resulting in fewer risk factors selection. Candidates obtaining the maximum average F1-score from the 10-folds cross-validation was chosen as the best . All the conducted tests were two-tailed with a p-value level of 0.05.
3 Results
3.1 Summary of collected samples
The sample collection summary is described in Supplementary Table S1. No different proportion was observed between males and females in either the case or control groups ( > 0.999). The ages ranged from 19 to 86, with a mean of 53.1 years for the case group and 50.5 years for the control group ( = 0.12). The proportion of self-reported ethnicity was also similar between the two cohorts, with Bugis and Makassar dominating both groups ( = 0.86). Other recorded local ethnicities were Mandar, Toraja, Palu, Kolaka, and even ethnic groups from outside of South Sulawesi residing in the city of Makassar.
Assessing from the patient’s health, a mean difference was observed in body weight ( < 0.001). However, a significant difference was not observed in body height ( = 0.35). The case group had a higher proportion of smokers ( < 0.001). Patients in both groups rarely exercised ( = 0.176). The control group had more access to the nearest health center than the case group ( < 0.001). Defecation location other than the lavatory significantly increased CRC incidence ( < 0.001). Marital status also had a significant association with CRC incidence ( = 0.007), with higher proportion of married patients in the CRC cohort. Regarding education level, both groups reported similar proportions at every level, rendering the risk factor insignificant ( = 0.493). The same notion occurs in the patient’s occupation in both cohort ( = 0.51). Univariate analysis revealed that body weight, smoking habit, defecation location, marital status, and distance to the nearest health center significantly contributed to CRC incidence, with the -value <0.001, except for marital status where -value = 0.007. Supplementary Table S1 summarizes the obtained -values for each risk factor. For continuous risk factors analyzed with the Mann-Whitney U test, instead of the mean and standard deviation, median and standard deviation are shown as well.
3.2 Significantly-associated nongenetic risk factors
The risk factors previously described in Supplementary Table S1 were inputted into the L1 logit model to determine significantly-associated nongenetic risk factors to CRC incidence. The nominal risk factors were encoded using one-hot encoding before insertion into the model. Unlike nominal risk factors, the ordinal risk factors have a rank or order that needs to be preserved, in which these risk factors were encoded through label encoding, with higher value representing higher level. The nominal risk factors were gender, ethnicity, defecation location, marital status, smoking habit, and occupation, while the ordinal risk factors were exercise habit and education level. Fisherman occupation caused a quasi-separation leading to their exclusion. From the optimization process, the L1 logit model with 0.08 had the highest F1-score of 75.5%. Despite having more risk factors, logit model with 0.08 exhibited lower average F1 score (Figure 1). Meanwhile, logit model with 0.08 was too sparse, with all risk factors shrunken to 0 in 0.01.
FIGURE 1
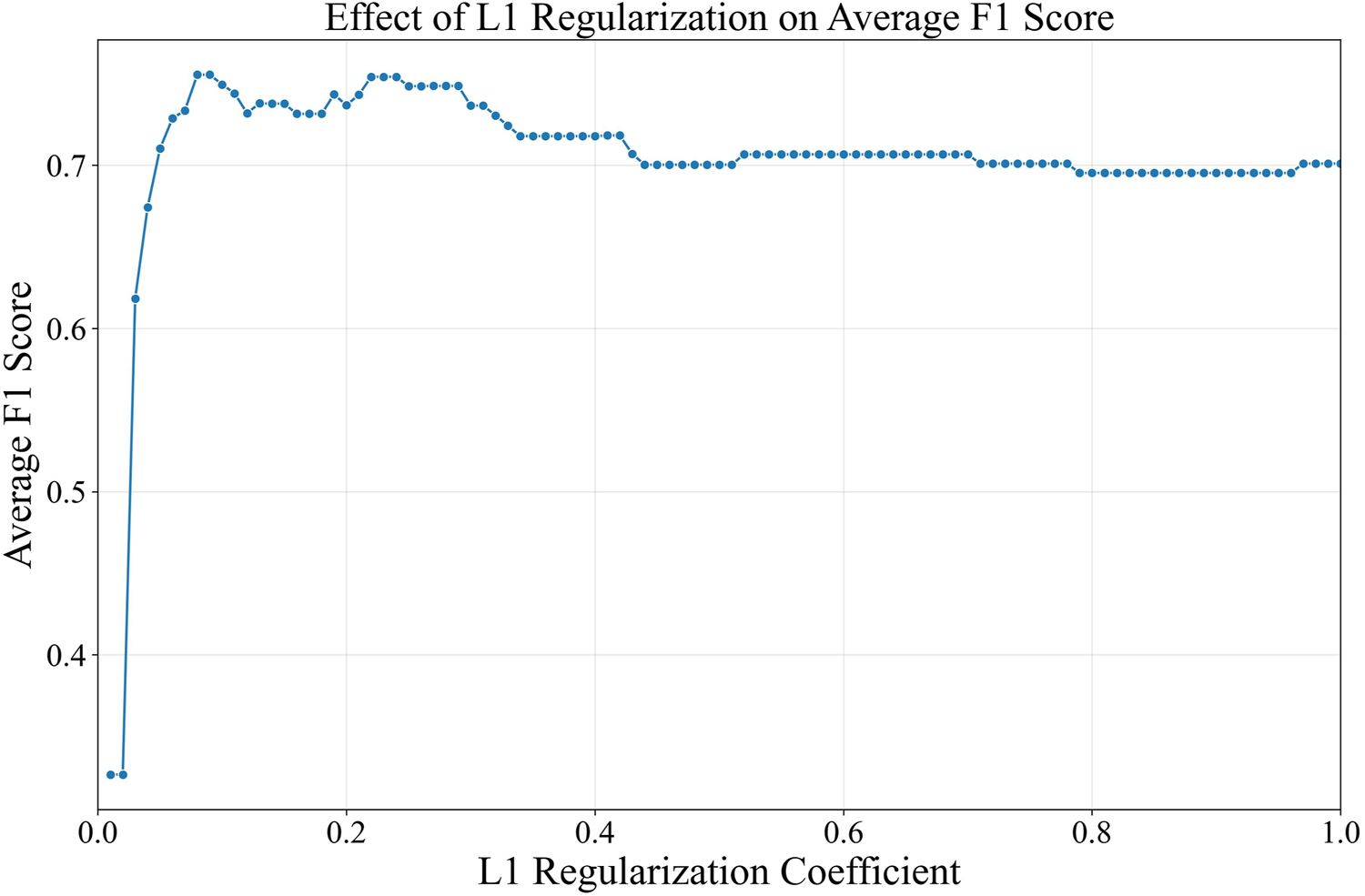
Regularization coefficient and its average F1 score from 10-folds cross-validation.
Further diagnostics of the L1 logit with 0.08 is shown by the Area Under the Curve (AUC) score of each Receiver Operating Characteristic (ROC) curve (Figure 2). Each ROC represents a fold in 0.08. The AUC scores for each cross-validation fold vary, with the minimum AUC generated by the fourth cross-validation fold (0.74) and the maximum generated by the sixth cross-validation fold (0.97). As shown in Figure 2, the average AUC score from the 10-fold cross-validation is 0.85, with only a 0.07 standard deviation. This average AUC score exhibits the L1 logit robustness in discriminating the CRC cases and controls in the population, specifically with 0.08 and despite the small sample size used. The risk factors from the L1 logit with 0.08 were then refitted to unregularized logit (summary in Table 1) to obtain the odds ratios.
FIGURE 2
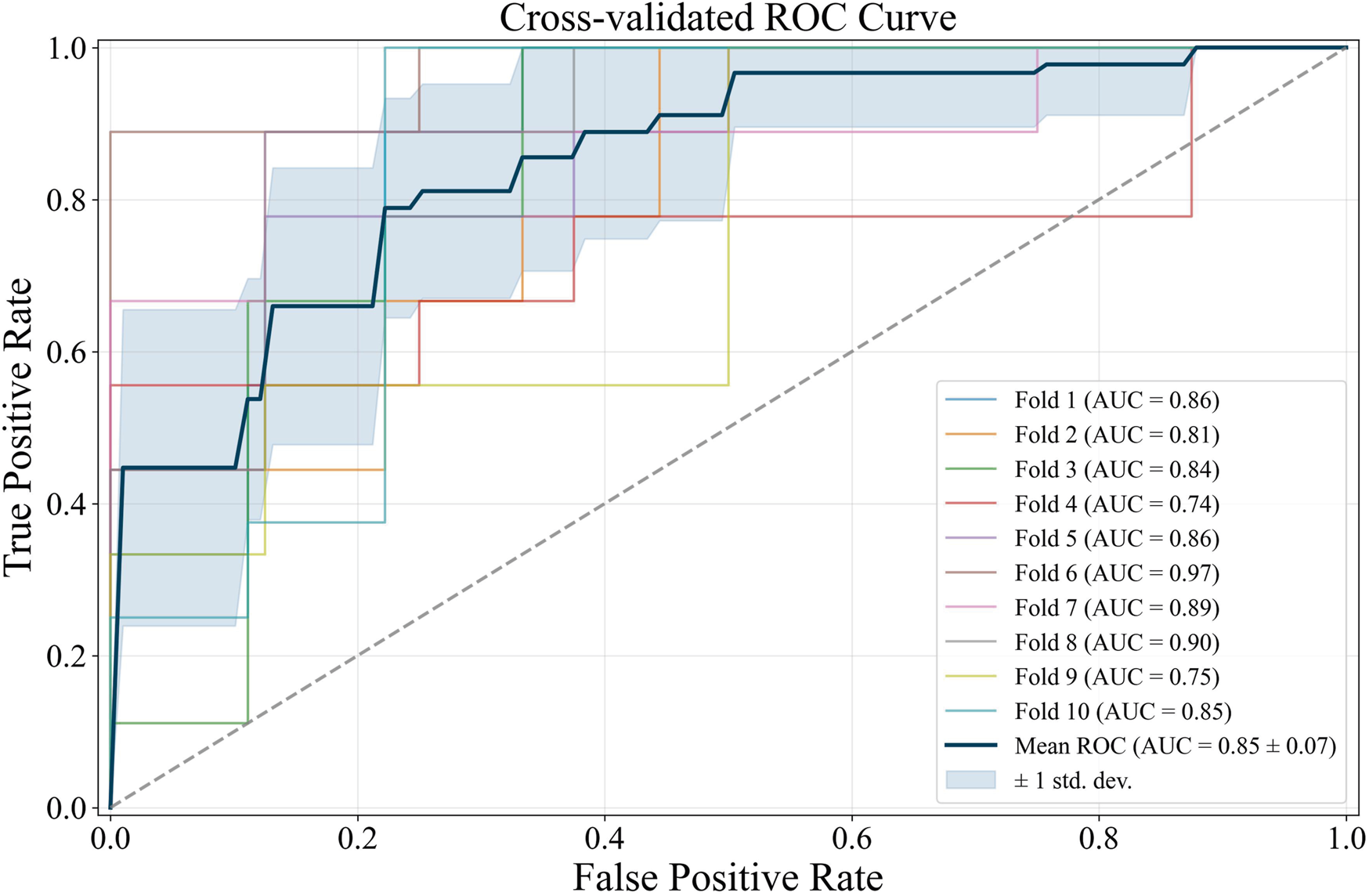
ROC curve of L1 logit with 0.08.
TABLE 1
| Risk factors | Coefficient | Standard deviation | -value |
|---|---|---|---|
| Weight | 0.030 | 0.008 | <0.001a |
| Smoking Habit | |||
| No | −1.125 | 0.397 | 0.005a |
| Marital Status | |||
| Married | −1.830 | 0.763 | 0.017a |
| Education Level | 0.598 | 0.151 | <0.001a |
| Distance to Nearest Health Center | 0.364 | 0.106 | 0.001a |
Logit summary of the refitted risk factors.
indicates significant difference between the case and control groups for that particular variable.
The effect measure plot of the odds ratios is shown in Figure 3. A 1-kg increase in a patient’s weight increased the odds ratio of CRC incidence by 3%. A patient with no regular smoking habit had an odds ratio decrease of 67.5%. Patients with lower education levels recorded an 81.9% increase in CRC incidence odds ratio. A married patient had a CRC incidence odds ratio decrease by 83.9%. An increase of 1 km to the nearest health center increased the odds ratio of CRC incidence by 81.9%. None of the adjusting risk factors were associated with CRC incidence in the South Sulawesi population.
FIGURE 3
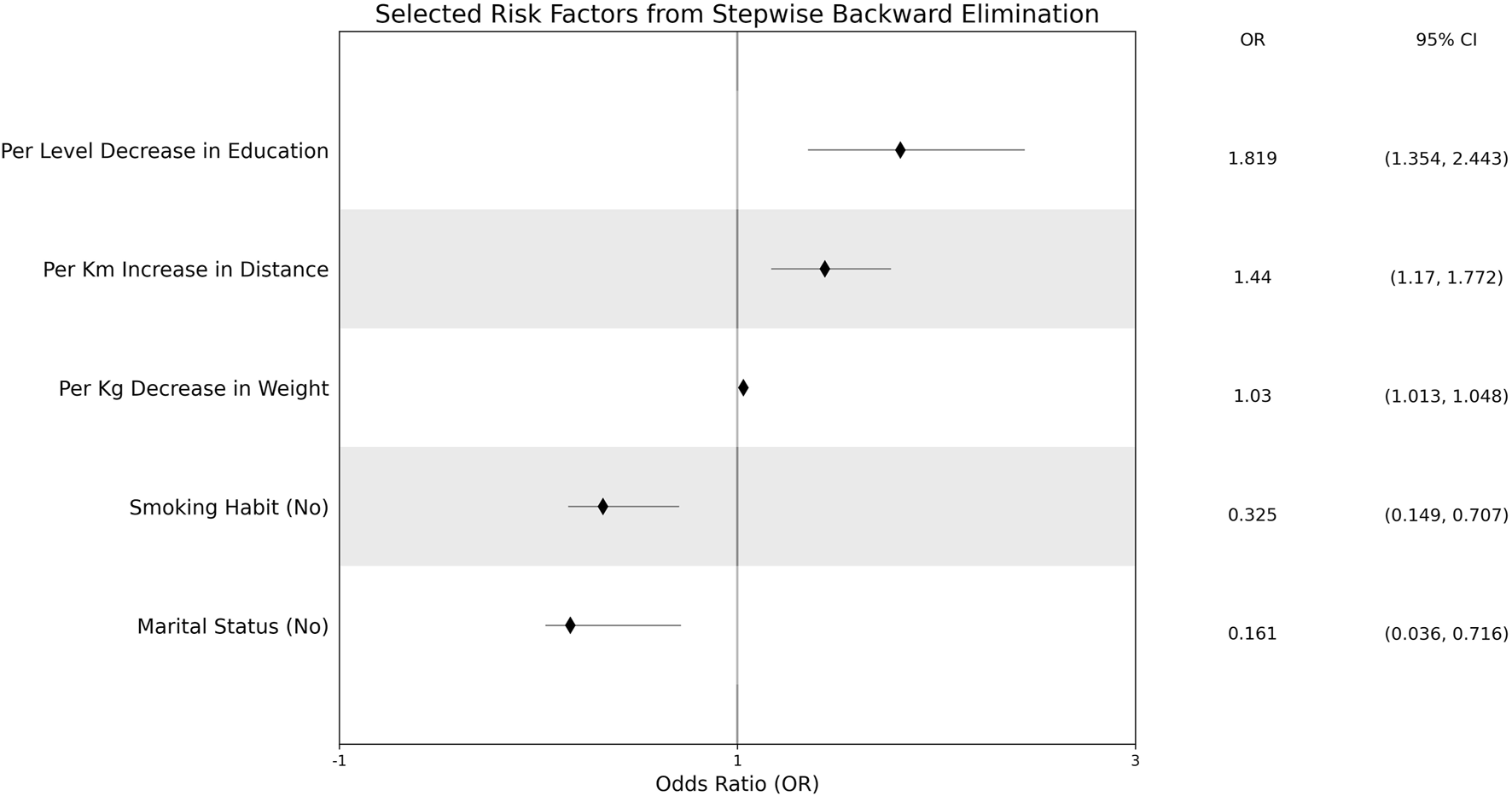
Effect measure plots of significantly-associated risk factors.
4 Discussions
In many studies involving diverse populations, smoking habit, either active or passive smoking, is significantly associated with CRC (20, 34, 35). The association is reinforced genetically by the discovery of an associated SNP from the cell adhesion molecule 2 (CADM2) gene, especially in GG and GA allele, that increases CRC risk (36). Based on these results, the significant association between smoking habit and CRC incidence in the South Sulawesi population, in which the absence of smoking habit decreases the chances of CRC (OR = 0.325; 95% CI 0.149–0.707) (Figure 3), and vice versa, is predictable and expected. Particularly in this study, the chi-square test on gender and smoking habit found that smoking was more prevalent in males ( < 0.001), which is proven by the same population distribution in other studies of tobacco use in the South Sulawesi population (37, 38).
Based on Supplementary Table S1, the higher education risk factor alone is independent from CRC incidence ( = 0.491). However, since education level is treated as an ordinal risk factor in the logit model, a Mann-Whitney U test was also conducted to assess the mean difference in education level in the two cohorts. When applied to the ordinal education level, the Mann-Whitney U test showed a mean difference among the two cohorts, with the education level of the control group averaged in senior high school graduates and the other in junior high school graduates ( = 0.007). There is no consensus regarding the relationship between education level and CRC incidence. A study in different regions of Indonesia reports socioeconomic factors, including higher education level, to decrease the odds of CRC incidence (39). The increasing odds ratio of education level in this study (OR = 1.819; 95% CI 1.354–2.443) (Figure 3) shows the prevalence of CRC in lower education patients, which aligns with that in other countries (40, 41). Nonetheless, there are other countries that have reported the opposite (42, 43).
Similar to education level, in previous studies, marital status (OR = 0.161; 95% CI 0.036–0.716) shows a diverse association with CRC incidence, with no information on the confounding risk factor if it is associated (41, 44). However, examined from the dietary intake risk factors (29), the confounding factor of marital status in this study is reheated food consumption (Fisher’s exact = 0.012). A married patient consumes reheated food more often (chi-square = 0.007). In unmarried patients, the condition is not observed (Fisher’s exact = 0.25) (Table 2). It is speculated that married patients in the city of Makassar, who are older than unmarried patients (Mann-Whitney U < 0.001), might consume reheated food for faster food serving or other economic factors, such as less frequent food shopping. Nevertheless, due to the sparsity in unmarried group, further study of the dietary intakes in older patients is required.
TABLE 2
| Marital status | Reheated foods consumption | CRC incidence | -value | Univariate test | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Married | Seldom | 23 | 57 | 0.007a | chi-square |
| Often | 63 | 10 | |||
| Unmarried | Seldom | 2 | 9 | 0.25 | Fisher’s exact |
| Often | 1 | 0 | |||
Contingency table of marital status and reheated foods consumption.
indicates significant difference between the case and control groups for that particular variable.
Patients residing in remote areas near the city of Makassar have limited access to healthcare facilities, especially to ones with comprehensive facilities, which increases the chances of CRC incidence (OR = 1.44; 95% CI 1.17–1.772). The high-quality healthcare facilities in the city of Makassar, based on Google reviews, are densely centralized in Rappocini, Panakkukang, Mariso, Manggala, Ujung Tanah, and Makassar districts (45), which are all located in Southwest Makassar, the central area of Makassar. Therefore, the geographical distribution is imbalanced, with the quality and number of healthcare facilities in Southwest Makassar districts outperforming and outnumbering the healthcare facilities in Tamalanrea and Biring Kanaya, two of the most populated districts in Northeast Makassar, which are also rural districts. A case-control study using other populations has supported the notion (41) that rural area residence contributes significantly to CRC incidence. A study of colon cancer incidence in Yogyakarta, another province of Indonesia, shows no significance of either rural or urban area residence with CRC incidence (26). However, unlike in the city of Makassar, the healthcare facilities in Yogyakarta, especially the hospital networks, are highly comprehensive, even in the rural areas (46). Apart from having easier access to health center facilities, patients residing in urban areas also tend to have more awareness regarding CRC screening and prevention, as proven by studies from other regions (47, 48).
An inverse association was observed between weight and CRC incidence (OR = 1.03; 95% CI 1.013–1.048), which differs from many previously conducted studies (49, 50). However, the inverse is caused by the late measurement of patient weight since it is recorded once the patient has been diagnosed at CRC stage III or IV, as also shown in our previous studies (28, 29).
Based on the identified risk factors, further CRC screening initiatives in Makassar City should prioritize people with low education levels and people living in rural areas, where health access is limited. These two risk factors should also alert the government regarding economic and health infrastructure disparities across the region, which thrive diseases like CRC. Health institutions in the area should strengthen the campaign on smoking dangers and cessation, the danger of often consuming reheated foods, and promoting routine medical checkups in order to facilitate early detection and prevention of CRC.
5 Conclusion
As the first to analyze the CRC non-genetic risk factors in the South Sulawesi population, this study delineated the pertinent non-genetic risk factors that could contributed to the CRC incidence in South Sulawesi, especially the city of Makassar. A multivariate logit model was utilized to determine the significant risk factors of CRC incidence for the South Sulawesi population, adjusted by the patient’s age, gender, and ethnicity. Among the inputted health, socioeconomic, and behavioral risk factors, the significantly-associated ones were smoking habit, education level, distance to the nearest health center, marital status, and a patient’s weight. Lower education levels increased the chances of CRC incidence within the population. Unhealthy dietary intake from reheated foods was the influential driver for the significance of the marital status risk factor, with married patients consuming reheated foods more often than the unmarried ones. Similar to other studies that have linked regular smoking with CRC, smoking status was found to be a significant risk factor as well. Additionally, reflecting the state of unequal access to health centers in certain areas surrounding Makassar City, higher chances of CRC incidence was found in patients who inhabit rural areas. Initially, decreased weight was a significant risk factor, but this was due to the late weight and BMI measurements of the patients rather than an independent risk factor. Defecation location also lost its significance in the multivariate analysis. No association was found in this population between CRC incidence and other risk factors, such as exercise habits and a person’s occupation.
Statements
Data availability statement
The datasets presented in this article are not readily available because the dataset contains personal information related to the patient privacy. Requests to access the datasets should be directed to bdsrc@binus.edu.
Ethics statement
The studies involving humans were approved by Hasanuddin University Ethical Committee with registration number UH 15040389. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
UM: Conceptualization, Data curation, Writing – original draft. MH: Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review and editing. CP: Writing – review and editing. AB: Formal Analysis, Writing – review and editing. AI: Data curation, Writing – original draft. JB: Conceptualization, Supervision, Writing – review and editing. IY: Conceptualization, Supervision, Writing – review and editing. BP: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge and thank Ronald E. Lusikooy and Arham Arsyad for their contributions in the collection of patient information and data used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/or.2025.1589655/full#supplementary-material
References
1.
Hutasoit G Miskad U Akil F Cangara MH Dahlan H Yamin A et al Snail expression as a prognostic factor in colorectal adenocarcinoma. Asian Pac J Cancer Prev (2024) 25(9):3143–9. 10.31557/apjcp.2024.25.9.3143
2.
Fanali C . Cancer stem cells in colorectal cancer from pathogenesis to therapy: controversies and perspectives. WJG (2014) 20(4):923. 10.3748/wjg.v20.i4.923
3.
Leedham SJ Brittan M McDonald SAC Wright NA . Intestinal stem cells. J Cell Mol Med (2005) 9(1):11–24. 10.1111/j.1582-4934.2005.tb00333.x
4.
Brittan M Wright NA . The gastrointestinal stem cell. Cell Prolif (2004) 37(1):35–53. 10.1111/j.1365-2184.2004.00299.x
5.
Elzagheid A Buhmeida A Korkeila E Collan Y Syrjänen K Pyrhönen S . Nuclear β-catenin expression as a prognostic factor in advanced colorectal carcinoma. WJG (2008) 14(24):3866. 10.3748/wjg.14.3866
6.
Ghaleb AM Yang VW . The pathobiology of Krüppel-like factors in colorectal cancer. Curr Colorectal Cancer Rep (2008) 4(2):59–64. 10.1007/s11888-008-0011-4
7.
Yoshikawa K Shimada M Miyamoto H Higashijima J Miyatani T Nishioka M et al Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol (2009) 44(11):1113–7. 10.1007/s00535-009-0110-2
8.
Tyagi A Sharma AK Damodaran C . A review on Notch signaling and colorectal cancer. Cells (2020) 9(6):1549. 10.3390/cells9061549
9.
Jordahl KM Shcherbina A Kim AE Su YR Lin Y Wang J et al Beyond GWAS of colorectal cancer: evidence of interaction with alcohol consumption and putative causal variant for the 10q24.2 region. Cancer Epidemiol Biomarkers and Prev (2022) 31(5):1077–89. 10.1158/1055-9965.epi-21-1003
10.
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians (2024) 74(3):229–63. 10.3322/caac.21834
11.
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians (2021) 71(3):209–49. 10.3322/caac.21660
12.
Santucci C Mignozzi S Malvezzi M Boffetta P Collatuzzo G Levi F et al European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann Oncol (2024) 35(3):308–16. 10.1016/j.annonc.2023.12.003
13.
Siegel RL Wagle NS Cercek A Smith RA Jemal A . Colorectal cancer statistics, 2023. CA: A Cancer J Clinicians (2023) 73(3):233–54. 10.3322/caac.21772
14.
Loomans-Kropp HA Umar A . Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol (2019) 2019:1–9. 10.1155/2019/9841295
15.
Vuik FE Nieuwenburg SA Bardou M Lansdorp-Vogelaar I Dinis-Ribeiro M Bento MJ et al Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut (2019) 68(10):1820–6. 10.1136/gutjnl-2018-317592
16.
Chen FW Sundaram V Chew TA Ladabaum U . Advanced-stage colorectal cancer in persons younger than 50 Years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol (2017) 15(5):728–37.e3. 10.1016/j.cgh.2016.10.038
17.
Akimoto N Ugai T Zhong R Hamada T Fujiyoshi K Giannakis M et al Rising incidence of early-onset colorectal cancer — a call to action. Nat Rev Clin Oncol (2021) 18(4):230–43. 10.1038/s41571-020-00445-1
18.
Luo Q Lew JB Steinberg J Worthington J Yu XQ Caruana M et al Trends in colon and rectal cancer mortality in Australia from 1972 to 2015 and associated projections to 2040. Sci Rep (2022) 12(1):3994. 10.1038/s41598-022-07797-x
19.
Swartjes H Sijtsma FPC Elferink MAG Van Erning FN Moons LMG Verheul HMW et al Trends in incidence, treatment, and relative survival of colorectal cancer in The Netherlands between 2000 and 2021. Eur J Cancer (2024) 205:114104. 10.1016/j.ejca.2024.114104
20.
Pardamean CI Sudigyo D Budiarto A Mahesworo B Hidayat AA Baurley JW et al Changing colorectal cancer trends in asians: epidemiology and risk factors. Oncol Rev (2023) 17:10576. 10.3389/or.2023.10576
21.
Awedew AF Asefa Z Belay WB . Burden and trend of colorectal cancer in 54 countries of Africa 2010–2019: a systematic examination for Global Burden of Disease. BMC Gastroenterol (2022) 22(1):204. 10.1186/s12876-022-02275-0
22.
Abreu Lopez BA Pinto-Colmenarez R Caliwag FMC Ponce-Lujan L Fermin MD Granillo Cortés AV et al Colorectal cancer screening and management in low- and middle-income countries and high-income countries: a narrative review. Cureus (2024) 16(10):e70933. 10.7759/cureus.70933
23.
Ferlay J Ervik M Lam F Laversanne M Colombet M Mery L et al Global cancer observatory. Cancer Today (2024). Available online at: https://gco.iarc.who.int/media/globocan/factsheets/populations/360-indonesia-fact-sheet.pdf.
24.
Makmun D Simadibrata M Abdullah M Syam AF Shatri H Fauzi A et al Colorectal cancer patients in a tertiary hospital in Indonesia: prevalence of the younger population and associated factors. World J Clin Cases (2021) 9(32):9804–14. 10.12998/wjcc.v9.i32.9804
25.
Budianto A Andarini S Hariyanti T Musliha N . Exploring the correlation between ethnicity and health- seeking behavior for colorectal cancer in east java, Indonesia: a case study of arek, mataraman, and pendalungan ethnic groups. Asian Pac J Cancer Prev (2023) 24(6):1931–42. 10.31557/apjcp.2023.24.6.1931
26.
Puspitaningtyas H Hutajulu SH Fachiroh J Anggorowati N Sanjaya GY Lazuardi L et al Diverging likelihood of colon and rectal cancer in Yogyakarta, Indonesia: a cross sectional study. PLoS ONE (2024) 19(3):e0301191. 10.1371/journal.pone.0301191
27.
Lukman K Muhammad A Ghozali M Nugraha P Sribudiani Y Nursabur BM . Epidemiological and clinicopathological characteristics of colorectal cancer patients in tertiary hospital in West Java. Clin Epidemiol Glob Health (2024) 28:101688. 10.1016/j.cegh.2024.101688
28.
Yusuf I Pardamean B Baurley JW Budiarto A Miskad UA Lusikooy RE et al Genetic risk factors for colorectal cancer in multiethnic Indonesians. Sci Rep (2021) 11(1):9988. 10.1038/s41598-021-88805-4
29.
Nurlaila I Hidayat AA Budiarto A Mahesworo B Purwandari K Pardamean B . Dietary intake as determinant nongenetic factors to colorectal cancer incidence and staging progression: a study in South Sulawesi population, Indonesia. Nutr Cancer (2021) 73(11–12):2523–31. 10.1080/01635581.2020.1839516
30.
Agresti A . Introduction to categorical data analysis. Wiley (2007).
31.
Kim TK . T test as a parametric statistic. Korean J Anesthesiol (2015) 68(6):540. 10.4097/kjae.2015.68.6.540
32.
Corder GW . Nonparametric statistics: a step-by-step approach. Wiley (2014).
33.
Tibshirani R . Regression shrinkage and selection via the lasso. J R Stat Soc Ser B: Stat Methodol (1996) 58(1):267–88. 10.1111/j.2517-6161.1996.tb02080.x
34.
Yang C Wang X Huang C Yuan W Chen Z . Passive smoking and risk of colorectal cancer: a meta-analysis of observational studies. Asia Pac J Public Health (2016) 28(5):394–403. 10.1177/1010539516650724
35.
Huang YM Wei PL Ho CH Yeh CC . Cigarette smoking associated with colorectal cancer survival: a nationwide, population-based cohort study. JCM (2022) 11(4):913. 10.3390/jcm11040913
36.
Carreras-Torres R Kim AE Lin Y Díez-Obrero V Bien SA Qu C et al Genome-wide interaction study with smoking for colorectal cancer risk identifies novel genetic loci related to tumor suppression, inflammation, and immune response. Cancer Epidemiol Biomarkers and Prev (2023) 32(3):315–28. 10.1158/1055-9965.epi-22-0763
37.
Amiruddin R Darmawangsa D Jumriani J Awaluddin A Azizah N . Smoking behaviors of street children in makassar 2013. MJHR (2015) 19(2). 10.7454/mjhr.v19i2.5176
38.
Al-Madin ARM Razak HA Darmawansyah S Maidin H Astuti P et al Community compliance regarding No-smoking area policy: belief control analysis and tobacco use habits in society the Bugis tribe (Pare-Pare city and sidrap regency) and the makassar tribe (Gowa-Takalar regency) in South Sulawesi. Pharmacognosy J (2023) 15:301–6.
39.
Budianto A Andarini S Hariyanti T Muslihah N . The prediction of colorectal cancer: perspective of smoking and socioeconomic influence of culture in East Java with SEM analysis. Nurture (2024) 18(2):315–27. 10.55951/nurture.v18i2.614
40.
Li L Fang YJ Abulimiti A Huang CY Liu KY Chen YM et al Educational level and colorectal cancer risk: the mediating roles of lifestyle and dietary factors. Eur J Cancer Prev (2022) 31(2):137–44. 10.1097/cej.0000000000000697
41.
Mint Sidi Ould Deoula M Huybrechts I El Kinany K Boudouaya H Hatime Z El Asri A et al Behavioral, nutritional, and genetic risk factors of colorectal cancers in Morocco: protocol for a multicenter case-control study. JMIR Res Protoc (2020) 9(1):e13998. 10.2196/13998
42.
Savijärvi S Seppä K Malila N Pitkäniemi J Heikkinen S . Trends of colorectal cancer incidence by education and socioeconomic status in Finland. Acta Oncologica (2019) 58(11):1557–63. 10.1080/0284186x.2019.1652340
43.
Wernly S Semmler G Schaffler-Schaden D Flamm M Aigner E Datz C et al The association between educational status and colorectal neoplasia: results from a screening cohort. Int J Colorectal Dis (2023) 38(1):91. 10.1007/s00384-023-04383-z
44.
Quang LN Hien NQ Quang NT Chung NT . Active lifestyle patterns reduce the risk of colorectal cancer in the north of vietnam: a hospital-based case–control study. Cancer Control (2019) 26: 1073274819864666. 10.1177/1073274819864666
45.
Yudono A Afrianto F Hariyanto AD . The evaluation of geographical health facilities structure in makassar city, Indonesia. IJERPH (2023) 20(6):5210. 10.3390/ijerph20065210
46.
Sadali MI Rijanta R Mutaali L Kurniawan A Study of the service functions of health facilities in Yogyakarta Special Province. E3S web of conferences, Che OmarRSri SumantyoJTWhiteBCardenas TristanAHaryonoEHizbaronDRet al editors (2021) 325:07006. 10.1051/e3sconf/202132507006
47.
Harmy M Norwati D Noor NM Amry A . Knowledge and attitude of colorectal cancer screening among moderate risk patients in west Malaysia. Asian Pac J Cancer Prev : APJCP (2011) 12(8):1957–60.
48.
Schliemann D Ramanathan K Ibrahim Tamin NSB O’Neill C Cardwell CR Ismail R et al Implementation of a home-based colorectal cancer screening intervention in Malaysia (CRC-SIM). BMC Cancer (2023) 23(1):22. 10.1186/s12885-022-10487-6
49.
Jochem C Leitzmann M . Obesity and colorectal cancer. In: PischonTNimptschK, editors. Obesity and cancer. Cham: Springer International Publishing (2016). Available online at: http://link.springer.com/10.1007/978-3-319-42542-9_2.
50.
Paragomi P Zhang Z Abe SK Islam MR Rahman MS Saito E et al Body mass index and risk of colorectal cancer incidence and mortality in Asia. JAMA Netw Open (2024) 7(8):e2429494. 10.1001/jamanetworkopen.2024.29494
Summary
Keywords
colorectal cancer, nongenetic, south sulawesi population, risk factors, lifestyle, socio-demographic
Citation
Miskad UA, Henry MM, Pardamean CI, Budiarto A, Irwan A, Baurley JW, Yusuf I and Pardamean B (2025) Colorectal cancer in south sulawesi: a case-control study for nongenetic risk factors. Oncol. Rev. 19:1589655. doi: 10.3389/or.2025.1589655
Received
07 March 2025
Accepted
14 May 2025
Published
30 May 2025
Volume
19 - 2025
Edited by
Almu'Atasim Khamees, Ministry of Health, Jordan
Reviewed by
Gabriel Madeira Werberich da Silva, National Cancer Institute (INCA), Brazil
Sam Agatre Okuonzi, Ministry of Health, Uganda
Updates
Copyright
© 2025 Miskad, Henry, Pardamean, Budiarto, Irwan, Baurley, Yusuf and Pardamean.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Upik A. Miskad, upik.miskad@med.unhas.ac.id
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.