- 1Department of Medical Oncology, Fiona Stanley Hospital, Perth, WA, Australia
- 2Department of Nucelar Medicine, Fiona StanleyHospital, Perth, WA, Australia
Fibroblast Activation Protein (FAP) has emerged as a critical player in cancer biology, particularly in shaping the tumour microenvironment (TME) and influencing immunotherapy outcomes. FAP-positive cancer-associated fibroblasts (CAFs) play multiple roles in tumour progression and immune modulation. FAP, predominantly expressed on CAFs, contributes significantly to extracellular matrix remodelling, angiogenesis, and the creation of an immunosuppressive milieu. There are complex interactions between FAP-positive CAFs and various components of the immune system, highlighting their impact on T cell function and macrophage polarisation. This makes FAP a promising target for cancer therapy and potentially as a biomarker for immunotherapy treatment response. This review highlights the clinical challenges to target FAP and also addresses the heterogeneity of CAFs with the need for more refined characterisation to enhance therapeutic strategies and future research directions.
1 Introduction
Immunotherapy has significantly transformed the landscape of cancer treatment by introducing a range of innovative strategies, including checkpoint inhibitors, CAR T-cell therapies, and personalised cancer vaccines. These advances in immunotherapy have shown considerable promise in improving patient outcomes but also face notable limitations due to their varying efficacy across different cancer types and among diverse patient populations. The variation in treatment response, coupled with the high costs associated with these therapies and the potential for severe, sometimes life-threatening side effects, underscore the critical need for accurate and reliable prediction of immunotherapeutic outcomes. This requirement for precision in forecasting responses is essential not only to enhance therapeutic efficacy but also to minimise adverse effects and optimise patient care in oncology (1, 2).
Recent advances in the field of oncology have significantly deepened our understanding of the tumour microenvironment (TME) and its crucial role in modulating responses to various therapies, including immunotherapy. The inherent complexity of the TME, shaped by its diverse cellular and molecular constituents, plays a pivotal role in influencing the efficacy of immunotherapies, either by promoting or inhibiting immune evasion and tumour growth. Among the most critical elements within the TME are the cancer-associated fibroblasts (CAFs). Predominantly prevalent in solid tumours, CAFs can make up to 90% of the cellular mass in certain cancers. They are known for their ability to secrete a range of cytokines and growth factors that can significantly reshape the landscape of immune surveillance and alter the overall responses to therapy, making them attractive targets in the development of new therapeutic strategies (3, 4). Fibroblast Activation Protein (FAP), a pivotal marker and mediator expressed by CAFs, has emerged as a significant diagnostic, therapeutic, and prognostic target due to its multifaceted roles in the tumour microenvironment. This review focusses on opportunities in targeting FAP and the unique challenges in the TME especially with immunotherapy treatment.
2 Cancer-associated fibroblasts (CAFs)
2.1 Origin and heterogeneity
Cancer-associated fibroblasts (CAFs) are pivotal elements within the tumour microenvironment, originating from diverse sources that contribute to their significant heterogeneity. These sources include local activation of resident fibroblasts, recruitment of bone marrow-derived mesenchymal stem cells, and transitions from epithelial and endothelial cells through processes known as epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndMT) (5–7). These varied origins contribute to the spectrum of functional capabilities of CAFs observed across different tumour types and individual cancers.
The heterogeneity of CAFs is further delineated by their expression of specific markers, which vary based on their origin and the local tumour environment. Common markers include alpha-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), and vimentin, which are indicative of their activated state and mesenchymal origin, aiding in distinguishing CAFs from normal fibroblasts. Additionally, more specific markers like S100A4 and PDGFRβ have been identified, helping to classify CAFs into subpopulations such as myofibroblastic CAFs and inflammatory CAFs, each associated with distinct functions within the tumour stroma, contributing variably to cancer progression and response to therapy (8, 9).
2.2 Functions in the tumour microenvironment
CAFs shape the tumour microenvironment by remodelling the extracellular matrix (ECM), supporting angiogenesis, and modulating immune responses. They secrete ECM components and matrix metalloproteinases (MMPs), which restructure the tumour stroma, increasing stiffness and invasiveness. This remodelling promotes tumour growth and invasion while also enhancing angiogenesis through VEGF release, sustaining the tumour’s nutrient and oxygen supply (5, 10)
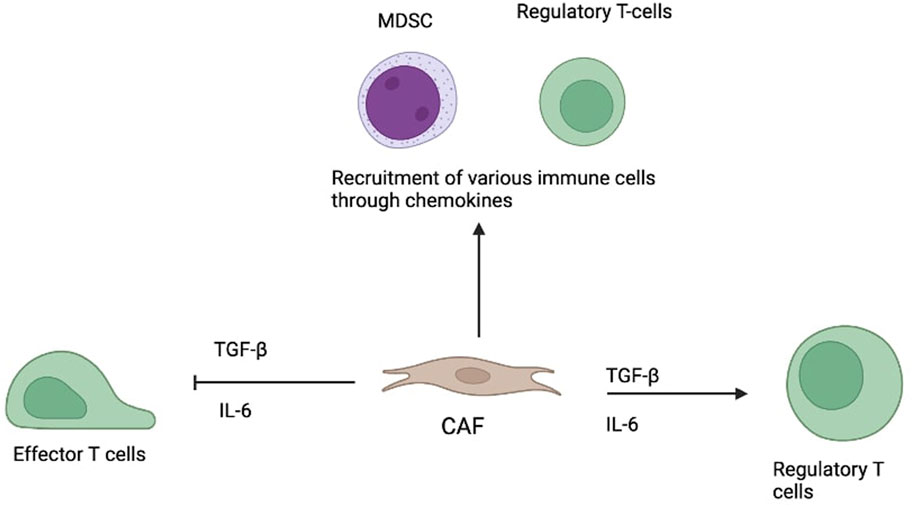
A major function of CAFs is immune modulation. They release cytokines like TGF-β and IL-6, suppressing effector T cells and encouraging regulatory T cell (Treg) expansion. CAFs also produce chemokines that attract immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and Tregs, creating an environment that allows tumour cells to thrive and evade immune detection. This immunosuppressive role significantly affects the success of immunotherapies (6–8, 11).
Interestingly, some CAFs secrete decorin, a protein that inhibits tumour growth and metastasis. This dual role reflects the complex, context-dependent nature of CAFs in tumour biology (10).
Given their multifaceted influence (see Figure 1), CAFs present promising targets for cancer therapy—particularly in boosting immunotherapy effectiveness by disrupting their tumour-supportive functions (5–7).
2.3 History and structural details of fibroblast activation protein
Fibroblast activation protein (FAP) was first identified by Rettig et al in the mid-1980s while studying cell surface antigens to characterise activated fibroblasts (12). They used a monoclonal antibody called F19, which detected an antigen on various cell types, including epithelial cancer cells, soft tissue sarcomas, granulation tissue in wound healing and foetal mesenchymal fibroblasts. This antigen was named “FAP” due to its strong expression on activated fibroblasts but not on normal fibroblasts or epithelial tumours (13).
The protein structure of FAP includes several key domains, namely, the large extracellular domain, transmembrane domain and a short cytoplasmic tail. The extracellular domain contains the catalytic alpha/beta-hydrolase domain, which houses the catalytic domain and the eight-bladed beta-propeller domain, which is important for the protein’s structure and function (13, 14). Within the catalytic domain, FAP possesses a catalytic triad typical of serine proteases, consisting of Serine (S624), Aspartate (D702) and Histidine (H734). This triad is crucial for its enzymatic activities, including both its dipeptidyl peptidase and endopeptidase functions. These serine proteases work together to catalyse the hydrolysis of peptide bonds. The serine residue in FAP’s active site acts as a nucleophile, enabling the cleavage of N-terminal Pro-X peptide bonds, whereX represents any amino acid except proline or hydroxyproline (13).
2.3.1 Substrates of fibroblast activation protein
FAP exerts its effects through both dipeptidyl peptidase and unique endopeptidase activities, particularly targeting collagen types I and III after initial breakdown by matrix metalloproteases. This highlights its role in tissue remodelling and fibrosis (8, 9).
FAP also cleaves α2-antiplasmin (α2-AP), enhancing its inhibition of plasmin and slowing fibrinolysis, which promotes scar formation. The cleaved α2-AP binds fibrin 13 times faster, earning FAP the name antiplasmin-cleaving enzyme (APCE) (9, 12).
Additionally, FAP inactivates Fibroblast Growth Factor 21 (FGF21), a hormone vital for regulating glucose, lipid, and energy metabolism, and for protecting cells from inflammation and immunometabolic stress (9, 13).
2.3.2 Enzymatic activity of fibroblast activation protein
Fibroblast activation protein (FAP) exhibits dual enzymatic activity: dipeptidyl peptidase and endopeptidase. The dipeptidyl peptidase activity cleaves Pro-X bonds at the N-terminus of substrates, such as neuropeptide Y and brain natriuretic peptide, thereby influencing neuropeptide signalling and cardiovascular regulation. Endopeptidase activity, unique to FAP, cleaves Gly-Pro-X sequences in denatured proteins such as collagen types I and III, aiding extracellular matrix (ECM) remodelling, tumour invasion, and fibrosis. FAP also enhances α2-antiplasmin fibrinolysis inhibition and inactivates fibroblast growth factor 21 (FGF21), thereby impacting metabolism and immune regulation. These enzymatic functions highlight FAP’s critical role in ECM remodelling and disease progression, establishing its potential as a therapeutic target (15, 16).
2.4 Fibroblast activation protein expression in normal tissue, benign and malignant pathology
2.4.1 Fibroblast activation protein expression in normal tissues and benign disease
In healthy adult tissues, FAP expression is minimal or absent in organs like the uterus, cervix, placenta, breast, and skin. However, FAP can be selectively expressed during tissue remodelling in conditions like wound healing, embryogenesis, inflammation, and fibrosis (17).
FAP is upregulated on activated fibroblasts during wound healing. Keloid scars contain more FAP-positive fibroblasts than normal skin (18). In liver fibrosis, FAP is prominently expressed on hepatic stellate cells (HSCs), particularly in fibrotic septa near inflammation. These FAP-positive HSCs—typically α-SMA-negative—are thought to represent a fibrosis-driving subpopulation and serve as a stronger marker than GFAP (19, 20).
In Crohn’s disease, FAP is significantly upregulated in myofibroblasts within intestinal strictures—an effect not seen in ulcerative colitis. Immunohistochemistry and imaging confirm FAP activity in fibrostenotic regions (21, 22).
Arthritis also shows FAP upregulation. In osteoarthritis, chondrocyte surface FAP is elevated, with FAPI PET-CT scans demonstrating uptake in affected joints (21, 23).
Cardiovascular conditions like atherosclerosis and myocardial infarction show FAP expression in aortic smooth muscle cells and peri-infarct zones. FAP imaging can also detect early chemotherapy-induced myocardial injury (21).
IgG4-related disease, a fibrotic condition, demonstrates broad FAP expression, with imaging detecting more sites than symptoms suggest. Some benign tumours, like angiomyolipoma and solitary fibrous tumours, also show low-level FAP uptake compared to malignant lesions (21).
2.4.2 Fibroblast activation protein expression in malignant disease
FAP is expressed in many cancers and contributes to tumour progression and metastasis. It is found on CAFs within the tumour stroma and, in some cases, on tumour cells themselves. FAP is commonly seen on fibroblasts surrounding epithelial cancers such as those of the skin, breast, prostate, colon, pancreas, and in sarcomas (24). Tumour cells expressing FAP include pancreatic adenocarcinoma, sarcoma, oesophageal and gastric cancers, colorectal cancer, mesothelioma, breast ductal adenocarcinoma, oral squamous cell carcinoma, glioma, ovarian, and cervical cancers (25). This specific localisation in both stroma and tumour cells makes FAP a promising prognostic and therapeutic target.
2.5 Functions of fibroblast activation protein in tumour biology
2.5.1 Role in tumour invasion, metastasis, and immune evasion
FAP has been shown to promote tumour invasion through several mechanisms. FAP is associated with α3β1 integrin which allows FAP to localise to invadopodia and enhance extracellular matrix degradation and invasion. Studies with ovarian cancer cells showed that the inhibition of α3β1 integrin reduced FAP-induced proliferation and migration (15, 26). Besides that, FAP can directly affect cell motility and migration via its enzymatic activity with the PTEN/PI3K/Akt and Ras-ERK pathway (27).
FAP plays a vital role in angiogenesis by contributing to the reorganisation of ECM which helps to promote endothelial cell invasion and capillary growth (15). Studies involving gastric cancer biopsies showed increased micro-vessel density with cancers of higher FAP expression (28). FAP is localised around the invadopodia of endothelial cells and the endothelial cells of developing microvascular systems in multiple malignancies (15).
Moreover, FAP plays a significant role in mediating immune evasion within the TME. FAP is expressed by CAFs which contribute to immune suppression in the TME directly by promoting regulatory T cells (Tregs) and tumour-associated macrophages via secreted cytokines and indirectly by ECM remodelling and creating a physical barrier (29, 30). Pancreatic cancer mouse models depleted of CAFs showed improved efficacy of checkpoint inhibitor therapy, confirming the role of CAFs in TME immune suppression. The chemokine (C-X-C motif) ligand 12 (CXCL12) was suggested to be responsible for this process and is produced by FAP-positive CAFs (31). Another study suggested FAP expressing macrophages induces immunosuppression by releasing heme oxygenase-1, which creates carbon monoxide, which suppresses the pro-apoptotic effects of TNFα on endothelial cells (15, 32).
2.6 Cancer associated fibroblasts, fibroblast activation protein and the immune microenvironment
2.6.1 Immune modulation by cancer associated fibroblasts
FAP positive (FAP+) CAFs have a substantial impact on the tumour immune microenvironment, influencing the behaviour and efficacy of immune cells in several mechanism.
1. Cytokine secretion: FAP + CAFs contribute to the creation of an immunosuppressive tumour microenvironment. They can inhibit the activity and proliferation of T cells, which are crucial for the immune response against tumours. This effect is often mediated through the secretion of immunosuppressive cytokines such as interleukin-6 (IL-6) and transforming growth factor-beta (TGF-β) (33–35).
2. Chemokine Secretion: FAP + CAFs secrete various chemokines that critically alter the recruitment and distribution of immune cells within the tumour microenvironment. For example, FAP + CAFs produce CXCL12, which has a dual role in attracting stromal cells and excluding effector T cells from tumour sites, thereby facilitating an immunosuppressive environment conducive to tumour growth. Additionally, CAFs can produce CCL2 (MCP-1), which attracts myeloid-derived suppressor cells (MDSCs) and macrophages that further support tumour growth and suppress anti-tumour immune responses (31, 36).
3. Modulation of Macrophages: FAP + CAFs influence macrophage polarization, promoting the differentiation of macrophages towards an M2-like phenotype. M2 macrophages are associated with tissue repair and tumour progression, as they produce anti-inflammatory cytokines and support angiogenesis and remodelling of the extracellular matrix (19, 37, 38).
4. Physical Barrier Formation: By remodelling the extracellular matrix, FAP + CAFs can physically impede the penetration of effector immune cells, such as cytotoxic T lymphocytes, into the tumour mass. This dense extracellular matrix can act as a physical barrier that limits the accessibility of immune cells to cancer cells (39–41).
5. Interaction with Other Immune Checkpoints: FAP + CAFs can affect the expression of other immune checkpoints on the surface of tumour cells or immune cells. For instance, they can promote the expression of PD-L1 on tumour cells, which interacts with PD-1 on T cells to inhibit their activation and function (42–44).
6. Modulation of Antigen Presentation by CAFs: CAFs play a significant role in regulating antigen presentation within the tumour microenvironment. These stromal cells can directly interact with dendritic cells (DCs) and other antigen-presenting cells (APCs), or they can modulate these cells’ functions indirectly through the secretion of cytokines and growth factors. This interaction can either enhance or suppress the immune responses, depending on the signals and the context within the tumour stroma, thereby influencing both the initiation and the propagation of anti-cancer immune activity (45–47).
2.7 Impact of FAP positive cancer associated fibroblasts on immunotherapy
CAFs are a heterogeneous group with multiple subsets, each playing distinct roles in the TME. The CAF-S1 subset has been identified as particularly important in immunosuppression. CAF-S1 fibroblasts attract T lymphocytes to the tumour site, enhance the survival of CD4+CD25+ T cells, and facilitate their transformation into CD25+FOXP3+ regulatory T cells (Tregs). Furthermore, CAF-S1 fibroblasts augment the immunosuppressive capabilities of Tregs, enabling them to inhibit the proliferation of effector T cells more effectively. In contrast, CAF-S4 fibroblasts do not exhibit these immunosuppressive properties (48).
Current therapeutic strategies targeting CAF-associated pathways focus on modulating the immunosuppressive effects of these cells. However, given the heterogeneity of CAFs and their complex interactions within the TME, further research is needed to develop more targeted approaches that can selectively inhibit pro-tumorigenic CAF subsets while preserving the anti-tumour functions of other fibroblast populations.
Strategies targeting CAF-associated immunosuppression include the depletion of CAFs, restoration of their quiescent phenotype, inhibition of effector molecules, and ECM remodelling. Simlukafusp alfa is an immunocytokine that binds FAP on tumour-associated fibroblasts and enhances immune cell activity by increasing antibody-mediated cytotoxicity through PD-L1 checkpoint inhibition. It is currently being tested in combination with anti-PD-1 therapy in Phase II trials for advanced melanoma, renal cell carcinoma, and pancreatic ductal adenocarcinoma, showing promising in vitro and in vivo results. Talabostat, a FAP inhibitor, is under investigation in advanced solid tumours alongside anti-PD-1 therapy, aiming to modulate TME-associated immunosuppression.
3 Challenges and future directions
3.1 Understanding the heterogeneity of cancer associated fibroblasts
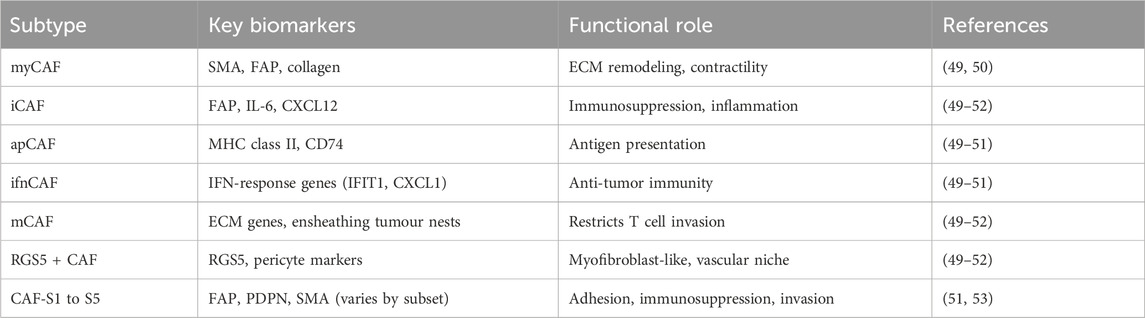
It is a major challenge to define CAFs and their sub-populations and delineate their specific functions in cancer tumorigenesis as they can originate from a variety of cells. The classification of CAF subtypes also varies depending on the specific type of cancer being studied. For example, CAF-N (normal) and CAF-D (divergent) were described in human oral squamous cell carcinoma (OSCC), CAF-A and CAF-B in colorectal tumours and four subtypes CAF-S1 to CAF-S4 in human breast adenocarcinomas. The more common and unified classification of CAF based on molecular features has been suggested with the following major subtypes: myofibroblastic CAF (myCAF), inflammatory CAF (iCAF), interferon-response CAF (ifnCAF), antigen-presenting CAF (apCAF), matrix CAF (mCAF), RGS5+ CAF and CAF-S1 to CAF-S5 (49–53). See summary Table 1.
FAP has been thought to be a potential broad biomarker for CAF and been proven to play an important role in cancer growth. FAP positive CAFs are thought to be instrumental in the development of immunosuppressive TME and high expression of FAP has been associated with poorer prognosis in various cancers. Consequently, FAP has attracted significant attention as a potential focus for developing therapeutic interventions and identifying biomarkers.
Despite numerous studies and technological advances, identifying a single biomarker that can definitively identify all CAFs in each tumour has proven challenging. The heterogeneity of CAFs makes it difficult to identify a single biomarker for all subtypes, while overlapping markers with other cell types complicate precise identification. CAFs demonstrate remarkable plasticity, transitioning between different states during tumour progression, and their functions and phenotypes vary based on tumour type, stage, and microenvironmental cues. The proportion and characteristics of CAF subpopulations evolve over time as tumours progress, and different subtypes show varying spatial distributions within tumours, adding another layer of complexity (54, 55).
Cancer-associated fibroblasts (CAFs) are dynamic components of the tumour microenvironment (TME), playing a crucial role in coordinating interactions between cancer cells and host matrix responses. The TME contributes significantly to CAF heterogeneity, with diverse subpopulations emerging in response to various environmental factors (55). This plasticity allows CAFs to adapt their phenotypes to environmental cues. Notably, different CAF subsets exhibit distinct spatial distributions within the tumour, highlighting the microenvironment’s influence on their localisation (54). Furthermore, the microenvironment influences metabolic interactions between CAFs and cancer cells, impacting tumour progression. CAFs also play a role in extracellular matrix (ECM) remodelling, both responding to and shaping the TME (54).
Future research directions to address these challenges include utilising advanced single-cell analysis techniques, validating findings using complementary methodologies such as CyTOF, multiplex flow cytometry, and multiplex immunostaining, and functional validation using various in vitro and in vivo model systems to understand the biological significance of proposed CAF subpopulations. Establishing a standardised classification system, investigating the role of the tumour microenvironment in shaping CAF heterogeneity and function, exploring metabolic interactions between CAFs and cancer cells, and studying ECM remodelling will contribute to a more comprehensive understanding of CAF biology and its impact on tumour progression (54).
3.2 Optimizing fibroblast activation protein targeted therapies
3.2.1 FAP as a biomarker for immunotherapy
FAP could be a potential biomarker to predict response to immunotherapy treatment. Higher FAP levels were found to correlate with poorer response and clinical outcomes in bladder urothelial carcinoma and cutaneous melanoma patients undergoing treatment with immune checkpoint inhibitors (56). On the other hand, pre-clinical mouse models with head and neck cancer show that absence of FAP positive CAF surprisingly did not affect tumour progression or sensitise tumours to combination cisplatin and anti-PD1 treatment. FAP positive CAF were also not found to increase tumour progression or recurrence in mouse models. It is possible that there may be a mismatch between gene and protein expression as FAP gene expression was negatively associated with outcomes (57). More studies are required to study and confirm FAP as a potential biomarker for immunotherapy response in various types of cancer.
3.2.2 Challenges in FAP-targeted therapies
Despite initial interest in targeting FAP for cancer treatment, a phase II exploratory trial of monoclonal antibody sibrotuzumab targeting FAP in metastatic colorectal cancer was discontinued early as it did not show efficacy (58). Unsurprisingly, another trial with FAP inhibitor talabostat in metastatic colorectal cancer patients also proved ineffective. There were no objective responses seen in all 28 participants in the trial. Laboratory analysis showed significant but incomplete inhibition of FAP enzymatic activity in the blood (59). The failure of these trials may be attributed to the heterogeneous nature of cancer-associated fibroblasts (CAFs) and the function of fibroblast activation protein (FAP), which can promote tumorigenesis in certain tumours while inhibiting it in others. Therefore, targeted treatment alone against FAP may not be effective until we are able to delineate FAP subtypes accurately and perform personalised targeted treatment. However, with an improved understanding of the tumour microenvironment, current research focuses on combination therapies to optimize FAP-targeted approaches, particularly involving the immune system. These include combining FAP inhibitors with immunotherapy and developing FAP-targeted CAR-T cells to target cancer-associated fibroblasts. A phase II basket study combining talabostat and immune checkpoint inhibitor pembrolizumab also showed limited efficacy without any objective response (60). While FAP-CAR-T cells show promise in activating the immune system and eliminating target cells, concerns about on-target off-tumour toxicity due to low-level FAP expression in healthy tissues persist. Ongoing clinical trials are investigating FAP-CAR-T cells, both alone and in combination with immunotherapy or other targets like Nectin-4, to address these challenges and improve efficacy (59, 61).
3.2.3 Advances in FAP theranostics
FAP is an attractive target for molecular imaging for cancer as it is minimally expressed in normal tissues hence is a perfect target for theranostics. Radionuclide therapy targeting FAP such as 177Lu-EB-FAPI is being investigated and the first-in-human trial for metastatic radioiodine-refractory thyroid cancer was conducted in 12 patients with objective response rate of 25% (62). This early phase study showed that this radioligand therapy is safe and paves the way for future radioligand studies. Newer strategies could investigate combining immunotherapy treatment with radiotherapy which could potentially increase infiltration by cytotoxic T-cells within the TME hence enhancing the potency of immunotherapy. Pre-clinical studies using LNC1004 is supportive of this strategy and is shown to upregulate tumour PD-L1 expression (63).
3.3 Personalized treatment approaches
FAP positive CAFs present both challenges and opportunities for targeted cancer therapies. Promising strategies have emerged, including FAP-activated prodrugs that selectively target tumour stroma, inhibition of specific signalling pathways involved in CAF-cancer cell crosstalk, and repurposing existing drugs like losartan for modulating the tumour microenvironment. Pre-clinical studies indicate that losartan, an angiotensin II receptor type 1 antagonist, demonstrates potential in reducing cancer-associated fibroblast (CAF) activity due to its anti-fibrotic properties (64, 65).
Further research is needed in several areas to advance CAF-targeted therapies. These include validating alternative biomarkers for CAF subtypes, understanding CAF-TME interactions across various cancer types, conducting longitudinal studies on CAF dynamics and subtype interconversion, exploring combination therapies with CAF-targeted approaches, and developing advanced 3D models to replicate complex tumour microenvironment interactions (66–68). Addressing these research gaps will be crucial for developing personalised therapy approaches that characterise CAF subtypes, comprehensively analysing the tumour microenvironment and focusing on tumour-supportive CAFs rather than broad depletion.
4 Conclusion
In conclusion, FAP-positive cancer-associated fibroblasts (CAFs) are pivotal in orchestrating the complex interplay within the tumour microenvironment that crucially influences cancer progression. Due to their heterogeneity, CAFs play diverse roles, from extracellular matrix remodelling and angiogenesis to intricately modulating the immune landscape. FAP, a salient marker of CAFs, is emerging as a critical diagnostic and therapeutic target. Its role in immune modulation is particularly compelling as it facilitates the creation of an immunosuppressive environment that can shield the tumour from immune surveillance. This makes FAP not only a target for traditional therapies but also a potential linchpin in combination with emerging immunotherapies.
However, harnessing the full potential of targeting FAP-positive CAFs faces several challenges. Key among these is the need for a more refined characterization of CAF subtypes to tailor therapies more precisely and to avoid the broad-brush effects that could inadvertently promote tumour progression. Moreover, the off-target effects of FAP-directed therapies necessitate cautious development to ensure safety and efficacy.
Future research should focus on advancing imaging techniques that can accurately identify and monitor FAP expression dynamically within the tumour milieu. Improving CAF classification systems will enhance our understanding of their roles and interactions within the tumour, guiding more effective combination therapies. By addressing these challenges, targeting FAP-positive CAFs holds the promise of crafting more nuanced and potent strategies in cancer therapy. Continued investigation into this field is essential and promises to substantially advance our capabilities in cancer treatment, particularly in the era of immunotherapy, ultimately improving patient outcomes and expanding the horizons of precision medicine.
Author contributions
HL: Writing – original draft, Methodology, Conceptualization, Writing – review and editing. ZA-O: Writing – original draft, Methodology, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gonzales Carazas, MM, Pinto, JA, and Casado, FL. Biological bases of cancer immunotherapy. Expert Rev Mol Med (2021) 23:e3. doi:10.1017/erm.2021.5
2. Peterson, C, Denlinger, N, and Yang, Y. Recent advances and challenges in cancer immunotherapy. Cancers (2022) 14(16):3972. doi:10.3390/cancers14163972
3. Mao, X, Xu, J, Wang, W, Liang, C, Hua, J, Liu, J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer (2021) 20(1):131. doi:10.1186/s12943-021-01428-1
4. Junttila, MR, and de Sauvage, FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature (2013) 501(7467):346–54. doi:10.1038/nature12626
5. Xing, F, Saidou, J, and Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (2010) 15(1):166–79. doi:10.2741/3613
6. LeBleu, VS, and Kalluri, R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Models and Mech (2018) 11(4):dmm029447. doi:10.1242/dmm.029447
7. Kalluri, R. The biology and function of fibroblasts in cancer. Nat Rev Cancer (2016) 16(9):582–98. doi:10.1038/nrc.2016.73
8. Peltier, A, Seban, RD, Buvat, I, Bidard, FC, and Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: from single cell analysis to whole-body imaging. Semin Cancer Biol (2022) 86:262–72. doi:10.1016/j.semcancer.2022.04.008
9. Nurmik, M, Ullmann, P, Rodriguez, F, Haan, S, and Letellier, E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer (2020) 146(4):895–905. doi:10.1002/ijc.32193
10. De, P, Aske, J, and Dey, N. Cancer-associated fibroblast functions as a road-block in cancer therapy. Cancers (Basel) (2021) 13(20):5246. doi:10.3390/cancers13205246
11. Swann, JB, and Smyth, MJ. Immune surveillance of tumors. J Clin Invest (2007) 117(5):1137–46. doi:10.1172/jci31405
12. Xin, L, Gao, J, Zheng, Z, Chen, Y, Lv, S, Zhao, Z, et al. Fibroblast activation protein-α as a target in the bench-to-bedside diagnosis and treatment of tumors: a narrative review. Front Oncol (2021) 11:648187. doi:10.3389/fonc.2021.648187
13. Mousavi, MJ, Karami, J, Alimohammadi, M, Solaymani-Mohammadi, F, and Rezaei, N. Fibroblast activation protein (FAP): a key modulator of the cancer microenvironment. In: N Rezaei, editor. Handbook of cancer and immunology. Springer International Publishing (2022). p. 1–23.
14. Aertgeerts, K, Levin, I, Shi, L, Snell, GP, Jennings, A, Prasad, GS, et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein α. J Biol Chem (2005) 280(20):19441–4. doi:10.1074/jbc.c500092200
15. Fitzgerald, AA, and Weiner, LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev (2020) 39(3):783–803. doi:10.1007/s10555-020-09909-3
16. Lv, B, Xie, F, Zhao, P, Ma, X, Jiang, WG, Yu, J, et al. Promotion of cellular growth and motility is independent of enzymatic activity of fibroblast activation protein-α. Cancer Genomics Proteomics (2016) 13(3):201–8. Available online at: https://cgp.iiarjournals.org/content/13/3/201.long.
17. Altmann, A, Haberkorn, U, and Siveke, J. The latest developments in imaging of fibroblast activation protein. J Nucl Med (2021) 62(2):160–7. doi:10.2967/jnumed.120.244806
18. Dienus, K, Bayat, A, Gilmore, BF, and Seifert, O. Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res (2010) 302(10):725–31. doi:10.1007/s00403-010-1084-x
19. Yang, AT, Kim, YO, Yan, XZ, Abe, H, Aslam, M, Park, KS, et al. Fibroblast activation protein activates macrophages and promotes parenchymal liver inflammation and fibrosis. Cell Mol Gastroenterol Hepatol (2023) 15(4):841–67. doi:10.1016/j.jcmgh.2022.12.005
20. Levy, MT, McCaughan, GW, Abbott, CA, Park, JE, Cunningham, AM, Müller, E, et al. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology (1999) 29(6):1768–78. doi:10.1002/hep.510290631
21. Dendl, K, Koerber, SA, Kratochwil, C, Cardinale, J, Finck, R, Dabir, M, et al. FAP and FAPI-PET/CT in malignant and non-malignant diseases: a perfect symbiosis? Cancers (2021) 13(19):4946. doi:10.3390/cancers13194946
22. Rovedatti, L, Di Sabatino, A, Knowles, CH, Sengupta, N, Biancheri, P, Corazza, GR, et al. Fibroblast activation protein expression in Crohn’s disease strictures. Inflamm Bowel Dis (2011) 17(5):1251–3. doi:10.1002/ibd.21446
23. Luo, Y, Pan, Q, Yang, H, Li, F, and Zhang, F. Inflammatory arthritis induced by anti-programmed death-1 shown in 68Ga-FAPI PET/CT in a patient with esophageal carcinoma. Clin Nucl Med (2021) 46(5):431–2. doi:10.1097/rlu.0000000000003608
24. Liu, F, Qi, L, Liu, B, Liu, J, Zhang, H, Che, D, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS One (2015) 10(3):e0116683. doi:10.1371/journal.pone.0116683
25. Sedo, A, Mateu, R, Zubal, M, Kotackova, L, and Sedo, A. Targeting fibroblast activation protein in cancer ndash Prospects and caveats. Front Biosci Landmark Ed (2018) 23(10):1933–68. doi:10.2741/4682
26. Yang, W, Han, W, Ye, S, Liu, D, Wu, J, Liu, H, et al. Fibroblast activation protein-α promotes ovarian cancer cell proliferation and invasion via extracellular and intracellular signaling mechanisms. Exp Mol Pathol (2013) 95(1):105–10. doi:10.1016/j.yexmp.2013.06.007
27. Wang, H, Wu, Q, Liu, Z, Luo, X, Fan, Y, Liu, Y, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis (2014) 5(4):e1155. doi:10.1038/cddis.2014.122
28. Gao, LM, Wang, F, Zheng, Y, Fu, ZZ, Zheng, L, and Chen, LL. Roles of fibroblast activation protein and hepatocyte growth factor expressions in angiogenesis and metastasis of gastric cancer. Pathol Oncol Res (2019) 25(1):369–76. doi:10.1007/s12253-017-0359-3
29. Hou, CM, Qu, XM, Zhang, J, Ding, TT, Han, W, Ji, GC, et al. Fibroblast activation proteins-α suppress tumor immunity by regulating T cells and tumor-associated macrophages. Exp Mol Pathol (2018) 104(1):29–37. doi:10.1016/j.yexmp.2017.12.003
30. Barrett, R, and Puré, E. Cancer-associated fibroblasts: key determinants of tumor immunity and immunotherapy. Curr Opin Immunol (2020) 64:80–7. doi:10.1016/j.coi.2020.03.004
31. Feig, C, Jones, JO, Kraman, M, Wells, RJB, Deonarine, A, Chan, DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A (2013) 110(50):20212–7. doi:10.1073/pnas.1320318110
32. Arnold, JN, Magiera, L, Kraman, M, and Fearon, DT. Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1. Cancer Immunol Res (2014) 2(2):121–6. doi:10.1158/2326-6066.cir-13-0150
33. Higashino, N, Koma, YI, Hosono, M, Takase, N, Okamoto, M, Kodaira, H, et al. Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma. Lab Invest (2019) 99(6):777–92. doi:10.1038/s41374-018-0185-6
34. Cremasco, V, Astarita, JL, Grauel, AL, Keerthivasan, S, MacIsaac, K, Woodruff, MC, et al. FAP delineates heterogeneous and functionally divergent stromal cells in immune-excluded breast tumors. Cancer Immunol Res (2018) 6(12):1472–85. doi:10.1158/2326-6066.cir-18-0098
35. Kraxner, A, Braun, F, Cheng, WY, Yang, THO, Pipaliya, S, Canamero, M, et al. Investigating the complex interplay between fibroblast activation protein α-positive cancer associated fibroblasts and the tumor microenvironment in the context of cancer immunotherapy. Front Immunol (2024) 15:1352632. doi:10.3389/fimmu.2024.1352632
36. Yang, X, Lin, Y, Shi, Y, Li, B, Liu, W, Yin, W, et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res (2016) 76(14):4124–35. doi:10.1158/0008-5472.can-15-2973
37. Chen, S, Morine, Y, Tokuda, K, Yamada, S, Saito, Y, Nishi, M, et al. Cancer-associated fibroblast-induced M2-polarized macrophages promote hepatocellular carcinoma progression via the plasminogen activator inhibitor-1 pathway. Int J Oncol (2021) 59(2):59. doi:10.3892/ijo.2021.5239
38. Miao, Y, Deng, Y, Liu, J, Wang, J, Hu, B, Hao, S, et al. Anti-cancer effect of targeting fibroblast activation protein alpha in glioblastoma through remodeling macrophage phenotype and suppressing tumor progression. CNS Neurosci Ther (2023) 29(3):878–92. doi:10.1111/cns.14024
39. Kaur, A, Ecker, BL, Douglass, SM, Kugel, CH, Webster, MR, Almeida, FV, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov (2019) 9(1):64–81. doi:10.1158/2159-8290.cd-18-0193
40. Sorokin, L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol (2010) 10(10):712–23. doi:10.1038/nri2852
41. Joyce, JA, and Fearon, DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science (2015) 348(6230):74–80. doi:10.1126/science.aaa6204
42. Cheng, Y, Li, H, Deng, Y, Tai, Y, Zeng, K, Zhang, Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis (2018) 9(4):422. doi:10.1038/s41419-018-0458-4
43. Inoue, C, Miki, Y, Saito, R, Hata, S, Abe, J, Sato, I, et al. PD-L1 induction by cancer-associated fibroblast-derived factors in lung adenocarcinoma cells. Cancers (Basel) (2019) 11(9):1257. doi:10.3390/cancers11091257
44. Li, Z, Zhou, J, Zhang, J, Li, S, Wang, H, and Du, J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer (2019) 145(7):1946–57. doi:10.1002/ijc.32278
45. Cheng, JT, Deng, YN, Yi, HM, Wang, G, Fu, B, Chen, W, et al. Hepatic carcinoma-associated fibroblasts induce Ido-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis (2016) 5(2):e198. doi:10.1038/oncsis.2016.7
46. Hsu, YL, Hung, JY, Chiang, SY, Jian, SF, Wu, CY, Lin, YS, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget (2016) 7(19):27584–98. doi:10.18632/oncotarget.8488
47. Papadas, A, Huang, Y, Cicala, A, Dou, Y, Fields, M, Gibbons, A, et al. Emerging roles for tumor stroma in antigen presentation and anti-cancer immunity. Biochem Soc Trans (2023) 51(6):2017–28. doi:10.1042/bst20221083
48. Costa, A, Kieffer, Y, Scholer-Dahirel, A, Pelon, F, Bourachot, B, Cardon, M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell (2018) 33(3):463–79.e10. doi:10.1016/j.ccell.2018.01.011
49. Cumming, J, Maneshi, P, Dongre, M, Alsaed, T, Dehghan-Nayeri, MJ, Ling, A, et al. Dissecting FAP+ cell diversity in pancreatic cancer uncovers an interferon-response subtype of cancer-associated fibroblasts with tumor-restraining properties. Cancer Res (2025) 85:2388–411. doi:10.1158/0008-5472.CAN-23-3252
50. Kazakova, AN, Lukina, MM, Anufrieva, KS, Bekbaeva, IV, Ivanova, OM, Shnaider, PV, et al. Exploring the diversity of cancer-associated fibroblasts: insights into mechanisms of drug resistance. Front Cell Dev Biol (2024) 12:1403122. doi:10.3389/fcell.2024.1403122
51. Cords, L, Tietscher, S, Anzeneder, T, Langwieder, C, Rees, M, de Souza, N, et al. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat Commun (2023) 14(1):4294. doi:10.1038/s41467-023-39762-1
52. Forsthuber, A, Aschenbrenner, B, Korosec, A, Jacob, T, Annusver, K, Krajic, N, et al. Cancer-associated fibroblast subtypes modulate the tumor-immune microenvironment and are associated with skin cancer malignancy. Nat Commun (2024) 15(1):9678. doi:10.1038/s41467-024-53908-9
53. Mathieson, L, Koppensteiner, L, Dorward, DA, O’Connor, RA, and Akram, AR. Cancer-associated fibroblasts expressing fibroblast activation protein and podoplanin in non-small cell lung cancer predict poor clinical outcome. Br J Cancer (2024) 130(11):1758–69. doi:10.1038/s41416-024-02671-1
54. Chen, Y, McAndrews, KM, and Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol (2021) 18(12):792–804. doi:10.1038/s41571-021-00546-5
55. Pei, L, Liu, Y, Liu, L, Gao, S, Gao, X, Feng, Y, et al. Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer (2023) 22(1):29. doi:10.1186/s12943-023-01731-z
56. Ma, C, Xi, S, Sun, H, Zhang, M, and Pei, Y. Identifying the oncogenic roles of FAP in human cancers based on systematic analysis. Aging (2023) 15(14):7056–83. doi:10.18632/aging.204892
57. Liu, R, Li, H, Liu, L, Yu, J, and Ren, X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol and Ther (2012) 13(3):123–9. doi:10.4161/cbt.13.3.18696
58. Hofheinz, RD, al-Batran, SE, Hartmann, F, Hartung, G, Jäger, D, Renner, C, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Oncol Res Treat (2003) 26(1):44–8. doi:10.1159/000069863
59. Narra, K, Mullins, SR, Lee, HO, Strzemkowski-Brun, B, Magalong, K, Christiansen, VJ, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol and Ther (2007) 6(11):1691–9. doi:10.4161/cbt.6.11.4874
60. Ahmed, J, Janku, F, Karp, DD, Piha-Paul, SA, Tsimberidou, AM, Yap, TA, et al. A phase 2 basket study of talabostat, a small-molecule inhibitor of dipeptidyl peptidases, administered in combination with pembrolizumab in patients with advanced solid cancers. Cancer (2025) 131(3):e35728. doi:10.1002/cncr.35728
61. Bughda, R, Dimou, P, D’Souza, RR, and Klampatsa, A. Fibroblast activation protein (FAP)-Targeted CAR-T cells: launching an attack on tumor stroma. ImmunoTargets Ther (2021) 10:313–23. doi:10.2147/itt.s291767
62. Fu, H, Huang, J, Zhao, T, Wang, H, Chen, Y, Xu, W, et al. Fibroblast activation protein-targeted radioligand therapy with 177Lu-EB-FAPI for metastatic radioiodine-refractory thyroid cancer: first-in-human, dose-escalation study. Clin Cancer Res (2023) 29(23):4740–50. doi:10.1158/1078-0432.ccr-23-1983
63. Zhao, L, Pang, Y, Zhou, Y, Chen, J, Fu, H, Guo, W, et al. Antitumor efficacy and potential mechanism of FAP-targeted radioligand therapy combined with immune checkpoint blockade. Signal Transduction Targeted Ther (2024) 9(1):142. doi:10.1038/s41392-024-01853-w
64. Brennen, WN, Rosen, DM, Wang, H, Isaacs, JT, and Denmeade, SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. JNCI J Natl Cancer Inst (2012) 104(17):1320–34. doi:10.1093/jnci/djs336
65. Mainetti, LE, Rico, MJ, Kaufman, CD, Grillo, MC, Guercetti, J, Baglioni, MV, et al. Losartan improves the therapeutic effect of metronomic cyclophosphamide in triple negative mammary cancer models. Oncotarget (2020) 11(32):3048–60. doi:10.18632/oncotarget.27694
66. Sahai, E, Astsaturov, I, Cukierman, E, DeNardo, DG, Egeblad, M, Evans, RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. doi:10.1038/s41568-019-0238-1
67. Arima, Y, Matsueda, S, and Saya, H. Significance of cancer-associated fibroblasts in the interactions of cancer cells with the tumor microenvironment of heterogeneous tumor tissue. Cancers (Basel) (2023) 15(9):2536. doi:10.3390/cancers15092536
Keywords: fibroblast activation protein (FAP), cancer associate fibroblasts (CAFs), tumour microenvironment (TME), immunotherapy, cancer
Citation: Lee HH and Al-Ogaili Z (2025) Fibroblast activation protein and the tumour microenvironment: challenges and therapeutic opportunities. Oncol. Rev. 19:1617487. doi: 10.3389/or.2025.1617487
Received: 24 April 2025; Accepted: 08 July 2025;
Published: 16 July 2025.
Edited by:
Run Shi, Nanjing medical university, ChinaReviewed by:
Mauro Francesco Pio Maiorano, University of Bari Aldo Moro, ItalyGopinath Prakasam, University of Texas Southwestern Medical Center, United States
Copyright © 2025 Lee and Al-Ogaili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hsing Hwa Lee, bGVlaHNpbmdod2FAZ21haWwuY29t
†These authors have contributed equally to this work
 Hsing Hwa Lee
Hsing Hwa Lee Zeyad Al-Ogaili
Zeyad Al-Ogaili