- 1Department of Audiology, San José State University, San José, CA, United States
- 2Division of Audiology, Department of Otolaryngology - Head and Neck Surgery, University of California, San Francisco, San Francisco, CA, United States
The study aimed to determine whether a self-fitting algorithm for hearing aids could produce outcomes comparable to those achieved with professionally fitted hearing aids. Involving up to 40 subjects aged 18 to 80, the research compared two fittings: one conducted by a professionally trained audiologist Best Practice Fit (BP-FIT) and one using a self-fitting software (SELF-FIT). Subjects completed both fittings, with Real Ear Measures and Quick Speech In Noise (QuickSIN) measures taken before field use of either fitting. The subjects were randomly assigned to start with either SELF-FIT or BP-FIT, remaining unaware of their condition throughout the trial. After 2 weeks of hearing aid use in each condition, subjects provided subjective reports of perceived benefit (via the APHAB survey) and had their hearing aids reprogrammed for the remaining condition. The study assessed the efficacy through objective (REM), behavioral (QuickSIN), and subjective (APHAB) measures. In summary, the study findings reveal that the SELF-FIT hearing aid system performed similar to the BP-FIT across multiple domains. This includes objective measures of self-fitting hearing aid output assessed through REM, with a difference of <5 dB SPL between fitting conditions, behavioral evaluation of speech understanding in background noise via QuickSIN, with a difference of <2.7 dB SNR between fitting conditions, and subjective assessment of efficacy as reported by the user via APHAB, with a difference of <10% between fitting conditions.
1 Introduction
Hearing loss is a prevalent condition that significantly impacts quality of life. Traditional audiological approaches involve audiologist-best practice-fitted hearing aids (BP-FIT), which are effective but can be inaccessible due to high costs and limited availability. The recent introduction of over-the-counter (OTC), self-fitting hearing aids (SELF-FIT) presents a potential paradigm shift in hearing care. These devices aim to address accessibility and affordability issues, enabling users to fit and adjust hearing aids independently (De Sousa et al., 2023).
Recent regulatory changes, such as the US Food and Drug Administration's (FDA) Reauthorization Act of 2017, have facilitated the emergence of OTC hearing aids. These devices, characterized by automated fitting processes and user-friendly interfaces, offer a promising alternative to traditional methods (National Institute on Deafness and Other Communication Disorders, 2022). Previous studies have explored various aspects of self-fitting hearing aids, including different approaches to user-selected settings (Humes, 2019; Sabin et al., 2020).
The effectiveness of SELF-FIT devices has been the subject of extensive research. A study conducted by De Sousa et al. (2023) compared self-fitting OTC hearing aids with remote support to audiologist-fitted hearing aids. Their findings suggest that the outcomes of self-fitted devices at 6 weeks post-fitting were comparable to those achieved through best practice fittings that use real ear measures. This raises important questions about the future role of self-fitting hearing aids in addressing the hearing care needs of a broader population.
Our study builds upon this foundation, aiming to further evaluate the clinical performance of SELF-FIT hearing aids in comparison to BP-FIT models. We focus on objective audiological measurements, behavioral responses, and reported subjective benefits to comprehensively assess these innovative devices. Our research seeks to contribute to the growing body of evidence supporting the efficacy of SELF-FIT hearing aids and to explore their potential in transforming hearing care access and affordability.
2 Methods
2.1 Participants
Our study enrolled 40 adult participants from a community of individuals experiencing mild-to-moderate hearing loss. We emphasized recruiting participants with prior experience using hearing aids but also accepted a small number from the same demographic who had limited or no hearing aid experience. Enrollment was equally divided between two sites: 20 participants at San Jose, CA (Site 1), and 20 participants at San Francisco, CA (Site 2).
The study's inclusion criteria comprised participants meeting the following specifications: age within the range of 18 to 80 years, mild-to-moderate hearing loss within the 500–4,000 Hz spectrum according to the World Health Organization's (WHO) classification, a minimum of 1 year of prior experience with hearing aids, proficiency in operating a laptop/desktop for internet browsing and online survey participation, and proficiency in English for effective communication and survey completion.
Conversely, exclusion criteria stipulated the exclusion of individuals with any reported history of significant neurological deficits, such as stroke or Alzheimer's disease, or those demonstrating an incapacity to maintain alertness during initial testing.
Comprehensive details regarding participant demographics, characteristics, and subgroup analyses such as randomization groups are delineated in Supplementary Tables 1–4. Briefly, the 4 Frequency Pure Tone Average (PTA) for all 80 combined ears was 41.45 dB HL, consistent with mild to moderate hearing loss. Specifically, 8.75% of ears had slight hearing loss, 32.5% had mild hearing loss, and 58.75% had moderate hearing loss. Additionally, the average Word Recognition Score (WRS) was 91.48%, with 68.75% of ears demonstrating normal or excellent scores, 18.75% with good scores, 3.75% with fair scores, 8.75% with poor scores, and none with very poor scores. See Supplementary Tables 1–4 for more details.
2.2 Study design
This clinical trial employed a repeated measures crossover design. Participants were exposed to two conditions: SELF-FIT and BP-FIT with use of receiver-in-canal (RIC) hearing aids with single vent domes, each for a period of 2 weeks, without a washout period between them (Figure 1). The objective was to assess the effectiveness of these conditions in real-world settings, understanding that not including a washout period meant participants' listening environments remained consistent throughout the trial.
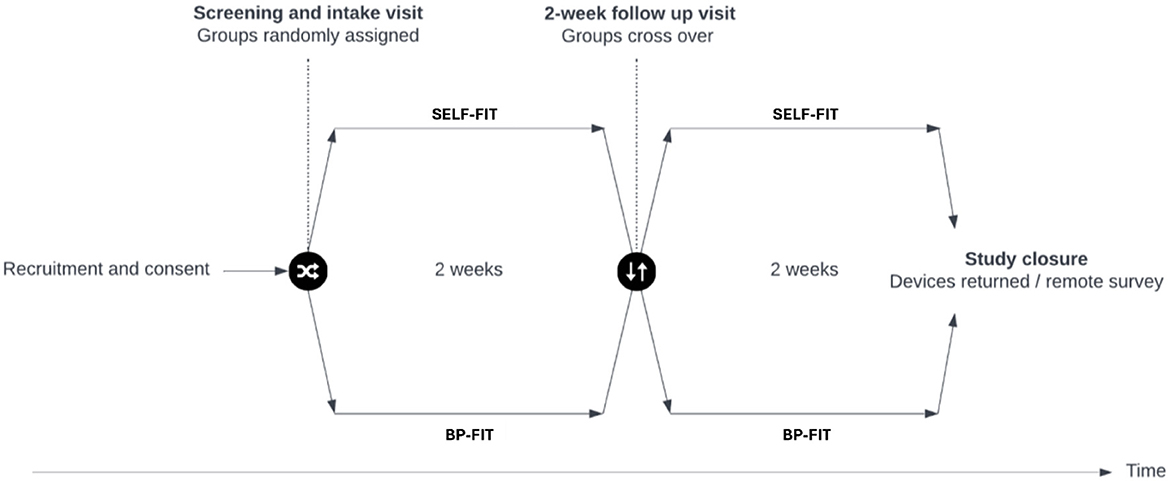
Figure 1. Visual representation of cross-over design used to complete this study. A randomized, cross-over design was used to complete this clinical trial. Objective and behavioral measures were completed at the intake visit while subjective measures were completed at approximately 2 weeks and 4 weeks after the intake visit to report on use of each hearing aid condition (SELF-FIT and BP-FIT) during the trial.
Participants initially completed a survey to provide baseline information about their hearing history. This was followed by an audiologic evaluation during their first visit to confirm their trial candidacy based on the inclusion criteria. Participants who met these criteria were then randomized into two groups. One group started with the BP-FIT condition and then switched to SELF-FIT, while the other group began with SELF-FIT and then moved to BP-FIT. Objective and behavioral measures were taken at the intake visit, and subjective measures were collected after 2 and 4 weeks to assess the effectiveness of each hearing aid condition.
Taking into account clinical best practices, in accordance with the guidelines set forth by the American Speech-Language-Hearing Association (ASHA, 2022), it is essential to assess whether outcomes align with this clinical model when evaluating over-the-counter (OTC) self-fitting hearing aids. Similar research completed previously (Food and Drug Administration, 2022) relied on pairwise comparisons combined with a non-inferiority analysis to evaluate potential differences between self-fitting hearing aids and professionally fit hearing aids. A crucial consideration is whether the outcomes obtained are comparable. Self-reported hearing aid outcomes serve as standard measures in hearing aid trials, particularly due to their correlation with consistent usage of hearing aids (Dornhoffer et al., 2020). This trial was conducted through a partnership between San Jose State University and an anonymous industry partner, which provided support in terms of funding, participants, equipment, and logistics.
2.3 Data collection and analysis
The efficacy analysis incorporated all enrolled participants, capturing objective, behavioral, and subjective data with exception to one set of missing APHAB data for a participant seen at the SJ site due to a deviation from protocol. The analysis included Real Ear Measure (REM) data for both ears of each participant, QuickSIN speech recognition test results, and responses to the APHAB survey.
A non-inferiority analysis was utilized to compare the experimental SELF-FIT with the BP-FIT across these three types of measures, similar to previous research (Food and Drug Administration, 2022). We set specific criteria for efficacy for each measure and applied a Bonferroni correction to address the issue of multiple comparisons in our statistical analysis, with correction factor of m = 3 applied to p-values computed in non-inferiority tests. For Real Ear Measures, a difference of 5 dB or less was set as criteria for efficacy. For QuickSIN, a difference of 2.7 dB SNR or less was set as criteria for efficacy. For APHAB, a difference in global benefit scores of 10% or less was set as the criteria for efficacy.
2.3.1 Efficacy subgroup analyses
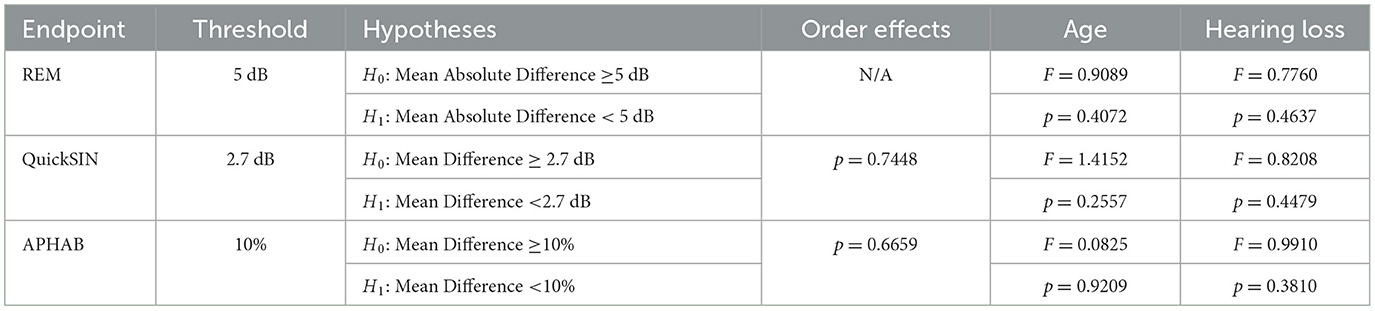
Another set of analyses was conducted to validate the main findings. These supplementary analyses examined the potential impact of the order of data collection for endpoints of interest, severity of hearing loss, and age groups (see Supplementary Tables 3, 4 for more details).
These additional analyses are presented for primary and secondary endpoints. In short, no statistically significant differences were found among any subgroups evaluated. Table 1 summarizes these statistical findings for each endpoint.
2.4 Professional audiogram and real ear measures
During their initial study visit, each participant underwent a basic audiological evaluation, including pure-tone audiometry and word recognition tests using the GSI AudioStar Pro at the San Jose site and the Interacoustics Equinox 2.0 at the SF site. For the REMs using Audioscan-Verifit2, we assessed the hearing aid output levels in participants' ears for both the SELF-FIT and BP-FIT conditions. The SELF-FIT settings were generated using a user-directed self-fitting algorithm while BP-FIT settings were based on professional audiometric thresholds and adjusted to meet NAL-NL2 gain targets with NOAH interface. The REMs in both conditions were compared across a range of input levels of soft at 55 dB, moderate at 65 dB, and loud at 75 dB.
2.5 Quick Speech in Noise test
Participants completed the QuickSIN test under both hearing aid conditions to estimate their ability to discern speech in noisy environments, without changing the position of the participant in their chair and keeping the hearing aid placement consistent for both conditions.
2.6 Abbreviated Profile of Hearing Aid Benefit
Participants completed the APHAB survey twice, based on their experiences with the SELF-FIT and BP-FIT settings. This survey was designed to evaluate subjective hearing aid benefits in typical listening scenarios, offering insights into the practical effectiveness of the hearing aids configured in both SELF-FIT and BP-FIT modes.
3 Results
3.1 Objective measure of hearing aid output
As a primary endpoint, participants (N = 40) completed Real Ear Measures, an objective measure of output levels while the hearing aids are worn by the user, to evaluate measured output between the two conditions of interest.
The examination of Real Ear Measures concentrated on frequencies crucial for speech understanding, specifically within the range of 500 to 4,000 Hz. This analysis included octave and interoctave frequencies at 500, 750, 1,000, 1,500, 2,000, 3,000, and 4,000 Hz.
To calculate the mean absolute difference between the SELF-FIT and BP-FIT conditions, a series of pairwise measurement differences were first computed by subtracting each pair of corresponding REM responses for a given participant ear (e.g., the BP-FIT response for each participant, ear, input level, and frequency was subtracted from the SELF-FIT response for the corresponding matching participant, ear, input level, and frequency). The absolute value of these pairwise differences were averaged across all input levels (55, 65, and 75 dB SPL) and all frequencies between 500–4,000 Hz, yielding a mean absolute difference value for each participant ear.
Data collected from 20 participants (or 40 ears) were then analyzed for each site—at San Francisco (SF) and San Jose (SJ)—as well as an analysis of the 40 participants (or 80 ears) in the study population as a whole. Taking the study population together, we have found that the mean absolute difference (MAD), which was required to be 5 dB or less to pass the non-inferiority analysis, was 2.90 dB (± 1.43, 95% CI: [2.58, 3.21]) and is within the 5 dB margin of the acceptance criteria (p < 0.0001) (Figure 2A). These data confirm that the SELF-FIT is not inferior to the BP-FIT according to study criteria.

Figure 2. Overall outcomes for Real Ear Measures, QuickSIN, and APHAB. (A) Mean absolute differences (MAD) in hearing aid output as measured for SELF-FIT and BP-FIT conditions by Real Ear Measures (REM), with each point in the box plot representing the MAD for each ear fit in the study (N = 80). (B) Overall speech intelligibility results compared across two conditions: SELF-FIT and BP-FIT. Differences in SNR Benefit scores as measured by QuickSIN (N = 40 participants). (C) Overall derived global APHAB difference scores measured across two conditions: SELF-FIT and BP-FIT (N = 39 participants). Using these derived global difference scores, positive values indicate a relatively better outcome for the BP-FIT while negative values indicate a relatively better outcome for the SELF-FIT.
Examining this more closely, it is possible that individuals with different degrees of hearing loss may demonstrate different outcomes due to the nature of hearing aid programming with one type of coupler to the ear (in this case, a single vent dome). As such, severity of hearing loss was evaluated as a potential predicting factor of outcomes of interest, using groups defined by the WHO guidelines as is consistent throughout this report. It was observed that there were no statistically significant differences between groups with differing hearing loss severities (Figure 3A, Table 1; p = 0.4637).
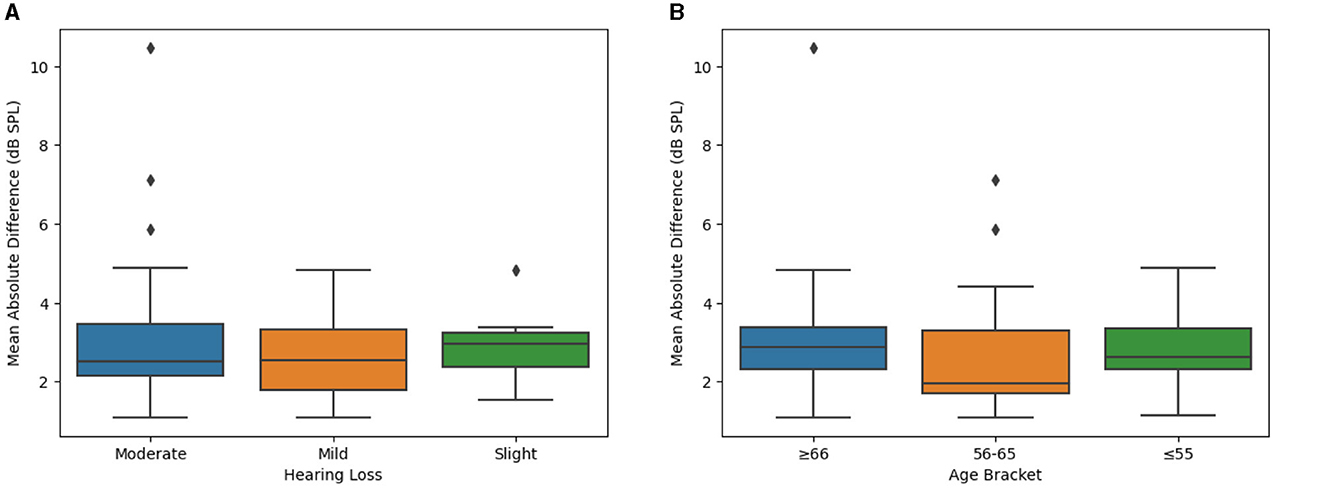
Figure 3. Difference between SELF- and BP-FIT for Real Ear Aided Responses when examining different hearing loss groups and age brackets. (A) Mean absolute difference in real ear aided response (by hearing loss). Using hearing loss groups as defined by the WHO, Real Ear Measures are compared using box plots to evaluate potential differences which may contribute to overall outcomes. (B) Mean absolute difference in real ear aided response (by age bracket). Real Ear Measures are compared across age brackets using box plots to evaluate potential significant differences which may contribute to overall outcomes.
Finally, age differences could contribute to an individual's relative success with the SELF-FIT due to a number of confounding factors, despite the applied inclusion and exclusion criteria (e.g., familiarity and comfort with computer or touch screen use, associated health issues, etc.). Accordingly, the primary endpoint was also analyzed to compare outcomes across age groups. It was observed that there were no statistically significant differences between age groups (Figure 3B, Table 1; p = 0.04072). Taken together, there were no significant differences in the outcomes of the primary endpoint (e.g., Real Ear Measures) due to clinical site, severity of hearing loss, or age group.
3.2 Behavioral measure of speech recognition
Participants (N = 40) completed a behavioral measure of speech recognition in noise as a secondary endpoint to compare the SELF-FIT and BP-FIT. Generalized speech-in-noise (SIN) recognition skills were assessed using the QuickSIN (Killion et al., 2004). Each participant completed two sets of QuickSIN lists for each condition. Using the final averaged QuickSIN scores for each condition, a singular difference score was computed by subtracting the SELF-FIT score from the BP-FIT score for each participant, indicating the disparity in QuickSIN scores between the two conditions.
These final difference values between the SELF-FIT and the BP-FIT were then averaged across participants at each site or across the full study population as appropriate. A difference of 2.7 dB SNR between two conditions is considered a critical difference (Mueller, 2016); therefore, the threshold for success when comparing the SELF-FIT and BP-FIT was set to 2.7 dB SNR. The mean difference in QuickSIN scores between the SELF-FIT and BP-FIT configurations was 0.15 dB SNR Loss (±1.97, 95% CI: [−0.48, 0.78]) and is within the 2.7 dB SNR Loss margin of the acceptance criteria (p < 0.0001) (Figure 2B). As seen in Figure 2B, use of the SELF-FIT was non-inferior to the BP-FIT when evaluating speech understanding in background noise.
Degree or severity of hearing loss could also contribute to different speech intelligibility outcomes when comparing the SELF-FIT and BP-FIT. Accordingly, severity of hearing loss was evaluated as a potential factor of the secondary endpoint, using groups defined by the WHO guidelines as is consistent throughout this report. It was observed that there were no statistically significant differences between groups with differing hearing loss severities (Figure 4A, Table 1; p = 0.4479).
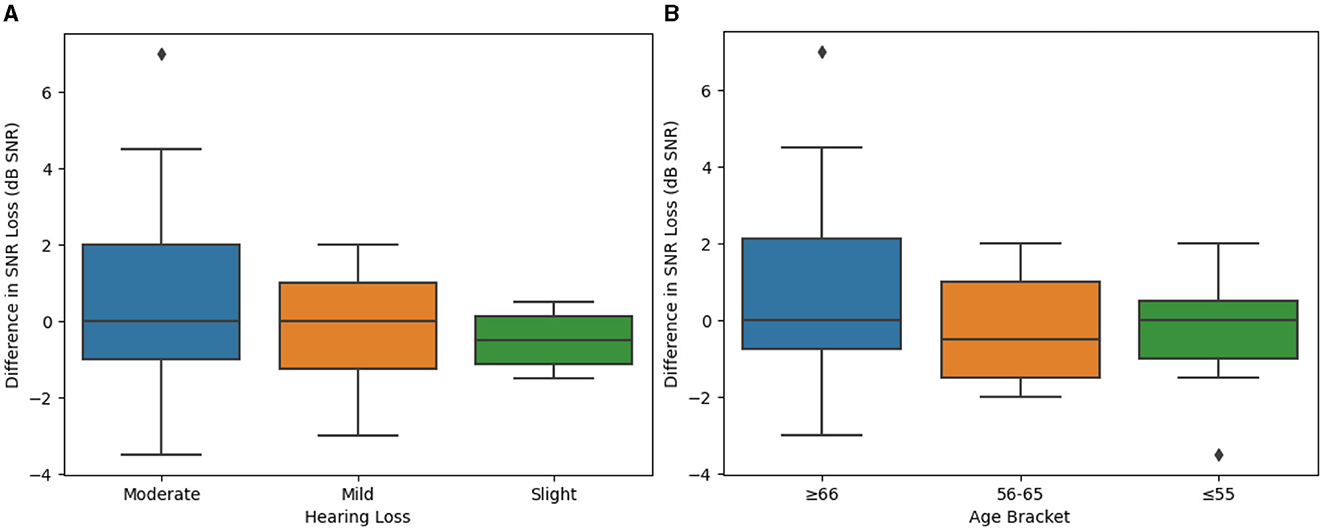
Figure 4. Difference between SELF- and BP-FIT for Speech in Noise responses when examining different hearing loss groups and age brackets. (A) Mean difference in speech intelligibility (by hearing loss). QuickSIN results grouped by degree of hearing loss (slight, mild, or moderate) are compared using box plots to evaluate potential significant differences which may contribute to overall outcomes. (B) Difference in speech intelligibility (by age bracket). QuickSIN results grouped by age bracket (55 years of age or younger, 56–65 years of age, or 66 years of age or older) are compared using box plots to evaluate potential significant differences which may contribute to overall outcomes.
Finally, age differences could contribute to an individual's relative success with the SELF-FIT. Accordingly, the secondary endpoint of speech intelligibility was also analyzed to compare outcomes across age groups. It was observed that there were no statistically significant differences between age groups (Figure 4B, Table 1; p = 0.2557).
Taken together, there were no significant differences in the outcomes of the secondary endpoint that evaluated behavioral measures of speech intelligibility due to clinical site, presentation order, degree of hearing loss, or age bracket.
3.3 Subjective report of perceived benefit
As previously described, the APHAB was used to collect subjective reports of perceived benefit in response to 24 prompt statements. For each prompt statement, participants (N = 39) select between an array of semi-quantitative categorical variables to approximate how often they experienced different problems while wearing hearing aids. These categories included the following: Always (99%), Almost Always (87%), Generally (75%), Half-the-time (50%), Occasionally (25%), Seldom (12%), or Never (1%). The traditional analysis approach was used to evaluate these reported scores for each completed APHAB survey; see Cox and Alexander (1995) for more details.
Each participant completed the APHAB twice: once after 2 weeks using the SELF-FIT hearing aid settings and once after 2 weeks using the BP-FIT hearing aid settings (note that approximately half of participants had the order of conditions used in the field reversed to counter-balance for potential order effects). These two conditions are then compared against each other to determine if the SELF-FIT yielded results that were non-inferior to the BP-FIT.
The APHAB yields a set of scores in a percentage of reported problems. Therefore, a lower percent value on the APHAB indicates fewer problems and greater perceived benefit as reported by the participant; meanwhile, raw APHAB scores with higher percent values would indicate a greater number of problems. These raw APHAB scores were then transformed into one comparison value for each participant, with overall box plot results plotted in Figure 4. Note that raw APHAB scores are not presented in this report; only transformed comparison values are presented in this report for direct comparison within participants.
In this comparison (see Figure 2C), the BP-FIT was subtracted from the SELF- FIT, which yielded a set of transformed APHAB scores that include positive and negative percent values, then averaged for each participant. For these transformed APHAB scores, a positive value indicates that the reported problems under the BP-FIT were fewer than the SELF-FIT; therefore, a positive value indicates a favorable outcome for the BP-FIT. Meanwhile, using these transformed APHAB scores, a negative value indicates that the reported problems under the BP-FIT were greater than the SELF-FIT; therefore, a negative value indicates a favorable outcome for the SELF-FIT.
When there is a difference of 10% or more favoring the same hearing aid when using the global score, these results can indicate with a high degree of certainty that there is a meaningful difference between two hearing aid conditions (Cox and Alexander, 1995). The presented non-inferiority analysis uses this same 10% difference threshold to compare the SELF-FIT and BP-FIT.
The mean difference in global APHAB scores between the SELF-FIT and BP-FIT configurations was −0.51% (±10.22, 95% CI: [−3.82, 2.81]) and is within the 10% margin of the acceptance criteria (p < 0.0001) (Figure 2C). As seen in Figure 2C, the overall perceived benefit of hearing aids in everyday listening environments was not significantly different when evaluating each participant's reported difference between the SELF-FIT and BP-FIT.
Of particular interest is the presentation order of the SELF-FIT and BP-FIT during field use evaluation of this secondary endpoint as it is possible that the user could experience acclimatization during the first 2 weeks using the hearing aids. Order of field use (whether an individual was assigned to the SELF-FIT condition or the BP-FIT condition for the first 2 weeks of use) was randomly assigned throughout the study to mitigate potential differences in this outcome. However, order effects were further evaluated here by regrouping the APHAB outcomes by individuals who completed the SELF-FIT field trial first and those who completed the BP-FIT field trial first. It was observed that there were no statistically significant differences between these two ordered groups (Figure 5, Table 1; p = 0.7448).
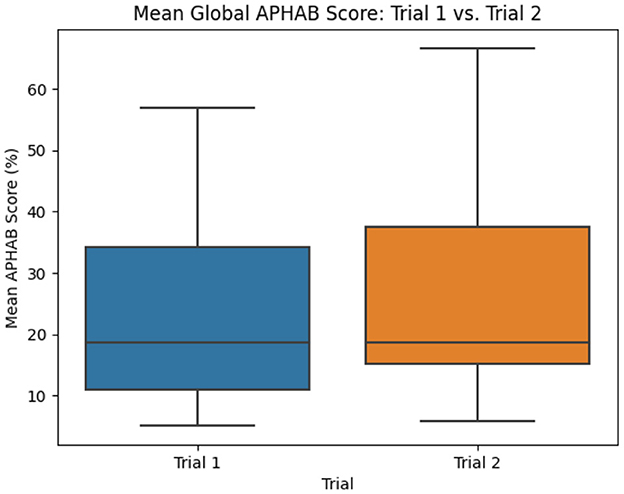
Figure 5. Mean difference in global APHAB scores (by Trial Order). APHAB results grouped by order of field use (SELF-FIT evaluated first or BP-FIT evaluated first) are compared using box plots to evaluate potential significant differences which may contribute to overall outcomes.
Degree or severity of hearing loss could also contribute to different perceived benefit outcomes when comparing the SELF-FIT and BP-FIT. Accordingly, severity of hearing loss was evaluated as a potential factor of this secondary endpoint that evaluates perceived benefit, using groups defined by the WHO guidelines as is consistent throughout this report. It was observed that there were no statistically significant differences between groups with differing hearing loss severities (Figure 6A, Table 1; p = 0.3810).
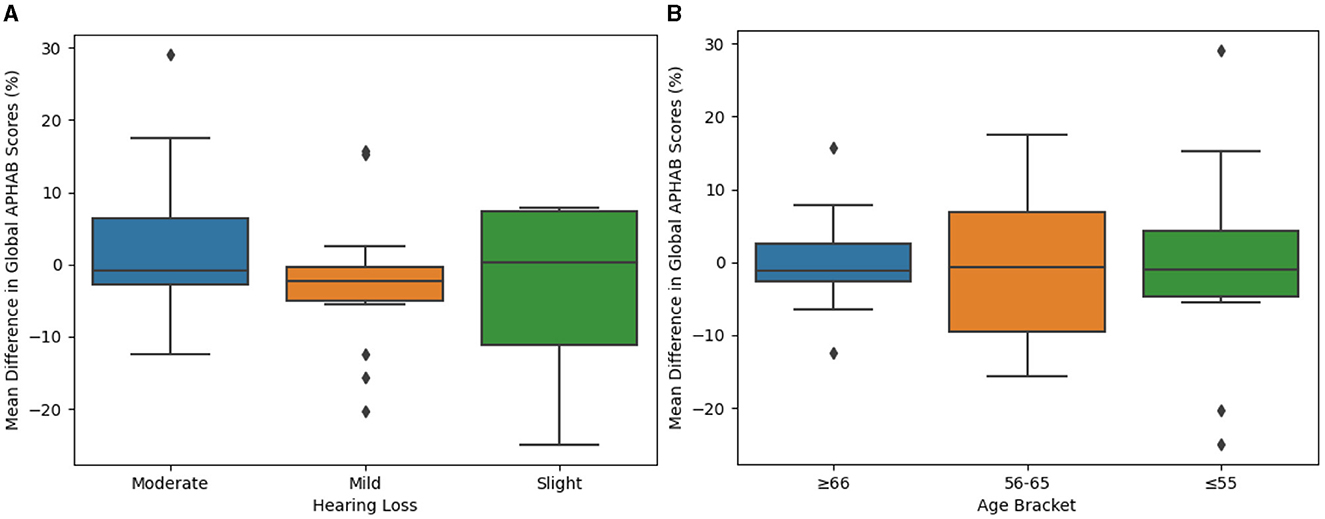
Figure 6. Difference between SELF- and BP-FIT for global APHAB scores when examining different hearing loss groups and age brackets. (A) Mean difference in global APHAB scores (by hearing loss). Global APHAB results grouped by degree of hearing loss (slight, mild, or moderate) are compared using box plots to evaluate potential significant differences which may contribute to overall outcomes. (B) Mean difference in global APHAB scores (by age bracket). Global APHAB results grouped by age bracket (55 years of age or younger, 56–65 years of age, or 66 years of age or older) are compared using box plots to evaluate potential significant differences which may contribute to overall outcomes.
Finally, age differences could contribute to an individual's perceived success with the SELF-FIT. Accordingly, the secondary endpoint of perceived benefit was also analyzed to compare outcomes across age groups. It was observed that there were no statistically significant differences between age groups (Figure 6B, Table 1; p = 0.9209).
Taken together, there were no significant differences in the outcomes of the secondary endpoint that evaluated perceived self-reported benefit due to clinical site, order of field use, degree of hearing loss, or age bracket.
4 Discussion
The findings from our study contribute significantly to the growing body of research on self-fitting hearing aid fitting procedures. The analysis of our primary endpoint, Real Ear Measures (REMs), aligns with recent studies suggesting that technological advancements in hearing aids, particularly in self-fitting algorithms, are increasingly capable of producing outcomes comparable to professional fittings (Sabin et al., 2020; De Sousa et al., 2023; Manchaiah et al., 2023; Maidment et al., 2024). Our results demonstrate that users completing the SELF-FIT procedure experience hearing aid output levels similar to those obtained via a BP-FIT. Access to SELF-FIT technology therefore can allow for greater user autonomy in their hearing aid fitting process (Sabin et al., 2020; Brice and Almond, 2022) without sacrificing an adequate fit when compared to a hearing aid fit using best practices.
In the realm of speech understanding in complex environments, as measured by QuickSIN, our findings echo the observations of Mackersie et al. (2020), Sabin et al. (2020), and De Sousa et al. (2023) who reported that user-oriented fittings could achieve results comparable to professionally fitted devices in challenging auditory situations. This is particularly relevant in the current landscape where ease of use and user adaptability are paramount in hearing aid technology (Convery et al., 2019; Maidment et al., 2024). Our study reinforces the notion that SELF-FIT procedures can offer non-inferior outcomes in speech recognition in noisy environments when compared to traditional BP-FIT methods.
The subjective experiences measured through the APHAB survey in our study are noteworthy, as our analysis indicates that the SELF-FIT procedure offers a level of benefit that users perceive as comparable to that provided by professional fittings. This aligns with the other findings which demonstrate comparable objective and behavioral outcomes between the two conditions.
Moreover, our study's comprehensive approach, including the assessment of hearing loss severity and age-related factors, adds to the nuanced understanding of hearing aid fittings. The lack of significant differences in outcomes due to these variables suggests a broad applicability of SELF-FIT procedures across a population of people with mild-to-moderate hearing loss (Oliver, 2017; Sabin et al., 2020; Blustein et al., 2022; De Sousa et al., 2023). This is consistent with the findings of Sabin et al. (2020) and De Sousa et al. (2023) as well as other recent studies that have also underscored the potential of self-fitting hearing aids to mitigate accessibility and customization disparities (Brice et al., 2023; Glista et al., 2023; Perez-Heydrich et al., 2023).
In conclusion, this study illuminates the advancing field of hearing aid technology, particularly the effectiveness of SELF-FIT procedures. These findings reveal that self-fitting hearing aids are not only comparable to professionally fitted ones in terms of clinical performance but also excel in offering user-friendly and adaptable solutions. This indicates a promising future for hearing aid technology, enhancing accessibility and potentially transforming the experiences of those with hearing impairments.
Looking ahead, further research should explore the differences in the perceived patient experience of these self-fitting service models with the best practices, audiologist-delivered model. It is crucial to understand how the availability of self-fitting, over-the-counter (OTC) hearing aids influences their adoption among the intended users. Greater access to well-functioning technology that improves an individual's quality of life may increase overall diversity through greater inclusion of less-frequently-represented demographics in the hearing aid population.
Further, OTC hearing aids may require ongoing support from audiologists or other hearing care professionals in order to ensure continued success with hearing aids (Perez-Heydrich et al., 2023; Swanepoel et al., 2023), for example beyond the 4 weeks evaluated in this study. Additional timely studies as well as public-facing educational materials will be needed to not only inform patients of the differences between professionally fit and OTC hearing aids, but also to help patients filter through the now-growing list of OTC options—all while continuing to provide the highest quality of care in all other aspects of the audiologist's scope of practice.
5 Limitations
While this study unveils significant findings, limitations must be acknowledged. A single device for both SELF-fitting and BP-fit procedures was utilized for this study; while this kept the study well-controlled, it potentially limited generalizability of these findings to other self-fitting devices. This is an important consideration as varied devices and fitting approaches may yield diverse outcomes, which warrants further research.
Moreover, the sample size for this study was insufficient to allow the subgroup analyses (e.g., age, hearing loss severity) to reveal their impact on self-fitting outcomes.
Additionally, data logging was not available for the study, and participants were required to rely on memory to assess their duration of use. Finally, outcomes were observed 2–3 weeks post-fitting; future research studies that are longer in duration would be required to elucidate longitudinal patient outcomes when using self-fitting technology. Considering physiological limitations—including presence of cerumen, collapsing ear canals, dexterity issues, structural abnormalities, and more—is crucial to understanding when this technology may or may not be successful among certain patient populations. This emphasizes the need for further exploration in self-fitting interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB board at San Jose State University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RL: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by an anonymous hearing aid manufacturer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2024.1397604/full#supplementary-material
References
ASHA (2022). American Speech Language and Hearing Association (ASHA). Hearing aids for adults. Available online at: https://www.asha.org/Practice-Portal/Professional-Issues/Hearing-Aids-for-Adults/ (accessed October 15, 2022).
Blustein, J., Weinstein, B. E., and Chodosh, J. (2022). Over-the-counter hearing aids: what will it mean for older Americans? J. Am. Geriatr. Soc. 70, 2115–2120. doi: 10.1111/jgs.17781
Brice, S., and Almond, H. (2022). Is teleaudiology achieving person-centered care: a review. Int. J. Environ. Res. Public Health 19:7436. doi: 10.3390/ijerph19127436
Brice, S., Saunders, E., and Edwards, B. (2023). Scoping review for a global hearing care framework: matching theory with practice. Semin. Hear. 44, 213–231. doi: 10.1055/s-0043-1769610
Convery, E., Keidser, G., Hickson, L., and Meyer, C. (2019). Factors associated with successful setup of a self-fitting hearing aid and the need for personalized support. Ear Hear. 40, 794–804. doi: 10.1097/AUD.0000000000000663
Cox, R. M., and Alexander, G. C. (1995). The abbreviated profile of hearing aid benefit. Ear Hear. 16, 176–186. doi: 10.1097/00003446-199504000-00005
De Sousa, K. C., Manchaiah, V., Moore, D. R., Graham, M. A., and Swanepoel, D. W. (2023). Effectiveness of an over-the-counter self-fitting hearing aid compared with an audiologist-fitted hearing aid: a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 149, 522–530. doi: 10.1001/jamaoto.2023.0376
Dornhoffer, J. R., Meyer, T. A., Dubno, J. R., and McRackan, T. R. (2020). Assessment of hearing aid benefit using patient-reported outcomes and audiologic measures. Audiol. Neurotol. 25, 215–223. doi: 10.1159/000506666
Food and Drug Administration (2022). BHA100 Series Braun® Clear™ Hearing Aid 510(k) Submission K212609. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf21/K212609.pdf (accessed March 7, 2024).
Glista, D., Schnittker, J. A., and Brice, S. (2023). The Modern hearing care landscape: toward the provision of personalized, dynamic, and adaptive care. Semin. Hear. 44, 261–273. doi: 10.1055/s-0043-1769621
Humes, L. E. (2019). The World Health Organization's hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int. J. Audiol. 58, 12–20. doi: 10.1080/14992027.2018.1518598
Killion, M. C., Niquette, P. A., Gudmundsen, G. I., Revit, L. J., and Banerjee, S. (2004). Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 116, 2395–2405. doi: 10.1121/1.1784440
Mackersie, C. L., Boothroyd, A., and Garudadri, H. (2020). Hearing aid self-adjustment: effects of formal speech-perception test and noise. Trends Hear. 24:2331216520930545. doi: 10.1177/2331216520930545
Maidment, D. W., Nakano, K., Bennett, R. J., Goodwin, M. V., and Ferguson, M. A. (2024). What's in a name? A systematic review and meta-analysis to assess the effectiveness of non-medical amplification devices in adults with mild and moderate hearing losses. Int. J. Audiol. 23:1–10. doi: 10.1080/14992027.2024.2321184
Manchaiah, V., Sharma, A., Rodrigo, H., Bailey, A., De Sousa, K. C., and Swanepoel, D. W. (2023). Hearing healthcare professionals' views about over-the-counter (OTC) hearing aids: analysis of retrospective survey data. Audiol. Res. 13, 185–195. doi: 10.3390/audiolres13020018
Mueller, H. G. (2016). “Signia expert series: Speech-in-noise testing for selection and fitting of hearing aids: worth the effort,” in Audiology, 18336.
National Institute on Deafness and Other Communication Disorders (2022). Congressional Justification FY 2022. Department of Health and Human Services, National Institutes of Health. Available online at: https://www.nidcd.nih.gov/sites/default/files/2021-06/nidcd-fy-2022-cj-508c.pdf (accessed March 7, 2024).
Oliver, A. (2017). Objective comparative analysis of self-fit personal sound amplification products (PSAPs) using three types of fitting protocols: out-of-the-box self-fit, advanced-user self-fit, and audiologist fit. Doctoral dissertation, Towson University.
Perez-Heydrich, C. A., Zenczak, C., Roque, L., Ryan, C., Agrawal, Y., and Sayyid, Z. N. (2023). The role of hearing professionals for over-the-counter hearing aids. Front. Audiol. Otol. 1:1167853. doi: 10.3389/fauot.2023.1167853
Sabin, A. T., Van Tasell, D. J., Rabinowitz, B., and Dhar, S. (2020). Validation of a self-fitting method for over-the-counter hearing aids. Trends Hear. 24:2331216519900589. doi: 10.1177/2331216519900589
Keywords: self-fitting algorithm, professionally fitted hearing aid, real-ear measurements, Quick Speech In Noise (QuickSIN), subjective assessment, mild-to-moderate hearing loss
Citation: Yellamsetty A and Lewis RM (2024) Evaluation of outcomes in a clinical trial: comparing self-fit hearing aids and hearing aids fit with best practices. Front. Audiol. Otol. 2:1397604. doi: 10.3389/fauot.2024.1397604
Received: 07 March 2024; Accepted: 20 June 2024;
Published: 12 July 2024.
Edited by:
Jorge Humberto Ferreira Martins, Polytechnic Institute of Porto, PortugalReviewed by:
Antonio Vasco Oliveira, Polytechnic Institute of Porto, PortugalDavid Tomé, Polytechnic of Porto (P.Porto), Portugal
Copyright © 2024 Yellamsetty and Lewis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anusha Yellamsetty, YW51c2hhLnllbGxhbXNldHR5QHNqc3UuZWR1
 Anusha Yellamsetty
Anusha Yellamsetty Rebecca M. Lewis
Rebecca M. Lewis