- 1Berman Institute of Bioethics, Johns Hopkins University, Baltimore, MD, United States
- 2de-bi, co., Greencastle, United States
- 3School of Medicine, Johns Hopkins University, Baltimore, MD, United States
- 4Carey Business School, Johns Hopkins University, Baltimore, MD, United States
- 5Binghamton University, Binghamton, NY, United States
- 6Black Woman Blockchain Council, Rockville, MD, United States
- 7Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, United States
- 8Department of Radiation Oncology, Mayo Clinic, Rochester, MN, United States
Introduction: Organoids are living, patient-derived tumor models that are revolutionizing precision medicine and drug development, however current privacy practices strip identifiers, thereby undermining ethics, efficiency, and effectiveness for patients and research enterprises alike. Decentralized biobanking “de-bi” applies non-fungible tokens (NFTs) to empower privacy-preserving specimen tracking and data sharing for networks of scientists, donors, and physicians. We design, develop, and demonstrate a functional de-bi platform for a real-world organoid biobank.
Methods: Ethnography of the organoid biobanking ecosystem was performed in 2022–2023, with site visits, interviews, focus groups, and structured observations of stakeholder interactions. An initial ERC-721 prototype was developed and tested, informing the design of a comprehensive NFT model. Web and mobile app prototypes were developed with a suite of ERC-1155 protocols representing ecosystem constituents as NFTs. We demonstrated the platform with publicly available Human Cancer Models Initiatives organoids to establish proof-of-concept for decentralized biobanking as the foundation of a democratized biomedical metaverse, or “biomediverse.”
Results: Scientists revealed key challenges for organoid research and development under policy, scientific, and economic constraints of the life science landscape. We advanced decentralized biobanking as a blockchain overlay network solution with potential to overcome barriers, enhance utility and unlock value by uniting collaborators in a privacy-preserving biomediverse. Dedicated smart contracts created “soulbound” NFTs as de-identified digital twins of patients, physicians, and scientists in a networked organoid ecosystem. We modeled biospecimen collection, processing, and distribution, including generation and expansion of organoids, via an auditable on-chain mechanism. Key features included the ability to bootstrap the digital twin NFT model onto an established organoid biobank, visibility of patient-linked biospecimens and related research activities for all ecosystem participants, as well as tooling for multisided data exchange. Implementing de-bi with ERC-1155 showed potential to minimize gas costs of on-chain activity vs ERC-721, though complementary layer-2 solutions will be essential for economic viability.
Conclusion: Decentralized biobanking has the potential to enhance efficiency, increase translational impact and drive research discovery through implementation of NFT digital twins for organoid research networks. Importantly, this approach also bolsters ethical practices by fostering inclusion, ensuring transparency, and enhancing accountability across the research ecosystem. Next steps include live pilot testing, market design research to align stakeholder incentives, and technical solutions to support a sustainable, scalable and mutually rewarding biomediverse.
1 Introduction
Human tissues and biofluids are essential raw materials for advancing precision medicine and drug development. However, current biobanking practices obtain prospective informed consent, then strip patient identifiers from specimens for privacy purposes, consequently disconnecting patients from the scientific progress, clinical insights, and ongoing value derived from their flesh and blood (Office for Civil Rights, U.S. Department of Health and Human Services, 2012). This process dehumanizes donors providing little to no visibility into how their contributions are being used and creating missed opportunities to share clinically relevant findings, financial compensation, or other benefits with the very individuals whose tissue, trust and generosity propels research forward (Gross et al., 2022). Meanwhile, this regulatory paradigm promulgates a fragmented biobanking ecosystem in which donors, specimens and scientists are siloed within and across institutions, with no streamlined mechanism to track and share bioassets or integrate real-time research activities relevant to specific individuals or cohorts (Simeon-Dubach et al., 2020).
In alignment with Weidener and Spreckelsen, 2024, we adopt their proposed definition of Decentralized Science: “Decentralized Science (DeSci) represents a collaborative and decentralized approach to science, leveraging technological and infrastructural advancements such as Distributed Ledger Technology (DLT), Web3, cryptocurrencies, and Decentralized Autonomous Organizations (DAOs) to enable permissionless, open, and inclusive participation, facilitating collective governance, equitable incentivization, unrestricted access, shared ownership, and transparent funding of the scientific process.”
(Weidener and Spreckelsen, 2024; Lesavre et al., 2021).1 We are advancing decentralized biobanking as a bioethics-informed platform that leverages nonfungible tokens (NFTs) to keep patients connected to their specimens and resulting derivatives, restoring provenance and enabling engagement, while preserving privacy. This mixed-methods study describes the design and development of a functional decentralized biobanking “de-bi” prototype as an NFT framework with potential to enhance ethics, efficiency and effectiveness of human cancer model research. Our application utilizes the ERC-1155 token standard and the NCI-funded Human Cancer Models Initiative to demonstrate assimilation of a decentralized approach for a real-world organoid research community.
2 Materials and methods
2.1 Next-generation biobanking context
Organoids are 3D, living cellular models derived from an individual patient’s tumor that may be grown, copied, shared, and used by scientists at multiple institutions over time.2 Patient-derived organoids provide a high-fidelity, long-lived and functional platform to study tissue physiology and pathology in remarkable detail. Stunning images of these human cancer models are captured in the process, showcasing their uniqueness and documenting treatment responses, as often featured in corresponding publications.
Since debuting in 2009, organoid technology has emerged as a gold standard for studying human disease, with dual applications for generalizable drug development and precision medicine for the individual donor (Guillen et al., 2022; Sachs et al., 2017). Dissemination of proprietary methods, as well as licensing and adaptation of tissue culture protocols has prompted a flourishing cottage industry of next-generation cancer model activity at international research institutes with necessary facilities, expertise, and access to patient tissues (Zhu et al., 2022). However, creating organoids is time consuming, resource intensive, and highly specialized labor, and thus largely occurs within dedicated model development centers, each of which cultivates proficiency in the idiosyncrasies of specific cancers and tissue types. Copies are then distributed for research use within and across institutions through collaborative research protocols or transfer agreements (MTAs), under a variety of academic and commercial business arrangements (Van Wichelen, 2023). The cost to produce or procure a single copy of a validated organoid model is several thousand USD, not including shipping and supplies.
We performed ethnography of a large, established institutional biobanking platform with a substantial breast cancer organoid collection at the intersection of a major U.S. hospital and research university. Site visits, interviews and in-depth discussions explored the affordances, constraints and operations of the U.S. organoid ecosystem (IRB00019273 - Non-Fungible Tokens for Ethical, Efficient and Effective Use of Biosamples). We engaged stakeholders within our local organoid pipeline, including all humans-in-the-loop that handle fresh patient tissues as they travel from the operating suite to the clinical pathology laboratory, through a biospecimen clearinghouse, an organoid development center, and ultimately to a scientist’s bench in affiliated research institutions (QRC 3958- Breast Cancer Supply Chain Analysis, Biobank Token Model Development, and Initial Pre-Pilot Testing with UPMC Patients). Complementary surveys, interviews and community engagement with breast cancer patients informed our understanding of specimen donors’ incentives and interests regarding organoids derived from their tissues (STUDY22010118 - Patient Views, Preferences and Engagement in Next-Generation Biobank Research; STUDY22020035 - Decentralized Biobanking “de-bi”: Exploring Patients Interests in Feedback, Education, Follow-up, Engagement and Tokens of Appreciation Regarding Biobank Donation via Mobile and Web Applications). Detailed methods and results of the human subjects research that informs this study are reported elsewhere (e.g., Hood et al., 2024).
2.2 Decentralized biobanking for patient-centered organoids
Decentralized biobanking is a novel conceptual and technical approach that combines bioethical first principles of autonomy, beneficence and justice with web3 tools to embed transparency, accountability, and inclusion in the biomedical research ecosystem (Gross and Miller, 2019). Core to our ethos is the imperative to build a biomedical metaverse or “biomediverse” that closes gaps in legacy research infrastructure stemming from outdated policies that utilize “de-identification,” removal of personal identifiers, as a strategy for protecting privacy while promoting learning (Far et al., 2023; Gross et al., 2022; Gross and Miller, 2021). Consequently, patients are disconnected from their donated biospecimen with little to no visibility into how their contributions are being used and restricting any opportunities for meaningful engagement in on-going research. Decentralized biobanking, “de-bi” for short, mints nonfungible tokens (NFTs) as de-identified digital twins of people, protocols and assets in a blockchain overlay network for real-world, physical biospecimen research activity, forging a democratized peer-to-peer platform for the biomediverse (Figure 1) (Sanchez et al., 2024; Hasan et al., 2023). By empowering donors as stakeholders in biospecimen research, we promote unprecedented donor inclusion by facilitating privacy-protected engagement between patients and scientists which wasn’t previously possible, enabling sustainable collaboration in shared discovery.
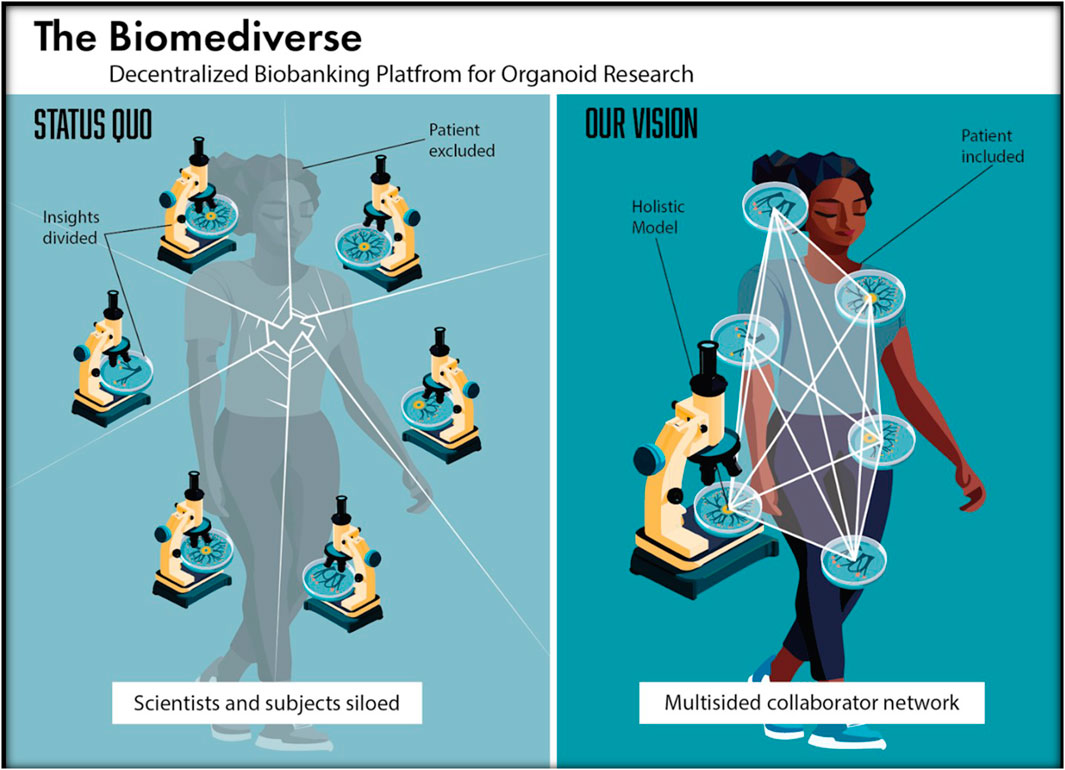
Figure 1. The Biomediverse: Status Quo vs Our Vision for Decentralized Biobanking for Organoid Research Networks. 1) Status Quo (left) illustrates how current policies that disconnect patients from research on their donated samples can create siloed datasets and disconnects between scientists researching the same individual, preventing collaboration across the fragmented ecosystem and hindering harmonization of multimodal data, which is critical for unlocking precision medicine and increasing translational impact on the individual, who is shown behind the broken glass; here, the patient is greyed out to show how she is not a primary consideration or active partner in the process; 2) Our Vision (right) illustrates the potential for networked collaboration platform that supports peer-to-peer connections between all nodes working on the same case-based specimens or organoid models; the patient is illuminated, indicating their recognition and involvement as a stakeholder in the human cancer model research ecosystem, in which she is a central figure.
Our foundational exploration of organoid biobanking highlighted challenges related to exclusion of patients after initial informed consent (Gross et al., 2021). Thus, we designed and developed an initial proof-of-concept prototype for decentralized biobanking that applied ERC-721 NFTs to empower patients to track donated specimens and engage with scientists via a privacy-preserving social network for science (Sanchez et al., 2024). The de-bi prototype encompassed activities from informed consent to specimen collection, organoid generation, distribution of copies for research, and ongoing patient engagement. Mobile app features for de-identified patients, physicians, and scientists were demonstrated with a synthetic dataset simulating the breast cancer organoid ecosystem.
2.3 Requirements gathering for organoid scientists
Decentralized biobanking is a multi-sided ecosystem. Successful empowerment of patients as stakeholders with programmable transparency and ongoing engagement in organoid research requires our solution to overcome barriers and meet needs of professional biobankers and scientists (Somiari and Somiari, 2015). A comparative overview of these barriers and the corresponding solutions from our proposed decentralized biobanking model is presented in Table 1. Design research methods explored incentives and challenges for those who generate and study organoids to inform technical implementation. To maximize feasibility of our decentralized approach, we sought to develop components that may be implemented for existing biobanks and research protocols, ensuring compatibility with established workflows, informed consent and de-identification practices (García et al., 2017).
We observed a series of virtual breast cancer organoid working group sessions where scientists from across the United States would meet monthly to identify common challenges and interests, share knowledge, protocols, and techniques, and seek to cultivate generalizable insights and desiderata for approaches to optimize efficiency and effectiveness within their niche. The most salient ethnographic findings were validated through discussions, interviews, prototype demonstrations and alignment exercises with subject matter experts. We also interviewed oncologists, surgeons, pathologists, biobankers, IRB members, institutional leadership and patient advocates from leading academic institutions, with particular emphasis on breast cancer use cases. Observational data, interviews, surveys, and focus groups were performed under multiple IRB protocols and a corresponding Quality Improvement protocol. Our concurrent research revealed that patients strongly favor receiving personalized research results, support public-private partnerships that expedite therapy development, and express interest in financial returns from research involving their tissue samples. Feedback from institutional stakeholders revealed a highly motivated field of subject matter experts who are passionate about advancing science for the sake of improving health and wellbeing overall, specifically addressing morbidity and mortality for cancer. Three key barriers emerged for the ethics, efficiency, and effectiveness of the established organoid research ecosystem which informed our approach. We found that scientists feel uneasy when not sharing information that patients would want to know, particularly when clinically actionable results emerged from their translational research. Knowledge sharing about successful and unsuccessful methodologies was also limited among investigators, leading to unnecessary duplication of expensive, time-consuming experiments and negatively impacting operational efficiency. The effectiveness of research aimed at driving discovery and precision medicine was often hindered by insufficient clinical annotation of specimens across longer time periods as well as the lack of viable, compatible specimens for continued experimentation with established organoids. A notable example is that many scientists identified the desire to co-culture organoids with peripheral blood mononuclear cells (PBMCs) as especially valuable, to replicate native immunologic conditions in the organoid model.
To demonstrate applicability under the current biobank regulatory regime, we developed our prototype with publicly available organoids, for which there is a community of users that may be yet unknown to one another. We designed and developed our de-bi prototype with data and images from the Human Cancer Models Initiative (HCMI) an active biobanking platform which distributes patient-derived organoids to academic and industry users around the world.
2.4 Real-world organoid biobank setting
Launched in 2016, the Human Cancer Models Initiative (HCMI) is an international collaboration devoted to the mission of creating a collection of 1,000 unique, next-generation human tissue–derived tumor models. The models have stringent quality controls and extensive associated clinical data, molecular profiles, and genomic annotation, all harmonized and accessible through NCI’s Genomic Data Commons.3 The HCMI prioritizes generation of models from underrepresented patient populations, rare tumor types, and for cancers which lack precision therapy, emphasizing the imperative for updated, high-fidelity models as essential to improving survival (Tonsing-Carter et al., 2023).
The HCMI consortium includes the National Cancer Institute (NCI), Cancer Research UK (CRUK), Hubrecht Organoid Technology (HUB), and Wellcome Sanger Institute (WSI), the Broad Institute and the Cold Spring Harbor Laboratory. These sites pool resources to create a robust organoid biobank as a “community resource” for cancer research, available to any user, through a combination of non-profit funding, donated patient specimens and clinical data, public research institution facilities, deep disciplinary expertise, advanced genomic sequencing technologies, and dedicated information systems for data storage and analysis.4 Partnership with a designated non-profit commercial entity, ATCC, enables HCMI organoids to be manufactured, preserved, and distributed for academic and commercial use in basic research, target screening, and drug development. Figure 2 maps the HCMI supply chain, with three institutional layers of abstraction between the donor’s bedside and the scientist’s bench.
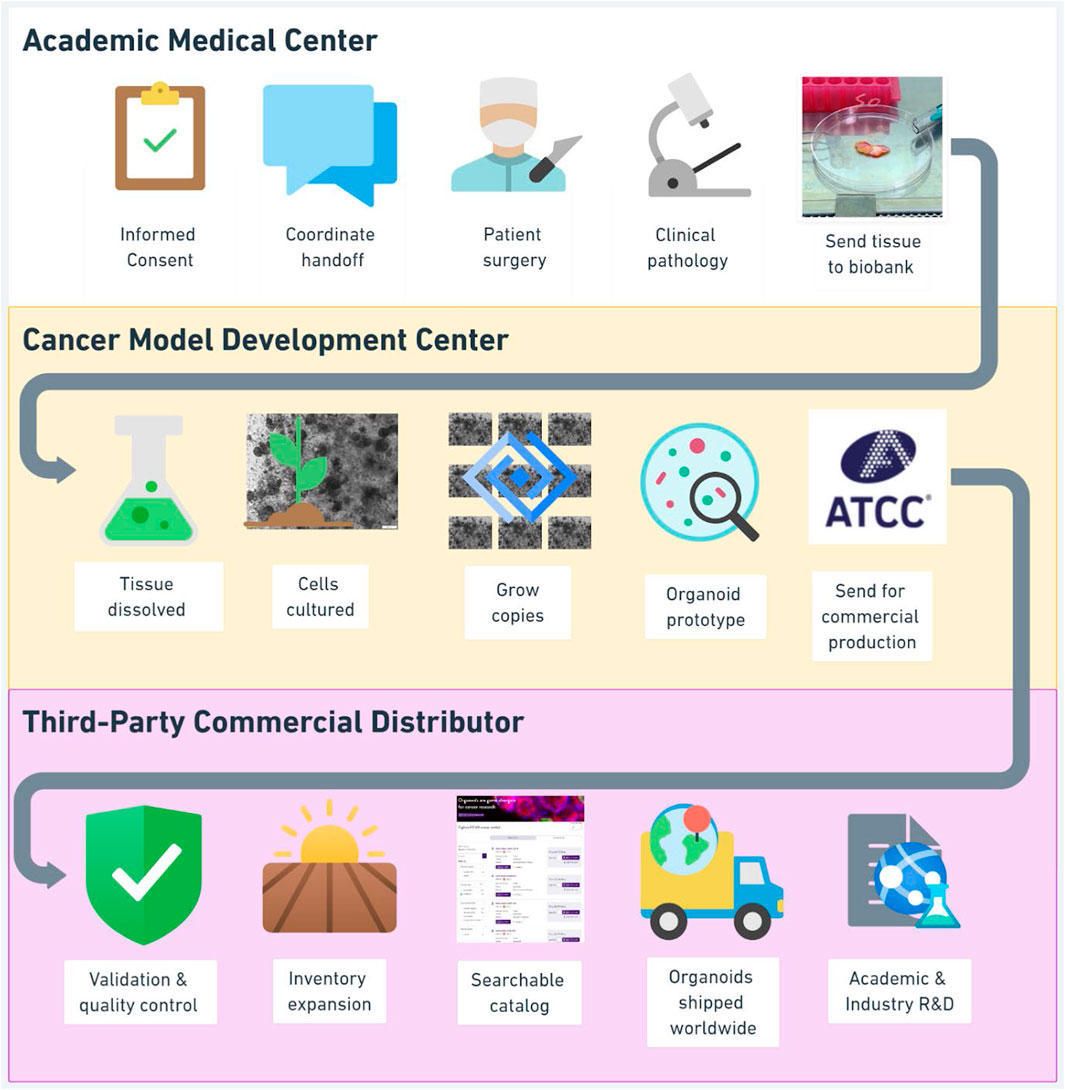
Figure 2. Human cancer model initiative organoid supply chain. 1) Academic Medical Center, the setting of clinical care, informed consent and biospecimen procurement; 2) Cancer Model Development Center, one of four highly specialized affiliated non-profit research institutions where patient derived organoids are initially produced; 3) Third-Party Commercial Distributor (American Type Culture Collection), where established organoids are validated, expanded, and distributed to academic and commercial customers.
Data for our proof-of-concept prototype was accessed through public NCI and ATCC websites. To validate and refine our assumptions, we interviewed principal investigators from 3 of the 4 NCI-supported HCMI sites, ATCC representatives, as well as competing commercial and academic organoid suppliers, biotechnology companies developing human cancer models, investors involved in or evaluating the organoid market, and over a dozen senior, mid, and junior-level scientists who obtain organoids for their research from outside institutions. To complete our realistic data model, we sought to include a corresponding biobank inventory of patient-linked tissue and biofluids that would typically complement an organoid biobank. We used sample data from the Breast Disease Research Repository (BDRR) at the Pitt Biospecimen Core, which feeds into the local organoid biobanking platform we were studying to case-match HCMI donor patients to representative specimens that had been collected from organoid donors.
2.5 Decentralized biobanking system design
Decentralization, Incentivization, Innovation and Advancement, and Collective Ownership have emerged as Guiding Principles of DeSci, described as actionable guidelines for designing practical implementation of our solution (Weidener and Spreckelsen). Decentralization focuses on establishing a privacy preserving overlay network atop the existing organoid research ecosystem that maps relationships between donors, samples, and institutional stakeholders to create pathways for meaningful knowledge exchange and collaboration. The data exchanged through these networks between donors and institutional stakeholders are mutually beneficial, naturally incentivizing reciprocation by each party. The patient-enriched multi-modal datasets, in addition to creating opportunities for serial sample donation, accelerate innovation and advancement of organoid development and precision medicine research. Finally, we can envision that collective ownership be applied to all contributors if any resulting discoveries have commercial applications and yield financial rewards, and the established channels which enabled collaboration may also be integral in disseminating those rewards.
2.5.1 Blockchain selection
DeSci applications utilize various layer 1 solutions, most notably Ethereum, Solana, Polkadot, and Avalanche. We chose to develop this de-bi prototype on the most popular public and permissionless Ethereum Network, in accordance with NFT protocol features and ethos optimized for decentralized finance, democratic shared governance, and pro-social collaboration.5 These core aspects make Ethereum ideal for uniting a global scientific community that transcends national and institutional barriers, and which provides a robust, well-established, and resilient network (Israni and Shah, 2023).
While Ethereum lacks the speed and scalability of the alternative layer 1 solutions, the use case of decentralized biobanking emphasizes low volume, high impact transactions of ecosystem participants and physical bioassets (e.g., tissues, blood), for which the utmost trust, certainty and reliability is required. We anticipate that full-scale implementation of decentralized biobanking will require the addition of layer 2 solutions, which we prioritize for further research and development.
Of note, most blockchain prototypes for healthcare and biobanking applications make use of private or permissioned blockchains such as Hyperledger Fabric, due to sensitivity of healthcare data and established benefits for enterprises seeking to maximize control and security of on-chain activity (Kimura et al., 2024; Ncube et al., 2020). Our approach is able to depart from this strategy for two principal reasons. First, we implement our solution for publicly available organoids and datasets that have been de-identified, and therefore meet the criteria for unrestricted sharing outside the purview of HIPAA and the IRB oversight mandates of the Common Rule (Maloy and Bass, 2020). Second, we allow that the specimens, organoids, and keys connecting de-identified specimens to respective individuals are maintained off-chain, necessarily as three-dimensional matter for the bioassets, and functionally, as already in place for the respective academic medical centers and cancer model development centers (Figure 2) involved in our HCMI use case (Mell and Yaga, 2024).6
2.5.2 Nonfungible token standard selection
When de-bi was initially conceived in 2021, ERC-721 was the gold-standard for NFTs, with mass adoption throughout the explosion in popularity of tokenized digital art and collectibles (Gupta et al., 2024; Arora et al., 2021).7 However, implementation in the initial de-bi prototype highlighted limitations, as the ERC-721 standard only supports one token collection within a single contract, requiring multiple smart contract deployments for complex ecosystems like de-bi, which necessarily involves multiple token collections with unique properties. In this setting, an ERC-721 approach requires redundant bytecode on Ethereum and imposes limitations on aspects of desired functionality for a comprehensive biobanking model.
To address these challenges, the ERC-1155 Multi-Token introduced flexibility to implement multiple collections of fungible, semi-fungible, and non-fungible tokens with a single smart contract (Radomski et al., 2018).8 The ability to batch mint tokens was especially appealing as it appeared to offer an essential enablement for our application of the de-bi approach to established biospecimen and organoid collections, with many assets needing to be registered retrospectively upon onboarding. Additionally, the standard enabled innovative mechanisms to represent rights over a specific asset through strategies like fractional ownership, which may prove useful for accurately representing multi-stakeholder interests in complex assets, like organoids, which are generated and deployed via cross-institutional collaborations (Mell and Yaga, 2024). Alternatively, a composable NFT standard, ERC-998, which was advanced to extend the ERC-721 functionality, enables NFTs to own other fungible and nonfungible tokens.9 Despite potential benefits from a taxonomic standpoint, we chose to apply the ERC-1155 standard for this prototype to prioritize streamlining and minimizing costs of implementation given baseline fiscal sustainability challenges for established biobanks.
2.5.3 Technology stack
The following open-source tools, frameworks, and standardized protocols to develop our decentralized application prototype.
• Solidity: Programming language used for writing the Ethereum smart contract that manages public data shared across all users and agents
• NodeJS: open-source, cross-platform JavaScript runtime environment used with Hardhat to initially deploy Smart Contracts
• Hardhat: Platform for writing, compiling, debugging, and deploying Smart Contracts
• Infura Hosted Node: An Ethereum node as a service which is hosted by Infura that enables blockchain transactions
• VueJS: An approachable, performant and versatile JavaScript framework for building web user interface
• Laravel 9: PHP backend which manages the application’s business logic through connections with external API’s and transactions sent to the deployed smart contracts via web3 packages and an Infura Hosted Node.
• MySQL: Relational database management system (RDBMS) which represents institutional bioasset data from HCMI and BDRR datasets.
• Firestore DB: NoSQL document database used to store in app activity and data uploaded by scientists
2.5.4 Token classification
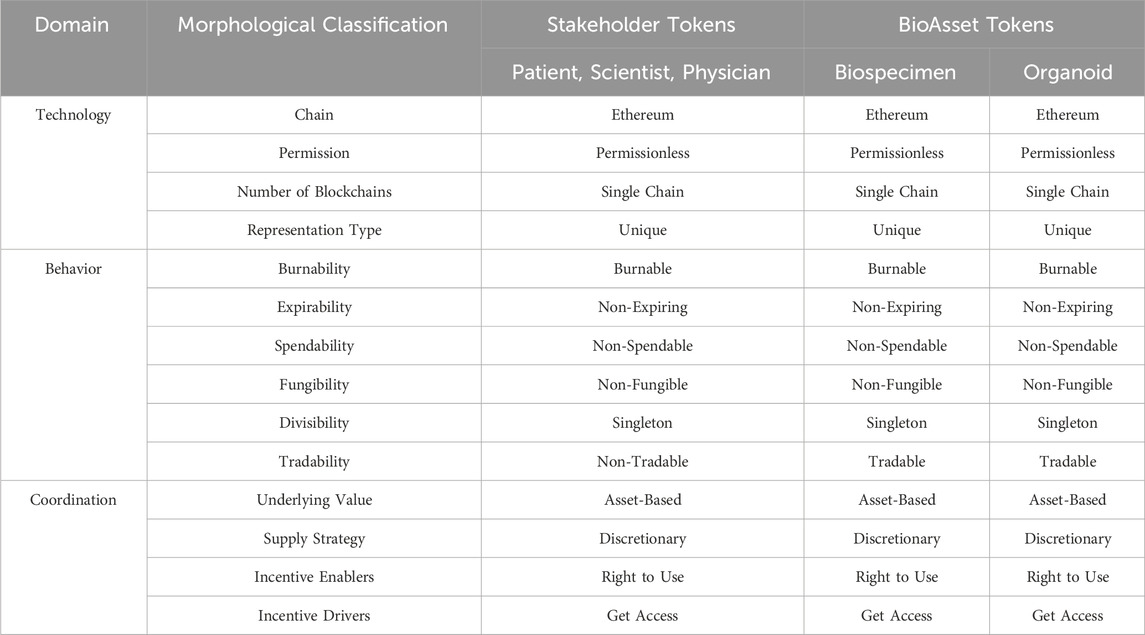
To refine the decentralized biobanking model for the organoid ecosystem, we describe our tokens using the Morphological Token Classification Framework (Freni et al., 2022). Created to ensure completeness, consistency, and adequate comparability, this framework is characterized by 14 dimensions, grouped into 3 domains, and nearly 5 million possible combinations. The NFT digital twin framework developed incorporates two classes of tokens: Stakeholder Tokens, representing patients, scientists, and physicians, and Bioasset Tokens, representing biospecimens, such as tissue, blood, and urine, as well as multi-generational organoid derivatives (Appendix 1).
2.6 System architecture
Four core assumptions enabled creation of our model NFT digital twin framework and system architecture.
1. Every user has a wallet that can store digital Assets from Ethereum through Metamask as a third-party provider (Lesavre et al., 2021).
2. Biobank protocol is represented by an authorized account with approved funds to cover related on-chain transactions.
3. Biobank account deploys Patient Contract, Scientist Contract, Physician Contract and SamplesOrganoids Contract as the owner.
4. Metamask wallet accounts have private keys which are securely stored as environment variables, which can be accessed by our back-end application to automatically sign transactions on behalf of the institutional biobank account through pre-programmed logic.10
Figure 3 demonstrates the system architecture, which enabled integration across three key domains: 1) Real-world off-chain context; 2) Service Application, and 3) NFT digital twin framework. The off-chain component included web and mobile user-facing client applications for patients and scientists (shown), as well as biobankers and physicians (not shown), facilitated by a MetaMask wallet interface. MySQL databases were used to simulate direct access to the HCMI organoid biobank and corresponding physical and digital biospecimen repositories. The service application utilized a NodeJS runtime environment coupled with hardhat for deploying initial Smart Contracts, a Laravel backend that processes blockchain-related service requests by sending a transaction to an Infura-hosted node, which broadcasts transactions to the remaining nodes in the system, and a cloud-hosted Firebase Database and API for storing all off-chain data, such as user records and in-app activity logs.
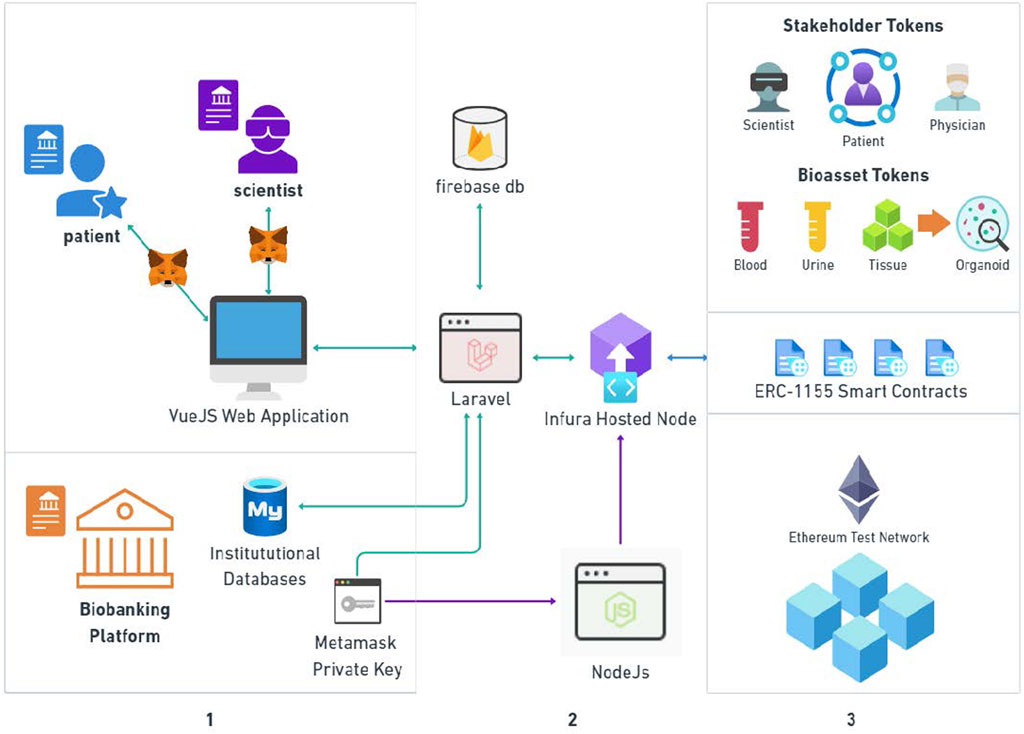
Figure 3. Decentralized biobanking for human cancer models initiative: System architecture diagram. This diagram illustrates three domains integrated within our solution architecture: 1) Real-world: off-chain context, including web-based user-facing applications with MetaMask wallet interface for patients and scientists (shown), and biobankers and physicians (not shown), plus MySQL databases simulating direct access to the HCMI biobank; 2) Service Application: A NodeJS runtime environment coupled with hardhat for deploying initial Smart Contracts, a Laravel backend that processes blockchain-related service requests by sending a transaction to an Infura-hosted node, which broadcasts transactions to the remaining nodes in the system, and a cloud-hosted Firebase Database and API for storing all off-chain data, such as user records and in-app activity logs; and, 3)NFT digital twin framework: NFTs representing stakeholders and specimens, respective ERC 1155 smart contracts, hosted on Rinkeby and Sepolia Ethereum test networks.
On-chain elements included NFTs representing all stakeholders and specimens, respective ERC-1155 smart contracts, hosted on the Ethereum Rinkeby and Sepolia test networks. Our implementation included four smart contracts, one for each stakeholder class and a comprehensive contract for biospecimen lifecycle management Activities enabled by each smart contract are illustrated in Table 2, including the minting and status confirmation of stakeholder tokens, as well as biospecimen to organoid lifecycle transactions.
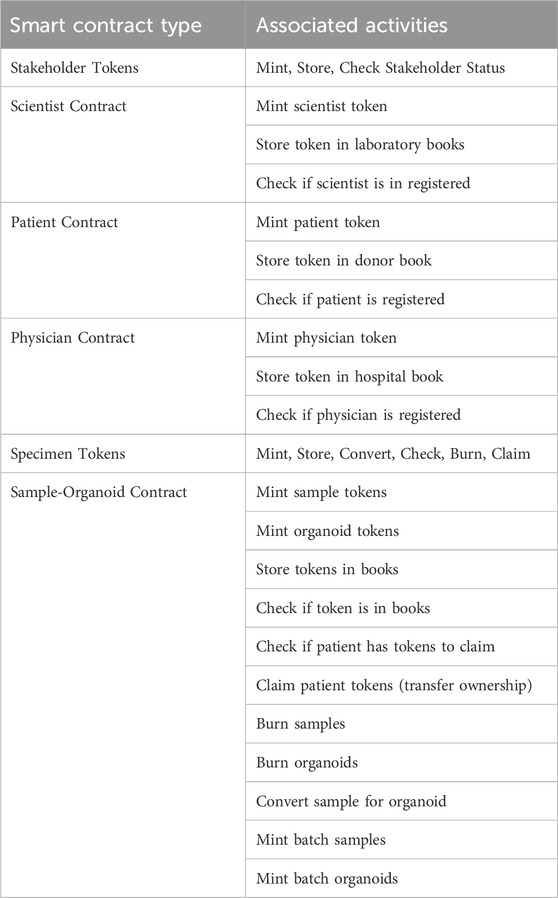
Table 2. Decentralized biobanking for NFT digital twins organoid ecosystem: Smart contracts and associated activities.
2.7 Feasibility analysis
Feasibility was measured by assessing system features with requirements identified in preliminary stakeholder interviews, alignment of the NFT framework properties against designed token classifications, preliminary cost analysis, as well as comparing the expected vs actual results of smart contract functionality. The prototype demonstration deployed smart contracts to the Rinkeby test network in 2022. As Rinkeby was subsequently deprecated, feasibility analysis was performed by deploying and verifying prototype functionality and efficiency via hardhat to the Sepolia Ethereum test network.
Evaluation of costs associated with deploying the ERC-1155 smart contracts was measured against deployment of ERC-721 smart contracts required to perform similar network actions. Transaction costs of both standards for smart contract deployment, mints, and transfers were recorded, analyzing across a range of scenarios pertinent to our use case. Because ERC-721 does not natively support batch transfers, we sent multiple transactions for token minting and transfers, then tallied up the total transaction costs to compare against the unique functions in the ERC-1155. As gas cost variability is significant, especially on a test network, we calculated transaction costs using a standard 25 gwei unit cost and actual # gas units used (Zarir et al., 2021).
Finally, we survey key areas for further research and development that will be required to advance a functional solution for real-world organoid biobanks and related research communities.
3 Results
3.1 Decentralized biobanking conceptual model
Figure 1 illustrates the consequences of current organoid supply chains and downstream research activities, and the role for our proposed solution. The image shows how copies of an organoid generated from a single individual may be simultaneously studied by multiple laboratories. However, each investigator’s view is a “Sybil,” an incomplete living model of the patient’s experience (Ali et al., 2023). These functional collaborators each receive de-identified physical and digital copies of the personalized model, but do not know about one another or have a way to share insights in real-time (Figure 1, left). Collective person-level learnings are fragmented, hindering the potential for a comprehensive human cancer model that accurately reflects a continuously evolving shared state of truth. The potential precision medicine utility is also forsaken by the status quo, as scientists have no way to communicate findings that may be actionable with the patient whose model they are studying.
Critically, leaving dissemination of findings to the peer-reviewed literature is neither timely nor adequate for clinical or scientific purposes, as these distributed research activities unfold in parallel over several years. If work is published, the individual who could have benefitted may have already died and countless hours and resources may have been misspent trying the same ineffective strategies. To solve this dilemma, we proposed the concept of a “biomediverse,” or biomedical metaverse, as a privacy-preserving collaboration platform for dynamic, real-time data sharing between various scientists studying the same organoids, biobanks collecting specimens, as well as the respective patient and physicians. The right side of Figure 1 illustrates how the various nodes may be connected in a synergistic NFT digital twin framework, yielding a comprehensive network that accounts for the research activities at all end user sites, and considers the patient as a stakeholder in the process.
3.1.1 Data model
The functional de-bi application was developed with a subset of HCMI organoids for which there were two or more models per patient, e.g., HCM-CSHL-0247-C18-A (ATCC® PDM-256™) and HCM-CSHL-0247-C18-B (ATCC® PDM-277™).11 This allowed us to illustrate a system for connecting scientists studying the same organoid, as well as those studying “sister” organoid models that “share a donor.” De-identified frozen specimens from local organoid biobank donors were case-matched from the BDRR population to simulate a comprehensive patient-linked bioasset model. Table 3 demonstrates the real-world patients, biospecimens, and organoids represented in our NFT digital twin model for the de-bi prototype.
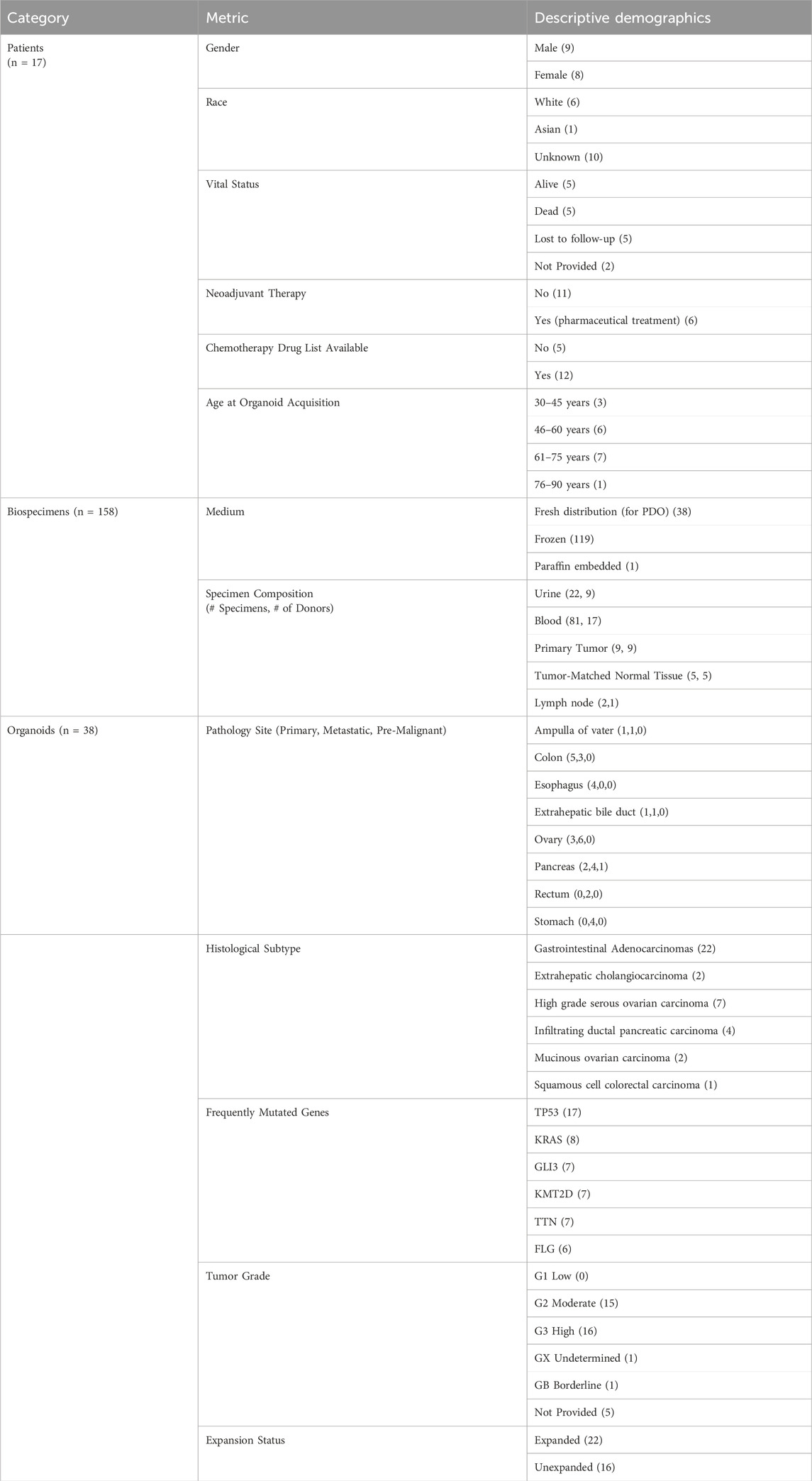
Table 3. Descriptive demographics of patients, biospecimens and organoids in decentralized biobanking model ecosystem.
3.2 Solution objectives and requirements
Our prototype sought to facilitate transparent sharing of organoids, validation of experiments across institutions, and a pathway that would enable the patient, their family and physicians to have access to data from all labs studying their specimens. As the direct link between patients, as biospecimen donors, and scientists, as users of biospecimens and their derivatives, our prototype was designed with biobanks as the nucleus for the biospecimen research ecosystem, serving as the site of organoid generation and as a settlor account that manages most of the activities for the decentralized exchange.
We make biospecimen collection, processing, and distribution transparent for all stakeholders, with a visible map of specimens and organoids derived from a unique individual patient and of all scientists studying that person. The advanced decentralized biobanking solution had three principal components: 1) NFT digital twin ecosystem upgrade; 2) Biospecimen lifecycle management functions, and; 3) Research collaboration tools. Table 4 outlines each technical component, relevant product requirements and implementation details.
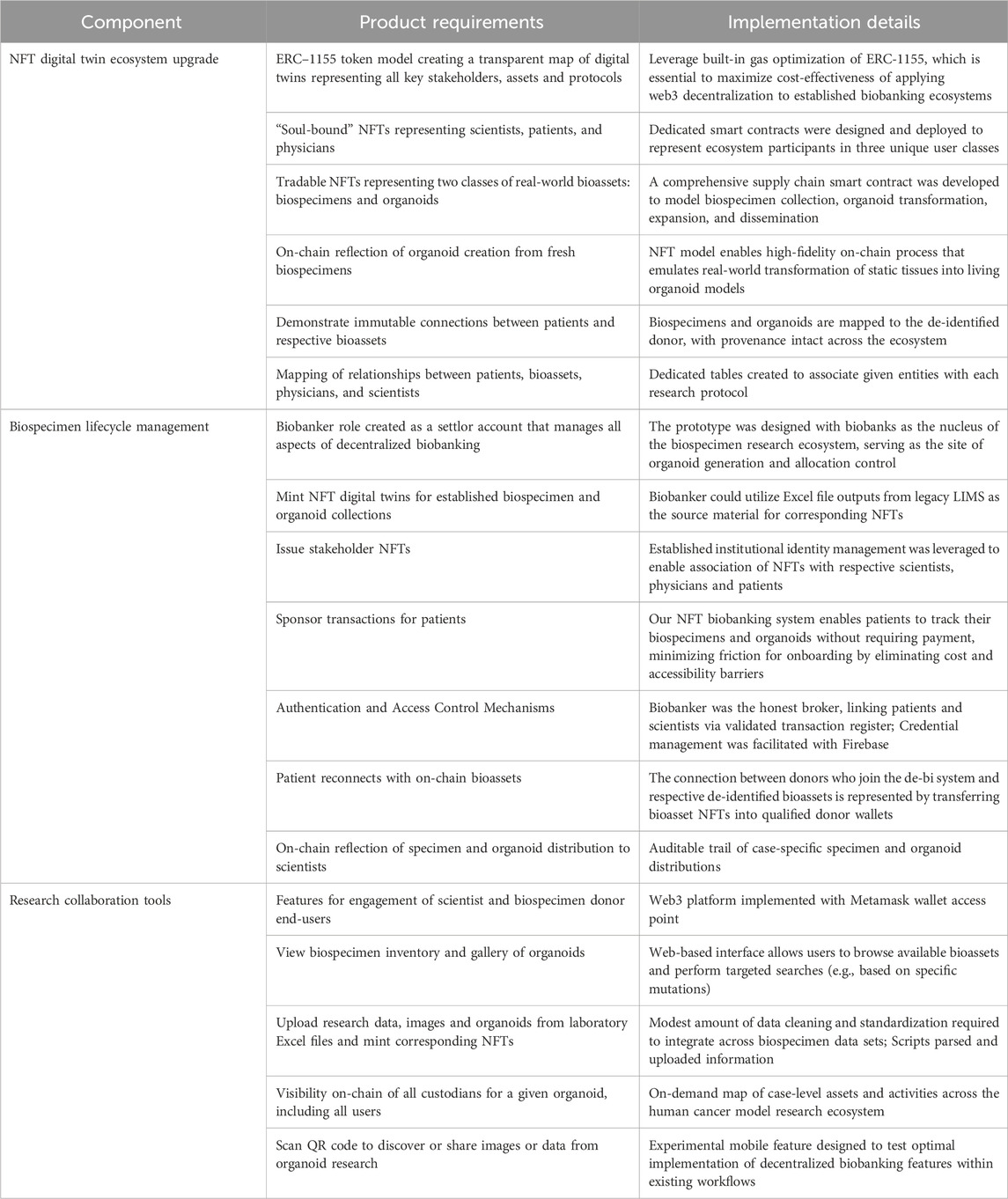
Table 4. Decentralized biobanking prototype for NFT organoid ecosystem: Technical components, product requirements, and implementation details.
3.2.1 NFT digital twin ecosystem
We improved upon the decentralized biobanking NFT framework by using ERC-1155 to create a digital twin map of all stakeholders, assets and relationships in the real-world organoid biobank. Our upgraded system deployed tokens representing scientists, patients and physicians as Soul Bound NFTs (SBTs) (Weyl et al., 2022). A stand-alone SBT is a token that is non-fungible and non-transferable, bound to a single user account that represents their decentralized identity, signifying the unique nature of individual identities within the biomediverse. Here, we enforced this restriction by modifying the logic within our smart contracts. OpenZeppelin enables this process by providing hooks, which “are simply functions that are called before or after some action takes place. They provide a centralized point to hook into and extend the original behavior.”13 We modify the_beforeTokenTransfer () hook to restrict transfers outside of minting and burning tokens.
By contrast, bioasset tokens representing donated specimens and organoid derivatives are implemented as transferrable NFTs, consistent with the ability to relocate these assets to different stewards and users, while simultaneously retaining immutable provenance from a specific de-identified donor. Critical to this aspect of the digital twin framework was the need to model collection of tissues and transformation of these finite assets into replicable living organoid models. This proved challenging to implement, as we attempted multiple avenues to converting an organoid derivative into a “parent” organoid entity with multiple nonfungible “daughter” copies. Serialization was an important component of the copies, which are created in a series of passages, with unique 3D properties that may have irreducible variation. Importantly, the organoid validation and expansion process implemented at ATCC for the commercial scale organoid products is designed to eliminate this variability as a key component of uniform productization. Thus we adopted a model in which we were able to indicate successive organoid generations, though NFTs representing individual copies within each generation were semi-fungible as executed in this prototype.
3.2.2 Biospecimen lifecycle management
3.2.2.1 Biospecimen creation
Institutional biobanks may or may not have an established Laboratory Information Management Systems (LIMS) Software for specimen collection, processing, storage and information management. Some biobanks use open source tools such as OpenSpecimen, proprietary in-house developed solutions, such as Biospecimen Inventory and Operations System (BIOS), while others even within the same institution may rely on excel spreadsheets and manual data entry. Our implementation considers these manual workflows revealed by stakeholder engagement and aims to ease the adoption of our platform by offering an intuitive Excel file upload mechanism, which triggers the automated process of minting digital twin NFTs representing the physical specimens on a given biobanks’ ledger, with adaptations for those under custody vs those previously distributed.
Biobank receives samples → creates records in an excel file → signs into our web application, uploads the file → and batch mints tokens for all samples by automatically calling SC function mintSampleBatch (). The biobank that initially mints these tokens is established as the “owner” of the tokens until claimed by respective donors. The term owner is a standard designation on non-fungible token smart contracts to display the wallet address which currently has custody of the NFT.
3.2.2.2 Issuing stakeholder tokens
As these tokens act as verifiable credentials, users must be validated to establish a linkage with their Ethereum Account in order to receive their identifying NFT. This verification will typically occur via each user’s respective institutions as trusted entities.
For researchers, this verification occurs internally as this token is representative of their status as an investigator for an IRB approved research study. The biobank account creates and issues a Researcher NFT via the mintResearcherToken () function. For patients, this identity verification will occur offline by comparing patient provided information against existing consented donors. A Patient NFT represents a donor’s participant in the biobank protocol, which is minted by the biobank via the mintPatientToken () function.
After successful token distribution, each user’s account address and NFT ID will be securely stored within an institutional database and linked to relevant records. The gas and fees required to complete these transactions will be sponsored by the biobank, typically with grant funds awarded for their IRB approved biobank protocol. Our intention is that patients should not incur any costs related to on-chain transactions, but biobanks may choose to pass these costs along to commercial and/or academic scientists during the commercial exchange of samples acquired for research.
3.2.2.3 Authentication and access control
Our web application uses an open source protocol called WalletConnect which provides Software Development Kits (SDKs) for enabling users to connect their wallets (i.e., Metamask) to decentralized applications. Connecting a Metamask wallet is the mechanism in which users sign-in to the platform. To authenticate the connected account, our application makes a read-only call to retrieve the mapping of all stakeholder account addresses that possess Patient or Researcher NFTs. The user will only be successfully signed in If the connected wallet possesses the appropriate token, otherwise they will receive an Access Denied error.
3.2.2.4 Patient claims sample token
If authenticated patients have samples that are currently stored within the biobank, or have been previously distributed for research through the banking protocol, then they will be invited to claim their bioasset tokens. This can be done through the “Claim Ownership” button displayed next to a list of the unclaimed bioasset tokens associated with the patient’s account address. When clicked, the application sends a signed transaction using the biobank account private keys to call the patientClaimSamples () smart contract function.
Unlike the Patient or Researcher NFTs associated directly with a user’s role inside of approved protocols, the tokens representing donated biospecimens and the derivative organoids are transferable. So upon successful completion, the biobank will have transferred all of the bioasset tokens to the patient’s account and update the mappings to list the biobank account address as the “manager” and transferring the “owner” designation to the patient account address.
3.2.2.5 Sample consumption and organoid creation
When the biobank distributes the physical samples to an investigator for research, the manager property of the Sample NFTs is updated with the researcher’s account address. This is the mechanism that expands the on-chain connection to the patient whose wallet holds the digital twin NFTs of the physical assets they’re researching. Researchers will also be able to upload their excel files to trigger automated database update and on-chain transactions, however the logic would be programmed differently depending on how the physical biospecimens are being used in the study.
If a sample is used completely for this research, then the sample tokens are “burned” by transferring them to a wallet which can’t be accessed by anyone, like the “Null Address”: 0x0000000000000000000000000000000000000000 (Radomski et al., 2018). This effectively removes the NFT from circulation, though its existence and history can still be viewed due to the immutable nature of Ethereum, maintaining the permanent connection with its donor. The Sample NFTs would also be burned if the physical biospecimen were processed to generate an organoid, but then new organoid NFTs would be minted and issued to the patient account by the researcher. All of these operations are triggered by calling the sampleToOrganoid () function. This function is atomic, meaning all of the operations are reverted without any state change unless all steps are successful.
Until this point, the biobank has been initiating and sponsoring all of the transactions within our platform. Though because the organoid creation process occurs strictly within the study protocol which it was approved for, the responsibility shifts to the organoid researchers. Additionally, the researchers will receive a request from Metamask that they must manually approve to send the required transactions to the smart contract. This manual approval is required because, unlike the biobank account, we don’t store the private keys for the researcher’s account to send transactions on their behalf.
3.2.3 Research collaboration tools
Key features included the visibility of patient-linked bioassets and related research activities for all participating biobanks, scientists, donors and physicians, plus tooling for multisided data exchange (Shabani, 2018). Figure 4 shows web-based user interfaces created to enable scientists to engage with specimen donors, view specimen inventory and organoid gallery images. Engagement with the blockchain component of the system was facilitated with a MetaMask wallet, as shown in Figure 4B. We also illustrate how scientists may add their specimen data and organoids.
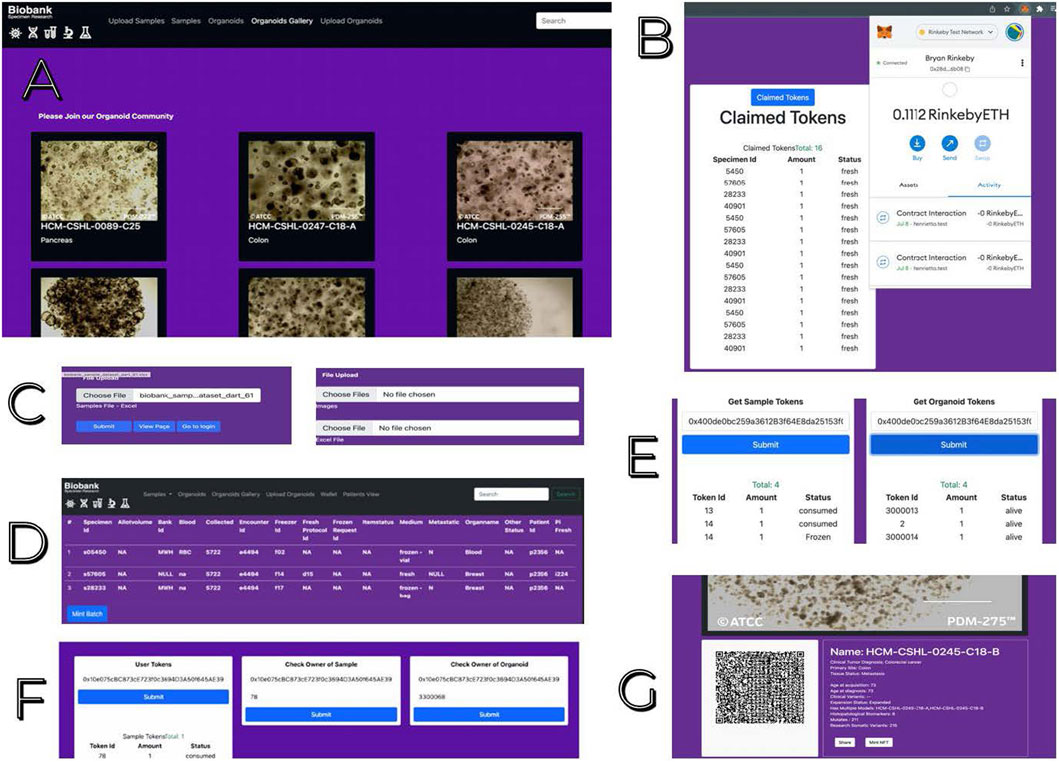
Figure 4. Web User Interfaces for Decentralized Biobanking Platform for Organoid Research Networks (A). View and search gallery of organoids from the Human Cancer Models Initiative. (B) Metamask wallet access demonstrating tokens claimed by participating patient. (C) Upload Excel of specimen inventory or organoid biobanking with corresponding jpgs. (D) ERC-1155 feature for batch minting NFTs for uploaded specimens or organoids. (E) Tool enabling scientists to seek additional biospecimens or organoids derived from the same individual whose specimens or organoids they are currently studying. (F) Biobanker workspace for allocation of user tokens to scientists, patients or physicians, as well as auditable mapping of case-level bioasset distributions. (G) App for scientists to mint an NFT to share images and ongoing research activities related to their use of a designated Human Cancer Models Initiative organoid. Available in mobile.
The resulting transparency and traceability of our NFT digital twin ecosystem and biospecimen management functions creates an interconnected biomediverse in which scientists can connect with other scientists studying the same models or donors in a mutually beneficial exchange of knowledge, practices, and experiments. This engagement can be initiated either through encrypted messaging between wallet addresses or through Etherscan to continue offline, but our prototype also provides tools that help facilitate this engagement within the web application. Scientists can share a web page with details of the organoids that they are studying by sending a QR code. We envision that a live implementation will need to provide scientists with the ability to customize permissions related to the visibility of this data (i.e., restricting visibility only to other accounts connected to organoid tokens stemming from the same origin).
3.2.3.1 System schematic
Figure 5 shows a functional unit of the de-bi ecosystem, demonstrating the level of an individual HCMI donor with two patient-derived organoids, as represented above, with copies distributed to multiple scientists. The schematic represents synergistic aggregation of cumulative insights from independent scientists who are studying the same person in parallel, with iterative integration of assets and insights at the level of an individual biospecimen donor and their respective disease models. The patient and their oncologist are ongoing contributors to and beneficiaries of the process, demonstrating an ethical, collaborative research environment which abides by the guiding principles of deSci.
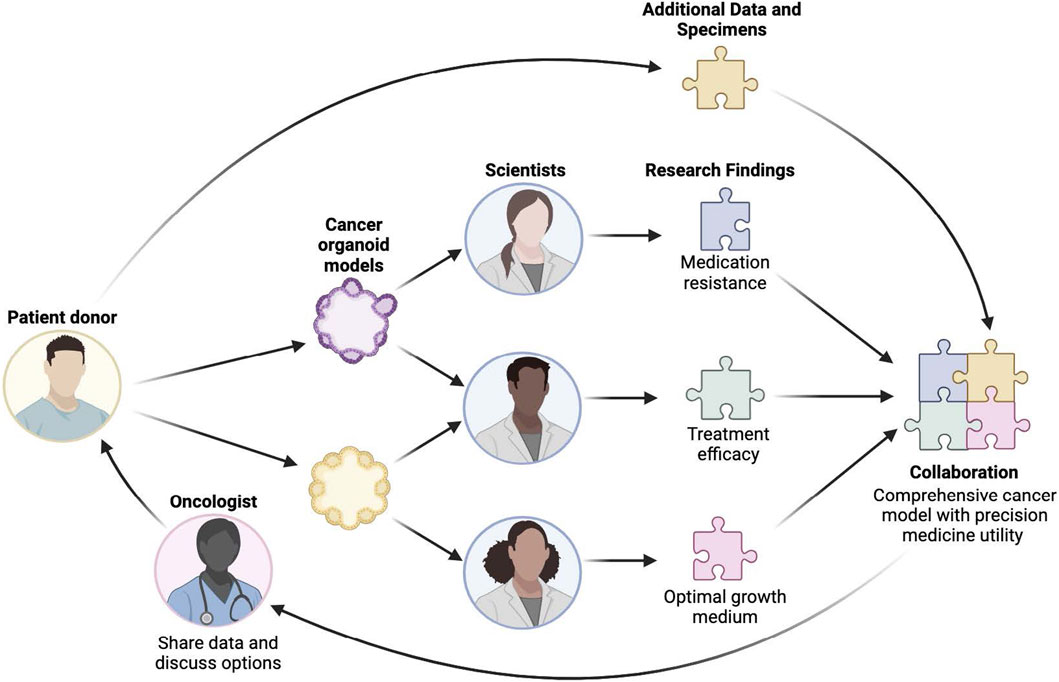
Figure 5. Functional unit of decentralized biobanking ecosystem: HCMI organoid use case. Illustrates the relationships between a patient, the organoids derived from their tumor tissues, the scientists (and respective research teams) who study them, their oncologist, and an overlay network that supports longitudinal collaboration across this ecosystem, in which insights and data from each participant are integrated into a comprehensive model with precision medicine potential.
3.3 Cost analysis
Table 5 illustrates the results of cost comparison across ERC-721 vs ERC-1155 for three representative activities within the de-bi prototype. ERC-1155 provided cost savings as compared to ERC-721 across the board, with greatest efficiency gains with smart contract deployments and higher-order transactions. This was critical for showing preliminary economic feasibility of implementation of a decentralized biobanking approach to existing organoid biobanks. For context, ATCC’s website lists price of $3,779.00 per organoid copy, with ≥1.0 × 10 cells per vial, and an added $1,520 for required supplies (ATCC, 2025).
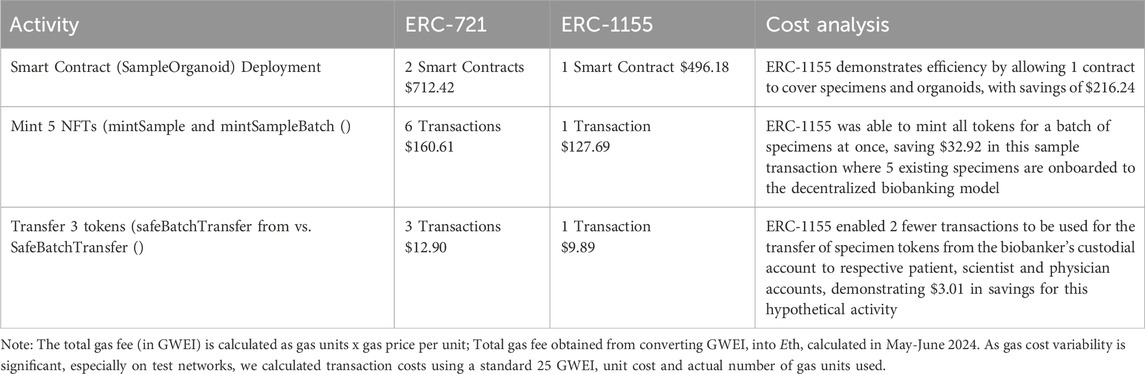
Table 5. Cost analysis for NFT digital twin framework with applications for decentralized organoid biobanking model.
4 Discussion
A functional decentralized biobanking “de-bi” application prototype was developed to advance our NFT framework for organoid research networks. Our novel DeSci approach is demonstrated for a real-world organoid biobank, illustrating how a democratized biospecimen asset layer may be implemented for established biobanking platforms, with implications for systemic ethics, efficiency and effectiveness. We demonstrate potential to bootstrap our platform for the HCMI Biobank, which provides human cancer models as a common pool resource for the international cancer research community in a manner that builds value and unlocks opportunities for collaboration to enrich ongoing activities.
First, independent of the feasibility of the proposed blockchain solution, this study identifies and articulates a critical gap in the biomedical research ecosystem that is exemplified by the human cancer model use case. We illuminate shortfalls of traditional biospecimen research paradigms emerging from current privacy practices, and highlight potential for decentralized science (DeSci) approaches to solve these challenges by applying NFTs to promote coordinated action, incentive alignment, data sharing and open collaboration that is efficient, effective and structurally just (Weidener and Spreckelsen, 2024).
The proof-of-concept prototype described here advances decentralized biobanking “de-bi” as a platform technology that forges a biomedical metaverse, or “biomediverse” with embedded ethical principles of transparency, accountability, and inclusion (Gross et al., 2023; Evangelatos et al., 2020). Our model unites scientists and biospecimen donors in a common platform that overcomes barriers for dynamic bench to bedside collaboration emerging from legacy research ethics, policies and infrastructures that have lagged behind scientific progress in next-generation biobanking (Bledsoe, 2017). By providing a mechanism for collective action, we can enable patients to benefit from timely translation and to enhance ongoing research via continued contributions of specimens and clinical data (Mak et al., 2021; Bot et al., 2019). Simultaneously, we offer a path for scientists to combine forces, enabling more comprehensive, living cancer models to evolve via integration of multimodal data from a variety of parallel sources.
Here, we elaborate an NFT digital twin framework via a functional decentralized application with smart contract features designed to meet needs and overcome constraints of biobankers and scientists who grow and study patient-derived organoids (Vasiliu-Feltes et al., 2023). Through ethnography, interviews, observations of group activities and site visits, we gained an understanding of day-to-day workflows in the biospecimen/organoid asset management pipelines, which guided our development towards functionality that could be automated or easily adopted without substantial effort or additional permissions by downstream users (Camburn et al., 2017; Harte et al., 2017). Additional feedback detailing common pain points and challenges in organoid research and development drove the implementation of the biospecimen lifecycle management and research collaboration features.
We found that a platform-based approach was essential, as organoid research is conducted in silos, and the complex, time-and-resource-intensive processes for cultivating and studying individual cancer models pose challenges whose solutions require coordination among those studying the same model systems (Baird et al., 2023; Horbach and Halffman, 2017; Markovic and Markovic, 1998). Research collaboration features that allow shared learning of failed experiences and successful solutions to common challenges may help optimize efficiency of research, unlock opportunities for translational impact, and mitigate waste. By demonstrating applicability to HCMI organoids, the de-bi approach may generalize well to any academic or industry organoid research activities, as copies distributed across the biomediverse can continue to enrich a holistic model via an overlay network.
Flexibility of the ERC-1155 standard presented several benefits, while also contributing to added complexity and increased responsibility for developers. As anticipated, ERC-1155 displayed the ability for gas cost reduction, specifically by minimizing the number of smart contracts and number of transactions required to implement our solution (Koutmos, 2023). The main challenge we found in transitioning from ERC-721 to ERC-1155 was that the latter’s methods for creating NFTs and mixing them with fungible tokens are neither specified nor standardized, potentially creating confusion for users and leading to potential unexpected behavior during future live deployment.
4.1 Strengths
Our solution was aimed at planting the roots of a decentralized system capable of emerging from an off-chain legacy model in which centralized governance rules the day and biospecimen research is shrouded in secrecy. Thus, feasibility and acceptability of our approach for implementation as an overlay network for legacy biobanking platforms prompted us to focus on a technical solution wherein a single entity was granted the ability to manage a majority of the biospecimen lifecycle activities. This approach enables automation and a reliable mechanism to maintain provenance between digital assets and real-world assets, simulating biobankers’ current mental models, regulatory paradigms and workflows (Krishnasamy and Gopalakrishnan, 2023). To mitigate privacy concerns and prevent corruption or inaccessibility of shared data, our solution applied data minimization techniques in which stakeholder and specimen NFTs and the relationships between them will remain on chain, while all sensitive donor and research data remain on institutional servers (Sanchez et al., 2024; Hasan et al., 2023). Importantly, the resulting modular biobanking infrastructure yields a composable model that can allow the value distributed across multiple biobanks to coalesce and enjoy progressive network effects over time (Yaraghi et al., 2013).
Use of soul-bound tokens (SBT’s) in our prototype was a provocative choice, as biobanking as an industry is predicated on the ability to treat biospecimens as transferable assets. Core to the ethos of decentralized biobanking, we view biospecimens as truly nonfungible assets, immutably linked to their human source (Boers et al., 2018). We describe a digital approach to reinstating the individual’s right to own their specimens, while unlocking a more efficient marketplace system for maximizing value and utility for use in research (Henkel and Maurer, 2010). Additionally, the platform implemented Sponsored Transactions for biospecimen donors, as we take the position transparency regarding use of donated biospecimens is a fundamental right that should be afforded for all patients, in recognition of the value that they provide through their biospecimen contributions (Spector-Bagdady et al., 2018).
4.2 Technical limitations
The prototype met all functional requirements as outlined if used as intended, however, significant improvements must be made to enforce proper use and restrict unintended behavior. For instance, additional steps are needed to enforce non-fungibility of the tokens deployed, as this process is not standardized for ERC-1155. To ensure non-fungibility, token ID#s must be unique. Our deployed ERC-1155 smart contracts were designed to create NFTs by applying a detailed struct for each token type, which included unique ID#s related to the specific stakeholders and assets, which were then appended onto an on-chain mapping.
Principal limitations of this approach include the fact that there is no mandate preventing duplication of the unique ID#s, enabling potential duplicates. For example, keeping the “_value” or “_amounts” argument in the standard mint and mintBatch functions enables minting of multiple tokens for a single ID each time these functions are called. As a result, the tokens created with this implementation will be non-fungible if users only input strictly unique IDs, but additional restrictions must be placed within the smart contract to ensure expected behavior. Technical solutions for this vulnerability will be essential for real-world implementation especially those intended to span multiple institutions. Implementation could be improved by verifying that the unique specimen_id does not already exist before minting, otherwise it reverts. Additionally, removing the “amount” as an input to enforce the minting of only 1 quantity for each unique token will further ensure non-fungibility. A potentially more efficient and private alternative approach would be to use the split id bits strategy mentioned in EIP-1155.
Additionally, viable access control mechanisms are needed, as Account-Based access mechanisms for this prototype limited permissions to one specific user with manual lookups via a frontend list in ERC-1155, as there is no default lookup for token owners by single address vs the ERC-721 standard. Here, the access control mechanism was only implemented for access to web applications, however smart contract functions themselves are not protected with specific role permissions or specific users. For actual implementation, permissions to calling important functions should be restricted to the appropriate stakeholders on the contract explicitly (Ismail et al., 2021). Access control mechanisms on the front-end web application will not prevent users from accessing the contract either on Etherscan or programmatically.
Alternatively, explicit role-based access control may be programmed into smart contract functionality to protect against misuse, for example, with Token Gating (Mell and Yaga, 2024). Gupta et al. (2024) clarifies, “NFT Token Gating refers to the practice of restricting access to specific websites, services, or applications based on the ownership of NFTs”. In this context, it involves verifying the presence of NFTs in a user’s wallet to grant or deny access to relevant websites.” Notably, downsides of account-based access control must be considered, given potential for loss of account control with improper use (Naicker and Moodley, 2024; Sookhak et al., 2020). For this reason, we may introducioe a standard method for leveraging centralized servers and systems for backup access. With an NFT-based access mechanism, centralized recovery mechanisms to transfer accounts and oversee transactions may be necessary, particularly given the stakes of real-world bioassets (Kumar and Venkatesh, 2022).
Within our technical framework, Biobank contracts are deployed as the owner of Patient Contracts, Researcher Contracts, and SamplesOrganoids Contracts, affording control over other contract types. In doing so, the Biobank contract is granted the ability to modify or manipulate these dependent contracts, posing a security risk if compromised. In address of security concerns, a multi-signature wallet requiring approval from multiple parties or dedicated governance contracts for each dependent contract could be implemented (Aitzhan and Svetinovic, 2016). In distributing ownership of contracts, the risk associated with a single point of failure is mitigated (Hu, 2022). Additional security measures to be considered for future research and development involve Privacy-Enhancing Techniques such as zero-knowledge proofs, blind signatures, and ring signatures, and off-chain privacy measures, potentially advanced as privacy group networks, community-controlled networks, and secure computation functions.
4.3 Next steps
Although the proof-of-concept for our prototype is established, true feasibility and acceptability among target users must be validated via live deployment. Our preliminary research and prototyping have focused domestically on organoid research and biobanking in the United States. However, issues resulting from donor exclusion and lack of transparency are relevant in other regions. For example, studies about biobank governance in Europe and Canada demonstrate lack of transparency as 29 (42%) of 69 biobanks failed to provide any information on governance or established procedures (Gille et al., 2021). Given the multitude of international stakeholders, institutions, and interests represented across the organoid research ecosystem, scalability challenges are paramount to address for successful deployment of a decentralized biobanking platform to harmonize activities across scientists, biobanks, physicians, and donors (Krishanasmamy et al., 2023). Importantly, our decentralized biobanking model is applicable to structural inefficiencies and ethical concerns inherent not only in the U.S. but also in international biobanking systems. Future efforts should therefore explicitly evaluate its feasibility and effectiveness in diverse regulatory and cultural contexts to demonstrate its broad applicability and global benefit.
Scaling our proposed technical overlay solution for harmonizing and enhancing organoid research will focus on deployment at an individual network level, tailoring technical approaches to the needs of specific stakeholders (Hansen and Özkil, 2020). To do so, multiple pilots will be deployed to a general, standardized, and composable solution that can be readily implemented and later integrated across institutions. Additionally, scaling a de-bi platform for organoid research for live implementation also necessitates minimizing metadata stored on chain to minimize gas fees and maximize return on investment (Zarir et al., 2021). Development of layer 2 solutions for live implementation will be necessary in scaling the platform and NFT framework for the multitude of specimen transactions and decentralized biobanking use cases.
Other challenges for implementation of our proposed platform include usability for blockchain applications, for which additional research and development will be required to ensure accessibility for patients and scientists alike, e.g., via account abstractions and related techniques. Further, our approach to leveraging manually uploaded Excel spreadsheets to empower individual ecosystem participants to take action from within established power structures and centralized institutional controls (Weyl et al., 2022). However, this workaround is not user-friendly, subject to bias and corruption, and necessarily limits sustainability and scalability for scientists and biobankers, for whom such data work could become overly burdensome, and hinders trustworthiness for patients and physicians, who require more robust assurances of truth (Sookhak et al., 202). APIs and oracle-based solutions will be necessary for long-term viability, though we demonstrate potential for motivated community members to bootstrap the process by simply exporting and uploading Excel files and images at will.
Finally, the decentralized biobanking network platform illustrated here represents the foundational rails, rules and tools needed to support more coordinated biospecimen research activities. Critically, additional rules and tools are needed to overcome misalignment of incentives and ensure compliance with established regulatory frameworks, including HIPAA, the Common Rule and GDPR, for those operating in the highly centralized, fragmented and dehumanized biospecimen research economy (Camilo, 2019). Biobanking and organoid research currently intersect across four relevant policy domains which may be challenged by the disruptive potential of our proposed system: 1) Informed Consent, 2_De-identification, 3) Return of Research Results, and 4) Biospecimen Ownership. Advancement of the existing legal protections and regulatory frameworks must consider potential liabilities for current stakeholders of reconnecting donors to their specimens and develop responsible transition strategies to mitigate against any unintended consequences for institutions (Sabharwal et al., 2025). Further work is necessary to realize decentralized biobanking as our proposed solution for advancing ethics, efficiency, and effectiveness in organoid research, including live pilot implementation under IRB-approved protocols, as well as further system integration and refinement of the event driven, cross-institutional supply chains (Bager et al., 2022). The adoption of our approach at scale requires extensive market design research for establishing comprehensive and sustainable incentive structures and governance frameworks which are composable across multiple contexts. Research activities should include exploration of mixed fungible and nonfungible token strategies with varying utility for incentivizing ongoing collaboration among network participants as well as the role of DAOs in attributing to governance of, collective ownership in, and distribution of any tangible rewards generated from network activities towards true democratization of biospecimen research.
5 Conclusion
The “de-bi” prototype fundamentally reshapes the organoid research landscape by leveraging decentralized science and blockchain technology. This innovative approach not only addresses critical inefficiencies in traditional biobanking but also enhances the ethical foundations and operational efficacy of biomedical research. The implementation of this system across the organoid research ecosystem is poised to enhance collaborations by enabling real-time data sharing and integration, streamline processes through automated tracking and verification, and significantly accelerate scientific advancements and their clinical applications (Maher and Khan, 2022). Looking ahead, rigorous pilot testing and continuous refinement of the system are essential to ensure scalability, usability, and compliance with regulatory standards. However, particular challenges, such as potential disincentives for adoption and the complexity of integrating new technologies into established systems, highlight the necessity for additional market design and policy research. These efforts are essential to address stakeholder concerns and align incentives, complementing the technical pilot testing. Despite the challenges ahead, the transformative potential of this model for stakeholders in the biomedical community underscores its critical importance and promising future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Detailed technical methods and results described herein are focused on non-human subjects technology design and development with publicly available de-identified model data. This study is informed by work performed under the IRB-approved human subjects research protocols and Hospital Quality Improvement activities listed below. The participants in each of the protocols below provided informed consent or waived informed consent, in accordance with the specific methods described within the respective IRB/QI protocols. IRB00019273 - Non-Fungible Tokens for Ethical, Efficient and Effective Use of Biosamples (Johns Hopkins University) STUDY22010118 - Patient Views, Preferences and Engagement in Next-Generation Biobank Research (University of Pittsburgh) STUDY22020035 - Decentralized Biobanking “de-bi”: Exploring Patients Interests in Feedback, Education, Follow-up, Engagement and Tokens of Appreciation Regarding Biobank Donation via Mobile and Web Applications (University of Pittsburgh QRC 3958- Patient-facing Biobank Platform Development QI Proposal for Beckwith Award – Breast Cancer Supply Chain Analysis, Biobank Token Model Development, and Initial Pre-Pilot Testing with UPMC Patients (University of Pittsburgh Medical Center).
Author contributions
MG: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing, Data curation. AD: Data curation, Formal Analysis, Investigation, Validation, Visualization, Writing – review and editing. MM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review and editing. EB: Formal Analysis, Methodology, Validation, Writing – review and editing, Investigation. ME: Investigation, Validation, Visualization, Writing – review and editing. OO: Investigation, Writing – review and editing, Methodology, Software, Validation, Writing – original draft. JK: Funding acquisition, Supervision, Writing – review and editing. RM: Formal Analysis, Investigation, Supervision, Writing – review and editing. WS: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this research was provided by the Emerson Collective/Yosemite Award #90097947, titled “Non-Fungible Tokens (NFTs) for Ethical, Efficient Biospecimen Use” (“Prime Award”) and the University of Pittsburgh Chancellors Gap Award from Pitt Innovation Institute.
Acknowledgments
The models and the data are from the Human Cancer Models Initiative (HCMI) www.cancer.gov/ccg/research/functional-genomics/hcmi; dbGaP accession number phs001486. Funding from Emerson Collective/Yosemite and University of Pittsburgh Chancellors Gap Award from Pitt Innovation Institute. We appreciate support of Professor Balaji Palanisami who oversaw our foundational technical blockchain research and Roberto Bellido who facilitated early prototyping.
Conflict of interest
Authors MG, WS, RM, JK, OO, and MM are shareholders in de-bi, co. ME worked as a consultant for de-bi, co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbloc.2025.1510429/full#supplementary-material
Footnotes
1https://ethereum.org/en/desci/
2https://www.roche.com/stories/modeling-the-future#a9efc235-a2a8-4780-8c10-d84b2161fcbb
3https://portal.gdc.cancer.gov/projects/HCMI-CMDC
4https://hcmi-searchable-catalog.nci.nih.gov/
5https://ethereum.foundation/ethereum
6https://github.com/centrifuge/paper-privacy-enabled-nfts
7https://ethereum.org/en/developers/docs/standards/tokens/erc-721/
8https://eips.ethereum.org/EIPS/eip-1155
9https://eips.ethereum.org/EIPS/eip-998
11https://www.atcc.org/products/pdm-256
References
Aitzhan, N. Z., and Svetinovic, D. (2016). Security and privacy in decentralized energy trading through Multi-Signatures, blockchain and anonymous messaging streams. IEEE Trans. nn Secure Comput. 15 (5), 840–852. doi:10.1109/tdsc.2016.2616861
Ali, S. E., Tariq, N., Khan, F. A., Ashraf, M., Abdul, W., and Saleem, K. (2023). BFT-IOMT: a Blockchain-Based trust mechanism to mitigate SyBiL Attack using fuzzy logic in the internet of medical things. Sensors 23 (9), 4265. doi:10.3390/s23094265
Arora, A., Kanisk, N., and Kumar, S. (2021). “Smart contracts and NFTs: non-Fungible tokens as a core component of blockchain to be used as collectibles,” in Lecture notes on data engineering and communications technology, 401–422. doi:10.1007/978-981-16-3961-6_34
Bager, S. L., Düdder, B., Henglein, F., Hébert, J. M., and Wu, H. (2022). Event-based supply chain network modeling: blockchain for good coffee. Front. Blockchain 5. doi:10.3389/fbloc.2022.846783
Baird, A. M., Westphaen, C. B., Blum, S., Nafria, B., Knott, T., Sergeant, I., et al. (2023). How can we deliver on the promise of precision medicine in oncology and beyond? A practical roadmap for action. Health Sci. Rep. 6 (6), e1349. doi:10.1002/hsr2.1349
Bledsoe, M. J. (2017). Ethical legal and social issues of biobanking: past, present, and future. Biopreserv. Biobank. 15 (2), 142–147. doi:10.1089/bio.2017.0030
Boers, S. N., Van Delden, J. J. M., and Bredenoord, A. L. (2018). Organoids as hybrids: ethical implications for the exchange of human tissues. J. Med. Ethics. 45 (2), 131–139. doi:10.1136/medethics-2018-104846
Bot, B. M., Wilbanks, J. T., and Mangravite, L. M. (2019). Assessing the consequences of decentralizing biomedical research. Big Data Soc. 6 (1), 205395171985385. doi:10.1177/2053951719853858
Camburn, B., Viswanathan, V., Linsey, J., Anderson, D., Jersen, D., Crawford, R., et al. (2017). Design prototyping methods: state of the art in strategies, techniques, and guidelines. Des. Sci. 3, e13. doi:10.1017/dsj.2017.10
Camilo, J. (2019). Blockchain-based consent manager for GDPR compliance. Open Identity Summit, 165–170. Available online at: https://dblp.uni-trier.de/db/conf/openidentity/openidentity2019.html#VargasC19.
Far, S. B., Rad, A. I., Bamakan, S. M. H., and Asaar, M. R. (2023). Toward Metaverse of everything: opportunities, challenges, and future directions of the next generation of visual/virtual communications. Netw. Comput. Appl. 217, 103675. doi:10.1016/j.jnca.2023.103675
Freni, P., Ferro, E., and Moncada, R. (2022). Tokenomics and blockchain tokens: a design-oriented morphological framework. Blockchain Res. Appl. 3 (1), 100069. doi:10.1016/j.bcra.2022.100069
García, F., Pedreira, O., Piattini, M., Cerdeira-Pena, A., and Penabad, M. (2017). A framework for gamification in software engineering. J. Syst. Softw. 132, 21–40. doi:10.1016/j.jss.2017.06.021
Gross, M., Hood, A. J., and Sanchez, W. L. (2023). Blockchain technology for ethical data practices: decentralized biobanking pilot study. Am. J. Bioeth. 23 (11), 60–63. doi:10.1080/15265161.2023.2256286
Gross, M., and Miller, R. C. (2021). Protecting privacy and promoting learning: blockchain and privacy preserving technology should inform new ethical guidelines for health data. Health Technol. 11 (5), 1165–1169. doi:10.1007/s12553-021-00589-9
Gross, M. S., Hood, A. J., and Miller, R. C. (2021). Nonfungible tokens as a blockchain solution to ethical challenges for the secondary use of biospecimens: viewpoint. JMIR Bioinform Biotechnol. 2 (1), E29905. doi:10.2196/29905
Gross, M. S., Hood, A. J., Rubin, J. C., and Miller, R. C. (2022). Respect, justice and learning are limited when patients are deidentified data subjects. Learn Health Syst. 6 (3), e10303. doi:10.1002/lrh2.10303
Gross, M. S., and Miller, R. C. (2019). Ethical implementation of the learning healthcare system with blockchain technology. Blockchain Healthc. Today 2. doi:10.30953/bhty.v2.113
Guillen, K. P., Fujita, M., Butterfield, A. J., Scherer, S. D., Bailey, M. H., Chu, X., et al. (2022). A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3 (2), 232–250. doi:10.1038/s43018-022-00337-6
Gupta, P., Nigar, N., Paswan, R., Singh, R. K., and Kumar, S. (2024). Non-fungible token integration for secure access control, monetization, and real-world applications. SSRN Electron. J. doi:10.2139/ssrn.4754956
Hansen, C. A., and Özkil, A. G. (2020). From Idea to Production: a retrospective and longitudinal case study of prototypes and prototyping strategies. J. Mech. Des. 142 (3). doi:10.1115/1.4045385
Harte, R., Glynn, L., Rodríguez-Molinero, A., Baker, P. M., Schard, T., Quinlan, L. R., et al. (2017). A Human-Centered Design Methodology to enhance the usability, human factors, and user experience of connected health systems: a Three-Phase methodology. JMIR Hum. Factors 4 (1), e8. doi:10.2196/humanfactors.5443
Hasan, H. R., Madine, M., Yaqoob, I., Salah, K., Jayaraman, R., and Boscovic, D. (2023). Using NFTs for ownership management of digital twins and for proof of delivery of their physical assets. Future Gener. comput. Syst. 146, 1–17. doi:10.1016/j.future.2023.03.047
Henkel, J., and Maurer, S. M. (2010). Network effects in biology R&D. Am. Econ. Rev. 100 (2), 159–164. doi:10.1257/aer.100.2.159
Hood, A., Macis, M., Mendoza Cevantes, D., Bear, T., Kahn, J., Atkinson, J. M., et al. (2024). Privacy, policy, and profits: survey of patient values and Preferences for research on de-identified Biosamples. Advance. doi:10.31124/advance.24155085.v1
Horbach, S. P. J. M., and Halffman, W. (2017). The ghosts of HeLa: how cell line misidentification contaminates the scientific literature. PLoS ONE 12 (10), e0186281. doi:10.1371/journal.pone.0186281
Hu, V. C. (2022). Blockchain for access control systems. Gaithersburg, MD: National Institute of Standards and Technology (NIST), 28. Available online at: https://nvlpubs.nist.gov/nistpubs/ir/2022/NIST.IR.8403.pdf.
Ismail, A., Wu, Q., Toohey, M., Choon Lee, Y., Dong, Z., and Zomaya, A. Y. (2021). “TRABAC: a tokenized Role-Attribute based access control using smart contract for supply chain applications,” in 2021 IEEE International Conference on Blockchain (Blockchain), Melbourne, Australia, 06-08 December 2021, 584–589. doi:10.1109/blockchain53845.2021.00088
Israni, D. K., and Shah, M. K. (2023). Blockchain: a decentralized, persistent, immutable, consensus, and irrevocable system in healthcare. Boca Raton, FL: CRC Press eBooks, 48–71. doi:10.1201/9781003408246-3
Koutmos, D. (2023). Network activity and ethereum gas prices. J. Risk Financ. Manag. 16 (10), 431. doi:10.3390/jrfm16100431
Krishnasamy, S., and Gopalakrishnan, B. N. (2023). Moving beyond proof of concept and pilots to mainstream: discovery and lessons from a reference framework and implementation. Blockchain Healthc. Today 6 (2). doi:10.30953/bhty.v6.280
Kumar, R., and Venkatesh, K. (2022). “Centralized and decentralized data backup approaches,” in AISC, 687–698. doi:10.1007/978-981-16-5652-1_60
Lesavre, L., Varin, P., and Yaga, D. (2021). Blockchain networks: token design and management overview (NISTIR 8301). Gaithersburg, MD: U.S. Department of Commerce, National Institute of Standards and Technology, 84. Available online at: https://nvlpubs.nist.gov/nistpubs/ir/2021/NIST.IR.8301.pdf.
Maher, M., and Khan, I. (2022). From sharing to selling. Blockchain Healthc. Today 5. doi:10.30953/bhty.v5.184
Mak, B. C., Addeman, B. T., Chen, J., Papp, K. A., Gooderham, M. J., Guenthen, L. C., et al. (2021). Leveraging blockchain technology for informed consent process and patient engagement in a clinical trial pilot. Blockchain Healthc. Today 4. doi:10.30953/bhty.v4.182
Maloy, J. W., and Bass, P. F. (2020). Understanding broad consent. Ochsner J. 20 (1), 81–86. doi:10.31486/toj.19.0088
Markovic, O., and Markovic, N. (1998). Cell cross-contamination in cell cultures: the silent and neglected danger. In Vitro Cell. Dev. Biol. Anim. 34 (1), 1–8. doi:10.1007/s11626-998-0040-y
Mell, P., and Yaga, D. (2024). Non-fungible token security (NISTIR 8472). Gaithersburg, MD: National Institute of Standards and Technology, Available online at: https://nvlpubs.nist.gov/nistpubs/ir/2024/NIST.IR.8472.pdf.
Naicker, D., and Moodley, M. (2024). Challenges of user data privacy in self-sovereign identity verifiable credentials for autonomous building access during the COVID-19 pandemic. Front. Blockchain 7. doi:10.3389/fbloc.2024.1374655
Ncube, T., Dlodlo, N., and Terzoli, A. (2020). “Private blockchain networks: a solution for data privacy,” in 2nd International Multidisciplinary Information Technology and Engineering Conference (IMITEC), Kimberley, South Africa, 25-27 November 2020, 1–8. doi:10.1109/IMITEC50163.2020.9334132
Radomski, W., Cooke, A., Castonguay, P., Therien, J., Binet, E., and Sandford, R. (2018). ERC-1155: multi token standard. Ethereum Improvement Proposals, no. 1155. Available online at: https://eips.ethereum.org/EIPS/eip-1155.
Sabharwal, K., Hutler, B., Eifler, M., and Gross, M. S. (2025). Decentralized biobanking for transparency, accountability, and engagement in biospecimen donation. J. Health Care Law Policy. 28 (1), 1–25. Available online at: https://digitalcommons.law.umaryland.edu/jhclp/1460.
Sachs, N., de Ligt, J., Kopper, O., Gogola, E., Bounova, G., Weeber, F., et al. (2017). A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172 (1–2), 373–386.e10. doi:10.1016/j.cell.2017.11.010
Sanchez, W., Lindler, L., Miller, R. C., Hood, A., and Gross, M. S. (2024). Non-fungible tokens for organoids: decentralized biobanking to empower patients in biospecimen research. Blockchain Healthc. Today 7 (1). doi:10.30953/bhty.v7.303
Shabani, M. (2018). Blockchain-based platforms for genomic data sharing: a de-centralized approach in response to the governance problems? J. Am. Med. Inf. Assoc. 26 (1), 76–80. doi:10.1093/jamia/ocy149
Simeon-Dubach, D., Poehrl, M. H., Hoffman, P., and Puchois, P. (2020). Enhancing cooperation between academic biobanks and biomedical industry: better mutual understanding and new collaborative models are needed. Biopreserv. Biobanking. 18 (2), 144–149. doi:10.1089/bio.2019.0095
Somiari, S. B., and Somiari, R. I. (2015). The Future of Biobanking: a conceptual look at how biobanks can respond to the growing human biospecimen needs of researchers. Adv. Exp. Med. Biol. 864, 11–27. doi:10.1007/978-3-319-20579-3_2
Sookhak, M., Jabbarpour, M. R., Safa, N. S., and Yu, F. R. (2020). Blockchain and smart contract for access control in healthcare: a survey, issues and challenges, and open issues. J. Netw. Comput. Appl. 178, 102950. doi:10.1016/j.jnca.2020.102950
Spector-Bagdady, K., De Vries, R. G., Gornick, M. G., Shuman, A. G., Kardia, S., and Platt, J. (2018). Encouraging participation and transparency in biobank research. Health Aff. 37 (8), 1313–1320. doi:10.1377/hlthaff.2018.0159
Tonsing-Carter, E., Agarwal, R., Kya, C. W., Perz-Mayoral, J., Soria, C. T., and Zenklusen, J. C. (2023). Abstract 4681: human Cancer Models Initiative (HCMI): a community resource of next-generation cancer models and associated data. Cancer Res. 83 (7_Suppl. ment), 4681. doi:10.1158/1538-7445.am2023-4681
U.S. Department of Health and Human Services (2012). Guidance regarding methods for de-identification of protected health information in accordance with the health insurance portability and accountability act (HIPAA) privacy rule. Available online at: https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html.
Van Wichelen, S. (2023). After biosovereignty: the material transfer agreement as technology of relations. Soc. Stud. Sci. 53 (4), 599–621. doi:10.1177/03063127231177455
Vasiliu-Feltes, I., Myrea, M., Zhang, C. Y., Wood, T. C., and Thornley, B. (2023). Impact of blockchain-digital twin technology on precision health, pharmaceutical industry, and life sciences: conference proceedings, Conv2X 2023. Blockchain Healthc. Today 6 (2). doi:10.30953/bhty.v6.281
Weidener, L., and Spreckelsen, C. (2024). 'Decentralized science (DeSci): definition, shared values, and guiding principles. Front. Blockchain. 7. doi:10.3389/fbloc.2024.1375763
Weyl, E. G., Ohlhaver, P., and Buterin, V. (2022). Decentralized society: finding Web3’s soul. SSRN Electron. J. doi:10.2139/ssrn.4105763
Yaraghi, N., Du, A. Y., Sharman, R., Gopal, R. D., Ramesh, R., Singh, R., et al. (2013). Professional and geographical network effects on healthcare information exchange growth: does proximity really matter? J. Am. Med. Inf. Assoc. 21 (4), 671–678. doi:10.1136/amiajnl-2012-001293
Zarir, A. A., Oliva, G. A., Jiang, Z. M., and Hassan, A. E. (2021). Developing cost-effective blockchain-powered applications. ACM Trans. Softw. Eng. Methodol. 30 (3), 1–38. doi:10.1145/3431726
Zhu, L., Fan, Y., Huang, X., Chen, T., Xu, X., Xu, F., et al. (2022). Patent bibliometric analysis for global trend of organoid technologies in the past decade. iScience 25 (8), 104728. doi:10.1016/j.isci.2022.104728
Appendix 1 Nonfungible token digital twin framework: morphological classification of stakeholder and bioasset tokens
Keywords: organoids, decentralized science (DeSci), biospecimens, bioethics, human cancer models, nonfungible tokens (NFTs), blockchain technology, decentralized biobanking
Citation: Gross M, Dewan A, Macis M, Budd E, Eifler M, Odeniran O, Kahn J, Miller RC and Sanchez W (2025) Decentralized biobanking platform for organoid research networks. Front. Blockchain 8:1510429. doi: 10.3389/fbloc.2025.1510429
Received: 12 October 2024; Accepted: 03 June 2025;
Published: 02 July 2025.
Edited by:
Peter Kieseberg, St. Pölten University of Applied Sciences, AustriaReviewed by:
Andrea Lavazza, Pegaso University, ItalyFrancois Sicard, University College London, United Kingdom
Copyright © 2025 Gross, Dewan, Macis, Budd, Eifler, Odeniran, Kahn, Miller and Sanchez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marielle Gross, bWdyb3NzMjNAamhtaS5lZHU=; William Sanchez, d2lsbEBkZS1iaS5jbw==
 Marielle Gross
Marielle Gross Ananya Dewan
Ananya Dewan Mario Macis
Mario Macis Eve Budd5
Eve Budd5 Robert Clell Miller
Robert Clell Miller William Sanchez
William Sanchez