- 1Department of Architecture, Dokuz Eylul University, Izmir, Türkiye
- 2Department of Biochemical Engineering, University College London, London, United Kingdom
- 3Bartlett School of Environment, Energy and Resources, University College London, London, United Kingdom
Mould growth adversely impacts the structural integrity of buildings, human health and indoor air quality. Traditional mould prevention in buildings primarily focuses on humidity control and ventilation, while the potential of lighting as a non-chemical strategy remains underexplored. This research examines how various lighting configurations affect the growth dynamics of two common indoor mould species found in UK dwellings. In this study, cultures were incubated under continuous illumination with either long-wavelength (650–700 nm) or short-wavelength (435–465 nm) LED light alongside dark conditions at two water activity levels (aw 0.95 and aw 0.91). Mycelial growth was quantified by measuring colony diameter and dry cell weight, and spore concentration was measured using a hemacytometer. Results showed that for Aspergillus Niger, long-wavelength light significantly increased both mycelial growth and conidia production in both high and low-water activities. Short-wavelength light irradiation resulted in the lowest conidia production, suggesting that short wavelengths could inhibit spore formation. For Cladosporium sphaerospermum, long wavelength light also increased conidia production; however, it only resulted in higher mycelial growth in higher water activity media. This study shows that incorporating spectrally optimised lighting systems into building designs could generate mould-resistant conditions and minimise the need for chemical treatments while enhancing indoor air quality. Further research is recommended to investigate broad-spectrum lighting with a wide range of wavelengths on mould growth to determine the most effective spectral conditions for mould prevention in the built environment.
1 Introduction
The presence of mould in buildings may not only compromise the structural and aesthetic integrity of buildings, but it also affects the durability and strength of materials (Viitanen, 2011), but also pose significant risks to occupants’ physical and mental health. Mould growth and dampness are prevalent in the UK, with reports estimating that anywhere between 4% and 27% of the housing stock is affected (BEIS, 2021; Department of Communities and Local Government, 2015). The physical impact of mould on humans is well reported, showing that prolonged exposure to mould spores can cause respiratory problems such as asthma, allergic rhinitis, and bronchitis (Caillaud et al., 2018). These effects are particularly harmful to vulnerable populations such as children, the elderly and those with chronic or underlying health conditions (Jones, 2023). Previous studies have shown that the presence of dampness and mould in homes also significantly impacts the mental health of residents due to increased stress and anxiety, damage to possessions, social isolation and unpleasant living conditions (Liddell and Guiney, 2015).
Mould growth in buildings is affected by several factors, including humidity, temperature, building materials and nutrient availability (Brambilla and Sangiorgio, 2020). Common mould types isolated from the built environment include Cladosporium sp. (Segers et al., 2015) Aspergillus sp. (Mycol et al., 2016), Penicillium sp. and Alternaria sp. (Pyrri et al., 2023). These mould types are prevalent due to their ability to grow in conditions with warm temperatures and high humidity/moisture, like those typically present in the built environment (Du et al., 2021). Despite overlapping environmental preferences and health risks, these moulds exhibit unique characteristics regarding substrate selection and adverse health consequences.
Traditionally, mould growth in the built environment has been managed through moisture control and ventilation strategies (World Health Organization, 2009). Ventilation approaches encompass both natural ventilation mechanisms, such as opening windows and mechanical systems, such as extraction from wet rooms (Brambilla and Sangiorgio, 2020). Relative humidity is also frequently maintained using dehumidifiers or HVAC systems designed to regulate moisture levels within the indoor environment; however, these systems require frequent maintenance (Haleem Khan and Mohan Karuppayil, 2012). Chemical treatments can also be used; however, they have been shown to be ineffective in eradicating mould contamination and pose environmental risks due to the toxic substances they contain (Peitzsch et al., 2012).
1.1 The influence of light on mould growth
Light plays a vital role in regulating biological processes (Professional, 2017). Light sensing in mould species has been shown to influence the growth, sporulation and expression of secondary metabolites and pigmentation (Purschwitz et al., 2006; Nmom et al., 2021). Previous studies have explored the application of light to inhibit mould growth for various industries, such as agriculture and food production, through targeted, monochromatic wavelengths (Rasiukevičiūtė et al., 2021; Begum et al., 2009). Specific wavelengths have been found to affect the growth and spore formation across different species. Short ultra-violet UVC wavelengths of 253 nm and 222 nm were able to influence the mycelial growth and spore formation of Aspergillus Niger (Narita et al., 2020). From the visible range, both short wavelengths of 445 nm and long wavelengths between 625 nm and 740 nm were found to reduce the production of a secondary metabolite, mycotoxin, in Aspergillus westerdijkiae (Cheong et al., 2016). Using specific wavelengths to prevent or minimise mould in indoor settings presents a significant opportunity. Light-emitting diodes (LEDs) offer great control over the spectrum emitted, allowing for wavelength-tailored spectra. For built environment applications, they are particularly attractive as they are now the most common light fitting for housing globally because of their energy and cost efficiency (Cheong et al., 2016). Previous studies have explored the influence of light on fungal development. For example, Fanelli et al. (2012) examined the role of visible light on the growth and conidiation of Aspergillus niger, showing that specific wavelengths can affect both sporulation and secondary metabolite production. Similarly, Priesterjahn et al. (2020) demonstrated that spectral conditions and water activity jointly influence the growth dynamics and mycotoxin production of Aspergillus species. Lam et al. (2022) further highlighted that short-wavelength daylight reduced the viability of indoor pathogens on building surfaces, indicating the potential of lighting design as a non-chemical strategy for microbial control. These studies provide foundational insights into the relationship between light spectra and fungal behaviour, underscoring the relevance of the current research.
This study aims to examine how two common indoor mould species, Aspergillus niger and Cladosporium sphaerospermum, are affected by wavelengths at either end of the visible light spectrum, specifically long wavelength (red) and short wavelength (blue), alongside dark conditions. The research investigates the practical implications of lighting design in indoor environments by exploring the effects of these light wavelengths on mould growth and examining how variations in water activity interact with light to influence mould development. This approach could offer a cost-efficient, low-maintenance alternative to traditional mitigation strategies, contributing to sustainability and healthier living environments by reducing mould growth and improving indoor air quality.
2 Methodology
This research aims to examine how spectral distribution influences the germination, growth, and sporulation of two common indoor mould species found in buildings. The experiments were conducted under controlled laboratory conditions. The experiment was conducted in three consecutive stages to examine how specific light wavelengths influenced mould growth. The experimental framework is presented in Figure 1. The initial stage involved preparing and cultivating the preliminary spore suspensions. In the following stage, mould growth dynamics were observed under the specified experimental settings. In the last stage, a detailed quantitative assessment was carried out, measuring colony diameter, dry cell weight and spore concentration.
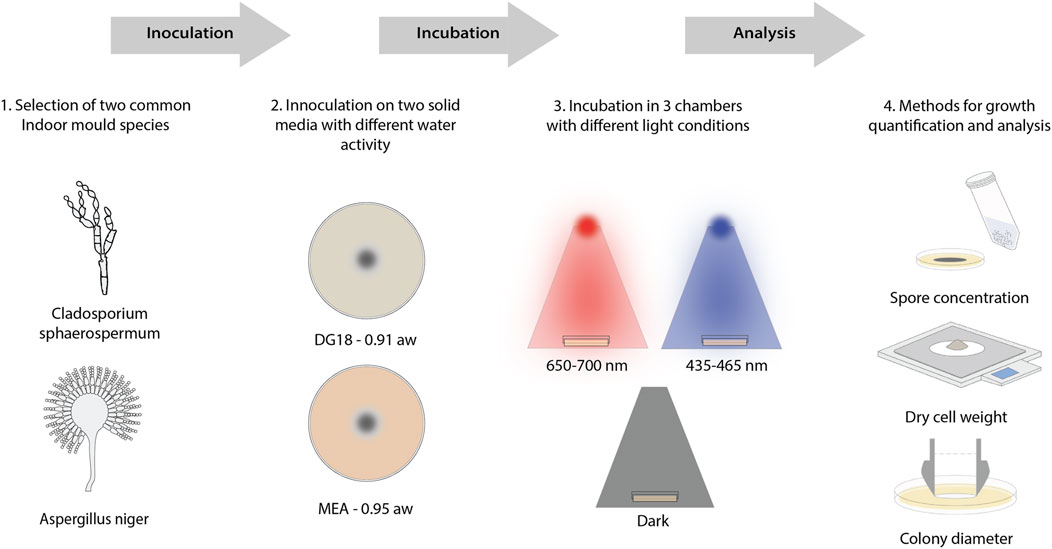
Figure 1. 1. Two common house mould species are chosen, Aspergillus Niger and Cladosporium sphaerospermum. 2. Spore solution from the two species is inoculated onto two media types with lower and higher water activity. 3. Plates are then incubated in LED light chambers at different peak wavelengths with a dark chamber as the control. 4. Quantitative analysis methods are used to measure the spore concentration, the dry cell weight of the biomass, and the colony diameter after 7 days.
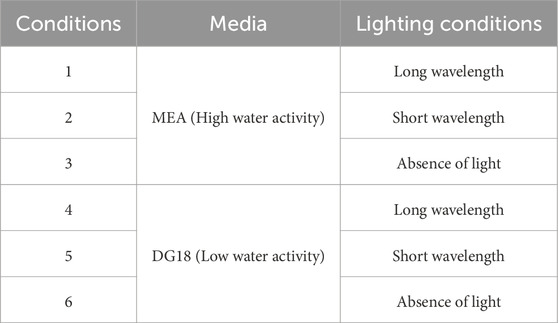
In the experimental setup, spore suspensions from the chosen mould species were inoculated onto two types of solid growth media with varying amounts of water activity: Malt Extract Agar (MEA) has higher water activity (0.95 aw), whereas Dichloran Glycerol Agar (DG18) has lower water activity (0.91 aw). The inoculation plates were then exposed to three lighting conditions—long wavelength illumination (650 nm–700 nm), short wavelength LED illumination (435 nm–465 nm), and complete darkness as a control parameter.
Aspergillus niger and Cladosporium sphaerospermum were cultivated under six different conditions, as shown in Table 1, and cultures were collected for investigation after incubation. The morphological structure of each obtained culture was observed using a Keyence VHX 7000 microscope at ×150 magnification. Each culture’s colony diameter, dry cell weight, and spore concentration were subsequently measured and recorded using triplicate parallel samples.
2.1 Experimental setup
2.1.1 Mould species
This study focused on two common indoor mould species, Aspergillus niger and Cladosporium sphaerospermum because of their widespread presence in the built environment and their significant influence on human health.
2.1.1.1 Aspergillus Niger
Aspergillus Niger, a common mould type found in both natural and urban environments (Costa et al., 2016) is a known cause of various respiratory diseases in the built environment. However, it demonstrates a high tolerance for survival in diverse environmental conditions and is also resistant to intense UV-C radiation (Cortesão et al., 2020). Furthermore, visible light intensity has been shown to induce morphological changes in Aspergillus Niger, such as biofilm formation and the production of melanin, a UV-resistant pigment (Sun et al., 2021). Although previous research had limited information on the impact of light wavelengths on the development and spore formation of Aspergillus Niger, short-wavelength light has been proven to play a key role in boosting conidiation levels and affecting mycotoxin production (Fanelli et al., 2012).
2.1.1.2 Cladosporium Sphaerospermum
Cladosporium sphaerospermum, another commonly encountered mould species in built environments, is distinguished from other species by its distinct and noticeable appearance (Al Hallak et al., 2023). Similar to Aspergillus Niger, it causes respiratory issues in the indoor environment; however, it is more recognised for being a prominent fungal aeroallergen, contributing to illnesses such as asthma. Cladosporium sphaerospermum can adapt to extreme environmental conditions, enabling this species to survive under challenging circumstances, such as low water activity and high radiation levels (Segers et al., 2015). Previous studies also highlighted the importance of Cladosporium sphaerospermum because individuals with allergies to other fungus species may experience allergic reactions to Cladosporium sphaerospermum due to protein similarities, a phenomenon known as serum cross-reactivity (Yew et al., 2016).
2.1.2 Culture preparation and conditions
The mould strains used in this research were Aspergillus niger (IMI 165984d) and Cladosporium sphaerospermum (IMI 84420) were supplied by CABI as A-freeze dried cultures. The strains were initially cultivated on MEA plates and then incubated at 25°C for 30 days to promote sporulation. Spores were extracted by flooding each plate with 10 mL of phosphate-buffered saline (PBS) containing 0.5% tween and filtering the suspension through a 40 μm cell strainer to remove hyphal fragments. The suspension was then centrifuged and resuspended in PBS/Tween solution. This process was repeated three times to ensure the purity of the spore suspension. Spore concentrations were measured with a hemacytometer, and the produced suspensions were stored at 4°C.
Cultures were inoculated onto two media types. MEA media with high water activity was prepared by combining 30 g/L malt extract, 10 g/L NaCl, and 15 g/L agar in distilled water. DG18 media with low water activity was prepared by mixing Dichloran Glycerol Agar base (Sigma Aldrich) and 110 g of glycerol per 500 mL of media. After preparation, both media were sterilised at 121°C for 15 min to ensure sterility. 10 μL of the spore suspension was pipetted onto the prepared media to inoculate culture plates. The inoculation plates were incubated in three chambers to investigate how light wavelengths and water activity affected mould growth.
The use of MEA and DG18 media to create high and low water activity conditions, respectively, follows standard practices in previous studies examining xerotolerant fungi and indoor mould growth (Segers et al., 2015; Narita et al., 2020). Similarly, spore suspension and inoculation protocols were adapted from established fungal microbiology methods. While some elements of the methodology are adapted, the combined experimental design using controlled visible light wavelengths and water activity levels is novel and has not been previously applied in the context of built environment fungal studies.
Temperatures were consistently monitored in each chamber using HOBO UX100-001 USB Temperature Data Loggers to minimise the confounding effects of thermal radiation from light sources. The average temperature was 21.39°C during the experiment, with a standard deviation of 0.73°. The light source positioning was optimised to ensure uniform exposure across all culture plates, avoiding intensity gradients or shading.
2.1.3 Lighting conditions
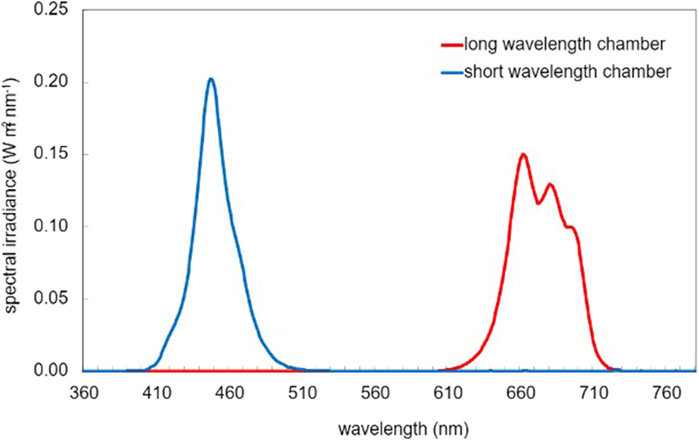
In this study, cultures were incubated in two chambers at different wavelength ranges: long-wavelength (650 nm–700 nm, 7.4 W/m2) and short-wavelength (435 nm–465 nm, 6.6 W/m2) (Figure 2), while the third chamber remained dark as a control.
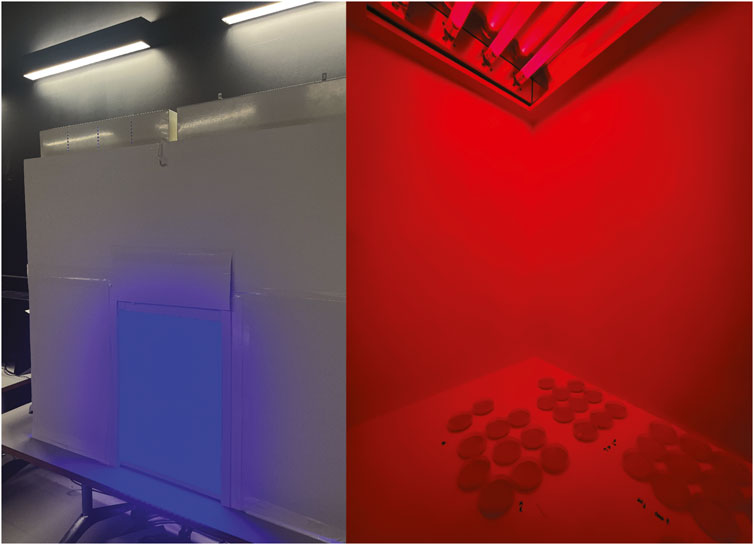
Figure 2. The left image shows the outside of the short-wavelength chamber. During the experimental period, the chamber’s entrance is sealed. The right image shows the interior of the long-wavelength chamber. Culture plates are 91 cm away from the light source.
The selected wavelength ranges represent the longest and shortest wavelengths the equipment could provide, covering the visible spectrum with red at one end and blue/violet at the other. The peak for the red wavelength is slightly broader, as multiple channels were required to achieve an irradiance level comparable to that of the blue light, approximately 7 W/m2 (Figure 3). While a higher irradiance would have been preferable, achieving this would have necessitated the inclusion of a broader range of wavelengths.
2.2 Data collection
Each culture’s dry cell weight (DCW) and spore concentration were quantified. Colonies were carefully removed from the plates (Figure 4) and placed in sample tubes. The tubes were flooded with PBS containing 0.5% tween and gently detached from the hyphae with a sterile loop. The spore suspension was pipetted off the surface and strained with a 40 μL strainer to remove hyphae debris 3 times. Spores were quantified using a haemocytometer. Colonies were carefully removed from plates and placed in separate centrifuge tubes to quantify DCW. The tubes were heated to 90°C to dissolve the agar before being poured over pre-weighed oven-dried Whatman 542 filter paper using a vacuum pump. The samples were then placed in a 30°C oven until dry. Both wet and dry weights were recorded.
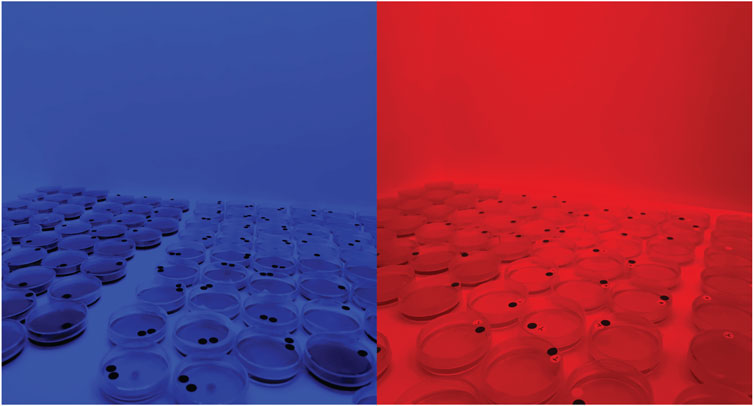
Figure 4. Images showing the cultures during incubation in the chambers with short wavelength (left) and long wavelength (right).
Plates from each condition were removed each day and observed under a microscope at ×150 magnification. The average colony diameter was calculated using a set of callipers to measure each colony in three directions. This process was repeated in triplicate to ensure reliability and consistency.
2.3 Data analysis
This experiment had three independent variables: mould species (Cladosporium sphaerospermum and Aspergillus niger), light wavelengths (long wavelength, short wavelength, and dark), and water activity (high and low). The dependent variables of this experiment, which were considered indications of mould growth, were colony diameter, dry cell weight, and spore concentration. These variables were then tested using appropriate statistical methods to assess not only the individual effects of each factor but also the combined influence of their interaction on mould growth. In addition to those quantitative variables, the morphological development of cultures was also observed and imaged using a Keyence VHX 7000 digital microscope.
Two-way ANOVA was performed for each dataset using JMP Pro 18 to investigate the influence of light wavelength and water activity on colony diameter, dry cell weight, and spore concentration in two mould species, with the dependent and independent variables summarised in Table 2. This analysis enabled the assessment of the impact of light and water activity on mould growth and their combined interaction. In addition to these quantitative measurements, the morphological structure of the fungal cultures was observed microscopically; however, these visual observations were qualitative in nature and could not be included in the statistical analysis.
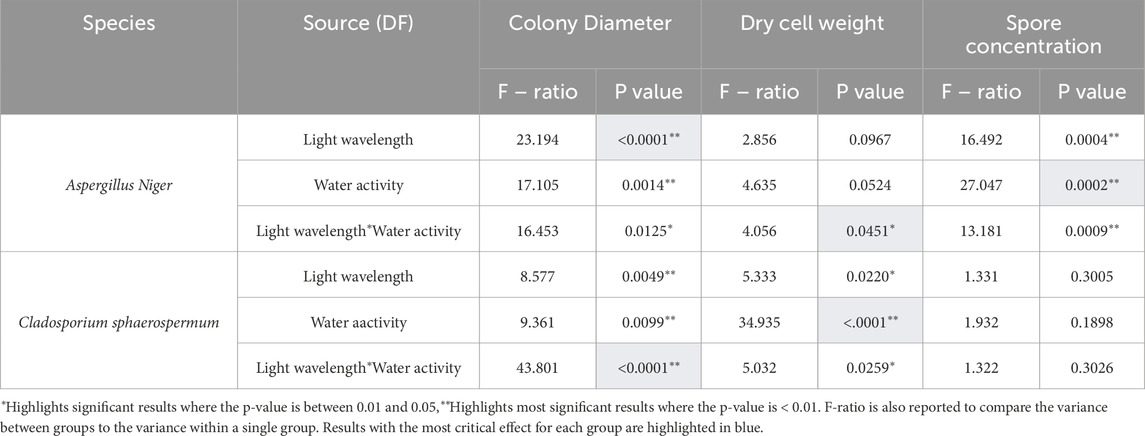
Table 2. Results from two-way ANOVA performed on each mould species, Aspergillus Niger and Cladosporium sphaerospermum.
3 Results
3.1 Morphology
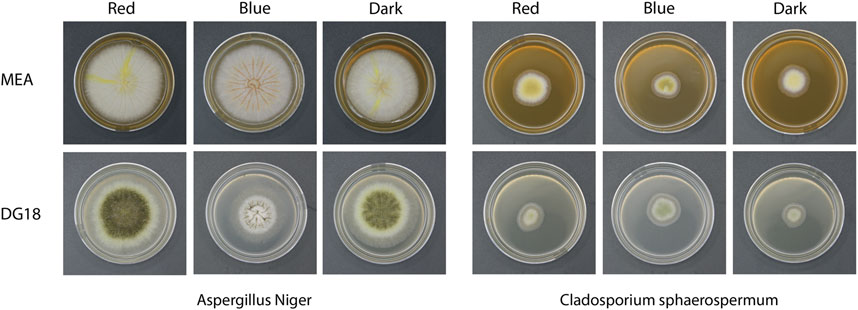
Inoculated media were cultivated under long wavelength (650 nm–700 nm, 7.4 W/m2), short wavelength (435 nm–465 nm, 6.6 W/m2), and no light (dark) as a control. After 7 days of incubation in each condition, samples were collected, photographed, and examined under a microscope (Figure 5) to determine the morphological differences between culture conditions.
Light wavelength was found to influence the morphology of Aspergillus niger. Groups cultivated in long-wavelength light and dark conditions produced yellow pigment. On MEA media, colonies produced yellow radial stripes, while DG18 hyphae were yellow. Under short-wavelength light, white colonies formed in both media types with no visible colouration. On DG18 media, short-wavelength light suppresses spore production and development compared to long-wavelength light or dark conditions. DG18 media grew more slowly than MEA media under all light conditions, with smaller, sparser hyphae and dense conidia for the long wavelength and dark groups. Spores produced on DG18 media were black, contrasting with the light brown spores formed on MEA under the long wavelength light.
The growth and sporulation of Cladosporium sphaerospermum presented fewer significant changes in the size and appearance of colonies developed under each condition. MEA-grown colonies were yellow, but DG18-grown colonies were pale green. The colonies were smaller and with fewer visible spore heads due to the germination and development rates being substantially slower than those of Aspergillus niger. As shown in Figure 6, the DG18 media resulted in a significantly lower concentration of spores, with predominantly hyphae. In contrast, MEA media resulted in a denser accumulation of spores.
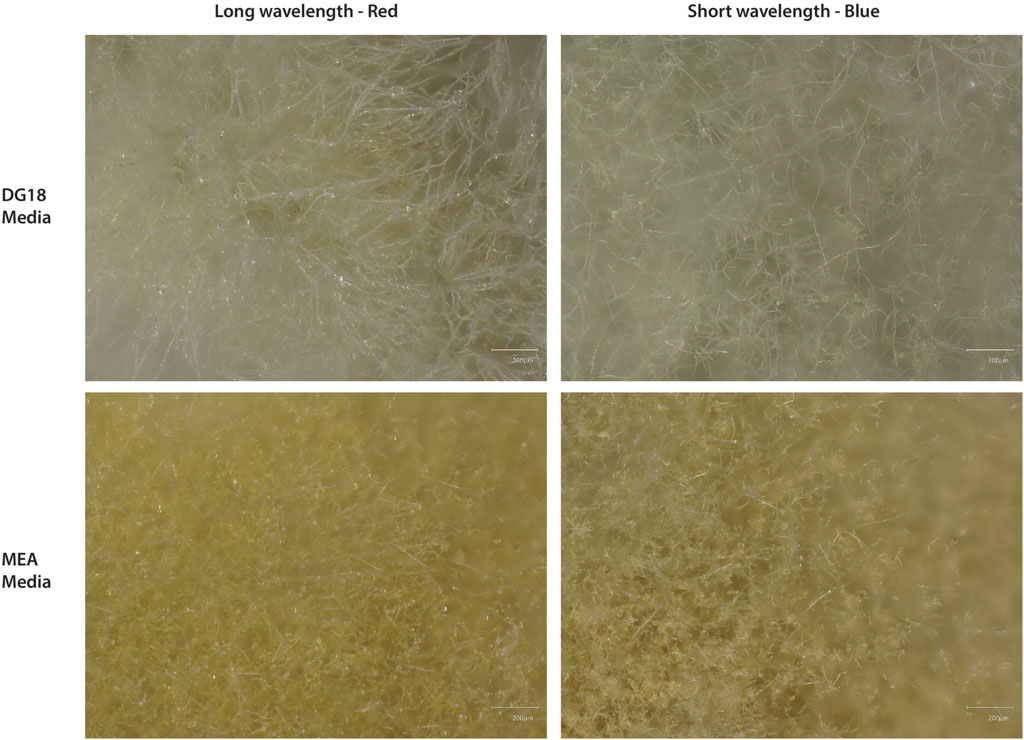
Figure 6. Microscope images showing the difference in hyphae and spore density from the centre of a Cladosporium sphaerospermum colony cultured on each media type. The top images show the hyphae after incubation under long wavelengths (left) and short wavelengths (right). The Bottom show the development of hyphae and spore heads under long wavelengths (left) and short wavelengths (right). Under both wavelengths, the MEA produced denser hyphae with spore heads. The DG18 did not develop as many spore heads with less dense and lighter colour hyphae.
3.2 Colony diameter
The average of four directional measurements using callipers across each fungal colony was used to determine the colony diameter, and the mean of the four readings was then recorded. The samples were collected in triplicate.
Long-wavelength (red) light consistently promoted Aspergillus niger mycelial growth, resulting in the largest colony diameters across all conditions (Figure 7). In contrast, dark conditions generally led to smaller colony diameters than red light, suggesting that the absence of light slows down mould growth. Short-wavelength (blue) light resulted in the smallest colony diameters in most conditions, particularly in DG18 media, indicating its potential effectiveness in reducing mould growth. Additionally, higher water activity levels enhanced growth across all lighting conditions. The most pronounced effect of water activity was observed under blue light, where an increase in water activity led to a 10.45 mm larger colony diameter, while the variation in red-light conditions was minimal (1.25 mm).
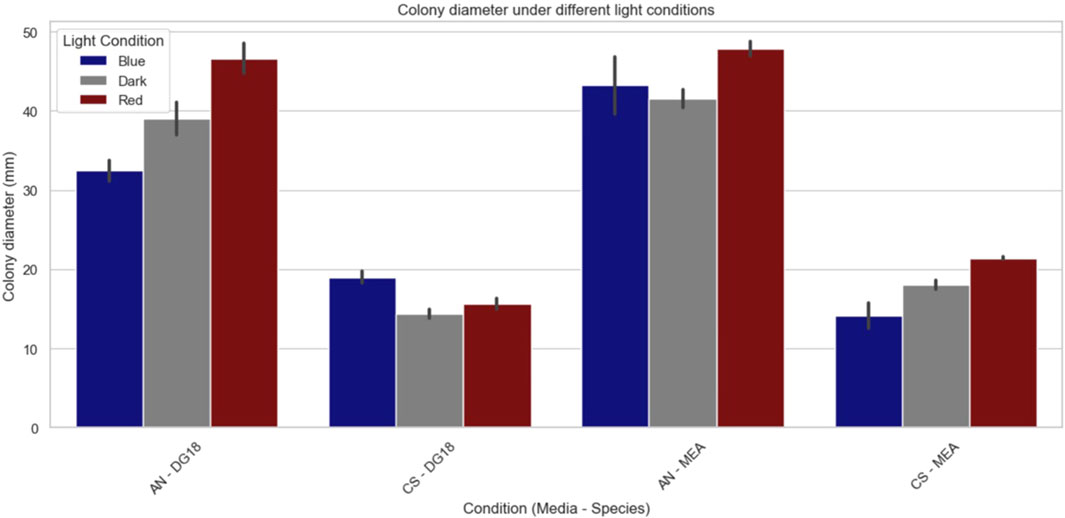
Figure 7. Colony diameter for each condition (AN, Aspergillus Niger; CS, Cladosporium sphaerospermum; DG18, Low water activity media; MEA, High water activity media).
The impact of light on colony growth seems more evident in media with lower water activity. DG18 media showed a 35.85% difference in colony diameters between the largest group (long wavelength light conditions) and the smallest group (short wavelength light conditions). On MEA media, there was only a 15.35% difference in colony diameter between the largest group (long wavelength light) and the smallest group (dark conditions). The main effect ANOVA analysis revealed that water activity (F1,12 = 17.1056, p = <0.0014) and light wavelength (F2,12 = 23.1943, p = <0.0001) had a significant effect on colony diameter for Aspergillus niger (Table 2).
Higher water activity appeared to increase the growth rate of Cladosporium sphaerospermum colonies cultivated under long-wavelength light and in dark circumstances, resulting in larger colony diameters on MEA media. In contrast, samples cultivated under short-wavelength light showed larger colonies on DG18 than on MEA media. Colonies were much smaller than those in the Aspergillus niger group, indicating that Cladosporium sphaerospermum grew at a slower rate. The main effect of ANOVA analysis (Table 2) despite both significant factors, light wavelength (F2,12 = 8.5770, p =0.0049) had a more substantial influence on colony diameter than water activity (F1,12 = 9.3618, p =0.0099).
3.3 Dry cell weight
Due to the variability in the morphology between groups, the species’ growth was also assessed according to their actual biomass; thus, dry cell weight was measured. Dry cell weight was measured by extracting the mycelial mats from agar and filtering them using the Whatman 542 filter paper, as described in the methods section. The samples were collected in triplicate.
The findings revealed that Aspergillus niger, under long-wavelength light, produced the highest average biomass in both media conditions. Under short wavelength light, Aspergillus niger produced the lowest overall biomass for the DG18 medium, whereas under dark conditions produced the lowest biomass for MEA media.
Aspergillus niger colonies grown on DG18 media under long wavelength and dark light conditions produced more biomass than colonies grown on MEA media. However, two-way ANOVA analysis (Table 2) revealed no significant effect on dry cell weight from either light wavelength (F2,12 = 2.8560, p = 0.967) or water activity (F1,12 = 4.6359, p = 0.0524).
Cladosporium sphaerospermum produced the highest biomass on MEA media when exposed to short-wavelength light, while DG18 media were exposed to long-wavelength light (Figure 8). Cladosporium sphaerospermum cultures cultivated on DG18 media showed greater overall biomass than those grown on MEA media in all light conditions.
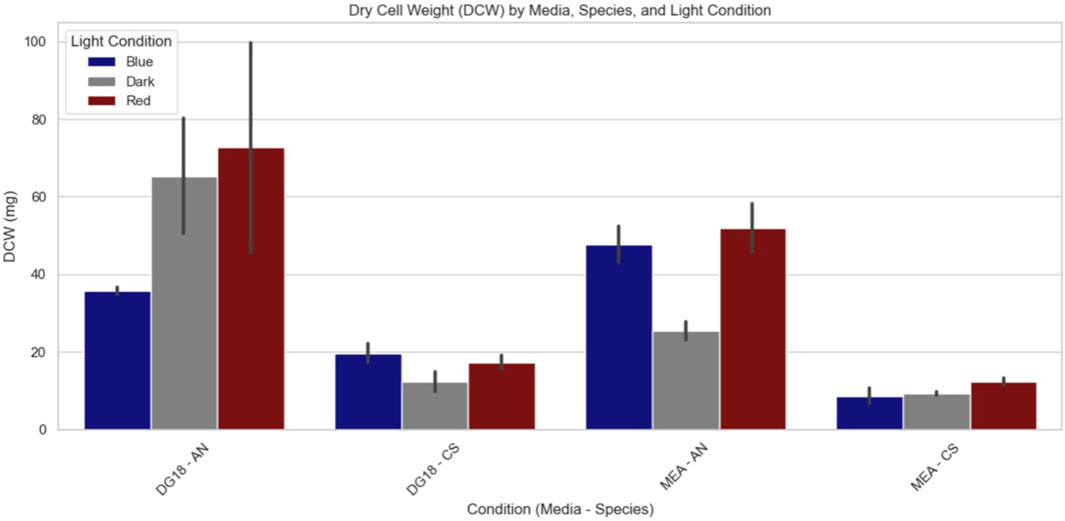
Figure 8. Dry cell weight measurements after incubation (AN, Aspergillus Niger; CS, Cladosporium sphaerospermum; DG18, Low water activity media; MEA, High water activity media).
Cladosporium sphaerospermum culture on DG18 media also produced the most significant fluctuation in dry cell weight between each light wavelength, implying that the low water activity exacerbated the influence of light on biomass. Two-way ANOVA analysis (Table 2) revealed that, despite both factors being significant, water activity (F1,12 = 34.9355, p = <0.0001) had a more substantial effect on dry cell weight than light wavelength (F2,12 = 5.3333, p = <0.0220).
3.4 Spore concentration
Spores were collected by flooding plates with 3 mL of PBS containing 0.5% tween. The suspension was then filtered through a 40 μm cell strainer to remove hyphae debris. Spore suspensions were then diluted and counted with a haemocytometer. Samples for each condition were taken in triplicate.
There was a significant variation in the resulting spore concentrations of each culture condition. As shown in Table 3, Aspergillus niger colonies grown in long-wavelength light resulted in higher spore density for both media conditions, with a concentration per ml of 6.18 × 106 on MEA and 5.17 × 107 on DG18. In contrast, short wavelength appeared to suppress spore formation on DG18 and MEA media compared with a spore concentration of 1.68 × 106 spores per ml in MEA and 5.31 × 105 in DG18.
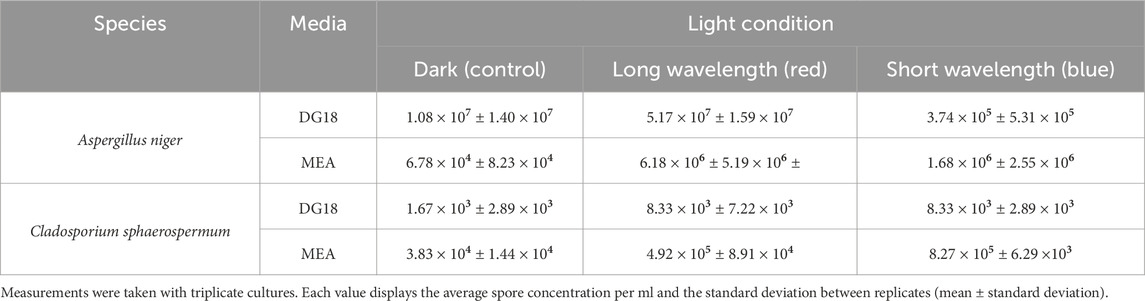
Table 3. The result was spore concentrations after incubation in 3 different lighting conditions: long wavelength–red light, short wavelength–blue light, and darkness.
DG18 media appeared to increase spore production in Aspergillus niger in comparison to MEA media implying that low water activities play an essential role in spore development. The main effect ANOVA analysis (Table 2) revealed that water activity had a slightly more significant impact on the recorded spore concentration of the Aspergillus niger samples (F1,12 = 27.0477, p = 0.0002) than light wavelength (F2,12 = 16.4923, p = 0.0004.
Long-wavelength (red) light incubation resulted in the highest conidia concentrations for Cladosporium sphaerospermum in both media, with 8.33 × 103 spores in DG18 and 4.92 × 105 spores in MEA. In contrast, dark conditions suppressed spore production, leading to lower concentrations of 1.67 × 103 in DG18 and 3.83 × 104 in MEA. Dark conditions also appeared to produce considerable variability in the measured values between samples, reflected in the large standard deviations of recorded spore concentrations. The variable spore concentrations between cultures from the same condition can be seen in Figure 9.
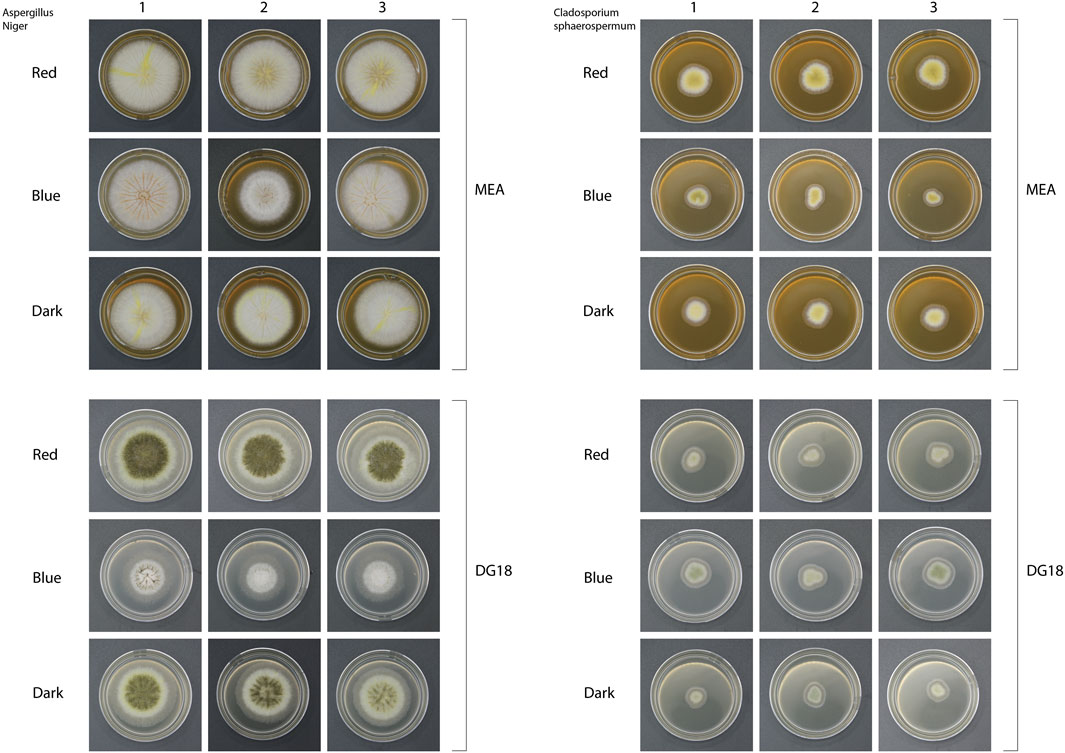
Figure 9. Images showing triplicate samples from each culture condition after 7 days of incubation with Aspergillus Niger shown on the left and Cladosporium Sphaerosperum on the right. Aspergillus Niger colonies grew significantly larger than the Cladosporium Sphaerosperum colonies. The Images show dense conidia production of Aspergillus niger on DG18 media under long wavelength (Red) and Dark conditions. Blue light conditions on DG18 and cultures in all light conditions on MEA produced fewer visible spores.
Unlike Aspergillus niger, Cladosporium sphaerospermum produced higher spore densities on MEA under all lighting conditions than on DG18. However, a two-way ANOVA analysis (Table 2) indicated that neither light wavelength (F2,12 = 1.3313, p = 0.3005) nor water activity (F1,12 = 1.9320, p = 0.1898) had a statistically significant effect on Cladosporium sphaerospermum spore concentration. Cladosporium sphaerospermum produced significantly lower spore densities than Aspergillus niger. Some colonies had not yet produced spore heads at the time of collection (after 7 days of incubation) due to their slower growth rate, as shown in the microscope photos in Figure 6.
3.5 Interaction between light wavelength and water activity
A two-way ANOVA was performed on each species to see how light wavelength and water activity combination influenced colony diameter, dry cell weight, and spore concentration (Table 4). The analysis demonstrated a statistically significant interaction between light wavelength and water activity on colony diameter for both species, Aspergillus niger (F2,12 = 16.4530, p = 0.0125) and Cladosporium sphaerospermum (F2,12 = 43.8012, p < 0.0001).

Table 4. Results from two-way ANOVA performed on each mould species (−: p-value >0.05, +: p-value between 0.01 and 0.05, ++: p-value <0.01).
There is a statistically significant interaction between light wavelength and water activity for Aspergillus niger’s spore concentration (F2,12 = 13.1810, p = 0.0009), colony diameter (F2,12 = 16.4530, p = 0.0125) and dry cell weight (F2,12 = 4.0565, p = 0.0451). The interaction effect for Aspergillus niger adversely influenced colony diameter and spore concentration since light wavelength and water activity had more substantial effects on their own. In contrast, the interaction promoted dry cell weight since neither factor alone was statistically significant on its own.
The interaction effect for Cladosporium sphaerospermum was also significant for colony diameter (F2,12 = 43.8012, p < 0.0001) and dry cell weight (F2,12 = 5.0323, p = 0.0259), but not for spore concentration (F2,12 = 1.3227, p = 0.3026). The interaction effect for Cladosporium sphaerospermum influenced colony diameter more than the individual impacts of light or water activity alone. A promoting effect of the interaction was also observed for dry cell weight. However, both light wavelength and water activity individually had a more influential impact, suggesting that the combination contributed to growth, but to a lesser extent than each factor alone. In contrast, neither the interaction nor the individual effects significantly influenced spore concentration, indicating that spore production was not notably affected by light wavelength, water activity, or their combination.
Images of fungal colonies (Figure 9) clearly show the morphological differences between cultures from each condition. This supports the statistical data demonstrating how the interaction between light wavelength and water activity influences fungal growth and sporulation. These results highlight the importance of considering the combined effect of light wavelength and water activity, as their interaction can either promote or suppress mould growth depending on the species and growth parameters. These findings suggest that mould growth should be evaluated by considering the combined effects of multiple environmental factors, such as light wavelength, water activity, and media type, rather than assessing each factor in isolation, as this better reflects real-world conditions in the built environment.
3.6 Key findings
The findings demonstrated that when Aspergillus niger was grown in low-water activity media, however, the combination of short-wavelength light and low-water activity effectively slowed down mycelial and conidia growth. In the same species, long-wavelength light stimulated mycelial growth and conidia formation in low and high-water activity conditions. Light wavelength had the most significant effect on colony diameter (p < 0.0001), whereas water activity seemed to have the most significant impact on spore concentration (p = 0.0002). The interaction between light wavelength and water activity had a higher influence on dry cell weight than the main effects (p < 0.0451).
Long-wavelength light was found to promote mycelial growth and spore formation of Cladosporium sphaerospermum in high-water activities. In contrast, short-wavelength light was found to increase biomass in low-water activities. The interaction between light wavelength and water activity had the most significant influence on colony diameter (p < 0.0001), whereas the water activity had the most critical effect on dry cell weight (p < 0.0001). Neither light wavelength (p = 0.3005) nor water activity (p = 0.1989) significantly affected spore concentrations.
4 Discussion
4.1 Mould growth dynamics under different light conditions
In this study, Aspergillus niger and Cladosporium sphaerospermum were exposed to various light conditions at different water activity levels. Mould growth and sporulation were then evaluated based on morphological characteristics, colony diameter, dry cell weight, and spore concentration differences. The wavelength of light significantly influenced the growth and sporulation of two species.
Long-wavelength light (650 nm–700 nm) showed an enhancing impact on both species’ colony growth and biomass production by influencing critical mechanisms for the development and proliferation of mould in indoor environments, such as activating photo-responsive metabolic pathways, increasing metabolic activity and promoting sporulation (Fanelli et al., 2012). This is consistent with previous research conducted by Priesterjahn et al. (2020), which found that visible light, particularly at certain wavelengths, can significantly affect fungal development and mycotoxin production in Aspergillus flavus and Aspergillus parasiticus under low water activity conditions. This implies a synergistic effect of spectral control and humidity regulation in managing fungal risks in buildings.
In contrast, colony diameters were slightly smaller when exposed to short-wavelength light (about 435 nm–465 nm) than those developed under long-wavelength light, although exhibiting more remarkable growth than those exposed to complete darkness. Lam et al. (2022) showed similar inhibitory effects, finding that sunshine with shorter wavelengths dramatically lowered fungal growth rates on interior surfaces. This highlights the possibility of controlled lighting conditions as a passive technique for managing indoor mould.
This finding reveals that short wavelengths do not promote development as much as long wavelengths, despite playing a role in stimulating essential metabolic processes for mould development (Nmom et al., 2021). Exposure to light, regardless of its wavelength, stimulates mould growth more than darkness. This finding emphasises the importance of light, as darkness inhibits metabolic processes essential for mould growth and reproduction (Lyu et al., 2023). The findings suggest that controlling light exposure and spectrum tuning could effectively mitigate mould in the built environment.
4.2 Growth and morphological responses of Aspergillus niger and Cladosporium sphaerospermum to light wavelength
When exposed to long-wavelength light, Aspergillus niger demonstrated faster growth than Cladosporium sphaerospermum, with larger colony diameters and more spores. This observation aligns with the findings by Fanelli et al. (2012), who reported that Aspergillus niger appears to thrive in illuminated environments, using light to increase its metabolic activities and reproductive capabilities. These findings are similar to those of Lam et al. (2022), who found that microbial growth was highest in darker or poorly lit environments. This highlights the influence of light not only on fungal growth rate but also on its morphological behaviour in real-world settings.
Conversely, Cladosporium sphaerospermum exhibited a significantly slower growth rate under almost all conditions, as supported by the findings (Segers et al., 2015). Similarly, Aspergillus niger demonstrated substantial structural variations under different light wavelengths, including changes in colony size, texture, and colour. In contrast, Cladosporium sphaerospermum exhibited more subtle morphological changes, likely due to its slower growth. This could be explained by the tendency of Cladosporium sphaerospermum to prioritise survival over rapid proliferation, exhibiting adaptive behaviours against enduring biological and physical challenges, such as postponing reproduction in response to unfavourable circumstances. Therefore, a longer experimental duration is necessary to evaluate this species’ responses to various light conditions. The variation in the reactions of these two species to different conditions highlights the importance of species-specific traits in mould dynamics, emphasising the requirement for tailored experimental designs that consider their growth rates and behavioural adaptations.
4.3 Mould growth dynamics under different water conditions
The findings of this study demonstrated that the differences in the responses of these two species were not limited to only light conditions but also to substrates with different water activities. Unlike Malt Extract Agar (MEA), mould growth was more noticeable on Dichloran Glycerol Agar (DG18), likely due to DG18’s lower water activity interacting with light exposure. Previous research also indicated that reduced water activity can enhance the inhibitory effects of specific light wavelengths, highlighting the complex interaction between environmental factors in mould proliferation (Segers et al., 2015). These findings show that environmental stresses, such as reduced moisture and controlled lighting, may be used to restrict mould development in the built environment.
The interaction between light wavelength and water activity significantly influenced mould growth, particularly for Aspergillus niger, where lighting conditions played a more dominant role in low water activity conditions. This aligns with previous research suggesting that fungi use light as an environmental cue, with photoreceptors regulating sporulation and hyphal growth (Priesterjahn et al., 2020; Nmom et al., 2021). A study (Hage Mogensen and Holm, 2024) has also found that combining light spectrum and environmental controls, such as daylight-optimised architectural elements or UVC-integrated building systems, can result in considerable reductions in indoor mould development. These techniques highlight new prospects for integrated environmental design compatible with sustainability and indoor health objectives.
However, the impact of the interaction between light and water activity remains insufficiently explored. In contrast, Cladosporium sphaerospermum exhibited species-specific adaptations, with short-wavelength light promoting biomass in low-water activity conditions and long-wavelength light being more effective in high-water activity. These findings emphasise the complexity of environmental factors influencing mould growth, as different species exhibit different adaptive behaviours to their surroundings. Therefore, it is crucial to consider both light and substrate conditions in mould management, highlighting the need for further research into the mechanisms that regulate these interactions.
4.4 Limitations and future research
While this study demonstrates that light wavelength significantly influences mould growth, further research should investigate the specific metabolic and genetic pathways in which they are involved. Although light wavelength and water activity influenced mould growth, their interaction appeared less significant than their individual effects. Future studies should, therefore, explore this relationship carefully to determine whether environmental factors operate independently or synergistically under certain conditions.
This study was also limited by the differing adaptive behaviours of species under challenging conditions. Cladosporium sphaerospermum exhibited a much slower growth rate than Aspergillus niger, affecting the assessment of its growth patterns and morphological adaptations. Therefore, future research should extend the experimental duration for Cladosporium sphaerospermum to better understand its growth patterns and morphological adaptations to light conditions. Long-term studies are necessary to evaluate fungal adaptation, potential resistance to continuous light exposure, and their effects under conditions that mimic real-world environments. Since specific wavelengths can either suppress or promote fungal growth, future research should systematically explore a broader range of spectral conditions to determine the most effective combinations for mould prevention.
4.5 Practical applications for indoor mould management
This study highlights the potential of spectral tuning as a non-chemical approach to mitigating mould in indoor environments. Spectrally optimised lighting systems could serve as a control layer alongside humidity regulation and ventilation. Future research should explore how such systems can be practically integrated into existing building management strategies.
Although daylight’s UV spectrum is known to influence mould growth, its application in mould control remains underexplored. Optimising daylight exposure through architectural design, strategic window placement, and light-reflective materials could help enhance its effectiveness while minimising moisture-related risks.
While daylight and specific spectral lighting have shown potential to inhibit mould growth, current indoor environmental quality (IEQ) regulations do not comprehensively address lighting as a parameter for mould prevention. Most standards focus primarily on humidity and ventilation. This study’s findings highlight a regulatory gap and suggest an opportunity for future work to contribute to prescriptive standards for indoor lighting in moisture-prone areas. Such integration of light-based strategies into IEQ frameworks could enhance risk awareness among building stakeholders and provide a more holistic approach to mould management. Thus, light-based mould prevention strategies can be developed and effectively implemented in real-world built environments to contribute to healthier indoor environments without chemical treatments.
5 Conclusion
This study investigated the influence of different light conditions on the growth dynamics of Aspergillus niger and Cladosporium sphaerospermum, revealing that spectral properties of light significantly impact mould development. Long-wavelength light promoted both mycelial growth and conidia production, whereas short-wavelength light, while not entirely inhibiting growth, had a suppressive effect on sporulation. The findings also demonstrated that water activity is a vital factor in mould growth; however, its interaction with light conditions varied by species, indicating that environmental factors may act differently independently and in combination under specific circumstances. These results highlight the potential of spectrally optimised lighting systems as a non-chemical strategy for mould prevention in indoor environments. Implementing these systems in the building environment could complement humidity and ventilation control to prevent moisture-related problems, particularly in moisture-prone areas such as basements, museums, and hospitals.
Although this study offers valuable insights, real-world studies are recommended since its lab-based limitations may not fully capture real-world environments. The difference in adaptive behaviours of different species, particularly the slower growth rate of Cladosporium sphaerospermum, suggests that extended experimental durations and tailored methodologies are necessary for a more comprehensive understanding of mould responses to light. Future research should investigate the long-term effects of light exposure and the interaction between lighting and environmental factors such as humidity and temperature. Thus, light-based mould prevention strategies can be developed and effectively implemented in real-world built environments to contribute to healthier indoor environments without chemical treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GIT: Writing – original draft, Formal Analysis, Writing – review and editing, Visualization, Data curation. EH: Formal Analysis, Data curation, Methodology, Writing – review and editing, Investigation, Writing – original draft, Visualization. EB: Conceptualization, Writing – review and editing, Supervision, Methodology, Writing – -original draft, Resources. HA: Supervision, Methodology, Investigation, Writing – review and editing, Conceptualization, Resources, Project administration, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AN, Aspergillus niger; CS, Cladosporium sphaerospermum; aw, water activity; MEA, Malt Extract Agar; DG18, Dichloran Glycerol Agar; DCW, dry cell weight; PBS, phosphate-buffered saline; LED, light-emitting diode; UV-C, ultraviolet C light; nm, nanometer; W/m2, watts per square meter; °C, degrees Celsius.
References
Al Hallak, M., Verdier, T., Bertron, A., Roques, C., and Bailly, J. D. (2023). Fungal contamination of building materials and the aerosolization of particles and toxins in indoor air and their associated risks to health: a review. Toxins (Basel). 15 (3), 175. doi:10.3390/toxins15030175
Begum, M., Hocking, A. D., and Miskelly, D. (2009). Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. Int. J. Food Microbiol. 129 (1), 74–77. doi:10.1016/j.ijfoodmicro.2008.11.020
BEIS (2021). Energy Follow up Survey: thermal comfort, damp and ventilation. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1018726/efus-thermal.pdf.
Brambilla, A., and Sangiorgio, A. (2020). Mould growth in energy efficient buildings: causes, health implications and strategies to mitigate the risk. Renew. Sustain. Energy Rev. 132 (July), 110093. doi:10.1016/j.rser.2020.110093
Caillaud, D., Leynaert, B., Keirsbulck, M., and Nadif, R.mould ANSES working group (2018). Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 27 (148), 170137. doi:10.1183/16000617.0137-2017
Cheong, K. K., Strub, C., Montet, D., Durand, N., Alter, P., Meile, J. C., et al. (2016). Effect of different light wavelengths on the growth and ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae. Fungal Biol. 120 (5), 745–751. doi:10.1016/j.funbio.2016.02.005
Cortesão, M., de Haas, A., Unterbusch, R., Fujimori, A., Schütze, T., Meyer, V., et al. (2020). Aspergillus Niger spores are highly resistant to space radiation. Front. Microbiol. 11 (April), 560–612. doi:10.3389/fmicb.2020.00560
Costa, C. P., Gonçalves Silva, D., Rudnitskaya, A., Almeida, A., and Rocha, S. M. (2016). Shedding light on Aspergillus Niger volatile exometabolome. Sci. Rep. 6 (May), 27441–27513. doi:10.1038/srep27441
Du, C., Li, B., and Yu, W. (2021). Indoor mould exposure: characteristics, influences and corresponding associations with built environment—a review. J. Build. Eng. 35 (174), 101983. doi:10.1016/j.jobe.2020.101983
Fanelli, F., Schmidt-Heydt, M., Haidukowski, M., Geisen, R., Logrieco, A., and Mulè, G. (2012). Influence of light on growth, conidiation and the mutual regulation of fumonisin B2 and ochratoxin A biosynthesis by Aspergillus Niger. World Mycotoxin J. 5 (2), 169–176. doi:10.3920/WMJ2011.1364
Hage Mogensen, E., and Holm, C. K.(2024). Intermittent low-intensity far-UVC irradiation inhibits growth of common mold below threshold limit value.
Haleem Khan, A. A., and Mohan Karuppayil, S. (2012). Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 19 (4), 405–426. doi:10.1016/j.sjbs.2012.06.002
Jones, C. L. (2023). Environmental and clinical mould spore risk thresholds. J. Bacteriol. Mycol. Open Access 11 (1), 44–48. doi:10.15406/jbmoa.2023.11.00342
Lam, M. I., Vojnits, K., Zhao, M., MacNaughton, P., and Pakpour, S. (2022). The effect of indoor daylight spectrum and intensity on viability of indoor pathogens on different surface materials. Indoor Air 32 (7), 130766–e13112. doi:10.1111/ina.13076
Liddell, C., and Guiney, C. (2015). Living in a cold and damp home: frameworks for understanding impacts on mental well-being. Public Health 129 (3), 191–199. doi:10.1016/j.puhe.2014.11.007
Lyu, J., Pitt, M., and Broyd, T. (2023). An assessment of the influencing factors promoting the development of mould in buildings, A literature review. J. Soft Comput. Decis. Anal. 1 (1), 161–180. doi:10.31181/jscda11202320
Mycol, C. M., Fungi, I., Branch, R. I., Diseases, I., and Road, K. (2016). Aspergillus species in indoor environments and their possible occupational and public health hazards. Curr. Med. Mycol. 2 (1), 36–42. doi:10.18869/acadpub.cmm.2.1.36
Narita, K., Aasno, K., Naito, K., Sasaki, M., Morimoto, Y., Igarashi, T., et al. (2020). Ultraviolet C light with wavelength of 222 nm inactivates a wide spectrum of microbial pathogens. J. Hosp. Infect. 105 (3), 459–467. doi:10.1016/j.jhin.2020.03.030
Nmom, F. W., Amadi, L. O., and Ngerebara, N. N. (2021). Influences of light regimes on reproduction, germination, pigmentation, pathogenesis and overall development of a variety of filamentous fungi – a review. Asian J. Biol. 11 (3), 25–34. doi:10.9734/ajob/2021/v11i330144
Peitzsch, M., Bloom, E., Haase, R., Must, A., and Larsson, L. (2012). Remediation of mould damaged building materials - efficiency of a broad spectrum of treatments. J. Environ. Monit. 14 (3), 908–915. doi:10.1039/c2em10806b
Priesterjahn, E. M., Geisen, R., and Schmidt-Heydt, M. (2020). Influence of light and water activity on growth and mycotoxin formation of selected isolates of aspergillus flavus and aspergillus parasiticus. Microorganisms 8 (12), 2000–2015. doi:10.3390/microorganisms8122000
Professional, L. (2017). Bio-effective lighting for humans, livestock and plants. Available online at: https://www.led-professional.com/resources-1/articles/bio-effective-lighting-for-humans-livestock-and-plants (Accessed November 12, 2023).
Purschwitz, J., Müller, S., Kastner, C., and Fischer, R. (2006). Seeing the rainbow: light sensing in fungi. Curr. Opin. Microbiol. 9 (6), 566–571. doi:10.1016/j.mib.2006.10.011
Pyrri, I., Stamatelopoulou, A., Pardali, D., and Maggos, T. (2023). The air and dust invisible mycobiome of urban domestic environments. Sci. Total Environ. 904 (June), 166228. doi:10.1016/j.scitotenv.2023.166228
Rasiukevičiūtė, N., Brazaitytė, A., Vaštakaitė-Kairienė, V., Kupčinskienė, A., Duchovskis, P., Samuolienė, G., et al. (2021). The effect of monochromatic LED light wavelengths and photoperiods on botrytis cinerea. J. Fungi 7 (11), 970. doi:10.3390/jof7110970
Segers, F. J. J., Meijer, M., Houbraken, J., Samson, R. A., Wösten, H. A. B., and Dijksterhuis, J. (2015). Xerotolerant cladosporium sphaerospermum are predominant on indoor surfaces compared to other cladosporium species. PLoS One 10 (12), e0145415–15. doi:10.1371/journal.pone.0145415
Sun, W., Ying, Y., Chen, J., and Yu, B. (2021). Light signaling regulates Aspergillus Niger bio fi lm formation by. Mol. Biol. Physiol. 12 (1), 1–13. doi:10.1128/mbio.03434-20
Viitanen, H. (2011). Moisture and bio-deterioration risk of building materials and structures. Mass Transf. - Adv. Asp. doi:10.5772/21184
World Health Organization (2009). WHO guidelines for indoor air quality:dampness and mould. Copenhagen: WHO Regional Office for Europe. Available online at: https://www.euro.who.int (Accessed March 31 2025).
Yew, S. M., Chan, C. L., Ngeow, Y. F., Toh, Y. F., Na, S. L., Lee, K. W., et al. (2016). Insight into different environmental niches adaptation and allergenicity from the Cladosporium sphaerospermum genome, a common human allergy-eliciting Dothideomycetes. Sci. Rep. 6 (26), 27008–27013. doi:10.1038/srep27008
Keywords: mould growth, light wavelength, water activity, built environment, sustainability, indoor air quality, indoor environmental quality (IEQ)
Citation: Izmir Tunahan G, Hetherington E, Barrett E and Altamirano H (2025) Exploring the impact of light wavelength on indoor mould growth. Front. Built Environ. 11:1602552. doi: 10.3389/fbuil.2025.1602552
Received: 29 March 2025; Accepted: 28 April 2025;
Published: 02 June 2025.
Edited by:
Yazmin Mack-Vergara, Technological University of Panama, PanamaReviewed by:
Nishant Raj Kapoor, Academy of Scientific and Innovative Research (AcSIR), IndiaJohn Ogundiran, University of Coimbra, Portugal
Copyright © 2025 Izmir Tunahan, Hetherington, Barrett and Altamirano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ella Hetherington, ZWxsYS5oZXRoZXJpbmd0b24uMTlAdWNsLmFjLnVr
 Gizem Izmir Tunahan
Gizem Izmir Tunahan Ella Hetherington
Ella Hetherington Edward Barrett3
Edward Barrett3