- 1Engineering Systems Management, College of Engineering, American University of Sharjah, Sharjah, United Arab Emirates
- 2Department of Civil Engineering, College of Engineering, American University of Sharjah, Sharjah, United Arab Emirates
- 3Director of the Project Delivery Institute and Director of the Construction Management Program, Cleveland State University, Cleveland, OH, United States
- 4College of Architecture Art and Design, American University of Sharjah, Sharjah, United Arab Emirates
This systematic literature review explores the critical role of microbiomes in the built environment (MoBE) and their impact on public health, particularly in pathogen transmission and infection control. Microbial communities in indoor and outdoor spaces are shaped by architectural design, ventilation, human occupancy, and other environmental factors. The COVID-19 pandemic has intensified the need to understand airborne, surface, waterborne, and other transmission pathways to mitigate disease spread. Despite advancements in microbial ecology, gaps remain in integrating findings with architectural and urban planning strategies. Future research should incorporate smart technologies, study long-term MoBE dynamics, explore sustainable building materials, and assess climate change impacts on microbial compositions. Strengthening policies on microbial risk management, air quality, and sanitation in high-occupancy environments, addressing these gaps, and fostering interdisciplinary collaboration will help create healthier, safer, and more resilient built environments aligned with global sustainability and public health goals.
1 Introduction
The built environment (BE) is regarded as man’s natural habitat that encompasses structures such as buildings, transportation systems, and all the physical surroundings constructed by humans (Kamble et al., 2024; Bruno et al., 2022; Gilbert and Stephens, 2018; Xie et al., 2023). These spaces host distinct microbial ensembles, called the “microbiome,” that differ from most microbial communities existing in other natural environments (Gilbert and Stephens, 2018; Xie et al., 2023). Numerous microorganisms can be found in BEs, but little is understood about these intricate microbial communities, their ecological function, or how they affect human health (Gilbert and Stephens, 2018; Kelley and Gilbert, 2013). As a result, there has been a recent widened interest in characterizing and analyzing the microbiomes in indoor and outdoor built environments (Prussin and Marr, 2015).
The microbiome of the built environment (MoBE) refers to the microbial communities harboured by human-constructed environments, including houses, offices, public buildings, cars, roads, and public transport, but also drinking water treatment plants and other human-built spaces (Bruno et al., 2022). However, researchers deduced that microbial communities differ significantly amongst these different environments (Prussin and Marr, 2015). As explained by Adams et al. (2014) and Yang et al. (2025), airborne and surface-deposited bacteria vary significantly between household spaces such as kitchens, bedrooms, and toilets, with distinct taxa and functional capacities identified across surfaces. Their findings emphasize the need for space-specific metagenomic microbial monitoring and hygiene practices. However, the coevolution of humans with microbes has been adversely impacted by diminished connection to nature and overly sanitized or sterilized environments, resulting in insufficient early human exposure to microbial diversity (Bruno et al., 2022), emphasizing the importance of not only understanding transmission pathways but also exploring in-depth the effective strategies to mitigate their health impacts.
Since people spend over 90% of their time indoors, the indoor component of the BE is significant when studying microbiomes (Kelley and Gilbert, 2013; Prussin and Marr, 2015; Höppe and Martinac, 1998). The emphasis on indoor microbial ecosystems is critical, as this is where human-microbial interactions are most frequent and impactful on health outcomes. While the BE supports modern population needs, it also contributes to long-term health challenges, such as rising infections, autoimmune diseases, and antibiotic resistance. Advanced molecular techniques allow for more nuanced understanding of these communities, revealing their structure, functional capabilities, and contributions to the indoor environmental quality (Gilbert and Hartmann, 2024).
Quesada-García et al. (2023) and Rice (2019) in this context, as we explore architectural history, we find that environmental design principles have always been linked to human wellbeing, a concept that is still relevant in today’s health-centered architecture. This section discusses early insights, showing how historical design approaches influence contemporary strategies to cultivate healthier, more resilient environments that harmoniously integrate microbial and human health.
Historically, architectural design was a key mechanism for promoting public health, emphasizing features like ventilation, natural light, and hygiene to prevent the spread of infectious disease (Brown et al., 2016). Ancient communities recognized the influence of architecture and building design on public health, with cities and civic structures intentionally constructed to reduce humidity and air stagnation (Bruno et al., 2022). As discussed by King (2001), the Hippocratic Corpus, dated in the 4th and 5th century BC, contains one of the earliest known assessments of humidity and airflow and their impact on the seasonal surges of infectious illnesses. Furthermore, without prior knowledge about microbes or the root causes of infectious diseases, the Roman engineer and architect Vitruvius (1st century BC) recommended constructing cities distant from wetlands and mosquitoes, and on elevated altitudes. When describing the ideal configuration for a theatre, he connected the building’s design to airflow and to the residents’ health (Mead, 1996). Additionally, in modern societies, preceding the discovery and adoption of antibiotics and vaccinations, many diseases were treated with natural remedies (Bruno et al., 2022). A prime example is the sanatorium movement, which originated in Europe and the United States of America in the late 1800s, where before the discovery of medications for tuberculosis, sanatoria, spaces intended to isolate, and treat patients were developed which were characterized by high hygienic standards and an abundance of sunlight and fresh air (Mccarthy, 2001). These new clinical settings and design attributes foreshadowed modern architectural designs that were reflected by the Swiss architect Le Corbusier, who noted in “The City of Tomorrow and Its Planning” in the year 1929 that the unplanned chaos of medieval towns gave rise to subpar housing and ineffective transit (Le Corbusier, 1987; Bruno et al., 2022) Le Corbusier further managed to foster a new consciousness regarding how cities should be envisioned, with naturally lit indoor areas, clean surfaces, and functionally designed outdoor areas. These elements, alongside their aesthetic value, represented modernist concerns about the restorative and healing powers of nature (Bruno et al., 2022). Modernist architects like Tony Garnier and Le Corbusier were significantly influenced by the proposition regarding buildings as “health machines,” as they planned their buildings to allow natural light and fresh air to foster the wellbeing of their occupants (Brown et al., 2016).
Building on these insights, integrating environmental microbiology with public health and building science allows for a more holistic understanding of how the BE can support or hinder human wellbeing (Rice, 2019; Quesada-García et al., 2023). As Rice (2019) discusses, historical architectural strategies, such as maximizing daylight, fresh air, and spatial hygiene, were deeply rooted in disease prevention long before germ theory was fully established. These foundational principles are resurging in contemporary practice, as seen in the growing movement for “healthy architecture” that designs buildings with microbiological and physiological factors in mind (Quesada-García et al., 2023). The alignment of these disciplines provides a rational framework for designing spaces that not only meet functional and aesthetic goals, but also promote microbial balance and mitigate infection risks. More recently, focusing research on the MoBE has improved human health resilience and supports key Sustainable Development Goals (SDGs), such as SDG 3: Good Health and Wellbeing and SDG 11: Sustainable Cities and Communities (Fagunwa and Olanbiwoninu, 2020; Fagunwa and Olanbiwoninu, 2020; Brown et al., 2016). Researchers have employed diverse methodologies to investigate the microbiomes of various BEs, including classrooms (Qian et al., 2012; Kembel et al., 2012; Meadow et al., 2014), homes (Jeon et al., 2013; Dunn et al., 2013; Flores et al., 2012), offices (Chase et al., 2016; Hewitt et al., 2012), hospitals (Kelley and Gilbert, 2013), museums (Gaüzère et al., 2014), nursing homes (Rintala et al., 2008), retail spaces (Hoisington et al., 2016), and subways (Robertson et al., 2013; Xiong et al., 2023; Afshinnekoo et al., 2015; Fagunwa and Olanbiwoninu, 2020). As noted by Prussin and Marr (2015), microbial communities in the BE originate from multiple sources, including human occupants, pets, indoor plants, plumbing systems, HVAC units, mold, resuspended dust, and outdoor air. These diverse reservoirs contribute varying proportions of bacteria, viruses, and fungi to indoor bioaerosols, with specific microbial species often traceable to particular sources (Prussin and Marr, 2015).
Furthermore, the Healthy Building framework established by the Harvard T.H. Chan School of Public Health delineates nine foundational elements—ventilation, air quality, thermal health, water quality, moisture, dust and pests, noise, safety, and lighting and views—that individually and interactively shape indoor environments, impacting the physiological and psychological wellbeing of building occupants (Lam et al., 2022).
Today, “Bio-informed” design is garnering the attention of architects, while scientists can render the emerging field of microbiology within the BE both timely and applicable, thus showing the potential to address critical issues related to health and safety, enhance building resilience, and contribute to sustainability efforts, particularly through the mitigation of biological degradation in building materials (Fagunwa and Olanbiwoninu, 2020; Brown et al., 2016). Thus, this research on the MoBE presents opportunities for collaboration between design science and related disciplines.
Moreover, the COVID-19 pandemic underscored the urgent need to establish protocols for reducing cross-infection in indoor environments to mitigate the risk of potentially lethal and infectious respiratory viruses (Morens et al., 2023; Hodson, 2022). The CLEAN 2020 virtual science and innovation summit, held in August 2020, convened leaders from a range of disciplines to assess the complexities of Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2) transmission within the BE, the findings of which are presented by Martinez and Morrow (2020) as well as by Morrow et al. (2021). The summit sought to evaluate the current state of knowledge regarding the factors influencing viral transmission and control, identifying research coordination opportunities to tackle COVID-19. It emphasized addressing research gaps and coordinating resources for safe facility reopening. The discussion highlighted an integrated approach combining environmental microbiology, building science, transmission science, and social science. Key findings emphasized advancing knowledge on viral persistence, transport mechanisms, and effective mitigation strategies. Additionally, the summit called for significant investment in research, bio-surveillance, and collaboration among stakeholders to lower indoor transmission risks and ensure safer BEs. Further, recent research into engineered probiotic platforms, such as the use of Escherichia coli Nissle 1917 to deliver antiviral nanobodies, highlights the growing role of microbiome-based interventions in managing respiratory pathogens like SARS-CoV-2, offering complementary insights to environmental mitigation strategies (Kamble et al., 2025).
In addition to architectural design and material choices, the composition and dynamics of MoBEs are significantly influenced by external environmental conditions, such as temperature, humidity, geographic location, air pollution, and local sanitation practices (Argyropoulos et al., 2023; Abdin and Mahmoud, 2024; Leung et al., 2019; Dietz et al., 2020).
Consequently, this review aims to synthesize the current knowledge on the various modes of transmission and their significance in developing effective strategies to mitigate health risks within BEs, stressing the importance of integrating microbiome research with architectural and public health approaches to create safer and more resilient spaces. To this end, the review is structured around two guiding research questions:
1. What are the primary transmission pathways of pathogens in indoor and outdoor built environments, and how can they be effectively mitigated?
2. How has the COVID-19 pandemic influenced research on microbiomes in the built environment (MoBE), particularly regarding pathogen transmission?
Building on these research questions, the review comprises of two methodological components: a bibliometric analysis to trace the evolution of MoBE-related research, especially in the wake of the COVID-19 pandemic, and a thematic literature review that synthesizes key findings related to pathogen transmission and environmental design. This combined approach provides both quantitative insight into research trends and a qualitative understanding of the mechanisms by which microbiomes influence health in the BE. These methodological components are elaborated in the subsequent section, providing the foundation for the analyses and insights discussed throughout this review.
2 Methods
The methodology employed in this review consists of: (1) an advanced bibliometric analysis conducted via VOSviewer, and (2) an exhaustive critical review of 43 rigorously selected peer-reviewed publications. The bibliometric analysis establishes a comprehensive framework for understanding the evolution of research trends, with particular emphasis on the impact of the COVID-19 pandemic on investigations concerning MoBE (the results of this in-depth study were presented at a conference as a separate publication and is only cited here). Following this, the systematic literature review synthesizes key insights from these selected studies, aiming to elucidate the mechanisms of pathogen transmission within BEs.
The first phase of this methodological framework entailed a detailed bibliometric analysis designed to explore the ramifications of the COVID-19 pandemic on scholarly inquiries into microbial dynamics in the BE. For this purpose, the Scopus database was utilized due to its extensive repository of peer-reviewed academic literature across diverse disciplines. A refined search using the keywords, “built environment” AND (virus OR pathogens OR bacteria OR microbio* OR microb* OR microbiota OR microorganisms), yielded 1,056 documents (1992–2025). After filtering for English-language and fully published documents, 1,043 entries were retained.
To further narrow the scope to engineering-related studies on the BE, and exclude medical/genetic content, documents containing terms such as “Microbiology,” “Microflora,” “Genetics,” “RNA 16S,” “Classification,” “Metagenomics,” “RNA, Ribosomal, 16S,” “Phylogeny,” “High Throughput Sequencing,” “Environmental Microbiology,” “DNA Extraction,” “Gene Sequence,” “DNA Sequence,” “Metagenome,” “Actinobacteria,” “Taxonomy,” “Prevalence,” “Metabolism,” “Firmicutes,” “Intestine Flora,” “Bioinformatics,” “Sequence Analysis, DNA,” “Real Time Polymerase Chain Reaction,” “Amplicon,” “RNA,” “Major Clinical Study,” “High-Throughput Nucleotide Sequencing,” “Chemistry,” “Bacterium Culture,” “Incidence” were excluded. This process refined the dataset to 583 documents, ensuring a precise alignment with the overarching research focus of this review.
Metadata from the selected documents, including titles, authors, publication years, journals, keywords, and abstracts, were meticulously extracted and exported into a format compatible with VOSviewer for an in-depth bibliometric analysis. VOSviewer was then employed to construct and visualize a series of bibliometric networks, including co-authorship, co-occurrence, citation, bibliographic coupling, and co-citation networks. These visualizations facilitated the identification of key intellectual relationships and research trajectories within the field. Various analyses grouped related items, allowing the delineation of significant research domains and the identification of emergent themes.
The second phase of the methodology comprised an in-depth and systematic literature review, focusing specifically on the transmission of pathogens within BEs. A targeted search was conducted using the keywords “built environment” AND “transmission” AND (“pathogens” OR “microbio*”), initially retrieving 98 documents. To ensure that only recent and pertinent studies were included, the search was limited to publications from 2019 to 2024, reducing the dataset to 67 documents. A further filter was applied to restrict the results to English-language publications, yielding 65 relevant studies. The dataset was then refined by excluding document types such as short surveys, errata, and letters, focusing solely on articles, reviews, books, and book chapters. This step further narrowed the list to 62 documents.
The literature screening for this study followed the PRISMA statement, ensuring systematic identification, selection, and evaluation of relevant literature, while providing reliability and reproducibility. An extensive screening of abstracts and full texts assessed publication alignment with the main focus on pathogen transmission in BEs. Consequently, 44 papers were identified as directly relevant, with one duplicate removed, resulting in a final selection of 43 peer-reviewed publications. Selected papers were in their final stages, ensuring only fully validated, peer-reviewed studies were considered. This literature corpus underwent analysis to extract insights into pathogen transmission mechanisms, environmental influences on microbial behaviour, and strategies for mitigating transmission in BEs. Figure 1 gives the PRISMA flow diagram.
Together, these complementary methods provided both a broad overview of the research landscape and a focused understanding of the factors influencing microbial transmission in BEs.
3 Results and discussion
3.1 Bibliometric analysis
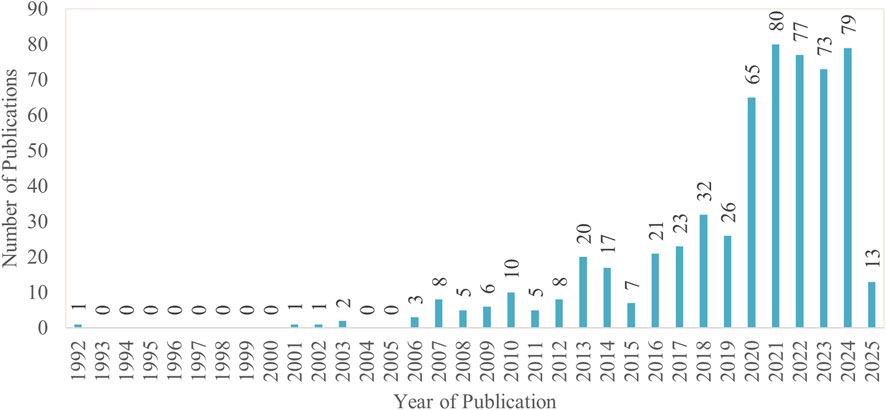
Between 1992 and 2025, 583 MoBE-related publications were retrieved from the Scopus database, as depicted in Figure 2. Research in this field remained scarce until 2009, with few publications appearing during this period. From 2010 onward, the number of studies gradually increased up to 2017. A sharp rise was observed in 2020, peaking at 80 publications in 2021. This aligns with the onset of the COVID-19 pandemic, which spurred interest around viral transmission. Although publication numbers have fluctuated in recent years, they remain high, indicating sustained research interest in this field.
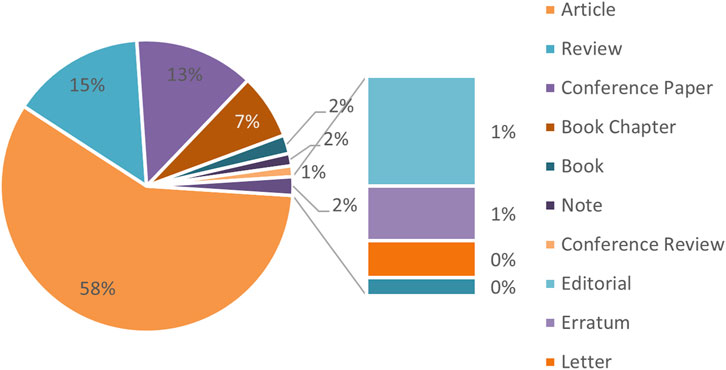
To add, Figure 3 categorizes these publications by type, showing that journal articles constitute the largest share (58%), followed by review articles (15%), conference papers (13%), book chapters (7%), books and notes (2% each). Additional document types include editorials, conference reviews, letters, errata, and short surveys.
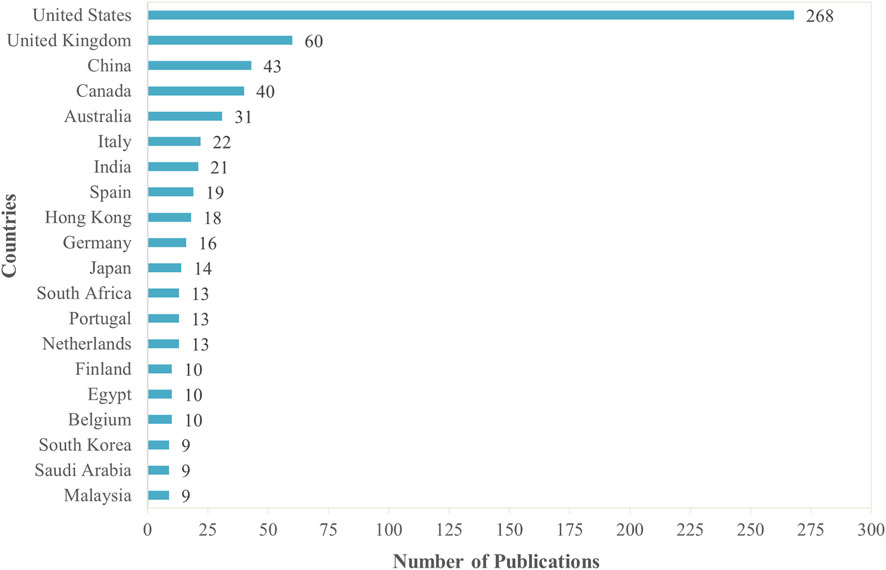
Furthermore, Figure 4 highlights the top 20 countries contributing to research on MoBE. The United States leads significantly with 268 publications, far surpassing the United Kingdom, which ranks second with 60 publications. China (43), Canada (40), and Australia (31) follow, indicating strong research output from these regions. European countries such as Italy (22), Spain (19), and Germany (16) also contribute notably. Asian nations including India (21), Hong Kong (18), Japan (14), and South Korea (9) reflect a growing interest in this field. Additionally, South Africa (13) stands out as the leading African contributor. The distribution of publications suggests that research on MoBE is predominantly driven by North America, Europe, and parts of Asia, with emerging contributions from other regions.
3.1.1 Co-occurrence analysis of all keywords and authors’ keywords
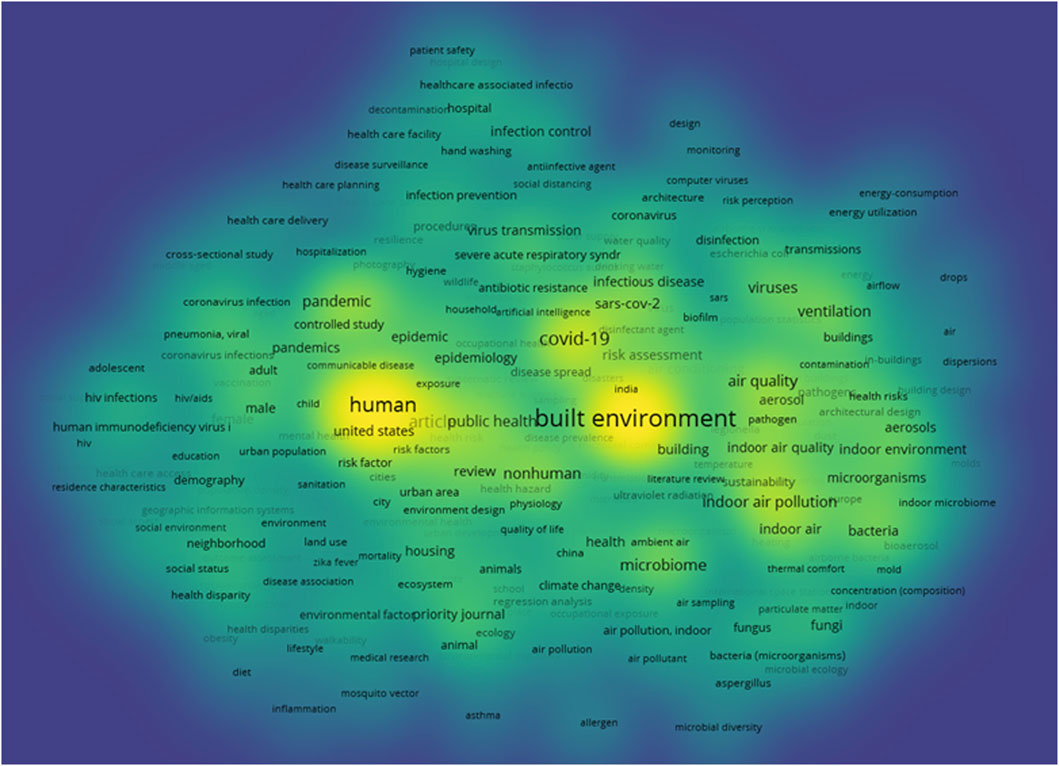
A co-occurrence analysis was performed using VOSviewer to examine keyword relationships based on their co-appearance in published documents. Out of 5,175 author-provided keywords, only 304 met the threshold of occurring more than five times. VOSviewer computed their total co-occurrence link strength, enabling the identification of the most significant keywords.
The density visualization analysis in VOSviewer (Figure 5) highlights the most frequently occurring and strongly connected keywords in the literature on MoBE. Prominent keywords such as “built environment,” “human,” and “COVID-19” appear with high intensity, indicating their central role in this research domain. The term “built environment” is the most dominant, reflecting its strong association with various subtopics, including “air quality,” “ventilation,” and “microbiome.” The density map also reveals the significant influence of COVID-19-related research, with high-frequency terms such as “pandemic,” “COVID-19,” “SARS-CoV-2,” and “virus transmission” having higher distinct intensity. Additionally, keywords related to public health, such as “hygiene” and “infection control,” appear prominently, demonstrating the growing focus on disease prevention and human health in BEs. The visualization further suggests an interdisciplinary trend, where environmental science, microbiology, and public health intersect, reinforcing the relevance of BE research in addressing contemporary health challenges.
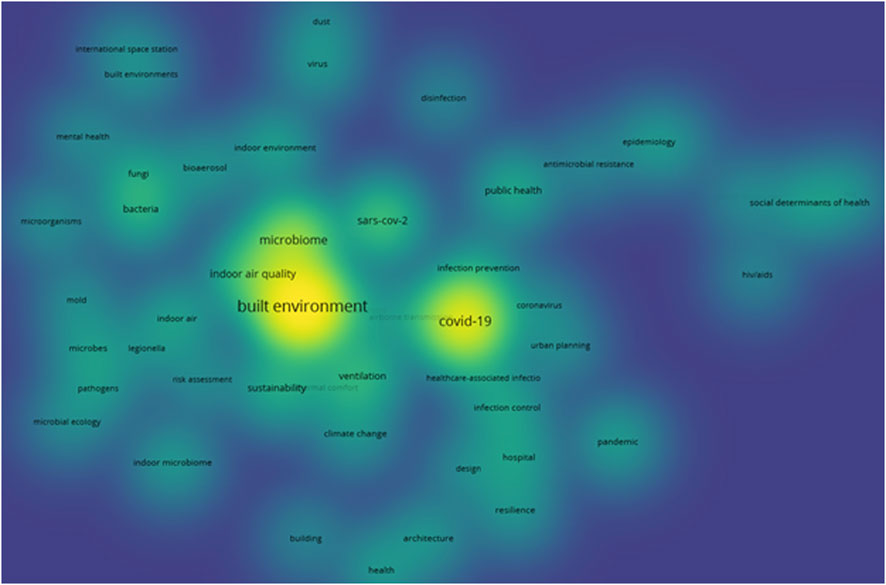
Similarly, a co-occurrence analysis of authors’ keywords was performed using VOSviewer and among the 1,821 keywords identified, only 50 met the threshold of appearing more than five times. The density visualization for this (Figure 6) further highlights the prominence of the keywords - “built environment,” “COVID-19,” and “microbiome,” as seen above, indicating their strong presence and central role in current research. The high-density areas also emphasize the growing interest in “SARS-CoV-2,” reflecting its significance in recent studies. In contrast, terms like “urban planning” and “architecture” appear with lower density, suggesting a gap in research on microbiomes at an urban scale.
3.1.2 Citation, bibliographic coupling and co-citation analysis of the sources
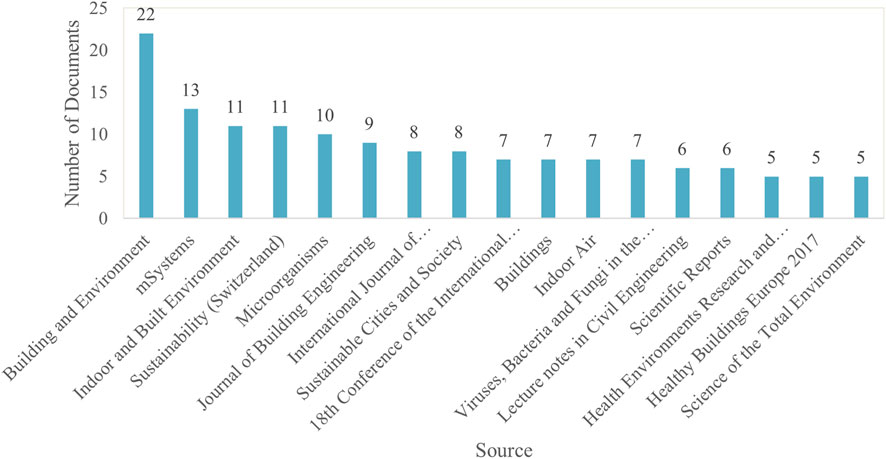
Citation Frequency. Among 363 publication sources, only 17 met the threshold of publishing more than five documents on MoBE. For these selected sources, VOSviewer calculated the total citation link strength to identify those with the strongest connections.
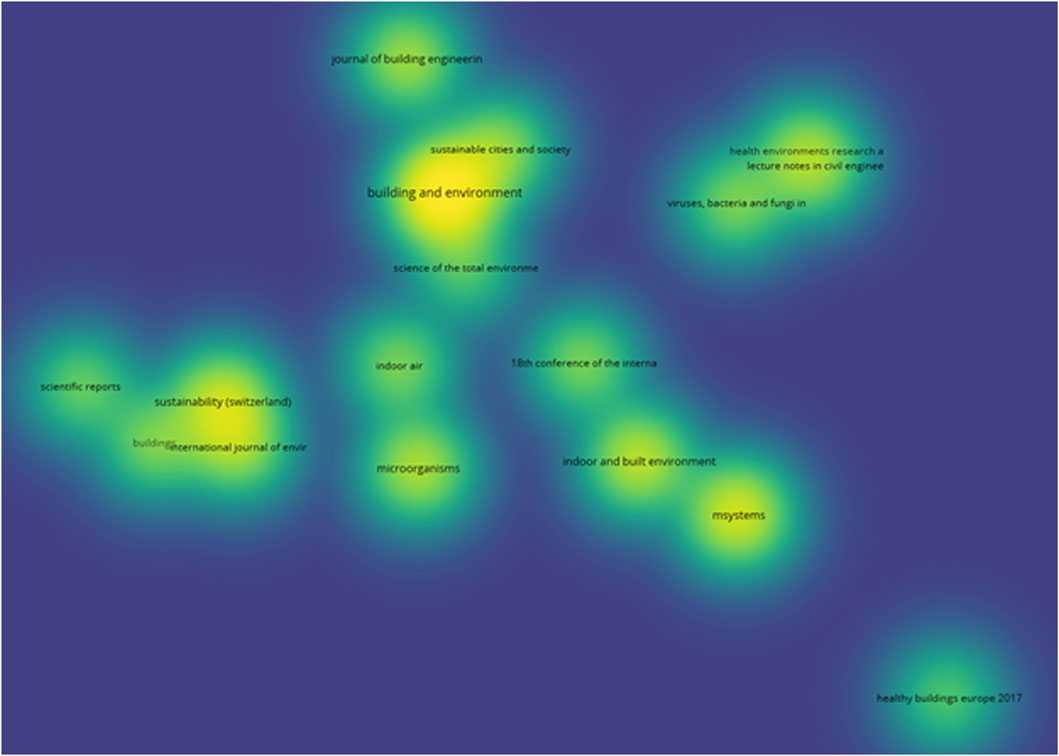
Figure 7 illustrates the top sources contributing to research on MoBE, identifying the most influential sources in the field. “Building and Environment” ranks highest with 22 publications, followed by “mSystems” with 13. “Indoor and Built Environment” and “Sustainability (Switzerland)” each have 11 publications, further emphasizing their impact. The variety of journals highlights the interdisciplinary nature of this research, spanning engineering, sustainability, environmental science, and microbiology.
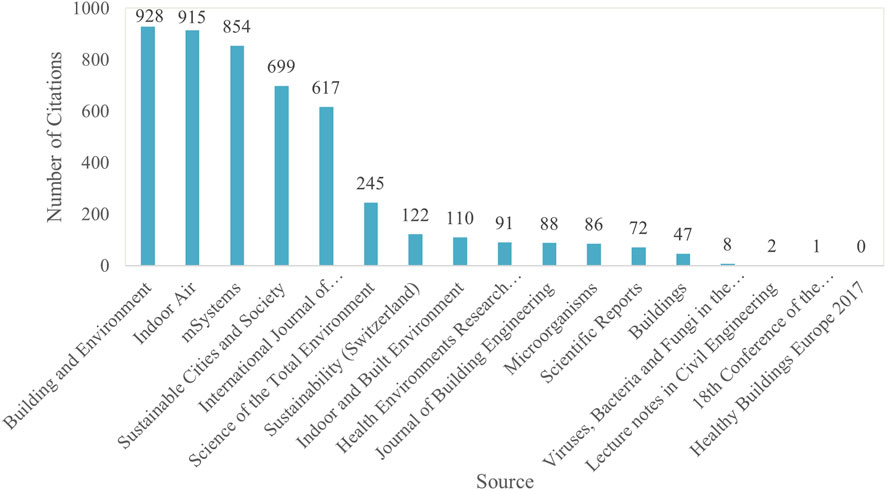
On the other hand, Figure 8 highlights the top sources with the highest number of cited journals on the topic of MoBE. “Building and Environment,” “Indoor Air,” “mSystems,” “Sustainable Cities and Society,” and the “International Journal of Environmental Research and Public Health” stand out with exceptionally high citation counts, ranging from 617 to 928.
Additionally, a bibliographic coupling analysis using VOSviewer identified the degree of thematic relatedness between sources based on shared references in their citations. Of the 363 sources, only 17 met the required threshold. In this context, the brightest area in the density visualization diagram (Figure 9) corresponds to “Building and Environment,” indicating highly shared references. Surrounding this core, “Indoor and Built Environment,” “mSystems,” “Sustainability,” and “Indoor Air” also appear as notable sources for researchers focusing on MoBE.
Moreover, a co-citation analysis was performed using VOSviewer to assess the interrelationship between sources based on the frequency with which they are cited together. However, no analysis could be generated, indicating that there are insufficient instances where two or more sources are cited together in the same documents. This could indicate a lack of direct connections or common references between the sources in the field, suggesting limited overlap in the literature or a fragmented research area with few interrelated studies.
In short, the growing recognition of the BE’s role in shaping microbial communities and influencing public health, particularly considering the COVID-19 pandemic, underscores the urgent need to better understand these dynamics. Despite substantial recent research into the presence, abundance, and diversity of microorganisms in the BE, as highlighted in the review by Li et al. (2021) and further emphasized by Bruno et al. (2022), research connecting BE attributes to its microbial communities is generally lacking. This gap is particularly important as pathogens emerge and environmental changes affect human-microbe interactions. The bibliometric analysis highlights the need for a more cohesive understanding of this field. Therefore, the systematic review seeks to fill these gaps by synthesizing existing literature and offering a comprehensive overview of the MoBE, to inform public health strategies, building design principles, and disease prevention efforts.
3.2 Modes of transmission of microbiomes in the built environment
Microbial communities found in indoor spaces are typically composed of bacteria, virus, fungi, and others, originating from human occupants, ventilation systems, and environmental surfaces. For example, Staphylococcus and Streptococcus species, commonly associated with skin and respiratory flora, are frequently found on high-contact surfaces like desks and doors. Fungal genera such as Aspergillus and Penicillium often thrive in damp conditions, including HVAC systems and bathrooms, where they influence indoor air quality and may pose respiratory risks (Gilbert and Stephens, 2018; Prussin and Marr, 2015; Jeon et al., 2013; Kim et al., 2022).
Understanding microbial transmission pathways, including microbial shedding and persistence in air, water, and on surfaces, is essential for evaluating associated risks and developing effective mitigation strategies (Martinez and Morrow, 2020; Morrow et al., 2021). Microbial pathogens spread through various transmission pathways, including direct contact, airborne particles, food or water contamination, blood, sexual contact, vector-borne, etc. (Argyropoulos et al., 2023). These can be broadly classified into contact transmission and non-contact transmission (Abdin and Mahmoud, 2024). Moreover, the BE plays a crucial role in the transmission dynamics of COVID-19 through factors like occupant density, human behaviour, spatial design, and ventilation (Dietz et al., 2020). SARS-CoV-2 can be transmitted through respiratory droplets, fomites, and aerosols, each shaped by different BE elements.
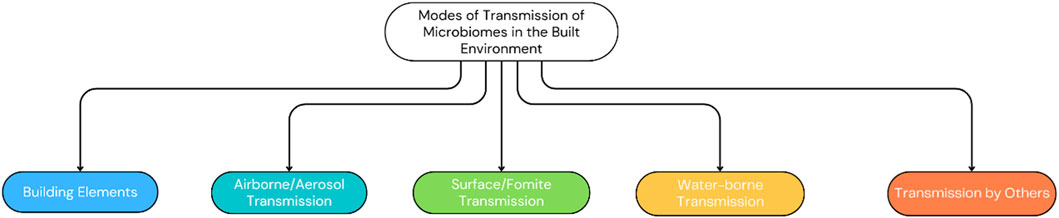
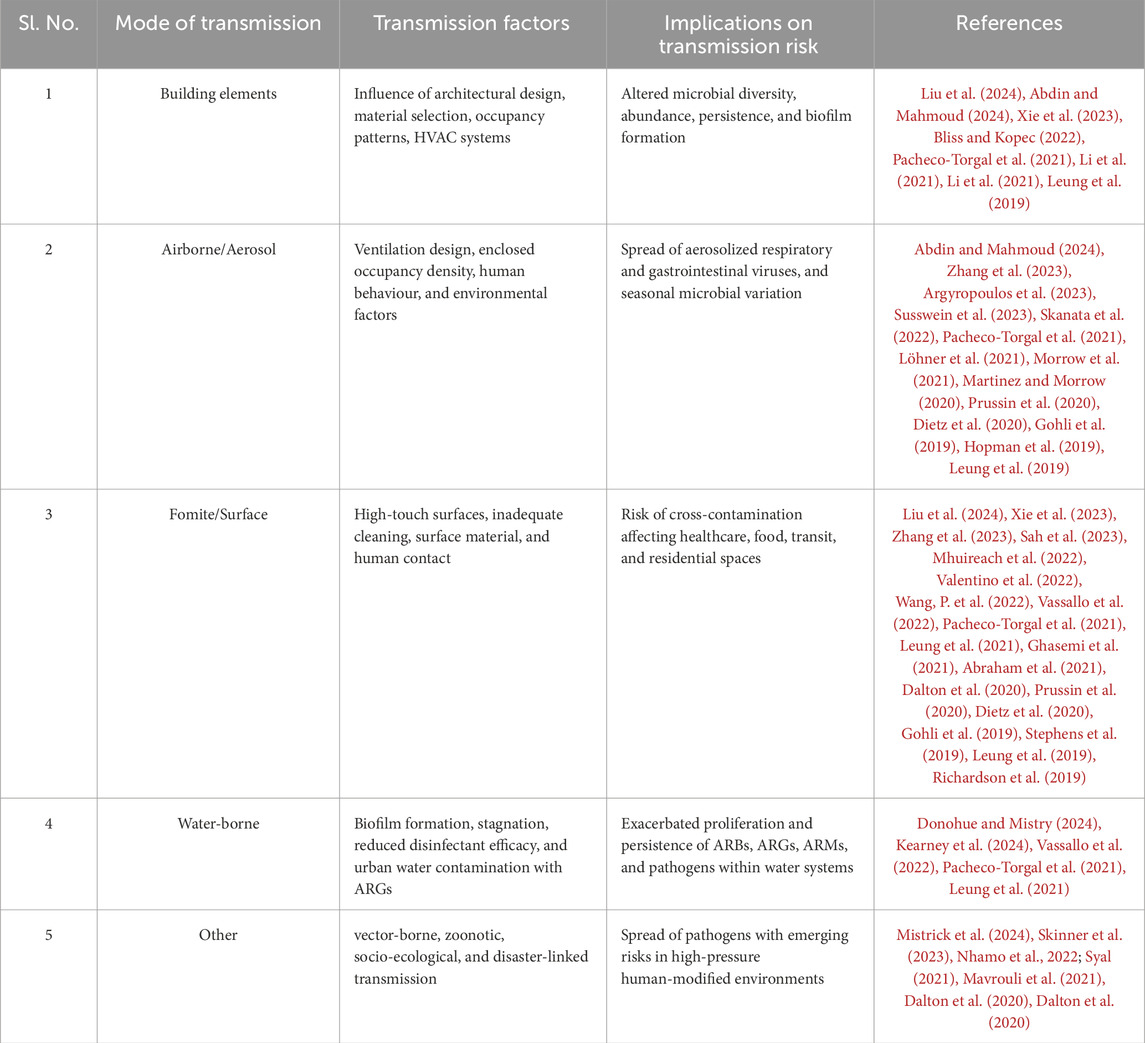
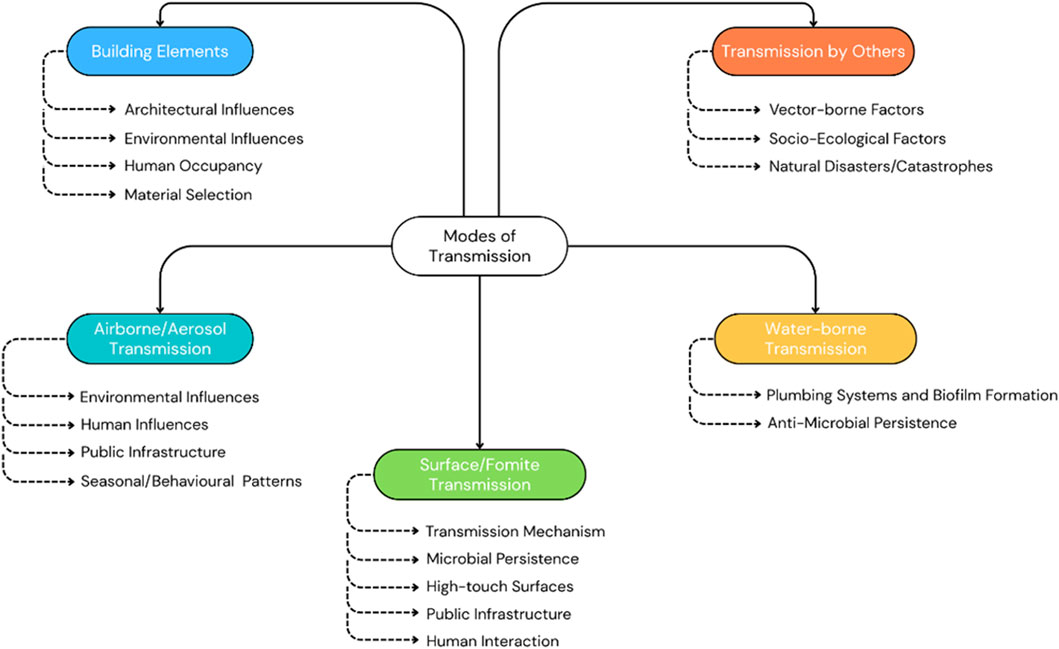
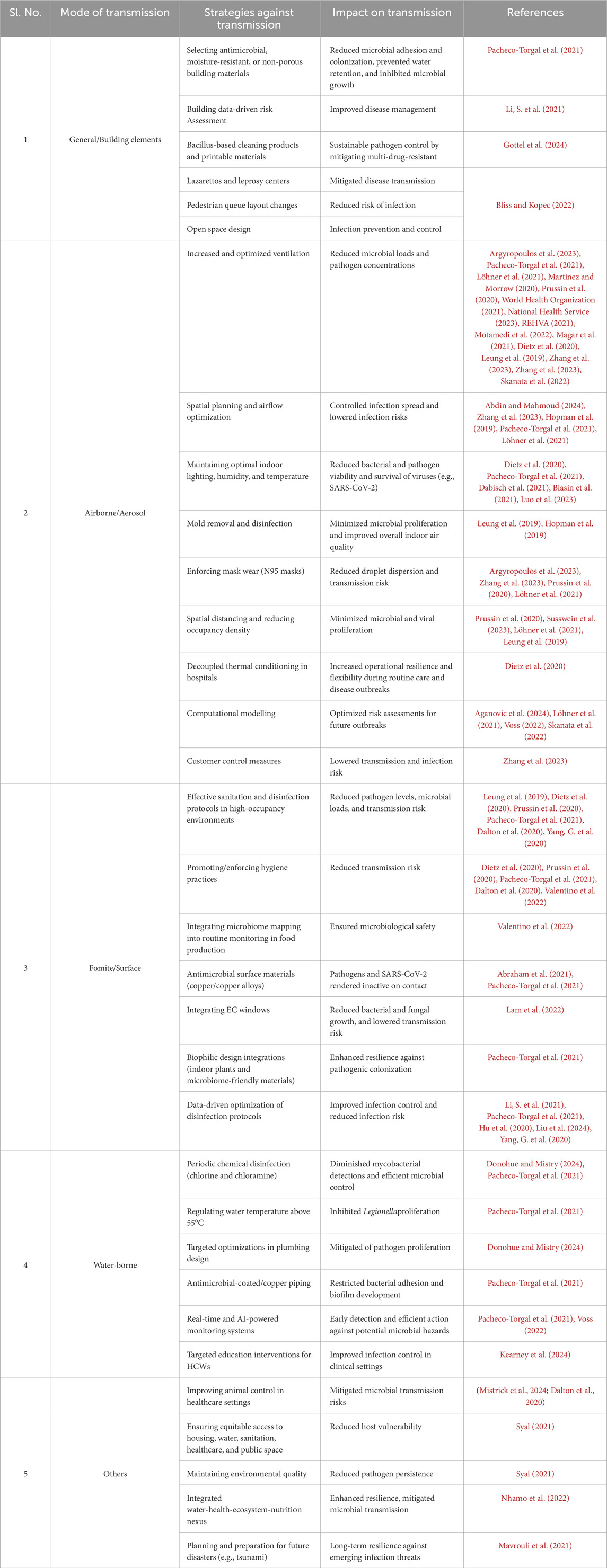
Figure 10 shows microbial transmission pathways within the BE, to inform effective control measures and public health strategies against pathogen spread. Building elements, including ventilation and occupant density, majorly influence the spread of microbial systems and are therefore included as a subsection here. Table 1 represents a summary of the literature in this section, categorized by transmission methods of the MoBE, while Figure 11 gives a summary of the various modes of transmission of microbiomes of the BE as recognized in this literature work.
3.2.1 Building elements
Buildings serve as dynamic microbial ecosystems, where the interplay of architectural design, human activity, ventilation systems, and environmental factors shapes the activity of microbial communities within indoor spaces. Table 1 summarizes these key determinants and their implications for microbial transmission risks.
3.2.1.1 Architectural and environmental influences
Design and environmental variables—including building typology, spatial configuration, ventilation type, and microclimatic conditions—play a pivotal role in shaping indoor microbial communities. Abdin and Mahmoud (2024) emphasize how environmental inputs like soil, vegetation, climate, and air velocity influence microbial influx and behaviour, while Li et al. (2021) underscore the absence of computational tools to model spatially distributed infection risks. Liu et al. (2024) and Leung et al. (2019) further demonstrate that spatial layout, occupancy patterns, room functionality, and ventilation systems significantly alter microbial diversity and abundance, with natural ventilation supporting more diverse communities compared to mechanical ventilation.
Although these studies confirm the spatial sensitivity of microbiome composition, a gap remains in integrating spatial analytics and predictive microbial modelling into mainstream building performance assessments. This limits architects and engineers from anticipating microbial dynamics in design decisions.
3.2.1.2 Human occupancy
Humans are one of the most significant microbial sources indoors. Liu et al. (2024) and Li et al. (2021) show that occupancy patterns influence microbial loading, with high-density use and varying activity levels reshaping microbiota in different zones. These human-associated microbes originate from the skin, oral, and gut microbiomes, and are redistributed through contact, bioaerosols, or dust (Li et al., 2021). Over time, the microbial fingerprint of indoor spaces is shaped by room function and disinfection schedules.
Interestingly, microbial transmission risk is also temporally dynamic, influenced by occupant movement patterns throughout the day (Liu et al., 2024). This highlights the need for time-based risk assessments alongside spatial analysis.
3.2.1.3 Material selection
Building materials function as reservoirs and vectors of microbial transmission. Pacheco-Torgal et al. (2021) point to porous materials, like drywall, insulation, and untreated wood, as conducive to microbial retention and biofilm formation, especially in humid environments. These materials sustain long-term microbial viability, persistence, and indirect transmission. Improper material maintenance exacerbates this risk.
Challenges lie in how common construction materials are rarely evaluated for microbial compatibility. While moisture-resistant materials offer better control, their use remains limited to specialized buildings.
Together, these findings establish buildings as more than passive environments—they are active agents in microbial ecology. Despite extensive documentation of microbial influences by spatial design, human presence, and material choice, policies continue to overlook microbial factors in architectural planning and performance metrics.
3.2.2 Airborne/aerosol transmission
Airborne transmission involves the spread of infectious agents through aerosols and fine respiratory droplets that remain suspended in the air over time and distance. This pathway has emerged as particularly critical in enclosed BEs, especially after the COVID-19 pandemic. As summarized in Table 1, understanding the mechanisms of this transmission mode is essential for resilient building design and ventilation policy.
3.2.2.1 Environmental and structural influences
Environmental and structural influences play a pivotal role in pathogen transmission within the BE. Argyropoulos et al. (2023) emphasize the significance of airborne transmission in microbiome dispersal, shaped by ventilation systems, airflow dynamics, temperature, and humidity. Inadequate or poorly maintained ventilation can unintentionally promote pathogen spread. Pacheco-Torgal et al. (2021) affirm that high humidity stabilizes droplet-based pathogens, while low humidity enhances aerosol persistence. Leung et al. (2019) highlight outdoor air and dust as key sources of indoor microbes, with geography and environmental factors shaping microbial profiles. The study also identifies that mechanical ventilation can reduce microbial loads, while moisture-rich spaces foster fungal growth. Similarly, Martinez and Morrow (2020), Morrow et al. (2021), and Skanata et al. (2022) highlight the role of aerosols in transmitting respiratory viruses, with particles that remain airborne, dispersing beyond 1–2 m (Wang, C. C. et al., 2021; Xie, X. et al., 2007; Dabisch et al., 2021). Abdin and Mahmoud (2024) stress the importance of grasping post-COVID-19 indoor infection dynamics, noting air stagnation, population density, and recirculation as key contributors, with pathogen buoyancy further influenced by particle mass, temperature, humidity, and human activity.
Consequently, it is imperative to examine the spatial and temporal pathogen dispersal patterns, alongside the development of science-based guidelines for safe indoor space utilization to mitigate future outbreaks.
3.2.2.2 Human influences
Human activities such as coughing, sneezing, speaking, or even moving through a space contribute to bioaerosol generation and resuspension. Notably, Dietz et al. (2020) stress that SARS-CoV-2 is primarily transmitted through droplets and aerosolized particles released from the mouth. Pacheco-Torgal et al. (2021) highlights airborne microbial dissemination as a critical concern in enclosed environments, where pathogenic aerosols persist depending on airflow and ventilation.
Löhner et al. (2021) found that sneezing expels particles at speeds up to 14 m/s, enabling widespread dispersion. Argyropoulos et al. (2023) and Leung et al. (2019) noted that even minor physical movement can resuspend settled particles, increasing exposure risk. Prussin et al. (2020) highlighted actions like flushing toilets or vomiting, which aerosolize gastrointestinal viruses like norovirus. Zhang et al. (2023) estimated an 18.7-fold increase in infection risk indoors due to suboptimal air movement. Meanwhile, Susswein et al. (2023) introduced GPS-derived mobility data, showing increased indoor occupancy during winter months, correlating with heightened transmission risk.
3.2.2.3 Public infrastructure
High-occupancy enclosed environments such as healthcare facilities and transit systems exhibit high aerosol transmission risk. Dietz et al. (2020) showed how confined, poorly ventilated public settings facilitate persistent aerosols. Gohli et al. (2019) found seasonal variation in airborne microbiota in subway systems, while Hopman et al. (2019) used genomic analysis to trace hospital infections to aerosolized particles from plumbing infrastructure.
In brief, the literature makes it clear that airborne transmission in BEs is a function of spatial design, environmental controls, and human behaviour. Despite these insights, policy translation remains weak, especially outside healthcare settings.
3.2.3 Surface/fomite transmission
Fomite transmission, the spread of pathogens via contaminated surfaces, materials, or objects, plays a significant role in infectious disease dynamics, including SARS-CoV-2, within the BE (Castaño et al., 2021; Leo et al., 2023; Tsang et al., 2023). These surfaces serve as reservoirs for pathogen transmission, posing a threat to human health (Sah et al., 2023). As detailed in Table 1, this transmission mode is influenced by surface type, material properties, cleaning protocols, human behaviour, and environmental conditions.
3.2.3.1 Transmission mechanism
Zhang et al. (2023) describe fomite transmission as a two-step process: the contamination of environmental surfaces and the subsequent acquisition of viral particles through physical contact. Surface contamination is attributable to the deposition of viral droplets originating from speech or coughing by infected individuals near surfaces, and the direct transfer of the virus via contact. However, quantifying transferred viral loads presents challenges due to the limited and heterogeneous quantities of viruses present in the hands of the infector.
Likewise, Stephens et al. (2019) reviews three primary methodologies employed to assess fomite-mediated microbial transmission and its ramifications for human health: (i) experimental quantification of microbial transfer (ii) mathematical modeling of microbial exchange in conjunction with other exposure pathways (e.g., direct contact and aerosol transmission), and (iii) epidemiological investigations on transmission dynamics. These approaches collectively contribute to a nuanced understanding of the mechanisms underlying microbial dissemination via fomites and other vectors.
3.2.3.2 Microbial persistence
Numerous studies indicate that pathogens, including SARS-CoV-2, can persist for hours to days on materials such as plastic, metal, glass, cloth, and skin (Dietz et al., 2020; Chin et al., 2020; Hirose et al., 2021; Doremalen et al., 2020). In this regard, Dietz et al. (2020) assert that fomites function as viral reservoirs, enabling indirect transmission when individuals interact with contaminated surfaces, with the persistence of the virus on plastic, cardboard, and stainless-steel lasting several hours to days. Further, Ghasemi et al. (2021) used molecular simulations to show that viral adhesion varies by material (e.g., aluminum, copper, copper oxide, polyethylene, and silicon dioxide), with the strongest binding on silicon dioxide and weakest on polyethylene. Polyethylene surfaces also facilitate viral transfer through water, whereas silicon dioxide surfaces cause viral damage through water.
3.2.3.3 High-touch surfaces
Pacheco-Torgal et al. (2021) emphasize that high-touch surfaces, including doorknobs, elevator buttons, desks, and countertops, are contact points for microbial transfer between individuals and the environment. Further, within hospital environments, the contamination of high-touch surfaces, including medical devices and hospital furnishings, is a pivotal factor in the dissemination of several pathogens, as elucidated by Dalton et al. (2020).
In the same way, Sah et al. (2023) investigated the profound health implications of microbial exchange between humans and the BE, within a laboratory handling SARS-CoV-2 samples during the COVID-19 pandemic. The researchers sampled a range of surfaces, including floors, benches, and sinks, across the laboratory over 3 months, revealing elevated bacterial diversity on the floors. The floors were primarily colonized by environmental bacteria, while benchtops exhibited a greater prevalence of human-associated microbial taxa.
3.2.3.4 Public infrastructure
Fomite transmission is a critical vector for pathogens, heightening the risk of cross-infections across healthcare environments, public spaces, and the food industry (Abraham et al., 2021). Liu et al. (2024) detected SARS-CoV-2 RNA on surfaces across restaurants, grocery stores, and healthcare facilities, underscoring its significance in indoor settings.
In this context, Abraham et al. (2021) elucidates that hospital-acquired infections (HAIs) remain a global health concern, with fomite transmission playing a significant role in community settings like healthcare settings, transportation hubs, and public gatherings. This underscores the necessity for enhanced infection control strategies during epidemic and pandemic situations. Similarly, a review by Dalton et al. (2020) highlights the critical role of the BE in pathogen transmission within healthcare settings, where the environment acts as a reservoir for multidrug-resistant organisms. Research finds that hospital design elements influence microbial transmission, with private room configurations and certain surface types associated with a reduced risk of HAIs, highlighting the importance of environmental factors in infection control.
Notably, fomite transmission extends beyond healthcare and public spaces to food-handling environments, where contaminated surfaces serve as vectors for pathogenic microorganisms and antibiotic-resistant bacteria. Accordingly, Valentino et al. (2022) highlight that microbial communities adapt to specific microclimatic conditions within these environments, often forming biofilms allowing them to persist on surfaces despite routine cleaning and disinfection, supporting the hypothesis that these environments may select for resistant and pathogenic microorganisms.
In addition, Wang et al. (2022) investigated fomite transmission, within an office setting, proposing that microbial invaders could be identified through microbial interaction networks, with contamination levels highest on hands and decreasing with geodesic distance from touchpoints, highlighting the critical role of human behavior in microbial dispersal and contamination dynamics. These findings support public health interventions addressing microbial interactions and anthropogenic factors alongside pathogen-specific disinfection measures.
Furthermore, several respiratory viruses, including the coronavirus, and coxsackie virus can survive on surfaces for several days, potentially facilitating infection if proper disinfection protocols are not followed (Kutter et al., 2018). Vassallo et al. (2022) highlight that densely populated urban environments are reservoirs for antibiotic-resistant bacteria (ARB) and genes (ARGs), its spread influenced by surface proximity, hygiene practices, and urban infrastructure. Airports, airplane cabins, shared facilities, and public transportation surfaces are key vectors for dissemination because of high-contact surfaces and inadequate sanitation. Similarly, urban green spaces, while beneficial, can host antimicrobial-resistant microorganisms (ARMs) due to atmospheric deposition and surface contamination from human-animal interactions.
Additionally, Leung et al. (2021) examined microbiomes and resistomes of public transit systems in six cities across three continents. Results indicate city-specific factors as primary determinants of microbiome diversity, revealing significant geographic variation in species, strain-level growth profiles, and resistance genes. The analysis suggests human skin, soil, and public transit surfaces are key sources of resistance genes, highlighting the need for further investigations to understand the factors shaping public transit systems microbiomes. Likewise, Gohli et al. (2019) confirm that subway surfaces are key vehicles for microbial transmission, due to constant interaction between people and the BE.
The COVID-19 pandemic reinforced the critical link between public health and the BE, revealing significant correlations between morbidity and mortality rates and factors such as housing quality, socioeconomic status, and urban infrastructure (Ghasemi et al., 2021). To foster resilient, sustainable urban development, there is a call for structural modifications to public spaces, transportation networks, and hygiene practices in cities, benefiting marginalized populations disproportionately affected by the pandemic. Despite advancements in understanding the virus’s transmission via surfaces, there remains a substantial gap in empirical data on nanoscale interactions between SARS-CoV-2 and various materials, highlighting the need for further research to clarify mechanisms of viral adhesion and its potential for surface transmission.
3.2.3.5 Human interaction
The interaction between human-associated microbiomes and indoor surfaces contributes to the dynamic exchange of microbial communities, potentially altering pathogen survival and transmission dynamics, as elucidated by Pacheco-Torgal et al. (2021). Liu et al. (2024) asserts that respiratory pathogens may be transmitted through direct contact, dust, or fomites when an individual contacts a contaminated surface and subsequently touches their facial, oral, or nasal regions. Likewise, Prussin et al. (2020) emphasize that gastrointestinal viruses are commonly transmitted via fomites like food, doorknobs, and electronic devices when individuals touch contaminated surfaces and transfer the virus to their mucosal membranes through hand-to-mouth contact.
The home BE predominantly harbors human-associated bacteria (human microbiomes) serving as microbial reservoirs (Xie et al., 2023). High-contact (e.g., sponges, toothbrushes, faucets, fridges, keyboards, pens, credit cards, cellphones, and keys) and low-contact surfaces (e.g., shower drains, countertops, sinks, walls, ceilings, floors, cutting boards, and stove knobs) demonstrate enhanced bacterial viability due to frequent microbial transfer from humans. However, Xie et al. (2023) suggest that most bacteria associated with human skin microbiomes are non-viable, indicating that transfers may result in diminished bacterial viability within the BE, with bacterial cultures in sterile settings displaying significantly higher average viability than those in non-sterile environments, weakening by 45% on sterile and 90% on non-sterile surfaces, over a week. These findings suggest that environmental exposure may reduce bacterial viability, contrasting initial expectations regarding high-contact surfaces.
Similarly, Richardson et al. (2019) explored the microbiota of a college dormitory, focusing on cohabitation’s impact on microbial communities. Their analysis identified distinct microbial interaction networks, with hands as the primary vector for transmission, while shoe-associated samples demonstrated more autonomous interaction patterns, highlighting the complexity of microbial dynamics in communal environments.
Humans are also identified by Leung et al. (2019), as key contributors to indoor microbiomes through skin-emitted microorganisms that form a “personalized microbial cloud” unique to individual occupants. However, well-ventilated or sparsely occupied spaces dilute this effect, resembling outdoor microbial communities. Factors including occupancy density, frequency, and ventilation strategies further influence human impact on surface transmission of microbiomes.
Moreover, Mhuireach et al. (2022) investigated bacterial transfer dynamics between indoor plants and human skin in a controlled climate chamber, revealing that soil-derived microbial taxa increased skin microbial diversity for at least 24 h post-contact, significantly influencing skin microbiome dynamics. This research highlights the health implications of interactions between indoor plant-associated and human-associated microbiomes, emphasizing the need for further investigation in urban living environments.
Therefore, fomite transmission is a recognized but often overlooked infection pathway in multiple BEs, including healthcare facilities, public spaces, food processing environments, and residential settings. While many studies have examined persistence and cleaning efficacy, few insights have influenced spatial or infrastructure-level policy. Further, as seen, viral fomite transmission gained attention during the COVID-19 pandemic. However, there remains comparatively limited empirical research focused on bacterial and other microbial communities deposited on surfaces in everyday BEs, showcasing a gap in the current literature.
3.2.4 Water-borne transmission
Waterborne transmission refers to the spread of microbial pathogens through contaminated water systems, posing significant risks in BEs where plumbing systems, biofilm formation, and disinfectant efficacy influence microbial persistence (Donohue and Mistry, 2024).
3.2.4.1 Plumbing systems and biofilm formation
Pacheco-Torgal et al. (2021) sheds light on plumbing systems as reservoirs for microbial colonization, with biofilm formation on pipe surfaces facilitating pathogen persistence, which later become aerosolized through water distribution points, including taps, and cooling towers, leading to inhalation-based transmission. Stagnant water within piping networks exacerbates microbial proliferation, particularly in sections of plumbing infrastructure where disinfectant residuals dissipate over time.
On a similar note, Donohue and Mistry (2024) emphasize that hot water systems within the BE, are optimal for the proliferation of pathogens, including nontuberculous mycobacteria (NTM). The investigation concluded that heating water diminishes the efficacy of chemical disinfectants, like chlorine or chloramine, thereby raising the rate of microbial proliferation. The findings also revealed varying detection frequencies based on structural attributes, like age and size. Hence, it stresses the importance of rigorous disinfection protocols and targeted infrastructural adaptations within water systems.
To add, Kearney et al. (2024) highlight that the plumbing systems in healthcare infrastructures, including the u-bend or p-trap components in sinks, serve as reservoirs for carbapenemase-producing Enterobacterales (CPE) due to biofilm formation in areas that are challenging to decontaminate, exacerbating cross-contamination risks. Their empirical assessment of healthcare personnel highlighted the necessity for targeted interventions to fortify protocols for healthcare-associated infection (HCAI) transmission.
3.2.4.2 Anti-microbial resistance
Waterborne transmission is further complicated by antibiotic-resistant bacteria (ARB) and genes (ARGs) in urban water systems. Vassallo et al. (2022) highlight that airport wastewater treatment facilities and airplane sewage contain higher concentrations of ARGs than other urban wastewater sources. Sewer leaks, overflows, and using wastewater for irrigation in urban green spaces also aid the spread and persistence of ARMs (antibiotic-resistant microorganisms), emphasizing the need for improved water monitoring and management. Additionally, Leung et al. (2021) identifies wastewater as a key source of resistance genes that lead to transmission, especially in public transit systems.
In summary, waterborne transmission is an overlooked transmission pathway in BEs, influenced by plumbing systems, disinfectant efficacy, and biofilm formation. A review of studies from 2019 to 2024 reveals a limited number on waterborne transmission related to the selected keywords. This highlights the necessity for additional research to better understand and develop effective strategies that enhance public health resilience.
3.2.5 Transmission by other modes
Microbes and pathogens transmit not only via air, water, and fomites, but also through a complex interplay of factors that shape disease dynamics and microbial distribution in human populations.
3.2.5.1 Vector-borne factors
Zoonotic viruses, transmitted from wildlife to humans, have increasingly contributed to outbreaks, with interspecific transmission occurring through both direct contact and domesticated animals (Syal, 2021), as evidenced by the COVID-19 pandemic.
Habitat encroachment, fragmentation, and biodiversity loss from human activities contribute significantly to disease emergence, stressing wildlife hosts and enabling viral shedding and mutation. Rodents, key reservoirs for zoonotic pathogens, frequent human-modified environments due to land use changes, especially from agricultural development, which introduce novel pathogens. Thus, an investigation by Mistrick et al. (2024) elucidates that agricultural and synanthropic habitats exhibited heightened microbiome richness, diversity, and evenness in wild Peromyscus mice, relative to undeveloped forest habitats. Despite a low overall abundance of putative pathogenic bacteria, these pathogens were more common in agricultural settings.
Furthermore, Dalton et al. (2020) indicates that animals, including therapy and service animals in hospitals, are reservoirs and vectors for pathogens, facilitating the transmission of hospital-associated microorganisms, and the intersection between hospital and community microbial ecosystems.
3.2.5.2 Socio-ecological factors
The interplay between climate change and accelerated population growth has created significant environmental challenges, including resource depletion, ecological degradation, and shifts in socio-ecological interactions, as highlighted by Nhamo et al. (2022). These have facilitated the emergence and transmission of microbiomes with pathogenic potential. Modifications to the BE and ecological infrastructure established new pathways for pathogen transmission, leaving hosts more vulnerable. The COVID-19 pandemic illustrated how altered human-nature interactions exacerbate the transmission of infectious agents, threatening public health.
Similarly, predicting pathogen transmission due to increasing human-environment interactions helps anticipate risks and develop mitigation strategies. Vector-borne diseases (VBDs) exhibit varying ecological responses to environmental changes, influenced by interactions between vectors, hosts, and socio-ecological factors. Utilizing methodologies like cumulative pressure mapping and machine learning, Skinner et al. (2023) demonstrate that the human footprint—encompassing BEs, infrastructure, agricultural land, and population density—predicts VBD occurrence, with thresholds defining the transition from diseases associated with lower human pressures to higher human pressures. These responses underscore land-use transitions for shifting infectious disease burdens and public health interventions.
3.2.5.3 Natural disasters/catastrophes
Earthquake-induced tsunamis cause widespread destruction of both nature and BEs, exacerbating the risk of respiratory infections (RIs) through air, water, and surface transmissions. Mavrouli et al. (2021) reviewed 47 studies on post-tsunami disease emergence, revealing outbreaks of polymicrobial RIs, influenza, measles, and tuberculosis among survivors. Contributing factors to the persistence of these infections include overcrowded evacuation shelters, destroyed healthcare infrastructure, heightened pathogen exposure in flooded areas, regional endemic disease patterns, and insufficient vaccination coverage.
Consequently, understanding the complex interplay of ecological, environmental, and societal factors in pathogen transmission is essential for mitigating disease risks. Evidence emphasizes how habitat changes, human-wildlife interactions, land-use transitions, and catastrophic events influence the emergence and spread of infectious diseases. As global challenges accelerate, interdisciplinary research and public health strategies are crucial to minimizing threats to human health.
3.3 Remedies/strategies against transmission of MoBE
Effectively mitigating microbial transmission in BEs requires combining architectural design, engineering controls, hygiene protocols, and policy interventions to enhance public health resilience. The COVID-19 pandemic revealed the need for optimized ventilation, rigorous surface disinfection, and improved water management to prepare for future outbreaks. This section explores strategies and interventions for mitigating pathogen spread through transmission pathways, with emphasis on evidence-based solutions.
Figure 12 and Table 2 gives a summary of the strategies against the modes of MoBE transmission as recognized in this literature work.
3.3.1 General/building elements
3.3.1.1 Historical and contemporary design approaches
Bliss and Kopec (2022) explore historical and modern urban design strategies for infection control, from lazarettos and leprosy centers to contemporary pedestrian flow and open space design. Their book highlights lessons from COVID-19 for healthier environments post-pandemic, making it a valuable resource for advancements in architecture and urban planning.
3.3.1.2 Building material selection
To mitigate microbial proliferation and transmission through building materials, Pacheco-Torgal et al. (2021) asserts that selecting antimicrobial, moisture-resistant, or non-porous surfaces significantly reduces microbial adhesion and colonization, prevents water retention, and inhibits microbial growth. Maintaining optimal humidity levels, adequate ventilation, routine disinfection, and assessing microbial contamination on these materials contribute to effective management of biofilm formation and microbial persistence in the BE.
3.3.1.3 Data-driven risk assessment in BEs
Li et al. (2021) underscore the critical public health challenge of microbial pathogen transmission in high-occupancy BEs, intensified by the COVID-19 pandemic. They propose a computational framework integrating Building Information Modeling (BIM), occupancy data, and pathogen transmission models to assess room-level outbreak risks using building characteristics, occupant interactions, and hygiene practices. Furthermore, their web-based system facilitates real-time communication of outbreak risks, providing insights to improve infectious disease management in BEs, like limiting occupancy levels and adjusting facility usage schedules based on risk distribution across rooms.
3.3.1.4 Biocontrol/bioremedies
Gottel et al. (2024) examine the MoBE, encompassing diverse bacterial, archaeal, fungal, and viral communities linked to the BE, highlighting the significant risk of colonization by antibiotic-resistant pathogens via surface transmission or inhalation. The authors cite studies cataloguing microbial composition across BEs to guide in-vitro investigations focused on replicating conditions conducive to pathogen persistence.
Furthermore (Gottel et al., 2024), paves the way for developing and validating biocontrol strategies, like Bacillus-based cleaning products and printable materials to mitigate multidrug-resistant infections. The review proposes strategies to combat antibiotic-resistant pathogens and positions biocontrol as a viable alternative to traditional antimicrobial approaches, highlighting the efficacy of resilient Bacillus spores for sustainable pathogen control in BEs.
In summary, effectively mitigating microbial transmission in BEs requires a comprehensive approach combining architectural design, spatial planning, and technology. Historical and contemporary strategies highlight structural interventions, while data-driven solutions provide valuable insights for mitigating outbreak risks. Together, they highlight interdisciplinary research and innovation for creating resilient indoor environments post-COVID-19.
3.3.2 Airborne/aerosol transmission
Airborne transmission was acknowledged by the World Health Organization (WHO) as the major mode for the spread of respiratory infections, including SARS-CoV-2 (Lewis, 2022). Consequently, numerous studies explore mitigation strategies like modulating air temperature and humidity (Dabisch et al., 2021), the application of ultraviolet (UV) light (Biasin et al., 2021), and the lowering of air pH (Luo et al., 2023) for viral deactivation. Additionally, national health authorities such as the WHO and the Federation of European Heating, Ventilation, and Air Conditioning Associations (REHVA) have highlighted sufficient ventilation as an essential preventive strategy (World Health Organization, 2021; National Health Service, 2023; REHVA, 2021). Validated computational fluid dynamics (CFD) models show that higher ventilation rates disperse infectious aerosols more effectively, reducing cross-infection risk. (Motamedi et al., 2022; Magar et al., 2021). This subsection highlights some of the research in this area.
3.3.2.1 Ventilation and air filtration
Mitigating airborne and dust-borne transmission requires optimized ventilation systems. Mechanical ventilation with advanced filtration effectively reduces microbial loads, while natural ventilation promotes microbial exchange with outdoor air, lacking control over specific microbial components, as studied by Leung et al. (2019).
On a similar note, Argyropoulos et al. (2023) emphasize that natural ventilation is cost-effective but variable in airflow and may bring in contaminants, while mechanical ventilation provides better control and filtration, albeit at a higher financial cost. In both systems, the efficacy of pathogen removal is primarily determined by airflow rates and movement patterns; downward displacement (DV) with 4 ACH (Air Changes per Hour) is indicated as most effective, while upward DV may heighten infection risk. Furthermore, personalized ventilation (PV) enhances air quality and mitigates transmission risk but loses efficiency in multi-story buildings due to natural ventilation. Notably, increasing ventilation rates above five ACH has lowered pathogen concentrations, but presents challenges for sustained implementation.
Likewise, Dietz et al. (2020), Pacheco-Torgal et al. (2021), Löhner et al. (2021) and Prussin et al. (2020) says mitigating airborne diseases like COVID-19 requires optimizing ventilation to increase air exchange rates and dilute microbial concentrations. Implementing fresh air ventilation, portable air purifiers, high-MERV (Minimum Efficiency Reporting Values)-rated filters and High-Efficiency Particulate Air (HEPA) filtration in HVAC systems effectively remove airborne pathogens, while UV-C (ultraviolet light with wavelengths between 100 and 280 nm) lighting in high-risk areas and Ultraviolet germicidal irradiation (UVGI) provides extra protection against microbes but needs precise placement to prevent harm to occupants. Contrastingly, Zhang et al. (2023) found enhancing filter efficiency to be the most consequential intervention, effectively reducing transmission risk in a supermarket to 0.33% when at maximum efficiency.
Additionally, the CLEAN 2020 summit emphasizes the necessity for a multifaceted approach to reduce viral transmission in indoor environments, as highlighted by Martinez and Morrow (2020) and Morrow et al. (2021). The summit advocates that eliminating the infectious agent is most effective, with building design and engineering measures like enhanced ventilation, filtration, transmission barriers, UVGI, and increased outdoor air dilution reducing viral exposure. The Summit also underscored the importance of developing long-term cleaning strategies that balance decontamination with human health, material integrity, and environmental impact.
3.3.2.2 Environmental and structural controls
Architectural design plays a critical role in pathogen dissemination, as highlighted by Zhang et al. (2023), who used agent-based modelling to simulate respiratory pathogen transmission in a supermarket. Interventions like multiple exits (lowered infection risk to 0.31%) and shelf layouts with multiple checkout lanes (risk reduced to 0.26%) decreased airborne transmission. Integrating strategies—structural, environmental, and human behavioural controls—resulted in 8 h without infections, signifying the importance of spatial planning and airflow optimization in controlling airborne pathogens.
Again, Abdin and Mahmoud (2024) illustrate how architectural design directly influences public health outcomes through curbing the spread of infection, by considering key factors such as airflow, recirculated air, and occupant proximity. Additionally, Pacheco-Torgal et al. (2021) also asserts that increasing natural ventilation through architectural design modifications, such as operable windows and ventilation shafts, further enhances indoor air quality and minimizes the risk of aerosol-based transmission.
Furthermore, environmental factors such as air temperature, relative humidity, and airflow patterns significantly influence pathogen viability, as elucidated by Dietz et al. (2020). The authors assert that maintaining optimal indoor lighting and temperature conditions is pivotal to controlling viral transmission. Research suggests that daylight exposure can reduce bacterial viability and influence the survival of viruses such as SARS-CoV-2. Moreover, Pacheco-Torgal et al. (2021) and Dietz et al. (2020) emphasized that modulating air temperature and maintaining indoor humidity levels between 40% and 60% can help reduce pathogen viability and transmission.
Moreover, Leung et al. (2019) recommend strategies such as maintaining robust ventilation, and addressing fungal and mold growth using environmentally sustainable mold removal products, coupled with strategies to control indoor humidity.
Additionally, Hopman et al. (2019) underscore the risk of airborne transmission of Carbapenemase-producing Pseudomonas aeruginosa in hospital environments, advocating for enhanced disinfection protocols for hospital drains, redesigning plumbing systems to minimize microbial reservoirs, and reconsidering the placement of sinks and showers in high-risk areas to limit infection spread.
3.3.2.3 Personal protective measures
The SARS-CoV-2 pandemic has underscored the importance of face masks in mitigating the transmission of respiratory droplets. Argyropoulos et al. (2023) indicates that masks, particularly N95 respirators, reduce droplet dispersion, although factors such as leakage and mask type influence their efficacy. This is confirmed by computational fluid dynamics (CFD) and large eddy simulation (LES) studies, though smaller droplets can persist in the air for prolonged durations. Prussin et al. (2020) also report interventions, like enforcing face masks and spatial distancing measures, as pivotal in minimizing the dissemination of aerosolized viral particles within BEs. Zhang et al. (2023) further concluded that implementing universal mask-wearing was the most efficacious intervention, reducing the infection risk to 0.08%.
Additionally, Dietz et al. (2020) highlight that protective environment (PE) rooms, which are positively pressurized to protect immunocompromised patients, can facilitate the migration of aerosols into high-traffic corridor spaces when doors are opened. In contrast, airborne infection isolation (AII) rooms using negative pressurization may expose occupants to adjacent airborne pathogens. Thus, hospital designs should decouple thermal conditioning from ventilation systems to increase operational resilience and flexibility during routine care and disease outbreaks.
3.3.2.4 Advanced modelling and data-driven strategies
Computational modelling is instrumental in optimizing infection control strategies. Aganovic et al. (2024) introduced a novel quanta-independent approach to address the limitations of the Wells-Riley model, which, despite its extensive use for modelling airborne transmission risk and assessing indoor infection control efficacy, is highly sensitive to variations in viral load, potentially leading to inaccurate risk predictions. The new approach concentrates on removal rates, room volume, and occupancy duration, thus providing a more robust assessment under steady-state and non-steady-state conditions.
Likewise, Löhner et al. (2021) reviews advancements in high-fidelity modelling for pathogen propagation, transmission, and mitigation within BEs, using differential equations to simulate airflow, particle dynamics, and UV radiation in indoor spaces and HVAC systems. Coupled with computational methods for crowd and fluid dynamics, these provide improvements in simulating aerosol transmission among moving pedestrians, contributing to the design of safer indoor environments, optimization of HVAC systems, and enhancement of protective measures, thereby strengthening resilience against future infectious disease outbreaks.
Moreover, Voss (2022) explores the integration of AI-powered surveillance systems, real-time diagnostics, and automation, as transformative tools in infection prevention and control (IPC). The research underscores the necessity for enhanced collaboration between acute and long-term care settings, advocating for a unified regional network in IPC. It emphasizes the role of engineering solutions in mitigating transmission risks, particularly of airborne and waterborne pathogens.
Additionally, a novel methodological framework, employing an aerosolized bacteriophage and its host, was developed by Skanata et al. (2022), to detect viable viral particles. This approach found viable particles to traverse distances up to 18 feet within 15 min in a classroom equipped with advanced HVAC systems, with dispersal notably attenuated when relative humidity exceeded 40%. The method is adaptable for diverse virus-host systems, providing a quantifiable measure of airborne transmission in BEs.
Further, Makris et al. (2024) presented the ICEE (Infection Control’s Energy Efficiency) index, a novel metric developed to evaluate the effectiveness of ventilation strategies in mitigating airborne pathogen transmission while accounting for energy demands, using a coherent analytical framework. Thus, the study provides essential insights for designing ventilation systems.
3.3.2.5 Behavioural and occupancy controls
Recent research during COVID-19 highlights that reducing indoor occupancy, rather than mobility, is crucial in lowering airborne disease transmission (Susswein et al., 2023). Behavioural interventions promoting outdoor activity, especially in urban areas, have effectively reduced case rates, with regional variations influencing seasonal transmission patterns. Thus, Susswein et al. (2023) offers critical empirical insights into the relationship between human behaviour, the BE, and infection risk, thereby informing more effective public health strategies for managing seasonal and pandemic respiratory pathogens.
Similarly, Löhner et al. (2021) suggest behavioural interventions like avoiding exhalation wake paths, wearing N95 masks, regulating pedestrian traffic, and utilizing plexiglass barriers or face shields to reduce transmission risks, though their efficacy depends on proper design. In addition, Zhang et al. (2023) assert that customer control measures—such as tripling entry intervals at supermarkets—demonstrated notable efficacy, reducing the infection risk to 0.10%. In their study, customer control was the most effective out of other strategies, lowering the infection risk to a mere 0.04% by minimizing interactions between susceptible and infected individuals, surpassing sanitary measures in efficacy. Leung et al. (2019) also suggested regulation of occupant density and frequency as instrumental in minimizing microbial proliferation and improving overall indoor air quality.
In short, the effectiveness of biocidal treatments must be rigorously assessed to ensure both efficacy and safety. Moving forward, continued research and innovation in air quality management, coupled with evidence-based design improvements, will be essential in enhancing indoor environments.
3.3.3 Surface/fomite transmission
3.3.3.1 Sanitation protocols and hygiene practices
Mitigating fomite-mediated transmission necessitates rigorous sanitation measures. While Zhang et al. (2023) primarily focus on airborne transmission and its related interventions, the acknowledgment of surface-based transmission routes as a critical pathway for SARS-CoV-2 spread in indoor environments implies the importance of comprehensive fomite-based mitigation strategies, including regular disinfection of high-touch surfaces and promoting hand hygiene.
Additionally, cleaning practices are effective in significantly reducing microbial loads and their transmission, with a single wipe of a wet cloth eliminating most bacteria and viruses, while disinfectants decrease pathogen levels, as emphasized by Leung et al. (2019). However, microbial recolonization can occur swiftly, as evidenced by their studies, highlighting the necessity of more frequent cleaning protocols in high-occupancy environments, to mitigate the risk of microbial transmission and to maintain hygiene standards.
Likewise, Dietz et al. (2020) emphasize that to mitigate fomite transmission, building operators must implement rigorous surface sanitation protocols, employing alcohol-based sanitizers and bleach solutions in high-contact areas like sinks, toilets, and communal workspaces. Research shows that hand sanitizers with 62%–71% ethanol effectively deactivate SARS-CoV-2. Continuous surface disinfection in shared spaces, strategic placement of hand hygiene stations, and clear signage promoting handwashing reduce the risk of fomite-mediated transmission.
Similarly, Prussin et al. (2020) advocate for frequent and systematic disinfection of high-contact surfaces using virucidal agents with proven efficacy against enveloped viruses, and for the promotion of stringent hand hygiene practices, including routine handwashing with antimicrobial soap and the use of alcohol-based hand sanitizers. Moreover, the integration of real-time environmental monitoring systems assesses contamination dynamics and informs adaptive cleaning regimens, particularly in high-occupancy and healthcare settings.
Additionally, Valentino et al. (2022) underscore the ineffectiveness of sanitation protocols in vegetable processing facilities at eradicating hazardous and antibiotic-resistant microorganisms from food contact surfaces. The authors suggest that widespread biocide use may increase selection pressure for resistant strains. These findings underscore the importance of incorporating microbiome mapping into routine monitoring frameworks within food production environments, alongside strict hygienic practices during vegetable harvesting and processing, to enhance food operators’ capacity and ensure microbiological safety and overall food quality.
To add, Sah et al. (2023) examine clinical laboratory microbiota, mapping microbial communities within these contexts, hence, enriching the understanding of potential sources of laboratory-acquired infections and supporting the refinement of safety protocols aimed at safeguarding healthcare workers.
Similarly, Dalton et al. (2020) emphasize that effective strategies for controlling microbial transmission in hospital settings require a comprehensive, integrative approach that targets the hospital environment, including manual cleaning protocols, no-touch disinfection technologies (e.g., UV irradiation), and architectural modifications. However, human error and microbial resistance often limit their success, reinforcing the need for multifaceted approaches, such as human-centered interventions, particularly hygiene practices like patient decolonization and healthcare worker hand hygiene.
Furthermore, Pacheco-Torgal et al. (2021) stress the importance of utilizing EPA-registered biocidal agents for surface disinfection, supplemented by behavioural interventions such as hand hygiene promotion and minimizing unnecessary contact with shared surfaces, to ensure the effective inactivation of microbial contaminants on frequently touched surfaces.
3.3.3.2 Material innovations for pathogen reduction
Advancements in antimicrobial surface materials provide promising solutions for infection control. On this note, Abraham et al. (2021) highlights copper and its alloys as effective antimicrobial agents, rendering pathogens like E. coli O157, nosocomial pathogens, and SARS-CoV-2 inactive on contact. Using copper-based surfaces in healthcare facilities, food industries, and public spaces may significantly reduce pathogen transmission, emphasizing the importance of further investigation into copper materials as an infection control strategy, especially during the COVID-19 pandemic. Pacheco-Torgal et al. (2021) further underscores the adoption of surface materials with antimicrobial properties or self-disinfecting capabilities, such as copper alloys or nanostructured coatings.
3.3.3.3 Environmental and structural modifications
BEs are critical in transmitting infectious diseases, with ventilation rates, humidity, and surface characteristics significantly influencing pathogen viability and spread. Lam et al. (2022) found that electrochromic (EC) windows, which adjust daylight intensity, significantly reduced bacterial growth by up to 100% and fungal growth by up to 42% on surfaces like PVC, polystyrene, and glass. These findings highlight the importance of integrating EC windows in healthier indoor environments like offices, homes, aviation, and healthcare settings while suggesting that indoor daylight exposure can potentially serve as an effective surface disinfection alternative.
In addition, Pacheco-Torgal et al. (2021) emphasize integrating biophilic design principles, such as the introduction of beneficial microbial communities through indoor plants or microbiome-friendly materials, to further contribute to a healthier indoor microbiome and enhanced resilience against pathogenic colonization.
3.3.3.4 Technology and data-driven disinfection solutions
Considering the pandemic, there is a pressing need for automated, intelligent, and precise disinfection solutions, including autonomous disinfection robots utilizing ultraviolet (UV) technology (Yang, G. et al., 2020), to mitigate pathogen transmission and prevent infectious disease outbreaks. In this regard, Liu et al. (2024) proposed a novel enhanced fomite-based pathogen transmission model to evaluate infection risks from disinfection robots’ schedules and human interactions. A mixed-integer programming model optimized disinfection schedules and routes, showing significant efficacy in reducing infection risk in buildings with fewer than 50 rooms. This research highlights the potential for reducing exposure to infectious diseases and addressing community transmission hotspots while offering scalability and adaptability across diverse facility types, configurations, and sizes.
Similarly, Hu et al. (2020) address the risk of pathogen transmission in mass gatherings like hospitals, schools, and airports by introducing a robotic disinfection framework that utilizes simultaneous localization and mapping for autonomous navigation, deep learning algorithms to identify and map contaminated areas, and ultraviolet light for effective decontamination. Its efficacy is validated through simulations and studies, showing its potential to enhance infection control measures in high-density BEs.
The application of data analytics in infection control is further proposed by Li et al. (2021), using Building Information Modeling (BIM), occupancy data, and pathogen transmission models to identify high-risk areas and optimize cleaning protocols. Key strategies include frequent surface disinfection, with five daily cleanings reducing pathogen reproduction rates by over 50%, enhanced hand hygiene practices, occupancy management to minimize contamination risks, and tailored cleaning schedules for high-traffic areas, like classrooms. A web-based system facilitates real-time risk communication, enabling proactive adjustments to facility usage and hygiene protocols based on localized contamination patterns.
Briefly, the evidence underscores the necessity of integrating stringent sanitation, antimicrobial materials, environmental modifications, robotic technologies, and data-driven strategies to reduce fomite-mediated disease transmission.
3.3.4 Water-borne transmission
3.3.4.1 Chemical disinfection and water treatment
The selection of chemical disinfectants within water systems significantly influences the persistence of mycobacterial species (Donohue and Mistry, 2024). Donohue and Mistry (2024) discuss the importance of the disinfectant choice and its corresponding residual concentration in the management of mycobacterial proliferation within hot water systems, to effectively safeguard public health in BEs. Although chlorine effectively diminishes mycobacterial detections, its high reactivity results in rapid dissipation at elevated temperatures. Conversely, chloramine, characterized by its lower reactivity, diminishes more gradually under heat; however, efficient microbial control depends on extended contact times, and it is less efficacious against multiple waterborne pathogens.
In the same way, Pacheco-Torgal et al. (2021) assert that ttemperature regulation within water systems is a critical factor, with hot water systems being maintained above 55 °C to inhibit Legionella proliferation. Routine flushing of stagnant water and periodic disinfection using chlorine-based or alternative biocidal treatments also reduce microbial colonization in pipes.
3.3.4.2 Engineering interventions for water safety
Effective management of waterborne pathogen risks requires integrating engineering solutions into plumbing systems. Accordingly, Donohue and Mistry (2024) emphasize that identifying contamination-prone areas—like biofilm formation sites, prolonged water stagnation zones, and extended distances between water heaters and point-of-use taps—enables targeted interventions toward optimizing plumbing design which can help address pathogen proliferation within water systems.
Additionally, utilizing materials such as copper or antimicrobial-coated piping is said to restrict bacterial adhesion and biofilm development by Pacheco-Torgal et al. (2021). Also, implementing real-time microbial monitoring systems can facilitate early detection and prompt remediation of potential microbial hazards within plumbing networks.
3.3.4.3 Collaborative infection prevention and control (IPC) measures
Voss (2022) advocates for a unified regional infection prevention and control (IPC) network, enhancing collaboration between acute and long-term care settings. The study emphasizes the role of engineering solutions, including optimized ventilation and water safety measures, in mitigating airborne and waterborne pathogen transmission risks. Furthermore, it explores the integration of AI-powered surveillance systems, real-time diagnostics, and automation to revolutionize IPC by enabling more efficient, data-driven responses to infections.
3.3.4.4 Education and awareness for healthcare workers
The role of healthcare workers (HCWs) in infection control is pivotal in preventing waterborne pathogen transmission, especially in clinical settings. Kearney et al. (2024) highlight a survey conducted between 2022 and 2023, which revealed that IPC HCWs exhibited higher awareness than non-IPC HCWs towards environmental transmission risks, and better adherence to liquid waste disposal practices. These findings emphasize the importance of targeted educational interventions to improve waste management practices among non-IPC HCWs.
Conclusively, waterborne transmission poses public health challenges, necessitating a multifaceted approach. Effective chemical disinfection strategies, engineering modifications, collaborative IPC frameworks, and educational programs help reduce pathogen persistence in water systems. As healthcare and BEs evolve, integrating innovative technologies and evidence-based interventions will be essential in safeguarding water quality and preventing disease transmission.
3.3.5 Transmission by other modes
3.3.5.1 Vectors of zoonotic pathogens
Rodents serve as potential reservoirs for zoonotic pathogens, with their interactions in agricultural and urban environments influencing disease transmission. Mistrick et al. (2024) underscore the importance of investigating zoonotic pathogens in synanthropic rodents and other wildlife, which are essential for delineating their roles as reservoirs and vectors for pathogen spillover at the interface between human and wildlife populations, especially in light of the COVID-19 pandemic which is believed to be spread from bats to an intermediary animal and then to humans (Amin, 2020).
Additionally, the role of animals as potential vectors for hospital-associated pathogens remains understudied as emphasized by Dalton et al. (2020). Guidelines for animal control in healthcare settings are primarily based on human data and need validation in animal populations. A One Health approach—considering human, animal, and environmental reservoirs—is essential for developing evidence-based interventions that largely address microbial transmission risks in healthcare environments.
3.3.5.2 Pandemic resilience and urban planning through socio-ecological interactions
Syal (2021) examines pandemics, like COVID-19, through a socio-ecological resilience framework, identifying five key urban planning and policy issues impacting disease persistence and management. These include integrating urbanization, land use, animal husbandry, and biodiversity conservation into policies to mitigate zoonotic and climate-related risks; ensuring equitable access to housing, water, sanitation, healthcare, and public spaces to lower vulnerability; maintaining environmental quality to limit pathogen persistence; building redundancy in supply chains and data systems for resilience during disruptions; and promoting decentralized infrastructure management and participatory governance for pandemic responses. The paper emphasizes understanding cities as integrated social-ecological systems for long-term resilience.
In addition, Nhamo et al. (2022) advocate for an integrated water-health-ecosystem-nutrition nexus to mitigate microbiome transmission risks. This approach stresses enhancing resilience and preparedness by addressing factors including sanitation, nutrition, and adaptive capacity, supporting SDG 3 (good health and wellbeing), SDG 6 (clean water and sanitation), and SDG 13 (climate action). By fostering sustainable socio-ecological relationships, this framework aims to minimize pathways for microbiome transmission and promote healthier environments.
3.3.5.3 Natural disasters/catastrophes
To mitigate the numerous microbial transmission risks posed by tsunamis, Mavrouli et al. (2021) emphasizes the importance of implementing disaster preparedness and response plans that address these challenges, including designing evacuation centres, strengthening healthcare facilities, enhancing disease surveillance, and raising awareness about preventive measures and the importance of vaccination.
Thus, pathogen transmission via non-traditional vectors demands a holistic, interdisciplinary approach. Surveillance, urban planning, hospital infection control, and sustainable public health strategies collectively contribute to mitigating these risks. Strengthening these measures ensures long-term resilience against emerging infectious threats.
4 Conclusion and future directions
This in-depth literature review highlights the crucial role of microbiomes in the built environment (MoBE) in shaping public health, particularly concerning pathogen transmission and infection control. The findings highlight that microbial communities within built spaces are shaped by the interplay of architectural design, ventilation systems, human occupancy, and environmental factors. The COVID-19 pandemic has further emphasized the necessity of understanding airborne, surface, and waterborne transmission pathways to mitigate disease spread in enclosed environments.
This review reveals that MoBE research has advanced in understanding microbial transmission but still lacks in connecting microbial ecology with architecture and urban planning strategies. Mitigation strategies, including improved ventilation, antimicrobial surfaces, disinfection protocols, and water management, vary in effectiveness based on context. Interdisciplinary work among microbiologists, engineers, architects, and public health experts is crucial for designing healthier, resilient BEs.
Moving forward, future research should focus on integrating smart technologies like real-time microbial monitoring, artificial intelligence (AI), and Internet of Things (IoT) technologies to enhance microbial surveillance and infection control in BEs. Long-term studies are needed on MoBE dynamics, as current research mainly assesses short-term changes; these studies are crucial for tracking microbial community shifts and their health impacts. Additionally, exploring sustainable, biophilic, and self-sanitizing building materials can promote healthy microbial ecosystems and reduce pathogen risks. Understanding how climate factors like temperature, humidity, and urbanization impact microbial compositions in BEs will be vital in adapting public health strategies. Furthermore, strengthening policies on microbial risk management, air quality, and water sanitation in high-occupancy settings such as hospitals, schools, and transit systems is imperative.
Despite increasing interest in understanding MoBE, there remains a lack of universally standardized protocols for microbial monitoring across diverse building types. Current research often varies in sampling strategies, sequencing platforms, and data interpretation methods, making cross-study comparisons difficult. Thus, the development of reliable, scalable, and reproducible protocols for microbiome sampling and analysis, especially with regards to building types, will be critical for translating MoBE insights into practical tools for health-focused architectural and urban policy.
By addressing these research gaps and fostering interdisciplinary collaborations, the field of MoBE can contribute significantly to creating healthier, safer, and more resilient BEs that align with global sustainability and public health goals. Looking ahead, there is strong potential for MoBE research to inform actionable policies for sustainable and health-oriented building design. Insights from microbial ecology and pathogen transmission studies in BEs can guide ventilation standards, surface material regulations, and spatial design norms that reduce pathogen persistence while supporting beneficial microbial communities. The integration of such knowledge into design and regulatory frameworks, such as the Healthy Buildings initiative or urban green infrastructure policies, can shape future construction and renovation practices. Embedding microbiome awareness into architecture, engineering, and public health planning ensures that buildings are not only structurally efficient but also biologically supportive of human health. Moreover, combining building design parameters, occupancy patterns, and microbiome profiling holds promise for predictive risk modelling, enabling real-time assessments of pathogen exposure and guiding targeted interventions in diverse built settings. As a result, MoBE research is well-positioned to play a transformative role in shaping resilient, evidence-based design policies for the post-pandemic world.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TJ: Methodology, Conceptualization, Investigation, Data curation, Writing – review and editing, Writing – original draft, Visualization. SA: Data curation, Writing – original draft, Conceptualization, Investigation, Methodology. MM: Methodology, Supervision, Writing – review and editing, Conceptualization, Funding acquisition, Investigation, Visualization, Project administration. SB: Conceptualization, Methodology, Writing – review and editing, Supervision. NZ: Writing – review and editing, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge the help and support from the American University of Sharjah (FRG24-C-E59). The work in this paper was supported, in part, by the Open Access Program from the American University of Sharjah. This paper represents the opinions of the authors and does not mean to represent the position or opinions of the American University of Sharjah.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdin, T. A. R., and Mahmoud, A. H. A. (2024). Lessons from the coronavirus pandemic: a review of how the disease spreads in indoor spaces. Int. J. Low-Carbon Technol. 19, 90–101. doi:10.1093/ijlct/ctad077
Abraham, J., Dowling, K., and Florentine, S. (2021). Can copper products and surfaces reduce the spread of infectious microorganisms and hospital-acquired infections? Materials 14 (13), 3444. doi:10.3390/ma14133444
Adams, R. I., Miletto, M., Lindow, S. E., Taylor, J. W., and Bruns, T. D. (2014). Airborne bacterial communities in residences: similarities and differences with fungi. PLoS One 9 (3), e91283. doi:10.1371/journal.pone.0091283
Afshinnekoo, E., Meydan, C., Chowdhury, S., Jaroudi, D., Boyer, C., Bernstein, N., et al. (2015). Geospatial resolution of human and bacterial diversity with city-scale metagenomics. Cell Syst. 1 (1), 72–87. doi:10.1016/j.cels.2015.01.001
Aganovic, A., Kurnitski, J., and Wargocki, P. (2024). A quanta-independent approach for the assessment of strategies to reduce the risk of airborne infection. Sci. Total Environ. 927, 172278. doi:10.1016/j.scitotenv.2024.172278
Amin, P. (2020). How did coronavirus get transferred from animals to humans? Scientists may have an answer.
Argyropoulos, C. D., Skoulou, V., Efthimiou, G., and Michopoulos, A. K. (2023). Airborne transmission of biological agents within the indoor built environment: a multidisciplinary review. Air Qual. Atmos. Health 16 (3), 477–533. doi:10.1007/s11869-022-01286-w
Biasin, M., Bianco, A., Pareschi, G., Cavalleri, A., Cavatorta, C., Fenizia, C., et al. (2021). UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 11 (1), 6260. doi:10.1038/s41598-021-85425-w
Bliss, A. M., and Kopec, D. (2022). Architectural factors for infection and disease control. 1st edn. New York: Routledge.
Brown, G. Z., Kline, J., Mhuireach, G., Northcutt, D., and Stenson, J. (2016). Making microbiology of the built environment relevant to design BioMed Central Ltd.
Bruno, A., Fumagalli, S., Ghisleni, G., and Labra, M. (2022). The microbiome of the built environment: the nexus for urban regeneration for the cities of tomorrow. Microorganisms 10 (12), 2311. doi:10.3390/microorganisms10122311
Castaño, N., Cordts, S. C., Kurosu Jalil, M., Zhang, K. S., Koppaka, S., Bick, A. D., et al. (2021). Fomite transmission, physicochemical origin of virus–surface interactions, and disinfection strategies for enveloped viruses with applications to SARS-CoV-2. ACS Omega 6 (10), 6509–6527. doi:10.1021/acsomega.0c06335
Chase, J., Fouquier, J., Zare, M., Sonderegger, D. L., Knight, R., Kelley, S. T., et al. (2016). Geography and location are the primary drivers of office microbiome composition. mSystems 1 (2), e00022-16. doi:10.1128/mSystems.00022-16
Chin, A. W. H., Chu, J. T. S., Perera, M. R. A., Hui, K. P. Y., Yen, H., Chan, M. C. W., et al. (2020). Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 1 (1), e10. doi:10.1016/S2666-5247(20)30003-3
Dabisch, P., Schuit, M., Herzog, A., Beck, K., Wood, S., Krause, M., et al. (2021). The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci. Technol. 55 (2), 142–153. doi:10.1080/02786826.2020.1829536
Dalton, K. R., Rock, C., Carroll, K. C., and Davis, M. F. (2020). One Health in hospitals: how understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 9 (1), 78. doi:10.1186/s13756-020-00737-2
Dietz, L., Horve, P. F., Coil, D. A., Fretz, M., Eisen, J. A., and van Den Wymelenberg, K. (2020). 2019 novel coronavirus (CoviD-19) pandemic: built environment considerations to reduce transmission. mSystems 5 (2), e00245-20. doi:10.1128/mSystems.00245-20
Donohue, M. J., and Mistry, J. H. (2024). Building size and disinfectant type influence the detection and concentration of Mycobacterium spp. in hot water plumbing. Sci. Total Environ. 927, 172112. doi:10.1016/j.scitotenv.2024.172112
Doremalen, N. V., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., et al. (2020). Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382 (16), 1564–1567. doi:10.1056/NEJMc2004973
Dunn, R. R., Fierer, N., Henley, J. B., Leff, J. W., and Menninger, H. L. (2013). Home life: factors structuring the bacterial diversity found within and between homes. PLoS One 8 (5), e64133. doi:10.1371/journal.pone.0064133
Fagunwa, O. E., and Olanbiwoninu, A. A. (2020). Accelerating the sustainable development goals through microbiology: some efforts and opportunities. Access Microbiol. 2 (5), acmi000112. doi:10.1099/acmi.0.000112
Flores, G. E., Bates, S. T., Caporaso, J. G., Lauber, C. L., Leff, J. W., Knight, R., et al. (2012). Diversity, distribution and sources of bacteria in residential kitchens. Environ. Microbiol. 15 (2), 588–596. doi:10.1111/1462-2920.12036
Gaüzère, C., Moletta-Denat, M., Blanquart, H., Ferreira, S., Moularat, S., Godon, J., et al. (2014). Stability of airborne microbes in the Louvre Museum over time. Indoor Air 24 (1), 29–40. doi:10.1111/ina.12053
Ghasemi, H., Yazdani, H., Fini, E. H., and Mansourpanah, Y. (2021). Interactions of SARS-CoV-2 with inanimate surfaces in built and transportation environments. Sustain. Cities Soc. 72, 103031. doi:10.1016/j.scs.2021.103031
Gilbert, J. A., and Hartmann, E. M. (2024). The indoors microbiome and human health. Nat. Rev. Microbiol. 22 (12), 742–755. doi:10.1038/s41579-024-01077-3
Gilbert, J. A., and Stephens, B. (2018). Microbiology of the built environment. Nat. Rev. Microbiol. 16 (11), 661–670. doi:10.1038/s41579-018-0065-5
Gohli, J., Bøifot, K. O., Moen, L. V., Pastuszek, P., Skogan, G., Udekwu, K. I., et al. (2019). The subway microbiome: seasonal dynamics and direct comparison of air and surface bacterial communities. Microbiome 7 (1), 160. doi:10.1186/s40168-019-0772-9
Gottel, N. R., Hill, M. S., Neal, M. J., Allard, S. M., Zengler, K., and Gilbert, J. A. (2024). Biocontrol in built environments to reduce pathogen exposure and infection risk. ISME J. 18 (1), wrad024. doi:10.1093/ismejo/wrad024
Hewitt, K. M., Gerba, C. P., Maxwell, S. L., and Kelley, S. T. (2012). Office space bacterial abundance and diversity in three metropolitan areas. PLoS One 7 (5), e37849. doi:10.1371/journal.pone.0037849
Hirose, R., Ikegaya, H., Naito, Y., Watanabe, N., Yoshida, T., Bandou, R., et al. (2021). Survival of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) and influenza virus on human skin: importance of hand hygiene in Coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 73 (11), e4329–e4335. doi:10.1093/cid/ciaa1517
Hodson, R. (2022). Preparing the world for the next pandemic. Nature 610 (7933), S33. doi:10.1038/d41586-022-03353-9
Hoisington, A., Maestre, J. P., Kinney, K. A., and Siegel, J. A. (2016). Characterizing the bacterial communities in retail stores in the United States. Indoor Air 26 (6), 857–868. doi:10.1111/ina.12273
Hopman, J., Meijer, C., Kenters, N., Coolen, J. P. M., Ghamati, M. R., Mehtar, S., et al. (2019). Risk assessment after a severe hospital-acquired infection associated with carbapenemase-producing Pseudomonas aeruginosa. JAMA Netw. Open 2 (2), e187665. doi:10.1001/jamanetworkopen.2018.7665
Höppe, P., and Martinac, I. (1998). Indoor climate and air quality. Int. J. Biometeorology 42 (1), 1–7. doi:10.1007/s004840050075
Hu, D., Zhong, H., Li, S., Tan, J., and He, Q. (2020). Segmenting areas of potential contamination for adaptive robotic disinfection in built environments. Build. Environ. 184, 107226. doi:10.1016/j.buildenv.2020.107226
Jeon, Y., Chun, J., and Kim, B. (2013). Identification of household bacterial community and analysis of species shared with human microbiome. Curr. Microbiol. 67 (5), 557–563. doi:10.1007/s00284-013-0401-y
Kamble, N. S., Bera, S., Bhedase, S. A., Gaur, V., and Chowdhury, D. (2024). Review on applied applications of microbiome on human lives. Bacteria 3 (3), 141–159. doi:10.3390/bacteria3030010
Kamble, N. S., Thomas, S., Madaan, T., Ehsani, N., Sange, S., Tucker, K., et al. (2025). Engineered bacteria as an orally administered anti-viral treatment and immunization system. Gut Microbes 17 (1), 2500056. doi:10.1080/19490976.2025.2500056
Kearney, A., Humphreys, H., and Fitzgerald-Hughes, D. (2024). Infection prevention and control policy implementation for CPE: a cross-sectional national survey of healthcare workers reveals knowledge gaps and suboptimal practices. J. Hosp. Infect. 145, 148–154. doi:10.1016/j.jhin.2023.12.007
Kelley, S. T., and Gilbert, J. A. (2013). Studying the microbiology of the indoor environment. Genome Biol. 14 (2), 202. doi:10.1186/gb-2013-14-2-202
Kembel, S. W., Jones, E., Kline, J., Northcutt, D., Stenson, J., Womack, A. M., et al. (2012). Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 6 (8), 1469–1479. doi:10.1038/ismej.2011.211
Kim, J., Han, S. J., and Yoo, K. (2022). Dust-associated bacterial and fungal communities in indoor multiple-use and public transportation facilities. Atmosphere 13 (9), 1373. doi:10.3390/atmos13091373
Kutter, J. S., Spronken, M. I., Fraaij, P. L., Fouchier, R. A., and Herfst, S. (2018). Transmission routes of respiratory viruses among humans. Curr. Opin. Virology 28, 142–151. doi:10.1016/j.coviro.2018.01.001
Lam, M. I., Vojnits, K., Zhao, M., MacNaughton, P., and Pakpour, S. (2022). The effect of indoor daylight spectrum and intensity on viability of indoor pathogens on different surface materials. Indoor Air 32 (7), e13076. doi:10.1111/ina.13076
Leo, B. F., Lin, C. Y., Markandan, K., Saw, L. H., Mohd Nadzir, M. S., Govindaraju, K., et al. (2023). An overview of SARS-CoV-2 transmission and engineering strategies to mitigate risk. J. Build. Eng. 73, 106737. doi:10.1016/j.jobe.2023.106737
Leung, M. H. Y., Tong, X., and Lee, P. K. H. (2019). Indoor microbiome and airborne pathogens. Compr. Biotechnol., 96–106. doi:10.1016/b978-0-444-64046-8.00477-8
Leung, M. H. Y., Tong, X., Bøifot, K. O., Bezdan, D., Butler, D. J., Danko, D. C., et al. (2021). Characterization of the public transit air microbiome and resistome reveals geographical specificity. Microbiome 9 (1), 112. doi:10.1186/s40168-021-01044-7
Lewis, D. (2022). Why the WHO took two years to say COVID is airborne. Nature 604 (7904), 26–31. doi:10.1038/d41586-022-00925-7
Li, S., Xu, Y., Cai, J., Hu, D., and He, Q. (2021). Integrated environment-occupant-pathogen information modeling to assess and communicate room-level outbreak risks of infectious diseases. Build. Environ. 187, 107394. doi:10.1016/j.buildenv.2020.107394
Li, S., Yang, Z., Hu, D., Cao, L., and He, Q. (2021). Understanding building-occupant-microbiome interactions toward healthy built environments: a review. Front. Environ. Sci. Eng. 15 (4), 65. doi:10.1007/s11783-020-1357-3
Liu, Z., Xu, Y., Jin, M., and Li, S. (2024). Disinfection robots scheduling and routing problem for healthy buildings. J. Build. Eng. 87, 108894. doi:10.1016/j.jobe.2024.108894
Löhner, R., Antil, H., Srinivasan, A., Idelsohn, S., and Oñate, E. (2021). High-fidelity simulation of pathogen propagation, transmission and mitigation in the built environment. Archives Comput. Methods Eng. 28 (6), 4237–4262. doi:10.1007/s11831-021-09606-6
Luo, B., Schaub, A., Glas, I., Klein, L. K., David, S. C., Bluvshtein, N., et al. (2023). Expiratory aerosol pH: the overlooked driver of airborne virus inactivation. Environ. Sci. Technol. 57 (1), 486–497. doi:10.1021/acs.est.2c05777
Magar, A., Joshi, M., Rajagopal, P. S., Khan, A., Rao, M. M., Sapra, B. K., et al. (2021). CFD simulation of the airborne transmission of COVID-19 vectors emitted during respiratory mechanisms: revisiting the concept of safe distance. ACS Omega 6 (26), 16876–16889. doi:10.1021/acsomega.1c01489
Makris, R., Kopic, C., Schumann, L., and Kriegel, M. (2024). A comprehensive index for evaluating the effectiveness of ventilation-related infection prevention measures with energy considerations: development and application perspectives. Indoor Air 2024, 9819794. doi:10.1155/2024/9819794
Martinez, K. F., and Morrow, J. B. (2020). Lessons learned in managing risk: tools and strategies for confident operations from the CLEAN 2020 summit. Toxicol. Industrial Health 36 (9), 736–742. doi:10.1177/0748233720971381
Mavrouli, M., Mavroulis, S., Lekkas, E., and Tsakris, A. (2021). Respiratory infections following earthquake-induced tsunamis: transmission risk factors and lessons learned for disaster risk management. Int. J. Environ. Res. Public Health 18 (9), 4952. doi:10.3390/ijerph18094952
Mccarthy, O. R. (2001). The key to the sanatoria. J. R. Soc. Med. 94 (8), 413–417. doi:10.1177/014107680109400813
Meadow, J. F., Altrichter, A. E., Kembel, S. W., Moriyama, M., O'Connor, T. K., Womack, A. M., et al. (2014). Bacterial communities on classroom surfaces vary with human contact. Microbiome 2 (1), 7. doi:10.1186/2049-2618-2-7
Mhuireach, G. Á., Fahimipour, A. K., Vandegrift, R., Muscarella, M. E., Hickey, R., Bateman, A. C., et al. (2022). Temporary establishment of bacteria from indoor plant leaves and soil on human skin. Environ. Microbiomes 17 (1), 61. doi:10.1186/s40793-022-00457-7
Mistrick, J., Kipp, E. J., Weinberg, S. I., Adams, C. C., Larsen, P. A., and Craft, M. E. (2024). Microbiome diversity and zoonotic bacterial pathogen prevalence in Peromyscus mice from agricultural landscapes and synanthropic habitat. Mol. Ecol. 33 (7), e17309. doi:10.1111/mec.17309
Morens, D. M., Park, J., and Taubenberger, J. K. (2023). Many potential pathways to future pandemic influenza. Sci. Transl. Med. 15 (718), eadj2379. doi:10.1126/scitranslmed.adj2379
Morrow, J. B., Packman, A. I., Martinez, K. F., Van Den Wymelenberg, K., Goeres, D., Farmer, D. K., et al. (2021). Critical capability needs for reduction of transmission of SARS-CoV-2 indoors. Front. Bioeng. Biotechnol. 9, 641599. doi:10.3389/fbioe.2021.641599
Motamedi, H., Shirzadi, M., Tominaga, Y., and Mirzaei, P. A. (2022). CFD modeling of airborne pathogen transmission of COVID-19 in confined spaces under different ventilation strategies. Sustain. Cities Soc. 76, 103397. doi:10.1016/j.scs.2021.103397
National Health Service (2023). How to avoid catching and spreading COVID-19 infection. Available online at: https://www.nhs.uk/conditions/covid-19/how-to-avoid-catching-and-spreading-covid-19/.
Nhamo, L., Mpandeli, S., Nhamo, S. P., Liphadzi, S., and Mabhaudhi, T. (2022). Enhancing sustainable human and environmental health through nexus planning. Water - Energy - Food Nexus Narrat. Resour. Secur. A Glob. South Perspective, 199–222. doi:10.1016/b978-0-323-91223-5.00012-5
Pacheco-Torgal, F., Falkinham, J. O., and Ivanov, V. (2021). Viruses, bacteria and fungi in the built environment: designing healthy indoor environments.
Prussin, A. J., and Marr, L. C. (2015). Sources of airborne microorganisms in the built environment. Microbiome 3, 78. doi:10.1186/s40168-015-0144-z
Prussin, A. J., Belser, J. A., Bischoff, W., Kelley, S. T., Lin, K., Lindsley, W. G., et al. (2020). Viruses in the built environment (VIBE) meeting report. Microbiome 8 (1), 1. doi:10.1186/s40168-019-0777-4
Qian, J., Hospodsky, D., Yamamoto, N., Nazaroff, W. W., and Peccia, J. (2012). Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air 22 (4), 339–351. doi:10.1111/j.1600-0668.2012.00769.x
Quesada-García, S., Valero-Flores, P., and Lozano-Gómez, M. (2023). Towards a healthy architecture: a new paradigm in the design and construction of buildings. Buildings 13 (8), 2001. doi:10.3390/buildings13082001
REHVA (2021). REHVA COVID-19 guidance document. Available online at: https://www.rehva.eu/fileadmin/user_upload/REHVA_COVID-19_guidance_document_V4.1_15042021.pdf.
Rice, L. (2019). The nature and extent of healthy architecture: the current state of progress. Int. J. Archit. Res. Archnet-IJAR 13 (3), 244–259. doi:10.1108/ARCH-11-2018-0005
Richardson, M., Gottel, N., Gilbert, J. A., and Lax, S. (2019). Microbial similarity between students in a common dormitory environment reveals the forensic potential of individual microbial signatures. mBio 10 (4), e01054-19. doi:10.1128/mBio.01054-19
Rintala, H., Pitkäranta, M., Toivola, M., Paulin, L., and Nevalainen, A. (2008). Diversity and seasonal dynamics of bacterial community in indoor environment. BMC Microbiol. 8, 56. doi:10.1186/1471-2180-8-56
Robertson, C. E., Baumgartner, L. K., Harris, J. K., Peterson, K. L., Stevens, M. J., Frank, D. N., et al. (2013). Culture-independent analysis of aerosol microbiology in a metropolitan subway system. Appl. Environ. Microbiol. 79 (11), 3485–3493. doi:10.1128/AEM.00331-13
Sah, G. P., Kovalick, G., Chopyk, J., Kuo, P., Huang, L., Ghatbale, P., et al. (2023). Characterization of SARS-CoV-2 distribution and microbial succession in a clinical microbiology testing facility during the SARS-CoV-2 pandemic. Microbiol. Spectr. 11 (2), e04509-22. doi:10.1128/spectrum.04509-22
Skanata, A., Spagnolo, F., Metz, M., Smyth, D. S., and Dennehy, J. J. (2022). Humidity reduces rapid and distant airborne dispersal of viable viral particles in classroom settings. Environ. Sci. Technol. Lett. 9 (7), 632–637. doi:10.1021/acs.estlett.2c00243
Skinner, E. B., Glidden, C. K., MacDonald, A. J., and Mordecai, E. A. (2023). Human footprint is associated with shifts in the assemblages of major vector-borne diseases. Nat. Sustain. 6 (6), 652–661. doi:10.1038/s41893-023-01080-1
Stephens, B., Azimi, P., Thoemmes, M. S., Heidarinejad, M., Allen, J. G., and Gilbert, J. A. (2019). Microbial exchange via fomites and implications for human health. Curr. Pollut. Rep. 5 (4), 198–213. doi:10.1007/s40726-019-00123-6
Susswein, Z., Rest, E. C., and Bansal, S. (2023). Disentangling the rhythms of human activity in the built environment for airborne transmission risk: an analysis of large-scale mobility data. eLife 12, e80466. doi:10.7554/eLife.80466
Syal, S. (2021). Learning from pandemics: applying resilience thinking to identify priorities for planning urban settlements. J. Urban Manag. 10 (3), 205–217. doi:10.1016/j.jum.2021.05.004
Tsang, T., Mui, K., and Wong, L. (2023). Computational fluid dynamics (CFD) studies on airborne transmission in hospitals: a review on the research approaches and the challenges. J. Build. Eng. 63, 105533. doi:10.1016/j.jobe.2022.105533
Valentino, V., Sequino, G., Cobo-Díaz, J. F., Álvarez-Ordóñez, A., De Filippis, F., and Ercolini, D. (2022). Evidence of virulence and antibiotic resistance genes from the microbiome mapping in minimally processed vegetables producing facilities. Food Res. Int. 162, 112202. doi:10.1016/j.foodres.2022.112202
Vassallo, A., Kett, S., Purchase, D., and Marvasi, M. (2022). The bacterial urban resistome: recent advances. Antibiotics 11 (4), 512. doi:10.3390/antibiotics11040512
Voss, A. (2022). BSAC vanguard series: the future of infection prevention and control: how COVID is already transforming our approach to antimicrobial resistance. J. Antimicrob. Chemother. 77 (3), 545–546. doi:10.1093/jac/dkab416
Wang, C. C., Prather, K. A., Sznitman, J., Jimenez, J. L., Lakdawala, S. S., Tufekci, Z., et al. (2021). Airborne transmission of respiratory viruses. Science 373 (6558), eabd9149. doi:10.1126/science.abd9149
Wang, P., Tong, X., Zhang, N., Miao, T., Chan, J. P. T., Huang, H., et al. (2022). Fomite transmission follows invasion ecology principles. mSystems 7 (3), e00211-22. doi:10.1128/msystems.00211-22
World Health Organization (2021). Roadmap to improve and ensure good indoor ventilation in the context of COVID-19. WHO Headquarters, Geneva: World Health Organization.
Xie, X., Li, Y., Chwang, A. T. Y., Ho, P. L., and Seto, W. H. (2007). How far droplets can move in indoor environments – revisiting the Wells evaporation–falling curve. Indoor Air 17, 211–225. doi:10.1111/j.1600-0668.2007.00469.x
Xie, J., Acosta, E. M., and Gitai, Z. (2023). Bacterial viability in the built environment of the home. PLoS ONE 18, e0288092. doi:10.1371/journal.pone.0288092
Xiong, G., Ji, L., Cheng, M., and Ning, K. (2023). Niche-based microbial community assemblage in urban transit systems and the influence of City characteristics. Microbiol. Spectr. 11 (2), e00167-23. doi:10.1128/spectrum.00167-23
Yang, G., Nelson, J., Murphy, R. R., Choset, H., Christensen, H., H. Collins, S., et al. (2020). Combating COVID-19—The role of robotics in managing public health and infectious diseases. Sci. Robotics 5 (40), eabb5589. doi:10.1126/scirobotics.abb5589
Keywords: microbiomes in the built environment, airborne, aerosol, surface, fomite, waterborne, microbial transmission
Citation: Joseph TM, Abdulmaksoud S, Mortula MM, Beheiry S and Zareen N (2025) Microbiomes of the built environment: a systematic literature review. Front. Built Environ. 11:1657297. doi: 10.3389/fbuil.2025.1657297
Received: 02 July 2025; Accepted: 12 August 2025;
Published: 29 August 2025.
Edited by:
Roberto Alonso González-Lezcano, CEU San Pablo University, SpainReviewed by:
Nitin Kamble, University of Cincinnati Medical Center, United StatesJinho Yang, Semyung University, Republic of Korea
Copyright © 2025 Joseph, Abdulmaksoud, Mortula, Beheiry and Zareen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tania M. Joseph, ZzAwMDk4NTY4QGF1cy5lZHU=
 Tania M. Joseph
Tania M. Joseph Sara Abdulmaksoud
Sara Abdulmaksoud Md. Maruf Mortula
Md. Maruf Mortula Salwa Beheiry3
Salwa Beheiry3 Nausheen Zareen
Nausheen Zareen