- Department of Psychiatry and Behavioral Science, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa, Japan
Lysosome is crucial for maintaining cellular homeostasis, but disintegrity of its limiting membrane affects the cell death fate. From 1972 to 1999, via the cytochemistry of cultured cells which were exposed to stresses, Brunk et al. defined lysosomal membrane permeabilization (LMP) as leakage through the ultrastructurally-intact limiting membrane. In 1996, via the electron microscopic analysis of the monkey hippocampal CA1 neurons after transient ischemia, Yamashima et al. first identified lysosomal membrane rupture (LMR) as an apparent disruption of the limiting membrane. To elucidate the mechanism of lysosomal cell death, it is indispensable to precisely differentiate LMP and its extensive form LMR. LMP indicates formation of ultrastructurally-undetectable, tiny pores at the lysosomal limiting membrane that allow selective leakage of lysosomal contents. LMP contributes to amplification of the cell death signal, and participates in apoptosis. In contrast, LMR indicates presence of larger holes that cause acute and massive leakage of hydrolytic cathepsin enzymes. LMR leads to the rapid and explosive vanishment of lysosomes, which proceeds along with vanishment of cells, i.e., necrosis. Each representative form of cell death is carried out in human diseases, depending upon the size and number of perforations, the amount of leakage, and the cellular context. The modality of the lysosomal membrane disintegrity, LMP or LMR, determines the cell death fate. It is likely that apoptosis occurs by the proteolytic activation of caspases via LMP, whereas necrosis occurs by the calpain-cathepsin cascade via LMR. This paper is to review ultrastructural evidences of LMR which were identified in diverse pathologic conditions of C. elegans, mice, monkeys, and humans. For elucidating mechanisms of each cell death in the organs affected by stresses, LMP and LMR should be precisely differentiated by electron microscopy. Herein, other lysosomal cell death such as pyroptosis and ferroptosis was discussed to make the difference clear. Ferroptosis might share very similar calpain-cathepsin cascade with necrosis.
1 Introduction
Lysosomes are membrane-bound vesicular structures which contain more than 60 acid hydrolytic enzymes including proteases, phosphatases, nucleases, glycosidases, peptidases, sulphatases, and lipases. Their main function is to degrade both macromolecules being internalized by endocytosis/phagocytosis and intercellular aged/damaged components. Hydrolytic enzymes break down complex macromolecules into amino acids within the lysosomal lumen for recycling. Since Christian de Duve discovered lysosomes in 1955 (de Duve et al., 1955), researchers made significant contributions to link lysosomes with cell death of eukaryotic cells. He was awarded Nobel prize in 1974 for the discovery of lysosomes. For ensuring the place of degradation within the acidic lumen at ∼pH4.5, the integrity of lysosomal limiting membranes is critical in order not to damage the cell via the leakage of hydrolytic enzymes into the cytoplasm. For this purpose, the lysosomal limiting membrane is approximately 8 nm thick, and has a highly-glycosylated transmembrane protein such as lysosome-associated membrane proteins 2 (LAMP-2). Both protect the membrane from inside not to be degraded by its own hydrolytic enzymes (Luzio et al., 2007; Saftig and Klumperman, 2009; Yamashima, 2024).
In normal conditions, lysosomal transcription factor EB (TFEB) is phosphorylated in the cytosol. At the lysosomal stress, TFEB is dephosphorylated and translocates to the nucleus, where it upregulates the transcription of coordinated lysosomal expression and regulation (CLEAR) genes. CLEAR genes encode hydrolases, lysosomal membrane proteins, and the v-ATPase proton pump complex, participating in the autophagosome formation (Sardiello et al., 2009). If the lysosomal membrane disintegrity is partial or if only a small subset of lysosomes is affected at the cellular stresses, lysophagy and TFEB-mediated responses are activated to ensure cell survival. Lysophagy senses pores being permeable to galectins at the lysosomal limiting membrane. The endosomal sorting complex required for transport proteins (ESCRT) can detect and repair small pores in the membrane (Skowyra, et al., 2018). In cases of more severe lysosomal disintegrity, however, such stress response mechanisms are unable to repair larger perforations, and result in cell death. Over the last 3 decades, the lysosome has emerged as an important executor of the cell death machinery such as necrosis, apoptosis, ferroptosis, and pyroptosis (Wang et al., 2018a) (Figure 1).
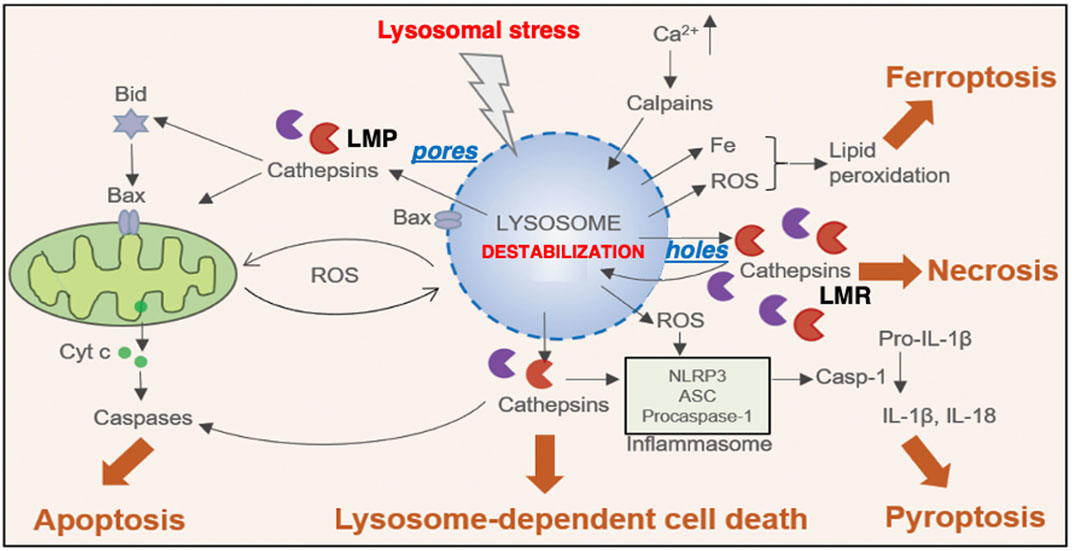
Figure 1. Relation between the lysosomal membrane disintegrity and the cell death. Different stimuli trigger lysosomal membrane-permeabilization (LMP) via pores and lysosomal membrane-rupture (LMR) via holes, that result in the translocation of cathepsins to the cytosol, leading to lysosome-dependent cell death. In some other instances, LMP and the selective cathepsin release engage in the activation of effectors, such as ROS, Bax and Iron and inflammasome that result in other types of cell death, such as apoptosis, pyroptosis and ferroptosis. In contrast, LMR results in the uncontrolled release of cathepsins and other lysosomal enzymes, cytosolic acidification and necrosis. ASC, apoptosis-associated speck-like protein containing a caspase-recruitment domain; Cyt c, cytochrome c; IL-1β, interleukin 1 beta; IL-18, interleukin-18; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; ROS, reactive oxygen species (Cited and adapted from Wang et al., 2018a).
Regardless of insults, cell types, organs, diseases, or species, leakage of the lysosomal content occurs via the disruption of the lysosomal limiting membrane. There are essentially two types of the lysosomal membrane disintegrity: ‘pores’ indicate the perforations being so small which could be made by a microneedle, and cannot be detected by electron microscopy, whereas ‘holes’ indicate the discontinuous lysosomal limiting membrane being detected on the electron microphotographs. The ultrastructurally-intact lysosomal limiting membrane shows permeabilization via formation of tiny pores (lysosomal membrane permeabilization, LMP). The massive leakage of the lysosomal content occurs through the apparent rupture (larger holes) of the lysosomal limiting membrane (lysosomal membrane rupture, LMR) (Figure 1). In response to acute intense insults, lysosomes fade away via the explosion-like rupture. Therefore, dissolving lysosomes are hardly detected during the phase of cell degeneration, and lysosomes in dying cells had been less frequently focused by the previous investigators (Yamashima et al., 1996; Yamashima, 2024).
By focusing on changes of the lysosomal membrane integrity with the time-lapse imaging and electron microscopic analyses in the primate tissues, herein, implications of LMP/LMR for the cell apoptosis/necrosis were discussed.
2 Permeabilization or rupture of lysosomal limiting membranes
More than half century ago, de Duve and Wattiaux proposed such a concept that cell degeneration and necrosis occur in pathological states via the leakage of hydrolytic enzymes from damaged lysosomes (de Duve and Wattiaux, 1966). Concerning the lysosomal leakage, it is likely that LMP induces the gradual and selective release of hydrolytic cathepsin enzymes, whereas LMR induces the rapid and massive release of cathepsins (Figure 1). The main questions to be addressed are (1) how does disintegrity of the lysosomal limiting membranes occur? (2) what specifically occurs after the formation of pores or holes at the lysosomal limiting membranes? (3) how do pores or holes participate in the execution of the representative cell death like apoptosis and necrosis?, and (4) how are they identified in the process of cell degeneration? To address these issues, it is indispensable to elucidate whether cell degeneration/death occurs via ultrastructurally-intact lysosomes.
In 1972, Brunk and Ericsson found by cytochemistry an extensive diffusion of lysosomal acid phosphatases through the ultrastructurally-intact lysosomal limiting membrane, using the cultured human glioma cells after the fixation at an improper osmotic pressure (Brunk and Ericsson, 1972). Presumably, pores allowing translocation of hydrolytic enzymes were formed artificially at the lysosomal limiting membranes by the improper fixation. In 1999, using a human T-leukemia cell line, Jurkat EG.1 cells, which were exposed to oxidative stresses, Brunk and Svensson first confirmed implication of LMP, i.e., leakage of acridine orange through the lysosomal limiting membrane (Brunk and Svensson, 1999) (Figure 2B, insert), which was not seen in the control (Figure 2A, insert). Due to its lysosomotropic properties, acridine orange accumulates mainly in the acidic vacuolar apparatus, preferentially in lysosomes, although to a minor degree in the cytosol and nucleus. As shown below, acridine orange leakage served as an indicator of both LMP in the process of apoptosis and dead cells after cell death is completed (Brunk et al., 1997). Since escaping detection from the electron microscopic observation, Brunk and his colleagues suggested implication of tiny pores at the lysosomal limiting membranes, i.e., constructed the concept of LMP, for the development of apoptosis (Figure 2B), which were confirmed by the subsequent researchers (Brunk et al., 1997; Brunk and Svensson, 1999; Li et al., 2000; Antunes et al., 2001; Kågedal et al., 2001).
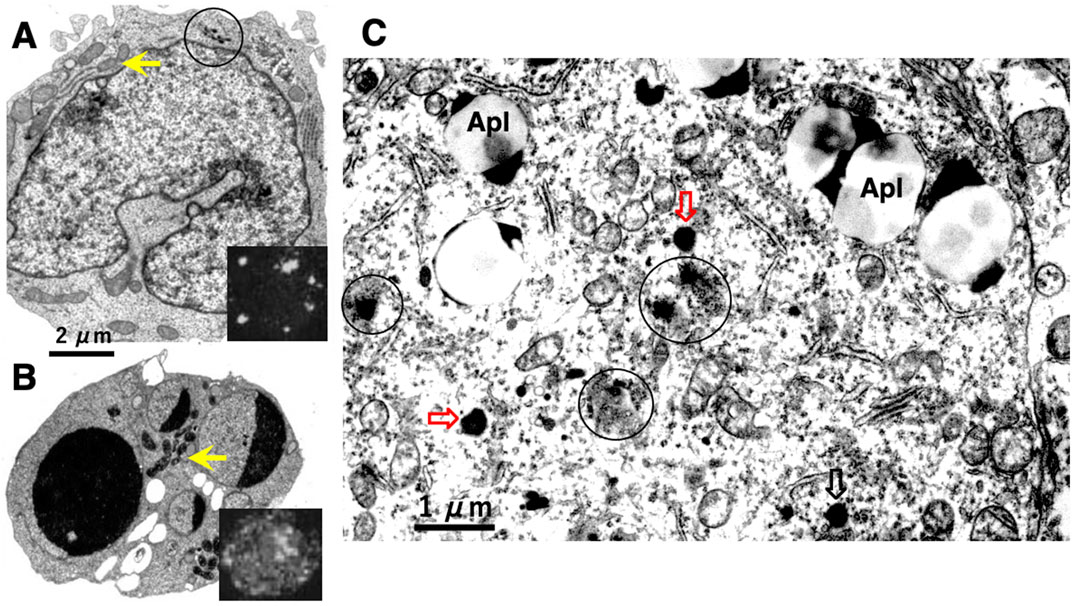
Figure 2. Electronmicroscopic photographs of Jurkat T-leukemia cells (A,B) and human cortical neuron of Alzheimer’s disease (C). The control Jurkat T-leukemia cell (A) is characterized by a folded nucleus with diffuse chromatin distribution, normal mitochondria (arrow), and a few lysosomes (circle). Following exposure to the anti-human Fas/APO-1/CD95 antibody, however, evidence of typical apoptosis such as nuclear chromatin condensation, fragmentation, and capping was seen (B). An increase of the mitochondrial electron density was confirmed after the insult (B, arrow), as compared to the control (A, arrow). Leakage of the lysosomal contents was not detected by electron microscopy. However, cells examined by confocal laser scanning microscopy after acridine orange staining, exhibited leakage of dye throughout the cytoplasm (B, insert, whitish cytoplasm), showing a marked contrast to the control (A, insert, black cytoplasm). These data indicated implication of LMP, but not of LMR, in the occurrence of apoptosis (Cited from Brunk and Svensson, 1999). In the human brain also, leakage of the lysosomal content was observed in the cortical neuron of Alzheimer’s disease (C, circles). As compared to the normal lysosomes which remained intact during degeneration (C, open arrows), affected lysosomes showing an irregular configuration were not bound by distinct membranes, with an apparent leakage of the lysosomal contents by LMR (C, circles). This showed a marked contrast to T-leukemia cells which exhibited LMP, but not LMR. Apl; autophagolysosomes (Cited from Yamashima et al., 2023d).
As LMP did not change the ultrastructure of lysosomes (Brunk and Ericsson, 1972), the interests in lysosomes, lysosomal membrane disintegrity and the resultant cell death gradually faded, and lysosomal involvement in cell death was overlooked thereafter. For a long time, the lysosome had been imprecisely considered a sturdy organelle that shows increased permeability, but does not show apparent rupture until the cell is already devitalized (Terman et al., 2006). It was believed that massive leakage of hydrolytic enzymes from injured lysosomes, if occurs, would be a late event in the terminal phase of cell degeneration or merely a postmortal alteration in autolytic cells.
In 1996, however, in the degenerating, but still alive, hippocampal cornu Ammonis 1 (CA1) neurons of Japanese macaque monkeys on days 3–5 after transient global brain ischemia, Yamashima et al. found by electron microscopy evidence of LMR forming larger holes which allow leakage of a massive amount of the lysosomal contents (Figure 3B, arrows), prior to the execution of necrosis (Yamashima et al., 1996; Yamashima et al., 1998). It showed a remarkable contrast to the normal lysosomes (Figure 3A, circles) in the non-ischemic CA1 neurons. This was the first description of LMR. Later, in 2012 and 2016, the similar LMR was confirmed in vitro in the phagolysosomes of both the cultured macrophages and alveolar macrophages of mice which were exposed to nanomaterials (Figure 4A, orange arrows). SCAV-3 is the C. elegans homologue of human lysosomal integral membrane protein type 2 (LIMP-2, also known as SCARB2) which serves as one of the key regulators of lysosomal membrane integrity. Li et al. also found that the loss of the scav-3 gene in C. elegans causes LMR of the lysosomal limiting membranes (Figure 4B, red arrows) (Li et al., 2016). Simultaneously, Yamashima and his colleagues confirmed presence of the similar leakage of the lysosomal contents in the degenerating cortical neuron of patients with Alzheimer’s disease (Figure 2C, circles). By the careful observation, double contour and/or irregular cofiguration of lysosomes indicating LMR were often detected in the vicinity of autophagolysosomes and degenerated mitochondria (Figure 2C, circles) (Yamashima, 2013; Yamashima et al., 2023d).
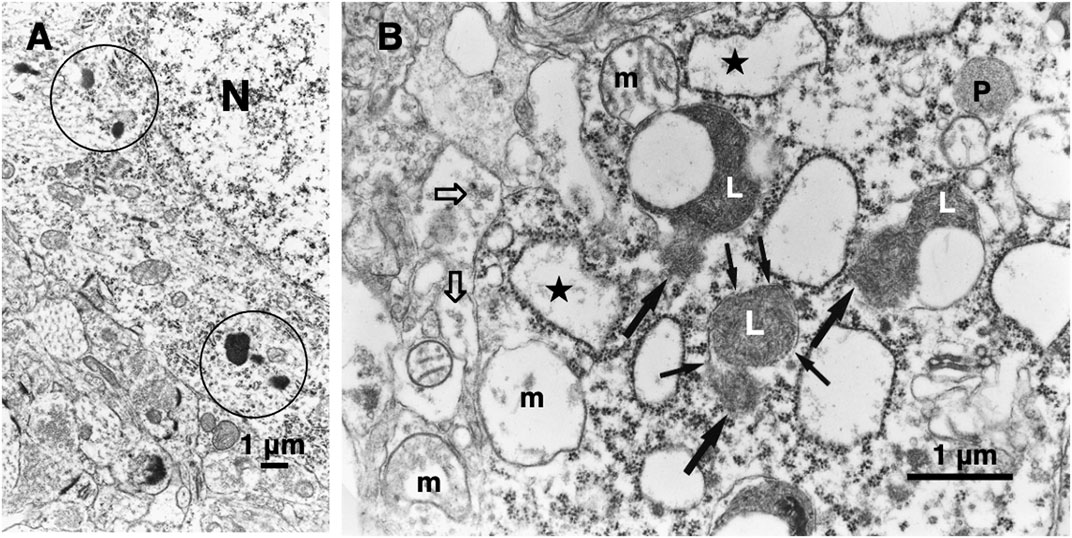
Figure 3. Electronmicroscopic photographs of the monkey hippocampal CA1 neuron before (A) and after (B) the transient ischemia. The non-ischemic CA1 neuron (A) shows normal lysosomes (circles). In contrast, the CA1 neuron after the transient ischemia shows apparent disruptions, i.e., LMR (B, small arrows) of the lysosomal limiting membrane with apparent leakage (B, large arrows) of the lysosomal content (L). Mitochondria (m) show marked disruptions of inner membranes, while rough ER (stars) show swelling. The synaptic vesicles (B, open arrows) are decreased, compared to the control (A). N, nucleus; P, peroxisome (cited from Yamashima et al., 1996; Yamashima et al., 2023d).
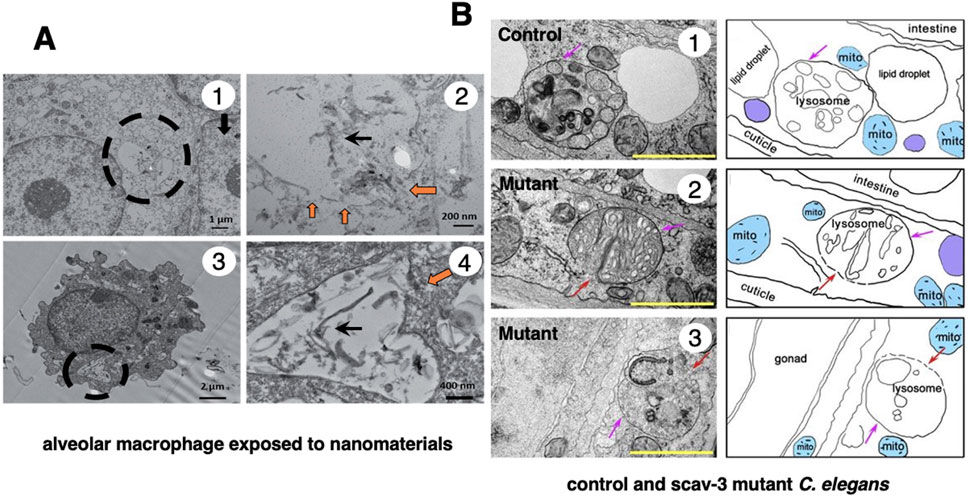
Figure 4. Ultrastructural evidence of LMR which was confirmed in the cultured mice macrophage (A) and the scav-3 mutant C. elegans (B). The cultured alveolar macrophage exposed to nanomaterials (A-1,3 circles, A-2,4 black arrows) show evidence of LMR (A-2,4, orange arrows) (Cited from Kodali et al., 2017). SCAV-3 is the C. elegans homologue of human lysosomal integral membrane protein type 2 (LIMP-2, also known as SCARB2) which serves as one of the key regulators of lysosomal membrane integrity. The loss of the scav-3 gene in C. elegans causes rupture of the lysosomal limiting membranes (B-2,3, red arrows). Purple arrows (B1-3) indicate intact portion of the lysosomal limiting membrane. LMR in the cultured cell and C. elegans is very similar to LMR found in the monkey brain (Figure 3B) (Cited from Li et al., 2016).
Nowadays, it is believed that a low level of cell stresses causes LMP and apoptosis by mitochondrial transmembrane potential loss or caspase activation (Bursch, 2001; Nylandsted et al., 2004; Kirkegaard, and Jäättelä, 2009). In contrast, necrosis is triggered via LMR by more intense, catastrophic events such as heat shock, ischemia, irradiation, or irreparable oxidative stress to the cell (Yamashima et al., 1996; Yamashima et al., 1998; Yamashima et al., 2020; Yamashima, 2000; Yamashima, 2013; Li et al., 2000; Kågedal et al., 2001; Syntichaki et al., 2002; Tardy et al., 2006; Terman et al., 2006). In the consecutive works independently done by Brunk’s group and Yamashima’s group, it was established that the extent of lysosomal disintegrity determines the cell death fate, i.e., a selective release of lysosomal contents results in apoptosis (Figure 2B) (Brunk and Ericsson, 1972; Brunk et al., 1997; Brunk and Svensson, 1999; Li et al., 2000; Antunes et al., 2001; Kågedal et al., 2001), whereas a massive lysosomal breakdown leads to necrosis (Yamashima et al., 1996; Yamashima et al., 1998; Oikawa et al., 2009; Yamashima and Oikawa, 2009; Yamashima, 2000; Yamashima, 2024).
This concept was recently confirmed by us in the cultured hepatocellular carcinoma cell line HepG2 which was exposed to lipid-peroxidation product, 4-hydroxy-2-nonenal (4-HNE), as compared to treatment with antibiotic chemotherapeutic agent, epirubicin (EPI) (Seike et al., 2022) (Figure 5). By the time-lapse imaging using LysoTracker (as shown by orange color), which is a highly-soluble small molecule that is retained in the acidic lysosomal compartment, induction of the lysosomal membrane disintegrity was studied (Supplementary Movie S1).

Figure 5. Time-lapse imagings showing LMR (upper) and LMP (lower). LysoTracker (as shown in orange) is a highly soluble small molecule that is retained within the acidic lysosomal compartments in normal conditions, but shows an extralysosomal leakage at pathological states generating ROS. Cultured HepG2 cells, the hepatocellular carcinoma cell line, were exposed to the synthetic 4-hydroxy-2-nonenal (4-HNE) or epirubicin (EPI). Inserts on each right top indicates morphological changes that were observed on the bright field imaging. The addition of 4-HNE resulted in the gradual reduction and loss of lysosomes (circle) prior to the occurrence of necrotic cell death (upper inserts). In contrast, lysosomes remained almost intact until the apoptotic cell death of EPI-treated cells (lower inserts). Please, note that orange color fades away in the early phase of necrosis, while remains until the end in apoptosis. This indicates that necrosis occurs via LMR, whereas apoptosis occurs via LMP. Blue, Hoechst in the nucleus. Orange, LysoTracker being retained within lysosomes and released around them (Cited from Seike et al., 2022).
The time-lapse imaging revealed that the addition of EPI to the HepG2 cultured cells caused cell shrinkage and formation of blebbing with most of the lysosomes preserved until the execution of apoptosis. As the lysosomal limiting membranes remained ultrastructurally intact, it is likely that gradual and selective leakage had occurred presumably through tiny pores in the limiting membrane. Although acridine orange showed extralysosomal leakage, it grossly remained within lysosomes until apoptosis is completed by EPI. The combined immunoreactivity of acridine orange within lysosomes and perilysosomal area was enlarged, as compared to the control lysosomes (Figure 5, lower column). Although lysosomes showed LMP, their structure grossly remained after the execution of apoptosis. In contrast, the addition of 4-HNE caused “bursting” cell death with all lysosomes fading away prior to the vanishment of the cytoplasm and nuclear chromatin via necrosis. 4-HNE treatment of HepG2 cells caused a rapid vanishment of lysosomes via the explosive shrinkage in the early phase of cell degeneration, as clearly shown in the Supplementary Movie S1. 4-HNE caused a distinct disruption of the lysosomal limiting membranes (LMR) with the rapid and massive leakage of LysoTracker into the cytoplasm (Seike et al., 2022). Consequently, all lysosomes disappeared in the early phase, prior to the execution of necrosis (Figure 5, upper column, circle). Most importantly, the intensity of lysosomal staining is not proportional to the extent of the lysosomal membrane disintegrity. In addition, tracing lysosomes until the execution of necrosis was impossible, whereas lysosomes could be grossly detected after the execution of apoptosis.
3 Lysosomal membrane permeabilization (LMP) and apoptosis
As mentioned above, ultrastructurally-undetectable, smaller perforations (pores) of the lysosomal limiting membrane cause a selective release of hydrolytic enzymes, whereas ultrastructurally-detectable, larger perforations (holes) cause a massive release of hydrolases (Figure 1). For example, smaller dextran molecules (MW, 10 and 40 kDa) are released from the lysosome via LMP, whereas larger dextran molecules (70 and 250 kDa) are retained (Bidere et al., 2003). Further, cationic nanoparticles initially induce the release of smaller cleaved-cathepsin D (∼27 kDa), followed by the larger cathepsin B (∼37 kDa) (Wang et al., 2018b). Nevertheless, the upper limit of size-selection alone does not always apply in all cell death models, because leakage of the bigger lysosomal protein like N-acetyl-β-glucosaminidase (150 kDa) was observed under certain experimental conditions (Ono et al., 2003; Kågedal et al., 2005; Blomgran et al., 2007). Both the extent of LMP and the subset of lysosomes affected by LMR probably direct the downstream cellular responses to determine the cell fate; survival or death, and apoptosis or necrosis (Figure 1). In this sense, electron microscopic analysis of the lysosomal limiting membrane is indispensable to implicate LMR in the given cell death model of each experimental paradigms. Since smaller perforations are hardly detected, LMP could not be detected by electron microscopy. One of the highly-sensitive methodology for detecting LMP would be the specific cytochemical procedure, for example, the lysosomal galectin puncta assay, which detects translocated galectins 1 and 3 much earlier than methods that monitor cathepsin release (Aits et al., 2015). The LysoTracker can also help distinguish the subset of damaged lysosomes from other intact lysosomes, as shown below.
Cathepsins are considered downstream mediators of the lysosomal cell death, but they can apparently initiate LMP. The promoting effect of LMP by cathepsins might be due to the intralysosomal degradation of highly-glycosylated lysosome-associated membrane proteins, i.e., the protective glycocalyx shield (Eskelinen et al., 2003; Fehrenbacher et al., 2008). Further, minor leakage of cathepsins activates LMP by cleaving sphingosine kinase 1 that maintains lysosomal membrane stability (Taha et al., 2005; Mora et al., 2010). LMP is induced also by phospholipase A2, which destabilizes purified lysosomes by degrading membrane phospholipids (Zhao et al., 2001; Zhao et al., 2003). LMP is triggered by a wide range of apoptotic stimuli such as death receptor activation, radiation, cytotoxic drugs, viral and bacterial proteins, oxidative stress, endoplasmic reticulum stress, proteasome inhibition, DNA damage, osmotic stress, and growth factor starvation (Guicciardi et al., 2004; Chwieralski et al., 2006; Stoka et al., 2007; Boya and Kroemer, 2008; Kirkegaard and Jäättelä, 2009). LMP activates apoptotic signaling and the intrinsic apoptosis pathway in the apoptosis-competent cells (Kågedal et al., 2001).
A key event in the execution of apoptosis is the release of apoptogenic factors from mitochondria. In 1998, Roberg and Öllinger discovered that a small amount of cathepsin D was released from lysosomes into the cytosol upon oxidative stress-induced apoptotic cell death (Roberg and Öllinger, 1998). Thereafter, the role of cathepsins as an executor of LMP-induced apoptosis was shown by the ability of microinjected cathepsin B or D to trigger mitochondrial outer membrane permeabilization (MOMP) (Roberg et al., 2002; Bivik et al., 2006). LMP-induced apoptosis is usually activated via MOMP, either by activating cathepsin-mediated cleavage of pro-apoptotic proteins (e.g., Bax, Bak, Bid, Bad, Bim, Noxa, and Puma), or by inhibiting cleavage of anti-apoptotic Bcl-2 homologue proteins (e.g., Bcl-2, Bcl-XL and Mcl-1) (Appelqvist et al., 2012; Cirman et al., 2004; Droga-Mazovec et al., 2008). Bax and Bak are pore-forming proteins that enable the release of apoptogenic factors such as cytochrome c from mitochondria. Cathepsins induce the proteolytic activation of substrates such as Bid and Bax, which in turn promotes MOMP and caspase activation (Aits and Jäättelä, 2013; Wang et al., 2018a). Based on recent findings, LMP may actually precede and contribute to Bax and Bak activation. During apoptosis, intracellular and extracellular perturbations converge in the MOMP-integration phase, resulting in the activation of caspases that serve as the final executors and dismantle cell components and nuclear DNA (Boya et al., 2003; Cirman et al., 2004; Droga-Mazovec et al., 2008; Appelqvist et al., 2012; Aits and Jäättelä, 2013; Wang et al., 2018b).
Interestingly, amyloid β shows structural similarities to pore-forming bacterial toxins, and triggers LMP by creating pores (Johansson et al., 2010). Recently, Zaretsky and Zaretskaia reported that amyloid fragment, specifically Aβ25-35, being mostly lipophilic and carrying a single positive charge, can effectively form non-selective membrane channels at the negatively-charged lysosomal membranes to facilitate Ca2+ efflux (Zaretsky and Zaretskaia, 2025). Activation of μ-calpain can occur not only by the daily intake of deep-fried foods containing abundant 4-HNE which can stimulate GPR40 to induce Ca2+ mobilization (Yamashima et al., 2023b). In addition, Aβ25-35-mediated lysosomal membrane channels would facilitate Ca2+ efflux into the cytoplasm (Zaretsky and Zaretskaia, 2025). It is conceivable that ‘non-selective membrane channels’ are consistent with ‘pores’ which cannot be detected by electron microscopy.
4 Lysosomal membrane rupture (LMR) and necrosis
Unlike mitochondria, lysosomes lack antioxidant enzymes such as superoxide dismutase, catalase, or glutathione peroxidase. Therefore, when ROS levels are high in the cell, ROS can readily cross and damage lysosomal limiting membranes. As lysosomes are rich in iron due to the degradation of iron-containing proteins, intralysosomal iron (Fe2+), generated from iron-containing metalloproteins, reacts with hydrogen peroxide (H2O2) in lysosomes. The Fenton reaction generates Fe3+ and highly-reactive hydroxyl radicals (HO·) to cause lipid peroxidation of the lysosomal membrane (Eaton and Qian, 2002; Kurz et al., 2006; Krenn et al., 2015). For example, macrophages that ingested silica particles with abundant surface-bound iron suffered from extensive lysosomal damage, whereas those that ingested silica particles pretreated with the pharmacologic iron chelator displayed only modest lysosomal leakage (Persson, 2005). Phagocytosis of iron complexes or iron-containing proteins increases lysosomal vulnerability, whereas a reduction in the intralysosomal reactive iron reduces lysosomal leakage and cell death (Garner et al., 1997; Yu et al., 2003). These data altogether indicate that iron is indispensable for inducing lysosomal membrane disintegrity.
4-HNE, previously described as a key inducer of LMR and necrosis, originates not only from dietary linoleic acid but also from cardiolipin in the mitochondrial inner membrane. Hydroxyl radicals induce peroxidation of cardiolipin and thereby generate endogenous 4-HNE. Since abundant ROS are produced during β-oxidation of the palmitate in mitochondria, an increased amount of 4-HNE is endogenously generated from linoleic acids which are involved in cardiolipin (Yamashima, 2024). 4-HNE being generated from both dietary and mitochondrial sources, forms adducts with different side chains in proteins, namely, Cys, His, Lys, and Arg. In a rat model with oxidative stress, Carbone et al. showed by mass spectral analysis that Hsp72 treated with 4-HNE caused adduct formation at Cys267 in the ATPase domain (Carbone et al., 2004). In addition, 4-HNE-adducted Hsp70 has been identified in the G93A-SOD1 transgenic mice, a model of familial amyotrophic lateral sclerosis (Perluigi et al., 2005). It is likely that modifications of Hsp72 or 70 (also called Hsp70.1 in humans) is closely related to diseases.
In 1962, the heat shock protein (Hsp) was discovered in Drosophila melanogaster after the heat shock (Ritossa, 1962). Later, it was demonstrated that, besides thermal stress, the expression of Hsp70 is induced by various insults, including ischemia, irradiation, infection, inflammation, and exposure to organic compounds and oxidants (Lindquist and Craig, 1988). Hsp70.1 is responsible for folding not only newly-synthesized polypeptides under physiological conditions but also misfolded proteins under cellular stresses. Hsp70.1 recruits damaged/aged proteins to the proteasome for turnover (Demand et al., 1998). Further, Hsp70.1 transports damaged/aged proteins to lysosomes for recycling amino acids via chaperone-mediated autophagy (Majeski and Dice, 2004), and, most importantly, contributes to preserving the lysosomal membrane integrity. Hsp70.1 exerts dual roles as a chaperone protein for the transport of garbage proteins and as a stabilizer for the lysosomal limiting membrane. The latter function is achieved by both sphingolipid composition and acid sphingomyelinase (EC3.1.4.12) (Gabande-Rodriguez et al., 2014). Acid sphingomyelinase resides inside lysosomal lumen and its hydrolytic activity is stabilized by bis(monoacylglycero)phosphate (BMP) (Linke et al., 2001). The Hsp70.1-BMP interaction enhances association of BMP with acid sphingomyelinase. This can activate this enzyme, and breakdown sphingomyelin to generate ceramide which protects the lysosomal membrane from rupturing (Heinrich et al., 2000; Kirkegaard et al., 2010; Yamashima, 2013; Yamashima et al., 2020). Accordingly, disorders of Hsp70.1 lead not only to autophagy failure but also to lysosomal disintegrity.
The hippocampal CA1 tissue on days 3 and 5 after transient brain ischemia showed a remarkable (3-9 fold) upregulation of Hsp70.1 on the 2-D oxyblot analysis (Figure 6A). Further, the Matrix-assisted laser desorption ionization-time of flight/time of flight analysis showed a decrease of its molecular weight from 157.20 to 113.12. This means that the specific oxidative injury ‘carbonylation’ occurred at the Arg469 of Hsp70.1 due to ROS being generated during the reperfusion phase (Figures 6B,C) (Oikawa et al., 2009; Yamashima and Oikawa, 2009; Yamashima et al., 2024). In addition, using various brain tissues of non-ischemic monkeys, the calpain-mediated cleavage of the carbonylated Hsp70.1 was demonstrated in vitro (Figure 6D) (Yamashima et al., 2014; Liang et al., 2016). Calpain alone without 4-HNE treatment, i.e., time point (‘0’) showed no cleavage of Hsp70.1, but calpain-mediated Hsp70.1 cleavage progressed time-dependently after incubation with 4-HNE (Figure 6D). Therefore, 4-HNE-induced carbonylation was considered indispensable to facilitate calpain-mediated cleavage of Hsp70.1 (Sahara and Yamashima, 2010; Yamashima et al., 2024).
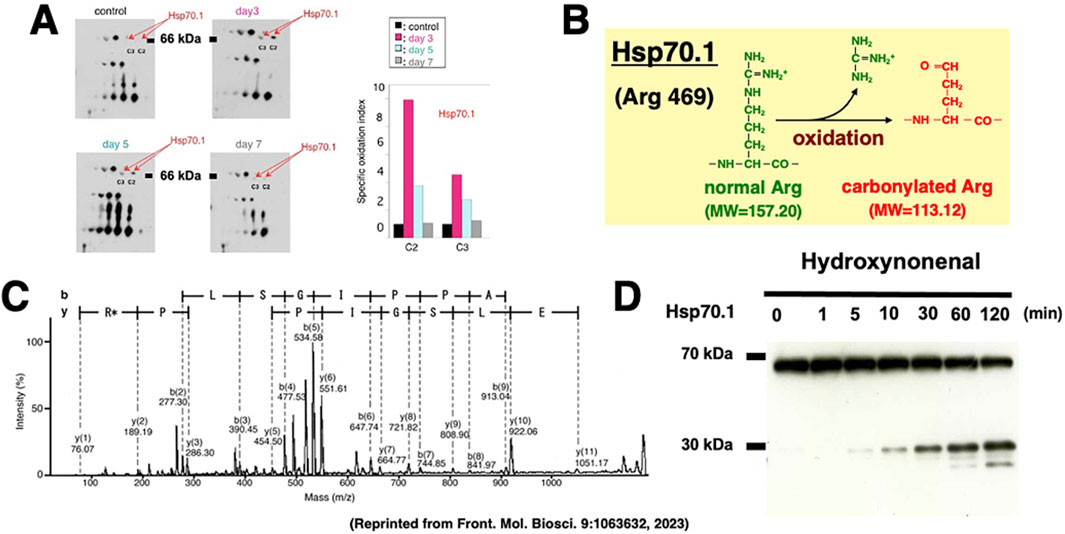
Figure 6. Upregulation, carbonylation, and cleavage of Hsp70.1 in the monkey hippocampal CA1 tissues after transient global brain ischemia. The proteomics analysis showed that Hsp70.1 carbonylation is upregulated on days 3 (pink) and 5 (blue) after ischemia at both C2 and C3 spots on the 2-dimensional electrophoresis (A), and carbonylation occurs at the key site Arg469 (R*) of Hsp70.1 (B,C). Calpain-mediated cleavage of carbonylated Hsp70.1 in vitro increases time-dependently after the 4-HNE treatment (D). Since Hsp70.1 exerts dual roles as a chaperone protein and a lysosomal stabilizer, its disorder causes not only autophagy failure but also cell degeneration/death via the lysosomal membrane disintegrity (Reprinted from Yamashima et al., 2023b).
Similar to the in-vitro experiments using the hippocampal tissues (Figure 6D), the Western blotting analysis of the liver of monkeys which were injected 4-HNE for 24 weeks (total amount 120 mg) also indicates that the calpain-mediated cleavage of Hsp70.1 was increased by 4-HNE in the living animals (Figure 7A) (Yamashima et al., 2023c). By the immunofluorescence analysis of the control liver, activated μ-calpain was observed in the Kupffer cells in the vicinity of sinusoids, whereas hepatocytes showed negligible immunoreactivity (Figure 7B). In contrast, after the 4-HNE injections, the hepatocytes showed a remarkable increase in the merged immunoreactivity of Hsp70.1 and activated μ-calpain (Figure 7C, yellow). Especially, CD68-positive Kupffer cells showed an extensive merged immunoreactivity of Hsp70.1 and activated μ-calpain (Figures 7C,D, circles). Perisinusoidal hepatocytes showed necrosis with a complete dissolution of cytoplasmic organelle (Figure 7E, circle), which was consistent with necrosis of CA1 neurons after transient ischemia (Yamashima et al., 1996). Morphological evidence of apoptosis such as apoptotic bodies and nuclear blebbings was scarcely observed in both neurons after transient ischemia and hepatocytes after the 4-HNE treatment. These in-vitro and in-vivo results suggested that activated μ-calpain interacted with Hsp70.1 after 4-HNE injections, which conceivably facilitated the calpain-mediated cleavage of carbonylated Hsp70.1. In parallel with hepatocyte degeneration/necrosis, cathepsin B immunoreactivity showed an increase in the cytoplasm of both hepatocytes (Figure 7G) and Kupffer cells, compared to the control (Figure 7F). The disintegration of lysosomes was much stronger in the Kupffer cells, compared to the hepatocytes. Most of the lysosomes in the Kupffer cells faded away due to LMR (Figures 7H,I, circles).
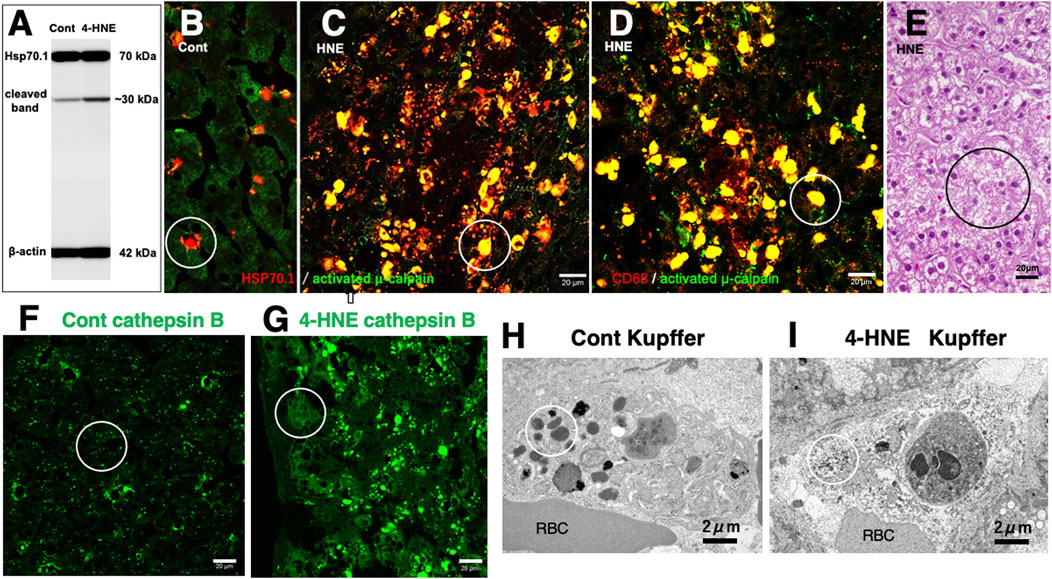
Figure 7. Possible interaction of activated μ-calpain with carbonylated Hsp70.1 in the monkey liver after the intravenous 4-HNE injections (5 mg/w × 24 weeks). On Western blotting, calpain-mediated cleavage of Hsp70.1 increases in the liver tissue after the 4-HNE treatment (A). By immunofluorescence histochemistry (B–D), merged immunoreactivity (yellow) of activated μ-calpain (green) and Hsp70.1 (red) is remarkably increased not only in hepatocytes (C, circle) but also in CD68-positive Kupffer cells (D, circle) after the 4-HNE treatment (C,D), as compared to the control (B). The extralysosomal release of cathepsin B (F,G, circles) in hepatocytes caused their necrotic cell death (E, circle). Apoptotic bodies were scarcely seen in both Kupffer cells and hepatocytes. Lysosomal loss occurs in the perisinusoidal Kupffer cells after the 4-HNE treatment (H,I, circles). RBC, red blood cell within the sinusoid (Cited and adapted from Yamashima et al., 2023d; Yamashima et al., 2023c).
The cathepsin release (Figure 8B, red circles, asterisks) was confirmed in the monkey CA1 neuron after transient ischemia by immunoelectron microscopic analysis of cathepsin B, as compared to the nonischemic CA1 neuron (Figure 8A, circle). This was consistent with the leakage of lysosomal contents as seen in the human Alzheimer neuron (Figure 2C, circles).
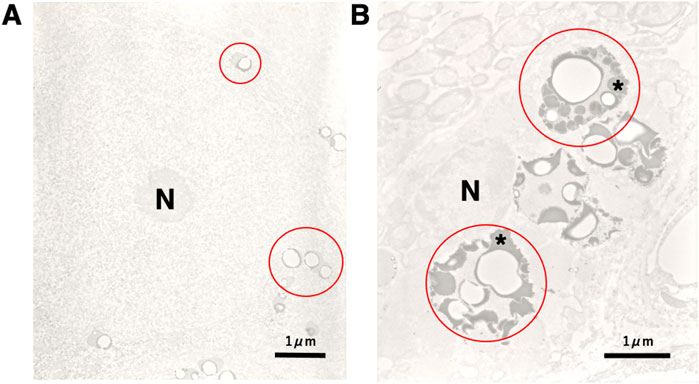
Figure 8. Electronmicroscopic photographs of cathepsin B imunohistochemistry in the monkey CA1 neuron before (A) and after (B) transient brain ischemia. Compared to the control CA1 neuron of the non-ischemic monkey (A, red circles), the CA1 neuron of the monkey on day 5 after transient brain ischemia (B) shows a remarkable increase of lysosomal cathepsin B immunoreactivity (as shown in black), i.e., leakage of the lysosomal content (B, red circles, asterisks) (without ultrastructural stainings). This finding is consistent with the rupture of the lysosomal limiting membranes of the postischemic CA1 neuron (Figure 3B, large arrows). Conceivably, the similar lysosomal rupture has occurred in the hepatocytes and Kupffer cells after the 4-HNE treatment (Figures 7G,I). N, nucleus (Cited and adapted from Yamashima et al., 1998).
Although the present review focused on two representative cell death to make the story simple, implications of LMP and/or LMR, if any, for the other types of cell death such as pyroptosis and ferroptosis etc., had better be briefly discussed (Figure 1). Pyroptosis shares some aspects of apoptosis and ferroptosis, and its concept was first proposed in 2001 (Cookson and Brennan, 2001). Pyroptosis originates from the Greek words of ‘pyro’ (fire or fever) and ‘ptosis’ (fall), symbolizing pro-inflammatory cell death. It takes place in immune cells by microbial infections, and is a highly inflammatory form of lytic programmed cell death. Pyroptosis plays a major role in innate immunity against intracellular pathogens and is triggered by intracellular danger signals that result in plasma membrane rupture and proinflammatory cytokine release. These processes promote the rapid clearance of various bacterial, viral, fungal and protozoan infections by removing intracellular replication niches and enhancing the host’s defensive responses. Since pyroptosis prompts the body to produce a strong inflammatory response, and induce immune phagocytosis, it is different from other type of cell death (Man et al., 2017; Wang et al., 2018a).
As morphologically, biochemically, and genetically distinct from non-apoptotic cell death, the concept of ferroptotic cell death (ferroptosis) was first suggested in 2012 (Dixon et al., 2012). Ferroptosis is one type of cell death resulting from the iron-dependent lipid peroxidation reactions. Lysosomal iron is a trigger of ferroptosis by catalysing generation of ROS which abstract a hydrogen from membrane phospholipids. Acidic nature of the lysosomal compartment, together with the presence of reactive iron and hydrogen peroxide, provides an ideal chemical environment to generate ROS (Cañeque et al., 2025). Accumulation of damaged phospholipids can eventually cause the loss of membrane integrity in such organelles as peroxisomes, mitochondria, endoplasmic reticulum, and lysosomes. However, the specific cellular source of highly-reactive lipid peroxides responsible for ferroptosis has remained subjects of ongoing debate. It is currently unclear whether and how individual organelles contribute to ferroptosis through impaired cell signaling, metabolism, and biosynthesis of specific biomolecules. It has been unelucidated at which cellular organelle lipid peroxidation is initiated and triggers ferroptosis. Intriguingly, Saimoto et al. recently demonstrated that lipid peroxidation at the lysosomal membrane triggers LMP during ferroptosis. Intralysosomal lipid peroxidation triggers iron leakage, fostering cell-wide lipid peroxidation by augmenting LMP (Saimoto et al., 2024).
As mentioned above, lipid peroxidation at the lysosomal membrane causes its disintegrity and necrosis when combined with the specific oxidative injury (carbonylation) of Hsp70.1 (Yamashima and Oikawa, 2009; Yamashima, 2023a; Yamashima, 2024). Hsp70.1 carbonylation is followed by its cleavage via activated μ-calpain, failure of Hsp70.1-BMP binding, and eventually destabilization of the lysosomal membrane. Accordingly, if considering implications of the Fenton reaction, ROS, lipid peroxidation reactions, Ca2+ signaling (Stejerean-Todoran et al., 2024), and the lysosomal membrane disintegrity, there are considerable similarities between the molecular mechanisms of necrosis and ferroptosis. It is tempting to speculate that the calpain-cathepsin cascade is working also in the occurrence of ferroptosis.
5 Summary
1. The available in-vitro and in-vivo experimental data which were obtained from C. elegans to primates, and from neurons to hepatocytes, combined together, indicate that calpain-mediated cleavage of the carbonylated Hsp70.1 may induce cell degeneration/death via LMR which was associated with the massive release of cathepsin enzymes.
2. The ‘calpain-cathepsin hypothesis (Figure 9)’ which was formulated by Yamashima et al., in 1998 and modified in 2009, can explain the mechanism of necrotic cell death of neurons, hepatocytes and Kupffer cells. Importantly, the ultrastructural evidence of LMR was found not after cell death or disease is completed, but during the progression phase of cell degeneration.
3. LMP can be detected by cytochemistry but not by electron microscopy, because of the difficulty of detecting tiny pores at the lysosomal limiting membrane. Since LMR may occur within minutes or hours being associated with lysosomal fading, we have a very restricted chance of finding very small numbers of LMR in the degenerating/dying cells at the given time point. In order to elucidate the cell death fate, careful and detailed electron microscopic analyses are indispensable to detect LMR.
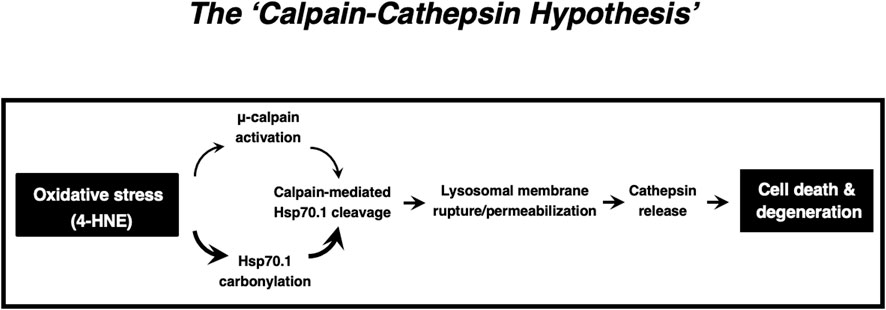
Figure 9. The ‘Calpain-Cathepsin Hypothesis’ explaining the molecular cascade from the oxidative stress (4-HNE) to cell death/degeneration via LMR/LMP (Cited from Yamashima et al., 1998; Yamashima and Oikawa, 2009; Yamashima, 2023a; Yamashima et al., 2023d).
Author contributions
TY: Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from Kiban-Kenkyu (B) (19H04029) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Acknowledgments
The author is deeply grateful for many colleagues participating in the monkey experiments from 1990 to 2025.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fceld.2025.1669955/full#supplementary-material
SUPPLEMENTARY MOVIE S1 | The Movie shows the mechanisms underlying hepatocyte death by stresses. 4-HNE or anti-cancer agent epirubicin (EPI) were added to the HepG2 cultured cells of hepatocellular carcinoma. Morphology of lysosomes was examined using time-lapse imaging by staining lysosomes with LysoTracker (as shown in orange), which is a highly soluble small molecule that is retained in acidic intracellular compartments. The addition of 4-HNE resulted in the gradual reduction and loss of lysosomes in the early phase of necrotic cell death. In contrast, lysosomes remained after the execution of apoptotic cell death of EPI-treated cells.
Abbreviations
ASC, apoptosis-associated speck-like protein containing a caspase-recruitment domain; BMP, bis(monoacylglycero)phosphate; CA1, cornu Ammonis 1; CLEAR, coordinated lysosomal expression and regulation; Cyt c, cytochrome c; EPI, epirubicin; ESCRT, endosomal sorting complex required for transport; 4-HNE, 4-hydroxy-2-nonenal; Hsp, heat-shock protein; Hsp70.1, heat-shock protein 70.1; IL-1β, interleukin-1β; IL-18, interleukin-18; LAMP-2, lysosome-associated membrane protein 2; LIMP-2, lysosomal integral membrane protein type 2; LMP, lysosomal membrane permeabilization; LMR, lysosomal membrane rupture; MOMP, mitochondrial outer membrane permeabilization; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; PUFA, polyunsaturated fatty acids; TFEB, transcription factor EB.
References
Aits, S., and Jäättelä, M. (2013). Lysosomal cell death at a glance. J. Cell Sci. 126 (9), 1905–1912. doi:10.1242/jcs.091181
Aits, S., Kricker, J., Liu, B., Ellegaard, A. M., Hämälistö, S., Tvingsholm, S., et al. (2015). Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 11 (8), 1408–1424. doi:10.1080/15548627.2015.1063871
Antunes, F., Cadenas, E., and Brunk, U. T. (2001). Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem. J. 356 (2), 549–555. doi:10.1042/0264-6021:3560549
Appelqvist, H., Johansson, A. C., Linderoth, E., Johansson, U., Antonsson, B., Steinfeld, R., et al. (2012). Lysosome-mediated apoptosis is associated with cathepsin D-specific processing of bid at Phe24, Trp48, and Phe183. Ann. Clin. Lab. Sci. 42 (3), 231–242. Available online at: https://www.annclinlabsci.org/content/42/3/231.full.pdf+html.
Bidère, N., Lorenzo, H. K., Carmona, S., Laforge, M., Harper, F., Dumont, C., et al. (2003). Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J. Biol. Chem. 278 (33), 31401–31411. doi:10.1074/jbc.M301911200
Bivik, C. A., Larsson, P. K., Kågedal, K. M., Rosdahl, I. K., and Öllinger, K. M. (2006). UVA/B-induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J. Invest. Dermatol. 126 (5), 1119–1127. doi:10.1038/sj.jid.5700124
Blomgran, R., Zheng, L., and Stendahl, O. (2007). Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J. Leukoc. Biol. 81 (5), 1213–1223. doi:10.1189/jlb.0506359
Boya, P., and Kroemer, G. (2008). Lysosomal membrane permeabilization in cell death. Oncogene 27 (50), 6434–6451. doi:10.1038/onc.2008.310
Boya, P., Andreau, K., Poncet, D., Zamzami, N., Perfettini, J. L., Metivier, D., et al. (2003). Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 197 (10), 1323–1334. doi:10.1084/jem.20021952
Brunk, U. T., and Ericsson, J. L. (1972). Cytochemical evidence for the leakage of acid phosphatase through ultrastructurally intact lysosomal membranes. Histochem. J. 4 (6), 479–491. doi:10.1007/BF01011128
Brunk, U. T., and Svensson, I. (1999). Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 4 (1-2), 3–11. doi:10.1179/135100099101534675
Brunk, U. T., Dalen, H., Roberg, K., and Hellquist, H. B. (1997). Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Rad. Biol. Med. 23 (4), 616–626. doi:10.1016/S0891-5849(97)00007-5
Bursch, W. (2001). The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 8, 569–581. doi:10.1038/sj.cdd.4400852
Cañeque, T., Baron, L., Müller, S., Carmona, A., Colombeau, L., Versini, A., et al. (2025). Activation of lysosomal iron triggers ferroptosis in cancer. Nature 642 (8067), 492–500. doi:10.1038/s41586-025-08974-4
Carbone, D. L., Doorn, J. A., Kiebler, Z., Sampey, B. P., and Petersen, D. R. (2004). Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 17 (11), 1459–1467. doi:10.1021/tx049838g
Chwieralski, C. E., Welte, T., and Bühling, F. (2006). Cathepsin-regulated apoptosis. Apoptosis 11 (2), 143–149. doi:10.1007/s10495-006-3486-y
Cirman, T., Orešić, K., Mazovec, G. D., Turk, V., Reed, J. C., Myers, R. M., et al. (2004). Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 279 (5), 3578–3587. doi:10.1074/jbc.M308347200
Cookson, B. T., and Brennan, M. A. (2001). Pro-inflammatory programmed cell death. Trends Microbiol. 9 (3), 113–114. doi:10.1016/s0966-842x(00)01936-3
de Duve, C., and Wattiaux, R. (1966). Functions of lysosomes. Ann. Rev. Physiol. 28, 435–492. doi:10.1146/annurev.ph.28.030166.002251
de Duve, C., Pressman, B. C., Gianetto, R., Wattiaux, R., and Appelmans, F. (1955). Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 60 (4), 604–617. doi:10.1042/bj0600604
Demand, J., Lüders, J., and Höhfeld, J. (1998). The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol. Cell Biol. 18 (4), 2023–2028. doi:10.1128/mcb.18.4.2023
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of non-apoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Droga-Mazovec, G., Bojič, L., Petelin, A., Ivanova, S., Romih, R., Repnik, U., et al. (2008). Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 283 (27), 19140–19150. doi:10.1074/jbc.M802513200
Eaton, J. W., and Qian, M. (2002). Molecular bases of cellular iron toxicity. Free Radic. Biol. Med. 32 (9), 833–840. doi:10.1016/s0891-5849(02)00772-4
Eskelinen, E. L., Tanaka, Y., and Saftig, P. (2003). At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13 (3), 137–145. doi:10.1016/s0962-8924(03)00005-9
Fehrenbacher, N., Bastholm, L., Kirkegaard-Sørensen, T., Rafn, B., Bøttzauw, T., Nielsen, C., et al. (2008). Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 68 (16), 6623–6633. doi:10.1158/0008-5472.CAN-08-0463
Gabandé-Rodríguez, E., Boya, P., Labrador, V., Dotti, C. G., and Ledesma, M. D. (2014). High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 21, 864–875. doi:10.1038/cdd.2014.4
Garner, B., Li, W., Roberg, K., and Brunk, U. T. (1997). On the cytoprotective role of ferritin in macrophages and its ability to enhance lysosomal stability. Free Radic. Res. 27 (5), 487–500. doi:10.3109/10715769709065788
Guicciardi, M. E., Leist, M., and Gores, G. J. (2004). Lysosomes in cell death. Oncogene 23, 2881–2890. doi:10.1038/sj.onc.1207512
Heinrich, M., Wickel, M., Winoto-Morbach, S., Schneider-Brachert, W., Weber, T., Brunner, J., et al. (2000). Ceramide as an activator lipid of cathepsin D. Adv. Exp. Med. Biol. 477, 305–315. doi:10.1007/0-306-46826-3_33
Johansson, A. C., Appelqvist, H., Nilsson, C., Kågedal, K., Roberg, K., and Öllinger, K. (2010). Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 15, 527–540. doi:10.1007/s10495-009-0452-5
Kågedal, K., Zhao, M., Svensson, I., and Brunk, U. T. (2001). Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359 (2), 335–343. doi:10.1042/0264-6021:3590335
Kågedal, K., Johansson, A. C., Johansson, U., Heimlich, G., Roberg, K., Wang, N. S., et al. (2005). Lysosomal membrane permeabilization during apoptosis-involvement of Bax? Int. J. Exp. Pathol. 86 (5), 309–321. doi:10.1111/j.0959-9673.2005.00442.x
Kirkegaard, T., and Jäättelä, M. (2009). Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 1793 (4), 746–754. doi:10.1016/j.bbamcr.2008.09.008
Kirkegaard, T., Roth, A. G., Petersen, N. H., Mahalka, A. K., Olsen, O. D., Moilanen, I., et al. (2010). Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463 (7280), 549–553. doi:10.1038/nature08710
Kodali, V. K., Roberts, J. R., Shoeb, M., Wolfarth, M. G., Bishop, L., Eye, T., et al. (2017). Acute in vitro and in vivo toxicity of a commercial grade born nitride nanotube mixture. Nanotoxicology, 11 (8). 1040–1058. doi:10.1080/17435390.2017.1390177
Krenn, M. A., Schürz, M., Teufl, B., Uchida, K., Eckl, P. M., and Bresgen, N. (2015). Ferritin-stimulated lipid peroxidation, lysosomal leak, and macroautophagy promote lysosomal “metastability” in primary hepatocytes determining in vitro cell survival. Free Radic. Biol. Med. 80, 48–58. doi:10.1016/j.freeradbiomed.2014.12.007
Kurz, T., Gustafsson, B., and Brunk, U. T. (2006). Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 273 (13), 3106–3117. doi:10.1111/j.1742-4658.2006.05321.x
Li, W., Yuan, X., Nordgren, G., Dalen, H., Dubowchik, G. M., Firestone, R. A., et al. (2000). Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 470 (1), 35–39. doi:10.1016/S0014-5793(00)01286-2
Li, Y., Chen, B., Zou, W., Wang, X., Wu, Y., Zhao, D., et al. (2016). The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity. J. Cell Biol. 215 (2), 167–185. doi:10.1083/jcb.201602090
Liang, H., Kurimoto, S., Shima, K. R., Shimizu, D., Ota, T., Minabe, Y., et al. (2016). Why is hippocampal CA1 especially vulnerable to ischemia? SOJ Biochem. 2 (2), 1–7. doi:10.15226/2376-4589/2/2/00114
Lindquist, S., and Craig, E. A. (1988). The heat-shock proteins. Annu. Rev. Genet. 22, 631–677. doi:10.1146/annurev.ge.22.120188.003215
Linke, T., Wilkening, G., Lansmann, S., Moczall, H., Bartelsen, O., Weisgerber, J., et al. (2001). Stimulation of acid sphingomyelinase activity by lysosomal lipids and sphingolipid activator proteins. Biol. Chem. 382 (2), 283–290. doi:10.1515/BC.2001.035
Luzio, J. P., Pryor, P. R., and Bright, N. A. (2007). Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8 (8), 622–632. doi:10.1038/nrm2217
Majeski, A. E., and Dice, J. F. (2004). Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 36 (12), 2435–2444. doi:10.1016/j.biocel.2004.02.013
Man, S. M., Karki, R., and Kanneganti, T. D. (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277 (1), 61–75. doi:10.1111/imr.12534
Mora, R., Dokic, I., Kees, T., Hüber, C. M., Keitel, D., Geibig, R., et al. (2010). Sphingolipid rheostat alterations related to transformation can be exploited for specific induction of lysosomal cell death in murine and human glioma. Glia 58 (11), 1364–1383. doi:10.1002/glia.21013
Nylandsted, J., Gyrd-Hansen, M., Danielewicz, A., Fehrenbacher, N., Lademann, U., Høyer-Hansen, M., et al. (2004). Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200 (4), 425–435. doi:10.1084/jem.20040531
Oikawa, S., Yamada, T., Minohata, T., Kobayashi, H., Furukawa, A., Tada-Oikawa, S., et al. (2009). Proteomic identification of carbonylated proteins in the monkey hippocampus after ischemia-reperfusion. Free Radic. Biol. Med. 46 (11), 1472–1477. doi:10.1016/j.freeradbiomed.2009.02.029
Ono, K., Kim, S. O., and Han, J. (2003). Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol. Cell Biol. 23 (2), 665–676. doi:10.1128/MCB.23.2.665-676.2003
Perluigi, M., Poon, H. F., Hensley, K., Pierce, W. M., Klein, J. B., Calabrese, V., et al. (2005). Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice – a model of familial amyotrophic lateral sclerosis. Free Radic. Biol. Med. 38 (7), 960–968. doi:10.1016/j.freeradbiomed.2004.12.021
Persson, H. L. (2005). Iron-dependent lysosomal destabilization initiates silica-induced apoptosis in murine macrophages. Toxicol. Lett. 159 (2), 124–133. doi:10.1016/j.toxlet.2005.05.002
Ritossa, F. (1962). A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18, 571–573. doi:10.1007/BF02172188
Roberg, K., and Öllinger, K. (1998). Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 152 (5), 1151–1156. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC1858594/.
Roberg, K., Kågedal, K., and Öllinger, K. (2002). Microinjection of cathepsin D induces caspase-dependent apoptosis in fibroblasts. Am. J. Pathol. 161 (1), 89–96. doi:10.1016/S0002-9440(10)64160-0
Saftig, P., and Klumperman, J. (2009). Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10 (9), 623–635. doi:10.1038/nrm2745
Sahara, S., and Yamashima, T. (2010). Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem. Biophys. Res. Commun. 393 (4), 806–811. doi:10.1016/j.bbrc.2010.02.087
Saimoto, Y., Kusakabe, D., Morimoto, K., Matsuoka, Y., Kozakura, E., Kato, N., et al. (2024). Lysosomal lipid peroxidation contributes to ferroptosis induction via lysosomal membrane permeabilization. Nat. Commun. 16, 3554. doi:10.1038/s41467-025-58909-w
Sardiello, M., Palmieri, M., di Ronza, A., Medina, D. L., Valenza, M., Gennarino, V. A., et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325 (5939), 473–477. doi:10.1126/science.1174447
Seike, T., Boontem, P., Kido, H., Yanagi, M., Yamamiya, D., Nakagawa, H., et al. (2022). Hydroxynonenal causes hepatocyte death by disrupting lysosomal integrity in non-alcoholic steatohepatitis. Cell. Mol. Gastroenterol Hepatol. 14 (4), 925–944. doi:10.1016/j.jcmgh.2022.06.008
Skowyra, M. L., Schlesinger, P. H., Naismith, T. V., and Hanson, P. I. (2018). Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360 (6384), eaar5078. doi:10.1126/science.aar5078
Stejerean-Todoran, I., Gibhardt, C. S., and Bogeski, I. (2024). Calcium signals as regulators of ferroptosis in cancer. Cell Calcium 124, 102966. doi:10.1016/j.ceca.2024.102966
Stoka, V., Turk, V., and Turk, B. (2007). Lysosomal cysteine cathepsins: signaling pathways in apoptosis. Biol. Chem. 388 (6), 555–560. doi:10.1515/BC.2007.064
Syntichaki, P., Xu, K., Driscoll, M., and Tavernarakis, N. (2002). Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419 (6910), 939–944. doi:10.1038/nature01108
Taha, T. A., Kitatani, K., Bielawski, J., Cho, W., Hannun, Y. A., and Obeid, L. M. (2005). Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J. Biol. Chem. 280 (17), 17196–17202. doi:10.1074/jbc.M413744200
Tardy, C., Codogno, P., Autefage, H., Levade, T., and Andrieu-Abadie, N. (2006). Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle). Biochim. Biophys. Acta 1765 (2), 101–125. doi:10.1016/j.bbcan.2005.11.003
Terman, A., Kurz, T., Gustafsson, B., and Brunk, U. T. (2006). Lysosomal labilization. IUBMB Life 58 (9), 531–539. doi:10.1080/15216540600904885
Wang, F., Gómez-Sintes, R., and Boya, P. (2018a). Lysosomal membrane permeabilization and cell death. Traffic 19 (12), 918–931. doi:10.1111/tra.12613
Wang, F., Salvati, A., and Boya, P. (2018b). Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 8, 170271. doi:10.1098/rsob.170271
Yamashima, T. (2000). Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62 (3), 273–295. doi:10.1016/s0301-0082(00)00006-x
Yamashima, T. (2013). Reconsider Alzheimer’s disease by the ‘calpain-cathepsin hypothesis’—a perspective review. Prog. Neurobiol. 105, 1–23. doi:10.1016/j.pneurobio.2013.02.004
Yamashima, T. (2023a). Implication of vegetable oil-derived hydroxynonenal in the lysosomal cell death for lifestyle-related diseases. Nutrients 15 (3), 609. doi:10.3390/nu15030609
Yamashima, T. (2024). 4-Hydroxynonenal from mitochondrial and dietary sources causes lysosomal cell death for lifestyle-related diseases. Nutrients 16 (23), 4171. doi:10.3390/nu16234171
Yamashima, T., and Oikawa, S. (2009). The role of lysosomal rupture in neuronal death. Prog. Neurobiol. 89 (4), 343–358. doi:10.1016/j.pneurobio.2009.09.003
Yamashima, T., Saido, T. C., Takita, M., Miyazawa, A., Yamano, J., Miyakawa, A., et al. (1996). Transient brain ischaemia provokes Ca2+, PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur. J. Neurosci. 8 (9), 1932–1944. doi:10.1111/j.1460-9568.1996.tb01337.x
Yamashima, T., Kohda, Y., Tsuchiya, K., Ueno, T., Yamashita, J., Yoshioka, T., et al. (1998). Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on 'calpain-cathepsin hypothesis'. Eur. J. Neurosci. 10 (5), 1723–1733. doi:10.1046/j.1460-9568.1998.00184.x
Yamashima, T., Mathivanan, A., Dazortsava, M. Y., Sakai, S., Kurimoto, S., Zhu, H., et al. (2014). Calpain-mediated Hsp70.1 cleavage in monkey CA1 after ischemia induces similar ‘lysosomal vesiculosis’ to Alzheimer neurons. J. Alzheimers Dis. Park. 4, 2. doi:10.4172/2161-0460.1000139
Yamashima, T., Ota, T., Mizukoshi, E., Nakamura, H., Yamamoto, Y., Kikuchi, M., et al. (2020). Intake of ω-6 polyunsaturated fatty acid-rich vegetable oils and risk of lifestyle diseases. Adv. Nutr. 11 (6), 1489–1509. doi:10.1093/advances/nmaa072
Yamashima, T., Seike, T., Mochly-Rosen, D., Chen, C. H., Kikuchi, M., and Mizukoshi, E. (2023b). Implication of the cooking oil-peroxidation product “hydroxynonenal” for Alzheimer’s disease. Front. Aging Neurosci. 15, 1211141. doi:10.3389/fnagi.2023.1211141
Yamashima, T., Mori, Y., Seike, T., Ahmed, S., Boontem, P., Li, S., et al. (2023c). Vegetable oil-peroxidation product ‘hydroxynonenal’ causes hepatocyte injury and steatosis via Hsp70.1 and BHMT disorders in the monkey liver. Nutrients 15, 1904. doi:10.3390/nu15081904
Yamashima, T., Seike, T., Oikawa, S., Kobayashi, H., Kido, H., Yanagi, M., et al. (2023d). Hsp70.1 carbonylation induces lysosomal cell death for lifestyle-related diseases. Front. Mol. Biosci. 9, 1063632. doi:10.3389/fmolb.2022.1063632
Yamashima, T., Mochly-Rosen, D., Wakatsuki, S., Mizukoshi, E., Seike, T., Larus, I. M., et al. (2024). Cleavage of Hsp70.1 causes lysosomal cell death under stress conditions. Front. Mol. Biosci. 11, 1378656. doi:10.3389/fmolb.2024.1378656
Yu, Z., Persson, H. L., Eaton, J. W., and Brunk, U. T. (2003). Intralysosomal iron: a major determinant of oxidant-induced cell death. Free Radic. Biol. Med. 34, 1243–1252. doi:10.1016/s0891-5849(03)00109-6
Zaretsky, D. V., and Zaretskaia, M. V. (2025). The framework for an integrative theory of Alzheimer’s disease. Curr. Alz. Res. 22, 179–204. doi:10.2174/0115672050381553250425062803
Zhao, M., Brunk, U. T., and Eaton, J. W. (2001). Delayed oxidant-induced cell death involves activation of phospholipase A2. FEBS Lett. 509, 399–404. doi:10.1016/s0014-5793(01)03184-2
Keywords: lysosome, calpain-cathepsin hypothesis, cell death, hydroxynonenal, apoptosis, necrosis, ferroptosis, pyroptosis
Citation: Yamashima T (2025) Lysosomal membrane-permeabilization (LMP) and -rupture (LMR) are distinct for cell death. Front. Cell Death 4:1669955. doi: 10.3389/fceld.2025.1669955
Received: 20 July 2025; Accepted: 20 August 2025;
Published: 12 September 2025.
Edited by:
Yun Fan, University of Birmingham, United KingdomReviewed by:
Natsuki Shinoda, The University of Tokyo, JapanAndrew Davidson, University of Glasgow, United Kingdom
Copyright © 2025 Yamashima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsumori Yamashima, eWFtYXNoaW1hMjE1QGdtYWlsLmNvbQ==
 Tetsumori Yamashima
Tetsumori Yamashima