- 1Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
- 2Alvin J. Siteman Cancer Center, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
- 3Division of Medical Oncology, Department of Medicine, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
- 4Program in Physical Therapy, Washington University School of Medicine in St. Louis, St. Louis, MO, United States
- 5Prevention Research Center, Brown School, Washington University in St. Louis, St. Louis, MO, United States
Background: Cancer-related cognitive decline (CRCD) is one of the most reported and debilitating symptoms associated with breast cancer treatment with no currently accepted treatment. Physical activity has emerged as a promising solution for maintaining cognitive health after cancer, with research suggesting that earlier intervention may be important for preventing or mitigating CRCD. There is a clear need to pilot the feasibility and efficacy of pragmatic physical activity interventions to promote cognitive health during active breast cancer treatment. The purpose of this study is to pilot test a home-based prehabilitation physical activity intervention aimed at preventing cognitive decline during chemotherapy, as well as assess the feasibility, acceptability and appropriateness of the intervention among patients.
Methods: This study is a two-arm, pilot randomized controlled trial in 40 adult patients newly diagnosed with breast cancer stages I-III who are scheduled to receive curative intent neo-adjuvant chemotherapy. Participants will be randomized 1:1 to a usual care waitlist control group or a home-based prehabilitation physical activity intervention delivered by a licensed physical therapist. The exercise group will receive an individualized, tapered exercise program comprised of home exercise sessions and virtual coaching calls. Both groups will receive activity monitors and be encouraged to maintain a healthy lifestyle during treatment. Participant adherence and adverse events will be assessed throughout the study.
Results: This research was supported by the Alvin J. Siteman Cancer Center through The Foundation for Barnes-Jewish Hospital (award No. 6257). The protocol was approved by Washington University's Protocol Review and Monitoring Committee and Institutional Review Board. Enrollment began in May 2023 and is anticipated to continue through July 2024.
Conclusions: This study will provide the necessary preliminary data to support larger trials investigating if and how physical activity can be incorporated into early rehabilitation strategies to prevent chemotherapy-related sequelae.
Trial registration: ClinicalTrials.gov, NCT05716542.
1 Introduction
More than 3.8 million women currently live with a history of breast cancer in the United States (DeSantis et al., 2019), and up to 75% of them report some degree of cognitive deficit during active treatment (Zimmer et al., 2016). Colloquially known as “chemo-brain,” cancer-related cognitive decline (CRCD) is defined as the loss of mental acuity associated with cancer and its subsequent treatment (Raffa, 2010). CRCD can present as impaired verbal and visual memory, attention, concentration, language, motor skills, multitasking, processing speed, and the ability to organize information (Raffa, 2010). CRCD is also pervasive, with approximately one third of breast cancer patients reporting lingering CRCD symptoms over a decade after treatment completion (Janelsins et al., 2014; Zimmer et al., 2016). Despite the importance of cognition for quality of life and cancer survival, there is little empirical evidence for effective CRCD treatments.
Maintaining sufficient levels of exercise is important to both prevent cancer and improve health post-diagnosis (Campbell et al., 2019; Patel et al., 2019; Schmitz et al., 2019). Exercise after breast cancer improves functional (Demark-Wahnefried et al., 2006; Campbell et al., 2019) and psychosocial health (Mustian et al., 2012; Mishra et al., 2014; Schmidt et al., 2015; Rogers et al., 2016; Campbell et al., 2019) and is associated with reduced risk of recurrence (Loprinzi et al., 2012; Courneya et al., 2014) and mortality (Holmes et al., 2005; Chen et al., 2011; Loprinzi et al., 2012). The benefits of exercise for cognitive function are emerging but incomplete (Zimmer et al., 2016; Campbell et al., 2019), largely due to a lack of cognition as a primary outcome in exercise trials, varying measures of cognitive function, and heterogeneous exercise dosing (Campbell et al., 2019). One randomized controlled aerobic exercise trial reported improvements in one domain of objectively-measured cognition, but not self-reported cognitive function, in breast cancer survivors after treatment completion (Hartman et al., 2018). Interestingly, improvements were only noted for those participants who were within 2 years of diagnosis. Another recently completed randomized controlled multicomponent exercise trial reported significant improvements in self-reported cognition and no change in objective measures (Koevoets et al., 2022). Other randomized controlled trials are currently in progress (Gentry et al., 2018; Kiesl et al., 2022), underscoring the burgeoning interest in the exercise-cognition relationship after cancer.
Despite these equivocal findings, evidence increasingly suggests that early intervention prior to or during chemotherapy (i.e., prehabilitation) may be important for cognitive function (Zimmer et al., 2016; Campbell et al., 2020; Salerno et al., 2021), while also reducing hospital length of stay, surgical complications, and overall healthcare costs (Silver and Baima, 2013; Silver, 2015; Stout et al., 2020). In breast cancer specifically, exercise interventions delivered during chemotherapy have noted improvements in health domains impaired by treatment, such as cardiac function, cognitive function, lymphedema, cardiorespiratory fitness, and musculoskeletal strength (Courneya et al., 2013, 2014; Furmaniak et al., 2016). We recently reported on the epidemiological association between higher levels of exercise before and during chemotherapy in patients with breast cancer, highlighting the potential cognitive benefits of early exercise intervention (Salerno et al., 2021).
However, implementation of prehabilitation programs during active breast cancer treatment is challenging for both patients and oncologists alike. Oncologists have limited (if any) time to systematically assess and advise patients on exercise behavior, and patients are often overwhelmed by a new cancer diagnosis and its multiple ensuing appointments. These factors converge to reduce enrollment in and adherence to exercise programs during chemotherapy; only between 25 and 50% of patients approached to participate in exercise trials during cancer treatment actually consent (van Waart et al., 2016). As a result, many rehabilitation programs are delivered after treatment completion, when health declines have already occurred. This current model waits too long to intervene. Leveraging existing healthcare professionals to increase exercise prior to or during treatment is an accessible and pragmatic approach to prehabilitation. Physical therapists (PTs) are rehabilitation clinicians who use a movement-based approach to treatment neurological, musculoskeletal, and cardiovascular conditions across populations (Bezner, 2015). Integrating PTs into oncology care is a cost-effective approach to improving function after cancer treatment (van Waart et al., 2018) and can increase uptake of positive health behaviors, largely due to increased awareness of benefits (Barnes et al., 2020) and trust in an oncologist-referred service (Lis et al., 2009).
1.1 Aims and hypotheses
The overall objective of this study is to pilot test an exercise intervention aimed at preventing cognitive decline during chemotherapy for breast cancer. The primary aim is to collect preliminary estimates of intervention efficacy on change in self-reported cognition. The secondary aim is to assess the intervention's feasibility, acceptability, appropriateness, and implementation potential among patients and the intervention PT using both quantitative (e.g., feasibility questionnaires) and qualitative (e.g., semi-structured interviews) methods. We will further explore potential mechanisms of the exercise-cognition relationship during treatment for breast cancer. We hypothesize that the exercise intervention will: (1) prevent a clinically meaningful decline in cognitive function in the intervention group compared with the control group, and (2) be feasible, acceptable, and appropriate to enrolled patients, with high levels of attendance.
2 Methods and analysis
2.1 Ethics and dissemination
The PROTECT (Prehabilitation to Revolutionize Oncology: Telehealth Exercise for Cognitive Triumphs) trial will be conducted in accordance with the Helsinki Declaration, and all participants will provide written informed consent prior to any participation. This study is approved by Washington University's Protocol Review and Monitoring Committee (PRMC) and institutional review board (HRPO #202302077), and preregistered at ClinicalTrials.gov (NCT05716542).
2.2 Study design
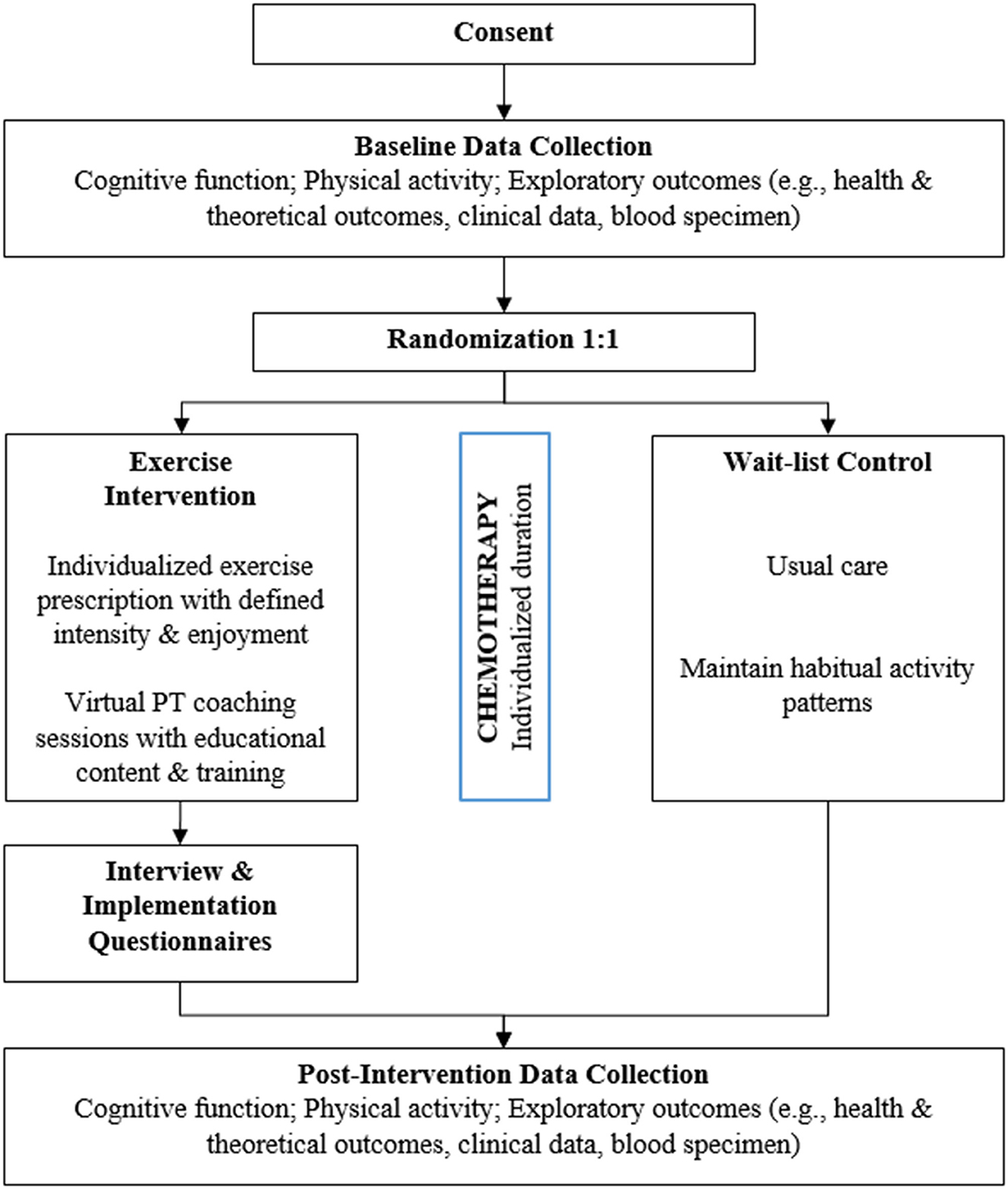
The PROTECT trial is a 2-arm pilot randomized controlled prehabilitation exercise intervention in patients newly diagnosed with breast cancer and scheduled to undergo curative-intent chemotherapy. Participants will be randomized 1:1 to either an individualized home-based aerobic exercise group delivered by a licensed PT (intervention group) or a wait-list usual care group (control group) (Figure 1).
2.3 Participants
Participants (N = 40) will be drawn from the breast cancer patient populations within our cancer center catchment area and randomized to the two arms by stratification of treatment (e.g., neoadjuvant vs. adjuvant) and age (e.g., <60 vs. ≥60 years of age) to have a relatively balanced representation of all eligible patient populations. Eligibility criteria include: (1) a physician-confirmed diagnosis of breast cancer (stage I-III); (2) female age 18 or older; (3) scheduled to receive curative-intent chemotherapy; (4) English speaking; (5) not currently participating in another physical activity research study; (6) low-active, defined as fewer than 2 days per week of at least 20 min of activity; (7) willing to sign the informed consent document, and; (8) medically cleared to participate in an exercise program by their oncologist (i.e., written attestation).
2.4 Recruitment
Women newly diagnosed with breast cancer stage I-III are identified through established partnerships with the clinical breast team at Siteman Cancer Center. Patients who match the eligibility criteria are flagged and either: (1) called by the research team, or (2) approached by a study team member in clinic. Patients are provided with a thorough description of the study and assessed for their interest. Eligible and interested patients who wish to participate are invited to consent procedures and scheduled for baseline assessments. Given the remote consent process and home-based intervention, this trial allows for enrollment across the local healthcare system and increases the potential to enroll patients who may have barriers to transportation or traditional work-related time constraints.
2.5 Randomization
Consenting and eligible participants are randomized using stratified randomization with varying block size, stratifying by cancer treatment (e.g., adjuvant vs. neoadjuvant) and age (e.g., <60, ≥60 years). Further covariates will be adjusted for in final analytic models. Block stratified randomization is generated by the study team using R, and the randomization table is uploaded to REDCap.
2.6 Procedures
2.6.1 Intervention group
Participants assigned to the intervention arm complete a home-based exercise intervention, with the goal of safely increasing their steps/day by incorporating moderate-to-vigorous physical activity (MVPA).
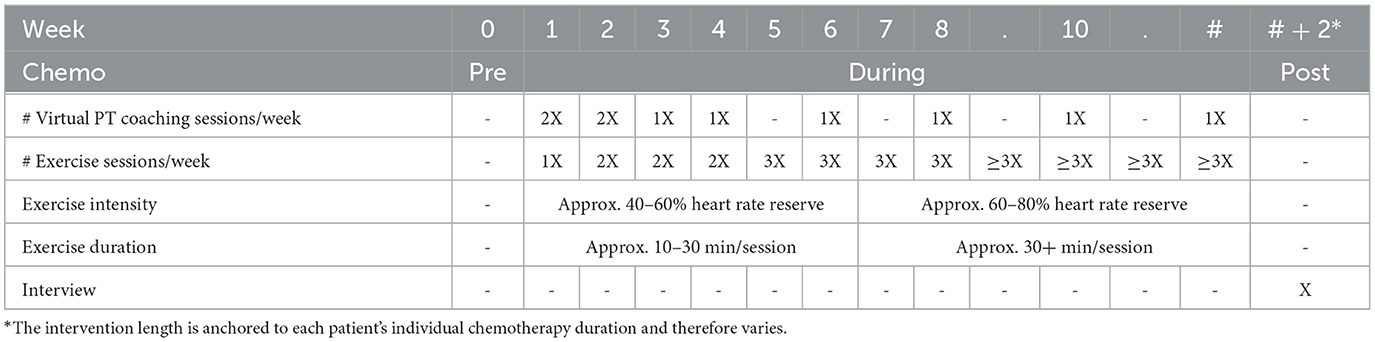
Intervention length is unique to each patient's chemotherapy duration and therefore varies; however, standards are maintained for frequency, intensity, and time. Table 1 details the intervention timeline and relevant components (described below).
2.6.1.1 Core intervention components
1. Virtual PT coaching sessions: The intervention PT meets with participants via teleconferencing technology (i.e., Zoom) or phone to provide educational content, support exercise maintenance, strategize new exercises, and troubleshoot emerging chemotherapy-related health declines. These coaching sessions are tapered in frequency to promote safety and support independence, a key ingredient in long-term behavioral maintenance. The first six sessions (weeks 0–4) contain educational content on the following topics:
• Session 1: Purpose of the study, value of exercise, exercise history & values.
• Session 2: Goal setting, tracking home exercise (i.e., home logs, heart rate training), safety.
• Session 3: Stages and maintenance of change, exercise barriers.
• Session 4: Self-knowledge, stress and time management.
• Session 5: Behavior change, self-talk, wellness.
• Session 6: Overview, relapse prevention.
After session 6 (week 4), participants meet with the intervention PT biweekly to gauge progress, identify solutions for new barriers, and readjust goals accordingly. Throughout the intervention, participants are trained on goal setting and self-regulation, identifying cues to action, and emphasizing the targets for self-efficacy (e.g., mastery experiences, social modeling, social persuasion, interpreting biological and psychological feedback) (Bandura, 2001).
2. Home exercise sessions: Participants will be prescribed home-based walking sessions at a moderate to vigorous intensity, given that the strongest effects on cognition appear to arise from MVPA rather than lighter intensity activities (Salerno et al., 2019, 2021). However, enjoyment is foundational for long-term behavior change (Lewis et al., 2016), therefore participants will strategize other aerobic MVPA behaviors with the intervention PT (e.g., walking, cycling) to maximize enjoyment and allow for flexibility if life- or cancer-specific barriers arise. Such an approach lays the groundwork for sustained exercise behavior well beyond intervention cessation. These home exercise sessions are progressive in time and intensity with set standards based on intervention week (see Table 1) to support safe progression through the program. Participants complete home exercise logs to record the exercise type, duration, heart rate, rating of perceived exertion (Borg, 1998), and enjoyment. The intervention PT then reviews these home logs with participants at the start of each virtual PT coaching session to ensure adherence and support goal setting.
3. A Fitbit wearable device: All participants receive (and keep) Fitbits with heart rate capabilities to support maintenance of the prescribed intensity during home exercise sessions and participants' self-monitoring of daily steps.
2.6.1.2 Individualized intervention components
Individualized components of the intervention are determined by the PT throughout the intervention to address treatment-specific barriers with participants as they arise (e.g., adding stretching exercises to help with lymphedema). Given that PTs are trained in identifying functional impairments, they are uniquely positioned to target individualized exercise barriers in a way that maximizes patient safety.
2.6.1.3 Interviews
A subsample of 10 patients from the intervention arm of the prehabilitation intervention and the intervention PT will participate in semi-structured interviews. All interviews will be recorded and guided by the Consolidated Framework for Implementation Research (CFIR) to assess intervention feasibility and satisfaction, as well as barriers and facilitators of implementation to inform strategies for future scalability (Damschroder et al., 2009). All interviews will be semi-structured, containing a combination of closed and open questions, and last no longer than 45 min to minimize burden. Questions will seek to understand intervention feasibility, acceptability, and appropriateness, as well as barriers and facilitators within all five domains of the CFIR: intervention characteristics, outer setting, inner setting, individual characteristics, and process. These qualitative interviews, combined with quantitative survey data, are essential for understanding the modifications required to successfully scale and broadly disseminate the PROTECT trial.
2.6.2 Control group
Participants in the control group proceed with their treatment regimen as prescribed (e.g., usual care). To prevent drop out and high attrition rates as well as promote healthy behavior, control group participants will receive an individualized home exercise program and up to two telehealth visits with the intervention PT after chemotherapy completion as well as a Fitbit device to wear throughout chemotherapy and eventually keep.
2.7 Assessments
Cognitive function and exploratory measures will be assessed at baseline (within 3 weeks of first chemotherapy infusion) and end of intervention (within 3 weeks of last day of final chemotherapy cycle). Feasibility outcomes will be assessed at end of intervention.
2.7.1 Cognitive function
Our primary cognitive function outcome variable is self-reported total cognition. We have previously demonstrated that this outcome is significantly affected by both chemotherapy and exercise (Janelsins et al., 2018; Salerno et al., 2021), and it has a benchmark for clinically meaningful change (i.e., ≥1/2 SD change over time) (Cheung et al., 2014; Janelsins et al., 2017). We are supplementing this outcome measure with a combination of validated objective cognitive measures; the International Cognition and Cancer Task Force recommends including both self-reported and objective measures of cognition in CRCD studies (Wefel et al., 2011). All cognitive function measures are collected using REDCap (self-reported) and the BrainBaseline app (objective) on a research iPad. BrainBaseline includes several tasks that assess domains of attention, memory, executive function, emotion and social cognition, and psychomotor speed. Each task consists of instructions, a practice session with accuracy feedback, and trials with no feedback. Participants are asked to respond to the task as quickly and as accurately as possible. This method allows for self-administered cognitive testing, an important component of designing pragmatic and sustainable trials aimed at improving cognition. All five objective measures of cognitive function listed below (i.e., spatial working memory paradigm, Stroop, n-back, Flanker, Trail Making Test) have been used consistently in the cancer literature and are considered valid and reliable tests (Wefel et al., 2011).
• Self-Reported Cognition: The Functional Assessment of Cancer Therapy-Cognition (FACT-Cog) yields a total score and four subdomains: impairments, abilities, noticeability, and quality of life (Jacobs et al., 2007; Lai et al., 2009). The FACT-Cog is a valid and reliable questionnaire with excellent internal consistency (Cronbach alphas for total score and subdomains >0.81) (Hajj et al., 2022).
• Spatial Processing: To assess spatial processing and working memory, participants will complete a modified version of the spatial working memory paradigm (Erickson et al., 2011).
• Inhibition: To assess cognition inhibition and interference, participants will perform a modified version of the Stroop Color Word Test (Stroop, 1935).
• Working Memory: A modified, serial n-back test (Nystrom et al., 2000; Takeuchi et al., 2011) that involves three consecutive parts called the 0-back, 1-back, and 2-back will be delivered.
• Attention: To measure attention and inhibition, participants will complete the Flanker task (Eriksen and Eriksen, 1974) with both congruent and incongruent trials.
• Processing Speed: Participants will perform the Trail Making Tests (TMT) A & B as described by Spreen (1998) to examine processing speed.
2.7.2 Feasibility outcomes
Participants in the intervention arm will complete three quantitative measures: Acceptability of Intervention Measure, Intervention Appropriate Measure, and Feasibility of Intervention Measure (Weiner et al., 2017). All have demonstrated sound psychometric properties and are essential for monitoring and evaluating the success of intervention feasibility and implementation efforts. We will further measure intervention adherence, defined as the number of virtual PT coaching sessions attended and home exercise sessions completed divided by the total number available. Semi-structured interviews (described above) will also be conducted with a subset of intervention participants with high (≥75% adherence; n = 5) and low adherence (<75% adherence; n = 5) and the treating PT at post-intervention to examine barriers and facilitators to implementing the intervention using the CFIR framework. Finally, we will assess reach, defined as the absolute number, proportion, and representativeness of enrolled patients compared with those contacted to participate but did not enroll.
2.7.3 Exploratory outcomes
The following measures are critical to collect as preliminary data for the development of a larger trial designed to test the efficacy of a prehabilitation exercise intervention on CRCD. While we are not powered to detect significant between-group changes in the factors below, they may serve as potential moderators of intervention adherence and/or effects. It will be important to identify how these factors change in response to the intervention, and can thus be targeted directly in a larger trial as potential mechanisms.
• Blood biomarkers: We collect blood from all participants to be analyzed for inflammatory markers (i.e., C-Reactive Protein, Insulin, Free Fatty Acid, and IL-6).
• Clinical factors: Factors associated with treatment are drawn from chart review and include: chemotherapy type, on-time treatment, treatment adherence, and dose reduction.
• Physical activity: Physical activity data are drawn from each participant's Fitbit device worn throughout the intervention period. Average daily steps and minutes of MVPA allow us to determine adherence to the intervention as described.
• Patient-reported outcomes: Patients complete a battery of patient-reported health and theoretical outcomes: self-reported physical activity (International Physical Activity Questionnaire; IPAQ) (Hagströmer et al., 2006), anxiety and depression (Hospital Anxiety and Depression Scale; HADS) (Snaith, 2003), fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue; FACIT-F) (Cella et al., 2021), pain (Brief Pain Inventory) (Cleeland and Ryan, 1994), quality of life (Functional Assessment of Cancer Therapy-Breast; FACT-B) (Brady et al., 1997), sleep (Pittsburgh Sleep Quality Index; PSQI) (Buysse et al., 1989), and self-reported health (Short Form-12 Health Survey; SF-12) (Ware et al., 1996); exercise self-efficacy (Exercise Self-Efficacy Scale; EXSE) (McAuley, 1993), walking self-efficacy (Self-Efficacy for Walking Scale-Duration; SEW_DUR) (McAuley et al., 2007a), barriers (Barriers Self-Efficacy Scale; BARSE) (McAuley, 1992), and outcome expectations (Multidimensional Outcome Expectations for Exercise Scale; MOEES) (Wójcicki et al., 2009). All patient-reported outcome measures are valid and reliable (Ware et al., 1996; Brady et al., 1997; Resnick and Jenkins, 2000; Bjelland et al., 2002; Craig et al., 2003; Webster et al., 2003; McAuley et al., 2007b; Hall et al., 2012; Akman et al., 2015) and have been used extensively in aging and cancer populations.
2.8 Statistical analysis
2.8.1 Sample size justification
2.8.1.1 Primary efficacy outcome
The primary efficacy outcome for our intervention is change in self-reported cognitive function at completion of chemotherapy relative to pre-chemotherapy. Assuming a 25% attrition rate (a conservative estimate based on our team's current research in this population), we plan to recruit 20 participants per arm, for a total of 40 breast cancer patients randomized 1:1 to each arm for at least 15 valid pre-post pairs per arm. For the primary efficacy endpoint, the average pre-post change comparison in self-reported cognition between arms, 15 per arm allows 80% power to detect an effect size (i.e., Cohen's D) of 1.06 based on 2-sided 2-sample t-test at alpha = 5%, calculated using PASS (v15). We acknowledge that this study only pilot tests efficacy with a small sample size of 15 per arm and thus may be underpowered.
2.8.1.2 Feasibility outcome
The primary feasibility outcome is quantitative acceptability. There are several competing factors driving desired sample size for our post-intervention interviews, including minimal sample sizes for theme saturation and pragmatic sample sizes due to time constraints (Vasileiou et al., 2018). A subsample of 10 participants from the intervention arm and the PT leading the intervention will participate in semi-structured interviews. We consider a response rate of approximately 50% as acceptable (i.e., over half of patients consider the intervention to be feasible and acceptable). The sample size was calculated to attain sufficient precision for 90% confidence interval (CI) to a proportion estimate. N = 10 allows us to estimate the desired 50% rate with a 2-sided 95% Wilson CI of 0.24 to 0.76 with a margin of error of 0.26.
2.8.2 Statistical plan
2.8.2.1 Efficacy outcome
For the efficacy outcomes, we will apply generalized linear mixed effects model or generalized estimating equation with the fixed effects of group (intervention vs. control), time (pre- & post-chemotherapy) and the group-by-time interaction. We will account for repeated measures in patients to determine differential changes in cognition over time within arms and the differential change difference between the intervention and control groups (i.e., the interaction arm and time effect). Estimation will be performed using restricted maximum likelihood (REML). Marginal adjusted means will quantify the between-group differences at the multiple time points, changes across chemotherapy, and between-group differences in these changes. Models will be adjusted for scientifically-based confounders, including body mass index, functional status, and chemotherapy duration. While we are not powered to conduct mediation tests of our hypothesized mechanistic factors (e.g., inflammatory blood markers, health outcomes), we will measure their between group changes over time to determine if our intervention affects these suspected mediators. Cohen's d effect sizes (Cohen, 1988) for between-group changes in cognition will be calculated to inform future larger trials. All analyses will be conducted in SAS, Version [9.4] or R (version 3.6.1).
2.8.2.2 Feasibility outcomes
For the feasibility outcomes, we will examine the means and frequencies of each quantitative response for adherence, acceptability, feasibility, and appropriateness (e.g., % of patients completing >75% of the home sessions, assessed by home logs; % of patients reporting, “The intervention seems possible;” “This intervention seems like a good match”). Semi-structured interviews assessing qualitative feasibility and implementation potential will be transcribed and subsequently analyzed for key themes using thematic analysis informed by CFIR contructs (Braun and Clarke, 2006), then comprehensively reported using the Consolidated Criteria for Reporting Qualitative Research (COREQ) (Tong et al., 2007). Emergent themes will be examined for desired modifications to the intervention. These modifications will be characterized using the updated Framework for Reporting Adaptations and Modifications-Enhanced (FRAME) (Stirman et al., 2019). The FRAME considers when and how modifications are warranted, reasons and goals for suggested modifications, how to maintain fidelity, and the impact on evidence-based interventions.
3 Results
This study was funded in December 2022 by the Alvin J. Siteman Cancer Center through The Foundation for Barnes-Jewish Hospital (award No. 6257). As of October 2023, seventeen patients have been enrolled; eight have been randomized to the intervention group, and nine have been randomized to the control group. Data collection began in May 2023 and is projected to continue through July 2024. Longitudinal data analysis will begin once all data collection is complete (e.g., once all patients have completed curative-intent neo/adjuvant chemotherapy).
4 Discussion
We propose to close significant scientific gaps through pilot testing a randomized controlled remote, PT-delivered exercise intervention during chemotherapy for breast cancer, and carefully assessing the feasibility of such a program and its effects on cognitive function. This trial includes robust, comprehensive, and understudied outcome measures (Janelsins et al., 2014) that will allow us to explore potential mechanisms of our intervention. The inclusion of both objective and self-reported cognition will allow us to fully explore the preliminary effects of exercise on cognitive function. We will also collect inflammatory blood markers, clinical data, and patient-reported outcomes. These measures are crucial for advancing our understanding of the exercise-cognition relationship during cancer treatment. To our knowledge, this study is one of the first to explore the remote delivery of an exercise intervention during curative-intent breast cancer chemotherapy designed to prevent cognitive decline, with comprehensive measures of cognitive function at both time points.
One key strength to this protocol is PROTECT's ability to address the persistent challenges in enrolling recently diagnosed breast cancer patients in exercise programs during chemotherapy (van Waart et al., 2016). Designed to be remote, it reduces patient burden and enhances trust and buy-in through its delivery by established clinicians. This design eliminates previously reported barriers that can reduce enrollment by over 60% (Wu et al., 2021). Many exercise interventions are delivered after chemotherapy completion when health outcomes and exercise levels have already significantly declined (Irwin et al., 2004; Bock et al., 2013; Nelson et al., 2020). This trial shifts the current cancer rehabilitation paradigm earlier on the treatment continuum to prevent CRCD. Our behavioral model provides patients with strategies to sustain positive health behaviors well into survivorship and prescribes individualized moderate-to-vigorous intensity activities that patients enjoy, which is foundational for long-term behavior change (Lewis et al., 2016). Each participant's intervention length is anchored to relevant cancer milestones (i.e., chemotherapy initiation), which allows us to answer critical questions about exercise and cognition around clinically meaningful treatment timeframes.
Importantly, this study combines behavioral science, physical therapy, and implementation science within the post-COVID-19 landscape. In response to COVID-19 pandemic, PT care shifted to telehealth with resounding success (Miller et al., 2021), highlighting increased accessibility of these clinicians and potential for long-term sustainability. Telehealth PT has been shown to improve activities of daily living, decrease hospitalization rates, and improve patient outcomes (Collins et al., 2019; LeDoux et al., 2020). Recent legislation has proposed direct access to PT care (APTA, 2020), with demonstrative safety (Piano et al., 2017; Piscitelli et al., 2018) and effectiveness (Hon et al., 2020). Such a movement highlights the potential for cancer patients' direct and sustained contact with a PT, a novel mechanism for long-term access to comprehensive survivorship care beyond the adjuvant setting (Pergolotti et al., 2019; Stout et al., 2019; Barnes et al., 2020).
Recruitment and attrition are serious concerns in any clinical trial, but especially so in this population so soon after a cancer diagnosis and while undergoing intensive treatment regimens. Our previous work has resulted in over 80% adherence to exercise interventions, largely due to our behavioral strategies, participant remuneration, and research staff-participant contact. We have designed this intervention to directly address significant limitations of previous studies (van Waart et al., 2016). If indeed attrition rates are high, our semi-structured interviews represent a prime opportunity to identify intervention strengths and weaknesses to better inform future trials that aim to improve cognitive function during breast cancer treatment.
5 Conclusion
As of today, there are no standardized exercise programs prescribed to treat or prevent CRCD. More research is needed to understand: (1) how exercise affects CRCD during treatment, and (2) best practices for intervention delivery to maximize efficacy and engagement. This study is poised to provide these necessary preliminary data to inform the development of larger survivorship trials. Depending on key feasibility findings, these future trials may range from tightly controlled to more pragmatic approaches to increasing exercise levels during chemotherapy. Unless and until these trials can be readily implemented into the standard of cancer care, patients will continue to lack access to sustainable health benefits. Identifying best practices for reducing CRCD while maximizing patient engagement after cancer can lead to a significant paradigm shift in the way we consider standard of care rehabilitation after cancer.
Ethics statement
The studies involving humans were approved by the Washington University in St. Louis Institutional Review Board (IRB) and the Protocol Review and Monitoring Committee (PRMC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ES: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing. CH: Methodology, Project administration, Writing—review & editing. DA: Methodology, Project administration, Writing—review & editing. LP: Methodology, Resources, Writing—review & editing. RD: Methodology, Writing—review & editing. MK: Methodology, Writing—review & editing. JL: Formal analysis, Methodology, Writing—review & editing. PC: Data curation, Project administration, Resources, Writing—review & editing. GC: Resources, Supervision, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Alvin J. Siteman Cancer Center through the Foundation for Barnes-Jewish Hospital (award No. 6257).
Acknowledgments
Our deepest thanks go to our research participants, without whom no clinical trial could be conducted.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akman, T., Yavuzsen, T., Sevgen, Z., Ellidokuz, H., and Yilmaz, A. U. (2015). Evaluation of sleep disorders in cancer patients based on pittsburgh sleep quality index. Eur. J. Cancer Care. 24, 553–559. doi: 10.1111/ecc.12296
APTA (2020). Direct Access Advocacy | APTA. Available online at: https://www.apta.org/advocacy/issues/direct-access-advocacy (accessed December 22, 2020).
Bandura, A. (2001). Social cognitive theory: an agentic perspective. Annu. Rev. Psychol. 52, 1–26. doi: 10.1146/annurev.psych.52.1.1
Barnes, C. A., Stout, N. L., Varghese Jr., T. K., Ulrich, C. M., Couriel, D. R., Lee, C. J., et al. (2020). Clinically integrated physical therapist practice in cancer care: a new comprehensive approach. Phys. Ther. 100, 543–553. doi: 10.1093/ptj/pzz169
Bezner, J. R. (2015). Promoting health and wellness: implications for physical therapist practice. Phys. Ther. 95, 1433–1444. doi: 10.2522/ptj.20140271
Bjelland, I., Dahl, A. A., Haug, T. T., and Neckelmann, D. (2002). The validity of the hospital anxiety and depression scale: an updated literature review. J. Psychosom. Res. 52, 69–77. doi: 10.1016/S0022-3999(01)00296-3
Bock, C., Schmidt, M. E., Vrieling, A., Chang-Claude, J., and Steindorf, K. (2013). Walking, bicycling, and sports in postmenopausal breast cancer survivors-results from a German patient cohort study. Psychooncology. 22, 1291–1298. doi: 10.1002/pon.3134
Brady, M. J., Cella, D. F., Mo, F., Bonomi, A. E., Tulsky, D. S., Lloyd, S. R., et al. (1997). Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J. Clin. Oncol. 15, 974–986. doi: 10.1200/JCO.1997.15.3.974
Braun, V., and Clarke, V. (2006). Using thematic analysis in psychology. Qual. Res. Psychol. 3, 77–101. doi: 10.1191/1478088706qp063oa
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiat. Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Campbell, K. L., Winters-Stone, K. M., Wiskemann, J., May, A. M., Schwartz, A. L., Courneya, K. S., et al. (2019). Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390. doi: 10.1249/MSS.0000000000002116
Campbell, K. L., Zadravec, K., Bland, K. A., Chesley, E., Wolf, F., and Janelsins, M. C. (2020). The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys. Ther. 100, 523–542. doi: 10.1093/ptj/pzz090
Cella, D., Yount, S., Chartash, S. M., Sengupta, N., and Grober, J. (2021). Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F). Available online at: www.facit.org (accessed March 16, 2021).
Chen, X., Lu, W., Zheng, W., Gu, K., Matthews, C. E., Chen, Z., et al. (2011). Exercise after diagnosis of breast cancer in association with survival. Cancer Prev. Res. 4, 1409–1418. doi: 10.1158/1940-6207.CAPR-10-0355
Cheung, Y. T., Foo, Y. L., Shwe, M., Tan, Y. P., Fan, G., Yong, W. S., et al. (2014). Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J. Clin. Epidemiol. 67, 811–820. doi: 10.1016/j.jclinepi.2013.12.011
Cleeland, C. S., and Ryan, K. M. (1994). Pain assessment: global use of the brief pain inventory. Ann. Acad. Med. Singapore. 23, 129–138.
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Larence Earlbaum Associates.
Collins, T. L., Yong, K. W., Marchetti, M. T., Miller, K. L., Booths, B., Falvey, J. R., et al. (2019). The value of home health physical therapy. Home Healthc. Now. 37, 145–151. doi: 10.1097/NHH.0000000000000760
Courneya, K. S., McKenzie, D. C., Mackey, J. R., Gelmon, K., Friedenreich, C. M., Yasui, Y., et al. (2013). Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. JNCI J. Natl. Cancer Inst. 105, 1821–1832. doi: 10.1093/jnci/djt297
Courneya, K. S., Segal, R. J., McKenzie, D. C., Dong, H., Gelmon, K., Friedenreich, C. M., et al. (2014). Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 46, 1744–1751. doi: 10.1249/MSS.0000000000000297
Craig, C. L., Marshall, A. L., Sjöström, M., Bauman, A. E., Booth, M. L., Ainsworth, B. E., et al. (2003). International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB
Damschroder, L. J., Aron, D. C., Keith, R. E., Kirsh, S. R., Alexander, J. A., Lowery, J. C., et al. (2009). Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Impl. Sci. 4, 50. doi: 10.1186/1748-5908-4-50
Demark-Wahnefried, W., Pinto, B. M., and Gritz, E. R. (2006). Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J. Clin. Oncol. 24, 5125–5131. doi: 10.1200/JCO.2006.06.6175
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A., et al. (2019). Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451. doi: 10.3322/caac.21583
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U S A. 108, 3017–3022. doi: 10.1073/pnas.1015950108
Eriksen, B. A., and Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149. doi: 10.3758/BF03203267
Furmaniak, A. C., Menig, M., and Markes, M. H. (2016). Exercise for women receiving adjuvant therapy for breast cancer. Cochr. Datab. Syst. Rev. 2016, CD005001. doi: 10.1002/14651858.CD005001.pub3
Gentry, A. L., Erickson, K. I., Sereika, S. M., Casillo, F. E., Crisafio, M. E., Donahue, P. T., et al. (2018). Protocol for Exercise Program in Cancer and Cognition (EPICC): A randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy. Contemp. Clin. Trials. 67, 109–115. doi: 10.1016/j.cct.2018.02.012
Hagströmer, M., Oja, P., and Sjöström, M. (2006). The international physical activity questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 9, 755–762. doi: 10.1079/PHN2005898
Hajj, A., Salameh, P., Khoury, R., Hachem, R., Sacre, H., Chahine, G., et al. (2022). Psychometric properties of the 37-item functional assessment of cancer therapy-cognitive function (FACT-Cog) scale. Future Oncol. 18, 3741–3753. doi: 10.2217/fon-2022-0438
Hall, K. S., Wójcicki, T. R., Phillips, S. M., and McAuley, E. (2012). Validity of the multidimensional outcome expectations for exercise scale in continuing-care retirement communities. J. Aging Phys. Act. 20, 456–468. doi: 10.1123/japa.20.4.456
Hartman, S. J., Nelson, S. H., Myers, E., Natarajan, L., Sears, D. D., Palmer, B. W., et al. (2018). Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory and motion study. Cancer. 124, 192–202. doi: 10.1002/cncr.30987
Holmes, M. D., Chen, W. Y., Feskanich, D., Kroenke, C. H., and Colditz, G. A. (2005). Physical activity and survival after breast cancer diagnosis. JAMA. 293, 2479–2486. doi: 10.1001/jama.293.20.2479
Hon, S., Ritter, R., and Allen, D. D. (2020). Cost-effectiveness and outcomes of direct access to physical therapy for musculoskeletal disorders compared to physician-first access in the united states: systematic review and meta-analysis. Phys. Ther. 101, pzaa201. doi: 10.1093/ptj/pzaa201
Irwin, M. L., McTiernan, A., Bernstein, L., Gilliland, F. D., Baumgartner, R., Baumgartner, K., et al. (2004). Physical activity levels among breast cancer survivors. Med. Sci. Sports Exerc. 36, 1484–1491. doi: 10.1249/01.MSS.0000074670.03001.98
Jacobs, S. R., Jacobsen, P. B., Booth-Jones, M., Wagner, L. I., and Anasetti, C. (2007). Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoetic stem cell transplant patients. J. Pain Symptom. Manage. 33, 13–23. doi: 10.1016/j.jpainsymman.2006.06.011
Janelsins, M. C., Heckler, C. E., Peppone, L. J., Ahles, T. A., Mohile, S. G., Mustian, K. M., et al. (2018). Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J. Clin. Oncol. 36, 3231–3239. doi: 10.1200/JCO.2018.78.6624
Janelsins, M. C., Heckler, C. E., Peppone, L. J., Kamen, C., Mustian, K. M., Mohile, S. G., et al. (2017). Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol. 35, 506–514. doi: 10.1200/JCO.2016.68.5826
Janelsins, M. C., Kesler, S. R., Ahles, T. A., and Morrow, G. R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry. 26, 102–113. doi: 10.3109/09540261.2013.864260
Kiesl, D., Kuzdas-Sallaberger, M., Fuchs, D., Brunner, S., Kommenda, R., Tischler, C., et al. (2022). Protocol for the exercise, cancer and cognition – the ECCO-study: a randomized controlled trial of simultaneous exercise during neo-/adjuvant chemotherapy in breast cancer patients and its effects on neurocognition. Front. Neurol. 13, 777808. doi: 10.3389/fneur.2022.777808
Koevoets, E. W., Schagen, S. B., De Ruiter, M. B., Geerlings, M. I., Witlox, L., Van Der Wall, E., et al. (2022). Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study). Breast Cancer Res. 24, 36. doi: 10.1186/s13058-022-01530-2
Lai, J. S., Butt, Z., Wagner, L., Sweet, J. J., Beaumont, J. L., Vardy, J., et al. (2009). Evaluating the dimensionality of perceived cognitive function. J. Pain Symptom. Manage. 37, 982–995. doi: 10.1016/j.jpainsymman.2008.07.012
LeDoux, C. V., Lindrooth, R. C., Seidler, K. J., Falvey, J. R., and Stevens-Lapsley, J. E. (2020). The impact of home health physical therapy on medicare beneficiaries with a primary diagnosis of dementia. J. Am. Geriatr. Soc. 68, 867–871. doi: 10.1111/jgs.16307
Lewis, B. A., Williams, D. M., Frayeh, A., and Marcus, B. H. (2016). Self-efficacy versus perceived enjoyment as predictors of physical activity behaviour. Psychol. Heal. 31, 456–469. doi: 10.1080/08870446.2015.1111372
Lis, C. G., Rodeghier, M., and Gupta, D. (2009). Distribution and determinants of patient satisfaction in oncology: a review of the literature. Patient Prefer. Adherence. 3, 287–304. doi: 10.2147/PPA.S6351
Loprinzi, P. D., Cardinal, B. J., Winters-Stone, K., Smit, E., and Loprinzi, C. L. (2012). Physical activity and the risk of breast cancer recurrence: a literature review. Oncol. Nurs. Forum. 39, 269–274. doi: 10.1188/12.ONF.269-274
McAuley, E. (1992). The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J. Behav. Med. 15, 65–88. doi: 10.1007/BF00848378
McAuley, E. (1993). Self-efficacy and the maintenance of exercise participation in older adults. J. Behav. Med. 16, 103–113. doi: 10.1007/BF00844757
McAuley, E., Blissmer, B., Katula, J., and Duncan, T. E. (2007a). Exercise environment, self-efficacy, and affective responses to acute exercise in older adults. Psychol. Health. 15, 341–355. doi: 10.1080/08870440008401997
McAuley, E., Morris, K. S., Doerksen, S. E., Motl, R. W., Liang, H., White, S. M., et al. (2007b). Effects of change in physical activity on physical function limitations in older women: mediating roles of physical function performance and self-efficacy. J. Am. Geriatr. Soc. 55, 1967–1973. doi: 10.1111/j.1532-5415.2007.01469.x
Miller, M. J., Pak, S. S., Keller, D. R., and Barnes, D. E. (2021). Evaluation of pragmatic telehealth physical therapy implementation during the COVID-19 pandemic. Phys. Ther. 101, 1–10. doi: 10.1093/ptj/pzaa193
Mishra, S. I., Scherer, R. W., Snyder, C., Geigle, P., and Gotay, C. (2014). Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncol. Nurs. Forum. 41, E326–E342. doi: 10.1188/14.ONF.E326-E342
Mustian, K. M., Sprod, L. K., Janelsins, M., Peppone, L. J., and Mohile, S. (2012). Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: a review. Oncol. Hematol. Rev. 8, 81–88. doi: 10.17925/OHR.2012.08.2.81
Nelson, S. H., Weiner, L. S., Natarajan, L., Parker, B. A., Patterson, R. E., Hartman, S. J., et al. (2020). Continuous, objective measurement of physical activity during chemotherapy for breast cancer: the activity in treatment pilot study. Transl. Behav. Med. 10, 1031–1038. doi: 10.1093/tbm/ibz079
Nystrom, L. E., Braver, T. S., Sabb, F. W., Delgado, M. R., Noll, D. C., Cohen, J. D., et al. (2000). Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 11, 424–446. doi: 10.1006/nimg.2000.0572
Patel, A. V., Friedenreich, C. M., Moore, S. C., Hayes, S. C., Silver, J. K., Campbell, K. L., et al. (2019). American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med. Sci. Sports Exerc. 51, 2391–2402. doi: 10.1249/MSS.0000000000002117
Pergolotti, M., Alfano, C. M., Cernich, A. N., Yabroff, K. R., Manning, P. R., de Moor, J. S., et al. (2019). A health services research agenda to fully integrate cancer rehabilitation into oncology care. Cancer. 125, 3908–3916. doi: 10.1002/cncr.32382
Piano, L., Maselli, F., Viceconti, A., Gianola, S., and Ciuro, A. (2017). Direct access to physical therapy for the patient with musculoskeletal disorders, a literature review. J. Phys. Ther. Sci. 29, 1463–1471. doi: 10.1589/jpts.29.1463
Piscitelli, D., Furmanek, M. P., Meroni, R., De Caro, W., and Pellicciari, L. (2018). Direct access in physical therapy: a systematic review. Clin. Ter. 169, e249–e260. doi: 10.7417/CT.2018.2087
Raffa, R. B. (2010). Is a picture worth a thousand (forgotten) words? Neuroimaging evidence for the cognitive deficits in “chemo-fog”/“chemo-brain.” J. Clin. Pharm. Ther. 35, 1–9. doi: 10.1111/j.1365-2710.2009.01044.x
Resnick, B., and Jenkins, L. S. (2000). Testing the reliability and validity of the self-efficacy for exercise scale. Nurs. Res. 49, 154–159. doi: 10.1097/00006199-200005000-00007
Rogers, L. Q., Courneya, K. S., Carter, S. J., Anton, P. M., Verhulst, S., Vicari, S. K., et al. (2016). Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res. Treat. 159, 283–291. doi: 10.1007/s10549-016-3945-2
Salerno, E. A., Culakova, E., Kleckner, A. S., Heckler, C. E., Lin, P. J., Matthews, C. E., et al. (2021). Physical activity patterns and relationships with cognitive function in patients with breast cancer before, during, and after chemotherapy in a prospective, nationwide study. J. Clin. Oncol. 39, 3283–3292. doi: 10.1200/JCO.20.03514
Salerno, E. A., Rowland, K., Kramer, A. F., and McAuley, E. (2019). Acute aerobic exercise effects on cognitive function in breast cancer survivors: a randomized crossover trial. BMC Cancer. 19, 371. doi: 10.1186/s12885-019-5589-1
Schmidt, M. E., Wiskemann, J., Armbrust, P., Schneeweiss, A., Ulrich, C. M., Steindorf, K., et al. (2015). Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int. J. Cancer. 137, 471–480. doi: 10.1002/ijc.29383
Schmitz, K. H., Campbell, A. M., Stuiver, M. M., Pinto, B. M., Schwartz, A. L., Morris, G. S., et al. (2019). Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 69, 468–484. doi: 10.3322/caac.21579
Silver, J. K. (2015). Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin. Oncol. Nurs. 31, 13–30. doi: 10.1016/j.soncn.2014.11.003
Silver, J. K., and Baima, J. (2013). Cancer prehabilitation. Am. J. Phys. Med. Rehabil. 92, 715–727. doi: 10.1097/PHM.0b013e31829b4afe
Snaith, R. P. (2003). The hospital anxiety and depression scale. Health Qual. Life Outc. 1, 29. doi: 10.1186/1477-7525-1-29
Spreen, O. A. (1998). Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford: Oxford University Press. Available online at: http://books.google.com/books/about/A_Compendium_of_Neuropsychological_Tests.html?id=CIy0_GH4ubICandpgis=1 (accessed December 11, 2013).
Stirman, S. W., Baumann, A. A., and Miller, C. J. (2019). The FRAME: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Impl. Sci. 14, 58. doi: 10.1186/s13012-019-0898-y
Stout, N. L., Silver, J. K., Alfano, C. M., Ness, K. K., and Gilchrist, L. S. (2019). Long-term survivorship care after cancer treatment: a new emphasis on the role of rehabilitation services. Phys. Ther. 99, 10–13. doi: 10.1093/ptj/pzy115
Stout, N. L., Silver, J. K., Baima, J., Knowlton, S. E., and Hu, X. (2020). “Prehabilitation: an emerging standard in exercise oncology,” in Exercise Oncology (Springer International Publishing), 111–143. doi: 10.1007/978-3-030-42011-6_6
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exper. Psychol. 18, 643. doi: 10.1037/h0054651
Takeuchi, H., Taki, Y., Hashizume, H., Sassa, Y., Nagase, T., Nouchi, R., et al. (2011). Effects of training of processing speed on neural systems. J. Neurosci. 31, 12139–12148. doi: 10.1523/JNEUROSCI.2948-11.2011
Tong, A., Sainsbury, P., and Craig, J. (2007). Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int. J. Qual. Heal Care. 19, 349–357. doi: 10.1093/intqhc/mzm042
van Waart, H., van Dongen, J. M., van Harten, W. H., Stuiver, M. M., Huijsmans, R., Hellendoorn-van Vreeswijk, J. A., et al. (2018). Cost–utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. Eur. J. Heal. Econ. 19, 893–904. doi: 10.1007/s10198-017-0936-0
van Waart, H., van Harten, W. H., Buffart, L. M., Sonke, G. S., Stuiver, M. M., Aaronson, N. K., et al. (2016). Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 25, 964–970. doi: 10.1002/pon.3936
Vasileiou, K., Barnett, J., Thorpe, S., and Young, T. (2018). Characterising and justifying sample size sufficiency in interview-based studies: systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 18, 148. doi: 10.1186/s12874-018-0594-7
Ware, J., Kosinski, M., and Keller, S. D. A. (1996). 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 34, 220–233. doi: 10.1097/00005650-199603000-00003
Webster, K., Cella, D., and Yost, K. (2003). The functional assessment of chronic illness therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual. Life Outc. 1, 1–7. doi: 10.1186/1477-7525-1-1
Wefel, J. S., Vardy, J., Ahles, T., and Schagen, S. B. (2011). International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12, 703–708. doi: 10.1016/S1470-2045(10)70294-1
Weiner, B. J., Lewis, C. C., Stanick, C., Powell, B. J., Dorsey, C. N., Clary, A. S., et al. (2017). Psychometric assessment of three newly developed implementation outcome measures. Impl. Sci. 12, 108. doi: 10.1186/s13012-017-0635-3
Wójcicki, T. R., White, S. M., and McAuley, E. (2009). Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J. Gerontol. B Psychol. Sci. Soc. Sci. 64, 33–40. doi: 10.1093/geronb/gbn032
Wu, F., Laza-Cagigas, R., Pagarkar, A., Olaoke, A., El Gammal, M., and Rampal, T. (2021). The feasibility of prehabilitation as part of the breast cancer treatment pathway. PMR 13, 1237–1246. doi: 10.1002/pmrj.12543
Keywords: exercise, prehabilitation, breast cancer, telehealth, cancer-associated cognitive decline, protocol
Citation: Salerno EA, Harriss C, Andrade DC, Peterson LL, Duncan RP, Kepper MM, Luo J, Creel P and Colditz GA (2023) Exercise during chemotherapy to prevent breast cancer-related cognitive decline: protocol for a pilot randomized controlled trial. Front. Cognit. 2:1289415. doi: 10.3389/fcogn.2023.1289415
Received: 05 September 2023; Accepted: 07 November 2023;
Published: 27 November 2023.
Edited by:
Diane K. Ehlers, Mayo Clinic Arizona, United StatesReviewed by:
Scherezade K. Mama, University of Texas MD Anderson Cancer Center, United StatesMeredith Minear, University of Wyoming, United States
Copyright © 2023 Salerno, Harriss, Andrade, Peterson, Duncan, Kepper, Luo, Creel and Colditz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth A. Salerno, ZS5zYWxlcm5vQHd1c3RsLmVkdQ==
 Elizabeth A. Salerno
Elizabeth A. Salerno Courtney Harriss1
Courtney Harriss1 Lindsay L. Peterson
Lindsay L. Peterson Maura M. Kepper
Maura M. Kepper Graham A. Colditz
Graham A. Colditz