- 1Climbers for Bat Conservation, Colorado Natural Heritage Program, Colorado State University, Fort Collins, CO, United States
- 2National Park Service/Bat Conservation International, Zion National Park, Springdale, UT, United States
- 3Access Fund, Boulder, CO, United States
- 4Colorado Parks and Wildlife, Grand Junction, CO, United States
Bats in North America have undergone unprecedented declines from various threats, including energy development, habitat loss, and disease. Mitigating these threats requires targeted conservation measures that address bat habitat and roosting requirements. One roosting structure commonly used by bats is caves. Human visitation to caves may cause disturbance to roosting bats through changes in noise, airflow, and temperature. Recreational climbing that occurs in and around caves may create vectors for disease transmission, disrupt bat roosting and physiology, and compromise bat conservation. However, managing certain climbing activities, such as climbing within caves and cave openings, and prolonged route development around cave openings, can ensure coexistence of recreational climbing and bat conservation. We outline bat biology at caves, potential stresses and threats to bats, and methods of managing climbing activity to conserve bats. In particular, we recommend limiting climbing activities within cave openings, and taking precautions to minimize disturbance based on distance from the cave opening. Finally, we advocate for collaboration between the climbing community and biologists to study and understand climbing impacts to bats.
1 Introduction
1.1 Bats and bat conservation
Bats are a diverse mammalian group with approximately 1,400 species worldwide (Burgin et al., 2018). Many of these species are insectivores and consume large quantities of arthropods (Kunz et al., 2011). Insect consumption by bats reduces pest control costs and reduces crop losses (Boyles et al., 2011), and likely reduces spread of insect-mediated diseases (Ghanem and Voigt, 2012). Other bats are nectar and fruit feeders, pollinating and dispersing seeds for over 500 plant species (Fleming et al., 2009; Lobova et al., 2009). Bat saliva of some species provide medicinal extracts for the treatment of stroke patients (Fernandez et al., 1999). Conservation of bat populations and their habitat has important ramifications for human economies and health (Frank, 2024).
Over the past 20 years, numerous threats have caused catastrophic declines in bat populations worldwide. Wind energy development has reduced bat populations by millions from direct mortality and habitat impacts (Arnett and Baerwald, 2013; Arnett et al., 2016; Kunz et al., 2007). Additionally, persecution and hunting, habitat loss, extreme weather events, and environmental contaminants have caused mass mortality events (O’Shea et al., 2016). In North America, a fatal fungal disease, called white-nose syndrome (WNS), has decimated populations of cave- and mine-roosting bats (Frick et al., 2016; Cheng et al., 2021). When WNS drastically reduced hibernating bat populations in eastern North America conservation strategies and management actions focused on protecting remnant bat populations in caves and mines (Foley et al., 2011). Because the fungus, Pseudogymnoascus destructans (Pd), that causes the disease (Lorch et al., 2013), can persist in cave and mine environments, human visitors may spread the disease and hasten bat population declines (Linder et al., 2010). Thus, decontamination protocols (http://www.whitenosesyndrome.org) and efforts to limit human access to important bat roosts have been implemented (Fascione, 2010). Although natural movements of bats may facilitate WNS spread, human-mediated dispersal is a concern (Frick et al., 2016), and humans were the likely mechanism for several cases of long-distance transmission in North America (Coleman and Reichard, 2014; Lorch et al., 2016).
1.2 Caves, bats, and recreation
A ‘‘cave’’ means any naturally occurring void, cavity, recess, or system of interconnected passages which occurs beneath the surface of the earth or within a cliff or ledge (including any cave resource therein, but not including any vug, mine, tunnel, aqueduct, or other manmade excavation) and which is large enough to permit an individual to enter, whether or not the entrance is naturally formed or manmade (Federal Cave Resource Protection Act. 1988).
Caves provide habitat for many unique lifeforms, including bats, and house incredible geologic features and anthropologic resources (Healy, 2007; Furey and Racey, 2016; Krajick, 2001; Niemiller and Taylor, 2019). For these reasons, many caves are protected through the Federal Cave Resources Protection Act of 1988 (16 USCS 4301 et seq., FCRPA). Caves are vital roosting resources for many bat species (Furey and Racey, 2016; Zagmajster, 2016), and these structures are habitat for some of the largest aggregations of bats in the world (McCracken, 2003). Compared to anthropogenic structures, such as mines and culverts, bats use caves more frequently and have higher site fidelity (Sherwin et al., 2000a; 2000b).
Caves can be extensive, and bats can be found in multiple regions. Although bats can be found deep within the dark interior of the cave (Dietz and Kiefer, 2016), they also use areas near the opening that are illuminated. Bats will roost inside the drip line, which is the line marking where rainfall reaches the ground, and further within the cave along the inner transitional zone or “twilight zone”, which is the area where daylight penetrates (White, 2016).
Historically, the biggest threat to cave-roosting bats from humans has been direct disturbance, such as fireworks, gunfire, open fires, poison, and direct persecution (harming or killing) bats (Clark, 1981; McCracken, 1989; O’Shea et al., 2016). Unintentional disturbance, such as human proximity, noise, temperature alterations, smoke, aerosol, modification of substrates, and removal of vegetation can alter bat roost site selection, cause bats to disperse, and deplete energy reserves (Ancillotto et al., 2019; Elliot, 2004; McCracken, 1989; Petrie, 1999; Sheffield et al., 1992; Thomas, 1995; Thomas et al., 1990). Installing gates can reduce human impacts; however, these gates may alter bat behavior or the internal roosting conditions (Martin et al., 2006; Pugh and Altringham, 2005). With the arrival of WNS, the greatest human-disturbance threat to cave-dwelling bats in North America is the human spread of Pd (Shelley et al., 2013). This threat prompted the development of national decontamination guidelines and guidelines for managing access to subterranean roosts (USFWS, 2016).
Because recreational climbers may access regions of caves that harbor Pd, there is the potential for human-mediated dispersal of Pd on climbing equipment and clothing. While humans are not biological vectors, they can passively transport Pd spores if proper decontamination measures are not followed (Ballmann et al., 2017). In addition, climbing activity near or within caves may disturb bats’ roosting and physiology. Thus, land managers in regions with high densities of caves and climbing opportunities are exploring management strategies to conserve bat populations and other resources. For example, some caves of the Deschutes National Forest, Oregon, experienced adverse impacts from humans, necessitating the development of guidelines for human activity at caves (Cave Resources Protection Restrictions - Forest Order 06-01-21-02; https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fseprd905238.pdf).
2 Potential impacts from climbing at caves
More than 4.7 million people engage in outdoor climbing (Cordell, 2012; OIA, 2019), which includes climbing vertical cliffs or boulders. However, outdoor climbing typically requires parking facilities, hiking trails, staging areas, route development and descending via rappelling or hiking. The activities most relevant for bat conservation are route development, climbing, descending, and activities near the openings of caves.
Most climbing at caves occurs at the opening and within the drip line. This activity has a lower risk for transmitting Pd than activities within the interior of caves because ultraviolet light and warmer temperatures create inhospitable conditions for Pd (Palmer et al., 2018). Although there is less potential to transmit Pd in these regions, human activity can disturb bats roosting in this region (Ancillotto et al., 2019). Potential climbing-caused disturbances include vegetation removal, substrate modification, noise, placement of gear in cracks, and chalk. Other indirect impacts include making fires (smoke/heat), spray-painting climbing bolts or hangers after installation (aerosol), playing music, and shouting or talking loudly around the cave opening and drip line (Ancillotto et al., 2019).
Route developers and climbers commonly remove vegetation or alter rock structures to make routes easier to climb. Vegetation removal, such as removing or trimming shrubs and trees, and modifying or removing rock structures near the opening of caves can alter internal air currents and temperatures within the cave, possibly affecting the suitability of bat roosting habitat (Tuttle and Stevenson, 1977). Chiseling rock may change crevice geometry, impact bat roosting habitat, and cause longer-ranging sound impacts that disturb bats. Bats, even those in torpor, can be sensitive to high-frequency sounds and human voices (Luo et al., 2014; Mann et al., 2002; Schaub et al., 2008). In a tourist cave, Cardiff et al. (2012) propose maintaining 12-m distance between cave visitors and roosting bats to minimize disturbance.
Climbing bolts are commonly drilled into rock walls to secure anchors for recreational climbing. Noise and vibration can increase the frequency of arousal of resting or torpid bats, depleting fat reserves or forcing them to flee (Speakman and Rowland, 1999). Armstrong (2010) suggests that drilling activity > 25 m from a cave opening, or 85 m from a bat roost within the cave poses minimal risk of disturbing bats. The noise and vibration caused by drilling anchors can be transmitted through the air and exceeds the level of regular conversation (68 dB) at 35 m (Figure 1). There is minimal data on how drill noise impacts bat behavior, but studies have demonstrated bat behavioral responses to other vibration sources, such as mineral exploration drilling (Armstrong, 2010; Brown and Berry, 2000), vibration noise from gas well compressor stations (Bunkley et al., 2015), and repeated blasting activity at a sand mine (Summers et al., 2023). The duration and magnitude of these resource extraction activities exceed those from hammer drills, so we recommend specific research to understand how handheld tool noise and vibration affect bats, and inform the proper placement of buffer distances to reduce impacts to bats. Additionally, high-frequency sounds, such as those produced by clanging metal climbing hardware may be inaudible to humans but may affect bat behavior (Thomas, 1995; Gomes and Goerlitz, 2020; Allen et al., 2021).
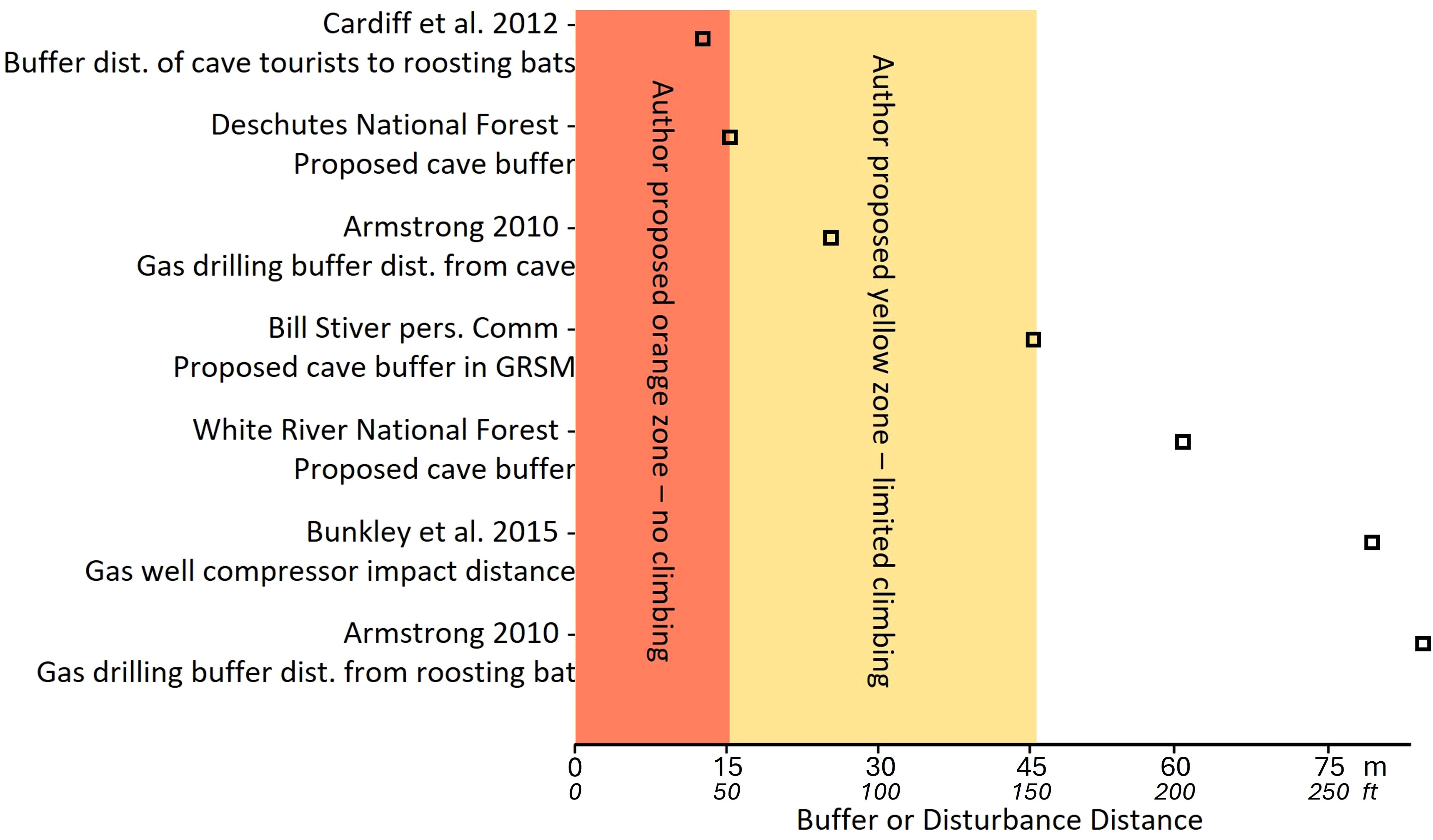
Figure 1. Published and unpublished proposed distances for buffering activities from caves and cave-roosting bats. Authors’ proposed distances for bufferingclimbing activity are identified by color bands in the figure. Sources: Armstrong (2010); Cardiff et al. (2012); Bunkley et al. (2015); Deschutes National Forest (2020); pers. comm. with Bill Stiver, Supervisory Wildlife Biologist, Great Smoky Mountains National Park, Gatlinburg, Tennessee. Figure by Z. Warren.
Due to the increased likelihood of encountering bats, climbing that occurs within the twilight zone or internal parts of a cave should be treated similarly to activities conducted by a caver. These darker, humid environments are more hospitable to Pd growth (Palmer et al., 2018). Thus, climbing equipment used or stored in these areas should be decontaminated following guidelines for caving equipment (USFWS, 2018; Schorr et al., 2021). Climbers should follow recommended guidelines for reducing conflict with bats in subterranean habitats, such as restricting access to areas where bats are roosting, minimizing disturbance to bats, prohibiting access to subterranean winter roosts when bats are present, and educating visitors and local communities about WNS and bat conservation (USFWS, 2016).
Chalk that climbers use to increase friction between fingers and rock surfaces can become aerosolized and blow into caves, which may present respiratory health risks to bats that are exposed to particulate matter (dos Santos Pedroso-Fidelis et al., 2020). The impacts of chalk dust on bat health may be similar to the respiratory and toxicity concerns for humans (Majumdar et al., 2012; Maruthi et al., 2017). A similar toxicity concern comes from spray-paint that may be used to mark or camouflage bolts in and around caves. The aerosol fumes can travel within a cave environment and disturb bats by causing them to awaken from torpor or leave the cave (Clark, 1981). In addition to becoming aerosolized in caves, climbing chalk may become permanently affixed to cave walls and interfere with bat roosting, and some cave management guidelines now prohibit the use of chalk (Petrie, 1999).
3 Actionable recommendations
Studies of recreational climbing and its potential impact on cave-roosting bat behavior or habitat use are needed. In lieu of research evaluating climbing impacts, we propose the use of buffer distances around cave openings to limit disturbance to bats. The buffer distances are meant to be conservative estimates to minimize disturbance to bats, but liberal enough to accommodate both climbing and caving recreation. We have relied on guidance from management agencies, published studies, and biological judgement. As studies of bats’ response to potential impacts becomes available, this guidance document should be modified to incorporate that understanding. Additionally, we believe partnerships between biologists, land managers, recreational users (climbers, cavers), and other stakeholders should use these recommendations to produce site-specific buffer distances. A collaborative, inclusive approach will be more broadly recognized and respected.
Several land management agencies have developed buffer distances to limit disturbance to bats. Great Smoky Mountains National Park, Tennessee, instituted a 45-m (150-ft) buffer around the White Oak Sink Cave opening (pers. comm. Bill Stiver, NPS). White River National Forest, Colorado, and Deschutes National Forest, Oregon proposed a 15-m (50-ft) climbing buffer around caves (Deschutes National Forest, 2020). We propose several zones of decreased climbing activity within a 45-m buffer distance from the nearest edge of the drip zone (Figures 2–4). We elaborate on the activities recommended with each buffer zone in Sections 3.2 – 3.4.
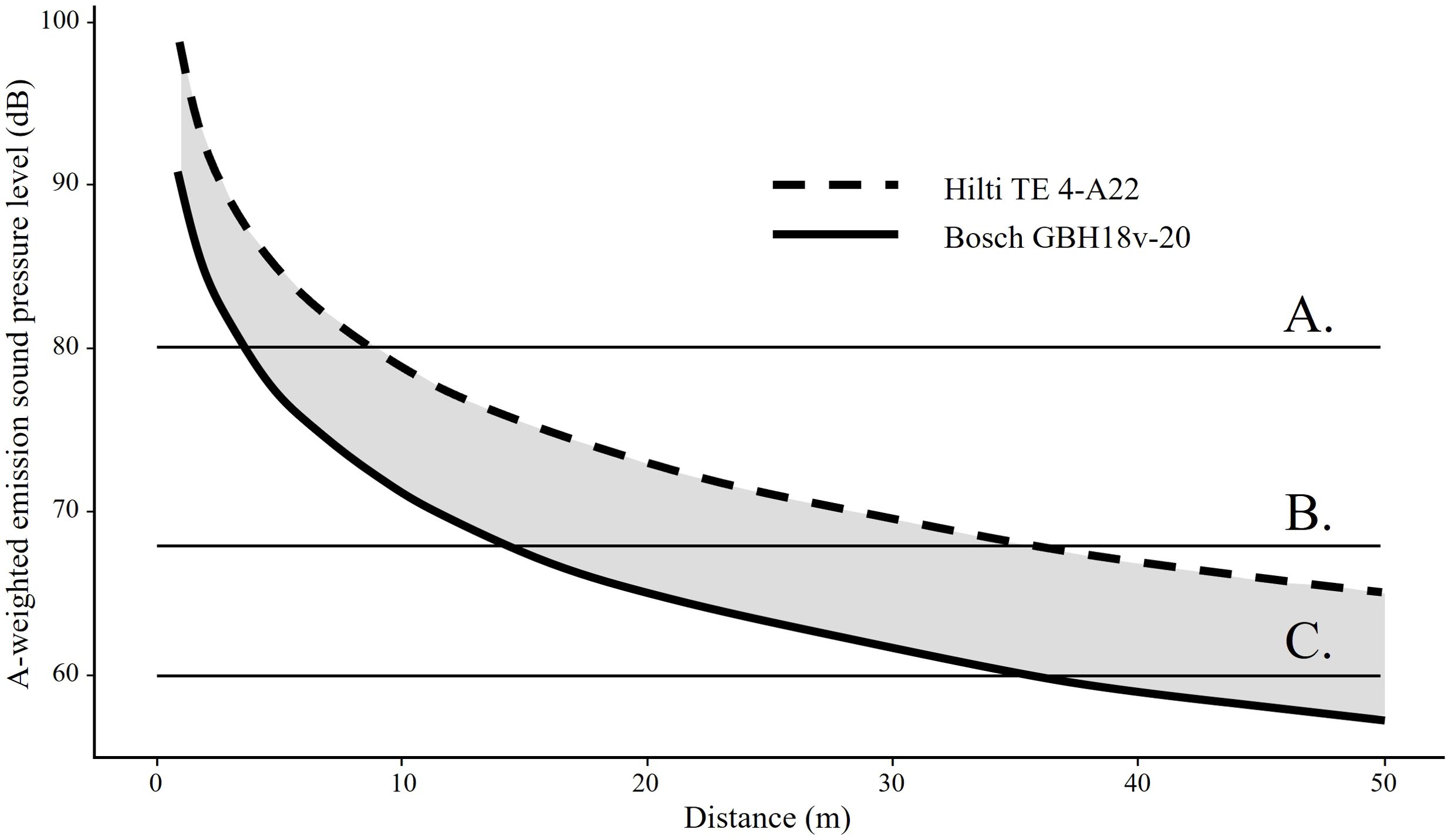
Figure 2. Sound attenuation of two common cordless hammer drills used by climbers to equip routes with bolts and hangers. The Hilti model emits a sound pressure level of 99 db while the Bosch reportedly emits a level of 91 db. The following reference levels are included for interpretation: (A) disruption of foraging in gleaning bats at 80 db (Schaub et al., 2008); (B) arousal of 50% of bats from torpor upon first exposure when played traffic sound at 68 dB (Luo et al., 2014); (C) normal conversation at 60 dB. 50 m = 164 ft. Figure by Z. Warren.
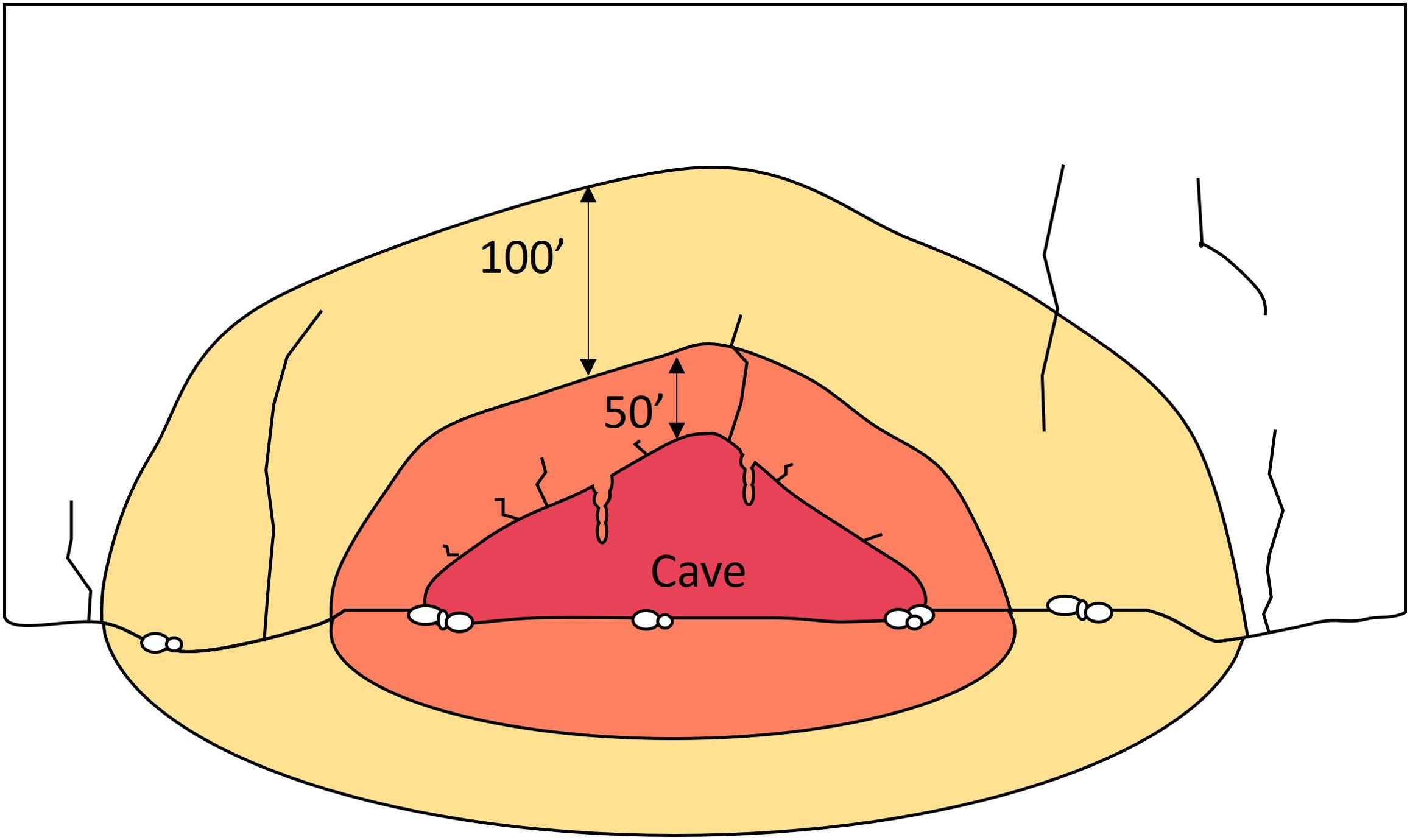
Figure 3. Diagram of proposed buffer distances away from cave opening to minimize disturbance of bats. See text for details. Figure by Z. Warren.
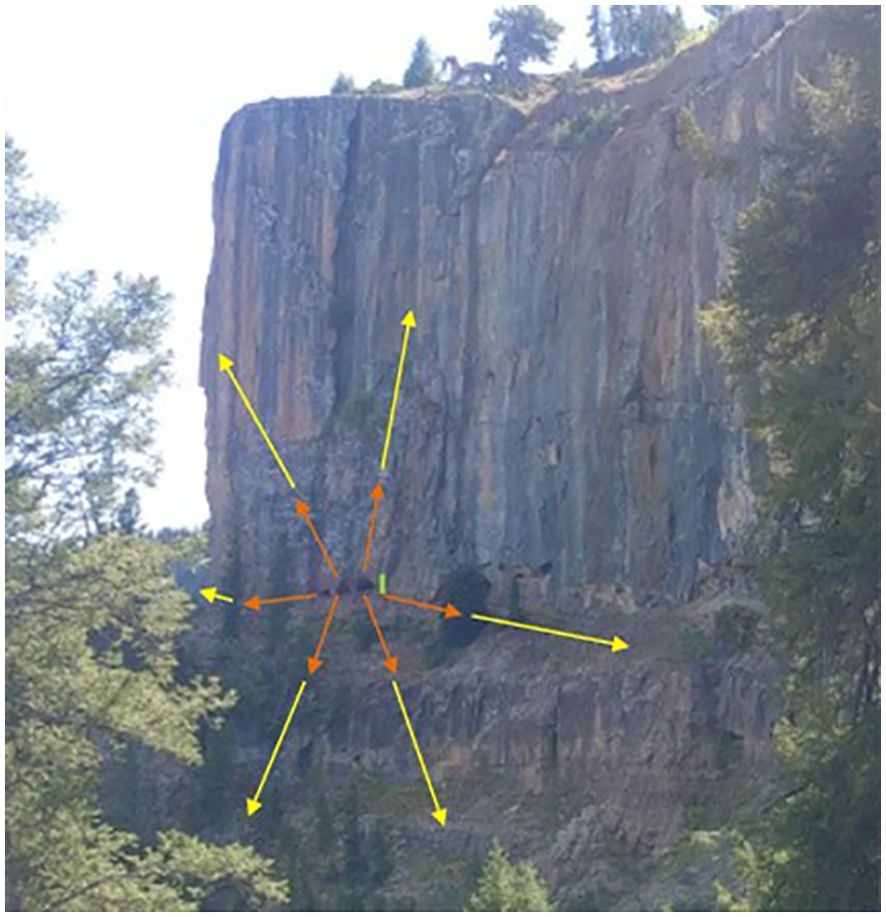
Figure 4. Diagram of proposed buffer distances displayed at a cave in Deep Creek Canyon, Colorado, used to avoid disturbance of bats with proposed 50-ft (orange) and 150-ft (yellow) buffers drawn (roughly to scale). The 150-ft buffer to the left is limited by the amount of cliff showing in the photograph. The green line represents the cave opening height. Photograph by Jennifer Zedalis of National Speleological Society.
3.1 Recommendations for climbing near caves where bats may roost
● Do not approach or touch bats.
● Be aware that human activities may disturb roosting bats. Disturbance of bat roosts in the winter can cause hibernating bats to awaken and exhaust needed energy reserves, and disturbance in the summer can increase pup and adult female mortality (Sheffield et al., 1992; López-Roig and Serra-Cobo, 2014; Ancillotto et al., 2019)
● Research a cave or cliff area to become aware of where bats may be roosting and in what seasons bats may use these areas. Climbers commonly report bat sightings on climbing resources, such as Mountain Project (www.mountainproject.com), The Crag (https://www.thecrag.com/), Summit Post (https://www.summitpost.org/), and Climbers for Bat Conservation (https://climbersforbats.colostate.edu/submit-view-climber-data/).
● Follow Pd decontamination protocols: Anyone entering caves or underground habitat should adhere to Pd decontamination protocols for their equipment or clothing to prevent the potential transmission of Pd.
● Report sightings of roosting bats to Climbers for Bat Conservation (https://climbersforbats.colostate.edu/submit-view-climber-data/). Observations, such as the number of bats, where they were located, if any dead bats were seen, or if white residue was seen on the face of the bat(s) are valuable.
● If any sick or dead bats are observed, take pictures of the bat(s) and the roost using minimal flash, record accurate location information, record date and time, leave the location immediately, and decontaminate equipment and clothing before entering another cave area. Report the sick or dead bat to local bat experts and resource managers at the appropriate state or provincial wildlife agency (https://www.whitenosesyndrome.org/contact/local-bat-expert), or U.S. Fish and Wildlife Service experts in your region (https://www.whitenosesyndrome.org/contact/region).
● Follow mindful climbing and recreating, which limits damage to rocks and the environment, such as “minimal impact”, “leave no trace”, and “clean climbing”. See resources available at Access Fund (https://www.accessfund.org/learn/the-climbers-pact).
● Use established routes instead of developing new ones.
● Do not chip rocks as this might disturb bats roosting in crevices and cracks, and do not remove organic material (“route cleaning”), such as vegetation, soil, or rocks.
● Climb in small groups to minimize damage to cliff habitat, and to reduce noise impacts that could disturb bats.
● Do not use speakers, cell phones, or radios to broadcast music or sounds as this noise may disturb roosting bats.
● Do not build fires because the smoke may flow into caves where bats are roosting
● Do not use aerosol paint to mark or “camouflage” anchors, fixed draws, bolts, or hangers. Chains and fixed draws should be painted off-site, allowed to dry, and then placed upon existing hangers. Hangers, which cannot feasibly be removed to paint, should be camouflaged using paint pens/markers, which do not release fumes as far as aerosol spray cans.
● If deemed necessary by the climbing community, biologists, and natural resource managers, use seasonal closures at high-priority conservation areas. Such closure designations should be evaluated and discussed collaboratively to achieve mutually-beneficial, conservation-oriented solutions.
● Encourage fellow climbers to voluntarily avoid areas known to be used by bats, and to always follow climbing procedures that minimize impacts to habitat. See Climbers for Bat Conservation website (https://climbersforbats.colostate.edu/) for more opportunities to get involved in bat conservation.
3.2 Recommendations for activities within cave opening (the drip zone; red zone; Figure 1)
● Do not rope climb or boulder in this area.
● Climbers should decontaminate equipment that spends time in recesses beyond direct sunlight and where the temperatures are cold (<19°C, see decontamination guidelines available at https://www.whitenosesyndrome.org/static-page/decontamination-information). These areas can harbor Pd and limiting WNS spread is critical.
3.3 Recommendations for activities within 15 m of cave opening (orange zone; Figure 1)
● Do not rope climb or boulder in this area.
● Do not bivy or stash gear here to limit prolonged activity near the opening.
● Do not clear or trim vegetation, which may negatively affect micro-climates within the cave.
3.4 Recommendations for activities from 15 m – 45 m of the cave opening (yellow zone; Figure 1)
● Limit noise by not clustering in large groups, and do not use sound emitting devices, like speakers.
● In temperate zones, such as North America, avoid climbing during the hibernation period to prevent disturbance of bats when most vulnerable to WNS.
● New “trad” routes can accessed if climbers use removable gear and do not require the use of loud drilling.
● Depending upon existing land restrictions, climbers can bivy, but fires should not be constructed.
● Do not bolt new routes or use cordless hammer drills within 45 m of cave opening.
4 Data collection and research to understand bat ecology at cave openings
4.1 Tools for land managers: assessing bat use after implementing buffers
Because there is a paucity of data on how bats respond to recreational activity, we recommend assessing bat responses to these suggested buffers. Assessing bat use and survival before and after implementation of buffers can illustrate the effectiveness of management strategies. Such research is difficult to conduct, so we recommend using metrics of bat use (see below) and roosting dynamics to document the effectiveness of climbing buffers.
Metrics, such as the following, can help evaluate bat behavior (Ancillotto et al., 2019):
● Time of initial bat emergence (minutes after sunset). When a colony is disturbed, the starting time for evening emergence can be delayed;
● Duration of emergence (difference in starting time of emergence and ending time). When a colony is disturbed, the length of time for emergence may be longer than normal;
● Degree of clustering during emergence (mean intensity of emergence = duration/number of inter-event intervals (Speakman et al., 1992)). Optimal bat emergence times are a trade-off between the risk of predation from visual predators, the need to hydrate, and the need to feed during optimal insect abundance (Jones and Rydell, 1994). Disturbance can make bat emergence times more varied and discontinuous, creating clustered emergences;
● Clustering, behaviors, and flight within the cave. The following metrics can be recorded with infrared video cameras and supplemental infrared light (Cardiff et al., 2012; O’Shea et al., 2003). Any video recording conducted within caves should only be done by or with the help of trained professionals that can safely access subterranean environments, avoid disturbance of bats, and properly decontaminate equipment. Two metrics that are easy to assess and have been used in other disturbance assessments (Ancillotto et al., 2019, but see Cardiff et al., 2012 for others) are:
Numbers of individuals roosting solitarily. When disturbed, bats tend to roost singly and in fewer clustered groups. For example, when machinery was activated outside of a roost, bats responded by taking flight in the cave and hanging in a different location, disrupting the group and making individuals appear more alert (Ancillotto et al., 2019); and
Numbers of interior flight events. Bats will take flight within the roost if they are disturbed leading to increased interior flight events. In some cases, bats may leave the cave during daylight hours, putting them at risk of predation.
4.2 Opportunities for collaborative research with climbing community
A great deal is unknown regarding how climbing activities may or may not impact cave-roosting bats. Collaborative research can address how these potential impacts affect bats, and inform land management plans and local climbing practices. Future research and surveys should address the following:
● What bat populations exist at a popular climbing cave? Interior and exterior bat surveys should be prioritized to identify bat habitat and the populations that may inhabit these areas. Once surveyed, regular follow-up surveys should be conducted to understand how use of the resource changes.
● What is the relationship between the amount of climbing that occurs at a cave area and the persistence, or lack thereof, of the bat population using the cave? If the bat population shows no response to nearby climbing, then buffer distances should be evaluated to determine if climbing restrictions can be relaxed.
● How do anthropogenic sounds from recreational climbing impact bats? Not all sounds affect bats equally. For example, in some settings vegetation noise and colony noise can arouse torpid bats more than traffic noise (Lou et al., 2014). How do sounds, such as drilling or the clanging of climbing gear affect bats? At what distance does drilling of bolts arouse bats from torpor and does this differ when bats are hibernating? Should areas within a dripline be a “quiet zone” to limit the potential impacts loud voices or cheering have on nearby bats?
● Does climbing chalk result in unfavorable roost conditions because of increased drying agents on the rock? Does chalk residue infiltrate cave environments on air currents? If so, how far does chalk dust penetrate the cave environment?
● How does Pd persist at the openings of caves or within the dripline? How do the cave opening conditions (aspect, temperature, humidity, air currents) and distance from the interior zone impact Pd persistence?
5 Discussion
5.1 The role of cave surveys in determining buffer distances at a cave?
Internal surveys provide critical insights into which caves bats use and help identify the locations and seasons of use by bats (Thorne, 1990). External surveys using videography equipment, infrared beams, or acoustic recorders provide data on bat abundances and seasonality of use (Furey and Racey, 2016). Knowledge from these surveys helps prioritize which caves should be managed to conserve bat populations. Failure to detect bats or the evidence of bats (guano, culled insect parts from feeding) does not mean bats are absent at a cave. A complete survey for bats is difficult, because of the small spaces and cryptic locations where bats roost. Additionally, not all rooms and crevices are accessible or known, making perfect detection difficult. Lastly, because bat surveys are conducted infrequently, failure to detect in a single survey does not mean the cave is not used during other seasons (Sherwin et al., 2000a; 2000b). Given that bats are present in and utilize many caves, we recommend applying climbing buffers around caves until multiple surveys confirm bats do not use the cave.
5.2 Caver and climber contributions to bat conservation
Recreational user groups and engaged citizen scientists are valuable contributors to bat conservation efforts (Gross et al., 2023; Hanauska-Brown et al, 2013). Cavers have long been an invaluable resource for understanding where bats roost and the relative population sizes of cave-roosting bats (Thorne, 1990; Hanauska-Brown et al., 2013). While cavers have been instrumental in understanding bat ecology, the presence of humans in caves can create challenges for conserving bat populations and cave resources (Furey and Racey, 2016; McCracken, 1989; Petrie, 1999; Sherwin et al., 2000). Recently, another recreational user group, climbers, are contributing observations of bats to understand the distribution and characteristics of the cliff habitats bats use (Davis et al., 2017). Climbers have a history of aiding species conservation efforts, contributing to the successful recovery of the Peregrine Falcon (Falco peregrinus) in North America (Access Fund, 2019; Stock, 2011). Rock climbing is quickly growing as a popular outdoor recreation activity (Bowker et al., 2012), and new routes are regularly being developed to accommodate the increasing recreational interest. However, information on the impacts of climbing on bats remains limited (Loeb and Jordice, 2018; Schorr et al., 2022; Wilson, 2019), and little is known about how climbing activities affect bats and cave ecosystems. The climbing community has only recently been engaged in bat conservation, but they are expanding biologists’ understanding of the roosting requirements of bats, and are identifying new roosting populations (Davis et al., 2017; Gross et al., 2023). Together, recreational users and land managers are developing collaborative solutions. In Deep Creek Canyon, Colorado, climbers, cavers, biologists, and land managers have agreed to work cooperatively to address increasing recreational use and cave resource conservation (USFS, 2024).
5.3 Conclusions
The rapid increase in popularity of recreational climbing presents challenges for land managers tasked with balancing public access and conservation of wildlife and natural resources. For those working to protect North America’s declining bat populations, key challenges include identifying bat roosting sites, understanding how bats use the landscape, and assessing the impacts of recreational activity on bat populations. Caves are a landscape feature known to harbor bat populations and provide climbing opportunities, and because cave openings are scarce and localized, proactive conservation measures at these locations may limit the impact to recreational climbing opportunities while maintaining bat habitat. We believe the recommendations suggested here strike a balance between conservation and recreational opportunities, but acknowledge that biologists, land managers, and climbers need to communicate and collaborate regularly to modify them as our collective understanding evolves.
Author contributions
RS: Conceptualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. KG: Conceptualization, Writing – original draft, Writing – review & editing. EM: Conceptualization, Writing – review & editing. DN: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the local climbing organizations that have contributed data on bat roosting ecology. We thank the caving and climbing communities that work to conserve the species that share the landscapes they enjoy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Access Fund (2019). Climbers partner with park service to protect zion’s peregrine falcons. Available online at: https://www.accessfund.org/news-and-events/news/climbers-partner-with-park-service-to-protect-zions-peregrine-falcons (Accessed October 12, 2023).
Allen L. C., Hristov N. I., Rubin J. J., Lightsey J. T., Barber J. R. (2021). Noise distracts foraging bats. Proc. R. Soc London B 288, 20202689. doi: 10.1098/rspb.2020.2689
Ancillotto L., Venturi G., Russo D. (2019). Presence of humans and domestic cats affects bat behavior in an urban nursery of greater horseshoe bats (Rhinolophus ferrumequinum). Behav. Processes 164, 4–9. doi: 10.1016/j.beproc.2019.04.003
Armstrong K. N. (2010). Assessing the short-term effects of minerals exploration drilling on colonies of bats of conservation significance: a case study near Marble Bar, Western Australia. J. R. Soc W. Aust. 93, 165–174.
Arnett E. B., Baerwald E. F. (2013). “Impacts of wind energy development on bats: implications for conservation,” in Bat evolution, ecology, and conservation. Eds. Adams R. A., Pedersen S. C. (Springer, New York), 435–456.
Arnett E. B., Baerwald E. F., Mathews F., Rodrigues L., Rodríguez-Durán, Rydell J., et al. (2016). “Impacts of wind energy development on bats: a global perspective,” in Bats in the anthropocene: conservation of bats in a changing world e. Eds. Voigt C. C., Kingston T. T. (Springer Open, Cham, Switzerland), 295–323.
Ballmann A. E., Torkelson M. R., Bohuski E. A., Russell R. E., Blehert D. S. (2017). Dispersal hazards of Pseudogymnoascus destructans by bats and human activity at hibernacula in summer. J. Wildl. Dis. 53, 725–735). doi: 10.7589/2016-09-206
Bowker J. M., Askew A. E., Cordell H. K., Betz C. J., Zarnoch S. J., Seymour L. (2012). Outdoor recreation participation in the United States - projections to 2060. Report to the U.S. Department of agriculture, forest service for 2010 renewable resources planning act assessment. (Athens, Georgia: U.S. Forest Service). Available at: https://www.srs.fs.usda.gov/pubs/gtr/gtr_srs150/gtr_srs150_105.pdf (Accessed October 15, 2023).
Boyles J. G., Cryan P. M., McCracken G. F., Kunz T. H. (2011). Economic importance of bats in agriculture. Science 332, 41–42. doi: 10.1126/science.1201366
Brown P. E., Berry R. D. (2000). “Renewed mining and reclamation: impacts on bats and potential mitigation,” in Proceedings of the 1997 national meeting of the american society for mining and reclamation. Eds. Brandt J. E., Galevotic J. R., Kost L., Trouart J. (American Society for Surface Mining and Reclamation, Austin, Texas), 196–201. Available at: https://www.asrs.us/past-asrs-meetings/1997-austin-tx-member/ (Accessed October 20, 2023).
Bunkley J. P., McClure C. J. W., Kleist N. J., Francis C. D., Barber J. R. (2015). Anthropogenic noise alters bat activity levels and echolocation calls. Global Ecol. Cons. 3, 62–71. Available at: https://www.sciencedirect.com/science/article/pii/S235198941400064X (Accessed September 10, 2023).
Burgin C. J., Colella J. P., Kahn P. L., Upham N. S. (2018). How many species of mammals are there? J. Mammal. 99, 1–14. Available at: https://academic.oup.com/jmammal/article/99/1/1/4834091 (Accessed November 1, 2023).
Cardiff S. G., Ratrimomanarivo F. H., Goodman S. M. (2012). The effect of tourist visits on the behavior of Rousettus Madagascariensis (Chiroptera: Pteropodidae) in caves of Ankarana, northern Madagascar. Acta Chiropterol. 14, 479–490. doi: 10.3161/150811012X661783
Cheng T. L., Reichard J. D., Coleman J. T. H., Weller T. J., Thogmartin W. E., Reichert B. E., et al. (2021). The scope and severity of white-nose syndrome on hibernating bats in North America. Cons. Biol. 35, 1586–1597. doi: 10.1111/cobi.13739
Clark D. R. Jr. (1981). “Bats and environmental contaminants: a review,” in Special scientific report no. 235 (U.S. Fish and Wildlife Service, Washington, D.C).
Coleman J. T. H., Reichard J. D. (2014). Bat white-nose syndrome in 2014: a brief assessment seven years after discovery of a virulent fungal pathogen in North America. Outlook Pest Manage. 25, 374–377. doi: 10.1564/v25_dec_08
Cordell H. K. (2012). Outdoor recreation trends and futures: a technical document supporting the forest service 2010 RPA assessment (Asheville, North Carolina: U.S Department of Agriculture Forest Service, Southern Research Station). Available at: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprd3843132.pdf (Accessed June 5, 2023).
Davis S. K., Schorr R., Kuhn B. (2017). “Climbers for Bat Conservation: methods in forming a novel partnership,” in Wildlife: perceptions, threats, and conservation. Ed. Ward C. (Nova Science Publishers, Hauppague, New York), 81–94.
Deschutes National Forest (2020). Cave resource protection forest order: draft environmental assessment. (Bend, Oregon: U.S. Department of Agriculture Forest Service, Deschutes National Forest, Bend, Oregon).
dos Santos Pedroso-Fidelis G., Resende Farias H., Antunes Mastella G., Appel Boufleur-Niekraszewicz L., Ferraz Dias., Alves M. C., et al. (2020). Pulmonary oxidative stress in wild bats exposed to coal dust: a model to evaluate the impact of coal mining on health. Ecotox. Environ. Safe. 191, 110211. doi: 10.1016/j.ecoenv.2020.110211
Elliot W. R. (2004). “Protecting caves and cave life,” in Encyclopedia of caves, 2nd. Eds. Culver D. E., White W. B. (Elsevier, Amsterdam), 458–467.
Federal Cave Resources Protection Act. (1988). 16 United States Code Chapter 63. Available online at: https://www.congress.gov/bill/100th-congress/house-bill/1975.
Fernandez A. Z., Tablante A., Beguín S., Hemker H. C., Apitz-Castro R. (1999). Draculin, the anticoagulant factor in vampire bat saliva, is a tight-binding, noncompetitive inhibitor of activated factor X. Biochim. Biophys. Acta 1434, 135–142. doi: 10.1016/S0167-4838(99)00160-0
Fleming T. H., Geiselman C. K., Kress W. J. (2009). The evolution of bat pollination: a phylogenetic perspective. Ann. Bot-London 104, 1017–1043. doi: 10.1093/aob/mcp197
Foley J., Clifford D., Castle K., Cryan P., Osterfeld R. S. (2011). Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conserv. Biol. 25, 223–231. doi: 10.1111/j.1523-1739.2010.01638.x
Frank E. G. (2024). The economic impacts of ecosystem disruptions: costs from substituting biological pest control. Science 38, https://doi.org/10.1126/science.adg0344. doi: 10.1126/science.adg0344
Frick W. F., Puechmaille S. J., Willis C. K. R. (2016). “White-nose syndrome in bats,” in Bats in the anthropocene: conservation of bats in a changing world. Eds. Voigt C. C., Kingston T. (Springer, Heidelberg, Germany), 245–262.
Furey N. M., Racey P. A. (2016). “Conservation ecology of cave bats,” in Bats in the anthropocene: conservation of bats in a changing world. Eds. Voigt C. C., Kingston T. (Springer, Heidelberg, Germany), 463–500.
Ghanem S. J., Voigt C. C. (2012). “Increasing awareness of ecosystem services provided by bats,” in Advances in the study of behavior. Eds. Brockmann H. J., Roper T. J., Naguib M., Mitani J. C., Simmons L. W. (Elsevier, Amsterdam), 279–302.
Gomes D. G.E., Goerlitz H. R. (2020). Individual differences show that only some bats can cope with noise-induced masking and distraction. PeerJ 8, e10551. Available online at: https://peerj.com/articles/10551/.
Gross E. E., Siebka M. E., Schorr R. A., Solomon J. N., Davis S. K. (2023). Climbers for Bat Conservation: creating a citizen science program in Red River Gorge, Kentucky. Front. Commun. 8. doi: 10.3389/fcomm.2023.1195796
Hanauska-Brown L., Maxell B., Bodenhamer H. (2013). Montana’s collaborative approach to the looming threat of white nose syndrome — an unlikely partnership. Nat. Speleo. Soc News 71, 22–23. Available at: https://fwp.mt.gov/binaries/content/assets/fwp/conservation/wildlife-reports/bats/nss-article-sept-2013-scanned-copy.pdf (Accessed July 6, 2023).
Healy P. F. (2007). The anthropology of Mesoamerican caves. Rev. Anthropol. 36, 245–278. doi: 10.1080/00938150701436636
Jones G., Rydell J. (1994). Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Philo. T. R. Soc B 346, 445–455. doi: 10.1098/rstb.1994.0161
Krajick K. (2001). Cave biologists unearth buried treasure. Science 293, 2378–2381. doi: 10.1126/science.293.5539.2378
Kunz T. H., Arnett E. B., Erickson W. P., Hoar A. R., Johnson G. D., Larkin R. P., et al. (2007). Ecological impacts of wind energy development on bats: questions, research needs, and hypotheses. Front. Ecol. Evol. 5, 315–324. doi: 10.1890/1540-9295(2007)5[315:EIOWED]2.0.CO;2
Kunz T. H., Braun de Torrez E., Bauer D., Lobova T., Fleming T. H. (2011). Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. (1), 1–38. doi: 10.1111/j.1749-6632.2011.06004.x
Linder D. L., Gargas A., Lorch J. M., Banik M. T., Glaeser J., Kunz T. H., et al. (2010). DNA-based detection of the fungal pathogen Geomyces destructans in soils from bat hibernacula. Mycologia 103, 241–246. doi: 10.3852/10-262
Lobova T. A., Geiselman C. K., Mori S. A. (2009). Seed dispersal by bats in the neotropics (New York: New York Botanical Garden Press).
Loeb S., Jodice P. (2018). Activity of southeastern bats along sandstone cliffs used for rock climbing. J. Fish Wildlife Manage. 9, 255–265. doi: 10.3996/032017-JFWM-020
López-Roig M., Serra-Coda J. (2014). Impact of human disturbance, density, and environmental conditions on the survival probabilities of pipistrelle bat (Pipistrellus pipistrellus). Pop. Ecol. 56, 471–480 doi: 10.1007/s10144-014-0437-2.
Lorch J. M., Miller L. K., Russell R. E., O’Connor M., Lindner D. L., Blehart D. S. (2013). Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Appl. Environ. Microb. 79, 1293–1301. doi: 10.1128/AEM.02939-12
Lorch J. M., Palmer J. M., Lindner D. L., Ballman A. E., George K. G., Griffin K., et al. (2016). First detection of bat white-nose syndrome in western North America. mSphere 1, e00148–e00116. doi: 10.1128/msphere.00148-16
Luo J., Markus Clarin B., Borissov I. M., Siemers B. M. (2014). Are torpid bats immune to anthropogenic noise? J. Exp. Biol. 217, 1072–1078. doi: 10.1242/jeb.092890
Majumdar D., Gajghate D. G., Pipalatkar P., Chalapati Rao C. V. (2012). Assessment of airborne fine particulate matter and particulate size distribution in settled chalk dust during writing and dusting exercises in a classroom. Indoor Built Environ. 21, 541–551. doi: 10.1177/1420326X11419691
Mann S. L., Steidl R. J., Dalton V. M. (2002). Effects of cave tours on breeding Myotis velifer. J. Wildlife Manage. 66, 618–624. doi: 10.2307/3803128
Martin K. W., Leslie D. M. Jr., Payton M. E., Puckette W. L., Hensley S. L. (2006). Impacts of passage manipulation on cave climate: conservation implications for cave-dwelling bats. Wildlife Soc B. 34, 137–143. doi: 10.2193/0091-7648(2006)34[137:IOPMOC]2.0.CO;2
Maruthi Y. A., Ramprasad S., Lakshamana Das N. (2017). Trace elemental characterization of chalk dust and their associated health risk assessment. Biol. Trace Elem. Res. 175, 466–474. doi: 10.1007/s12011-016-0769-1
McCracken G. F. (2003). “Estimates of population sizes in summer colonies of Brazilian free-tailed bats (Tadarida brasiliensis),” in Monitoring trends in bat populations in the United States and territories: problems and prospects (USGS-BRD information and technical report-2003-0003). Eds. O’Shea T. J., Bogan M. A. (U. S. Geological Survey, Fort Collins, Colorado), 21–30. Available at: https://pubs.usgs.gov/publication/itr030003 (Accessed April 10, 2023).
Niemiller M. L., Taylor S. J. (2019). “Protecting cave life,” in Encyclopedia of caves. Eds. White W. B., Culver D. C., Pipan T. (Academic Press, Cambridge, Massachusetts), 822–829.
O’Shea T. J., Bogan M. A., Ellison L. E. (2003). Monitoring trends in bat populations of the United States and territories: status of the science and recommendations for the future. Wildlife Soc B. 31, 16–29. Available at: https://www.jstor.org/stable/3784356 (Accessed April 3, 2023).
O’Shea T. J., Cryan P. M., Hayman D. T. S., Plowright R. K., Streicker D. G. (2016). Multiple mortality events in bats: a global review. Mammal Rev. 46, 175–190. doi: 10.1111/mam.12064
Outdoor Industry Association (OIA) (2019). 2019 outdoor participation report. (Boulder, Colorado: Outdoor Foundation).
Palmer J. M., Drees K. P., Foster J. T., Lindner D. L. (2018). Extreme sensitivity to ultraviolet light in the fungal pathogen causing white-nose syndrome of bats. Nat. Commun. 9, 35. doi: 10.1038/s41467-017-02441-z
Petrie G. (1999). “Conservation challenges: restoration of the caves of central Oregon,” in Proceedings of the 1997 national cave management symposium and 13th national cave management symposium. Ed. Stitt R. (National Speleological Society, Bellingham, Washington), 153–156.
Pugh M., Altringham J. D. (2005). The effect of gates on cave entry by swarming bats. Acta Chiropterol. 7, 293–299. doi: 10.3161/1733-5329(2005)7[293:TEOGOC]2.0.CO;2
Schaub A., Ostwald J., Siemers B. M. (2008). Foraging bats avoid noise. J. Exper. Bio. 211, 3174–3180. doi: 10.1242/jeb.022863
Schorr R., Goodwin K., Murdock E., Neubaum D., Warren Z. (2021). Climbers and bat conservation. The global climbing initiative best management practices for the development of climbing worldwide. Available online at: https://globalclimbing.org/best-practices (Accessed March 25, 2024).
Schorr R. A., Matthews M. D., Hoover B. A. (2022). Bat roosts along cliffs: using rock climbing surveys to understand roosting habitat of bats along the Front Range of Colorado. Acta Chiropterol. 24, 167–176. doi: 10.3161/15081109ACC2022.24.1.013
Sheffield S. R., Shaw J. H., Heidt G. A., McClenaghan L. R. (1992). Guidelines for the protection of bat roosts. J. Mammal. 73, 707–710. doi: 10.2307/1382051
Shelley V., Kaiser S., Shelley E., Williams T., Kramer M., Haman, et al. (2013). Evaluation of strategies for the decontamination of equipment for Geomyces destructans, the causative agent of white-nose syndrome (WNS). J. Cave Karst Stud. 75, 1–10. Available at: https://caves.org/wp-content/uploads/Publications/JCKS/v75/cave-75-01-01.pdf (Accessed October 2, 2023).
Sherwin R. E., Gannon W. L., Altenbach S., Striklan D. (2000a). Roost fidelity of Townsend’s big-eared bats in Utah and Nevada. T. W. Sec. Wil. 36, 15–20. Available at: https://www.wildlifeprofessional.org/western/transactions/transactions_2000_3.pdf (Accessed June 6, 2023).
Sherwin R. E., Stricklan D. (2000b). Roosting affinities of Townsend’s big-eared bat (Corynorhinus townsendii) in northern Utah. J. Mammal. 81, 939–947. doi: 10.1644/1545-1542(2000)081<0939:RAOTSB>2.0.CO;2
Speakman J. R., Bullock D. J., Eales L. A., Racey P. A. (1992). A problem defining temporal pattern in animal behaviour: clustering in the emergence behaviour of bats from maternity roosts. Anim. Behav. 43, 491–500. doi: 10.1016/S0003-3472(05)80107-1
Speakman J. R., Rowland A. (1999). Preparing for inactivity: how insectivorous bats deposit a fat store for hibernation. P. Nutr. Soc 58, 123–131. Available at: https://www.cambridge.org/core/services/aop-cambridge-core/content/view/5B11DEFEB906316640FF330E5B58204D/S0029665199000191a.pdf/preparing_for_inactivity_how_insectivorous_bats_deposit_a_fat_store_for_hibernation.pdf (Accessed September 10, 2023).
Stock S. (2011). Brink of extinction: climbers help bring peregrine falcons back to El Capitan. Vertical Times 92, 8–10. Available at: https://www.accessfund.org/vertical-times-magazine (Accessed October 22, 2023).
Summers J. L., White J. P., Kaarakka H. M., Hygnstrom S. E., Sedinger B. S., Riddle J., et al. (2023). Influence of underground mining with explosives on a hibernating bat population. Conserv. Sci. Pract. 5, e12849. doi: 10.1111/csp2.12849
Thomas D. W. (1995). Hibernating bats are sensitive to nontactile human disturbance. J. Mammal. 76, 940–946. doi: 10.2307/1382764
Thomas D. W., Dorais M., Bergeron J. M. (1990). Winter energy budgets and costs for arousals for hibernating little brown bats, Myotis lucifugus. J. Mammal. 71, 475–479. Available at: https://www.jstor.org/stable/1381967 (Accessed June 5, 2024).
Thorne J. (1990). Bats and cavers: the association between bats and cave explorers is a long one, with cavers increasingly lending a helping hand. BATS magazine 8, 10–14.
Tuttle M. D., Stevenson D. E. (1977). “Variation in the cave environment and its biological implications,” in National cave management symposium proceedings. Eds. Zuber R., Chester J., Rhodes D. (Adobe Press, Berkeley, California), 108–121.
United State Fish and Wildlife Service (USFWS) (2016). Recommendations for managing access to subterranean bat roosts to reduce the impacts of white-nose syndrome in bats. Available online at: https://s3.us-west-2.amazonaws.com/prod-is-cms-assets/wns/prod/25dbf390-1aa1-11ea-a154-67ca1cde5e5d-FINAL-Cave-Access-Advisory-2016.pdf (Accessed December 27, 2023).
United States Fish and Wildlife Services (USFWS) (2018). National white-nose syndrome decontamination protocol – version 09.13.2018. Available online at: https://www.whitenosesyndrome.org/mmedia-education/united-states-national-white-nose-syndrome-decontamination-protocol-april-2016-2 (Accessed December 27, 2023).
United States Forest Service (USFS) (2024). Memorandum of understanding between the Access Fund and the Colorado Cave Survey and the USDA Forest Service White River National Forest. FS Agreement No. 24-MU-11021500-005. Available online at: https://coloradocavesurvey.org/wp-content/uploads/2015/07/Final_2015-MOU-CCS-White-River_6.17.2015-2.pdf (Accessed March 24, 2024).
White W. B. (2016). “Entrances,” in Encyclopedia of caves. Eds. White W. B., Culver D. C., Pipan T. (Academic Press, Cambridge, Massachusetts), 380–386.
Wilson A. K. (2019). The impacts of rock climbing on the selection of roosts by bats and the influence of these mammals on the biodiversity and nutrient influx of cliff-face ecosystems (Dissertation. Greeley, Colorado: University of Northern Colorado).
Keywords: bats, caves, Chiroptera, cliffs, recreational climbing
Citation: Schorr RA, Warren ZA, Goodwin K, Murdock E and Neubaum DJ (2025) Collaborative conservation of cave-roosting bats: guidance on managing rock climbing near caves. Front. Conserv. Sci. 6:1411427. doi: 10.3389/fcosc.2025.1411427
Received: 05 April 2024; Accepted: 18 April 2025;
Published: 14 May 2025.
Edited by:
Mário Gabriel Santiago Santos, University of Trás-os-Montes and Alto Douro, PortugalReviewed by:
David R. Breininger, University of Central Florida, United StatesLisa Smith, Florida Fish and Wildlife Conservation Commission, United States
Thejanee Perera, University of Colombo, Sri Lanka
Copyright © 2025 Schorr, Warren, Goodwin, Murdock and Neubaum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert A. Schorr, cm9iZXJ0LnNjaG9yckBjb2xvc3RhdGUuZWR1
 Robert A. Schorr
Robert A. Schorr Zachary A. Warren2
Zachary A. Warren2