- Section of Plant Pathology, Mycology and Microbiology, Department of Botany, University of Kashmir, Srinagar, J&K, India
Dactylorhiza hatagirea maintain a symbiotic relationship with rhizospheric fungi in their lifecycle. Rhizospheric fungi have different roles during its growth and development. Although various rhizospheric fungi have been isolated from D. hatagirea, little is known about their specific effects on its growth and development. To understand the role of fungal species on growth parameters of D. hatagirea, we compared the effect of eight fungal species on growth parameters of D. hatagirea. viz. Trichoderma asperellum, Talaromyces falvus, Aspergillus candidus, Circinella muscae, Aspergillus flavus, Aspergillus niger, Cephalosporium acremonium and Trichoderma harzianum in the form of three treatments. Treatment (T1) comprised the combined application of Trichoderma asperellum, Talaromyces falvus, Aspergillus candidus and Circinella muscae. Treatment (T2) comprised the combined application of Aspergillus flavus, Aspergillus niger, Cephalosporium acremonium and Trichoderma harzianum. Another treatment (T3) comprised the combined application of T1 and T2. A separate set of plants which were un-treated with any fungal isolated served as control. Our results revealed that the tubers inoculated with T3 conferred the highest shoot length, tuber length, optimal fresh and dry matter yield, and greatly enhanced other growth parameters, length of inflorescence, number of flowers and specific leaf area. Treatment (T3) has a discernible impact on plant growth compared to the T1, T2 and control. The results revealed that these fungal species we used in the presented study of tested plant D. hatagirea promoted growth with different efficiencies. Our results also revealed that rhizospheric fungal associations with D. hatagirea showed development-dependent preference and hence may provide the basic knowledge for use of different fungal species in conservation of D. hatagirea at different elevations.
Introduction
A well-documented example of mutualism is the symbiotic relationship between fungi and land plants, where fungi facilitate the acquisition of essential mineral nutrients from the soil and deliver them to plants, while plants reciprocate by supplying photosynthates to fungi (Smith and Read, 2008). In the case of orchids, a compatible fungal association is critical for seed germination in natural habitats (Rasmussen, 1995). During the germination of orchid seeds and the early development of protocorms, the fungi transfer mineral nutrients to the orchid, a phenomenon termed “mycoheterotrophy” (Leake, 1994). Upon successful fungal colonization, hyphae invade and form coiled structures known as pelotons within the cortical cells of the orchid (Smith and Read, 2008). These pelotons undergo degradation and senescence, releasing nutrients into the orchid cells (Kuga et al., 2014). The cyclical formation and breakdown of pelotons are essential processes for the nutrient exchange between the orchid and its fungal symbiont (Dearnaley and Cameron, 2017; Fochi et al., 2017). Orchids display varying degrees of specificity in their fungal associations. Some mycoheterotrophic orchids, such as those in the Corallorhiza striata complex (Barrett et al., 2010) and Hexalectris species (Kennedy and Watson, 2010), exhibit a narrow fungal partner range. In contrast, other orchids engage with a broader spectrum of fungal taxa. For example, Cypripedium californicum forms associations with fungi from the families Tulasnellaceae, Ceratobasidiaceae, and Sebacinales (Sheferson et al., 2007). Similarly, fungal taxa such as Tulasnellaceae, Telephoraceae, Ceratobasidiaceae, Sebacinales, Russulaceae, and Clavulinaceae have been detected in Cymbidium goeringii and Cymbidium lancifolium (Ogura-Tsujita et al., 2012). Despite the broad fungal colonization observed in orchids, the effects of these associations on orchid growth and development can vary. For instance, in Vanilla, different fungal isolates from the roots exhibit variable impacts on plant growth and survival (Porras-Alfaro and Bayman, 2007).
D. hatagirea, also known by its common names such as salam panja in Hindi and Pahari, Panchangle in Nepali, Narmad in Shina and Kashmiri, is a perennial herb that thrives in sub-alpine to alpine regions. This plant has gained global recognition due to its numerous advantageous properties. It has been found effective in treating various disorders related to the urinary system (Ballabh et al., 2008), respiratory system, nervous system (Khajuria et al., 2017), digestive system (Tsering et al., 2017), skeletal system (Giri and Tamata, 2010), and reproductive system (Chauchan, 1990). Additionally, it has the ability to enhance the immune system to combat infectious diseases. The roles of various fungal species isolated from rhizosphere of D. hatagirea have never been tested systematically with in vivo methods. To understand the effects of various fungal species on ontogenetic stages in orchid, we compared the effect of eight fungal species of Trichoderma asperellum, Talaromyces falvus, Aspergillus candidus, Circinella muscae, Aspergillus flavus, Aspergillus niger, Cephalosporium acremonium and Trichoderma harzianum on their ability to promote growth by examining the growth parameters. Knowledge of rhizospheric fungal association in medicinal important D. hatagirea species would be helpful for propagation, commercial cultivation and conservation.
Materials and methods
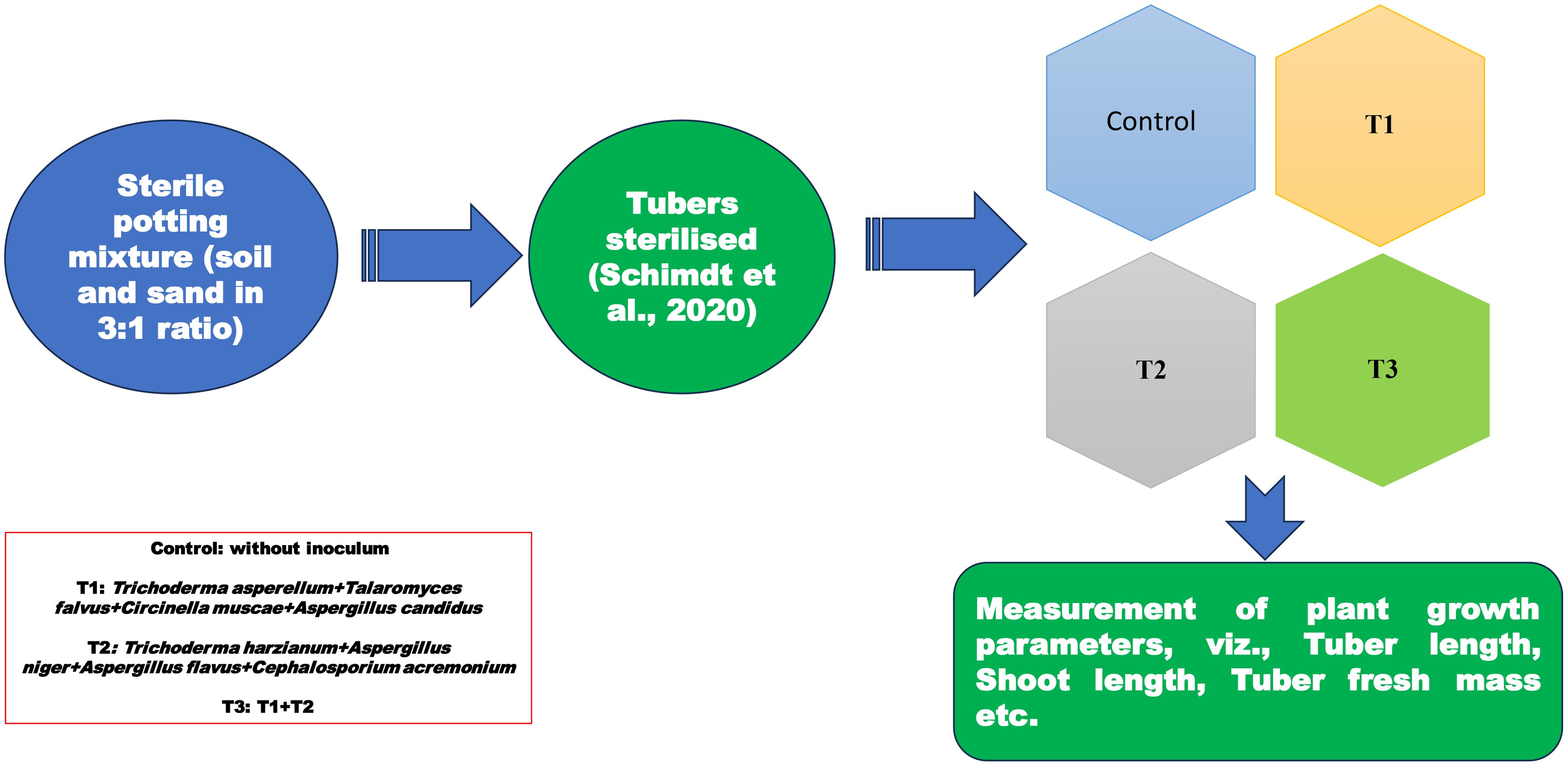
For the fungal inoculum, the fungal species were cultured in Potato dextrose agar (PDA for 5 days at 28°C. After this incubation period, the actively growing fungal plate was flooded with sterile water, and gentle agitation was employed to liberate the spores from the mycelia. The liberated spores were then carefully separated from the mycelial debris by passing them through a sieve and were then collected in a beaker. The spore concentration was then adjusted by dilution with sterile water to a final concentration of 104 to 106 spores/ml. The concentration of fungal spores was selected based on an OD reading of 0.1 of the suspension at 600 nm using a spectrophotometer (Figure 1).
Preparation of potting mixture
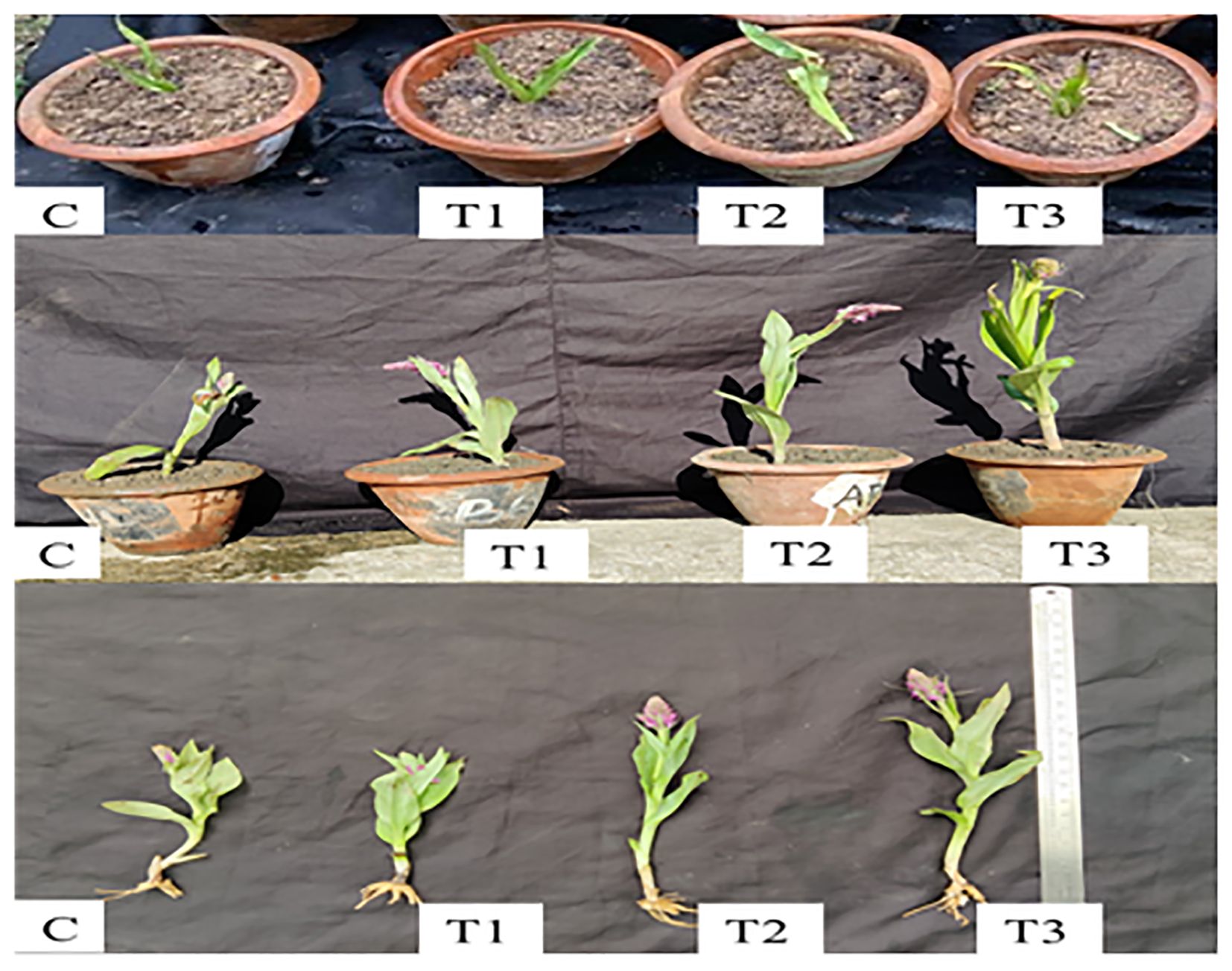
The potting mixture used in the experiment was carefully prepared to provide an optimal environment for seedling growth. It was composed of sterile soil and sand, combined in a 3:1 ratio. To ensure the absence of any living microorganisms or contaminants that could interfere with the experiment, the potting mixture was autoclaved thrice. Each autoclave cycle involved subjecting the potting mixture to high-pressure steam of 15 pascals at a temperature of 121°C for 20 minutes. Following the first autoclave cycle, the material was autoclaved again the following day. This allowed any remaining heat-resistant spores within the material to potentially germinate overnight and subsequently be eradicated. The third autoclave cycle was performed on the third day to ensure thorough eradication of any potential pathogens, including bacteria, fungi, or weed seeds. Tubers were sterilized in (2% NaOCl for 10 min followed by streptomycin (100 μg/ml) + cycloheximide (100 μg/ml) for 12 hrs. (Schmidt et al., 2021). After sterilization, the inoculated tubers were planted in pots (4 replicates for each treatment) filled with triple autoclaved soil and sand in a ratio of 3:1. The pots were placed in a green house and the pots were irrigated with double distilled water every alternate day (Figures 2, 3). Plants were grown in the green house for 3 months during which various observations like tuber length, tuber fresh mass, tuber dry mass, shoot lengths, shoot dry mass, shoot fresh mass, specific leaf area were recorded. The plants were allowed to reach the flowering stage, and the length of inflorescence and number of flowers were recorded before the termination of the experiment. Three treatments (T1, T2, T3) and Control were employed to investigate the effect of rhizospheric fungi on the growth characteristics.
Results
Effect of rhizospheric fungi on growth parameters of D. hatagirea
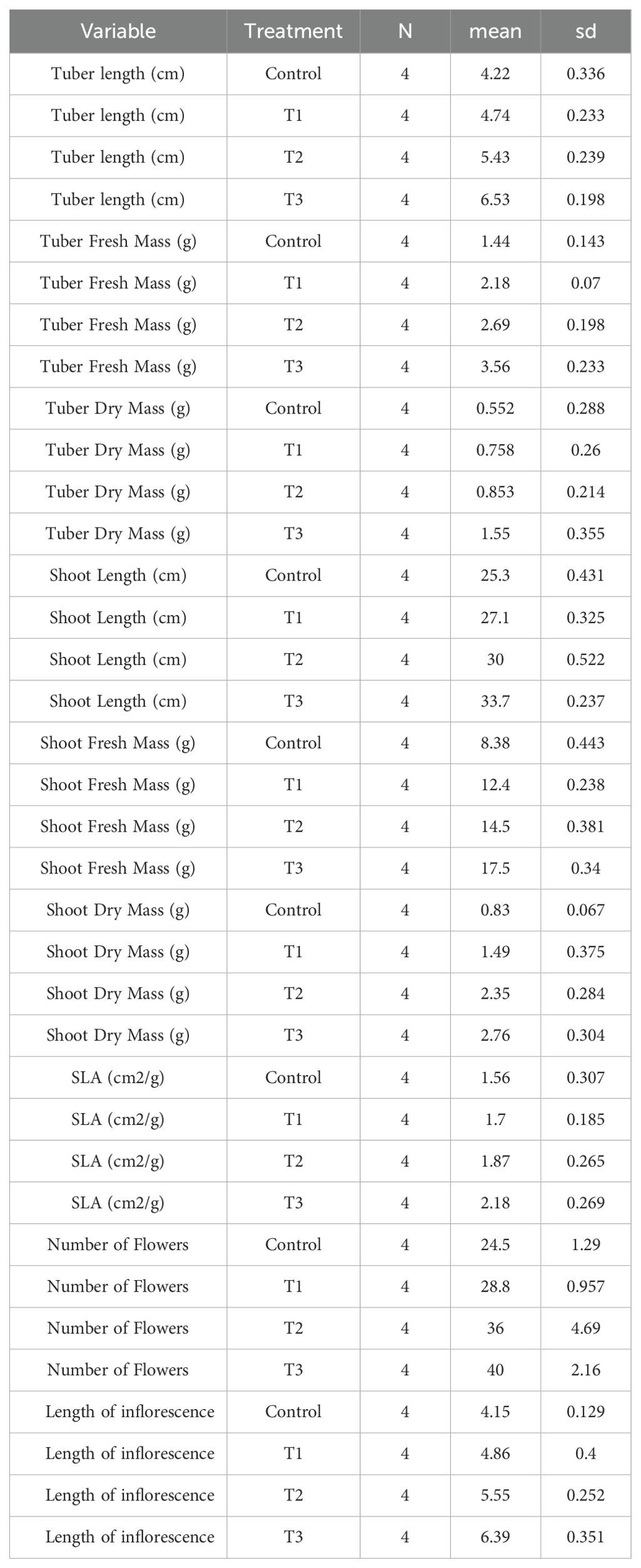
The effect of various treatments on growth parameters is shown in (Figure 4). Mycobial inoculants significantly improved different growth parameters. Tuber length was highest (6.52 cm) under T3 and lowest (4.21 cm) under control treatment (Table 1, Figure 4). ANOVA and post-hoc analysis revealed that the increase in tuber length under the T3 treatment is significantly higher compared to other treatments. Shoot length varied from a highest of 3.7 cm under T3 and lowest of 25.27 cm under control treatment. Like tuber and shoot length, tuber fresh mass was also highest (3.56 g/plant) under T3 and lowest (1.4 g/plant) under the control treatment (Table 2, Figure 4). Shoot fresh mass also differed under different treatments and was significantly higher (17.54 g/plant) under T3 and was lowest (8.3 g/plant) under control treatment (Table 2, Figure 4). ANOVA and post-hoc analysis revealed significant differences among all the treatment (Tables 2, 3). Further the number of flowers per plant ranged from 40 under T3 to 24.5 under control (Table 1, Figure 4). ANOVA and post-hoc analysis revealed that treatments such as T3 and T2, T1 and control were not significantly different from one another. Moreover, the specific leaf area was highest in T3 (2.32 cm2/gm) and lowest in control (1.56 cm2/gm). There were significant differences between T3 and control treatments, however no significant differences were found between T1 and T2. The effects of different treatments (T1, T2, T3) and Control, on tuber length, tuber fresh mass, tuber dry mass, shoot length, shoot fresh mass, shoot dry mass, specific leaf area (SLA), number of flowers, and length of inflorescence revealed significant differences in these growth parameters across the treatments, suggesting that the rhizospheric fungi have a discernible impact on plant growth compared to the control group (Table 1). Each parameter was measured in four replicates (n=4), and the results are presented as mean values with their respective standard deviations (SD).
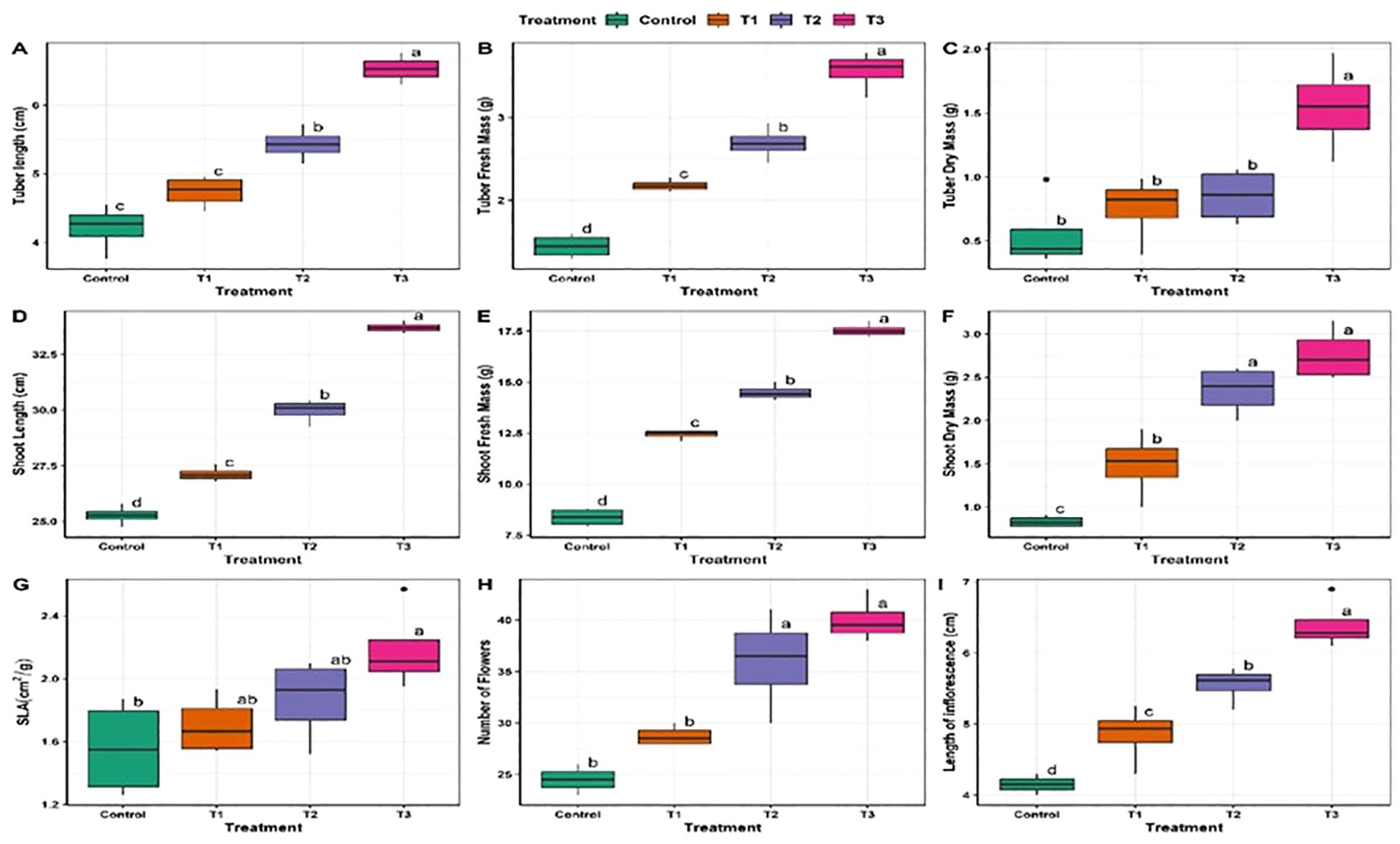
Figure 4. Effect of fungal species on plant growth parameters of D. hatagirea. Letters represent the significant differences at p < 0.05. Where (A): Tuber length, (B): Tuber fresh mass, (C): Tuber dry mass, (D): Shoot length, (E): Shoot fresh mass, (F): Shoot dry mass, (G): Specefic leaf area, (H): Number of flowers, (I): Length of Inflorescence.
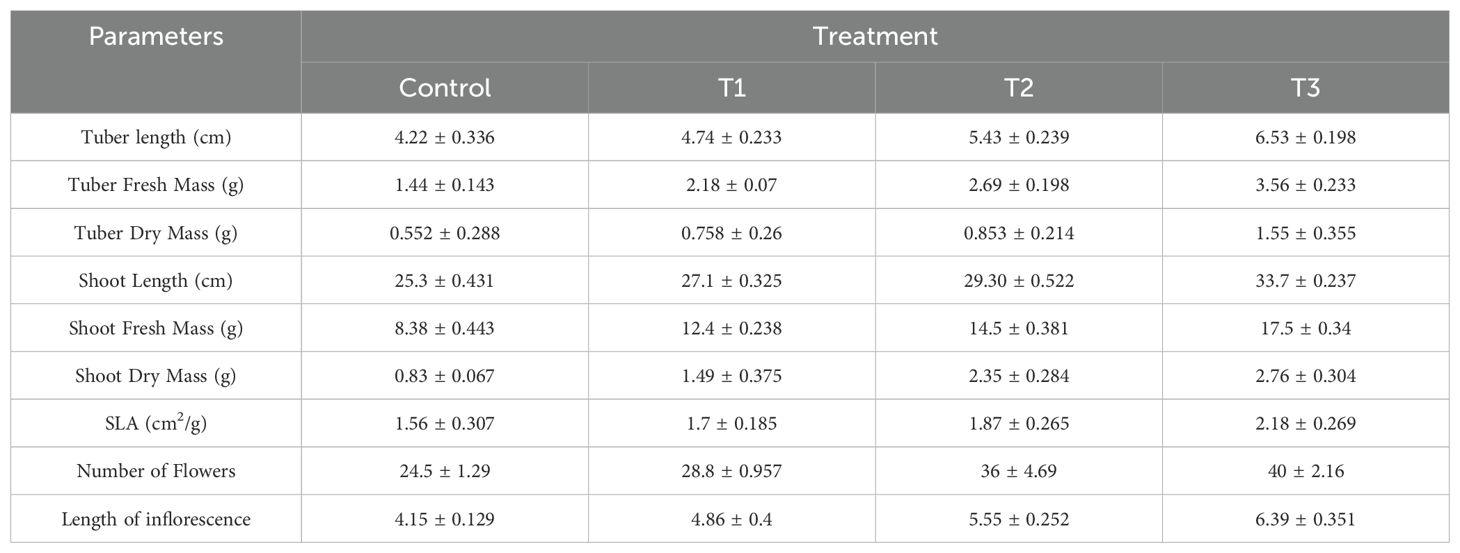
Table 1. Effects of different treatments (Control, T1, T2, T3) on tuber length, tuber fresh mass, tuber dry mass, shoot length, shoot fresh mass, shoot dry mass, specific leaf area (SLA), number of flowers, and length of inflorescence.
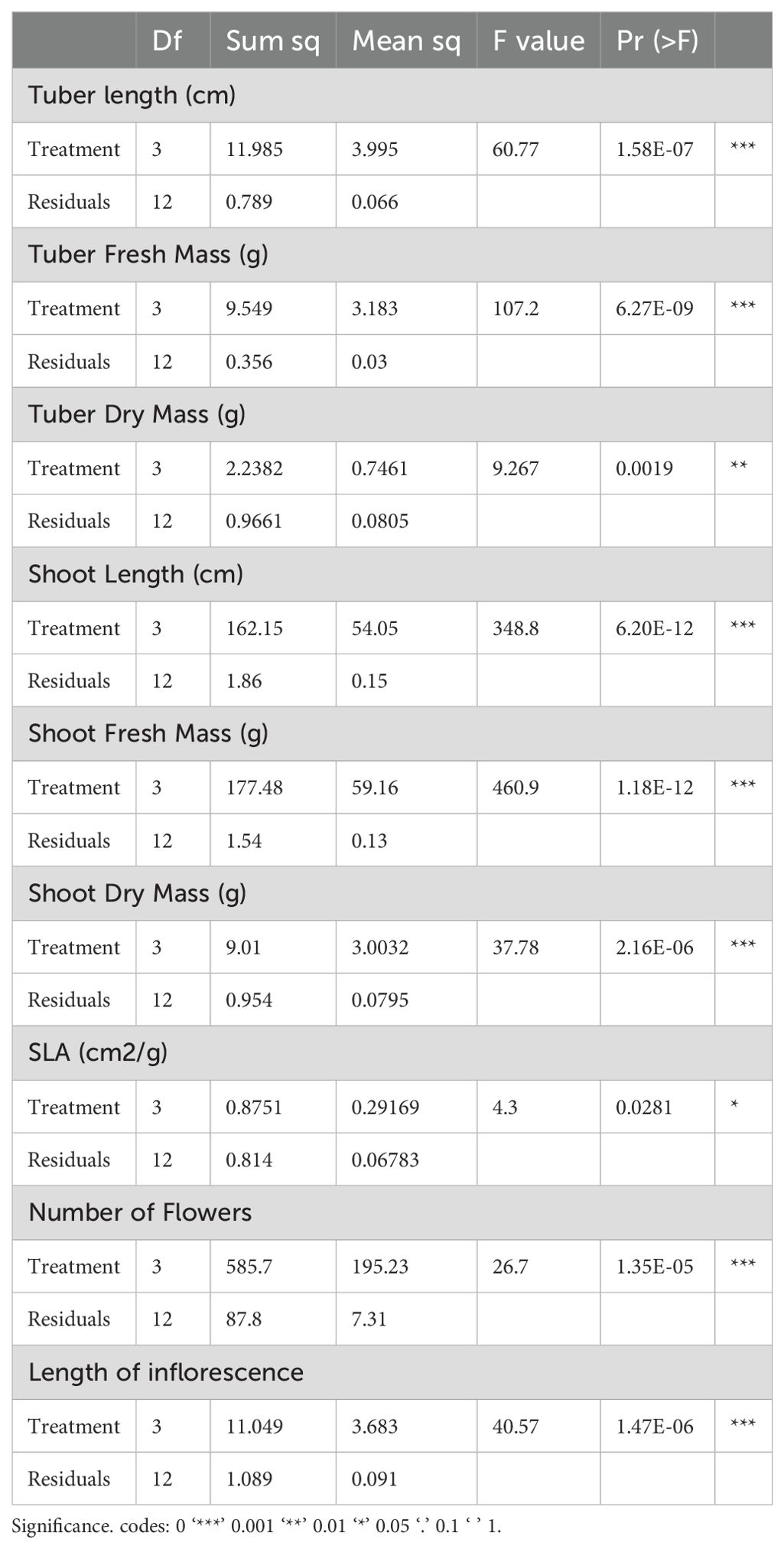
Table 3. The Table 4 displays the degrees of freedom (Df), sum of squares (Sum sq), mean squares (Mean sq), F values, and the p-values (Pr(>F)).
ANOVA tests conducted on various plant growth parameters, including tuber length, fresh and dry mass, shoot length, fresh and dry mass, specific leaf area (SLA), number of flowers, and length of inflorescence. The Table 3 displays the degrees of freedom (Df), sum of squares (Sum sq), mean squares (Mean sq), F values, and the p-values (Pr(>F)). Significant results are denoted by asterisks, with *** indicating p < 0.001, ** indicating p < 0.01, and * indicating p.
< 0.05. These results highlight where treatment effects are statistically significant, indicating a notable impact of the treatments on the respective growth parameters. Tukey’s pairwise comparisons for several plant growth parameters (Table 4), including tuber length, fresh and dry mass, shoot length, fresh and dry mass, specific leaf area (SLA), number of flowers, and length of inflorescence. The comparisons are made between different treatment groups (T1, T2, T3) and a control group. The columns show the differences (diff), lower and upper confidence limits (lwr, upr), and the adjusted p-values (p adj). Significant differences (p adj < 0.05) are highlighted to indicate where the treatments had a notable effect compared to the control or other treatments.
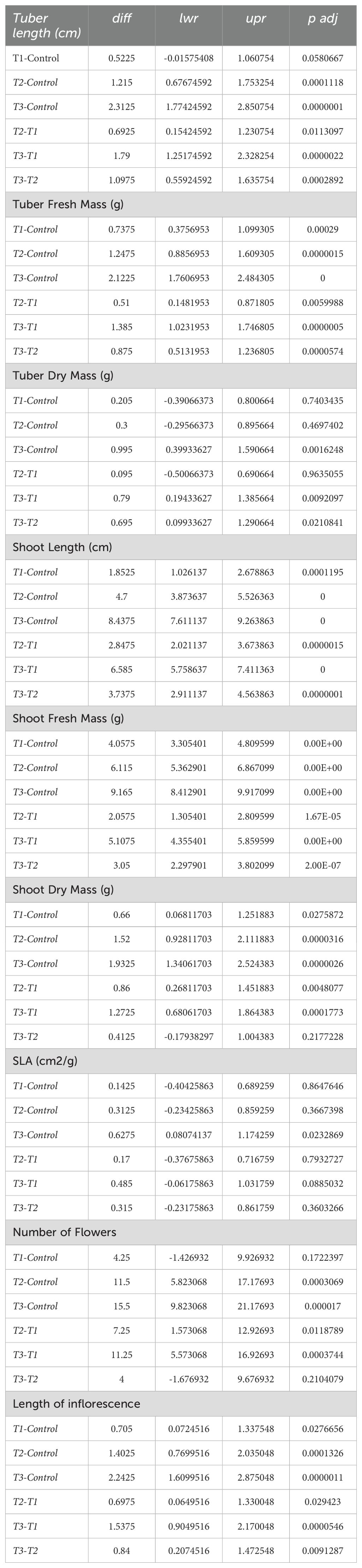
Table 4. Tukey’s pairwise comparisons for several plant growth parameters, including tuber length, fresh and dry mass, shoot length, fresh and dry mass, specific leaf area (SLA), number of flowers, and length of inflorescence.
Discussion
Fungi play a critical role in plant growth and development, particularly through symbiotic interactions where they supply essential soil minerals, such as nitrogen and phosphorus, in exchange for carbon-based compounds produced by the plant. These symbiotic fungi, particularly mycorrhizal fungi, form associations with the roots of over 90% of plant species and can fulfill up to 80% of the plant’s mineral nutrient requirements. Orchids, a diverse and ecologically significant family, are known for their complex fungal associations, which often involve clades such as Serendipitaceae (Sebacinales), Tulasnellaceae, and Ceratobasidiaceae (Batty et al., 2006; Valadares et al., 2011). These associations can vary in specificity, ranging from highly specialized relationships with a narrow group of fungal partners to more generalized interactions with multiple fungal taxa (Otero et al., 2002; Ma et al., 2003; McCormick et al., 2004; Sheferson et al., 2005; Suárez et al., 2006; Li et al., 2021). In orchids, fungi play a fundamental role in seed germination, protocorm development, and overall growth. Previous studies have demonstrated that specific fungal species can stimulate key growth parameters, including seed germination and early developmental stages of orchids (Wang et al., 2011; Zhao et al., 2013; Tian et al., 2022). This is particularly relevant for species like D. hatagirea, an orchid of high conservation and medicinal value, which forms specific associations with certain fungal species. In this study, we found that the tubers of D. hatagirea are compatible with eight fungal species, suggesting a specialized yet flexible symbiotic relationship.
In the context of plant growth enhancement, the addition of fungal inoculants such as Trichoderma asperellum, Talaromyces flavus, Aspergillus candidus, Circinella muscae, Aspergillus flavus, Aspergillus niger, Cephalosporium acremonium, and Trichoderma harzianum. these has been shown to significantly improve various growth parameters of D. hatagirea. Treatments involving these fungi (T1, T2, and T3) were evaluated for their effects on tuber length, tuber fresh and dry mass, shoot length, shoot fresh and dry mass, specific leaf area (SLA), number of flowers, and inflorescence length. The results revealed that all treatments led to substantial improvements in plant growth compared to the untreated control, with treatment T3 consistently showing the most pronounced effects across all measured parameters. Specifically, treatment T3 led to the greatest increases in tuber length, fresh and dry tuber mass, shoot length, fresh and dry shoot mass, SLA, number of flowers, and inflorescence length. Statistical analysis using ANOVA confirmed the significance of these effects, with p-values less than 0.001 for most parameters, indicating a strong correlation between fungal treatment and enhanced plant growth. Furthermore, Tukey’s pairwise comparisons highlighted significant differences between the control and all treatments, with T3 being consistently superior, underscoring the remarkable efficacy of this particular treatment in promoting plant development. These findings provide valuable insights into the specific fungal interactions that underpin the growth and productivity of D. hatagirea. The variation in growth responses to different fungal species suggests a development-dependent specificity, where certain fungal strains are more effective at different stages of plant growth. These results not only contribute to our understanding of orchid-fungal symbioses but also have practical implications for conservation and cultivation strategies for D. hatagirea. The identification of effective fungal partners could be leveraged in conservation programs aimed at enhancing the survival and growth of this species, as well as in agricultural practices for its sustainable production. The study highlights the critical role of fungal associations in the growth and development of D. hatagirea, demonstrating that the use of specific fungal strains, particularly in treatment T3, can substantially improve various growth parameters. These results lay the foundation for further research into the mechanisms of orchid-fungal interactions and offer practical applications in the conservation and propagation of D. hatagirea.
Conclusion
Rhizospheric fungi establish mutualistic associations with plant roots known as root-fungal associations, playing a vital role in every aspect of plant growth and development stages while enhancing resistance against diseases and environmental stressors. The fungal rhizosphere is an intelligent and sentient system with inherent ecological healing properties that functions as a living Internet, facilitating nutrient transport and communication. Moreover, fungi produce extracellular enzymes essential for crucial soil processes such as the breakdown of organic matter, mineralization, and nutrient cycling. fungi are eco-friendly, have a crucial function in plant nutrient uptake, and bolster their capacity to adjust to environmental adversities. The utilization of fungal inoculants can be considered an eco-friendly method of improving plant fitness. The study also demonstrates that fungal mycobionts significantly enhanced growth parameters, including tuber and shoot mass, leaf area, and flower number. Statistical analysis (ANOVA and Tukey’s tests) confirmed the efficacy of this fungal combination. The future implications of our study are conservation of endangered species like D. hatagirea and other rare or endangered plants, specific fungal associations can be leveraged to enhance growth, reproductive success, and in-situ or ex-situ conservation efforts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
BD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RQ: Data curation, Methodology, Project administration, Software, Visualization, Writing – original draft. AW: Supervision, Investigation, Validation, Writing – original draft, Writing – review & editing. MYB: Supervision, Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are highly thankful to Head, Department of Botany, University of Kashmir for providing better facilities for conducting research. The authors also thank the learned reviewers for their valuable suggestions to improve the quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ballabh B., Chaurasia O., Ahmed Z., Singh S. B. (2008). Traditional medicinal plants of cold desert Ladakh Used against kidney and urinary disorders. J. Ethnopharmacology. 2, 331–339.
Barrett C. F., Freudenstein J. V., Taylor D. L., Kõljalg U. (2010). Range wide analysis of fungal associations in the fully mycoheterotrophic Corallorhiza striata complex (Orchidaceae) reveals extreme specificity on ectomycorrhizal Tomentella (Thelephoraceae) across North America. Am. J. Bot. 97, 628–643.
Batty A. L., Brundrett M. C., Dixon K. W., Sivasithamparam K. (2006). New methods to improve symbiotic propagation of temperate terrestrial orchid seedlings from axenic culture to soil. Aust. J. Bot. 54, 367–374.
Dearnaley J. D., Cameron D. D. (2017). Nitrogen transport in the orchid mycorrhizal symbiosis-further evidence for a mutualistic association. New Phytol. 213, 10–12.
Fochi V., Chitarra W., Kohler A., Voyron S., Singan V. R., Lindquist E. A., et al. (2017). Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol. 213, 365–379.
Giri D., Tamata S. (2010). A general account on medicinal uses of D. hatagirea. New York Sci. J. 2, 78–79.
Kennedy A. H., Watson L. E. (2010). Species delimitations and phylogenetic relationships within the fully myco-heterotrophic Hexalectris (Orchidaceae). System Botany. 35, 64–76.
Khajuria A. K., Kumar G., Bisht N. S. (2017). Diversity with ethnomedicinal notes on Orchids: A case study of Nagdev Forest range, Pauri Garhwal, Uttarakhand, India. J. Medicinal Plants Stud. 1, 171–174.
Kuga U., Sakamoto N., Yurimoto H. (2014). Stable isotope imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 202, 594–605.
Leake J. R. (1994). The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 127, 171–216.
Li T., Yang W., Wu S., Selosse M. A., Gao J. (2021). Progress and prospects of mycorrhizal fungal diversity in orchids. Front. Plant Sci. 12, 646325.
Ma M., Tan T. K., Wong S. M. (2003). Identification and molecular phylogeny of Epulorhiza isolates from tropical orchids. Mycological Res. 107, 1041–1049.
McCormick M. K., Whigham D. F., O’Neill J. (2004). Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytol. 163, 425–438.
Ogura-Tsujita Y., Yokoyama J., Miyoshi K., Yukawa T. (2012). Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am. J. Bot. 99, 1158–1176.
Otero J. T., Ackerman J. D., Bayman P. (2002). Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. Am. J. Bot. 89, 1852–1858.
Porras-Alfaro A., Bayman P. (2007). Mycorrhizal fungi of Vanilla: diversity, specificity and effects on seed germination and plant growth. Mycologia 99, 510–525.
Rasmussen H. N. (1995). Terrestrial orchids-from seed to mycotrophic plant (Cambridge: Cambridge University Press).
Schmidt C. S., Mrnka L., Lovecká P., Frantík T., Fenclová M., Demnerová K., et al. (2021). Bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential for biocontrol of Sclerotinia and Phoma disease. Sci. Rep. 11, 3810.
Sheferson R. P., Taylor D. L., Weiss M., Garnica S., McCormick M. K., Adams S., et al. (2007). The evolutionary history of mycorrhizal specificity among lady’s slipper orchids. Evolution 61, 1380–1390.
Sheferson R. P., Weiß M., Kull T., Taylor D. L. (2005). High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium. Mol. Ecol. 14, 613–626.
Suárez J. P., Weiß M., Abele A., Garnica S., Oberwinkler F., Kottke I. (2006). Diverse tulasnelloid fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycological Res. 110, 1257–1270.
Tian F., Liao X. F., Wang L. H., Bai X. X., Yang Y. B., Luo Z. Q., et al. (2022). Isolation and identification of beneficial orchid mycorrhizal fungi in Paphiopedilum barbigerum (Orchidaceae). Plant Signaling Behav. 17, 2005882.
Tsering J., Tam N., Tag H., Gogoi B. J., Apang O. (2017). Medicinal orchids of arunachal pradesh: A review. Bull. Arunachal For. Res. 32, 1–16.
Valadares R., Pereira M., Otero J., Cardoso E. (2011). Narrow fungal mycorrhizal diversity in a population of the orchid Coppensia doniana. Biotropica 44, 114–122.
Wang H., Fang H. Y., Wang Y. Q., Duan L. S., Guo S. X. (2011). In situ seed baiting techniques in Dendrobium offcinale Kimura et Migo and Dendrobium nobile Lindl. the endangered Chinese endemic Dendrobium (Orchidaceae). World. J. Microbial Biotechnol. 27, 2051–2059.
Keywords: Dactylorhiza, fungal species, growth parameters, tuber, treatments
Citation: Wani AH, Qadir R, Bhat MY and Dar BA (2025) Effect of different mycobionts on growth parameters of Dactylorhiza hatagirea (D. Don) Soo: implications on conservation strategies. Front. Conserv. Sci. 6:1470018. doi: 10.3389/fcosc.2025.1470018
Received: 24 July 2024; Accepted: 25 March 2025;
Published: 16 April 2025.
Edited by:
Zeeshan Ahmad, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Amit Bahukhandi, Govind Ballabh Pant National Institute of Himalayan Environment and Sustainable Development, IndiaMuhammad Numan Khan, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Wani, Qadir, Bhat and Dar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bilal Ahmad Dar, Z3VsYmlsYWxib3RAZ21haWwuY29t
 Abdul Hamid Wani
Abdul Hamid Wani Bilal Ahmad Dar
Bilal Ahmad Dar