- Faculty of Science, Assam down town University, Guwahati, Assam, India
Introduction: The study examines the ecological and ethnobotanical significance of significance of Alternanthera philoxeroides (Mart) Griseb, commonly known as Alligator weed in Panikhaiti Gaon, a diverse locality in Kamrup Metropolitan district, Assam. The plant often plays a dual role as an ecological invader and a culturally significant species. This study aimed to assess the ecological impact and traditional knowledge associated with A. philoxeroides in the region.
Methods: Ecological data were collected using the quadrat method (1.5m x 1.5m) with 45 quadrats sampled across three sites. In addition to this, ethnobotanical data were obtained through semi-structured interviews with 45 local informants and supplemented via an online questionnaire. The ecological parameters, Relative Density (RD), Relative Frequency (RF), Relative Dominance (R dom), and Importance Value Index (IVI)—were assessed across these sites.
Results: In site 1, the RD, RF, R dom, and IVI values were 24.2, 56.1, 45.2, and 125, respectively, while in site 2, they were 45.6, 33.4, 12.8, and 91.8. Site 3 recorded values of 19.3, 33.9, 54.5, and 107.7. Biodiversity indices, including Shannon–Wiener diversity index (H) and Pielou’s evenness index (I), ranged from 0.21 to 0.75 across the sites, indicating variations in species distribution. Beyond its ecological role, A. philoxeroides holds cultural and traditional significance among the local communities. Ethnobotanical findings revealed a range of medicinal and culinary uses for the plant, highlighting its cultural relevance despite its invasive status.
Discussion: The study underscores the value of integrating traditional knowledge with scientific data to inform locally grounded conservation and resource management strategies.
Introduction
Alternanthera philoxeroides (Mart) Griseb, more commonly Alligator or pig weed, is a prominent invasive species native to South America (EPPO, 2016) known since 1897 in the USA (Kay and Haller, 1982), 1906 in New Zealand (Roberts and Sutherland, 1986), 1940s in Australia (Julien and Bourne, 1988), Europe in 1971 (Dupont, 1989) and Asia around 1999 (Jayasinghe, 2008). A. philoxeroides is now found throughout India predominantly in the states of Assam, Bihar, West Bengal, Tripura, Manipur, Andhra Pradesh, Karnataka, Maharashtra, Delhi and Punjab (Pramod et al., 2008).
Alternanthera philoxeroides is an amphibious species, with a preference for aquatic environments. Its adaptability to both waterlogged and terrestrial conditions contributes significantly to its invasive potential, enabling it to alter ecological dynamics, outcompete native species, and disrupt biodiversity across diverse ecosystems.
Morphologically, the stem is cylindrical, hollow, and buoyant (Figure 1a)—an essential adaptation for aquatic habitats. This trait allows the plant to float, spread laterally, and form thick vegetative mats on the water surface (Humane et al., 2015) (Figure 1b). These mats reduce dissolved oxygen levels, impede light penetration, and obstruct water flow, adversely affecting aquatic flora and fauna (Wang et al., 2008). Over time, such biomass accumulation can increase sedimentation rates and change the local hydrology, posing major ecological threats to wetland systems (Sosa and Julien, 2010) like those surrounding Guwahati, Assam.

Figure 1. Morphological adaptations of Alternanthera philoxeroides (Alligator weed) in aquatic and terrestrial environments. (a) Transverse section of an aquatic stem showing a hollow, buoyant structure that facilitates flotation. (b) Dense mat formation by aquatic forms of the weed, enabling lateral spread over water surfaces. not (c) Thickened and lignified stems in terrestrial forms, providing structural rigidity and anchorage in soil. (d) Aggressive terrestrial growth pattern characterised by rapid spread and ground coverage.
In terrestrial environments, A. philoxeroides undergoes structural modifications. The stem becomes thicker and more lignified, enhancing its rigidity and anchorage in soil (Figure 1c). Its aggressive growth suppresses (Figure 1d) native vegetation, leading to a decline in plant diversity. Despite its invasive character, the plant can also contribute positively to ecosystem function in certain scenarios. Its deep taproot system and lateral rhizomes stabilise soil, improve nitrogen content, and help prevent erosion, especially in degraded lands (Geng et al., 2007).
The weed’s root architecture is highly adaptive. In aquatic settings, adventitious roots emerge from the stem nodes, assisting nutrient uptake and providing buoyancy. These roots also anchor the plant mats to the substrate, increasing their stability (Julien and Stanley, 1999). Terrestrial forms, in contrast, develop a more robust taproot system and underground rhizomes, which can penetrate up to a meter deep (Sosa and Julien, 2010).
Leaf morphology demonstrates high phenotypic plasticity in response to environmental conditions. In aquatic environments, leaves are typically larger, darker, and thinner, maximising photosynthetic efficiency under low light. In contrast, terrestrial leaves tend to be smaller, thicker, and more rigid, reducing water loss through transpiration and enhancing drought resistance (Tao et al., 2009). This morphological adaptability plays a critical role in the plant’s ecological success across habitats.
Unlike many invasive species that rely primarily on seed dispersal, A. philoxeroides propagates almost exclusively through vegetative means. Although it can flower and produce seeds, viable seed formation is rare in its introduced ranges. Instead, it spreads rapidly via fragmentation, rhizomes, and growth from axillary buds (EPPO, 2016), making mechanical control difficult and enhancing its colonisation potential through water, wind, and anthropogenic activities.
Despite its classification as an invasive species, alligator weed holds significant traditional value as various communities utilise this weed due to its medicinal properties as a phytotherapeutic interventions (Kleinowski et al., 2016; Nahar et al., 2022). It serves as livestock fodder (Farooq et al., 2021) and has also been explored for bioremediation purposes such as wastewater treatment due to its absorption properties (Rane et al., 2015; Simmons et al., 2007; Yan et al., 2023). However, its utility is counterbalanced by documented toxicity in livestock, where ingestion has been associated with photosensitisation and liver damage (Bourke and Rayward, 2003).
In this study, we aim to assess the ecological status of A. philoxeroides in Panikhaiti gaon, Kamrup metropolitan district of Assam. The village covers an area of approximately 361.46 hectares and lies in the outskirts of Guwahati, well-known for its cultural heritage. The closest city, Guwahati, which is roughly 15 kilometres away from Panikhaiti Gaon, is situated on the south bank of the mighty river Brahmaputra. The village economy primarily relies on agriculture along with fishing and other small businesses. According to the 2011 census, the village population is over 1764 comprising of 928 male and 836 female (Office of the Registrar General & Census Commissioner, India, 2011). The population predominantly speaks Assamese, Bengali and Boro languages and has a literacy rate of 75.58%.
We focus on quantitative measurement of ecological parameters such as Relative Density (RD), Relative Frequency (RF), Relative Dominance (RDom), and Importance Value Index (IVI). To collect this data, we conducted quadrat-based vegetation sampling across three representative field sites encompassing both terrestrial and semi-aquatic habitats. These surveys were complemented by biodiversity indices including species richness and evenness, enabling a site-wise comparison of plant community structures.
In parallel, we carried out a comprehensive ethnobotanical survey involving 45 in-person interviews with community members; ranging from traditional herbal practitioners to local farmers, and supplemented this with responses from an online questionnaire. These interactions provided nuanced insights into the cultural and medicinal uses of A. philoxeroides, including culinary practices and preparation methods.
This dual-survey approach allowed us to integrate empirical ecological data with community-derived traditional knowledge, offering a more holistic understanding of A. philoxeroides in the local context. Through this combined ecological and ethnobotanical lens, we seek to bridge scientific assessments with indigenous perspectives, ultimately contributing to context-aware conservation strategies and sustainable rural livelihoods.
Materials and methods
Study area
Assam, the bio-geographical gateway to North-east India, has Bhutan and Arunachal Pradesh to its north, Manipur and Nagaland to its east, Tripura, Mizoram, Meghalaya and Bangladesh to the south and West Bengal to the west. The ethnobotanical study was conducted in the Panikhaiti region, located in Kamrup (Metro) district of Assam, India. This area is characterised by semi-urban settlements, surrounding hillocks, and patches of undisturbed vegetation. The geographic location falls approximately between 26.1609° to 26.1634° N latitude and 91.8385° to 91.8455° E longitude, under the WGS 84 coordinate reference system (EPSG:4326).
Three field sites were selected based on preliminary surveys and local knowledge of plant availability and usage (Table 1, Figure 2).
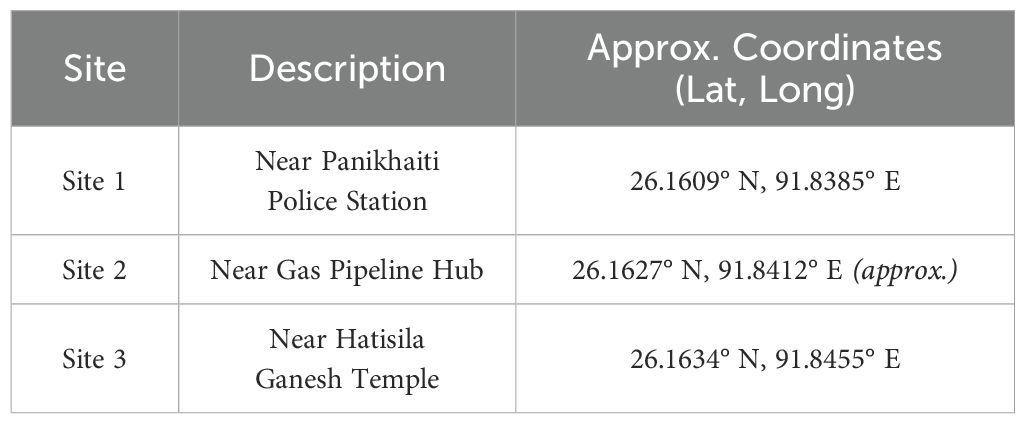
Table 1. Geographic coordinates and descriptions of the three surveyed sites in Panikhaiti Gaon, Kamrup (Metropolitan), Assam, selected for ecological and ethnobotanical assessment of Alternanthera philoxeroides .
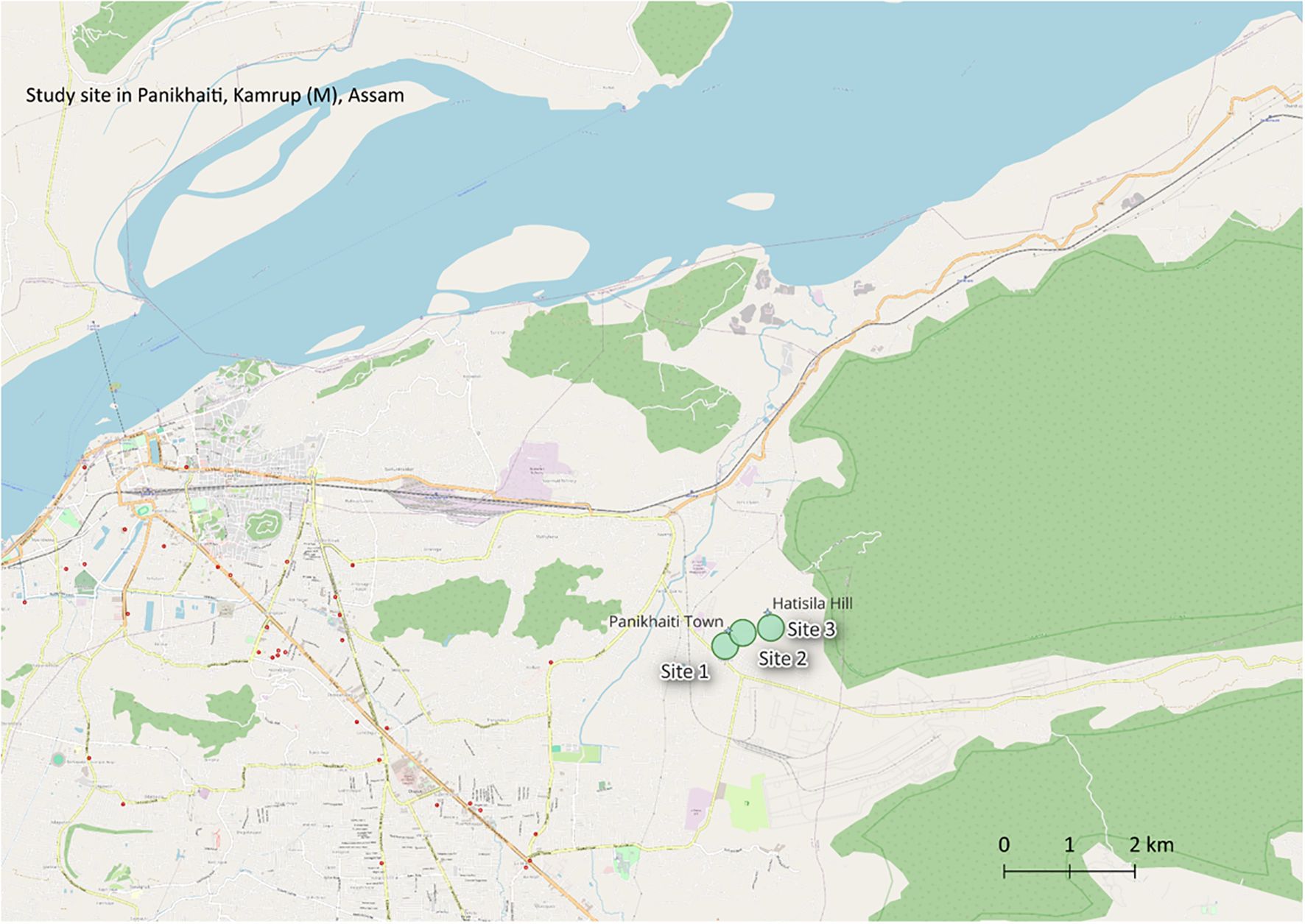
Figure 2. Map showing the ethnobotanical study sites near Panikhaiti, Assam. The locations include Site 1 (near Panikhaiti Police Station), Site 2 (Gas Pipeline Hub), and Site 3 (Hatisila Ganesh Temple), marked with their approximate geographic coordinates. Prominent landmarks such as Panikhaiti Town and Hatisila Hill are also displayed to provide spatial context.
Data collection
This study was primarily based on extensive field inventories carried out to evaluate the ecological status of A. philoxeroides, commonly known as Alligator weed. The quadrat method was employed to assess phyto-sociological parameters such as Relative Density (RD), Relative Frequency (RF), Basal Area (BA), Relative Dominance (RDom), and Importance Value Index (IVI). These parameters were calculated for A. philoxeroides as well as associated plant species across three different sites in the study area.
Vegetation sampling was conducted using standard quadrat sampling methods. A total of 45 quadrats (15 per site) were sampled across the three selected locations. At each of the three study sites, quadrats measuring 1.5 m × 1.5 m were randomly placed within ecologically representative zones to capture the diversity and abundance of plant species. Quadrats were spaced at intervals of 4 to 8 meters, strategically covering both terrestrial and semi-aquatic microhabitats in and around Panikhaiti Gaon, Kamrup Metropolitan district, Assam. This sampling was conducted during the months between April, 2022 to December, 2023 without temporal replication. Plant species associated with Alternanthera philoxeroides were identified using standard regional floras, online botanical databases, and local ethnobotanical knowledge contributed by community members familiar with the vegetation of the area.
Ethnobotanical information was gathered through semi-structured interviews and informal discussions with local residents, including traditional herbal healers and knowledgeable elders. Repeated visits were made to reliable informants to validate the information. A total of 45 participants were surveyed in person using a structured physical questionnaire. This questionnaire included sections on the local, vernacular, and scientific names of the plant, parts used, methods of preparation, and the ailments treated with A. philoxeroides (Cunningham, 2001; Tardío and Pardo-de-Santayana, 2008).
In addition to the physical surveys, an online Google Form was used to collect supplementary data. The digital questionnaire comprised sixteen questions, divided into sections covering personal information of the respondent, plant identity, ethnomedicinal uses of A. philoxeroides, and perceived medicinal efficacy. Of these, twelve were required questions, and four were optional explanatory responses. While demographic profiling was not possible for this group, the responses yielded novel ethnomedicinal uses and aligned well with in-person accounts. Voluntary sampling is common in exploratory ethnobotanical studies, and the number of responses is considered acceptable given the specific, use-focused scope of the form (Tongco, 2007).
The overall data collection strategy is illustrated in Figure 3.
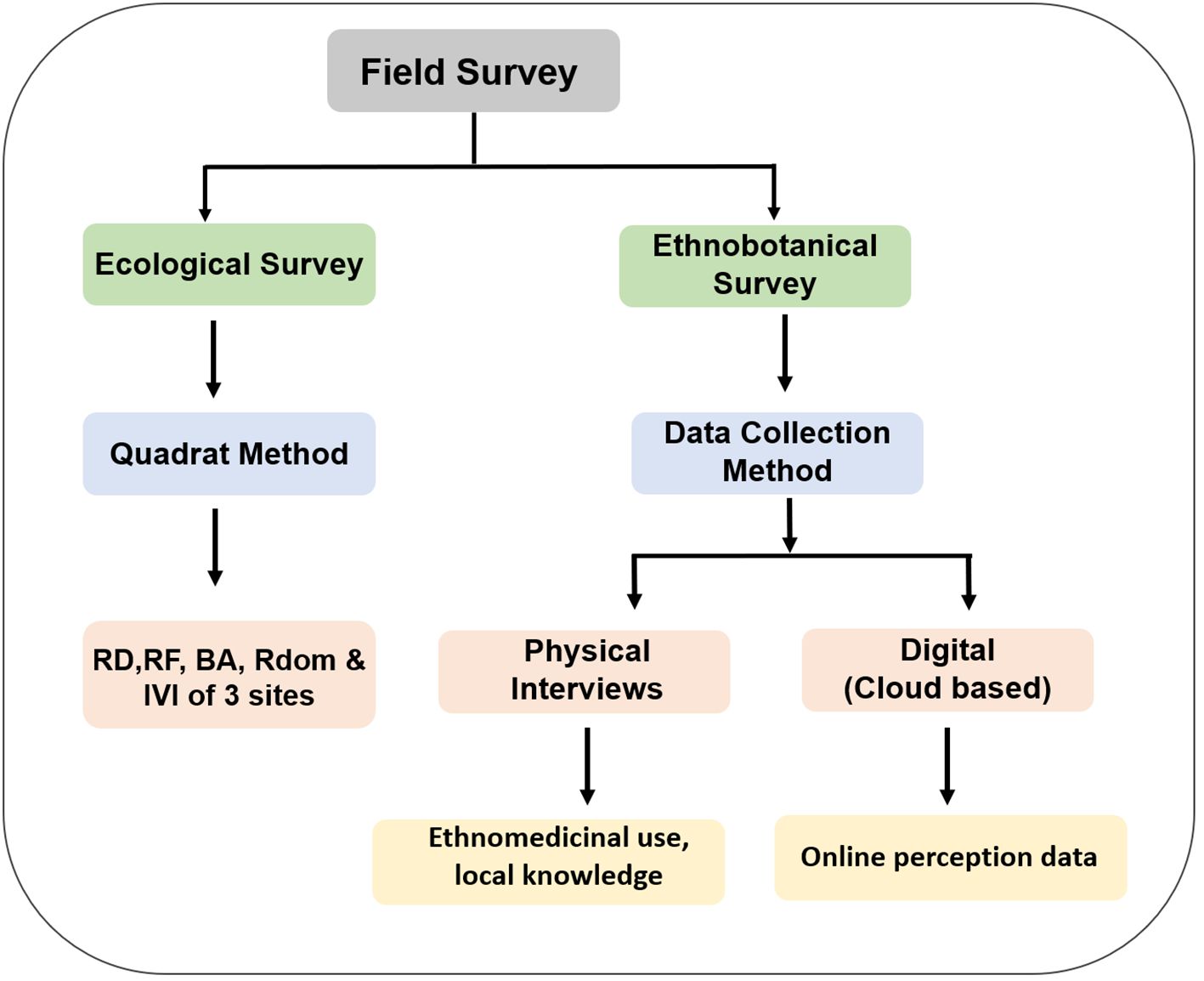
Figure 3. Flowchart representation of the data collection strategy used for ecological and ethnobotanical field surveys. The study employed two parallel approaches—ecological surveys and ethnobotanical surveys—to investigate the prevalence and traditional uses of Alternanthera philoxeroides. Ecological assessments involved quadrat sampling to quantify plant community structure through ecological parameters. Ethnobotanical data were gathered using structured physical questionnaires (n=45) and informal interviews with local informants. An online cloud-based questionnaire via Google Forms complemented in-person surveys, documenting traditional knowledge of Paani Matikaduri (A. philoxeroides) uses and preparation methods.
Data processing
The following standard ecological formulae were used to compute the phytosociological and biodiversity parameters:
E) Basal area (BA)
n = total no of organisms of a particular species.
N = total number of organisms of all species.
I) Species richness index (Dmg) (Margalef, 1951)
S = Number of species.
N = Total number of individuals in the community
J) Shannon and Weiner diversity index (H) (Shannon, 1948; Shannon and Weaver, 1949)
Pi = Proportion of Individuals of the community
LN = Natural logarithm
K) Pielou’s evenness index (I) (Pielou, 1966)
H = Number derived from Shannon’s diversity index
S = Total number of species
Data visualisation
Map-based visualisation of ethnobotanical collection sites was conducted using QGIS (v3.x). The geographical coordinates for three field sites, Site1, 2 and 3 were used to plot sampling locations. The Coordinate Reference System (CRS) was set to EPSG:4326 (WGS 84) to match the latitude-longitude data format. An OpenStreetMap XYZ basemap was added to provide geographical context.
Further, polygonal phytographs were constructed, using MS Excel, to visually compare phytosociological metrics, such as Importance Value Index (IVI), Relative Density (RD), Relative Frequency (RF), and Relative Dominance (RDom), across multiple plant species and sites.
Data visualisations were performed using RStudio (Posit Software, version 2024.12.1 Build 563, “Kousa Dogwood” Release). The demographic Sankey plot was generated using the networkD3 package in R. These visualisations were used to illustrate demographic distributions, ethnomedicinal data summaries, and ecological parameters computed during the study. Data derived from ethnobotanical questionnaires were summarised and plotted using the ggplot2 package. All codes used for data visualisation in this study is archived and accessible via Zenodo at https://doi.org/10.5281/zenodo.16080681.
Result
Numerous field surveys were conducted across different seasons from 2022 to 2023. These included ecological assessments using the quadrat method and ethnomedicinal data collection through structured questionnaires and informal conversations. The results are presented through a combination of tables, graphs, and narrative summaries, offering an integrated overview of ecological patterns and ethnobotanical knowledge associated with Alternanthera philoxeroides (Mart.) Griseb.
Ecology of Alternanthera philoxeroides
The field surveys conducted during 2022–2023 across three distinct sites in Panikhaiti Gaon revealed seasonal trends in the life cycle and growth patterns of A. philoxeroides. The shoots of this species were observed to undergo senescence during the colder months, from the end of December to early February, followed by gradual regeneration beginning in March. The plant showed prominent and active growth during the pre-monsoon and monsoon periods, thriving especially in semi-aquatic and moist terrestrial patches.
A total of 31 plant species, including A. philoxeroides, was recorded across the three sites, belonging to 17 different families and comprising a mix of herbs, shrubs, climbers, and trees. Site-wise species count revealed 11 species in Site 1, and 10 species each in Sites 2 and 3. The phyto-sociological parameters, Relative Density (RD), Relative Frequency (RF), Basal Area (BA), Relative Dominance (R Dom), and Importance Value Index (IVI), were calculated for A. philoxeroides and its associated species across all three sites and are presented in Tables 2–4 respectively. Site 1 (Figure 4a) recorded the highest Importance Value Index (IVI) for A. philoxeroides (125.5), suggesting greater ecological dominance compared to Site 2 (Figure 4b) and Site 3 (Figure 4c). These phytographs visually convey the relative abundance and distribution of the species across different plant families in the surveyed habitats.
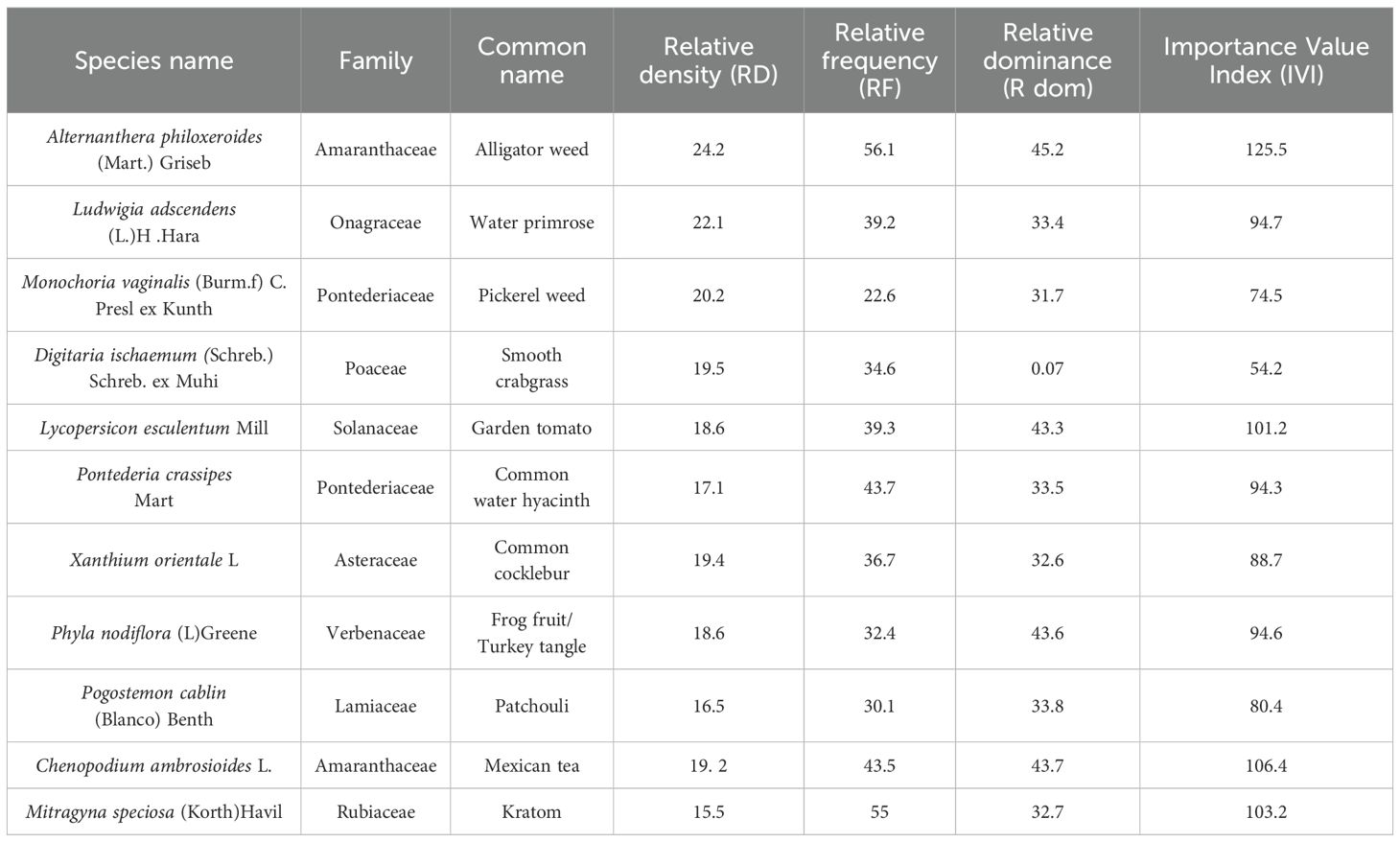
Table 2. Quantitative analysis of Phyto-sociological data of Alternanthera philoxeroides (Mart.) Griseb and its associate plants with families in Site 1.
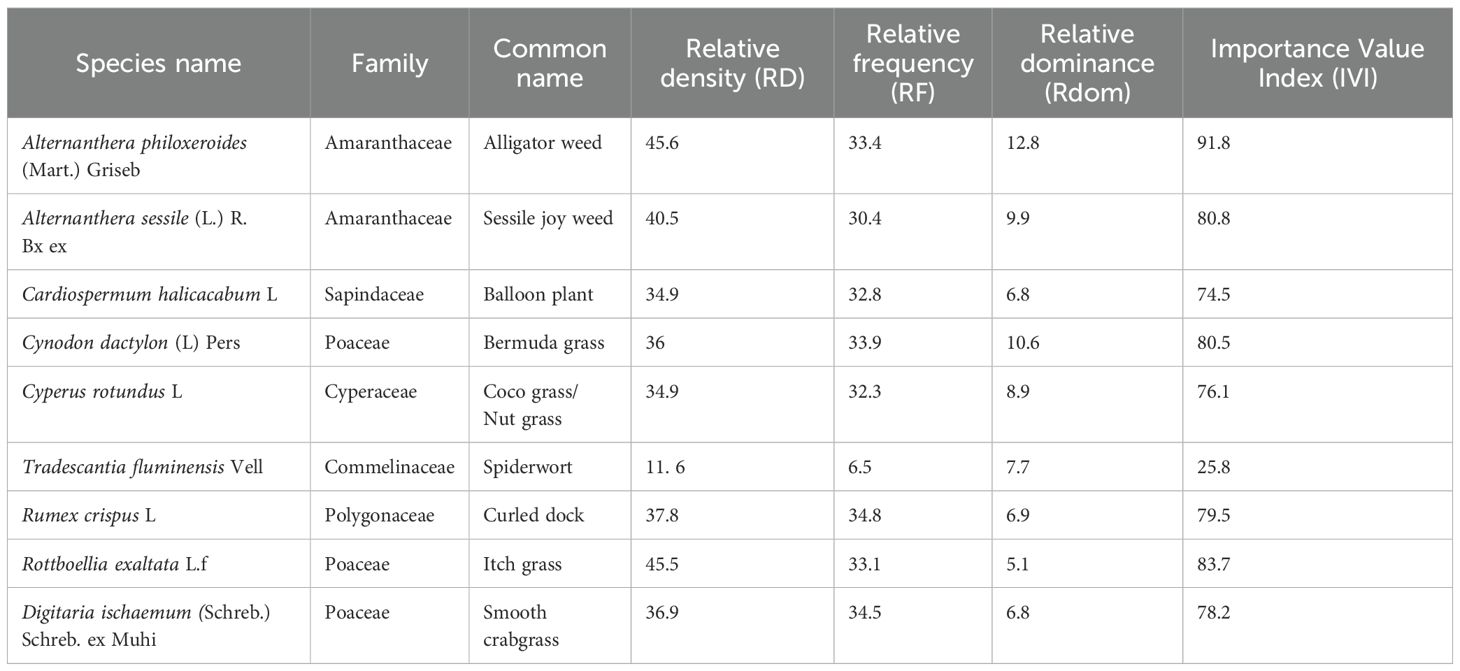
Table 3. Quantitative analysis of Phyto-sociological data of Alternanthera philoxeroides (Mart.) Griseb and its associate plants with families in Site 2.
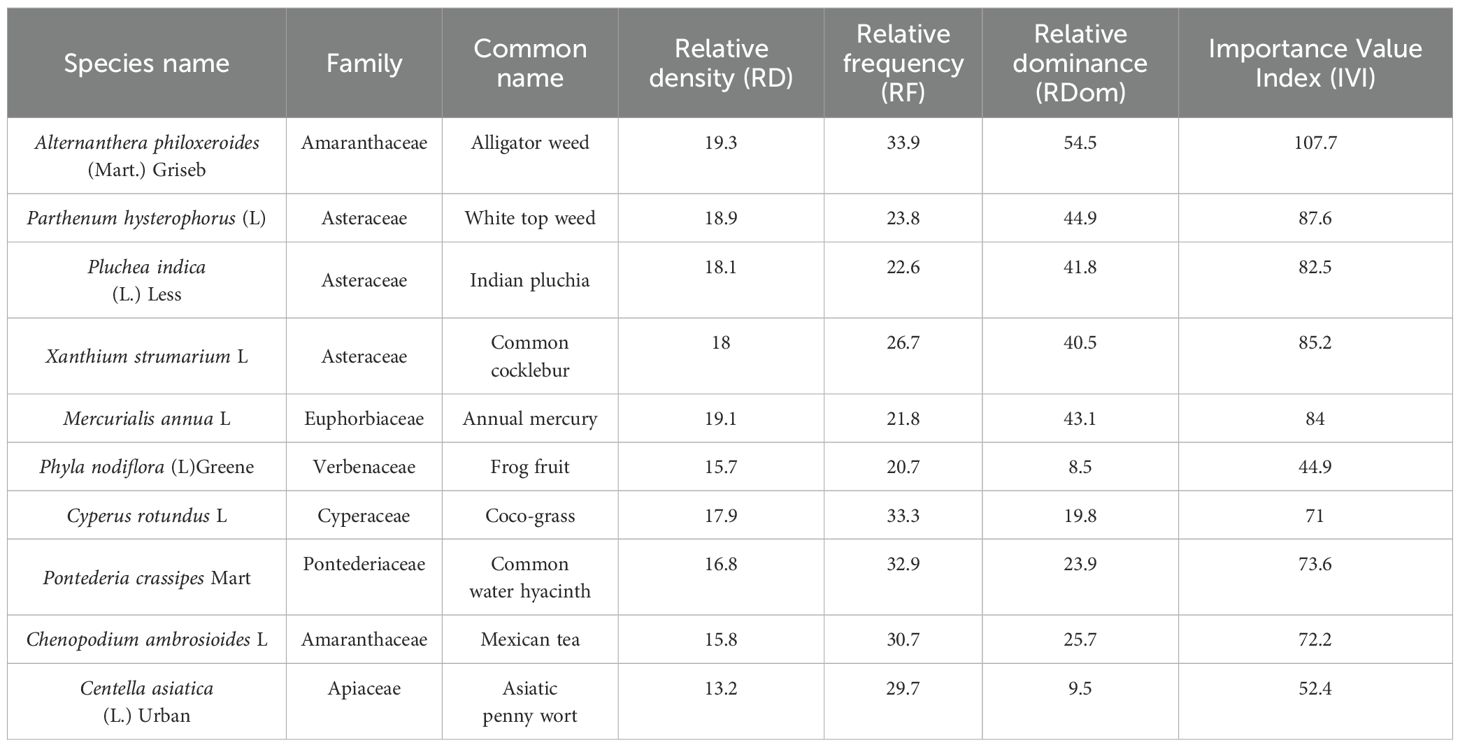
Table 4. Quantitative analysis of Phyto-sociological data of Alternanthera philoxeroides (Mart.) Griseb and its associate plants with families in Site 3.
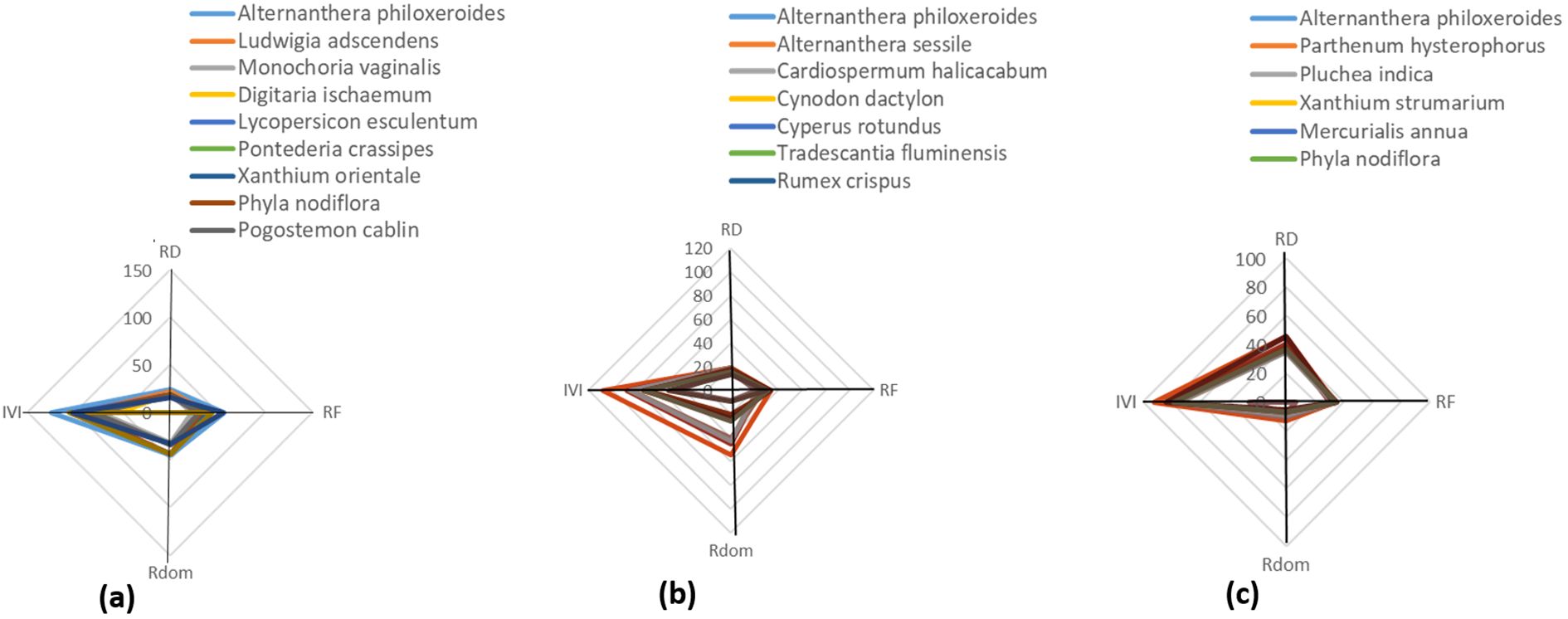
Figure 4. Polygonal phytographs representing plant community structures and phyto-sociological dynamics at three distinct sites (a–c) in Panikhaiti Gaon, Kamrup (Metropolitan), Assam, with a focus on Alternanthera philoxeroides (Mart.) Griseb. (a) Site 1: This site exhibited the highest ecological dominance of A. philoxeroides with an IVI of 125.5. The community included 11 species across 9 families. (b) Site 2: Comprising 10 species from 6 plant families, this site was characterised by a greater presence of grass and herbaceous species and A. philoxeroides had a moderate IVI. (c) Site 3: The plant community here consisted of 10 species across 7 families. Although A. philoxeroides remained a prominent species, its IVI was lower than at Site 1.
The associated plant families at each site varied in composition and dominance.
● Site 1: Amaranthaceae (2), Onagraceae (1), Pontederiaceae (2), Poaceae (1), Solanaceae (1), Asteraceae (1), Verbenaceae (1), Lamiaceae (1), Rubiaceae (1)
● Site 2: Amaranthaceae (2), Sapindaceae (1), Poaceae (3), Cyperaceae (1), Commelinaceae (1), Polygonaceae (1)
● Site 3: Amaranthaceae (2), Asteraceae (3), Euphorbiaceae (1), Verbenaceae (1), Cyperaceae (1), Pontederiaceae (1), Apiaceae (1)
Additionally, ecological diversity across the three sites was assessed using Simpson’s Diversity Index (D), Species Richness Index (Dmg), and Pielou’s Evenness Index (I). Notable variation was observed in species richness and evenness across sites. Site 1 exhibited the highest richness (Dmg = 3.45) but lowest evenness (I = 0.21), suggesting dominance by a few species. In contrast, Site 3 showed lower richness (Dmg = 1.78) but higher evenness (I = 0.63), implying a more balanced species distribution despite fewer taxa. In terms of overall ecological diversity, Shannon’s diversity index (H) and Simpson’s index (D) also showed site-specific variation (Table 5, Figure 5), though with smaller effect sizes compared to IVI and evenness. Site 1, despite having the highest species richness, exhibited low evenness and diversity, suggesting dominance by A. philoxeroides. In contrast, Site 3 showed a more balanced species distribution, likely influenced by reduced human activity and less soil disturbance near the temple site.
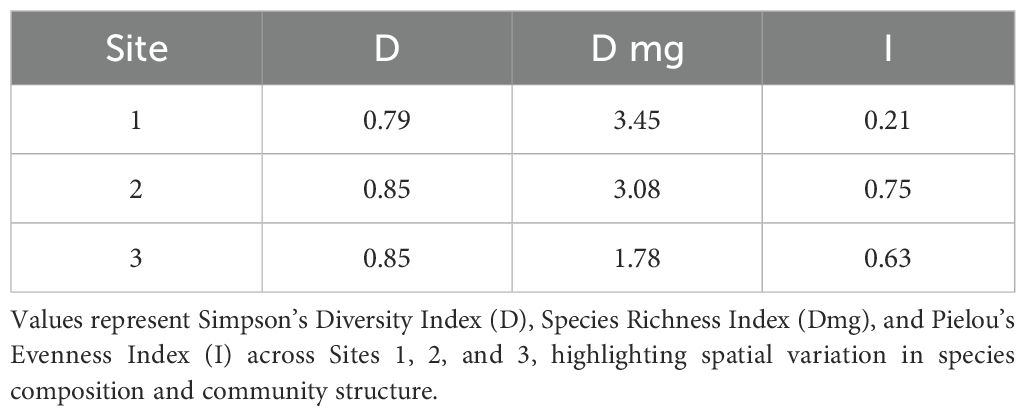
Table 5. Site-wise ecological diversity indices for A. philoxeroides and associated plant communities in Panikhaiti Gaon.
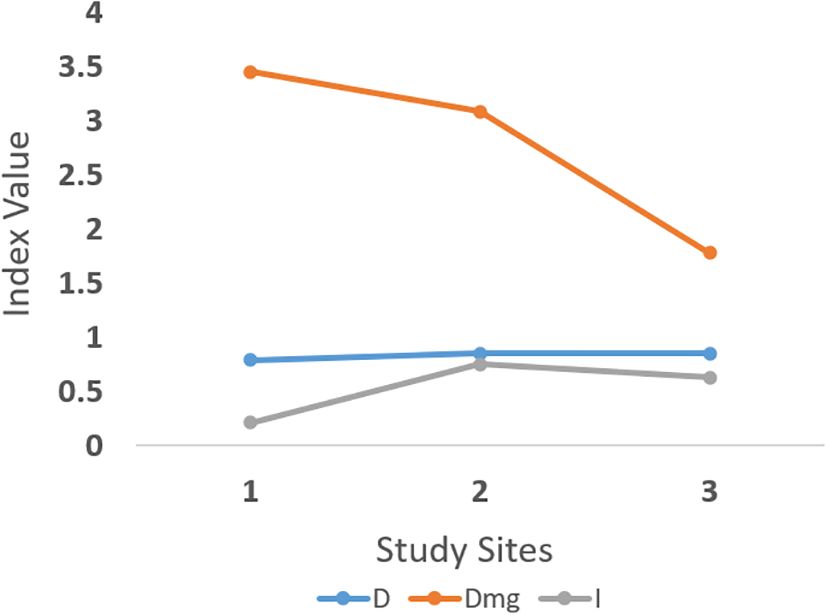
Figure 5. Comparison of ecological diversity indices across three surveyed sites. Simpson’s Diversity Index (D), Species Richness Index (Dmg), and Pielou’s Evenness Index (I) were calculated to assess biodiversity. Species richness (Dmg) shows a decreasing trend from Site 1 to Site 3, while diversity (D) and evenness (I) remain relatively stable. These metrics highlight spatial variation in biodiversity, possibly influenced by local ecological conditions and land-use patterns.
While statistical analysis could not be applied due to single-value summaries, these trends point to meaningful differences in plant community structure across sites. These values are compiled in Table 5 and visualised in Figure 5. Interestingly, several of the associated species were found to be of ethnobotanical relevance, some being used as leafy vegetables, while others were employed in traditional medicine by the local communities for treating a range of ailments.
One limitation of the present study is the absence of uninvaded control plots to assess directly the ecological impact of A. philoxeroides. Due to the widespread and patchy distribution of the plant across the study area, locating ecologically comparable sites free from its colonisation was not feasible. However, site-to-site comparisons revealed clear gradients in community composition, species richness, and dominance patterns, allowing for a relative assessment of its ecological influence. Future studies may benefit from a Before–After–Control–Impact (BACI) design or long-term monitoring of restored or managed sites where A. philoxeroides has been actively removed or suppressed, to capture its ecological footprint more explicitly.
Ethno-medicinal documentation
Ethno-medicinal data were documented through structured questionnaires and in-person discussions with local residents and traditional herbal practitioners of Panikhaiti Gaon. These offline conversations helped to preserve and record valuable indigenous knowledge. The demographic details of the participants involved in this study are presented in Table 6, and their distribution is illustrated in Figure 6. The Sankey plot reveals a diverse age and occupational composition among the 45 respondents from Panikhaiti Gaon. Participants spanned multiple age categories, with notable representation from both younger (students) and older (herbal healers and elders) generations. The occupation-based categorisation included housewives, domestic workers, shopkeepers, and traditional healers, indicating that ethnomedicinal knowledge is maintained and transmitted across a broad social spectrum. This diversity implies towards the communal nature of traditional knowledge.
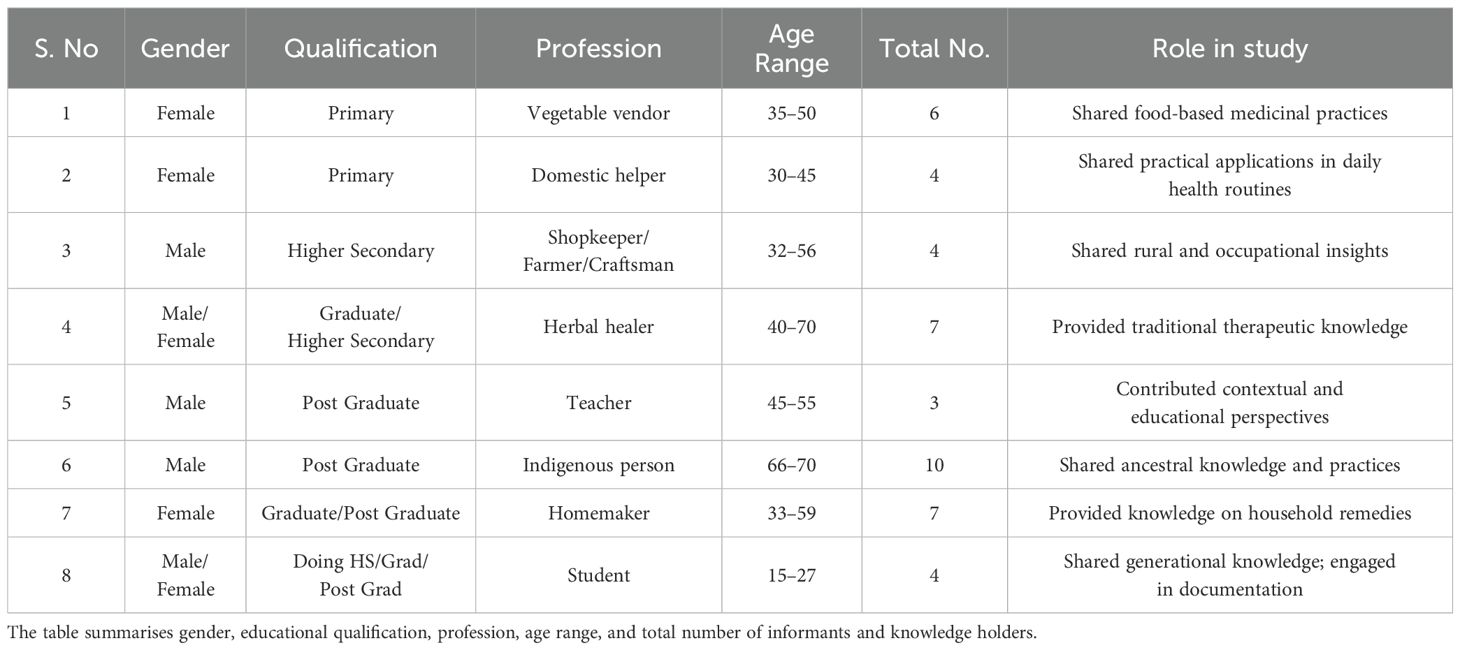
Table 6. Demographic profile of participants from Panikhaiti Gaon, Kamrup (Metropolitan), Assam, involved in the documentation of ethno-medicinal practices.
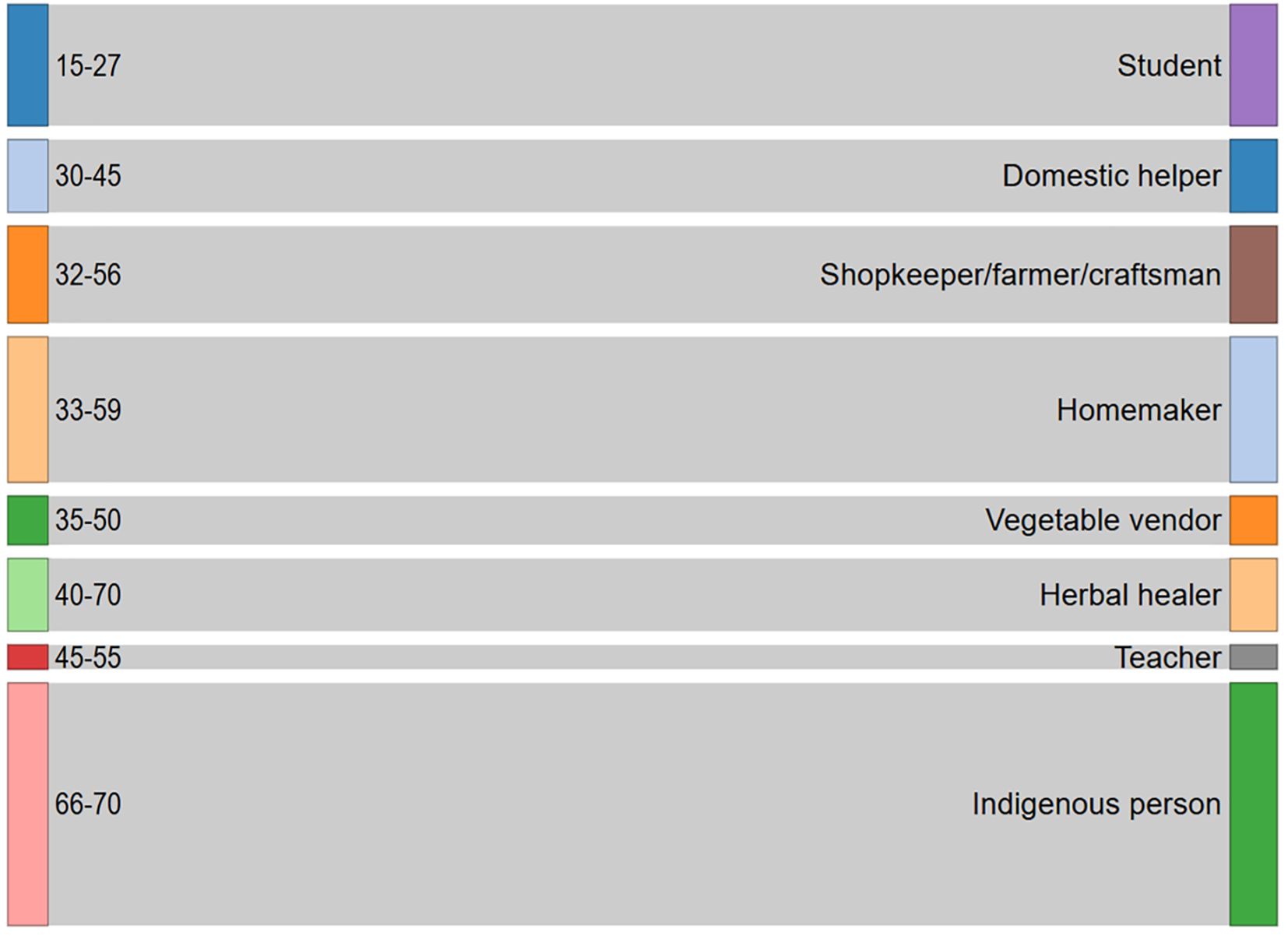
Figure 6. Sankey plot representing the demographic distribution of participants involved in the ethno-medicinal study conducted in Panikhaiti Gaon. The left-hand bars indicate age ranges of the participants, while the right-hand bars denote their primary occupations or social roles. The flow between age and occupation highlights the diversity of individuals contributing to the preservation and transmission of indigenous knowledge. These insights were gathered through structured questionnaires and personal interviews with local residents and traditional practitioners.
In addition to field interactions, responses were also collected through an online Google form, which helped document details such as which part of the plant is used, the method of preparation, and specific ailments treated. These ethnomedicinal insights, collected via the cloud-based survey from 26 respondents aged 18 to 70, are compiled in Table 7.

Table 7. Ethnomedicinal knowledge and traditional practices associated with Paani Matikaduri (Alternanthera philoxeroides) among respondents from Panikhaiti Gaon, Kamrup (Metropolitan), Assam, based on structured questionnaire data.
Figures 7a-c represents a set of pie charts summarising the core ethnomedicinal knowledge shared by respondents.
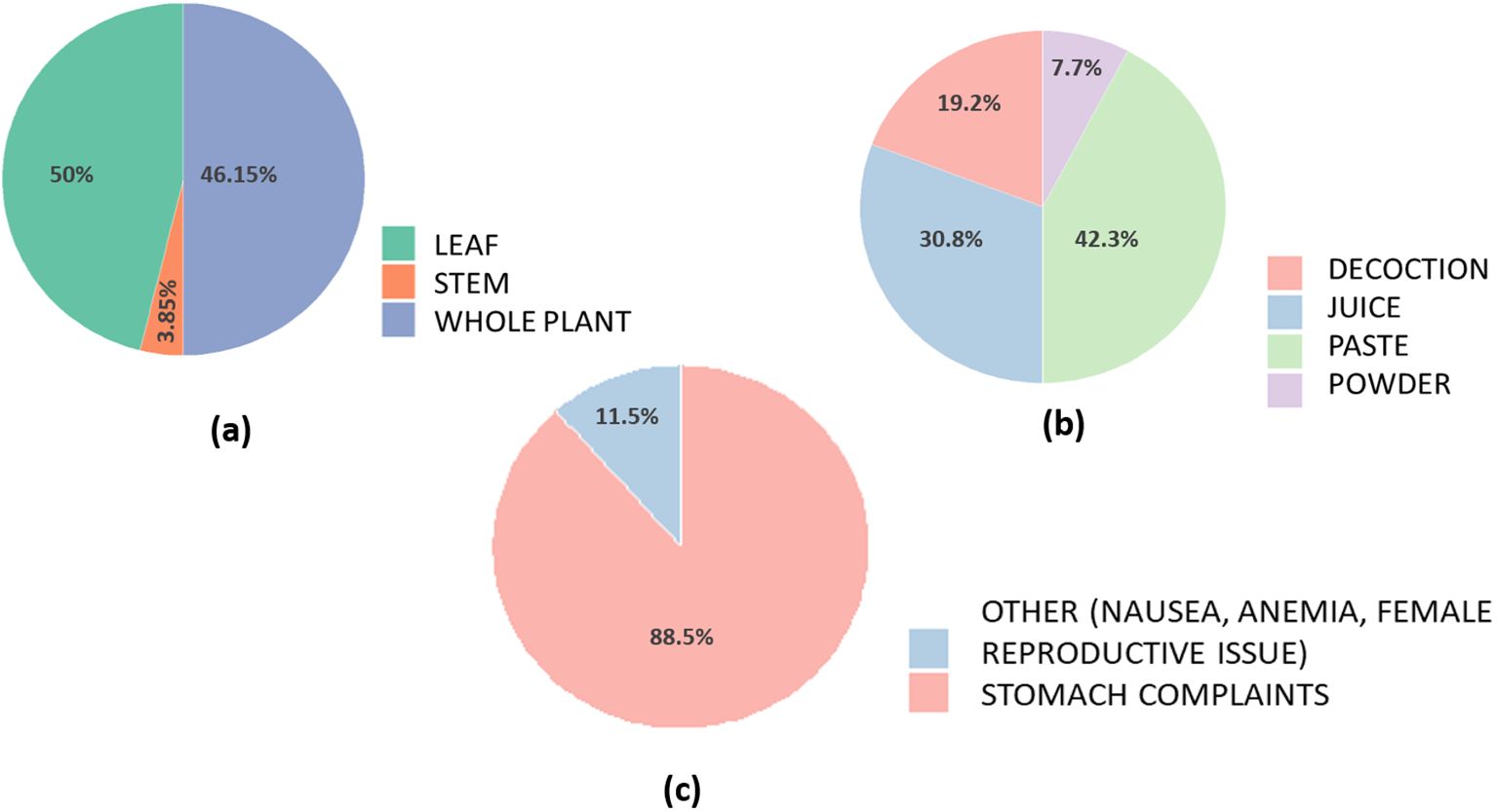
Figure 7. Ethnomedicinal knowledge and traditional practices related to A. philoxeroides among respondents in Assam. (a) Plant part used: Respondents most commonly utilised the whole plant, followed by leaves, and to a lesser extent, the stem, highlighting a preference for holistic use in traditional remedies. (b) Mode of preparation: The plant is primarily prepared as a paste or juice, with some also using decoctions or powder. (c) Ailments treated: The plant is predominantly used for stomach-related issues, while a few responses also indicate its use in treating nausea, anemia, and female reproductive health problems.
The residents of Panikhaiti Gaon actively use A. philoxeroides in the treatment of a range of ailments. Most commonly, the plant is used to manage gastrointestinal issues such as diarrhoea, jaundice, and appetite loss. It is also applied in cases of anaemia, asthma, and acute cough, with several individuals confirming its effectiveness. Many participants described the herb as highly beneficial and said they would recommend its use to future generations.
Different parts of the plant, leaves, stems, and roots, are used either independently or in combination. These parts are often processed into juice, paste, or decoction form, depending on the condition being treated. The majority of participants indicated the use of the whole plant (50%) in traditional remedies, followed closely by the leaves (46%), and to a lesser extent, the stem (4%). This preference underscores a holistic approach to plant usage in local healing systems. The most common methods of preparation included paste (42%) and juice (31%), with some respondents also reporting the use of decoctions (19%) and powdered forms (8%).
Traditional herbal healers stressed the importance of considering patient-specific factors, such as age, overall health status, and the duration of illness, for safe and effective treatment. Interestingly, the data suggests that adults and older individuals in the community are generally more informed and experienced in traditional medicinal practices.
Ethnobotanical uses- culinary applications of A. philoxeroides
In addition to its medicinal importance, A. philoxeroides holds a place in the local cuisine of ethnic communities in Assam. The leaves, stems, and sometimes even the roots of this plant are regarded as edible, and are used in various traditional dishes, particularly during periods of food scarcity. Communities in and around Panikhaiti Gaon prepare this wild edible plant by steaming its leaves and stems or consuming it as a spinach-like green alongside staple meals. Owing to its high nutritional and proteinaceous content, the plant is considered a wholesome dietary component. However, informants also noted that frequent or excessive consumption could potentially lead to mild digestive discomfort, suggesting a need for moderation. Traditional dishes include Alligator weed curry prepared with locally available freshwater fish species such as Magur (Clarias batrachus), Sol (Channa striata), and Kawai (Anabas testudineus), all of which are delicacies in Assamese households. In some regions, a variation of pork curry is also prepared using A. philoxeroides, blending indigenous flavors with seasonal availability. These culinary practices reflect the adaptive use of local biodiversity within the community, extending the relevance of A. philoxeroides from ecological concern to a culturally embedded resource.
Discussion
The use of Alternanthera philoxeroides in traditional medicine, in rural and indigenous communities, has been extensively explored. In Assam, particularly in Panikhaiti Gaon, the concept of A. philoxeroides holds cultural significance in the treatment of gynecological issues. Local women use it as part of home-based remedies, preserving an oral tradition passed down through generations. Similar practices have been reported across Asia and South America, where the plant is utilised for its antimicrobial, anti-inflammatory, and wound-healing properties (Tanveer et al., 2018; Wang et al., 2025).
In one study, the leaves of A. philoxeroides were found to contain secondary metabolites like flavonoids and phenolic acids, which contribute to their medicinal potential (Wang et al., 2025). This underscores the value of documenting such community-based plant knowledge, especially in light of increasing ecological threats to these resources.
While it has important traditional uses, A. philoxeroides is also a highly invasive species. It shows significant adaptability to various environments, terrestrial and aquatic, and can outcompete native flora. Its ecological behavior is influenced by water level, nutrient content, and soil characteristics, which makes it particularly successful in wetlands and floodplains. Research shows that under flooding conditions, the plant alters soil organic matter and microbial activity, which may impact surrounding biodiversity and soil health. Therefore, any medicinal use must be balanced with its ecological management. The disparity in species richness and evenness among the three sites may reflect underlying differences in habitat quality, disturbance levels, or competitive effects of A. philoxeroides. These preliminary findings support the need for quadrat-level replication and long-term ecological monitoring to confirm patterns through statistical testing.
Although ethnobotanical studies in India rarely explore A. philoxeroides as a food source, culinary uses have emerged in folk practices. Other recipes use the tender shoots as a green vegetable or in combination with potato and mustard paste, as seen in social media-based food documentation. Scientific interest in its edible potential is growing, particularly due to its fast-growing biomass and nutrient-rich profile, though more safety and toxicity studies are needed.
This study contributes valuable insight into the lesser-known traditional and ecological knowledge of A. philoxeroides. The contrasting roles of A. philoxeroides; as both a disruptive coloniser and a source of therapeutic value, underscore the need for integrative frameworks that consider ecological, pharmacological, and socio-cultural dimensions. Future studies would benefit from long-term monitoring of its ecological impact and integrating local knowledge into efficient management strategies.
The cultural significance of A. philoxeroides in Assamese communities indicates the need to further explore and document indigenous health systems. With a large population of Indian population being dependent on plant-based traditional prescriptions (Dey et al., 2021), these practices deserve scientific recognition and protection under biodiversity conservation and indigenous rights frameworks.
Conclusion
The high Important Value Index (IVI) of Alternanthera philoxeroides across all three study sites highlights its strong regrowth potential and ecological adaptability. Our ecological survey, encompassing parameters such as density, frequency, relative dominance, Simpson’s Diversity Index, species richness (Dmg), and Pielou’s Evenness Index, offers comprehensive insights into the species’ role within its plant community. These data underscore the importance of integrating ecological approaches in understanding and managing the growth dynamics of this species.
“Paani Matikaduri”, as it is known locally in Assam, holds significant ecological and ethnomedicinal value. Its affordability, adaptability, and cultural importance make it a promising candidate for both conservation and domestication. Ensuring its sustainable use requires targeted interventions, such as educating local farmers on nutrient management.
● To promote conservation and sustainable utilisation, we recommend the following:
● Awareness programs to preserve and transmit traditional knowledge
● Protected cultivation through home gardens or agroecological methods
● Tissue culture and propagation techniques for large-scale multiplication
● Integration of digital tools for biodiversity monitoring and conservation tracking
In conclusion, the ecological resilience and community-level utility of A. philoxeroides point towards the importance of evidence-based, locally informed strategies which balances sustainable use with invasive species control.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements, as it posed no risk to participants and did not collect sensitive personal information. Verbal informed consent was obtained from all participants prior to their involvement in the study, and participation was entirely voluntary. No personal identifiers were recorded.
Author contributions
NS: Writing – original draft, Visualization, Data curation, Formal analysis, Conceptualization, Methodology, Writing – review & editing, Validation. GD: Methodology, Validation, Conceptualization, Supervision, Writing – review & editing, Funding acquisition, Resources, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to the Department of Botany, Assam down town University, Panikhaiti, Guwahati, Assam, India for proving the facilities to carried out this work. The authors are grateful to the local community members of Panikhaiti Gaon, Kamrup (M), Assam, for their invaluable cooperation and willingness to share indigenous knowledge throughout the field surveys. The authors gratefully acknowledge Dr. Joorie Bhattacharya, Research Fellow, ICRISAT, India, for her valuable guidance during data analysis, insightful feedback on the manuscript and meticulous proof reading. We also sincerely thank the editors and reviewers for their constructive comments and suggestions, which greatly helped improve the quality and clarity of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bourke C. A. and Rayward D. (2003). Photosensitisation in dairy cattle grazing alligator weed (Alternanthera philoxeroides) infested pastures. Aust. Veterinary J. 81, 361–362. doi: 10.1111/j.1751-0813.2003.tb11515.x
Cunningham A. B. (Ed.) (2001). Applied ethnobotany: people, wild plant use and conservation. 1st ed (London, UK: Routledge).
Dey S., Das D., Chakraborty A., Roychoudhury S., Choudhury B. P., Choudhury A. P., et al. (2021). “Plant-based traditional herbal contraceptive use in India: safety and regulatory issues,” in Evidence based validation of traditional medicines: a comprehensive approach, Singapore: Springer Singapore 659–675.
Dupont P. (1989). An unpublished discovery by Emile Contre: Althernanthera philoxeroides in the Garonne Valley. Bulletin of the Botanical Society of the Centre-West, Niort, France, 20, 27–28.
EPPO (2016). Alternanthera philoxeroides (Mart.) griseb. EPPO Bulletin / Bulletin OEPP, 46(1), 8–13. doi: 10.1111/epp.12275
Farooq N., Mehmood A., Tanveer A., Nadeem M. A., Sarwar G., Abbas T., et al. (2021). Exploiting fodder and nutritive value of Alternanthera philoxeroides (alligator weed) at different times of harvest: a potential fodder weed. Pak J. Bot. 53, 1101–1106. doi: 10.30848/PJB2021-3(40)
Geng Y. P., Pan X. Y., Xu C. Y., Zhang W. J., Li B., and Chen J. K. (2007). Phenotypic plasticity and local adaptation in invasive Alternanthera philoxeroides. Biol. Invasions 9, 717–727. doi: 10.1007/s10530-006-9075-4
Humane P. T., Somkuwar S. R., and Chaturvedi A. (2015). Extending Habitat of an exotic aquatic weed Alternanthera philoxeroides (Mart) Griseb.in Maharashtra, India. IJRBAT. 6, 117–119. Available online at: http://www.vmsIndia.org. (Accessed February 2, 2025).
Jayasinghe H. (2008). Please don’t eat mallung leaf lookalike (Colombo, Sri Lanka: Sundaytimes). Available online at: http://www.sundaytimes.lk/080504/News/news0013.html.
Julien M. H. and Bourne A. S. (1988). Alligator weed is spreading in Australia. Plant Prot. Q. 3, 91–96. Control, Wallingford (GB).
Julien M. H. and Stanley J. N. (1999). The management of alligator weed, Alternanthera philoxeroides, in Australia: A review. Environ. Manage. 23, 453–465. doi: 10.1007/s002679900198
Kay S. H. and Haller W. T. (1982). Evidence for the existence of distinct Alligator weed biotypes. J. Aquat. Plant Manage. 20, 37–41.
Kleinowski A. M., Ribeiro G. A., Milech C., and Braga E. J. B. (2016). Potential allelopathic and antibacterial activity from Alternanthera philoxeroides. Hoehnea 43, 533–540. doi: 10.1590/2236-8906-26/2016
Margalef R. (1951). Diversidad de especies en las comunidades naturales. Publicaciones del Instituto Biología Aplicada 9, 5–27.
Nahar L., Nath S., and Sarker S. D. (2022). Malancha” [Alternanthera philoxeroides (Mart.) Griseb.]: a potential therapeutic option against viral diseases. Biomolecules 12(4), 582. doi: 10.3390/biom12040582
Office of the Registrar General & Census Commissioner, India (2011). Census of India 2011: primary census abstract data (Government of India: Ministry of Home Affairs, Government of India). Available online at: http://www.censusIndia.gov.in. (Accessed January 25, 2025).
Pielou E. C. (1966). The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144. doi: 10.1016/0022-5193(66)90013-0
Pramod K., Sanjay M., and Satya N. (2008). Alternanthera philoxeroides (Mart.) Griseb. An addition to Uttar Pradesh. J. Indian Botanical Soc. 87, 285–286.
Rane N. R., Chandanshive V. V., Watharkar A. D., Khandare R. V., Patil T. S., Pawar P. K., et al. (2015). Phytoremediation of sulfonated Remazol Red dye and textile effluents by Alternanthera philoxeroides: an anatomical, enzymatic and pilot scale study. Water Res. 83, 271–281. doi: 10.1016/j.watres.2015.06.046
Roberts L. I. N. and Sutherland O. R. W. (1986). “) Alternanthera philoxeroides (C. Martius) Grisebach, Alligator weed (Amaranthaceae),” in A review of biological control of invertebrates pests and weeds in New Zealand 1874 to 1987. Eds. Cameron P. J., Hill R. L., Bain J., and Thomas W. P. (Wallingford, United Kingdom: CAB International Institute of Biological), 325–330.
Shannon C. E. (1948). A mathematical theory of communication. Bell system Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Shannon C. E. and Weaver W. (1949). The mathematical theory of communication (USA, University of Illinois Press).
Simmons Z. D., Suleiman A. A., and Theegala C. S. (2007). Phytoremediation of arsenic and lead using alligator weed (Alternathera philoxeroides). Trans. ASABE 50, 1895–1900. doi: 10.13031/2013.23929
Sosa A. J. and Julien M. H. (2010). Alternanthera philoxeroides (Mart.) Griseb. (Alligator weed) (Wallingford, Oxfordshire, UK: CABI Compendium).
Tanveer A., Ali H. H., Manalil S., Raza A., and Chauhan B. S. (2018). Eco-biology and management of alligator weed [Alternanthera philoxeroides) (Mart.) griseb.]: a review. Wetlands 38, 1067–1079. doi: 10.1007/s13157-018-1062-1
Tao Y., Chen F., Wan K., Li X., and Li J. (2009). The structural adaptation of aerial parts of invasive alternanthera philoxeroides to water regime. J. Plant Biol 52, 403–410. doi: 10.1007/s12374-009-9051-9
Tardío J. and Pardo-de-Santayana M. (2008). Cultural importance indices: A comparative analysis based on the useful wild plants of southern cantabria (Northern Spain)1. Econ Bot. 62, 24–39. doi: 10.1007/s12231-007-9004-5
Tongco M. D. C. (2007). Purposive sampling as a tool for informant selection. Ethnobotany Res. Appl. 5, 147–158. doi: 10.17348/era.5.0.147-158
Wang Y., Li C., Zhao Y., Liu X., Wang Y., and Liu J. (2025). Effects of herb Alternanthera philoxeroides invasion on soil organic matter varied with flooding conditions in wetlands. Plant Soil, 1–13. doi: 10.1007/s11104-025-07282-0
Wang N., Yu F. H., Li P. X., He W. M., Liu F. H., and Dong M. (2008). Clonal integration supports the expansion from terrestrial to aquatic environments of the invasive herb Alternanthera philoxeroides. Plant Ecol. 199, 235–242. doi: 10.1007/s11258-008-9431-7
Keywords: Alternanthera philoxeroides (Mart) Griseb., ecological status, alligator weed, indigenous knowledge, Panikhaiti Gaon
Citation: Sharma N and Das G (2025) An approach to ecological balance and indigenous knowledge of Alternanthera philoxeroides (Mart.) Griseb in Panikhaiti village of Kamrup (Metropolitan) district of Assam. Front. Conserv. Sci. 6:1615630. doi: 10.3389/fcosc.2025.1615630
Received: 21 April 2025; Accepted: 21 July 2025;
Published: 13 August 2025.
Edited by:
Qingqing Cao, Shandong Jianzhu University, ChinaReviewed by:
Necmi Aksoy, Duzce University, TürkiyeAulia Ulmillah, Raden Intan State Islamic University, Indonesia
Copyright © 2025 Sharma and Das. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gunamoni Das, Z3VuYW1vbmkwOUBnbWFpbC5jb20=
 Nandini Sharma
Nandini Sharma Gunamoni Das
Gunamoni Das