- 1Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, Serdang, Selangor Darul Ehsan, Malaysia
- 2International Institute of Aquaculture and Aquatic Sciences (I-AQUAS), Universiti Putra Malaysia, Port Dickson, Negeri Sembilan, Malaysia
- 3Retired, Kuala Lumpur, Malaysia
Halophila beccarii Aschers. is a small, vulnerable seagrass species found throughout the Indo-West Pacific. In Malaysia, its distribution is highly fragmented and faces increasing threats from human activities and natural disturbances. Although it can tolerate various ecological conditions, its limited and scattered range raises significant conservation concerns. Long-term observations and monitoring were conducted from 1996 to 2025 at numerous coastal and estuarine sites within Peninsular Malaysia, Sabah, and Sarawak. Historical data and recent field surveys were combined to assess changes in distribution, population status, and habitat conditions. Halophila beccarii, once considered rare, was found at 15 locations across various intertidal habitats, ranging from freshwater to the brackish waters of mangrove river systems, shallow coastal lagoons, marine coastal areas, and subtidal shoals. It is commonly observed at depths of 1.0 to 2.0 meters and rarely exceeds 3.0 meters during high tide. The species thrives in temperatures from 25 to 38°C and salinity levels from 0 to 32 psu. Scattered patches or monospecific meadows of H. beccarii often occur alongside other seagrasses, such as Halodule pinifolia, macroalgae, seaside grasses, or mangroves, on substrates including sand, silty sand, loam, and calcareous mud. Declines are driven by reclamation, dredging, sand mining, sedimentation, monsoonal flooding, and coastal erosion. Disturbed habitats often shift to dominance by H. pinifolia, a more resilient species. These findings highlight H. beccarii’s vulnerability despite its ecological adaptability, reinforcing its current IUCN “Vulnerable” status and emphasizing the need for targeted conservation, seed bank protection, and adaptive monitoring strategies.
1 Introduction
Seagrasses are underwater marine angiosperms from the class Monocotyledoneae. They are the only group of flowering plants (monocotyledonous angiosperms) that have successfully adapted to life in marine and estuarine environments. Unlike algae, seagrasses possess true vascular tissues, including roots, stems, and reproductive organs such as flowers and seeds, allowing them to complete their entire life cycle submerged in seawater. Seagrasses play essential ecological roles by stabilizing sediments, improving water quality, cycling nutrients, and serving as vital nursery grounds for many marine organisms (Orth et al., 2006; Duarte, 2002). Evolutionary, they are thought to have derived from terrestrial monocots that recolonized the sea approximately 100 million years ago (Les et al., 1997). Globally, about 72 species are currently recognized, distributed in tropical and temperate shallow waters (Short et al., 2007).
In Malaysia, they form patches to meadows, usually growing in shallow intertidal riverbanks, subtidal rivers, inland lagoons, and around islands. Malaysia has 17 seagrass species; Enhalus acoroides, Thalassia hemprichii, Halophila beccarii, H. decipiens, H. major, H. minor, H. ovalis, H. nipponica, H. spinulosa, H. tricostata, Cymodocea rotundata, C. serrulata, Halodule pinifolia, H. uninervis, Syringodium isoetifolium, Thalassadendron ciliatum, and Ruppia maritima (Fortes et al., 2018; Muta Harah and Japar Sidik, 2024; Che Alias et al., 2024). Of the 17 seagrass species, H. beccarii has a disconnected and scattered distribution that faces mounting threats from human activities and natural disturbances (Japar Sidik and Muta Harah, 2011a; Japar Sidik et al., 2018).
Halophila beccarii is a monoecious, i.e., with male and female flowers either on the same erect shoot or separate shoots of the same plant. Its distribution is limited, found in intertidal sand and muddy areas of Korea Island, Penang, the northern Peninsula of Malaysia (Muta Harah and Japar Sidik, 2004), as well as in muddy riverbanks of the Pendas and Johor Rivers at Kampong Pendas in southern Johor (Muta Harah, 2023). On the east coast of Peninsula Malaysia, it is in shallow intertidal sand and mud areas within inland lagoons of Pengkalan Nangka in Kelantan, Paka, Kemaman, and Telaga Simpul in Terengganu. Halophila beccarii is commonly associated with mangroves. In riverine environments, H. beccarii experiences stable salinity levels ranging from 20 to 24 psu. Conversely, lagoon habitats showed significant salinity fluctuations, ranging from low levels of 0 to 0.5 psu during the monsoon season to brackish water conditions with salinity levels between 11 and 19 psu during the dry season (Japar Sidik et al., 2018). In Sarawak, East Malaysia, H. beccarii thrives in small patches located at the forefront of mangroves on muddy substrates.
Globally, H. beccarii is distributed across the Indo-West Pacific region and is widely reported from India (Untawale and Jagtap, 1977; Parthasarathy et al., 1988), Sri Lanka (Udagedara et al., 2017), Bangladesh (Billah et al., 2016; Hena et al., 2009), Malaysia (Fakhrulddin et al., 2013; Muta Harah et al., 1999; 2002), the Philippines (Liao and Geraldino, 2020), Thailand (Hiranphan et al., 2020), Myanmar (Aye et al., 2014), and Vietnam (Phan et al., 2017). It has also been recorded in the Andaman and Nicobar Islands (Savurirajan et al., 2015) and is described as one of the earliest colonizers in mangrove succession (Parthasarathy et al., 1988, Parthasarathy et al., 1991; Jagtap, 1991). Halophila beccarii tolerates high nutrient environments and fluctuating conditions in temperature, salinity, and tidal inundation (Greve and Binzer, 2004; Fakhrulddin et al., 2013). Despite its wide ecological tolerance, its distribution remains narrow and fragmented, with the IUCN Red List Categories and Criteria Version 3.1 classifying the species as Vulnerable (IUCN, 2011; Short et al., 2011; Geng et al., 2022), while some national assessments, such as in Sri Lanka, report it as Endangered or locally extinct in parts of the Philippines (Udagedara et al., 2017; Liao and Geraldino, 2020).
This research project on the inventory of marine plants and associated organisms is part of the Intensification in Priorities Research, funded by the Ministry of Science, Technology, and Environment Malaysia, the Japan International Cooperation Agency (JICA), the Japan Society for the Promotion of Science (JSPS), and the Forest City project. The taxonomic identification of H. beccarii was based on the criteria established by den Hartog (1970). This paper provides a historical perspective on the discovery and distribution of H. beccarii in Peninsular Malaysia and Sarawak, East Malaysia, drawing on both previous and current records. The significance of H. beccarii seagrass areas and the habitat disruption caused by natural factors and human activities affecting H. beccarii are also explored.
2 Materials and methods
Observation and in-situ field data were collected from February 1996 to April 2025 across multiple coastal locations in Peninsular Malaysia and Sarawak. From February 1996 to January 1999, three key sites, Sungai Kemaman, Sungai Paka Lagoon, and Pengkalan Nangka Lagoon were monitored almost monthly, providing high-resolution temporal data. Other sites were visited 2–5 times during this period for exploratory or confirmatory surveys. From 2011 onwards, long-term monitoring continued for several sites where H. beccarii populations persisted or reappeared. These included Paka Shoal, Pengkalan Nangka Lagoon, Sungai Kemaman, Pulau Korea, and Sungai Pendas, which were revisited one to two times per year up to 2025 to track species persistence and changes in habitat. Surveys were conducted during low to lowest spring tides, when seagrass beds were naturally exposed, or through snorkeling and SCUBA diving in subtidal zones.
The roving method was employed during sampling, using a 50 cm x 50 cm quadrat, to assess community composition. When available, complete plant specimens, including leaves, rhizomes, flowers, and fruits, were collected for morphological analysis. Measurements of morphological features included leaf blade length and width, petiole, and erect stem, taken with vernier calipers, (n=30). The morphometric measurements were analyzed using SPSS Statistics ANOVA to compare between locations. Reproductive structures were examined when present, with additional measurements for pedicel, tepal, anthers, ovary, hypanthium, style, fruits, and seeds. Specimens were preserved in two ways: (1) wet preservation in 5% formaldehyde (diluted from 37–40% commercial formalin at a 1:8 ratio with seawater), and (2) mounted as herbarium specimens following Menez et al. (1983). All site locations were recorded with handheld GPS units to document the spatial distribution of H. beccarii.
Environmental parameters, including temperature (°C), salinity (psu), pH, dissolved oxygen (DO, mg/L), and conductivity (µS/cm), were measured in situ at each site using a YSI 33 SCT meter and a YSI Professional Plus multiparameter instrument (YSI Inc., USA) for measurements collected from 2015 onward. Measurements were taken during both low and high tides, with three replicates per parameter at each station. The instruments were calibrated in the field following the manufacturer’s guidelines. Salinity measurements were conducted during both the monsoon and dry seasons to account for tidal and seasonal variation. In brackish systems such as Sungai Kemaman and Pengkalan Nangka, data collection focused on detecting fluctuations caused by freshwater inflow and tidal mixing. These measurements contributed to assessing the estuarine conditions where H. beccarii was found.
Sediment samples were analyzed using the standard hydrometer method (English et al., 1997), and soil texture was classified based on the USDA Textural Triangle (Gerakis and Baer, 1999). Water depth was estimated using Ebb Low Water Spring (ELWS) tide charts. Disturbances at the sites (e.g., reclamation, infrastructure, breakwaters) were documented with photographs during each survey.
Changes in seagrass extent were evaluated using historical mapping (before 2011) and field surveys conducted between 2015 and 2025. The seagrass area was estimated through GPS tracking of patch boundaries during low tide and aerial imagery, when available. Losses were identified by comparing the current extent to earlier records.
3 Results
3.1 Historical overview and records of Halophila beccarii
Halophila beccarii was first collected in 1867 by Italian botanist Odoardo Beccari from the Bintulu River (now Bintulu), Sarawak, East Malaysia (Beccari, 1904; den Hartog, 1970). This type specimen, later described by Ascherson in 1871, laid the foundation for recognizing H. beccarii, commonly known as Beccari’s seagrass or Ocean’s Turf seagrass, as a member of the Hydrocharitaceae family. Characterized by its rosette-forming, leafy shoots and short, creeping rhizomes, the species is well-adapted to colonize unstable substrates in brackish and estuarine environments (Figure 1).

Figure 1. (A) TYPE specimen of Halophila beccarii Aschers. 1871 in Neumayer, Borneo, Sarawak, in the mouth of the river Bintula, O. Beccari 11826 (=P.B. 3666) (L). The TYPE specimen photo was obtained with permission from the Rijksherbarium, Leiden, the Netherlands. (B) Dense H. beccarii forms thick growth. (C) Halophila beccarii ramets exhibit a modular arrangement. Each ramet is composed of modules, i.e., a piece of rhizome, which can be horizontal or vertical, a pair or bundle of leaves attached to the rhizome, and a root system. (D) Halophila beccarii (shown as green growth on mudflat surface, exposed during low tide) growing in brackish coastal water of the mangrove system.
The storyline of the discovery of H. beccarii in Malaysia (Figure 2) starts with the first collection made by Beccari in 1867 in Sarawak, East Malaysia. In Peninsular Malaysia, subsequent records of H. beccarii collection were noted by den Hartog (1970) from Sungai Tebrau and Jalan Skudai, Johor, in 1965. Our surveys confirmed the species’ presence along the east coast starting in 1992 at the mudflats of Sungai Kemaman, Terengganu. This was followed by successive discoveries in 1996 at Paka Lagoon, Paka River, Paka Shoal, and Chukai, and in 1997 at Telaga Simpul. During this period, field investigations also extended to Kelantan, where H. beccarii was observed at Pengkalan Nangka Lagoon, Pengkalan Nangka Shoal, Kampung Baru Nelayan, and Pantai Baru Lagoon. By 1999, its distribution expanded northward in Penang, observed at Seberang Prai and Korea Island. Rediscovery in Sarawak occurred much later, with documentation in Lawas after a 141-year gap from the original Bintulu collection. In Johor, the species reappeared at Sungai Pendas in 2015 and at Tanjung Surat, Kampung Nyior by 2021 (Figure 3), where it was found in both monospecific and mixed stands with Halophila ovalis.

Figure 2. The timeline of the discovery of seagrass, H. beccarii, associated with the mangrove ecosystem from 1867 to 2021.

Figure 3. New record of H. beccarii in 2015 at (A, B) shallow intertidal, in the mangrove area of Sungai Pendas, Johor, and (C, D) monospecific H. beccarii in 2021 at an intertidal mudflat in front of mangrove Tanjung Surat, Johor.
3.2 The distribution, present status, and disturbances of Halophila beccarii population
Halophila beccarii is mainly found along the east coast of Peninsular Malaysia, with scattered populations also along the southeast, south, and west coasts. This species has been observed in 15 areas, as shown in Figure 4. It is found as either monospecies or two species in the lagoon, subtidal island, intertidal mangrove association, intertidal sandy beach, subtidal shoal, and intertidal areas associated with coral. It occupies the middle to upper intertidal zone, shallow intertidal in the mangrove area, and intertidal mudflat in front of the mangrove.

Figure 4. The distribution of H. beccarii and other seagrass areas, associated habitats, and utilization by coastal communities and other users in Peninsular Malaysia, Sabah, and Sarawak. 1-Lagoon, 2-subtidal island, 3-intertidal mangrove association, 4-intertidal sandy beach, 5-subtidal shoal, 6-intertidal associated with coral. Aquaculturea, turtle sanctuaryb, traditional capture fisheriesc, dugong feeding groundd, and marine parke (Japar Sidik and Muta Harah, 2011b; Muta Harah and Japar Sidik, 2024).
Table 1 shows the changes in distribution and area coverage before 2011 and from 2015 to 2025, highlighting significant population declines or complete losses across many sites. The largest historical populations were recorded in Kemaman (17 ha), Telaga Simpul (28 ha), Paka Shoal (43 ha), and Pengkalan Nangka Lagoon (35 ha). However, many of these sites now show significantly reduced sizes or are completely lost. For example, sites such as Chukai, Paka River, and Kampung Baru Nelayan no longer have any H. beccarii present. Field visits between 2011 and 2025 confirmed the ongoing decline and disappearance of H. beccarii meadows at several locations, while only a few sites, such as Paka Shoal, Telaga Simpul, Pengkalan Nangka Lagoon, Korea Island, and Sungai Pendas, still support small patches.
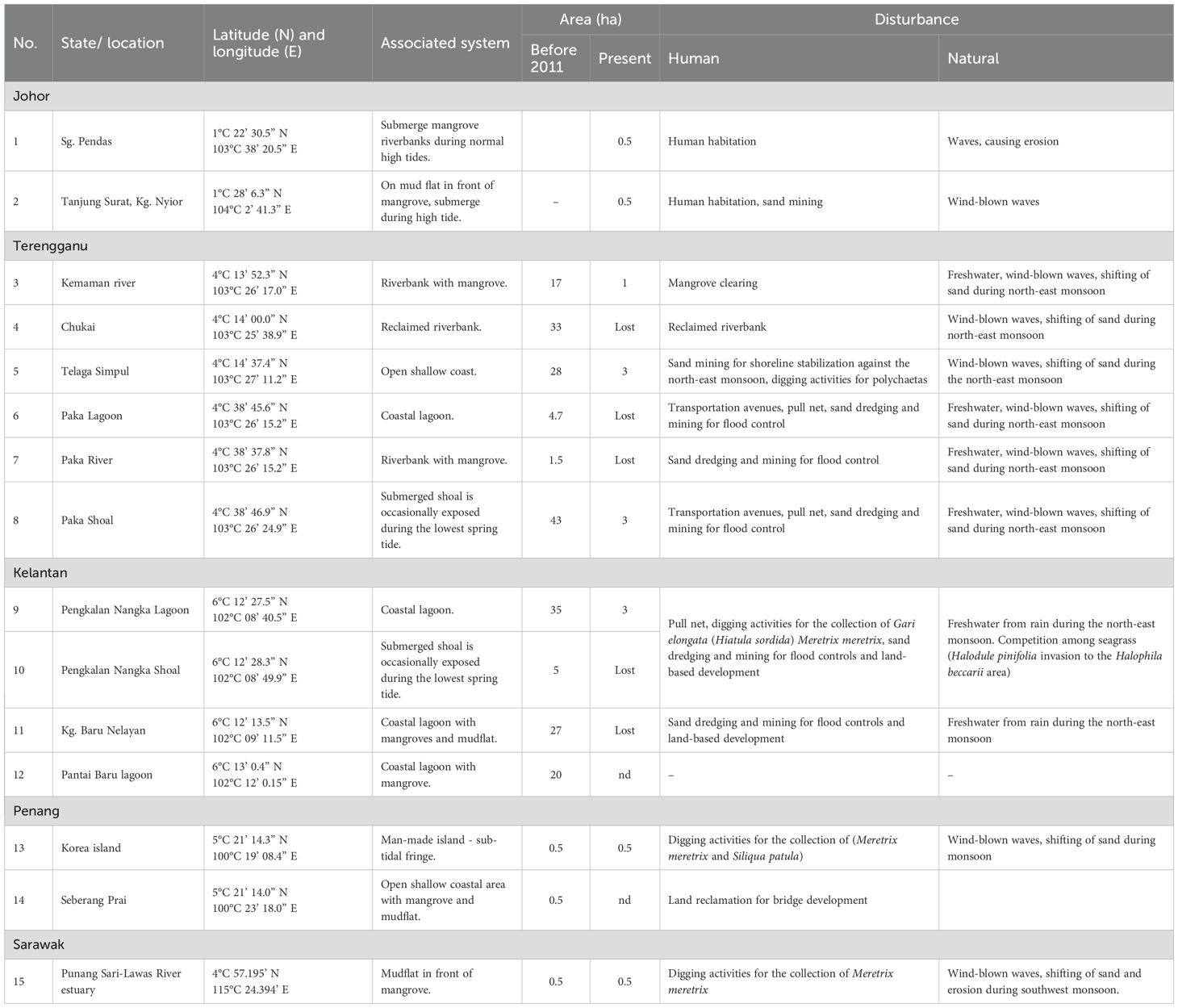
Table 1. Distribution area of Halophila beccarii in Peninsular Malaysia and Sarawak, with associated systems, area of previous and present seagrass, and identified disturbance factors.
The decline results from both human and natural disturbances. Human pressures include riverbank reclamation at Chukai, mangrove clearing at Kemaman, sand mining or dredging for flood control projects at Paka Shoal (Figures 5A, B). In Pengkalan Nangka, dredging replaced muddy substrate with sand, allowing H. pinifolia to colonize the area previously occupied by H. beccarii (Figure 5C). Additionally, deepening the shoals and road construction damaged H. beccarii in parts of Pengkalan Nangka and Kg Baru Nelayan (Figure 5D). Shellfish harvesting for Ostrea sp., Meretrix meretrix, Gari elongata (Figures 5E, F), and urban development in Seberang Prai also destroy the habitat of H. beccarii. Natural factors such as freshwater input during monsoons, wind-driven waves, and sediment movement also contribute to habitat instability in shallow estuarine areas. These findings, gathered over decades of monitoring and field visits, indicate a significant reduction in the range of H. beccarii in Malaysia.

Figure 5. Disturbances to H. beccarii ecosystems. (A) flood barrier construction, (B) ground view of H. beccarii meadow with flood sand barrier (arrow) at Paka Shoal, (C) H. beccarii growing separately from Halodule pinifolia after dredging replaced muddy substrate with sand, (D) sand dredging to deepen the shoals and road construction caused damage to H. beccarii in parts of Pengkalan Nangka and Kg Baru Nelayan. Shellfish digging (E) Ostrea sp., (F) Meretrix meretrix, and Gari elongata at Pengkalan Nangka also destroyed the H. beccarii.
3.3 Habitat description of Halophila beccarii
In Peninsular Malaysia, H. beccarii is distributed across various shallow intertidal zones with differing environmental and substrate conditions. The species thrives in areas influenced by the northeast monsoon, including the east coast sites of Terengganu (Kemaman River, Paka Lagoon) and Kelantan (Pengkalan Nangka Lagoon), where it shows notable seasonal changes in rainfall, salinity, and water temperature.
Table 2 presents the habitat characteristics, physical and chemical parameters of H. beccarii in various locations. This species inhabits a variety of intertidal environments, from freshwater zones during low tide to brackish waters at high tide. Environmental monitoring at key sites revealed that during the monsoon season, heavy rainfall, ranging from 300 to over 700 mm/month, causes reduced salinity, often approaching freshwater levels, especially during low tides. Temperatures remain warm, generally between 27 and 31°C, and total suspended solids (TSS) tend to rise, indicating increased turbidity. In contrast, during the dry season, rainfall is minimal, and salinity increases significantly, with peak measurements ranging from 30 to 38 psu, depending on the site and tide phase. Despite these changes, H. beccarii remains present, demonstrating its ecological adaptability and wide environmental tolerance.
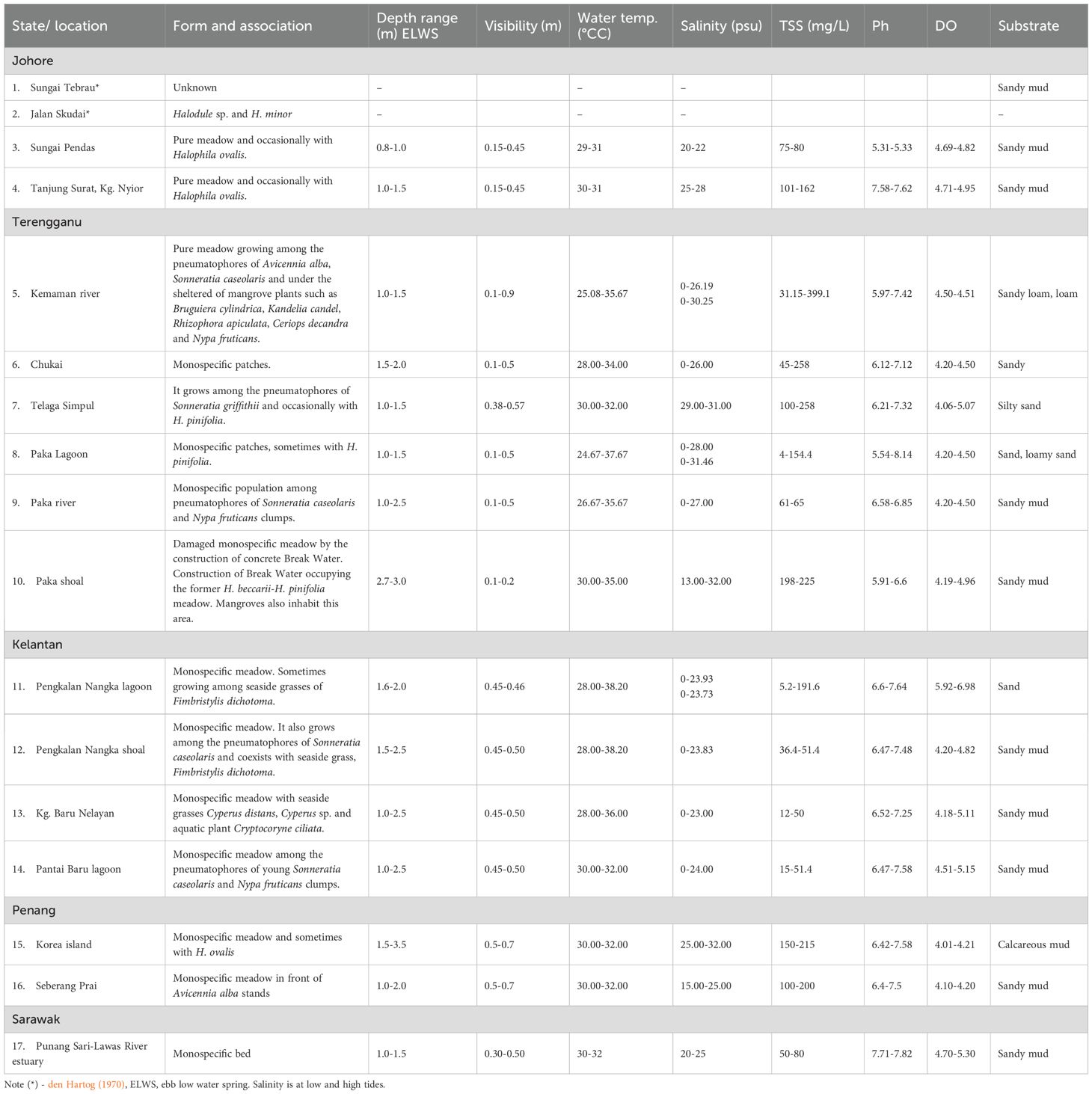
Table 2. Habitat characteristics, physicochemical conditions, and substrate types of the Halophila beccarii ecosystem in Malaysia.
Environmental variability is closely related to habitat type. In mangrove riverbank and mudflat systems such as Kemaman River and Sungai Paka, H. beccarii coexists with mangrove pneumatophores in warm, low-salinity environments with muddy or sandy-loam substrates. In these habitats, the species is usually found at depths ranging from 0.8 to 2.5 meters below the water surface (ELWS). The intertidal seagrass habitats are not entirely submerged but are exposed twice daily depending on the tides.
In protected coastal lagoons and estuaries, such as Pengkalan Nangka Lagoon, the species often forms pure stands or coexists with emergent freshwater species, including Fimbristylis dichotoma and Cryptocoryne ciliata. These areas exhibit fluctuating salinity levels ranging from 0 to 24 psu, warm waters with temperatures from 28 to 38°C, and sand to sandy-mud substrates.
Open shallow coastal areas, such as Telaga Simpul and Seberang Prai, have slightly higher salinity levels, ranging from 15 to 31 psu, and host H. beccarii on sandy-mud substrates at depths of 1.0 to 2.0 meters ELWS, often in association with other seagrasses like H. pinifolia. In contrast, shoals and reclaimed island habitats, including Korea Island and Paka Shoal, show evidence of colonization by H. beccarii in more disturbed or human-altered environments. Here, the species is found at greater depths of 2.7 to 3.5 meters ELWS, on calcareous or sandy mud substrates, and coexists with H. pinifolia. These areas exhibit high environmental variability, especially in salinity (13 to 32 psu) and temperature (30 to 35°C), yet still support healthy populations. The range of environmental conditions observed across these habitats demonstrates the adaptability of H. beccarii, especially in estuarine and monsoon-influenced ecosystems. This habitat-based classification, combined with seasonal environmental data, addresses reviewer concerns by showing how H. beccarii’s distribution is more influenced by its tolerance to fluctuations in salinity, temperature, and turbidity than by strict environmental thresholds. Additionally, the analysis of site-specific monsoon impacts highlights the importance of considering seasonal variability and hydrodynamic protection when interpreting ecological patterns of this vulnerable seagrass species.
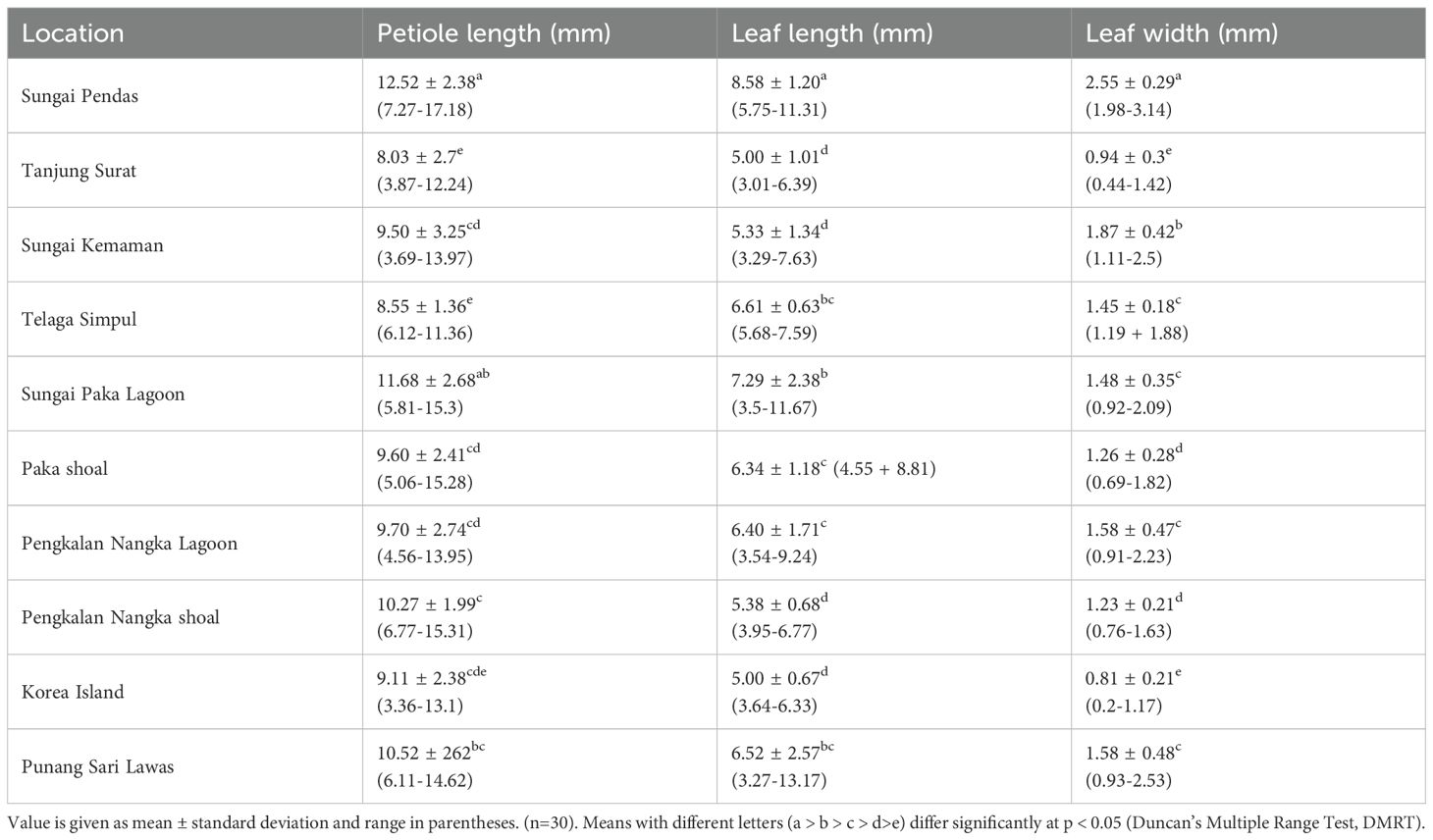
3.4 Life cycle of Halophila beccarii
Halophila beccarii is a small seagrass species characterized by its monoecious reproductive system and creeping rhizome with a single root per node that bears numerous root hairs. Morphometric measurement of vegetative morphology of H. beccarii from ten locations is illustrated in Table 3. The species features two protective scales: an upper transparent scale that covers the upright shoot and a lower scale that shields the rhizome. Each shoot produces 4 to 12 rosette leaves that are oblong to elliptical. Petiole lengths range from 3.36 to 17.18 mm, leaf lengths from 3.27 to 13.17 mm, and leaf widths from 0.20 to 3.14 mm across different locations in Malaysia. Leaf morphology varies across sites, likely due to local environmental factors and hydrodynamic exposure. The longest petioles were recorded at Sungai Pendas (12.52 ± 2.38 mm), and the widest leaf blades (2.55 ± 0.29 mm). These differences highlight H. beccarii’s ability to adapt to various hydrological and sedimentation conditions, especially in highly dynamic coastal and intertidal zones. The male flowers arise sub-terminally from short lateral branches within the rosette and have three pale brown tepals (1.82–2.91 mm × 0.55–2.18 mm) and three anthers (1.00–2.30 mm long). Female flowers emerge laterally, consisting of an ovoid ovary (0.55–1.82 mm × 0.18–0.86 mm) and 2–3 styles that can reach up to 13.01 mm in length. Typically, one fruit is produced per reproductive shoot, and it is ellipsoid to ovoid in shape, transparent, and contains 1 to 7 seeds. Each seed is globose, reticulate, and reddish-brown, measuring 0.64–1.40 mm × 0.45–1.09 mm.
Field observations and seedling studies reveal that H. beccarii exhibits both annual and perennial life cycles, which are affected by local hydrology and climate. At dynamic, monsoon-driven sites like Pengkalan Nangka Lagoon, Kelantan, and Paka Shoal, Terengganu, H. beccarii functions as an annual species, as illustrated in Figure 6. Plant cover completely disappears during the northeast monsoon from November to January, entering dormancy. Recolonization begins in February with seed germination (Figure 6A). The germinating seeds (Figure 6B) develop into seedlings (Figures 6C, D) from March to April, forming a network of rhizomes. Juvenile plants (Figure 6E) continue vegetative propagation, growing into mature plants that produce male (Figure 6F), female (Figure 6G) flowers, fruit (Figure 6H) and seeds (Figure 6I) from April to September. Between October and November, plant density decreases again, signaling another dormancy phase. From December to January, the meadow recolonizes through the germination of seeds and seedlings, ensuring its continued presence in the natural environment.
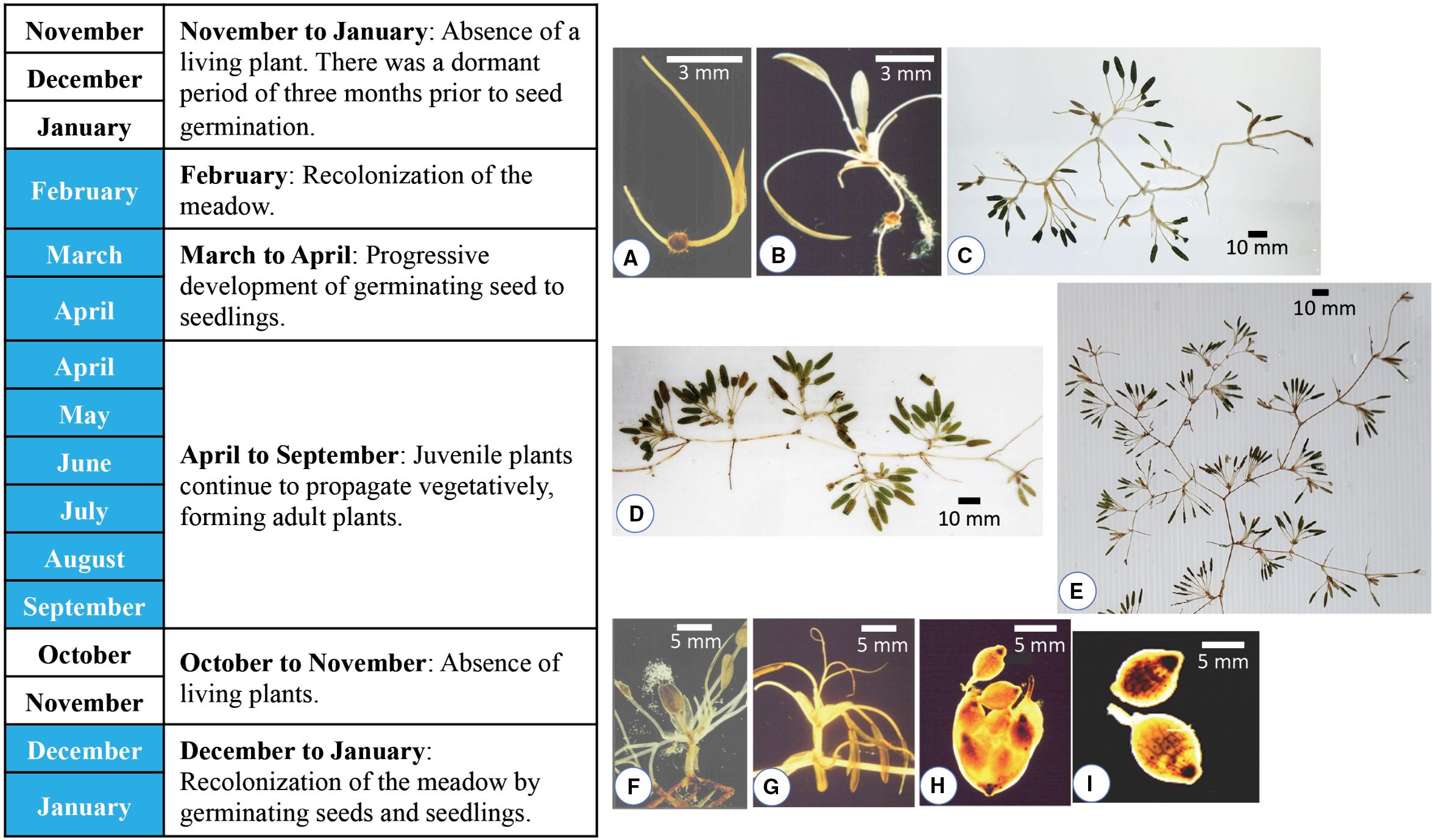
Figure 6. The growth cycle of annual plants from Pengkalan Nangka Lagoon, Kelantan, and Paka Shoal, Terengganu. (A) Recolonization of the meadow by germinating seed in February. Progressive development of (B) germinating seed to (C, D) seedlings with an array of rhizome network systems from March to April. (E) Juvenile plants continue to propagate vegetatively, forming adult plants that produce (F) male flower, (G) female flower, (H) fruit and (I) seeds from April to September.
Conversely, at more stable estuarine sites such as Sungai Kemaman (Terengganu), H. beccarii exhibits perennial traits, with populations existing year-round. Flowering and fruiting activities were observed almost every month except June for male flowers and May–June for female flowers. This persistence suggests a more stable and resilient population structure, supported by both vegetative spread and seed production. This dual strategy, perennial in stable estuarine habitats and annual in monsoon-affected shoals, reflects H. beccarii’s ecological flexibility. It allows the species to survive disturbances such as sediment shifts, freshwater influx, and seasonal dieback, although its long-term survival remains vulnerable to increased human activity and habitat deterioration.
4 Discussions
4.1 Distribution, habitat, and disturbances of vulnerable Halophila beccarii
Halophila beccarii is currently listed as Vulnerable under the IUCN Red List Categories and Criteria Version 3.1 (Short et al., 2011; IUCN, 2012), with an estimated global distribution of less than 2,000 km² (Geng et al., 2022; Chen et al., 2024). Although den Hartog (1970) described it as rare yet widely distributed across the Indo-West Pacific, recent evidence suggests its range has contracted significantly. The species is still reported in India (Jagtap and Untawale, 1981; Untawale and Jagtap, 1977; Jagtap, 1991; Parthasarathy et al., 1991), Thailand (Poovachiranon et al., 1994), Vietnam (Van Tien, 1998), and Hong Kong (Hodgkiss and Morton, 1978), but it has been declared locally extinct in the Philippines (Liao and Geraldino, 2020) and Endangered in Sri Lanka (Udagedara et al., 2017). Notably, the first record from Bangladesh was recently confirmed in the Andharmanik River estuary (Haque et al., 2024), extending the known distribution along the Bay of Bengal.
In Malaysia, H. beccarii shows a patchy and declining distribution, with recent occurrences primarily along the east coast and limited observations on the west coast. It tolerates a wide range of salinity (0–32 psu) and temperature ranges (25–38 °C), thriving in substrates ranging from sandy mud, silty clay, and calcareous substrates. Its physiological flexibility allows it to inhabit estuarine environments, where it coexists with seagrasses such as Halophila ovalis and Halodule pinifolia, macroalgae like Ulva intestinalis, and emergent species like Fimbristylis dichotoma and Cryptocoryne ciliata (den Hartog, 1970; Abhijith et al., 2019). However, despite this flexibility, H. beccarii typically persists only as scattered patches or monospecific meadows, indicating ecological vulnerability when disturbed. Experimental studies confirm its salinity tolerance in Malaysian estuaries (Fakhrulddin et al., 2019), but regional evidence shows limited resilience: low clonal diversity in Vietnam (Phan et al., 2017), strong habitat specialization in Hainan (Geng et al., 2022), and small, recently reported intertidal populations in Bangladesh (Haque et al., 2024).
Monsoonal flooding, coastal erosion, and activities such as land reclamation and pollution directly threaten its population stability. For example, severe flooding in Paka Shoal in 2014 resulted in the complete loss of seagrass due to sediment burial (Japar Sidik et al., 2018; Hossain et al., 2015). A recent visit to Pengkalan Nangka Lagoon reveals that, following the 2024 flooding event, the H. beccarii area has decreased. Seasonal changes in salinity and turbidity, especially during the northeast monsoon, further threaten meadow persistence along Malaysia’s east coast. These patterns emphasize that H. beccarii’s survival depends not only on its physiological tolerance but also on minimizing human impacts, protecting seed bank health, and improving adaptive monitoring to observe short-term ecological changes.
Human activities have also contributed to the decline of H. beccarii in Peninsular Malaysia. In areas such as Chukai, Paka Lagoon, Paka River, Terengganu, and Pengkalan Nangka shoal, as well as Kampung Baru Nelayan, Kelantan, the species is now absent. The habitat is affected by large-scale disturbances, including land reclamation, dredging to deepen boat channels, and sand mining for construction. Dredging naturally removes bed material and seagrasses, causing increased sedimentation that smothers seagrasses over large areas and nearby regions. This sedimentation displaces H. beccarii, allowing more resilient species such as H. pinifolia to grow and dominate the area.
In disturbed or sediment-rich habitats, H. pinifolia often becomes the dominant species due to its higher adaptability and resilience. For example, Lamit and Tanaka (2019) reported that H. pinifolia thrived across sites with different sediment types and nutrient conditions, showing the highest biomass and leaf length in areas with increased silt and clay content related to ongoing coastal development. This adaptability is further supported by its exceptional tolerance to low-light conditions. Longstaff and Dennison (1999) demonstrated that H. pinifolia could survive extended periods of near darkness with minimal biomass loss, a trait not observed in other seagrass species such as Heterozostera tasmanica (Bulthuis, 1983) and Posidonia sinuosa (Gordon et al., 1994), which declined rapidly under similar stress. Additionally, the life cycle of H. pinifolia, characterized by quick colonization through seed banks, provides an extra advantage in post-disturbance environments. As Unsworth et al. (2015) observed, these early successional species can stabilize sediments and promote recovery, often outcompeting more sensitive taxa like H. beccarii.
This vulnerability differs from more resilient taxa like E. acoroides and follows similar patterns observed in other small seagrass species, Halodule pinifolia and H. ovalis, which are also vulnerable to sedimentation and habitat alteration (Short et al., 2011; Yaakub et al., 2014). The ecological impacts are significant, as H. beccarii meadows serve as habitat for juvenile fish, invertebrate nurseries, and support coastal food webs (Waycott et al., 2009). Moreover, their ability to sequester carbon is reduced under low-light and turbid conditions (Premarathne et al., 2021; Mishra et al., 2021). Recovery at these disturbed sites appears slow and likely depends on seed banks, a pattern also observed in Bangladesh (Rakib et al., 2022).
On the west coast of Peninsular Malaysia, the absence of H. beccarii from previously recorded sites, such as Sungai Tebrau and Jalan Skudai, Johor (den Hartog, 1970), suggests possible extirpation. Recent reappearances in 2021 in Sungai Pendas, Johor, suggest that populations may persist in less-disturbed habitats. These findings highlight the importance of adaptive and site-specific monitoring strategies, particularly for cryptic or small-bodied seagrass species that are easily overlooked during conventional surveys. Using rotational monitoring combined with more frequent or community-based assessments could enhance detection and provide early warning signs of biodiversity changes. Although this study benefited from long-term revisit data, it also highlights the need for consistent, frequent monitoring to capture local dynamics and support effective conservation efforts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MZ: Data curation, Resources, Visualization, Conceptualization, Formal analysis, Project administration, Validation, Software, Writing – review & editing, Methodology, Supervision, Investigation, Writing – original draft, Funding acquisition. NS: Writing – review & editing, Methodology, Formal analysis, Visualization. JB: Conceptualization, Visualization, Investigation, Supervision, Methodology, Funding acquisition, Validation, Software, Project administration, Formal analysis, Resources, Data curation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for theresearch and/or publication of this article. This research was funded by an Industry Grant, Country Garden Pacificview Sdn. Bhd. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We would like to thank the Vice-Chancellor of Universiti Putra Malaysia for his encouragement and the facilities provided. The authors also thank the academic editor and three anonymous reviewers, whose constructive comments and input significantly improved the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abhijith R. P., Kaladharan P. K., Asokan K. V., Shilta M. T., Mahesh V., Zacharia P. U., et al. (2019). Present status of Halophila beccarii seagrass bed in Kadalundi Community Reserve. Mar. Fisheries Inf. Service; Tech. Extension Ser. 241, 22–23. Available online at: http://eprints.cmfri.org.in/id/eprint/14416 (Accessed July 30, 2025).
Aye A. A., Hsan A. M., and Soe-Htun U. (2014). The morphotaxonomy and phytosociolgy of Halophila beccarii (Family: Hydrocharitaceae) in Kalegauk Island, Mon State. Mawlamyine University Research Journal 5, 1–5. Available online at: https://www.researchgate.net/profile/U-Soe-Htun/publication/280824720.
Beccari O. (1904). Wanderings in the great forests of borneo (Oxford, New York: Oxford University Press).
Billah M. M., Hossain Z., Abu Hena M. K., Hoque A. R., Rahman M. M., Hoque M. M., et al. (2016). Saltmarsh and seagrass beds on the south-eastern coast of Bangladesh: vegetation characteristics and adjacent fisheries diversity. Zoology Ecol. 26, 313–322. doi: 10.1080/21658005.2016.1225364
Bulthuis D. A. (1983). Effects of in situ light reduction on density and growth of the seagrass Heterozostera tasmanica (Martens ex Aschers.) den Hartog in Western Port, Victoria, Australia. J. Exp. Mar. Biol. Ecol. 67, 91–103. doi: 10.1016/0022-0981(83)90137-5
Che Alias M. A., Zakaria M. H., Ramaiya S. D., Esa Y., Ab. Ghani N. I., and Bujang J. S. (2024). Morphological and genetic identification of Halophila species and a new distribution record of Halophila nipponica at the Tanjung Adang Laut shoal, Johor, Malaysia. PloS One 19, e0309143. doi: 10.1371/journal.pone.0309143
Chen S., Yuanfang P., Siting Q., and Guanglong Q. (2024). Assembly and comparative analysis of the multichromosomal mitochondrial genome of globally endangered seagrass Halophila beccarii. BMC Plant Biol. 24, 1–22. doi: 10.1186/s12870-024-05765-3
Duarte C. M. (2002). The future of seagrass meadows. Environ. Conserv. 29, 192–206. doi: 10.1017/S0376892902000127
English S., Wilkinson C., and Baker V. (1997). Survey manual for tropical resources (Townsville, Australia: AIMS).
Fakhrulddin M. I., Japar Sidik B., and Muta Harah Z. (2013). Halophila beccarii Aschers. (Hydrocharitaceae) responses to different salinity. J. Fisheries Aquat. Sci. 8, 462–471. doi: 10.3923/jfas.2013.462.471
Fortes M. D., Ooi J. L. S., Tan Y. M., Prathep A., Bujang J. S., and Yaakub S. M. (2018). Seagrass in Southeast Asia: A Review of status and knowledge gaps, and a road map for conservation. Botanica Marina 61, 269–288. doi: 10.1515/bot-2018-0008
Geng X., Cai Z., Jia S., Shen J., Tang D., Wang D., et al. (2022). Environmental Determinants of the distribution of Halophila beccarii Ascherson in Hainan Island, China. Sustainability 14, 13491. doi: 10.3390/su142013491
Gerakis A. and Baer B. (1999). A computer program for soil textural classification. Soil Sci. Soc. America J. 63, 807–808. doi: 10.2136/sssaj1999.634807x
Gordon D. M., Grey K. A., Chase S. C., and Simpson C. J. (1994). Changes to the structure and productivity of a Posidonia sinuosa meadow during and after imposed shading. Aquatic Botany 47, 265–275. doi: 10.1016/0304-3770(94)90057-4
Greve T. M. and Binzer T. (2004). “Which factors regulate seagrass growth and distribution,” in European seagrasses: An introduction to monitoring and management. Eds. Borum J., Krause-Jensen D., and Duarte C. M. (The M&MS project, Denmark), 19–23.
Haque M. A., Ullah M. R., Hasan M. M., Bosu A., Yasmin F., Islam M. A., et al. (2024). First report of seagrass (Halophila beccarii) from the mid-southern coast of Bangladesh, Bay of Bengal. Aquat. Bot. 190, 103727. doi: 10.1016/j.aquabot.2023.103727
Hena M. K., Hossain M. S., and Islam M. R. (2009). First report on the presence of seagrass (Halophila beccarii) in Bangladesh. Bangladesh J. Bot. 38, 127–130. doi: 10.1016/j.aquabot.2023.103727
Hiranphan R., Hiranphan P., Puangpairote T., Prathep A., and Eksomtramage L. (2020). Karyomorphology of three halophila species (Hydrocharitaceae, alismatales) from haad chao mai national park, trang province, Thailand. Chiang Mai Univ. J. Natural Sci. 47, 57–63. Available online at: https://www.thaiscience.info/Journals/Article/CMJS/10990743.pdf (Accessed July 30, 2025).
Hodgkiss I. J. and Morton B. (1978). Halophila beccarii Ascherson (Hydrocharitaceae)- A new record for Hong Kong with notes on other Halophila species. Memoirs Hong Kong Natural History Soc. 13, 28–32. Available online at: https://www.researchgate.net/publication/303605997_Halophila_beccarii_Ascherson_Hydrocharitaceae_-_A_new_record_for_Hong_Kong_with_notes_on_other_Halophila_species (Accessed June 16, 2025).
Hossain M. S., Japar Sidik B., Muta Harah Z., and Mazlan H. (2015). The application of remote sensing to seagrass ecosystems: An overview and future research prospects. Int. J. Remote Sens. 36, 61–114. doi: 10.1080/01431161.2014.990649
International Union for Conservation of Nature (IUCN). (2011). IUCN Red List of Threatened Species. Version 2011.1. IUCN, Gland, Switzerland. Available at: https://www.iucnredlist.org (Accessed July 30, 2025).
Jagtap T. G. (1991). Distribution of seagrasses along the Indian Coast. Aquat. Bot. 40, 379–386. doi: 10.1016/0304-3770(91)90082-G
Jagtap T. G. and Untawale A. G. (1981). Ecology of seagrass bed Halophila beccarii (Aschers) in Mandovi Estuary, Goa. Indian J. Mar. Sci. 4, 215–217. Available online at: https://drs.nio.res.in/drs/handle/2264/6713 (Accessed June 16, 2025).
Japar Sidik B. and Muta Harah Z. (2011a). “Seagrasses- diversity, values and why they are declining,” in Malaysia’s marine biodiversity: inventory and current status. Eds. Kamaruddin I., Mohamed C. A. R., Rozaimi M. J., Kee Alfian B. A. A., Fitra A. Z., and Lee J. N. (Department of Marine Park Malaysia, Putrajaya).
Japar Sidik B. and Muta Harah Z. (2011b). “Seagrasses in Malaysia,” in Seagrasses: resource status and trends in Indonesia, Japan, Malaysia, Thailand and Vietnam. Eds. Ogawa H., Japar Sidik B., and Muta Harah Z. (Department of Marine Park Malaysia, Putrajaya).
Japar Sidik B., Muta Harah Z., and Short F. T. (2018). “Seagrass in Malaysia: issues and challenges ahead,” in The wetland book II. Eds. Finlayson C., Milton G., Prentice R., and Davidson N. (Springer, Dordrecht), 975–1883. doi: 10.1007/978-94-007-4001-3_268
Lamit N. and Tanaka Y. (2019). Species-specific distribution of intertidal seagrasses along environmental gradients in a tropical estuary. Regional Stud. Mar. Sci. 29, 100671. doi: 10.1016/j.rsma.2019.100671
Les D. H., Cleland M. A., and Waycott M. (1997). Phylogenetic studies in Alismatidae, II: Evolution of marine angiosperms (seagrasses) and hydrophily. Systematic Bot. 22, 443–463. doi: 10.2307/2419820
Liao L. M. and Geraldino P. J. L. (2020). Has halophila beccarii ascherson (Alismatales, hydrocharitaceae) been locally extirpated in the Philippines? Trop. Natural History 20, 104–110. doi: 10.58837/tnh.20.1.206130
Longstaff B. J. and Dennison W. C. (1999). Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat. Bot. 65, 105–121. doi: 10.1016/S0304-3770(99)00035-2
Menez E. G., Philips R. C., and Calumpong H. P. (1983). Seagrass from the Philippines (Washington: Smithsonian Institution Press). Available online at: https://repository.si.edu/server/api/core/bitstreams/6606f7a4-4e84-4d54-b6d6-faf15325c276/content (Accessed June 1, 2025).
Mishra A. K., Khadanga M. K., Patro S., Apte D., and Farooq S. H. (2021). Population structure of a newly recorded (Halodule uninervis) and native seagrass (Halophila ovalis) species from an intertidal creek ecosystem. Lakes Reserv. Res. Manag. 26, e12376. doi: 10.1111/lre.12376
Muta Harah Z. (2023). “Inaugural lecture,” in Journey to the fascinating world of aquatic ecology (UPM Press, Universiti Putra Malaysia, Serdang, Selangor).
Muta Harah Z. and Japar Sidik B. (2004). Seagrass communities of Punang-Bt. Sari-Lawas River estuary, Sarawak, East Malaysia. Bio-Science Res. Bull. 20, 1–8.
Muta Harah Z. and Japar Sidik B. (2024). “Chapter 7. Seagrass to sanctuary: A blueprint for marine conversation. A description of seagrass ecosystem from the region- their diversity, current status and challenges in Malaysia,” in Tides of change: the middle bank marine sanctuary and the quest for a resilient. Eds. Zulfigar Y., Saito H., Norhanis R., and Aileen T. S. H. (P&Y Design Network, Penang), 132–136.
Muta Harah Z., Japar Sidik B., and Arshad A. (2002). Flowering, fruiting and seedling of annual Halophila beccarii Aschers. in Peninsular Malaysia. Bull. Mar. Sci. 71, 1199–1205. doi: 10.1016/S0304-3770(99)00040-6
Muta Harah Z., Japar Sidik B., and Hishamuddin O. (1999). Flowering, fruting and seedling of Halophila beccarii Aschers. (Hydrocharitaceae) from Malaysia. Aquat. Bot. 65, 199–207. doi: 10.1016/S0304-3770(99)00040-6
Orth R. J., Carruthers T. J. B., and Dennison W. C. (2006). A global crisis for seagrass ecosystems. BioScience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Parthasarathy N., Ravikumar K., Ganesan R., and Ramamurthy K. (1991). Distribution of seagrasses along the coast of Tamil Nadu, Southern India. Aquat. Bot. 40, 145–153. doi: 10.1016/0304-3770(91)90092-J
Parthasarathy N., Ravikumar K., and Ramamurthy K. (1988). Floral biology and ecology of Halophila beccarii Aschers. (Hydrocharitaceae). Aquat. Bot. 31, 141–151. doi: 10.1016/0304-3770(88)90044-7
Phan T. T. H., De Raeymaeker M., Luong Q. D., and Triest L. (2017). Clonal and genetic diversity of the threatened seagrass Halophila beccarii in a tropical lagoon: resilience through short distance dispersal. Aquat. Bot. 142, 96–104. doi: 10.1016/j.aquabot.2017.07.006
Poovachiranon S., Nateekanjanalarp S., and Sudara S. (1994). “Seagrass beds in Thailand,” in Proceedings third ASEAN Australia symposium on living coastal resources, volume 1: status reviews. Eds. Wilkinson C. R., Sudara S., and Chou L. M. (Australian Institute of Marine Science, Chulalongkom University, Bangkok, Thailand), 317–321.
Premarathne C., Jiang Z., He J., Fang Y., Chen Q., Cui L., et al. (2021). Low light availability reduces the subsurface sediment carbon content in Halophila beccarii from the South China Sea. Front. Plant Sci. 12, 664060. doi: 10.3389/fpls.2021.664060
Rakib M. R. J., Hossain M. B., Islam M. S., Hossain I., Rahman M. M., Kumar R., et al. (2022). Ecohydrological features and biodiversity status of estuaries in Bengal delta, Bangladesh: A comprehensive review. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.990099
Savurirajan M., Raj K. L., and Thiruchitrambalam G. (2015). A new record of the seagrass Halophila beccarii Ascherson from the Port Blair coast, Andaman and Nicobar Islands, India. Botanica Marina 58, 409–413. doi: 10.1515/bot-2014-0076
Short F. T., Beth P., Suzanne R. L., Kent E. C., Bandeira S, Japar Sidik B., et al. (2011). Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 144, 1961–1971. doi: 10.1016/j.biocon.2011.04.010
Short F., Carruthers T., Dennison W., and Waycott M. (2007). Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Udagedara S., Fernando D., Perera N., Tanna A., and Bown R. (2017). A first record of Halodule pinifolia Miki den Hartog, and new locality of nationally endangered Halophila beccarii Asch, from the eastern coast of Sri Lanka. Int. J. Aquat. Biol. 5, 328–335. doi: 10.22034/ijab.v5i5.358
Unsworth R. K. F., Collier C. J., Waycott M., McKenzie L. J., and Cullen Unsworth L. C. (2015). A framework for the resilience of seagrass ecosystems. Mar. pollut. Bull. 100, 34–46. doi: 10.1016/j.marpolbul.2015.08.016
Untawale A. G. and Jagtap T. G. (1977). A new record of Halophila beccarii (Aschers.) from Mandovi Estuary, Goa, India. Mahasagar Bull. Natl. Institute Oceanography 10, 91–93. doi: 10.1016/j.jembe.2007.06.012
Van Tien N. (1998). “Species composition and distribution of seagrasses in Vietnam,” in Proceedings of the third international seagrass biology workshop “Frontiers in seagrass research: A challenge of the new millenium (The Sulo Hotel & Marine Science Institute Cs, University of Philippines, Diliman).
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Keywords: vulnerable seagrass, Halophila beccarii, coastal development, dredging, sand mining, Malaysia
Citation: Zakaria MH, Syed NNF and Bujang JS (2025) The vulnerable seagrass Halophila beccarii Aschers. (Hydrocharitaceae) from Malaysia. Front. Conserv. Sci. 6:1648415. doi: 10.3389/fcosc.2025.1648415
Received: 17 June 2025; Accepted: 03 September 2025;
Published: 22 September 2025.
Edited by:
Mike van Keulen, Murdoch University, AustraliaReviewed by:
Jessica Pazzaglia, Anton Dohrn Zoological Station Naples, ItalyQuang Doc Luong, Hue University, Vietnam
Eduardo Gabriel Torres Conde, National Autonomous University of Mexico, Mexico
Copyright © 2025 Zakaria, Syed and Bujang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muta Harah Zakaria, bXV0YUB1cG0uZWR1Lm15
 Muta Harah Zakaria
Muta Harah Zakaria Nurul Nur Farahin Syed
Nurul Nur Farahin Syed Japar Sidik Bujang
Japar Sidik Bujang