- Columbia River Fish and Wildlife Conservation Office, U.S. Fish and Wildlife Service, Vancouver, WA, United States
The classification of a population as imperiled may lead to the development of a formal recovery plan with specific legal requirements. Rigorous recovery plans typically include criteria by which to gauge population recovery and monitoring plans to guide evaluations of whether criteria are achieved. Monitoring plans have traditionally focused on ecological characteristics. However, there is an increasing frequency of recovery plans and criteria focused on the reduction of threats. Traditional monitoring plans often match imperfectly with threats-based criteria. For example, Bull Trout in the U.S. are currently considered imperiled. Their recovery plan and criteria call for 75-100% of the primary threats to their persistence to be managed effectively. Incongruous with these criteria, monitoring plans to inform the recovery status of Bull Trout have generally focused on characteristics such as abundance, trends in abundance, distribution and connectivity. Bull Trout in the Elwha River are discussed as an example of threats being explicitly monitored and informing status. For species such as Bull Trout, the most useful monitoring plan for assessing recovery status would guide explicit and quantitative evaluations of threat scope and severity, determine how effectively threats are being managed, and be commensurate with the criteria for recovery.
Introduction
The number of species on earth appears to be greater than ever before (Ceballos et al., 2010). Despite this abundance, studies have reported large reductions in global biodiversity (Pimm et al., 2014) and an extinction crisis. In addition to species extinction, the extirpation of populations is of particular concern (Dirzo et al., 2022). Numerous taxa are part of the current extinction crisis. For example, significant declines have been reported in butterflies, birds and plants (Thomas et al., 2004) and it has been estimated that 33-50% of amphibian species (Stuart et al., 2004) and 60-75% of non-human primates have declining populations (Estrada et al., 2017). Declines in fish have also been reported with over 33% of species from the class Chondrichthyes classified as threatened (Dulvy et al., 2021) and modern extinction rates for freshwater fish in North America estimated to be more than 850 times background rates (Burkhead, 2012). Notably, freshwater fish species may be disproportionately at risk (Reid et al., 2019). Recent estimates from the IUCN red list of threatened species indicate the persistence of more than 25% of all species is likely in doubt (Gimenez, 1996; IUCN, 2025). Furthermore, extinction rates in the past 120 years appear disproportionately high, with current values likely 100- to 1,000-fold greater than background values (Ceballos et al., 2015a; 2015b).
Classification of a species as imperiled may lead to the development of a recovery plan or conservation initiative (hereafter, recovery plan). These recovery plans are sometimes formal with legal requirements (e.g. USFWS, 2018) and other times informal and reliant on voluntary participation (Minckley et al., 2003). Since planning and implementation can be time and cost prohibitive (Harris et al., 2012), many imperiled species often go without a formal recovery plan. For species that are classified as imperiled under the Endangered Species Act (ESA) of the United States of America (U.S.), it has been estimated that approximately 25% do not have a recovery plan, 50% have a recovery plan that took more than five years to complete after they were classified as imperiled, and 50% have recovery plans that are more than 20 years old (Malcom and Li, 2018). Globally, more than 90% of imperiled species may not be associated with any recovery plan (Wilcove and Master, 2005).
Criteria by which to evaluate conservation status and assess recovery efforts are typically included in recovery plans. Historically, these criteria have commonly been based on ecological characteristics associated with population persistence (e.g. trends in abundance). These characteristics are perhaps the most definitive expression of a population’s ability to persist and the best measures of its viability (White, 2000; Shaffer, 2019). However, these characteristics can be difficult and expensive to quantify and their expression may exhibit a lag relative to changes to the circumstances of a population. Furthermore, threats are often responsible for a particular population’s status. Partly for these reasons, it is becoming common for recovery plans to have explicit recovery criteria associated with the reduction of threats (Lawler et al., 2002; Troyer and Gerber, 2015). For example, under the ESA, a recovered condition and listing status of a species are ultimately determined through a five-factor analysis, each factor being associated with a threat (Sullins, 2001; Smith-Hicks and Morrison, 2021). The biological viability of a species is a critical component of a recovered condition, and assessments of recovery may include the ecological principles of representation, resiliency and redundancy. However, since a recovered condition often has a legal or administrative context, criteria associated with population viability are not necessarily equivalent to criteria for recovery (Wolf et al., 2015).
A monitoring plan is typically associated with a robust recovery plan and the recovery criteria within. To align with recovery criteria, monitoring plans are often focused on population and regulatory requirements (Gerber et al., 1999; Evansen et al., 2021). While valuable, monitoring plans based on ecological characteristics present several challenges, specifically that the expression of changes can be protracted, and these characteristics are not the ultimate problem to persistence. Threats are often not the focus of recovery criteria (Campbell et al., 2002) but the potential value of monitoring threats for recovery assessments has been recognized (Regan et al., 2008; Troyer and Gerber, 2015; NatureServe, 2024). Although a recovery plan and recovery criteria based solely on threats is relatively unusual, this approach is becoming more common (Gerber and Hatch, 2002). Precisely how to assess threats is not a standardized process and threats are often not primary factors to which status evaluations are most sensitive (NOAA, 2011). Assessments of the scope, severity, and how well threats are being managed have commonly been a qualitative process dependent on professional opinion. Although decisions based on the collective opinions of experts can be useful and are often necessary (Tonelli, 1999; Taylor, 2006), they can also be problematic and misleading (Mosleh et al., 1988; McKee et al., 1991; Van Der Fels‐Klerx et al., 2002; Orsi et al., 2011). The purpose of this perspective is to emphasize the potential value of and describe potential applications for implementing explicit and quantitative monitoring plans associated with recovery criteria based on threats. Bull Trout (Salvelinus confluentus) are used as an example. However, quantitatively monitoring threats may have utility for the conservation of other imperiled species.
Threats-based recovery criteria
Explicitly monitoring the scope and severity of a threat as well as how effectively it is being managed is logically necessary to assess the legal conservation status of Bull Trout. A quantitative evaluation of threats would reduce subjectivity and add objectivity to assessments of recovery and decisions regarding ESA status. Efforts to provide quantitative evaluations associated with threats are not unprecedented. They include relatively broad efforts to model (Ferson et al., 2000) and integrate the impacts and significance of threats (Sinnatamby et al., 2020) as well as relatively specific efforts such as quantifying the toxicity of herbicides (Fairchild et al., 2009). However, quantitative monitoring of the scope, severity and management of threats can be complex. It is unlikely that all threats to Bull Trout have been identified. Conversely, some that have been identified may not be a significant threat to persistence. Which are primary threats and most significant to the persistence of Bull Trout is not always known. Whether and how threats interact and result in synergistic effects is unclear. Although it may be possible to measure a change in the scope or severity of a given threat, there can be uncertainty regarding how Bull Trout will respond to these changes. Whether management and a change in scope and severity of a threat cause an effect in Bull Trout populations can also be difficult to determine. Relative to population viability and traditional measures of persistence, it is somewhat unusual for an explicit need to quantitatively monitor threats. However, it is clear this approach could be useful for assessing the recovered condition and ESA status of Bull Trout.
Potential threats to Bull Trout are numerous and vary within a given recovery unit, core area or local population. In addition, threats are often related, sometimes with a large amount of overlap (e.g. water management and water quality). As such, there is not one set of standard threats that can always be monitored to inform the recovery and status of Bull Trout. This is unlike ecological characteristics where, distribution, abundance, trends in abundance, connectivity, and genetic condition are standard metrics that are often monitored to assess population status. As such, this perspective does not attempt to be prescriptive about which threats should be monitored and approaches to monitoring. Instead, it proposes a five-step process to consider when developing a plan to monitor threats. Initially, (i) start with a clear identification of a potential threat (e.g. non-native species). Once a threat has been identified, (ii) assess whether the threat actually exists. If the threat is present, (iii) quantitatively assess the magnitude (or incidence) as well as the frequency (or prevalence) in the population. Next, (iv) seek to evaluate if and how the threat influences Bull Trout. At a minimum, this step would evaluate whether there is a relation between the scope (extent) and severity (level) of the threat, actions to manage the threat, and the response of Bull Trout populations. Ideally, this step would evaluate any cause-and-effect relationship between the level of the threat or management activity and population response. When possible, (v) test how Bull Trout respond to changing levels of threats. If there is a potential threat that cannot be ameliorated, monitoring it may not be a priority. However, monitoring to characterize its magnitude, frequency, scope and severity is still warranted. Rao et al. (2007) provides an overview of assessing threats for conservation. Ultimately, in the case of Bull Trout, the best monitoring plans would provide an explicit and quantitative assessment of the scope, severity and effectiveness of management associated with threats.
Bull Trout
Bull Trout are native to the North Pacific and Arctic ocean drainages (Haas and McPhail, 2001). They spawn from summer into fall, burying embryos in the gravel of cold, headwater streams (McPhail and Baxter, 1996). Fry emerge from the gravel, rear near spawning areas, then express a resident, fluvial, adfluvial or an anadromous life history (see McPhail and Baxter, 1996; Homel et al., 2008; Howell et al., 2016; Brenkman et al., 2019). Bull Trout have a relatively high fidelity to natal areas, generally spawning in streams where they hatched (Swanberg, 1997; Howell and Sankovich, 2012). Throughout their range, Bull Trout are considered an imperiled species (Gimenez, 1996). In 1998, the U.S. Fish and Wildlife Service (USFWS) began to protect Bull Trout, classifying those inhabiting the Klamath and Columbia Rivers as threatened pursuant to the ESA (USFWS, 1998). Ultimately, all Bull Trout throughout their range in the U.S. were provided protection. As a procedural outcome of the ESA, all Bull Trout in the U.S. are currently considered one Distinct Population Segment (DPS), or a single unit of conservation (USFWS, 1999).
A recovery plan for Bull Trout in the U.S. was published in 2015 (USFWS, 2015). Administratively, Bull Trout in the DPS are organized into six recovery units, each containing one or more core areas (generally aligned with metapopulation structure) that, in turn, are comprised of one or more local populations (USFWS, 2015; Figure 1). The recovery plan is focused on threats to the persistence of Bull Trout and recovery criteria are associated with these threats. Numerous threats to the persistence of Bull Trout have been identified (USFWS, 2012, 2015; USFWS, 2024). Some of these threats have been documented empirically while other putative threats have been inferred from observations. While not intended as an exhaustive list, threats to the persistence of Bull Trout include habitat degradation (Fraley and Shepard, 1989), barriers to migration (Rieman and McIntyre, 1995), non-native species (Leary et al., 1993), range contraction (Sinnatamby et al., 2020) and a rapidly changing climate (Rieman et al., 2007). The decline of Bull Trout populations has been attributed to many of these threats (Rieman et al., 1997). The recovery plan and criteria are centered around using professional judgement and quantitative evidence (if available) to i) identify the primary threats to Bull Trout and ii) determine how effectively these threats are being managed. In summary, the recovery criterion identified in the recovery plan are that 75-100% (dependent on the recovery unit) of the primary threats are managed effectively. The relative importance of a threat, what makes something a primary threat, and what determines whether management of that threat is effective, are not always defined clearly. Per the recovery plan, decisions associated with recovery of Bull Trout and status under the ESA are directly dependent on an assessment of threats.
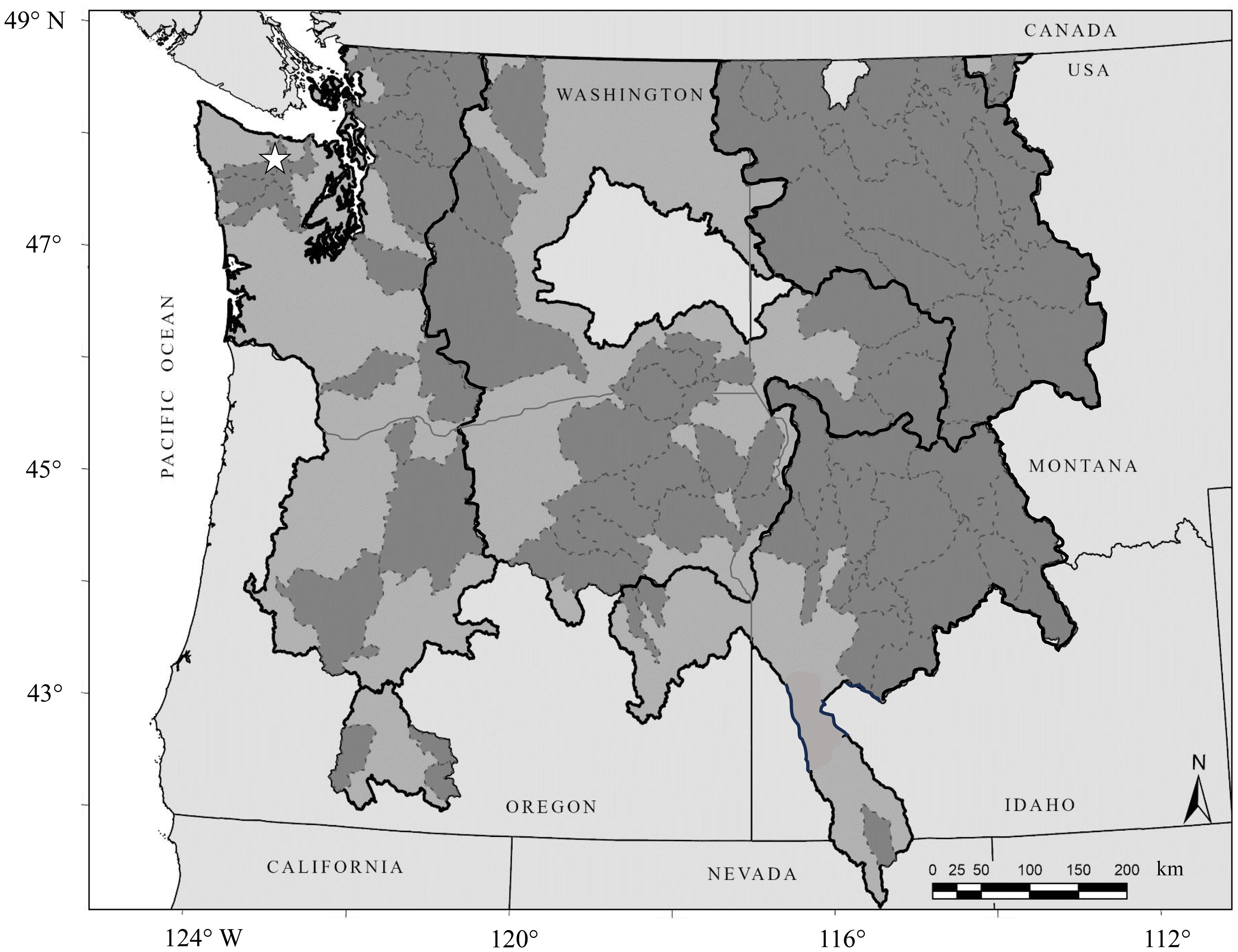
Figure 1. Distribution and organization of Bull Trout in the United States of America (U.S.). The coterminous Distinct Population Segment under the Endangered Species Act includes the entire distribution in the U.S. Recovery units are represented in light gray and outlined by thick, black lines. Core areas are represented in a dark gray and outlined by thin, dotted lines. ☆ identifies the Elwha River core area.
The Elwha River
In general, threats to the persistence of Bull Trout are numerous and each warrants consideration. When trying to categorize many of the threats to Bull Trout there can be significant overlap. Water management has been shown to be a potential threat to Bull Trout (Muhlfeld et al., 2012). Perhaps the most extreme example of water management threatening Bull Trout is an anthropogenic dam preventing natural flow, stranding fish, requiring them to move through turbines if they wish to pass or preventing them from passing. Irrigation diversions, culverts, and pumping operations are other examples of water management that can impact Bull Trout. Potential impacts include passage restriction, changes to water levels and temperature, as well as fragmentation or disconnection between water bodies. For example, Bull Trout often exhibit a migratory behavior during their life history. An inability to move freely through mainstem areas because of dams (see Barrows et al., 2016) and tributaries because of culverts (Shrimpton et al., 2008) or weirs (Sankovich and Whitesel, 2019) can be a significant threat. Various approaches can be used to monitor the threat of water management. These include assessing the relation between river regulation and fish movement (Muhlfeld et al., 2012), redd distribution (Barnett et al., 2013), fragmentation and genetic impacts (Neraas and Spruell, 2001), as well as metapopulation dynamics (Kaeding and Mogen, 2023). Numerous examples assessing water management or river regulation as a threat to Bull Trout exist (see Naman et al., 2022; Muhlfeld et al., 2003, 2012; Naman et al., 2022). Unsurprisingly, natural flow regimes appear to be the most protective of Bull Trout (Muhlfeld et al., 2003).
By way of demonstration, and as a simple example, this perspective examines the threat associated with water management and fish passage to Bull Trout in the Elwha River core area. The Elwha River is a core area located in the Olympic Peninsula of Washington where it flows directly into the Strait of Juan de Fuca (48.1474 latitude, -123.5648 longitude, Figure 1). It has an extant population of Bull Trout, at least a portion of which expresses an anadromous life history (Brenkman et al., 2019). Based on a 2005 assessment using the NatureServe process (USFWS, 2005), Bull Trout in this core area received a score of 0.94 on a scale of 5.50, were considered imperiled and at high risk of extinction. This status was driven by threats that were high in scope and moderate in severity, predominantly related to the presence of barriers to migration (two, impassable dams) and associated with a limited number of local populations. Management activities resulted in the threat of both dams being removed in 2014. Currently, there are no known passage barriers in the Elwha River and the potential for Bull Trout to access all habitat was presumably restored. Subsequent monitoring of passage revealed that Bull Trout responded to removal of the threat, migrated past the previous dam sites, and distributed throughout the river system (Brenkman et al., 2019; Duda et al., 2021). The resiliency, defined as the population’s ability to withstand stochastic disturbance (USFWS, 2016), of Bull Trout in this core area was recently rated as 3.44 on scale of 4.62, or high, based on the Species Status Assessment process (USFWS, 2024). Although the 2005 and 2024 assessments used slightly different approaches and scales, the results suggest a substantial and quantitative improvement in population status.
Ecological expression
The Recovery Plan for Bull Trout suggests that monitoring ecological characteristics, while not required, may also be useful to help inform recovery and listing status. Many recovery plans include recovery criteria associated with information on demographic or genetic assessments of populations (Himes Boor, 2014; Doak et al., 2015). The challenges and complexities associated with quantitatively monitoring threats highlight the potential utility of also monitoring traditional ecological characteristics. From an ecological perspective, Bull Trout and their populations integrate the impacts of all threats and express those in terms of demographic and genetic attributes. Quantitative population assessments represent the definitive, and potentially observable, expression of whether primary threats are diminishing in a manner that is beneficial (or increasing in a manner that is detrimental). Ultimately, the most conclusive way Bull Trout express the scope, severity and effectiveness of management associated with threats is how they respond in terms of these ecological characteristics. For example, following the removal of dams that threatened Bull Trout in the Elwha River and the subsequent movement of fish past these areas, Bull Trout increased in abundance and now exhibit peak densities in the upper rather than lower parts of the river system (Brenkman et al., 2019; Duda et al., 2021). Monitoring ecological characteristics to assess recovery can also be complex and challenging. In particular, it is important to recognize there can be a lag between, for example, a change in the management of a given threat and a detectable influence on Bull Trout populations (Whitesel et al., 2022; Mallet et al., 2024). Thus, demographic and genetic monitoring may not immediately reflect changes to the scope or severity of a threat. For Bull Trout it is worth reiterating, specific thresholds associated with assessments of ecological characteristics are not formal recovery criteria that must be monitored.
Discussion
Species across the planet may be experiencing an extinction crisis. Often associated with a species’ imperilment is the development of a plan to guide conservation efforts and criteria by which to assess their recovery. An explicit and quantitative monitoring plan is required to objectively and effectively evaluate whether recovery criteria are achieved. These plans are often based on traditional ecological characteristics of a population. However, it is not uncommon for recovery criteria to be based at least partly, and sometimes exclusively, on the threats to a species. In these cases, it follows that a monitoring plan focused on explicitly and quantitatively evaluating the threats to a species, or how effectively they are managed, is necessary to assess recovery. If criteria for recovery exist and are appropriate, the most useful monitoring plans for assessing recovery would be commensurate with and reflect these criteria. The logical contrapositive implies that if monitoring plans do not reflect these criteria, then criteria for recovery may not exist or be appropriate. Importantly, since ecological characteristics represent the integration and ultimate expression of threats to a population, the utility of monitoring traditional demographic or genetic metrics to assess recovery remains evident. This suggests recovery criteria would often be most appropriate when they include ecological characteristics.
Data availability statement
Under the OPEN Government Data Act, data are available from the U.S. Fish and Wildlife Service upon request. Contact the corresponding author or U.S. Fish and Wildlife Service, 911 NE 11th Avenue, Portland, Oregon, USA, 97232, +1-503-231-6120.
Author contributions
TW: Project administration, Writing – original draft, Funding acquisition, Conceptualization, Investigation, Formal Analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the U.S. Fish and Wildlife Service.
Acknowledgments
Genuine gratitude to those, unfortunately too numerous to list completely, with whom I have shared deliberations, and sometimes quixotic ideas, on conservation, monitoring, threats and the recovery of Bull Trout. Specific thanks to R.D. Nelle, R. Peters, J. Romine, P. Sankovich and J. Vazquez for help identifying this need, formative discussions, and manuscript review as well as J. Castro for espousing this work.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings and conclusions in this manuscript are those of the author and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
References
Barnett H. K., Paige D. K., and Belknap W. C. (2013). Impact of reservoir elevation during the spawning season on the distribution of Bull Trout redds. N. Amer. J. Fish. Manage. 33, 917–925. doi: 10.1080/02755947.2013.818081
Barrows M. G., Anglin D. R., Sankovich P. M., Hudson M., Koch R. C., Skalicky J. J., et al. (2016). Use of the Mainstem Columbia and Lower Snake River by Migratory Bull Trout: Data Synthesis and Analyses (Columbia River Fisheries Program Office, Vancouver, Washington: Report prepared for the U.S. Fish and Wildlife Service Report).
Brenkman S. J., Peters R. J., Tabor R. A., Geffre J. J., and Sutton K. T. (2019). Rapid recolonization and life history responses of Bull Trout following dam removal in Washington’s Elwha River. N. Amer. J. Fish. Manage. 39, 560–573. doi: 10.1002/nafm.10291
Burkhead N. M. (2012). Extinction rates in North American freshwater fishes 1900–2010. BioScience 62, 798–808. doi: 10.1525/bio.2012.62.9.5
Campbell S. P., Clark J. A., Crampton L. H., Guerry A. D., Hatch L. T., Hosseini P. R., et al. (2002). An assessment of monitoring efforts in endangered species recovery plans. Ecol. Appl. 12, 674–681. doi: 10.1890/1051-0761(2002)012[0674:AAOMEI]2.0.CO;2
Ceballos G., Ehrlich P. R., Barnosky A. D., García A., Pringle R. M., and Palmer T. D. (2015a). Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253. doi: 10.1126/sciadv.1400253
Ceballos G., Ehrlich A. H., and Ehrlich P. R. (2015b). The annihilation of nature: human extinction of birds and mammals. (Baltimore, Maryland: Johns Hopkins University Press).
Ceballos G., García A., and Ehrlich P. R. (2010). The sixth extinction crisis: Loss of animal populations and species. J. Cosmol. 8, 31.
Dirzo R., Ceballos G., and Ehrlich P. R. (2022). Circling the drain: the extinction crisis and the future of humanity. Phil. Trans. R. Soc B. 377, 20210378. doi: 10.1098/rstb.2021.0378
Doak D. F., Himes Boor G. K., Bakker V. J., Morris W. F., Louthan A., Morrison S. A., et al. (2015). Recommendations for improving recovery criteria under the US Endangered Species Act. BioScience 65, 189–199. doi: 10.1093/biosci/biu215
Duda J. J., Torgersen C. E., Brenkman S. J., Peters R. J., Sutton K. T., Connor H. A., et al. (2021). Reconnecting the Elwha River: spatial patterns of fish response to dam removal. Front. Ecol. Evol. 9, p.765488. doi: 10.3389/fevo.2021.765488
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31, 4773–4787. doi: 10.1016/j.cub.2021.08.062
Estrada A., Garber P. A., Rylands A. B., Roos C., Fernandez-Duque E., Di Fiore A., et al. (2017). Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 3, p.e1600946. doi: 10.1126/sciadv.1600946
Evansen M., Carter A., and Malcom J. (2021). A monitoring policy framework for the United States Endangered Species Act. Environ. Res. L. 16, p.031001. doi: 10.1088/1748-9326/abe0ea
Fairchild J. F., Feltz K. P., Sappington L. C., Allert A. L., Nelson K. J., and Valle J. (2009). An ecological risk assessment of the acute and chronic toxicity of the herbicide picloram to the threatened bull trout (Salvelinus confluentus) and the rainbow trout (Onchorhyncus mykiss). Archiv. Environ. Contam. Toxic. 56, 761–769. doi: 10.1007/s00244-008-9227-1
Ferson S., Burgman M., and Lee D. C. (2000). Assessing land-use impacts on bull trout using Bayesian belief networks. Quant. Meth. Conserv. Biol. 2000, 127–147. doi: 10.1007/0-387-22648-6_9
Fraley J. J. and Shepard B. B. (1989). Life history, ecology, and population status of migratory bull trout (Salvelinus confluentus) in the Flathead Lake and River system, Montana. Northw. Sci. 63, 33–143. doi: 10.2307/3763119
Gerber L. R., Demaster D. P., and Kareiva P. M. (1999). Gray whales and the value of monitoring data in implementing the US Endangered Species Act. Conser. Biol. 13, 215–1219. doi: 10.1046/j.1523-1739.1999.98466.x
Gerber L. R. and Hatch L. T. (2002). Are we recovering? An evaluation of recovery criteria under the US Endangered Species Act. Ecol. Appl. 12, 668–673. doi: 10.1890/1051-0761(2002)012[0668:AWRAEO]2.0.CO;2
Gimenez M. (1996). Salvelinus confluentus, In the: IUCN 2011, IUCN Red List of Threatened Species, Version 2011.2 (Switzerland: International Union for Conservation of Nature Gland). Available online at: www.iucnredlist.org.
Haas G. R. and McPhail J. D. (2001). The post-Wisconsinan glacial biogeography of bull trout (Salvelinus confluentus): a multivariate morphometric approach for conservation biology and management. Can. J. Fish. Aquat. Sci. 58, 2189–2203. doi: 10.1139/f01-139
Harris J. B. C., Reid J. L., Scheffers B. R., Wanger T. C., Sodhi N. S., Fordham D. A., et al. (2012). Conserving imperiled species: a comparison of the IUCN Red List and US Endangered Species Act. Conser. Lett. 5, 64–72. doi: 10.1111/j.1755-263X.2011.00205.x
Himes Boor G. K. (2014). A framework for developing objective and measurable recovery criteria for threatened and endangered species. Conser. Biol. 28, 33–43. doi: 10.1111/cobi.12155
Homel K., Budy P., Pfrender M. E., Whitesel T. A., and Mock K. (2008). Evaluating genetic structure among resident and migratory forms of bull trout (Salvelinus confluentus) in Northeast Oregon. Ecol. Freshw. Fish. 17, 465–474. doi: 10.1111/j.1600-0633.2008.00299.x
Howell P. J., Colvin M. E., Sankovich P. M., Buchanan D. V., and Hemmingsen A. R. (2016). Life histories, demography, and distribution of a fluvial bull trout population. Trans. Amer. Fish. Soc 145, 173–194. doi: 10.1080/00028487.2015.1105870
Howell P. J. and Sankovich P. M. (2012). An evaluation of redd counts as a measure of Bull Trout population size and trend. N. Amer. J. Fish. Manage. 32, 1–3. doi: 10.1080/02755947.2011.649192
IUCN (2025). The extinction crisis continues. Available online at: https://iucn.org/content/extinction-crisis-continues-apace (Accessed March 2025).
Kaeding L. R. and Mogen J. T. (2023). Climate-driven stream-flow impacts on bull trout metapopulation dynamics revealed by quantitative assessment of 22 years of tag–recapture data. Freshw. Biol. 68, 301–311. doi: 10.1111/fwb.14025
Lawler J. J., Campbell S. P., Guerry A. D., Kolozsvary M. B., O’Connor R. J., and Seward L. C. (2002). The scope and treatment of threats in endangered species recovery plans. Ecol. Appl. 12, 663–667. doi: 10.1890/1051-0761(2002)012[0663:TSATOT]2.0.CO;2
Leary R. F., Allendorf F. W., and Forbes S. H. (1993). Conservation genetics of bull trout in the Columbia and Klamath River drainages. Conserv. Biol. 7, 856–865. doi: 10.1046/j.1523-1739.1993.740856.x
Malcom J. W. and Li Y. W. (2018). Missing, delayed, and old: The status of ESA recovery plans. Conserv. Lett. 11, p.e12601. doi: 10.1111/conl.12601
Mallett M. C., Thiem J. D., Butler G. L., and Kennard M. J. (2024). A systematic review of approaches to assess fish health responses to anthropogenic threats in freshwater ecosystems. Conserv. Physiol. 12, coae022. doi: 10.1093/conphys/coae022
McKee M., Priest P., Ginzler M., and Black N. (1991). How representative are members of expert panels? Internat. J. Qual. Health Care 3, 89–94. doi: 10.1093/intqhc/3.2.89
McPhail J. D. and Baxter J. S. (1996). A review of bull trout (Salvelinus confluentus) life-history and habitat use in relation to compensation and improvement opportunities. Fish Management Report No. 104. (Victoria (British Columbia): British Columbia Ministry of Environment, Lands and Parks).
Minckley W. L., Marsh P. C., Deacon J. E., Dowling T. E., Hedrick P. W., Matthews W. J., et al. (2003). A conservation plan for native fishes of the lower Colorado River. BioScience 53, 219–234. doi: 10.1641/0006-3568(2003)053[0219:ACPFNF]2.0.CO;2
Mosleh A., Bier V. M., and Apostolakis G. (1988). A critique of current practice for the use of expert opinions in probabilistic risk assessment. Reliab. Engin. Syst. Saf. 20, 63–85. doi: 10.1016/0951-8320(88)90006-3
Muhlfeld C. C., Glutting S., Hunt R., Daniels D., and Marotz B. (2003). Winter diel habitat use and movement by subadult bull trout in the upper Flathead River, Montana. N. Amer. J. Fish. Manage. 23, 163–171. doi: 10.1577/1548-8675(2003)023<0163:WDHUAM>2.0.CO;2
Muhlfeld C. C., Jones L., Kotter D., Miller W. J., Geise D., Tohtz J., et al. (2012). Assessing the impacts of river regulation on native bull trout (Salvelinus confluentus) and westslope cutthroat trout (Oncorhynchus clarkii lewisi) habitats in the upper Flathead River, Montana, USA. River Res. Appl. 28, 940–959. doi: 10.1002/rra.1494
Naman S. M., Rosenfeld J. S., and Lannan A. S. (2022). Diel patterns of foraging and microhabitat use by sympatric rainbow trout and bull trout: implications for adaptive differentiation and instream flow assessment. Can. J. Fish. Aquat. Sci. 79, 223–233. doi: 10.1139/cjfas-2020-0475
NatureServe (2024). NatureServe conservation rank calculator. Available online at: https://www.natureserve.org/products/conservation-rank-calculator (Accessed July 7, 2024).
Neraas L. P. and Spruell P. (2001). Fragmentation of riverine systems: the genetic effects of dams on bull trout (Salvelinus confluentus) in the Clark Fork River system. Mol. Ecol. 10, 1153–1164. doi: 10.1046/j.1365-294X.2001.01269.x
NOAA (2011). Guidance for Monitoring Recovery of Pacific Northwest Salmon & Steelhead listed under the Federal Endangered Species Act. Available online at: https://cfw.nwcouncil.org/Content/AMS/files/NOAA_RME_GuidanceAppendices2011.pdf (Accessed February 15, 2025).
Orsi F., Geneletti D., and Newton A. C. (2011). Towards a common set of criteria and indicators to identify forest restoration priorities: An expert panel-based approach. Ecol. Indic. 11, 337–347. doi: 10.1016/j.ecolind.2010.06.001
Pimm S. L., Jenkins C. N., Abell R., Brooks T. M., Gittleman J. L., Joppa L. N., et al. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, p.1246752. doi: 10.1126/science.1246752
Rao M., Johnson A., and Bynum N. (2007). Assessing threats in conservation planning and management. Lessons Conserv. 1, 44–71. doi: 10.5531/cbc.linc.1.1.2
Regan H. M., Hierl L. A., Franklin J., Deutschman D. H., Schmalbach H. L., Winchell C. S., et al. (2008). Species prioritization for monitoring and management in regional multiple species conservation plans. Divers. Distrib. 14, 462–471. doi: 10.1111/j.1472-4642.2007.00447.x
Reid A. J., Carlson A. K., Creed I. F., Eliason E. J., Gell P. A., Johnson P. T., et al. (2019). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873. doi: 10.1111/brv.12480
Rieman B. E., Isaak D., Adams S., Horan D., Nagel D., Luce C., et al. (2007). Anticipated climate warming effects on bull trout habitats and populations across the interior Columbia River basin. Trans. Amer. Fish. Soc 136, 1552–1565. doi: 10.1577/T07-028.1
Rieman B. E., Lee D. C., and Thurow R. F. (1997). Distribution, status, and likely future trends of bull trout within the Columbia River and Klamath River basins. N. Amer. J. Fish. Manage. 17, 1111–1125. doi: 10.1577/1548-8675(1997)017<1111:DSALFT>2.3.CO;2
Rieman B. E. and McIntyre J. D. (1995). Occurrence of bull trout in naturally fragmented habitat patches of varied size. Trans. Amer. Fish. Soc 124, 285–296. doi: 10.1577/1548-8659(1995)124<0285:OOBTIN>2.3.CO;2
Sankovich P. M. and Whitesel T. A. (2019). Assessment of Bull Trout passage during operation of the Imnaha River weir 2018 Annual Progress Report. Report prepared for the U. S. Fish and Wildlife Service, Columbia River Fish and Wildlife Conservation Office, Vancouver, Washington. (Vancouver (Washington): U.S. Fish and Wildlife Service).
Shaffer M. L. (2019). “Population viability analysis,” in Challenges in the conservation of biological resources. Ed. Decker D. J. (New York (New York): Routledge), 107–118.
Shrimpton J. M., Cena C. J., and Clarke A. (2008). Response of bull trout (Salvelinus confluentus) to habitat reconnection through replacement of hanging culverts with bridges. BC J. Ecosys. Manage. 9, 71–79. doi: 10.22230/jem.2008v9n1a387
Sinnatamby R. N., Cantin A., and Post J. R. (2020). Threats to at-risk salmonids of the Canadian Rocky Mountain region. Ecol. Freshw. Fish. 29, 477–494. doi: 10.1111/eff.12531
Smith-Hicks K. N. and Morrison M. L. (2021). Factors associated with listing decisions under the US Endangered Species Act. Environ. Manage. 67, 563–573. doi: 10.1007/s00267-021-01452-3
Stuart S., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S. L., Fishman D. L., et al. (2004). Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786. doi: 10.1126/science.1103538
Sullins T. A. (2001). ESA, Endangered Species Act (Chicago, Illinois: American Bar Association Publishing).
Swanberg T. R. (1997). Movements of and habitat use by fluvial bull trout in the Blackfoot River, Montana. Trans. Amer. Fish. Soc 126, 735–746. doi: 10.1577/1548-8659(1997)126<0735:MOAHUB>2.3.CO;2
Taylor B. J. (2006). Factorial surveys: Using vignettes to study professional judgement. Brit. J. Soc. Work 36, 1187–1207. doi: 10.1093/bjsw/bch345
Thomas J. A., Telfer M. G., Roy D. B., Preston C. D., Greenwood J. J. D., Asher J., et al. (2004). Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303, 1879–1881. doi: 10.1126/science.1095046
Tonelli M. R. (1999). In defense of expert opinion. Academic medicine. J. Assoc. Amer. Med. Coll. 74, 1187–1192. doi: 10.1097/00001888-199911000-00010
Troyer C. M. and Gerber L. R. (2015). Assessing the impact of the US Endangered Species Act recovery planning guidelines on managing threats for listed species. Conserv. Biol. 29, 1423–1433. doi: 10.1111/cobi.12552
USFWS (U.S. Fish and Wildlife Service) (1998). Endangered and Threatened Wildlife and Plants: Emergency Listing of the Jarbidge River Population Segment of Bull Trout as Endangered (Portland (Oregon): U.S. Fish and Wildlife Service). Available online at: https://www.federalregister.gov/documents/1998/08/11/98-21550/endangered-and-threatened-wildlife-and-plants-emergency-listing-of-the-jarbidge-river-population.
USFWS (U.S. Fish and Wildlife Service) (1999). Determination of threatened status for Bull Trout in the coterminous United States. Available online at: https://www.federalregister.gov/documents/1999/11/01/99-28295/endangered-and-threatened-wildlife-and-plants-determination-of-threatened-status-for-bull-trout-in (Accessed November 24, 2024).
USFWS (U.S. Fish and Wildlife Service) (2005). Bull trout core area templates - complete core area by core area analysis. Eds. Fredenberg W. and Chan J. (Portland, Oregon: U.S. Fish and Wildlife Service), 1–660.
USFWS (U.S. Fish and Wildlife Service) (2012). “Bull trout recovery: monitoring and evaluation guidance, volume II,” in Report prepared for the U.S. Fish and Wildlife Service by the Bull Trout Recovery and Monitoring Technical Group (U.S. Fish and Wildlife Service, Vancouver, Washington).
USFWS (U.S. Fish and Wildlife Service) (2015). Recovery plan for the coterminous United States population of bull trout (Salvelinus confluentus) U.S. Fish and Wildlife Service, Portland, Oregon. Available online at: https://ecos.fws.gov/docs/recovery_plan/Final_Bull_Trout_Recovery_Plan_092915-corrected.pdf.
USFWS (U.S. Fish and Wildlife Service) (2016). USFWS Species Status Assessment Framework: an integrated analytical framework for conservation. (Version 3.4) (Washington, DC: U.S. Fish and Wildlife Service). Available online at: https://www.fws.gov/sites/default/files/documents/species-status-assesment-framework-2016-08-10.pdf.
USFWS (U.S. Fish and Wildlife Service) (2018). Recovery plan for the Gulf of Maine Distinct Population Segment of Atlantic salmon (Salmo salar) (East Orland, Maine: U.S. Fish and Wildlife Service, Maine Fish and Wildlife Service Complex). Available online at: https://media.fisheries.noaa.gov/dam-migration/final_recovery_plan2.pdf.
USFWS (U.S. Fish and Wildlife Service) (2024). Species Status Assessment for the Coterminous Distinct Population Segment of Bull Trout (Salvelinus confluentus), Version 1.1 (Boise, Idaho: U.S. Fish and Wildlife Service). Available online at: https://iris.fws.gov/APPS/ServCat/DownloadFile/255078.
Van Der Fels-Klerx I. H., Goossens L. H., Saatkamp H. W., and Horst S. H. (2002). Elicitation of quantitative data from a heterogeneous expert panel: formal process and application in animal health. Risk Anal. 22, 67–81. doi: 10.1111/0272-4332.t01-1-00007
White G. C. (2000). “Population viability analysis: data requirements and essential analyses,” in Research techniques in animal ecology: controversies and consequences (New York, Columbia University Press, New York), 288–331.
Whitesel T. A., DeHaan P. W., Doyle J., Adams B. A., and Sankovich P. M. (2022). Evaluating the success of a conservation reintroduction: The case of bull trout in the Wallowa River. Conserv. Sci. Pract. 4, p.e12674. doi: 10.1111/csp2.12674
Wilcove D. S. and Master L. L. (2005). How many endangered species are there in the United States? Front. Ecol. Environ. 3, 414–420. doi: 10.1890/1540-9295(2005)003[0414:HMESAT]2.0.CO;2
Keywords: Bull Trout, conservation, monitoring, recovery criteria, threats
Citation: Whitesel TA (2025) Monitoring to assess the recovery status of imperiled species should be commensurate with the criteria for their recovery: the case of Bull Trout. Front. Conserv. Sci. 6:1651127. doi: 10.3389/fcosc.2025.1651127
Received: 20 June 2025; Accepted: 18 August 2025;
Published: 19 September 2025.
Edited by:
David R. Breininger, University of Central Florida, United StatesReviewed by:
Eric Walther, University of Georgia, United StatesCopyright This work is authored by Timothy A. Whitesel on behalf of the U.S. Government and as regards Dr. Whitesel and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy A. Whitesel, dGltb3RoeV93aGl0ZXNlbEBmd3MuZ292
†ORCID: Timothy A. Whitesel, orcid.org/0000-0003-4452-2807
 Timothy A. Whitesel
Timothy A. Whitesel