- Department of Animal Sciences, Colorado State University, Fort Collins, CO, United States
The North American bison (Bison bison) is gaining prominence in agricultural production systems across North America; however, comprehensive species-specific management guidelines, particularly regarding nutrition, remain limited. Vitamins and minerals are critical components of animal nutrition, playing essential roles in health maintenance and productivity. As bison production expands, the demand for precise nutritional benchmarks tailored to the species becomes increasingly evident. This scoping review aimed to (1) synthesize existing data on vitamin and mineral concentrations in bison tissues and (2) identify gaps in the current knowledge base to enhance bison health, welfare, and productivity. The review evaluated literature addressing vitamin and mineral profiles across various tissues in bison, while highlighting emerging areas of research, including the influence of environmental conditions and feeding systems (e.g., grass- versus grain-finished diets) on micronutrient status. Furthermore, although limited data were available, initial findings suggest sex and age do not impact micronutrient concentrations in bison tissues. These results underscore the need for further targeted research to refine and advance our understanding of micronutrient status of bison in order to develop a robust baseline data set to begin experiments to determine mineral and vitamin requirements for bison.
1 Introduction
The North American bison (Bison bison), once a symbol of the North American plains, has become increasingly significant within agricultural systems across the US and Canada (Freese et al., 2007; USDA National Agricultural Statistics Service, 2022). With the growth of bison populations and their use in commercial and tribal production systems, there is increasing interest in their biology and nutritional management, especially under confined conditions that differ from traditional grazing environments (Galbraith et al., 2014; Hecker et al., 2021). However, current nutritional data are largely derived from wild populations or from studies on European bison (Bison bonasus) in European reserves (Richmond et al., 1977; Wolk and Krasinska, 2004). This leaves significant gaps in understanding the specific dietary needs of farmed bison.
Minerals and vitamins are fundamental to livestock health, influencing growth, immunity, reproduction, and overall performance (Puls, 1994; McDowell, 2006). Yet, research on mineral content in bison tissues has mostly focused on macro- and micro-mineral concentrations in organs like the liver and muscle, often using cattle as a comparative baseline (Marchello et al., 1989; Galbraith et al., 2006; Durkalec et al., 2018). Vitamins are even less studied, with limited work addressing their role beyond growth and meat quality, with a particular focus on vitamins A and E (Driskell et al., 2004; Galbraith et al., 2016; Wagner et al., 2023). Although studies in cattle show the importance of vitamins for immune support and antioxidant function, such insights have yet to be fully explored in bison, which differ in digestive physiology and natural diets (Delgiudice et al., 1994; Raynor et al., 2015).
As the bison industry grows, there is a clear need for species-specific nutritional data. This review aims to 1) synthesize available data on vitamin and mineral concentrations in bison tissues and 2) identify knowledge gap, to enhance bison health, welfare, and productivity (NFACC, 2017). As discussed later, there are limitations to this review. Specifically, this review encompasses both North American and European bison. This introduces variability, as these species most likely have different nutritional profiles. However, with the limited data available on mineral and vitamin status of North American bison, both species were included in this review.
2 Methods
This scoping review was completed using the methodological framework detailed by Arksey and O’Malley (2005) and expanded by Levac et al. (2010) and applied the reporting guidelines from the PRISMA checklist and flow diagram (Page et al., 2021).
2.1 Eligibility criteria
This scoping review focuses on two closely related bison species: the North American bison (Bison bison; Plains bison) and the European bison (Bison bonasus). Their shared genus (Bison) suggests important similarities, making them both interesting subjects for this study. Furthermore, the review encompasses both free-range and captive bison populations of all ages and sexes. The search prioritized studies investigating vitamin and mineral concentrations within various tissue types including all known vitamins and both macro and micro minerals. Tissue samples included were both external (e.g., skin, hair, and hooves) and internal (e.g., organs, blood, and bone) obtained either from live or recently deceased animals. Only papers with at least an abstract available in English were considered. Additionally, the focus was strictly on primary research, excluding reviews or secondary analyses. There are many terms used for bison. While “buffalo” is sometimes used interchangeably with “bison,” papers solely using “buffalo” without clarifying the species were examined to ensure the focus of the paper was on Bison bison or Bison bonasus. If the origin of the term “buffalo” was unclear and could not be definitively identified as referring to bison, the study was excluded from this review. Studies focusing on Cape buffalo (Syncerus caffer), water buffalo (Bubalus spp.), and/or Indian bison/buffalo (Bos gaurus) were not included in this study. Finally, the analysis solely considered raw or uncooked meat tissue samples, eliminating studies that analyzed cooked bison meat. When identifying statistical differences in data reported by researchers cited in the discussion section, P ≤ 0.05 was considered significant. P-values from cited literature were not included in the discussion section, therefore, the use of terms such as “greater” or “lesser” in the discussion section should be considered significant at the P ≤ 0.05 level.
2.2 Search strategy
Three academic databases were searched for this review: PubMed, CAB Abstract, and Web of Science. Filters were applied within each database to limit results to articles published in English and designated as primary research (excluding reviews or meta-analyses).
The core search concepts combined terms related to the target population (“North American bison” OR “European bison”) with the topic of interest (“vitamin* AND mineral* AND tissue*”). Finally, to ensure data accuracy, any studies referencing “water buffalo” were excluded from further analysis. Initial testing, using a few key reference papers for comparison, revealed a significant issue with the use of the term “buffalo”; using the word “buffalo” captured both many irrelevant and relevant studies. Consequently, we developed a tailored search string used for each database, ensuring optimal retrieval of relevant studies while minimizing the inclusion of unwanted “buffalo” references. Table 1 includes the databases utilized, as well as the complete search string used for procurement of articles for the scoping review. Databases and search string for the scoping review covering mineral and vitamin status within tissues of bison. This comprehensive search was conducted on March 1st, 2024.
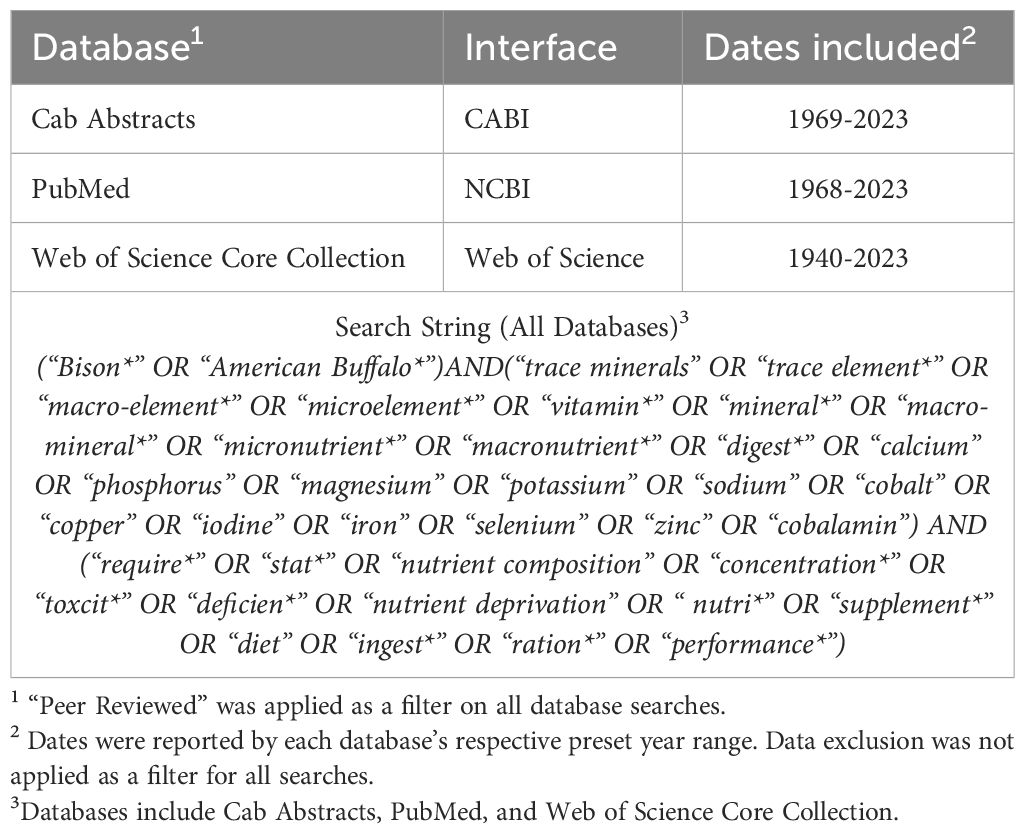
Table 1. Databases and search string for a scoping review covering vitamin and mineral concentrations within various tissues in bison.
2.3 Article screening
After each search was conducted, all retrieved articles were downloaded and imported into a reference management software (Zotero, Fairfax, VA). Following this, a two-stage screening process was implemented. In the first stage, two researchers independently reviewed all article titles and abstracts, discarding any studies that did not meet the inclusion criteria (Table 2). The population was either North American bison or European bison; “buffalo” with no indication of specific species was excluded. The text had to be original research and be available in English. Articles needed to evaluate mineral or vitamin concentrations in liver, kidney, muscle, blood, hair, bone, or other organs; studies that only evaluated cooked meat were not included. Next, the full texts were screened by both researchers using inclusion and exclusion criteria. Disagreements on article inclusion were resolved through discussion to ensure consensus. Finally, any relevant studies not captured by the database searches and reported via bibliography searches from original database search results were (n = 6) manually added to Zotero, ensuring a comprehensive pool of potential sources for the paper.

Table 2. Inclusion and exclusion criteria for article screening for the scoping review investigating mineral and vitamin status within tissues in bison.
2.4 Data charting and synthesis of results
Data extraction followed a two-step process. First, both reviewers collaboratively defined the key variables of interest to be extracted from the articles, including authors, year of publication, journal, population, sample size, and region of study. Other variables of interest that were recorded were categorized as stated in Table 3. Subsequently, one researcher systematically reviewed each article and extracted the identified variables. All extracted data were then compiled and organized in a Microsoft Excel spreadsheet. To facilitate analysis, the variables were categorized as either predictor or outcome variables. A “predictor” is any variable used to predict the value of another variable, called the “outcome”. Additionally, the number of articles investigating each individual variable was documented. Any duplicate articles were eliminated from the final counts utilizing Zotero software.
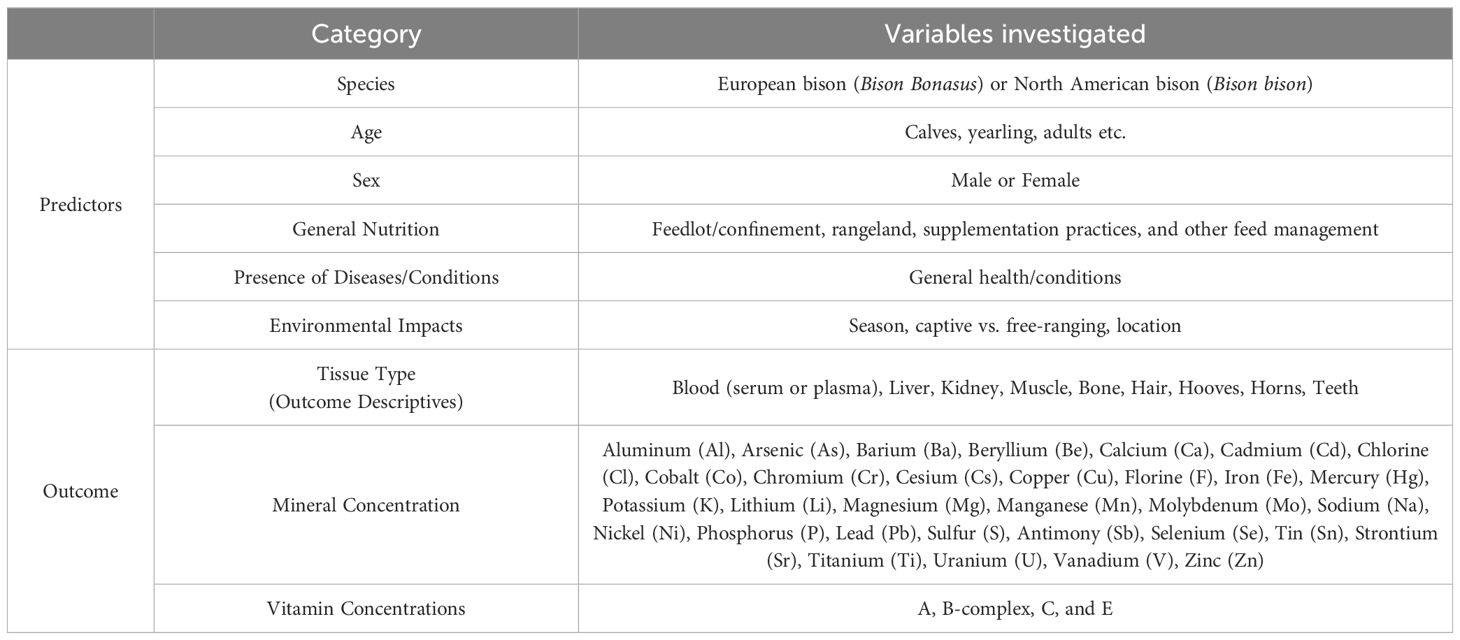
Table 3. Predictor and outcome categories from studies included in analysis (n = 51) for the scoping review.
3 Results
3.1 Characteristics of eligible studies
The initial search across PubMed, CAB Abstract, and Web of Science yielded a total of 1,039 articles. Duplicates were eliminated (n = 108) utilizing Zotero software. During title screening, 872 articles were excluded due to titles indicating irrelevance to the topic or the population of interest. An additional 13 articles were removed after abstract and full-text screening based upon not sampling correct tissue types, not investigating vitamin and mineral concentrations, not a primary research study, or not available in English. To ensure comprehensiveness, 6 relevant studies identified through other means (e.g., reference lists) were manually added. This selection process resulted in a final population of 51 articles deemed suitable for the objective of this scoping review. A flow diagram outlines in the search results (Figure 1).
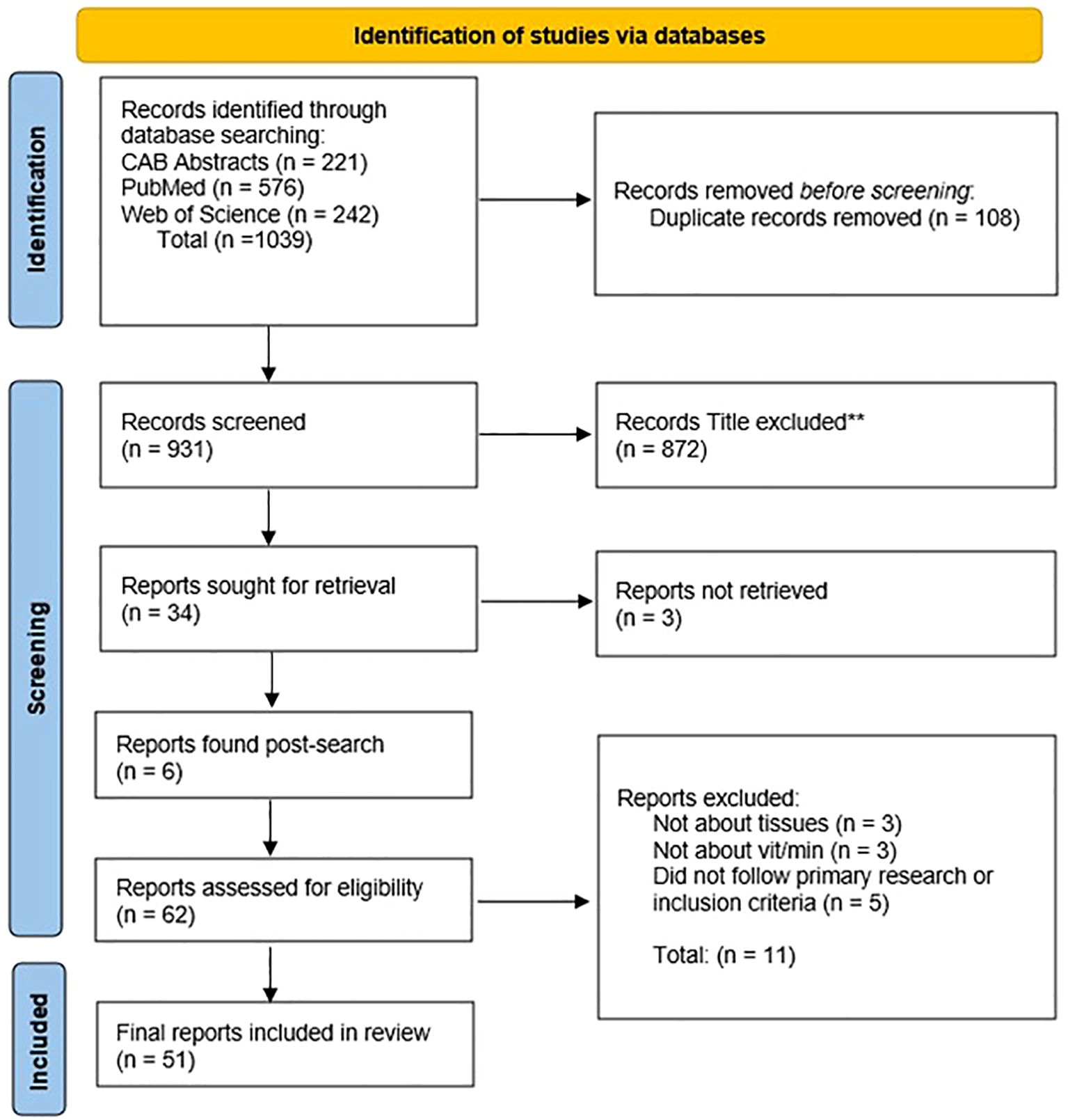
Figure 1. PRIMSA flow diagram outlining the identification and inclusion of articles representing mineral and vitamin status within tissues of bison. Flow chart modified from the PRIMSA 2020 Statement (Page et al., 2021).
The studies originated from diverse geographic locations, with a concentration in Poland (n =26), particularly the Białowieża Forest (n = 24). The United States (n = 18) also contributed a notable portion of studies, with representation from various states such as South Dakota (n = 7), Wyoming (n = 5), and Michigan (n = 5). Canada (n = 8) was another source, with studies from Alberta (n = 3) and other provinces. Publication dates ranged from 1975 to 2023, with a trend of studies from Europe collecting data during the winter months due to opportunistic herd culling of European bison.
Sample sizes in studies varied, ranging from 3 to 257 bison, with an average of approximately 72 animals per study. However, three studies did not report final sample numbers and one study provided data on bison cuts instead of whole animals. The study population identified a near-equal split between studies on European bison (n = 27) and North American bison (n = 25). This distribution mirrored the geographic spread, with European bison studies concentrated in Poland and Spain, and North American bison studies in the US and Canada.
Management practices varied across studies. Seven studies investigated both free-range and captive bison, while 27 studies focused on only free-range bison and 16 focused on captive/privately owned bison. Two studies did not specify the management type. The age of bison within studies was also varied, with a reported range of less than 1 year to 25 years of age. Calves (under 1 year) were included in 12 studies, while studies on bison aged 1–3 years (n = 38) and older than 3 years (n = 21) represented the majority of studies. However, 12 studies lacked age data.
Sex distribution within studies revealed a greater concentration focused on mixed-sex groups (n = 32). Ten studies focused solely on bulls, while only one study investigated solely females. The sex of the bison was not reported in 9 studies.
The studies offered a variety of dietary information. The most common diet category was “woodlands” (n = 26), encompassing elements such as moss, coniferous trees, root vegetables, and supplemental hay. Additionally, studies documented full concentrate diets with minimal forage (n = 13), rangeland grazing with minimal supplementation (n = 15), and diets with unreported composition (n = 2). One outlier study reported a mixed vegetable diet.
3.2 Predictors
A descriptive summary of predictors within each category of included studies was recorded and is displayed in Figure 2. Age (n = 37) and sex (n = 42) were the highly reported categories due to these factors contributing to mineral and/or vitamin baseline status. Studies also largely investigated diet or feed management (n = 49) and their subsequent mineral and vitamin concentrations within tissues due to diet being highly correlated with mineral and vitamin metabolism. Diet and feed are also very connected with whether the animals were captive or free-range.
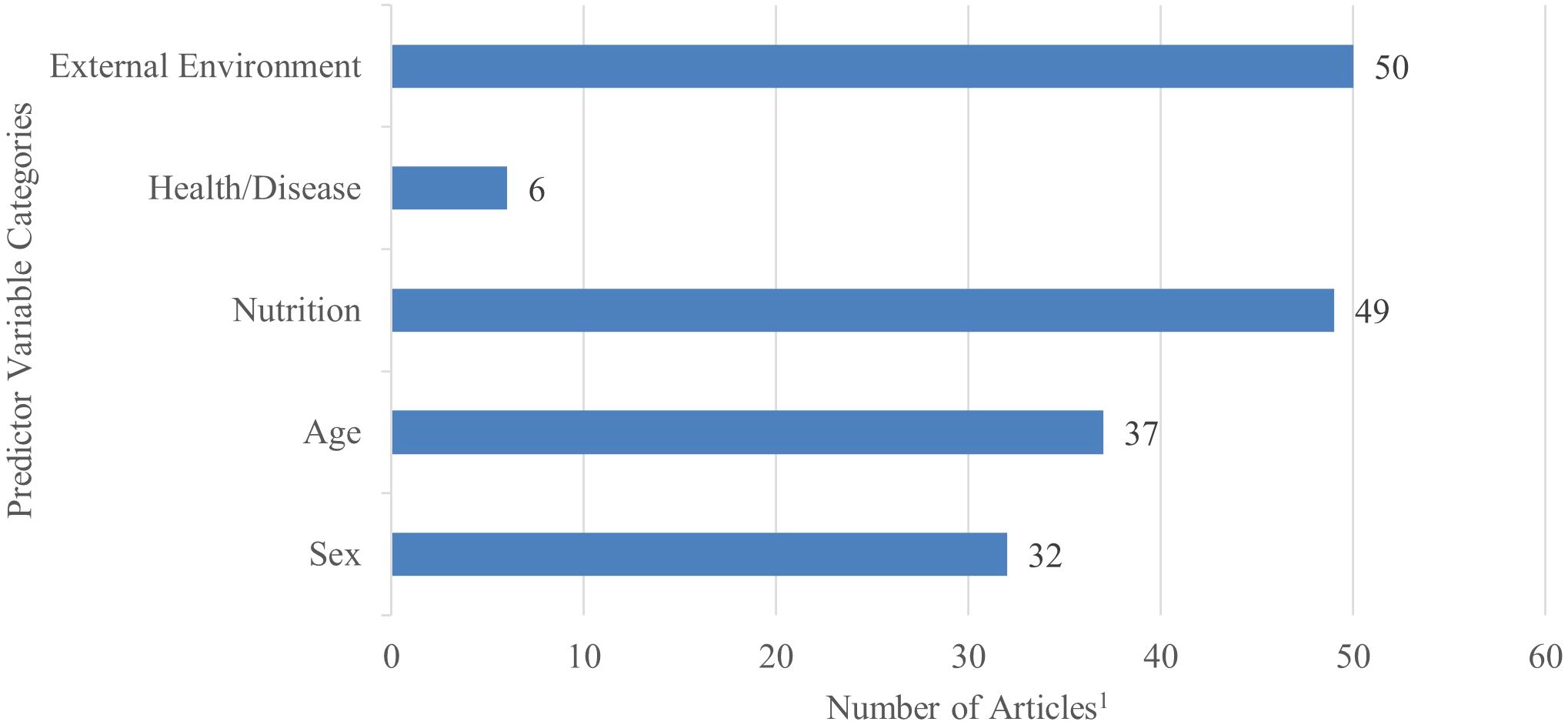
Figure 2. Number of studies per each predictor category comprised in the final scoping review analysis out of the total collection (n = 51). Several studies conveyed multiple factors within each category, therefore the total number of variables reported in each category represent more than total studies contained in the analysis.1.
3.3 Outcomes
After evaluating the 51 studies, 2 outcome groups were categorized: mineral concentrations and vitamin concentrations. Figure 3 demonstrates the division by tissue type for the studies within the scoping review. Figure 4 displays the distribution of minerals and vitamins analyzed in studies of interest for the review.
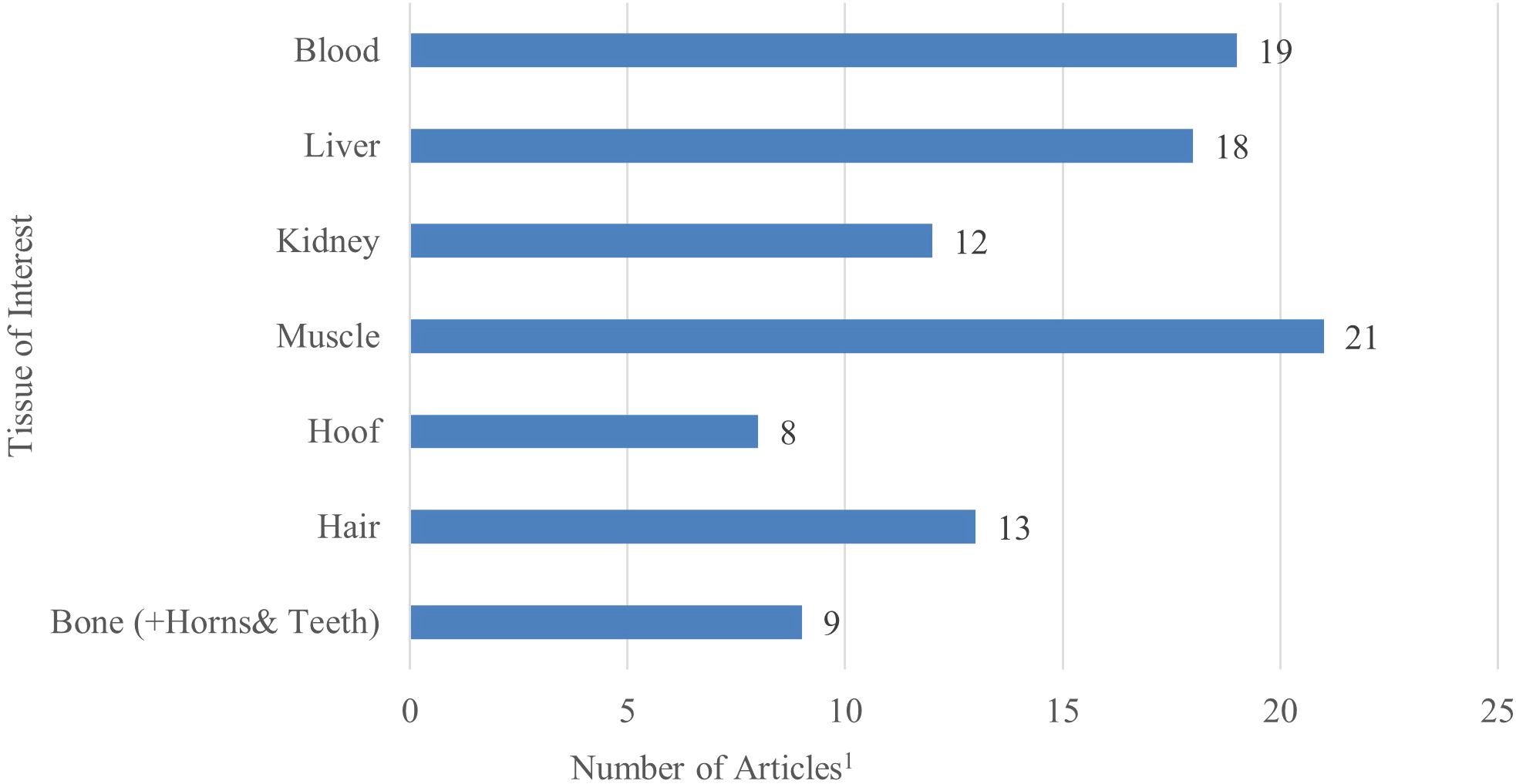
Figure 3. Number of studies per each outcome category of tissue of interest in the final scoping review analysis (n = 51). Several studies reported multiple outcomes within each category, therefore the total number of variables reported in each category represents more than total studies included in the analysis.1.
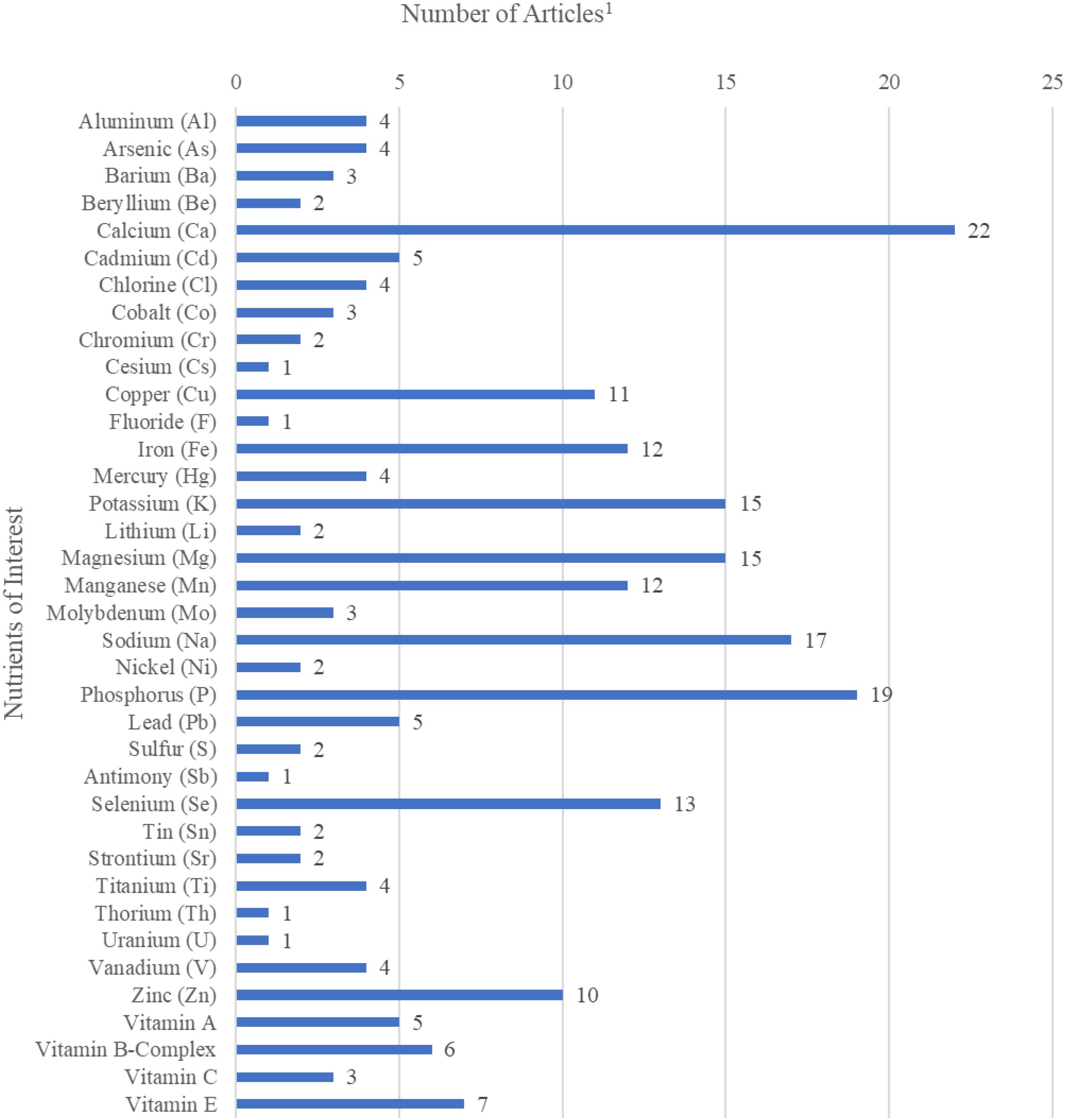
Figure 4. Number of studies per each outcome variable of nutrient of interest (mineral or vitamin) in the final scoping review analysis (n=51). Several studies reported multiple outcomes within each category, therefore the total number of variables reported in each category represents more than total studies included in the analysis.1.
4 Discussion
4.1 Factors and outcomes
The scoping review revealed that the majority of studies centered on the topics of nutrition and external environment (i.e., management practices) in bison populations (Dębska et al., 2006a, b, Kosla et al., 2011; Marchello et al., 1998; Sikarskie et al., 1990). This focus is likely due to the well-established link between an individual’s physiological status and its capacity for nutrient intake and forage utilization (Hawley and Peden, 1982). Research related to sex and age was the next most studied area, frequently overlapping with investigations into nutrition and management (Kosla et al., 2010; Peinado et al., 1999; Vestweber et al., 1991). Such studies highlight the interconnectedness of these factors, as age and sex are known to influence nutritional requirements, growth rates, and reproductive behaviors in bison (Church, 1988).
In contrast, health was identified as the least researched domain among the articles included in this review (Dębska et al., 2005; Klich et al., 2021; Rhodes et al., 2018; Wolk and Krasinska, 2004). The limited focus on health-related factors may be attributable to the challenges associating health condition with bison mineral and vitamin status. These elements are critical to overall health but remain understudied, likely due to the complexities involved in measuring their bioavailability and interactions within the bison’s unique physiology (Klich et al., 2021; Dębska et al., 2005). This gap in the literature suggests that future research exploring health-related variables could provide valuable insights, particularly in the context of improving conservation and management practices.
4.2 Factor and outcome relationships
The remainder of the discussion section is structured to examine mineral and vitamin concentrations across different tissues and their associations with relevant predictors as reported in included studies. Each section is organized by mineral or vitamin outcome and then is sub-sectioned by the main predictor of interest within the study. Within each mineral or vitamin category, tissue type outcomes are discussed in detail. Additionally, given that many studies included in this review rely on cattle reference ranges for comparative purposes, these comparisons are incorporated where applicable.
4.3 Minerals
4.3.1 Calcium and Phosphorus
4.3.1.1 Sex
Notably, calcium (Ca) and phosphorus (P) (typically reported as total Ca and P) concentrations in male and female bison did not differ in many of the studies. In general, Ca and P tissue concentrations of bison are rarely impacted by sex. For example, Zaugg et al. (1993) and Wołk and Józefczak (1988) both reported no differences in serum Ca concentrations between males and females in North American bison and European bison, respectively and similarly with concentrations of P. Comparable results were observed by Wolk and Krasinska (2004) and Wolf et al. (2004), with no sex differences in healthy European bison. Additionally, studies investigating Ca concentrations in various tissues also showed no differences between sexes (Kośla et al., 2010). When investigating hair concentrations of P in European bison, authors reported no difference in concentrations between sexes (Kosla et al., 2011). Witkowska and Kotik (1987) also reported no differences in P concentrations (inorganic P and total P) in longissimus muscle tissues between sexes of European bison. However, more recent research by Klich et al. (2021, 2023) noted that males have a tendency to have greater Ca concentrations in the liver compared to females. The authors hypothesized that this is possibly due to the increased Ca demand in females during lactation. These variations underscore the complexity of Ca metabolism in bison and indicate that while sex may not always be a critical factor, certain physiological states, such as milk production, could influence Ca distribution in females. Interestingly, meat cuts from North American bison bulls and heifers showed differences in Ca content within individual cuts, but no overarching sex differences for similar cuts (Galbraith et al., 2006). These findings collectively suggest that sex does not play a key role in influencing Ca and P concentrations across tissues and species, with any observed differences likely attributable to external factors rather than intrinsic sex differences.
4.3.1.2 Age
Calcium status across different ages of bison revealed varied findings, with most studies indicating no differences across ages, while P concentrations appear to be strongly influenced by age across several studies within this review, mostly attributed to the physiological demands associated with growth and development. For instance, Zaugg et al. (1993) reported greater serum P concentrations in calves (9.62 mg/dl) compared to bulls (8.38 mg/dl) and cows (5.5 mg/dl). Wołk and Józefczak (1988) observed that P concentrations decreased with age in European bison, with younger animals under two years old having greater inorganic P concentrations (3.5 mg/dl) than adults (2.8 mg/dl), a pattern echoed by Vestweber et al. (1991) in North American bison. Marler (1975) reported no difference in serum Ca concentrations between North American bison under 2 years (11.0 mg/dl) and those over 2 years (10.6 mg/dl). Similarly, a study on European bison by Wolk and Krasinska (2004) reported no age-based differences in ionized Ca concentrations across groups less than 1 year, 1–4 years, and over 4 years. However, there were some notable age-related patterns; Wolf et al. (2004) observed a difference in the Ca-to-K ratio between European bison calves (1.2) and adults (1.00) but no variation in serum P. Peinado et al. (1999) also reported no variation in serum P between calves and adult bison in their study. Vestweber et al. (1991) reported a decrease in serum Ca with advancing age in North American bison from 6 months to 23 months. Kośla et al. (2010) reported no difference in Ca concentrations across tissues (liver, kidney, rib, muscle, hair, hoof) between European bison under and over 1 year of age, except for muscle, where older bison had greater concentrations. Additionally, Dębska et al. (2006a) reported no Ca differences in hair, serum, liver, or kidney between European bison calves, youth, and adults, but reported a decrease in serum, hair, and kidney P concentrations with age. However, age differences in muscle P concentrations were observed by Witkowska and Kotik (1987), where younger European bison showed greater P content in Longissimus dorsi muscle. When authors also compared these values to beef cattle values, hair Ca concentrations were notably lower than those reported for beef cattle. Overall, these findings suggest that while Ca concentrations remain consistent across ages, specific physiological demands, such as growth or muscle development, may influence Ca distribution in certain tissues.
4.3.1.3 Nutrition
Studies investigating the relationship between nutrition and Ca and P status in bison focused largely on grass-fed versus grain-fed animals. Marchello and Driskell (2001) reported no difference in Ca content between grass- and grain-finished North American bison meat, but discovered that grass-finished North American bison P concentrations varied among cuts, with the top sirloin and top round containing greater amounts than ribeye cuts. Similarly, Marchello et al. (1989, 1998) reported Ca concentrations of 4.0-6.0 mg/100g wet weight in grain-fed North American bison. Further, Marchello et al. (1998) investigated North American bison fed concentrate diets, finding difference in P concentrations among cuts of ribeye, sirloin, top round, and clod. Dębska et al. (2006b) observed that captive European bison had greater serum Ca, hair Ca, and hair P concentrations compared to free-range counterparts, indicating for the authors that natural forage may offer more bioavailable Ca. Contradictory, Wolk and Krasinska (2004) speculated that their discovery of low Ca serum concentrations in European bison during winter might be due to Ca-deficient fodder in the Białowieża forest, a key habitat for European bison in Poland. Kośla (1993) also noted lower serum Ca concentrations in European bison compared to cattle despite appropriate fodder content, but both serum and hair concentrations of P results were within recommended ranges for ruminants. Additionally, Keith et al. (1978) reported no difference in serum Ca or P between North American bison fed high- and low-energy/nitrogen diets. Hawley and Peden (1982) further noted that in North American bison, P concentrations in serum increased with greater crude protein content in the diet and decreased with greater dietary energy concentrations, with bison consistently having greater serum P concentrations than cattle. Overall, while nutrition plays a role in Ca and P concentrations, most studies reported inconsistent results between feeding types.
4.3.1.4 Health and disease
Studies investigating the relationship between health conditions and Ca status in bison reveal few differences in Ca concentrations between healthy and diseased animals, with balanoposthitis being one of the most researched diseases reported in the review. Miller et al. (1989) reported no significant difference in serum Ca and P concentrations in North American bison compared to the cattle average reported in the study, and no variation between treatment groups for tuberculosis. The P and Ca concentrations in these bison fell within the reference ranges for cattle. Similarly, Wolk and Krasinska (2004) reported no significant difference in Ca concentrations between posthitis-affected European bison males and healthy counterparts. Wolf et al. (2004) also reported no significant Ca or P serum concentrations disparity between healthy European bison males and those with balanoposthitis. Dębska et al. (2005) noted that while Ca concentrations in bison with balanoposthitis were lower than cattle reference values, the difference between healthy and affected bison were not different for either Ca or P concentrations. Both values were within the reference ranges for cattle, reinforcing that common diseases did not significantly disrupt Ca or P homeostasis in bison. Investigating other diseases, Marczuk et al. (2015) reported no significant difference in serum P and Ca concentrations in European bison with chronic liver flukes compared to unaffected animals. Moreover, liver Ca concentrations showed no associations with comorbidities in European bison (Klich et al., 2023). Overall, the research suggests that certain disease conditions do not impact Ca concentrations in bison, with most findings showing minor variation between healthy and diseased individuals for both P and Ca concentrations.
4.3.1.5 External environment
Research on captive animals (versus free-range animals) shows that environmental conditions studied do not often result in differences in Ca concentrations, but do show differences of concentrations of P. Sikarskie et al. (1990) reported no difference in serum Ca concentrations between captive and free-range North American bison; however, observed a notable difference in serum P concentrations with captive bison having an average concentration of 6.5 mg/dl compared to 4.37 mg/dl in free-range bison. The authors attributed this difference to variations in protein intake and highlighted how management practices and nutrition can impact P status. For example, phytate in grain is known to bind P and reduce P availability and to some extent the availability of Zn in grazing herbivores. Although ruminants are thought to be less sensitive to phytate due to phytase activity provided by the rumen microorganisms, limited information is available regarding the phytase activity in the rumen of bison. Therefore, bison fed in captivity receiving, greater amounts of grain compared to grazing bison, may need an alternative management plan to ensure optimal mineral nutrition. Furthermore, site-specific variations in bison populations have been observed by Klich et al. (2021), reporting that European bison living in the Bieszczady Mountains had greater liver Ca concentrations as compared to a variety of other sites in Poland. The authors speculated this is potentially due to elevated Ca concentrations in mountainous water sources. Additionally, Dębska et al. (2006b) reported greater serum and hair Ca concentrations in captive European bison compared to free-range animals, though all values were below reference concentrations for cattle. Geographic differences between North American bison populations were also noted by Brzuski et al. (2010), who reported lower serum Ca concentrations in North American bison imported into Poland beginning in the early 2000’s compared to US North American bison, possibly due to limited pastureland variability or insufficient dietary supplementation in Poland. Witkowska and Kotik (1987) reported that P concentrations in muscle tissue varied due to external conditions such as time, year, and severity of environmental factors, suggesting that these variables play a critical role beyond just dietary influences. Hawley and Peden (1982) similarly reported that serum P concentrations in juvenile bison from Wood Buffalo National Park were greater in deceased individuals compared to live juveniles. Although the bison sampled were from the same geographic location, this study highlights the importance of the timing of collection, as it can significantly impact serum P concentrations. These findings suggest that while management and geo-location may influence Ca and P concentrations in bison, the differences are often subtle and may depend on specific and compounding environmental factors such as water mineral content, seasonality, and/or nutrition of available feedstuffs.
4.3.2 Sodium and Potassium
4.3.2.1 Sex
Multiple studies present varying results across different animal populations when investigating the effects of sex on sodium (Na) and potassium (K) concentrations. Zaugg et al. (1993) examined free-ranging North American bison in Yellowstone and reported that bulls had greater serum K concentrations compared to cows. These K concentrations were notably greater than those reported in other bison studies, which the researchers attributed to the momentary stress (handling of animals) temporarily affecting serum K concentrations, making it difficult to determine whether the differences between sexes are physiological or situational. However, they reported no differences in serum Na concentrations between male and female bison. Similarly, Wolk and Krasinska (2004) and Kosla et al. (2015) reported no sex differences in serum Na concentrations among European bison from the Białowieża Forest. The study from Kosla et al. (2015) did find correlations between Na and K concentrations in various organs, indicating complex interactions between these electrolytes. In contrast, Galbraith et al. (2006) identified differences in Na concentrations across different cuts of North American bison meat, with bulls showing greater Na concentrations in the bottom round and heifers in the clod and sirloin. Durkalec et al. (2018) further demonstrated sex differences in European bison liver Na concentrations. Likewise, Klich et al. (2021) analyzed liver concentrations of K and reported that female European bison had greater concentrations than males. These findings suggest that while sex may not always play a role in Na and K concentrations, certain contexts, such as tissue type or sampling methods may reveal differences between observed concentrations. Further studies controlling for stressors would be needed to better understand the inherent sex-related differences in K status in bison.
4.3.2.2 Age
The impact of age on Na concentrations in bison is variable across different studies and tissues. Several studies report no age-related differences in serum Na concentrations. For example, Wolk and Krasinska (2004) reported no difference in European bison Na concentrations, but reported an age-related decline in serum K, with < 1-year-old bison at 9.42 mmol/l, 1–4 years at 8.65 mmol/l, and adults over 4 years at 7.68 mmol/l. Sikarskie et al. (1990) similarly observed no difference in captive North American bison calf and adult serum concentrations of K or Na. Peinado et al. (1999) also reported no difference between adult and young European bison serum Na concentrations. However, some studies suggest that Na concentrations can vary with age in specific contexts. Vestweber et al. (1991) reported greater Na concentrations in North American bison calves compared to those aged 7–23 months and older bison, the authors suggesting that younger bison may retain more Na. However, the authors reported no differences for K concentrations across age. Similarly, Kosla et al. (2015) reported age-dependent differences in K but not Na concentrations in tissues, with variations in rib K between younger and older European bison. Other tissues like liver and kidney had minor differences for both K and Na concentrations. Dębska et al. (2006a) also identified differences in kidney Na concentrations across age groups in European bison, with calves exhibiting lower concentrations than adults. In free-ranging Yellowstone North American bison, Zaugg et al. (1993) reported serum K concentrations in adults ranging from 5.8 - 31.5 mmol/l and in calves from 8.1 - 19.4 mmol/l. These findings suggest that while age may not influence Na and K concentrations in serum, certain tissues, such as hair and kidneys, may have age-related differences.
4.3.2.3 Nutrition
The effect of nutrition on Na concentrations in bison differs depending on the specific tissues and dietary conditions studied. Marchello and Driskell (2001) reported no differences in Na concentrations between grass-fed and grain-fed North American bison in raw lean cuts. However, they did observe differences in Na content across various cuts of meat, with the ribeye and clod showing greater Na concentrations compared to the top round. Conversely, studies focusing on diet and serum Na concentrations showed little impact of nutrition. Keith et al. (1978) reported no difference in serum Na concentrations between North American bison fed high- and low-energy/nitrogen diets, suggesting that dietary variations in energy and nitrogen do not strongly influence Na concentrations in the serum. It was reported that bison on the high-energy diet had serum K concentrations ranging from 5.2 to 4.2 mmol/l, while those on the low-energy diet had slightly lower K concentrations, ranging from 4.6 to 4.4 mmol/l. However, Kośla (1993) reported that European bison feeding on forage low in Na had relatively high Na concentrations in their hair, indicating that dietary supplementation, such as hay, likely helped compensate for low natural Na intake. Dębska et al. (2006b) further observed that European bison had lower Na concentrations in both free-range and captive diets than recommended by NRC guidelines, yet hair Na concentrations were greater in captive animals compared to free-range ones, suggested by authors that Na intake in captivity may be greater. Differences in Na content across tissues, particularly in hair and certain cuts of meat, highlight the role that diet may play in specific contexts, such as mineral access or in response to dietary supplementation.
4.3.2.4 Health and disease
The impact of Na on the health of bison, particularly European bison, appears to be minimal in terms of mineral deficiencies and overall health status based on current available literature. Durkalec et al. (2018) investigated Na deficiencies in European bison and reported no differences in liver Na concentrations between captive and free-ranging animals, with concentrations remaining above the standard reference of 800 mg/kg wet weight. This suggests that Na deficiency is not a common health issue in either population. Additionally, Dębska et al. (2005) analyzed serum Na and K concentrations between European bison balanoposthitis-positive bison and healthy individuals. Researchers reported concentrations to be within the healthy range based on cattle reference standards with no differences between healthy animals and those diagnosed with balanoposthitis. Similarly, Wolk and Krasinska (2004) reported no difference in serum K concentrations between males diagnosed with balanoposthitis and healthy individuals. However, the study noted that elevated K concentrations, or hyperkalemia, can be associated with various health issues such as kidney disorders, pneumonia, pleurisy, and acute pancreatitis. Among the examined animals, kidney cysts were present in 18 out of 102 bison, and many were diagnosed with pulmonary helminthiasis, pneumonia, or bronchitis, often due to parasitic infections. These findings indicate that Na and K concentrations in both liver and serum are generally adequate and stable, regardless of health conditions or captivity status, suggesting that Na imbalances are not a significant factor in bison health, but further research is needed to fully understand the role of K in disease pathology in bison.
4.3.2.5 External environment
Sodium concentrations in bison appear to be minimally affected by environmental factors such as geologic location and general management practices, with most studies reporting no differences across various conditions. Sikarskie et al. (1990) reported greater serum K concentrations in free-range North American bison from South Dakota compared to captive North American bison in Michigan but reported no difference in serum Na concentrations. Similarly, Marchello et al. (1989) reported no differences in Na content between North American bison and beef in the longissimus muscle or across different cuts of bison meat, with Na concentrations remaining consistent across samples. Dębska et al. (2006b) reported that captive European bison had greater serum and hair K concentrations compared to free-range bison. The greater K concentrations in captive bison were likely due to supplemented feed, which provided more available K than the natural forage consumed by free-range animals. However, variations in Na concentrations by geologic location were observed in European bison. Klich et al. (2021) reported that Na concentrations in European bison varied by site location in Poland, with bison from Knysynska Forest having lower Na concentrations than those from Białowieża, Borecka, and Bieszczady regions. Potassium concentrations varied based on animal origin as well, with serum K concentrations differing across most regions in Poland, except for those from the Knysynska and Białowieża forests where no differences were observed. This suggests that local environmental factors, such as soil composition or forage quality, might influence Na availability in certain areas. Despite this, Klich et al. (2023) reported no difference in liver Na concentrations between bison from enclosed and free-range environments or between different sites (Białowieża and Smardzewice), indicating that enclosure or geographic site might not strongly impact internal Na concentrations in European bison. Overall, Na concentrations in bison are relatively stable across management systems, though some regional variations may occur for both K and Na due to local environmental conditions, as well as free-range animals possibly having greater K variations. One challenge in comparing Na and K status in grazing and captive bison is that in certain grazing situations, supplemental forage and salt are provided during the winter months in some studies, making it difficult to determine Na and K intake.
4.3.3 Magnesium
4.3.3.1 Sex
Studies investigating magnesium (Mg) status in bison across different sexes generally report minimal differences between males and females. In free-ranging European bison from the Białowieża Forest, Kosla et al. (2011) reported no sex difference in Mg concentrations in hair. Similarly, Durkalec et al. (2018) reported no sex-based differences in hepatic Mg concentrations. Although no direct link was reported between sex and Mg concentrations in the liver, the study did note positive relationships between Co and Mg, as well as negative correlations between Mg and Na. However, Klich et al. (2023) observed that females consistently had numerically lower hepatic concentrations of several elements, including Mg, compared to males, though the numerical differences were not statistically different for Mg alone. Furthermore, Klich et al. (2021) reported no correlation between Mg and other studied factors, such as sex or environmental conditions, in their examination of hepatic trace elements. Overall, while there are some observed trends of lower Mg concentrations in females, the differences are generally not significant, and Mg appears to be consistent across sexes in both hair and liver tissues.
4.3.3.2 Age
The effects of age on Mg status in bison show varied results across different tissues and age groups, with differences observed in some tissues but not others. For North American bison, Sikarskie et al. (1990) reported no difference in serum Mg concentrations between calves and adults. However, in European bison, Kosla et al. (2012) reported age-related differences in both hair and hoof Mg concentrations, with calves having greater Mg concentrations in hair and hoof compared to adults, while other tissues like muscle, liver, and kidney showed no significant variations. Similarly, Dębska et al. (2006a) reported differences in Mg concentrations in serum, hair, and liver across age groups, with calves having greater serum Mg, hair Mg and liver Mg compared to adults, whose concentrations were lower in all three tissues. However, no differences were observed in kidney Mg concentrations across ages. In contrast, Kosla et al. (2011) reported no age-related differences in Mg concentrations in hair among free-ranging European bison. Furthermore, Klich et al. (2021) observed no correlation between Mg and age or other factors in hepatic Mg concentrations. Overall, while some tissues, particularly hair and liver, exhibit age-related differences in Mg concentrations, others, like serum and kidney, show minimal variation, indicating that Mg distribution may vary with age depending on the tissue type.
4.3.3.3 Nutrition
Research analyzing the effects of nutrition on Mg status in bison revealed both subtle differences based on diet type and differences across different meat cuts. Marchello and Driskell (2001) reported that grass-finished North American bison had slightly greater Mg concentrations compared to grain-finished bison. Among grass-finished bison cuts, the ribeye had lower Mg concentrations compared to the top sirloin and top round. In European bison, Kośla (1993) analyzed the Mg content of the diet, including grasses, forbs, and supplemented hay, and reported it inadequate for the herd’s nutritional needs based on cattle and bison references. Although European bison serum Mg concentrations fell within cattle reference ranges, hair Mg concentrations were lower than those of black and brown cattle. Furthermore, Dębska et al. (2006b) observed that free-range European bison had greater serum Mg concentrations than captive bison, suggesting that natural forage might better meet Mg requirements than supplemented diets, especially as high potassium (K) in captive diets may negatively correlate with Mg absorption.
When investigating meat concentrations of Mg, Marchello et al. (1998) reported differences among grain-fed North American bison cuts, with clod showing lower Mg concentrations than ribeye, top sirloin, and top round (23.3 vs. 24.1, 24.5, and 24.8, mg/100 g wet weight, respectively). Overall, while nutritional conditions influence Mg concentrations in bison, variations in dietary sources, such as natural forage versus supplemented feed, play a key role in determining Mg status.
4.3.3.4 Health and disease
In the studies included, there were no Mg concentration differences between healthy and diseased individuals. In European bison euthanized in Poland due to clinical signs of illness, Klich et al. (2023) reported no difference in hepatic Mg concentrations between individuals with observed clinical signs or comorbidities. This suggests that in these instances illness did not substantially affect Mg storage in the liver. Similarly, Dębska et al. (2005) reported no difference in serum Mg concentrations between healthy European bison and those diagnosed with balanoposthitis, with both values falling within cattle reference ranges. These findings indicate that Mg concentrations in both the liver and serum remain stable regardless of disease presence, highlighting that Mg status is not a reliable biomarker for diagnosing or assessing health conditions in bison. The stability of Mg concentrations across different health status suggests that other elements or factors may be more sensitive indicators of disease in bison populations.
4.3.3.5 External environment
Sikarskie et al. (1990) reported no difference in serum Mg concentrations between captive North American bison in Michigan and free-range bison in South Dakota. Similarly, Durkalec et al. (2018) reported no difference in liver Mg concentrations in European bison based on management style, with captive herds having Mg concentrations of 92.42–184.19 mg/kg wet weight and free-ranging herds showing concentrations of 96.82–169.72 mg/kg wet weight. However, Dębska et al. (2006b) reported a difference in serum Mg concentrations between captive and free-ranging European bison, although hair Mg concentrations showed no variation between management types. In terms of site differences, Durkalec et al. (2018) noted no variation in liver Mg concentrations between European bison populations in different regions, such as Białowieża and Smardzewice. Marczuk et al. (2015) also observed that European bison had greater serum Mg concentrations than dairy cattle in the same region, but no difference compared to sheep, with the presence of liver flukes having no effect on Mg concentrations. Lastly, Marchello et al. (1989) reported that North American bison had greater Mg concentrations in lean muscle compared to beef cattle. Overall, while management and geographic factors show minimal effects on Mg concentrations, captive European bison tend to have lower serum Mg compared to their free-ranging counterparts.
4.3.4 Selenium
4.3.4.1 Sex
Studies investigating selenium (Se) status in bison across different sexes indicate minimal differences between males and females in Se concentrations across various tissues. In North American bison, Medeiros et al. (1993) reported no interaction between sex and Se concentrations in muscle samples. European bison research by Kosla et al. (2019) also revealed no sex-based differences in Se concentrations, with male and female hair Se concentrations being 0.140 μg/g and 0.134 μg/g dry weight, respectively. Similarly, Se concentrations in the liver and kidneys showed no sex-related variation. Durkalec et al. (2018) further confirmed this, reporting no differences in liver Se concentrations between females and males. Overall, these findings suggest that Se distribution in bison, whether in muscle, liver, kidney, or hair, does not differ by sex.
4.3.4.2 Age
Among the studies evaluating Se status in bison, only one paper addressed age differences. Kosla et al. (2019) reported that Se concentrations in the hair of European bison were numerically greater in calves (up to 1 year of age) at 0.142 μg/g dry weight compared to individuals over 2 years of age (0.126 μg/g). Similarly, Se concentrations in the kidneys were within reference ranges for cattle but lower than those reported for free-range and experimental animals, suggesting that the bison population studied may have been Se deficient. Overall, the limited focus on age in existing literature suggests a need for further research to understand how Se status may vary across different life stages of bison.
4.3.4.3 Nutrition
Selenium status in bison across different diets reveal notable differences influenced by regional soil Se concentrations and feeding practices. Medeiros et al. (1993) reported that North American bison had greater Se concentrations in muscle samples compared to cattle, with the high Se content linked to forage grown on Se-rich soils. Driskell et al. (1997) explored Se concentrations in various bison muscle cuts (clod: 26.356 μg/g, ribeye: 23.238 μg/g, top round: 27.204 μg/g, top sirloin: 25.059 μg/g) and reported that individual bulls had varying Se concentrations, with bulls from Se-rich areas like North Dakota having greater Se concentrations. This study emphasized that bison meat, especially from Se-rich areas, could serve as a rich source of Se.
Research by Marchello et al. (1998) reported no Se differences between bison cuts from animals fed concentrate diets. Driskell et al. (2004) reported no difference in Se concentrations between grain-finished and grass-finished North American bison bulls, though Se concentrations in ribeye were slightly greater in grain-finished animals. Marchello and Driskell (2001) noted that grass-finished bison had four times greater Se content in their meat compared to grain-finished bison, highlighting that Se content in bison meat is largely dependent on dietary sources, such as grass versus grain.
4.3.4.4 Health and disease
Schillhorn van Veen et al. (1991) reported that North American bison (n = 2) from Michigan, some of which died from suspected organophosphate poisoning, had liver Se concentrations lower than cattle reference ranges. One bison showed mild fatty infiltration in the liver and brain lesions, while others displayed no pathophysiological changes. Similarly, Rhodes et al. (2018) reported elevated Se concentrations in the liver of euthanized North American bison, with concurrent Cu deficiency likely contributing to increased Se absorption, further suggesting that mineral imbalances can exacerbate health issues. In European bison, Klich et al. (2023) identified that liver Se concentrations were greater in bison with more numerous comorbidities, such as lung pneumonia. Despite the increased Se concentrations, these animals still showed overall Se deficiency based on cattle reference values, which, along with Cu deficiency, likely contributed to their deteriorating health. Additionally, Dębska et al. (2005) reported no difference in serum Se concentrations between European bison with balanoposthitis and healthy individuals, though both values were lower than cattle references. The findings highlight the critical role of Se and other trace minerals like Cu in maintaining bison health, with deficiencies potentially leading to severe health consequences.
4.3.4.5 External environment
Selenium status in bison across different environmental and management conditions highlight significant variations depending on geographic location and management practices. Sikarskie et al. (1990) reported that North American bison from South Dakota had greater serum Se concentrations compared to those from Michigan, despite the Michigan bison receiving Se supplements and injections. Similarly, MacNeil et al. (1990) reported that North American bison from Wood Buffalo National Park had deficient liver Se concentrations and marginal kidney Se concentrations, while captive bison had Se concentrations within normal cattle reference ranges, with liver and kidney Se concentrations deemed adequate. In European bison, Klich et al. (2021) reported that animals from the Bieszczady region in Poland had greater liver Se concentrations compared to other sites, likely due to greater soil Se concentrations or better forage uptake. They also observed a strong correlation between Se and Cd concentrations. However, other studies, such as Durkalec et al. (2018), noted no differences in liver Se concentrations between European bison from different sites, such as Białowieża and Smardzewice, or between enclosed and free-range bison, though all animals sampled were reported to be Se deficient based on previous literature. Additionally, Clemens et al. (1987) reported that captive North American bison had serum Se concentrations similar to white-tailed deer but not pronghorn antelope in the same environment. Overall, these studies suggest that environmental factors like soil Se content and management practices influence Se status in bison, with some populations showing deficiencies despite supplementation efforts.
4.3.5 Manganese
4.3.5.1 Sex
Studies examining manganese (Mn) status in bison across different sexes generally show minimal differences between males and females, although some variations have been noted. Skibniewski et al. (2010) reported no sex-based differences in Mn concentrations in European bison from the Białowieża Primeval Forest across various tissues, including the liver, kidney, and hair. However, female European bison had slightly greater Mn concentrations in hair compared to males, while males had greater Mn concentrations in the hoof than females. In contrast, Klich et al. (2023) reported differences in liver Mn concentrations between sexes in European bison from Poland, while an earlier study by the same author (Klich et al., 2021) reported no relationship between sex and Mn concentrations in the liver. Similarly, Durkalec et al. (2018) observed no differences in liver Mn concentrations between female and male European bison in Poland. These findings suggest that while some minor variations in Mn concentrations may occur between sexes, concentrations are generally not different across sex classes.
4.3.5.2 Age
No reported articles in this review covered the effect of age on the concentrations of Mn.
4.3.5.3 Nutrition
Research highlights minimal differences between grass- and grain-finished diets, but significant variations in Mn concentrations between different cuts of meat and comparisons with beef. Marchello and Driskell (2001) reported no difference in Mn concentrations between grass-finished and grain-finished North American bison, with grass-finished meat showing Mn concentrations of 11.5 μg/100g wet weight and grain-finished meat at 13.4 μg/100g wet weight. However, among grass-finished bison cuts, Mn concentrations varied slightly, with the clod containing the greatest Mn concentrations and top round the lowest. Marchello et al. (1998) also observed that different bison muscle cuts had varying Mn concentrations, with sirloin greater than ribeye and top round. Comparatively, bison meat contains lower Mn than beef, with Marchello et al. (1989) reporting Mn concentrations of 0.007 mg/100g wet weight in bison and 0.013 mg/100g wet weight in beef.
4.3.5.4 Health and disease
The scoping review identified only one study investigating the relationship between health conditions and Mn status in bison, specifically focusing on European bison with balanoposthitis. In this study, Dębska et al. (2005) measured serum Mn concentrations in both healthy and balanoposthitis-diagnosed European bison, finding no difference between the two groups, with both having Mn concentrations of 0.12 μmol/l. Future work is needed to evaluate disease impacts on tissue Mn concentrations in bison.
4.3.5.5 External environment
Studies investigating Mn status in bison across different management environments and locations reveal notable variations depending on habitat and management conditions. MacNeil et al. (1990) compared Mn concentrations in liver and kidney tissues of North American bison from Wood Buffalo National Park and a game ranch, finding that the game ranch bison had greater Mn concentrations in both the liver and kidney compared to bison from Wood Buffalo National Park. Based on cattle nutritional standards, the game ranch bison were classified as having adequate Mn concentrations, while those from Wood Buffalo were marginal. In Poland, however, Mn concentrations showed no relation to site location. Klich et al. (2021) reported no differences in Mn concentrations across various locations, while Durkalec et al. (2018) observed no difference between European bison from Białowieża and Smardzewice (Mn liver concentrations: 0.240-4.35 mg/kg wet weight in Białowieża versus 2.4-3.34 mg/kg wet weight in Smardzewice). In terms of management conditions, Durkalec et al. (2018) reported different Mn concentrations between free-range and captive European bison, with Mn deficiencies more prevalent in free-range animals (37%) compared to captive ones (20%). The study further suggested that liver tissue may not be a reliable indicator of Mn deficiency and recommended measuring Mn concentrations in heart muscle or plasma for a more accurate assessment of Mn status in bison. These findings emphasize the influence of environmental and management factors on Mn concentrations, particularly with more deficiencies observed in free-range populations.
4.3.6 Iron
4.3.6.1 Sex
Research in iron (Fe) status in bison across different sexes show minimal differences between males and females. In Yellowstone, Zaugg et al. (1993) reported that serum Fe concentrations had no sex-based differences. Similarly, research by Kosla et al. (2013) on European bison reported no differences in Fe content across various tissues, including the liver, kidney, muscle, rib, and hoof, despite slightly greater Fe concentrations in females across most tissues, such as the liver and kidney. In contrast, male European bison had greater Fe concentrations in the hoof compared to females. Hair Fe concentrations also showed no difference between sexes (Kosla et al., 2011). Klich et al. (2021) also reported no sex-based relationship in hepatic Fe concentrations across different European bison sites in Poland. In terms of meat cuts, Galbraith et al. (2006) observed that sirloin had the greatest Fe concentration in bulls, with differences between specific cuts, such as bottom roll and clod. In heifers, the clod and sirloin Fe concentrations were also different. Overall, while some tissue-specific variations exist, sex does not appear to influence Fe status in bison within the articles reviewed.
4.3.6.2 Age
In North American bison from Yellowstone, Zaugg et al. (1993) reported that serum Fe concentrations were generally greater in calves compared to adults; the authors suggested that younger bison may have greater circulating Fe concentrations. Similarly, Kosla et al. (2013) reported age-related differences in Fe content in various tissues of European bison. Adult bison had greater Fe concentrations in the liver and muscle compared to calves, indicating to authors that Fe accumulates more in these tissues as bison age. However, in other tissues, such as the rib and hoof, the pattern was reversed, with calves having greater Fe concentrations in the rib and hoof. Kosla et al. (2011) also examined Fe concentrations in hair across age groups and reported no difference between calves and adults, authors stating that hair may not reflect the age-related changes in Fe seen in other tissues. Overall, these studies indicate that Fe concentrations in bison vary with age, particularly in the liver and muscle.
4.3.6.3 Nutrition
The impact of nutrition on Fe status in bison reveals minor differences based on diet type. Marchello and Driskell (2001) reported no difference in Fe concentrations between grass-finished and grain-finished North American bison. Across different cuts of grass-finished bison, the Fe content was consistent, with ribeye, top sirloin, and clod all approximately 3.0 mg/100g wet weight, except for ribeye and top round. However, differences between cuts were more pronounced in grain-finished bison. Marchello et al. (1998) reported that ribeye had lower Fe concentrations than sirloin, top round, and clod, indicating variation in Fe distribution across muscle types. Additionally, in a comparison of bison and beef, Marchello et al. (1989) reported that bison had greater Fe content in the longissimus muscle compared to beef, highlighting bison meat as a richer source of Fe. These findings suggest that while diet does not drastically affect Fe content in bison meat, there are notable differences between specific cuts and between bison and other meat sources like beef.
4.3.6.4 Health and disease
Studies exploring the relationship between health conditions and Fe status in bison suggest that Fe concentrations are generally stable across various disease states, with a few exceptions. Marczuk et al. (2015) reported that European bison diagnosed with chronic liver flukes showed no evidence of changes in liver Fe concentrations, while domestic ruminants in the same study displayed slight changes in Fe concentrations depending on the presence of liver flukes. Similarly, Klich et al. (2023) investigated comorbidities in European bison and reported that Fe concentrations in the liver did not correlate with any other elements or comorbid conditions, leading the researchers to exclude Fe from further analysis regarding disease relationships. In contrast, Dębska et al. (2005) reported a difference in serum Fe concentrations between European bison diagnosed with balanoposthitis and healthy individuals, with the diagnosed animals having greater Fe concentrations compared to cattle reference data. Although Fe concentrations remained stable in most cases, other trace elements like Cu and Se showed notable changes in relation to health conditions. Klich et al. (2023) observed that as the number of comorbidities increased, Cu concentrations decreased, contributing to a weakened state in the bison. They hypothesized that this imbalance in trace elements, combined with parasitic infestations, likely contributed to reduced weight and birth rates in the affected populations.
4.3.6.5 External environment
Findings for Fe status in bison across different management practices, environments, and locations reveal notable variations influenced by habitat conditions. MacNeil et al. (1990) compared North American bison from Wood Buffalo National Park and a game ranch, finding that Fe concentrations in the liver and kidney were greater in the game ranch bison compared to those from Wood Buffalo National Park. In comparison to cattle data, the nutritional status for Fe in bison was determined: from Wood Buffalo National Park, Fe concentrations were considered marginal, while the game ranch bison had adequate Fe concentrations in both liver and kidney. This suggests that management conditions, such as controlled feeding and habitat quality, may influence Fe concentrations in bison. In a separate study, Klich et al. (2021) analyzed liver Fe concentrations in European bison from four different sites in Poland, finding that only two of the sites had differences in Fe concentrations. Overall, these studies emphasize that both natural environments and management practices can affect the Fe nutritional status of bison.
4.3.7 Copper
4.3.7.1 Sex
The scoping review identified only one study investigating copper (Cu) status in bison evaluating sex effects. Durkalec et al. (2018) examined Cu concentrations in the liver of European bison from Poland and reported no differences between males and females. The study reported a Cu range of 0.870-29.22 mg/kg wet weight for females and 2.03-49.170 mg/kg wet weight for males, with both sexes exhibiting broad variation in Cu concentrations.
4.3.7.2 Age
No reported articles in this review covered the effect age on the concentrations of Cu.
4.3.7.3 Nutrition
Studies investigating the influence of nutrition on Cu status in bison highlight slight differences based on diet type and variations across different meat cuts. Marchello and Driskell (2001) reported that grass-finished North American bison had a nonsignificant increase in Cu concentrations in raw separable lean meat compared to grain-finished bison. The Cu content was also assessed across various cuts, including ribeye, top sirloin, top round, and clod, with no differences between cuts. In a separate study, Marchello et al. (1998) reported that for bison fed concentrate diets, the sirloin cut had greater Cu concentrations compared to other cuts such as ribeye and top round. Additionally, Marchello et al. (1989) compared Cu concentrations between bison and beef, finding that bison had lower Cu concentrations in the longissimus muscle compared to beef. These findings suggest that while diet (grass vs. grain) may not impact Cu concentrations in bison meat, there are notable differences between specific cuts and in comparison, to other meats like beef.
4.3.7.4 Health and disease
Papers examining the relationship between health conditions and Cu status in bison suggest that Cu deficiencies may be linked to various disease states, but the overall impact varies across different conditions. Schillhorn van Veen et al. (1991) reported that North American bison necropsied after death had low liver Cu concentrations, which were comparable to low Cu concentrations observed in cattle. Similarly, Rhodes et al. (2018) reported low liver Cu concentrations in euthanized North American bison, speculating that parasitic infestations, like coccidia, could interfere with Cu absorption, as well as other nutrients. The authors stipulated that this malabsorption could be further exacerbated by increased absorption of competing minerals such as Se, Mo, or Zn. In European bison, Dębska et al. (2005) reported no difference in serum Cu concentrations between individuals with balanoposthitis and healthy bison, with both groups falling within cattle reference ranges. However, Klich et al. (2023) observed that European bison with fewer lesions had greater hepatic Cu concentrations, suggesting that Cu deficiencies may be associated with greater susceptibility to comorbidities. These findings indicate that while Cu deficiency does not always correlate with specific diseases like balanoposthitis, it may play a role in the overall health and resilience of the animal.
4.3.7.5 External environment
Cu status in bison across different environments and management conditions highlight the influence of location, habitat, and human activities on Cu concentrations. Skibniewska et al. (2006) reported that European bison from Białowieża Forest had Cu concentrations below standard values in various tissues. These deficiencies have authors suggesting environmental factors in Białowieża lands may impact Cu absorption. Similarly, MacNeil et al. (1990) reported that North American bison from Wood Buffalo National Park had lower liver Cu concentrations compared to those from a game ranch, though kidney Cu concentrations were adequate in both populations. This discrepancy may reflect differences in diet and habitat management, as the game ranch provided a more controlled environment. Klich et al. (2021) further demonstrated how local agricultural practices affect Cu concentrations, finding that bison from the Knyszyńska region had lower hepatic Cu concentrations due to intensive farming, where treatments of nitrogen and P may dilute Cu content in crops. Meanwhile, Durkalec et al. (2018) reported no difference in Cu concentrations between bison from Białowieża and Smardzewice, nor between free-range and captive bison, though Cu deficiencies were widespread, affecting 85% of captive and 88% of free-range bison. These findings indicate that both natural and managed environments can have deficiencies due to environmental conditions and agricultural impacts.
4.3.8 Zinc
4.3.8.1 Sex
Studies evaluating zinc (Zn) status in bison across different sexes generally have not reported differences between males and females. Kosla et al. (2004) reported a tendency for European bison males to have greater Zn concentrations in various tissues, with liver Zn concentrations averaging 172.7 mg/kg wet weight in males compared to 134.6 mg/kg wet weight in females. Despite this trend, no sex-based differences were observed across tissues. Similarly, Durkalec et al. (2018) reported wide variations in liver Zn concentrations in European bison from Poland, ranging from 30.69 to 430.12 mg/kg wet weight in males and 33.68 to 99.57 mg/kg wet weight in females, but again, there were no differences between sexes.
4.3.8.2 Age
The scoping review revealed only one study investigating Zn status in bison across different age groups. Kosla et al. (2004) reported that Zn concentrations in the kidneys and liver tended to increase with age. In their study, older European bison (over two years) had greater Zn concentrations in the kidneys compared to younger bison.
4.3.8.3 Nutrition
Research investigating the effects of nutrition on Zn status in bison reveal variations in Zn concentrations based on diet type and meat cuts. Marchello and Driskell (2001) reported no difference in Zn concentrations between grass-finished and grain-finished North American bison, suggesting that diet type has minimal impact on Zn content in bison meat. However, Zn concentrations varied between different cuts of grass-finished bison, with the clod having the highest Zn content, different from the top sirloin and ribeye, while the top round had the lowest concentrations. Similarly, Marchello et al. (1998) observed differences in Zn concentrations across cuts of bison fed concentrate diets, with clod and ribeye differing from other cuts like top round. When comparing bison and beef, Marchello et al. (1989) reported that beef had greater Zn concentrations than bison.
4.3.8.4 Health and disease
Research examining Zn status in bison in relation to health and disease conditions reveals that Zn concentrations can fluctuate based on specific illnesses, though clear patterns are not always consistent across different conditions. In North American bison, Rhodes et al. (2018) reported elevated hepatic Zn concentrations in animals euthanized due to poor body condition, alongside a high presence of intestinal parasites. The authors concluded that this increase in Zn likely contributed to the observed Cu deficiency, as Zn can interfere with Cu absorption, compounding the animals’ health problems. Similarly, Klich et al. (2023) reported that European bison in Poland, euthanized for various clinical illnesses, showed greater Zn concentrations in the liver as the number of lesions increased, particularly in animals classified in the more severe lesion groups. The number of multiple types of lesions was strongly associated with greater Zn concentrations in hepatic tissue, suggesting a possible connection between disease severity and Zn metabolism. In contrast, Dębska et al. (2005) reported no difference in serum Zn concentrations between European bison with balanoposthitis and healthy bison, with both groups falling within cattle reference ranges. These findings indicate that while Zn concentrations may fluctuate in response to specific diseases or parasitic infections, the relationship between Zn and overall health in bison remains complex and may be influenced by interactions with other trace minerals, such as Cu.
4.3.8.5 External environment
The influence of external environment, management, and location on Zn status in bison highlights variations in Zn concentrations based on habitat and management conditions. MacNeil et al. (1990) compared North American bison from Wood Buffalo National Park and a game ranch, finding that liver Zn concentrations were greater in bison from Wood Buffalo than those from the game ranch, while kidney Zn concentrations were similar between both sites. In Poland, Klich et al. (2021) analyzed Zn concentrations in bison liver across multiple sites and reported differences, with the greatest concentrations in the Bieszczady Mountains and the lowest in the Knyszyńska Forest. The authors suggested that greater Zn concentrations in Bieszczady soils contributed to the difference, while in Knyszyńska, the lower Zn intake was linked to feeding on Zn-deficient crops, exacerbated by soil pH. Durkalec et al. (2018) reported no difference in liver Zn concentrations between Białowieża and Smardzewice, but they did report a difference between free-range and captive bison, with free-range animals showing greater Zn concentrations compared to captive ones. The authors hypothesize that Zn deficiencies were more prevalent in captive bison (37%) compared to free-range bison (3%) and suggest that natural foraging may better support adequate Zn intake.
4.3.9 Other minerals
4.3.9.1 Sex
Articles analyzing the mineral status of bison across different sexes reveal minimal differences. Durkalec et al. (2018) analyzed liver concentrations of various minerals in European bison and reported that most minerals, including aluminum (Al), cadmium (Cd), mercury (Hg), and lead (Pb), did not have differences between sexes. However, Ag concentrations were greater in males than females. Klich et al. (2023) also reported that males had greater hepatic concentrations of molybdenum (Mo) and nickel (Ni), with six elements, including these two, consistently lower in females. This trend of males having greater mineral concentrations was echoed in Kosla et al. (2016), who observed that strontium (Sr) and cesium (Cs) concentrations were greater in the hooves of males compared to females. In contrast, other studies reported no sex-based differences for minerals like P, titanium (Ti), vanadium (V), Sr, and barium (Ba) in hair (Kosla et al., 2010, 2011). Zaugg et al. (1993) also reported no differences in blood serum chlorine (Cl) concentrations between North American bison bulls and cows. Overall, while some elements, like silver (Ag), Mo, and Cs, show sex-based differences, the majority of minerals do not vary between male and female bison within the articles of this review.
4.3.9.2 Age
There are varying results of the impacts of age on other mineral status. For Cl concentrations in serum, Brzuski et al. (2010) reported that adult North American bison had greater Cl concentrations than calves, a trend also observed by Vestweber et al. (1991), who reported greater Cl concentrations in bison older than 24 months compared to those aged 7–23 months. In contrast, Zaugg et al. (1993) reported similar Cl ranges for adults and calves in Yellowstone bison. Klich et al. (2023) noted that only Cd and Ni concentrations in the liver showed correlations with age, while Kosla et al. (2010) reported greater vanadium concentrations in the hair of older European bison compared to calves. In muscle tissue, Sr was greater in adults than in calves, while Cs was greater in calves than in adults (Kosla et al., 2016). However, Sr concentrations in hooves were lower in adults compared to calves. Additionally, Shupe et al. (1987) linked the severity of dental fluorosis and wear in North American bison to both age and fluoride exposure. Overall, these findings suggest that age plays a variable role in mineral status, with some elements like Cd, Ni, V, and Sr showing age-related differences, while others like Cl display more subtle variations depending on the study.
4.3.9.3 Nutrition
Bison nutrition and mineral status only exhibited one study specifically investigated the impact of other trace elements in relation to diet. Wlostowski et al. (2006) examined the accumulation of Cd in the liver and kidneys of European bison and Polish cattle, finding greater Cd concentrations in bison tissues. The liver Cd concentration in European bison was 0.45 mg/kg wet weight, compared to just 0.20 mg/kg wet weight in cattle, while Cd concentrations in the kidneys were also markedly greater in bison versus cattle. The authors concluded that these differences were directly related to the greater concentrations of Cd reported in the grasses consumed by bison, which were twice as high as those grazed by cattle. This study highlights the strong correlation between dietary intake and trace element accumulation in tissues, emphasizing the need for further research into how different diets affect trace mineral accumulation in bison.
4.3.9.4 Health and disease
In this scoping review, one study specifically investigated the relationship between other trace elements, health problems, and mineral absorption. Rhodes et al. (2018) analyzed liver and fecal samples from North American bison and reported toxic concentrations of Se and Mo at 4.2 ppm, which were linked to deteriorating health conditions. The study suggested that the elevated concentrations of Mo, along with other trace elements, likely contributed to malabsorption issues, particularly for Cu, which is critical for various physiological processes. Additionally, the presence of parasites in the digestive systems of these animals further compounded the health problems by interfering with nutrient absorption. This research highlights the complex interplay between trace elements and overall health, indicating that imbalances in elements like Mo, combined with parasitic infections, can significantly affect mineral absorption and lead to worsening health outcomes in bison.
4.3.9.5 External environment
Findings for the influence of external environments, management styles, and geographic locations on the mineral status of bison indicate deviations in trace element concentrations across different habitats and management conditions. Durkalec et al. (2018) reported no differences in mineral concentrations—including Ag, Al, Cd, and Hg—between captive and free-ranging European bison, suggesting that management style (captive vs. free-ranging) does not impact the accumulation of these trace elements. However, when comparing liver mineral concentrations by geographic location, differences were reported for arsenic, Cr, Ni, and Pb. For instance, arsenic concentrations in the liver were greater in bison from Smardewice compared to those from Białowieża, and similar trends were observed for Cr, Ni, and Pb, with Smardewice bison generally exhibiting greater concentrations. Klich et al. (2021) also reported that Mo concentrations were lower in bison from the Bieszczady Mountains compared to other regions, likely due to the acidic soil in the region, which limits Mo uptake by plants. Other elements such as beryllium, Co, lithium, silicon, titanium, and Cd showed site-specific correlations, reflecting the strong influence of local environmental factors on mineral accumulation. Similarly, Kośla et al. (2006) reported greater Al concentrations in the hair and hoof compared to the rib, indicating tissue-specific accumulation patterns in free-ranging bison in Poland. In North American bison, MacNeil et al. (1990) compared trace element concentrations in bison from Wood Buffalo National Park and a game ranch, finding that while all mineral concentrations were adequate, bison from Wood Buffalo had greater Cd concentrations in the liver and kidney compared to ranch bison. The geographic and environmental factors influencing these mineral concentrations demonstrate how habitat, soil composition, and local forage can significantly affect trace element status in bison across different locations.
4.4 Vitamins
4.4.1 Sex
Two studies investigated the relationship between sex and vitamin A (retinol) concentrations in North American bison within this review, comparing heifers and bulls across different cuts of meat. Galbraith et al. (2006) reported that vitamin A concentrations varied among cuts but showed no differences within sex classes. The study also noted a positive correlation between vitamin A and trans-fat content in bison meat, suggesting a potential link between dietary fat profiles and retinol concentrations. This research underscores that while vitamin A concentrations may vary across different cuts and exceed previously recorded concentrations, sex does not appear to impact vitamin A status in bison meat. There were no reported articles in this review that covered the effect of sex on B-complex vitamins, or vitamins, C, or E concentrations in bison.
4.4.2 Age
No reported articles in this review covered the effect of age on vitamin concentrations in bison.
4.4.3 Nutrition
The effects of diet on vitamin A (retinol) and related metabolites in bison reveal notable differences based on nutrition type, particularly between grass- and grain-finished diets. Driskell et al. (2004) compared vitamin concentrations of ribeye cuts from grass- and grain-finished North American bison and reported that both retinol and alpha-tocopherol (vitamin E) differences were not significant, and undetectable limits of vitamin C. However, beta-carotene concentrations were greater in grass-finished bison compared to grain-finished bison, likely due to the natural seasonality and beta-carotene content of grasses. Grass-finished bison had lower riboflavin, but greater niacin compared to grain-finished bison, likely due to protein differences in feed and niacin’s reduced bioavailability in grains. Another study by Driskell et al. (1997) showed that vitamin A concentrations were similar across cuts (clod, ribeye, top round and top sirloin) in North American bison bulls ranged from 0.7-1.0 µg/100g wet weight across diets, with the greatest concentrations reported in bulls fed a diet rich in wheat, oats, and corn, whereas lower concentrations were reported in bison on wheat by-products. When comparing bison meat to other retail meats, bison exceeded beef and turkey in vitamin A but not pork or chicken, had greater thiamin, B6, and B12 than pork, chicken, and turkey, combined alpha- and gamma-tocopherols concentrations were lower than in beef, pork, chicken, and turkey, and vitamin C was undetectable. Marchello et al. (1998) also reported differences in vitamin A among bison meat cuts, reporting greater retinol in the sirloin compared to clod, variations in B6 and thiamin across cuts, with the top round having greater B6 and ribeye exhibiting lower thiamin concentrations, and no difference in alpha-tocopherol concentrations across cuts of ribeye and top round. Additionally, van Vliet et al. (2023) noted that pasture-raised North American bison had greater M. longissimus lumborum concentrations of carotene diol and alpha-tocopherol compared to grain-finished bison, greater concentrations of pyridoxine,a B6 metabolite, in grain-finished North American bison, and observed that grain-finished bison exhibited greater ascorbate, a vitamin C metabolite, than grass-finished animals. Although vitamin C is not essential for ruminants, greater ascorbate concentrations could enhance meat shelf stability and quality. Lakritz et al. (1998) tested for vitamin B and E loss in bison under gamma radiation for meat sterilization, finding no change in riboflavin or thiamin, thus supporting the nutritional stability of bison meat. Additionally, Lozicki et al. (2017) observed that musculus semitendinosus muscle collected from European bison had greater alpha-tocopherol concentrations than beef, but not compared to their hybrid crosses, Zubrons. In a direct comparison, Galbraith et al. (2016) reported that bison had greater alpha-tocopherol and total vitamin E concentrations than beef but lower alpha-tocotrienol concentrations. These studies underscore how diet, cut, and processing conditions can affect the vitamin profile in bison, but emphasize that vitamin A and E are more predominantly measured in muscle tissues within the field.
4.4.4 Health and disease
No reported articles in this review covered the effect of health/disease on vitamin concentrations in bison.
4.4.5 External environment
No reported articles in this review covered the effect of external environment on vitamin concentrations in bison.
5 Limitations
To the author’s knowledge, this is the first scoping review on vitamin and mineral concentrations within tissues of bison; however, this scoping review is subject to several important limitations. This review does not focus exclusively on a single bison species, encompassing both North American and European bison. This introduces variability, as these species may have different ecological, physiological, and nutritional profiles. Inconsistencies in terminology in the literature also pose a challenge; articles with unclear species designation were excluded from this review. This approach may have led to the omission of studies relevant to the population of interest, potentially limiting the breadth of data available for analysis. Additionally, differences in sampling procedures, analytical methods, and reporting standards were not accounted for, which could affect the comparability of findings. Furthermore, the choice of tissues analyzed in studies may not fully represent the overall mineral or vitamin status of the animals, as these concentrations are influenced by complex physiological interactions involving multiple tissues. Sampling bias is also a concern, particularly for European bison, as samples are often collected opportunistically due to the species’ protected status. These samples frequently come from animals culled due to illness, injury, or advanced age, which may not reflect the nutrient status of the broader population. Another limitation in grazing bison is that a complete inventory of what they consume (soil, tree bark, etc.) is not available. Thus, making it difficult to quantify total mineral and vitamin intake. Finally, variability in the terminology used to describe management practices adds complexity to the interpretation of results. Terms like “free-range,” “enclosed,” “captive,” and “ranched” were often used inconsistently or interchangeably across studies, complicating efforts to make meaningful comparisons of mineral and vitamin concentrations across different management contexts.
6 Conclusions
This scoping review highlights significant gaps in our understanding of mineral and vitamin concentrations within bison. Research on vitamins is minimal and has primarily focused on nutrient content of consumable products rather than examining vitamin concentrations across different tissues with interest in the status of the animal. There are notable gaps in research on trace elements as well; while studies have explored mineral concentrations in the context of grass-fed versus grain-fed diets, there is limited investigation into how these concentrations vary by health status, sex, or age. Moreover, few studies have examined blood serum mineral concentrations over time or across seasons, even though many bison reside and are raised in range environments where nutrient availability likely fluctuates. Seasonal and longitudinal studies could offer valuable insights into how mineral concentrations in bison shift in response to environmental changes. There is also an imbalance in research focus between North American bison and European bison. While North American bison studies emphasize the nutrient profile of meat, there is relatively less research attention on the assessment of overall mineral status of these animals. In contrast, research on European bison places a greater emphasis on mineral and vitamin status, likely due to the protected status of the species in Poland, where there is a strong focus on health monitoring. One challenge with conducting these studies is sample collection and that should be considered when developing future studies; sample collection at a given time and location may be more the result of opportunity than a planned research design. Overall, the current literature on bison nutrition reveals a limited focus on vitamin concentrations in tissues other than meat, and a need for more comprehensive studies on mineral and vitamin status across different contexts and life stages of bison populations.
Author contributions
CO: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. TE: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. LE-C: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was made possible through the Center of Excellence for Bison Studies, a partnership among South Dakota State University, the National Bison Association, and the National Buffalo Foundation and by Turner Institute for Ecoagriculture. Financial support was provided by the National Buffalo Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arksey H. and O’Malley L. (2005). Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32. doi: 10.1080/14681720520000407755
Brzuski P., Cywicka D., and Hedrzak M. (2010). Some blood biochemical parameter values in a herd of North American bison (Bison bison L.) from an experimental animal farm in Poland. Folia Biol. (Krakow) 58, 145–149. doi: 10.3409/fb58_3-4.145-149
Church D. C. (1988). The ruminant animal. Digestive physiology and nutrition (Englewood Cliffs, N.J: Prentice 511 Alan).
Clemens E. T., Meyer K. L., Carlson M. P., and Schneider N. R. (1987). Hematology, blood chemistry and selenium values of captive pronghorn antelope, white-tailed deer and North American bison. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 87, 167–170. doi: 10.1016/0742-8413(87)90198-8
Dębska M., Dymnicka M., Olech W., and Arkuszewska E. (2006a). Levels of macroelements in selected tissues and in the hair coat of the European bison, depending on the age of animals. Pol. J. Nat. Sci. Supplement 21, 601–606.
Dębska M., Dymnicka M., Olech W., and Arkuszewska E. (2006b). Nutrient content of feed and levels of some biochemical parameters and macroelements in the blood serum and hair coat of the European bison from captive and free-ranging herds in the Białowieża Forest. Pol. J. Nat. Sci. 21, 607–614.
Dębska M., Dymnicka M., and Olech-Piasecka W. (2005). Comparison of selected serum biochemical parameters of European bison (Bison bonasus) with balanoposthitis from the Białowieża Forest. Ann. Warsaw Agric. Univ. Anim. Sci. 42, 3–7.
Delgiudice G., Singer F., Seal U., and Bowser G. (1994). Physiological-responses of Yellowstone bison to winter nutritional deprivation. J. Wildl. Manage. 58, 24–34. doi: 10.2307/3809545
Driskell J., Marchello M., Giraud D., and Sulaeman A. (2004). Vitamin and selenium content of ribeye cuts from grass- and grain-finished bison of the same herd. J. Food Qual. 27, 388–398. doi: 10.1111/j.1745-4557.2004.00680.x
Driskell J., Yuan X., Giraud D., Hadley M., and Marchello M. (1997). Concentrations of selected vitamins and selenium in bison cuts. J. Anim. Sci. 75, 2950–2954. doi: 10.2527/1997.75112950x
Durkalec M., Nawrocka A., Krzysiak M., Larska M., Kmiecik M., and Posyniak A. (2018). Trace elements in the liver of captive and free-ranging European bison (Bison bonasus L.). Chemosphere 193, 454–463. doi: 10.1016/j.chemosphere.2017.11.050
Freese C. H., Aune K. E., Boyd D. P., Derr J. N., Forrest S. C., Cormack Gates C., et al. (2007). Second chance for the plains bison. Biol. Conserv. 136, 175–184. doi: 10.1016/j.biocon.2006.11.019
Galbraith J. K., Aalhus J. L., Juárez M., Dugan M. E. R., Larsen I. L., Aldai N., et al. (2016). Meat colour stability and fatty acid profile in commercial bison and beef. J. Food Res. 5, 92–101. doi: 10.5539/jfr.v5n5p92
Galbraith J., Rodas-González A., López-Campos Ó., Juárez M., and Aalhus J. (2014). Bison meat: Characteristics, challenges, and opportunities. Anim. Front. 4, 68–73. doi: 10.2527/af.2014-0036
Galbraith J., Hauer G., Helbig L., Wang Z., Marchello M., and Goonewardene L. (2006). Nutrient profiles in retail cuts of bison meat. Meat Sci. 74, 648–654. doi: 10.1016/j.meatsci.2006.05.015
Hawley A. W. L. and Peden D. G. (1982). Effects of ration, season, and animal handling on composition of bison and cattle blood. J. Wildl. Dis. 18, 321–338. doi: 10.7589/0090-3558-18.3.321
Hecker L. J., Edwards M. A., and Nielsen S. E. (2021). Assessing the nutritional consequences of switching foraging behavior in wood bison. Ecol. Evol. 11, 16165–16176. doi: 10.1002/ece3.8298
Keith E. O., Ellis J. E., Phillips R. W., and Benjamin M. M. (1978). Serologic and hematologic values of bison in Colorado. J. Wildl. Dis. 14, 493–500. doi: 10.7589/0090-3558-14.4.493
Klich D., Kitowski I., Lopucki R., Wiacek D., and Olech W. (2021). Essential differences in the mineral status of free-ranging European bison Bison bonasus populations in Poland: The effect of the anthroposphere and lithosphere. Sci. Total Environ. 757, 143926. doi: 10.1016/j.scitotenv.2020.143926
Klich D., Lopucki R., Kaczor S., Zwolak I., Didkowska A., Wiacek D., et al. (2023). Comorbidities and concentration of trace elements in livers of European bison from Bieszczady Mountains (Poland). Sci. Rep. 13, 5137. doi: 10.1038/s41598-023-31245-z
Kośla T. (1993). The contents of macro- and microelements in the fodder, blood serum and hair of European bison. Part I. Macroelements. Ann. Warsaw Agric. Univ. SGGW-AR Vet. Med. 17, 79–85.
Kośla T., Skibniewska E. M., and Skibniewski M. (2010). Calcium content in the tissues of European bisons from the Białowieża primeval forest in relation to sex and age. Med. Wet. 66, 63–65.
Kośla T., Skibniewski M., Skibniewska E. M., and Urbańska-Słomka G. (2006). Aluminum status in free living European bisons from Białowieża Primeval Forest. Presented at the. Polish J. Environ. Stud. 15, 374–377.
Kosla T., Skibniewska E., and Skibniewski M. (2011). The state of bioelements in the hair of free-ranging European bison from Białowieża Primeval Forest. Pol. J. Vet. Sci. 14, 81–86. doi: 10.2478/v10181-011-0012-0
Kosla T., Skibniewska E., Skibniewski M., Kolnierzak M., Lasocka I., and Kmiec H. (2019). Hair concentration of selenium in European bison in relation to sex and age, with regard to liver and kidney Se levels. Folia Biol.-Krakow 67, 99–108. doi: 10.3409/fb_67-3.10
Kosla T., Skibniewska E., Skibniewski M., and Urbanska-Slomka G. (2012). Magnesium concentrations in the tissues of free-ranging European bison. Magnes. Res. 25, 99–103. doi: 10.1684/mrh.2012.0312
Kosla T., Skibniewska E., and Urbanska-Slomka G. (2010). Strontium and barium content in the coat of free-living European bisons in Białowieża forest. Fresenius Environ. Bull. 19, 579–581.
Kosla T., Skibniewski M., and Skibniewska E. (2015). Content of sodium and potassium in tissues and organs of free-ranging European bisons. J. Elem. 20, 343–352. doi: 10.5601/jelem.2014.19.3.690
Kosla T., Skibniewski M., Skibniewska E., and Kolnierzak M. (2016). Total strontium and cesium concentrations in selected tissues of free-ranging European bison (Bison bonasus) in relation to age and sex. Trace Elements Electrolytes 33, 102–107. doi: 10.5414/TEX01429
Kosla T., Skibniewski M., Skibniewska E., and Urbanska-Slomka G. (2004). The zinc status in free living European bisons. Acta Aliment. 33, 269–273. doi: 10.1556/AAlim.33.2004.3.7
Kosla T., Skibniewski M., Skibniewska E., and Urbanska-Slomka G. (2013). The iron content in organs of free ranging European bison from the Białowieża herd. Ann. Anim. Sci. 13, 357–364. doi: 10.2478/aoas-2013-0015
Lakritz L., Fox J., and Thayer D. (1998). Thiamin, riboflavin, and α-tocopherol content of exotic meats and loss due to gamma radiation. J. Food Prot. 61, 1681–1683. doi: 10.4315/0362-028X-61.12.1681
Levac D., Colquhoun H., and O’Brien K. K. (2010). Scoping studies: advancing the methodology. Implement. Sci. 5, 69. doi: 10.1186/1748-5908-5-69
Lozicki A., Olech W., Dymnicka M., Florowski T., Adannczak L., Arkuszewska E., et al. (2017). Nutritive value and meat quality of domestic cattle (Bos taurus), zubron (Bos taurus x Bison bonasus) and European bison (Bison bonasus) meat. Agric. Food Sci. 26, 118–128. doi: 10.23986/afsci.60516
MacNeil J., Patterson J., Salisbury C., and Tessaro S. (1990). An investigation of the trace-element status of bison in Wood-Buffalo-National-Park and of ranch-raised bison in Saskatchewan, Canada. Int. J. Environ. Anal. Chem. 41, 99–104. doi: 10.1080/03067319008027353
Marchello M. J. and Driskell J. A. (2001). Nutrient composition of grass- and grain-finished bison. Great Plains Res. 11, 65–82.
Marchello M. J., Slanger W. D., Hadley M., Milne D. B., and Driskell J. A. (1998). Nutrient composition of bison fed concentrate diets. J. Food Compos. Anal. 11, 231–239. doi: 10.1006/jfca.1998.0583
Marchello M. J., Slanger W. D., Milne D. B., Fischer A. G., and Berg P. T. (1989). Nutrient composition of raw and cooked Bison bison. J. Food Compos. Anal. 2, 177–185. doi: 10.1016/0889-1575(89)90079-3
Marczuk J., Lutnicki K., Kurek L., and Brodzki P. (2015). Atypical changes in hematology and biochemistry of European bison and domestic ruminants with chronic fasciolosis. Med. Weter.-Vet. Med.-Sci. Pract. 71, 441–447. doi: 10.21521/mw.6025
Marler R. J. (1975). Some hematologic and blood chemistry values in two herds of North American bison in Kansas. J. Wildl. Dis. 11, 97–100. doi: 10.7589/0090-3558-11.1.97
McDowell L. R. (2006). Vitamin nutrition of livestock animals: Overview from vitamin discovery to today. Can. J. Anim. Sci. 86, 171–179. doi: 10.4141/A05-057
Medeiros L., Belden R., and Williams E. (1993). Selenium content of bison, elk, and mule deer. J. Food Sci. 58, 731–733. doi: 10.1111/j.1365-2621.1993.tb09346.x
Miller L. D., Thoen C. O., Throlson K. J., Himes E. M., and Morgan R. L. (1989). Serum biochemical and hematologic values of normal and Mycobacterium bovis-infected North American bison. J. Vet. Diagn. Invest. 1, 219–222. doi: 10.1177/104063878900100304
NFACC (2017). Code of practice for the care and handling of bison (National Farm Animal Care Council (NFACC). Available online at: https://www.nfacc.ca/codes-of-practice/bison.
Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi: 10.1136/bmj.n71
Peinado V., Celdrán J., and Palomeque J. (1999). Blood biochemistry values in some wild ruminants in captivity. Comp. Haematol. Int. 9, 175–181. doi: 10.1007/s005809970001
Puls R. (1994). Mineral levels in animal health: diagnostic data. 2nd ed (Clearbrook, B.C: Sherpa International).
Raynor E. J., Joern A., and Briggs J. M. (2015). Bison foraging responds to fire frequency in nutritionally heterogeneous grassland. Ecology 96, 1586–1597. doi: 10.1890/14-2027.1
Rhodes R., Topliff C., Kelling C., and Coon S. (2018). Malabsorption of copper and other nutrients as well as intestinal parasite infections cause health problems and lower birthrates in the North American bison. FASEB J. 32. doi: 10.1096/fasebj.2018.32.1_supplement.747.18
Richmond R. J., Hudson R. J., and Christopherson R. J. (1977). Bisoniana LXVI. Comparison of forage intake and digestibility by North American bison, yak and cattle. Acta Theriol. 22, 225–230. doi: 10.4098/AT.arch.77-17
Schillhorn van Veen T. W., Mullaney T. P., Trapp A. L., and Taylor A. F. (1991). Fatal reactions in bison following systemic organophosphate treatment for the control of Hypoderma bovis. J. Vet. Diagn. Invest. 3, 355–356. doi: 10.1177/104063879100300419
Shupe J. L., Christofferson P. V., Olson A. E., Allred E. S., and Hurst R. L. (1987). Relationship of cheek tooth abrasion to fluoride-induced permanent incisor lesions in livestock. Am. J. Vet. Res. 48, 1498–1503. doi: 10.2460/ajvr.1987.48.10.1498
Sikarskie J. G., Schillhorn van Veen T. W., van Selm G., and Kock M. D. (1990). Comparative blood characteristics of ranched and free-ranging North American bison (Bison bison). Am. J. Vet. Res. 51, 955–957. doi: 10.2460/ajvr.1990.51.06.955
Skibniewska E. M., Kośla T., Skibniewski M., and Urbańska-Słomka G. (2006). Copper concentration in chosen organs and tissues of free living European bison from Białowieża forest. Polish J. Environ. Stud. 15, 479–481.
Skibniewski M., Kosla T., and Skibniewska E. (2010). Manganese status in free ranging European bisons from Białowieża Primeval Forest. Bull. Vet. Inst. Pulawy 54, 429–432.
USDA National Agricultural Statistics Service (2024). 2022 census of agriculture (Washington, DC: U.S. Department of Agriculture). Available online at: https://www.nass.usda.gov/AgCensus.
van Vliet S., Blair A., Hite L., Cloward J., Ward R., Kruse C., et al. (2023). Pasture-finishing of bison improves animal metabolic health and potential health-promoting compounds in meat. J. Anim. Sci. Biotechnol. 14, 49. doi: 10.1186/s40104-023-00843-2
Vestweber J., Johnson D., and Merrill G. (1991). Hematological and blood-chemistry profiles of North American bison grazing on Konza Prarie of Kansas. J. Wildl. Dis. 27, 417–420. doi: 10.7589/0090-3558-27.3.417
Wagner J. J., Edwards-Callaway L. N., and Engle T. E. (2023). Vitamins and trace minerals in ruminants: Confinement feedlot. Vet. Clin. North Am. Food Anim. Pract. 39, 505–516. doi: 10.1016/j.cvfa.2023.06.005
Witkowska A. and Kotik T. (1987). Concentrations of creatine, creatinine and phosphorus in skeletal-muscles of the European bison. Acta Theriol. 32, 219–228.
Wlostowski T., Bonda E., and Krasowska A. (2006). Free-ranging European bisons accumulate more cadmium in the liver and kidneys than domestic cattle in north-eastern Poland. Sci. Total Environ. 364, 295–300. doi: 10.1016/j.scitotenv.2005.12.009
Wolf O., Glatzel P., Lehnen A., Streich W., and Frölich K. (2004). Blood values in the European bison (Bison bonasus) in relation to sex, age and balanoposthitis in bulls. Tierärztl. Prax. Ausgabe Grosstiere Nutztiere 32, 269–273.
Wołk E. and Józefczak E. (1988). Serum biochemistry of free-ranging European bison. Acta Theriol. 33, 47–56. doi: 10.4098/AT.arch.88-4
Wolk E. and Krasinska M. (2004). Has the condition of European bison deteriorated over last twenty years? Acta Theriol. 49, 405–418. doi: 10.1007/BF03192538
Keywords: bison, concentration, environment, health, mineral, nutrition, vitamin
Citation: Okoren C, Engle T and Edwards-Callaway L (2025) Vitamin and mineral status within various tissues of bison: a scoping review. Front. Conserv. Sci. 6:1677365. doi: 10.3389/fcosc.2025.1677365
Received: 31 July 2025; Accepted: 12 September 2025;
Published: 07 October 2025.
Edited by:
Jeff M. Martin, South Dakota State University, United StatesReviewed by:
Gerald Huntington, North Carolina State University, United StatesEllen Dierenfeld, World Wildlife Fund, United States
Philip Urso, South Dakota State University, United States
Copyright © 2025 Okoren, Engle and Edwards-Callaway. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lily Edwards-Callaway, bGlseS5lZHdhcmRzLWNhbGxhd2F5QGNvbG9zdGF0ZS5lZHU=
 Claire Okoren
Claire Okoren Terry Engle
Terry Engle Lily Edwards-Callaway
Lily Edwards-Callaway