- 1Department of Community Medicine, University of Calabar, Calabar, Nigeria
- 2HEAL Global Research Centre, Health Research Institute, University of Canberra, Canberra, ACT, Australia
- 3Cochrane Nigeria, Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital, Calabar, Nigeria
- 4Health and Demographics Surveillance System, Directorate of Research and Development, University of Calabar, Calabar, Nigeria
Background: The healthcare setting is a high-transmission-risk environment for COVID-19. Attending clinicians and patients are at risk of infection if measures are not established to secure the microbial safety of the health facility. Air cleaning technologies may deliver a safer clinical environment by depleting airborne viral concentrations.
Aim: This systematic review aims to assess the effectiveness of air-cleaning methods in preventing COVID-19 transmission in health facilities and the effectiveness of air cleaning rated by microbial depletion.
Method: This study is a rapid systematic review.
Results: No study assessed COVID-19 transmission relative to the air cleaning methods. HEPA filtration produced a more rapid and thorough removal of aerosols from health facilities. HEPA filtration showed mixed performance in removing COVID-19 viral RNA from a routine care ward and an intensive care unit (ICU). Meta-analyses could not be conducted due to dissimilarities in included studies.
Conclusion: The reviewed papers demonstrate that HEPA filtration hastens the depletion of aerosols from the indoor space in the health facility. Further translation of this finding to prevent COVID-19 transmission should assume the relevance of room occupancy density, virus-free outdoor air supply, recirculated filtered air, virus source strength, number of sources, and the use of facemasks by health workers and visitors to the health facility.
Introduction
The corona virus disease 2019 (COVID-19) was officially declared a pandemic by the World Health Organisation (WHO) in March of 2020 (1). The primary clinical manifestation of COVID-19 is a severe acute respiratory illness (2) which may progress to a viral pneumonia (3). The pathogen responsible for this disease is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a highly transmissible RNA virus.
The viron size is 60–140 nm (4). The dominant mode of transmission is by an inhalation of respiratory aerosols released by an infected person. Larger aerosols (5–100 µm) and small aerosol (<5 µm) (5) entrain large numbers of SARS-CoV-2 particles. The viability of these particles depends on their physical transport and biological inactivation which in turn determine the integrity of their genomic material, nucleoprotein, capsid and envelope. The ambient temperature, humidity, and ultraviolet irradiation affect the stability, transportation, and inactivation of viruses (6, 7). Temperature and humidity also influence the ability of the deposited virus to establish an infection in a potential host. Aerosols in the lower size differentiation have higher residence time in the air. At 5 µm, aerosols can stay suspended in still air at a height of 1.5 m for up to 33 min, at 1 µm, aerosols can remain suspended for more than 12 h. Aerosols of 100 µm remain suspended for 5 s and can be dispersed over a distance of 1–2 m, depending on the local airflow velocity. The larger aerosols settle faster under the influence of gravity (5), but the smaller aerosols, which remain longer in the air, are transported across greater distances (8) depending on their size, the initial velocity of the air stream bearing the aerosols, outdoor windspeed or indoor air drafts. Smaller aerosols also exhibit different deposition characteristics from larger aerosols. Aerosols larger than 5 µm tend to be deposited in different parts of the nasopharynx, but smaller aerosols penetrate deeper down the respiratory tree to settle on the alveoli and bronchioles where SARS-CoV-2 has stronger potential to colonise tissues, multiply, and establish infection. Smaller aerosols have also been demonstrated to contain more viruses (7).
The healthcare setting is a high-transmission risk environment for SARS-Cov-2 because patients and healthcare workers interact mostly in enclosed indoor spaces where the recirculating air tends to concentrate viral particles (9). The proximity of healthcare workers to the patients during clinical care further enhances viral transmissibility. The availability of an infective source(s) (patients in clinics, isolation wards, treatment rooms) guarantees a sustained release of the virus into the surrounding spaces. The SARS-Cov-2 has been detected in aerosols as far as 3 meters from infected patients (10). Moreover, medical and surgical procedures performed on patients can facilitate an increased release of infective aerosols; these aerosol-generating procedures (AGP) reinforce the transmission risk within the health facility. Therefore, healthcare workers (HCW), visitors to the health facility, and other patients who are uninfected by SARS-CoV-2, are at greater risk of infection. In the health facility, there is also the additional the risk of cross-infection with different variants of the virus which may cause infections with diverse clinical manifestations.
Reducing transmission risks in health facilities demands regular comprehensive decontamination of indoor hospital spaces (11). It has been recommended that rooms where COVID-19 patients have been managed and rooms used for AGPs be decontaminated between use for successive patients (12). This may involve a procedural mandate requiring these rooms to be left unused after an infected patient has received care until satisfactory decontamination is achieved (13). In addition to providing a safer indoor space in the health facility, decontamination protocols seek to prevent the outdoor egress of SARS-Cov-2 and accordingly prevent the transmission of the virus in the precinct of the health facility.
Specific recommendations exist for health facility indoor ventilation which seek to, among other things, limit air movements in between service areas and departments; attenuate and eliminate microbial, chemical, radioactive, and odoriferous contaminations; maintain the temperature and humidity demands which are conducive to the operations of different spaces (ASHRAE 2019). An important distinction of the ventilation systems of health facilities in comparison with office complexes, public buildings, schools, and aircrafts, for example, is the principle of zoning. Zoning achieves different ventilation system specifications in different department. Therefore, regular patients wards, isolation wards, intensive care units, operating theatres, recovery rooms, treatment rooms, nurses' stations, laboratories, sterilizing rooms, and administrative offices differ in environmental circulation components (ASHRAE 2019). In addition, health facilities are unique because they operate throughout the day every day of the year, so their air dilution and corresponding energy demands are enormous.
To create safer indoor air-quality conditions in the health facility, different procedures, protocols, and technologies can be deployed to facilitate decontamination (12). Engineering technologies in health facilities are designed to achieve differential air pressure gradients between spaces, control directional airflow, filter delivered air and exhausts in order to improve microbial air quality (ASHRAE 2019) (9). The heating, ventilation and air-conditioning (HVAC) system can be set to allow unidirectional airflows into high infection risk rooms by creating negative air pressure in these rooms. The HVAC uses a system of fans, ducts, and pressure differentials between rooms to set the airflow rate, direction, controlled-room-air diffusion, and air exchange and dilution (14). The system segregates rooms based on these mechanisms and correspondingly prevents uncontrolled diffusion of air between rooms. The airflow rate, air diffusion, air mixing, and flow direction have to be calibrated to facilitate efficient particles (viruses, bacteria, fungi, aerosols, particulates) removal (15). Air volumes which exit these rooms can then be sequestered through a single exhaust to remove airborne pathogens (9). Particle-removal effectiveness can be improved by integrating HEPA filters and other purification technologies (16).
Highly-efficient particle arrestance (HEPA) filters (commonly known as high-efficiency particulate air filters) have been fixed to air duct vents or used in portable HEPA filter air cleaners to accelerate indoor attrition of particulate air pollutants. The same technology has shown promising efficacy against indoor airborne viruses (5, 17). The HEPA-filter mechanism can be integrated with air-flow streams and air diffusion patterns generated by the HVACs to promote the transport of airborne viruses to the filters and their subsequent removal. Ultraviolet (UV) irradiation (18), ozone fumigation (19) can be used separately, as an alternative to HEPA filtration, or combined with HEPA filtration to inactivate airborne or trapped viruses and surface-impacted viruses (20).
The HEPA filters are composed of multiple layers of interlaced microfibers which trap airborne particles as air currents diffuse between the fibres. These filters have a 99.97% removal rate for size-segregated particles of 0.3 µm in diameter (17). Particle are removed by the combined mechanisms of impaction, interception, and diffusion. The mechanism largely depends on the particle size (17). Removed particles adhere to the filter microfibres by electrostatic attraction, capillary action and Van der Waals forces (5). The removal efficiency of these filters is rated using the Minimum Efficiency Reporting Values (MERVs) metric. The MERV rating expresses a filter's ability to trap and retain particles within the size range of 0.3–10 µm. The HEPA filter typically has a MERV rating ≥13 (15). Other air purification technologies, such as ultraviolet irradiation (21) and ozone fumigation are adapted to air cleaning because of their germicidal properties. These methods inactivate viruses by damaging their nucleic acids and biomolecules. Ultraviolet irradiation releases energy which denatures the genomic structure of the virus (18) while ozone releases free radicals which initiate a damaging powerful oxidizing action (22).
At the height of the COVID-19 pandemic, the morbidity and hospitalisation rates from this infection imposed such an extraordinary burden on the healthcare resources and manpower to the extent that admission to hospitals for in-patient care was selective. Persons with mild to moderate infection had to self-isolate at home. Healthcare workers faced the composite risk of acquiring the infection and the relentless workload. The WHO acknowledged the challenge the pandemic presented to human health and development; in particular, the threat to health-sector manpower. The year 2021 was therefore designated as the International Year of Health and Care Workers (YHCW) (23). Even as COVID-19 has evolved into an endemic infection, the imperative to guarantee indoor safety of the health facility and the protection of healthcare workers and patients from threats of local transmission justifies this systematic review of air cleaning methods. This review aims to assess the effectiveness of physical and chemical air purification methods (intervention) in preventing SARS-Cov-2 transmission in the health facility and compare them with equivalent ventilation provided by natural or mechanical modes (control). A secondary objective is to assess the effectiveness of air cleaning in health facilities.
Methods
Eligibility criteria
Criteria for including studies in this review:
Types of studies
This protocol (24) proposed to include studies which assessed incident SARS-Cov-2 infection rates in health facilities where air purification methods were used and compared these with control facilities without air cleaning. The proposed study designs were cohort studies, interrupted time series, and case-control studies. The electronic search did not reveal any study with these designs. We included experiments which compared the effectiveness of air-cleaning in the health facility to the more customary mechanical or natural ventilation. The rationale for this was that, although numerous studies exist whose investigators assessed the air cleaning effectiveness of HEPA filter-based devices, a significant proportion of these studies were conducted outside of the health facility in laboratories, rooms, offices, or other types of buildings.
Recommendations exist for health facility indoor ventilation, some of which are apply to specific areas of the hospital such as the operating theatre. These recommendations were developed with the aim to guarantee indoor comfort in the health facility as well as safety from microbial contamination. An assessment of the microbial-depleting efficacy of air cleaners in buildings designed with different ventilation objectives in mind or in experimental chambers where the ventilation settings maximise the air-cleaning performance, is unlikely to represent the functionality of these air cleaners in real-world healthcare settings. The studies included in this review were mostly “before and after” studies with self-control or “before and after” studies with parallel control.
Types of participants
We proposed to assess the differential transmission risk experienced by health-workers and in-patients in relation to the presence or absence of air-cleaning devices. As electronic searches did not reveal studies which examined transmissions, we could only focus on studies which assessed the effectiveness of different air cleaning technologies in healthcare settings such as hospital wards, dental hospitals, dental clinics, operating theatres, and treatment rooms.
Types of interventions
From the search results, the interventions which met the inclusion criteria were those in which HEPA filters in portable or fixed air-cleaning systems were used either alone or in combination with other air cleaning technologies. The comparators were mechanical or natural ventilation which met acceptable indoor air quality (IAQ) standards.
Types of outcome measures
Primary outcomes
The proposed primary outcome measures were the incidence rates of COVID-19 in healthcare workers and patients who were under admission for some other disease, and prevalence rates of COVID-19 infections in healthcare workers. No studies examined this outcome.
Secondary outcomes
The secondary outcome measure was the air cleaning effectiveness of the methods used in primary studies. We, therefore, examined the removal rate/efficacy of viruses from the indoor air.
Information sources and search strategy
On the 5th of September, 2022, we searched the following databases: The Cochrane Library—Central Register of Controlled Trials (CENTRAL) and Cochrane Database of systematic reviews; MEDLINE and EMBASE. In addition to these databases, we also assessed the references of all included studies for studies which met the inclusion criteria.
We applied no date or language restrictions to our search and we used the PRISMA guideline and flow diagram to report the search process and selection of studies. The search strategy included text words and controlled vocabulary for air cleaning/purifier technologies and Corona Virus Disease such as: “High Efficiency Particulate Arrestance”, “High Efficiency Particulate Air”, “Clean Air Delivery Rate”, “Heat Recovery Ventilator”, “Energy Recovery Ventilator”, HVAC, HEPA, ultraviolet, ozone, air filter, “COVID-19”, “SARS-CoV-2”, “Severe Acute Respiratory Syndrome Coronavirus 2”, “NCOV”, “2019 NCOV” and Coronavirus. For search strategy (MEDLINE) and terms used, see Supplementary S1.
Selection process
Study titles and abstracts from the search results were independently screened by two pairs of reviewers (C.E. and M.B.; M.C. and V.M.B.) using the eligibility criteria. Studies selected from the screening, or studies, which did not provide sufficient information to support an inclusion decision, were retrieved in full text for further assessment. The authors independently applied the inclusion criteria to the full-text reports using a pre-tested eligibility form. Publications were scrutinized to eliminate duplicated reports. The teams resolved disagreements between author pairs by broad discussions leading to consensus among team members. Excluded studies and the reasons for their exclusion were listed in a table.
Data collection process
We extracted data on the author names and year of publication, study title, status of study (published, unpublished), study objectives, study location, study design, type of health facility, and reference to coronavirus disease (covid-19, covid, sars-cov-2, 2019-ncov, coronavirus disease). We also extracted data on the type of room ventilation (natural or mechanical), meteorological conditions in the room (principally temperature and relative humidity), air exchange rate, medical procedure (which were likely to generate aerosol), artificial aerosol-generating procedure, type of intervention and implementation of co-interventions, air sampling techniques and analysis methods, duration of air sampling, outcome metric.
Two author pairs (C.E. and M.B.; M.C. and V.M.B.) independently extracted data using a customised and pre-tested data extraction form. Disagreements were resolved by discussions between all review authors. For each outcome the authors extracted either the air sampling metric with the corresponding standard deviation or surface sampling metric with standard deviation. We extracted results for both the intervention arm, and the comparator arm if that was available.
Risk of bias assessment
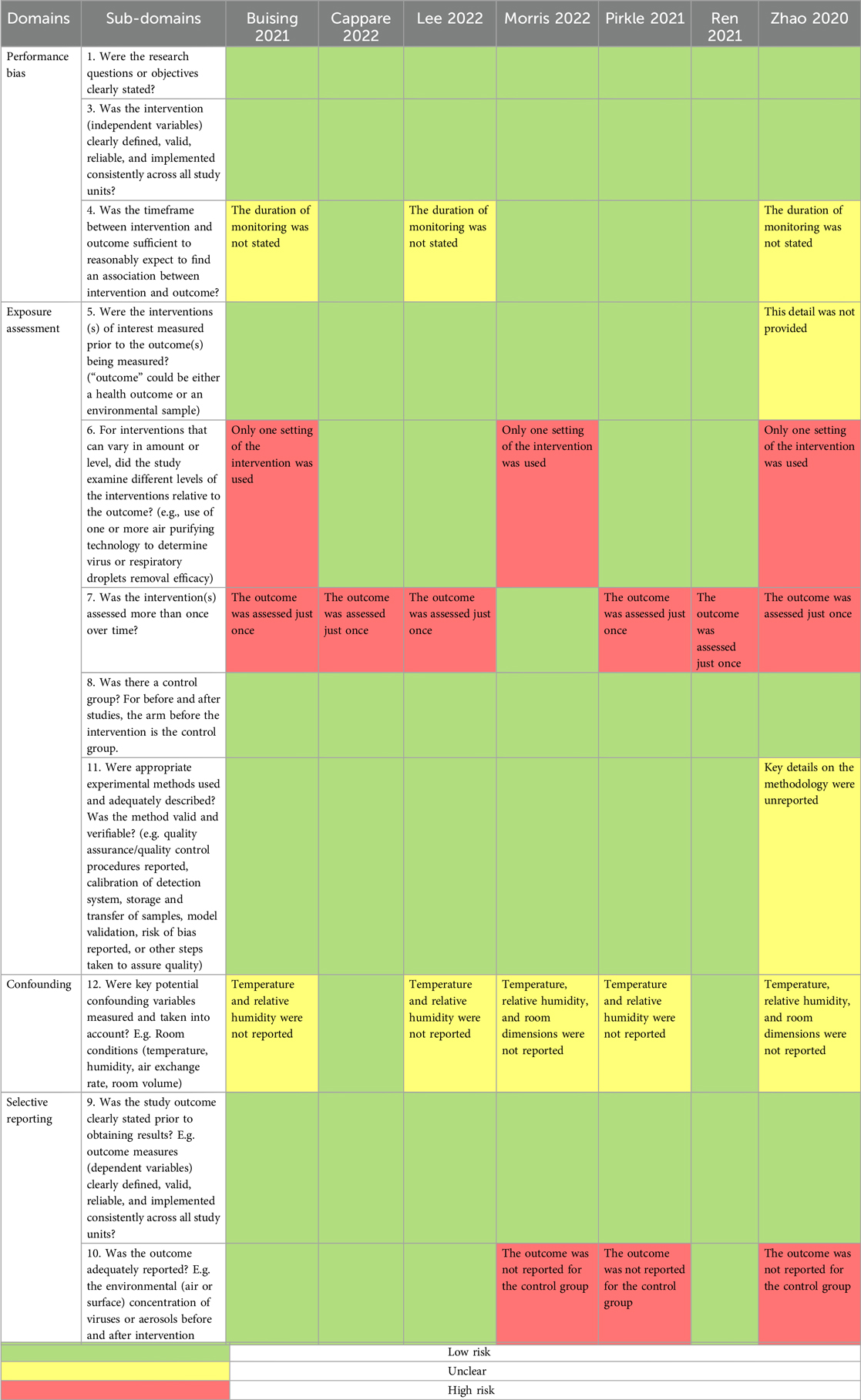
The author pairs independently assessed the risk of bias of each included study using a “Risk of bias” (ROB) assessment tool. Disagreements between author pairs were resolved by the lead author. It is notable that no epidemiologic study was retrieved and the included studies were interventional exposure assessment experiments. The risk of bias assessment tool was adapted from the Risk of Bias Assessment Instrument for Systematic Reviews Informing WHO Global Air Quality Guidelines (25) and the Principles and Framework for Assessing the Risk of Bias for Studies Included in Comparative Quantitative Environmental Systematic Reviews (26).
The risk of bias was assessed in 4 domains: performance bias, exposure assessment, confounding, and selective outcome reporting. Each domain had 1–5 subdomains which were represented by questions designed to elicit the pertinent methodological detail. Judgement calls of “yes”, “no”, and “unclear” were made to indicate a low, high, or unclear (moderate) risk of bias. If any subdomain was rated “unclear”, the entire domain was rated as having an unclear risk of bias if no rating of high risk of bias was made. However, if any subdomain had a rating of a high risk of bias, the entire domain was rated to have a high risk of bias regardless of other subdomains ratings. If the study had any domain rated as having a high ROB, then the entire study was rated as having a high ROB despite the ratings of other domains. Similarly, a study had a rating of unclear or moderate ROB if at least one domain was rated as being of moderate ROB, but none was rated as having a high ROB. A study was rated as being of low ROB if all the domains had low ROB.
Effect measures
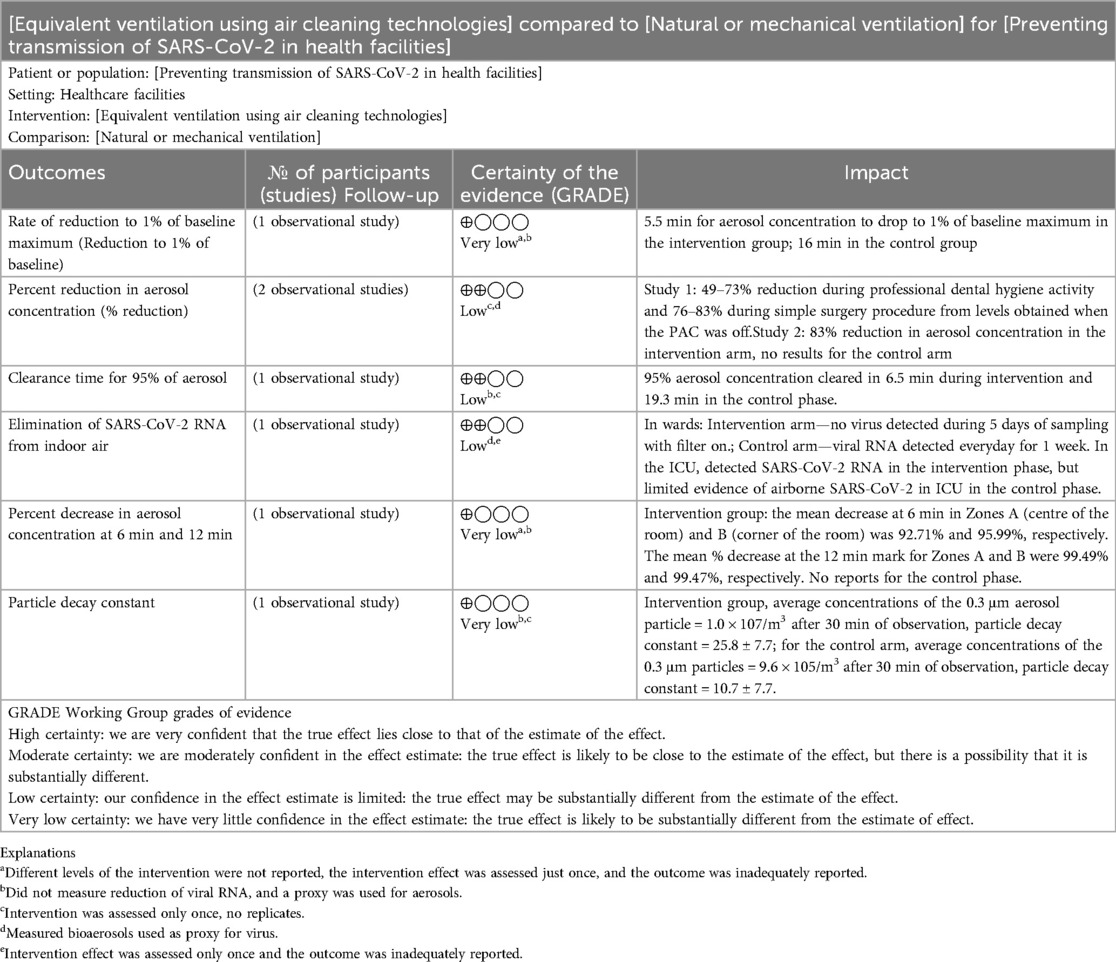
The measures of intervention effect varied across studies. In general, these metrics measured the rate and extent of depletion of SARS-CoV-2 viral particles or aerosols of human origin or their proxies. They included: the elimination of SARS-Cov-2 RNA; the rate of reduction of aerosol concentration to 1% of baseline maximum; percent reduction of particle concentration; clearance time for 95% of the aerosol concentration; percent reduction in aerosol concentration at 6 min and 12 min; and particle decay constant. In papers where investigators used measures which had similar labels, sometimes the mathematical equations for deriving those measures were different.
Synthesis methods
Dissimilarities in methodology and outcome metrics in the included studies prevented a meta-analysis of outcome measures. Furthermore, most of the studies provided only descriptive outcome measures without an inferential test statistic which assessed outcome differences between the intervention and control arms.
Reporting bias assessment
We checked to see if there were unexplained omissions of results from analyses which were mentioned in the methods section of the primary studies but were not accounted for in the results, discussion, or supplements.
Certainty assessment
The certainty of findings was assessed using the Grade Profiler (27).
Results
Search results
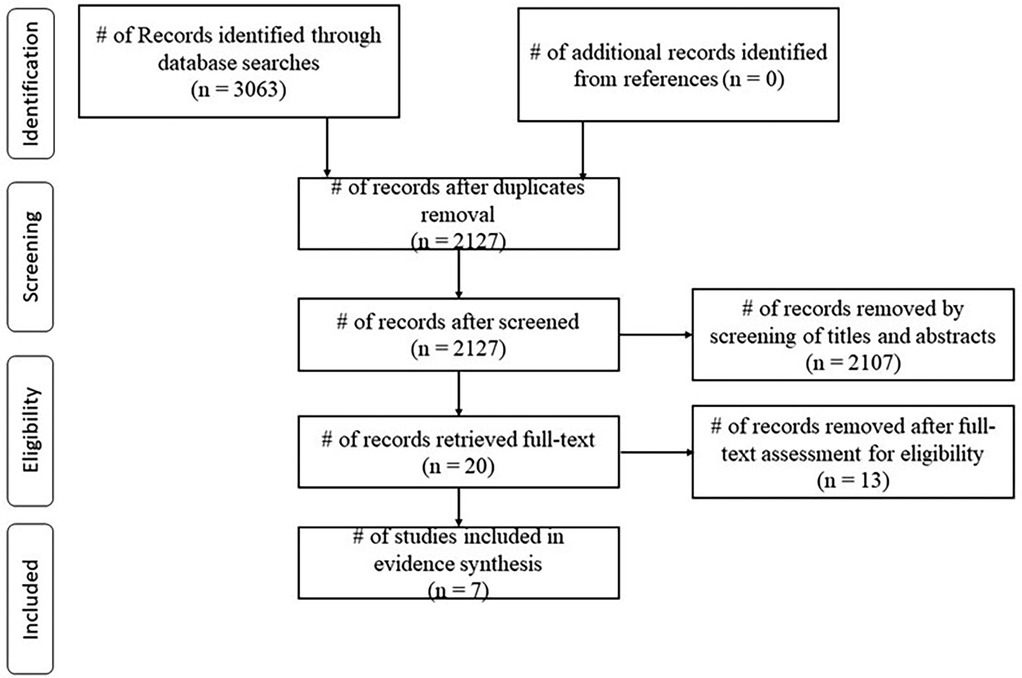
The electronic search yielded 3,063 references; from these 936 duplicates were removed. A further 2107 studies were removed by screening of titles and abstracts leaving 20 studies for full-text retrieval. Thirteen of these studies did not meet the inclusion criteria; only seven were suitable for data extraction. No additional studies were included from an assessment the references in the included studies (Figure 1). None of the included studies assessed the primary outcome of this systematic review which is the COVID-19 transmission change in the health facility due to air-cleaning.
Study selection
The included studies were conducted in Australia, China, Italy, Saudi Arabia, the United Kingdom, and the United States of America. All included studies used the before and after design. Thus, the pre-implementation arm of the study was the control, while the post-implementation arm was the intervention phase. Only one study (28) had a parallel control.
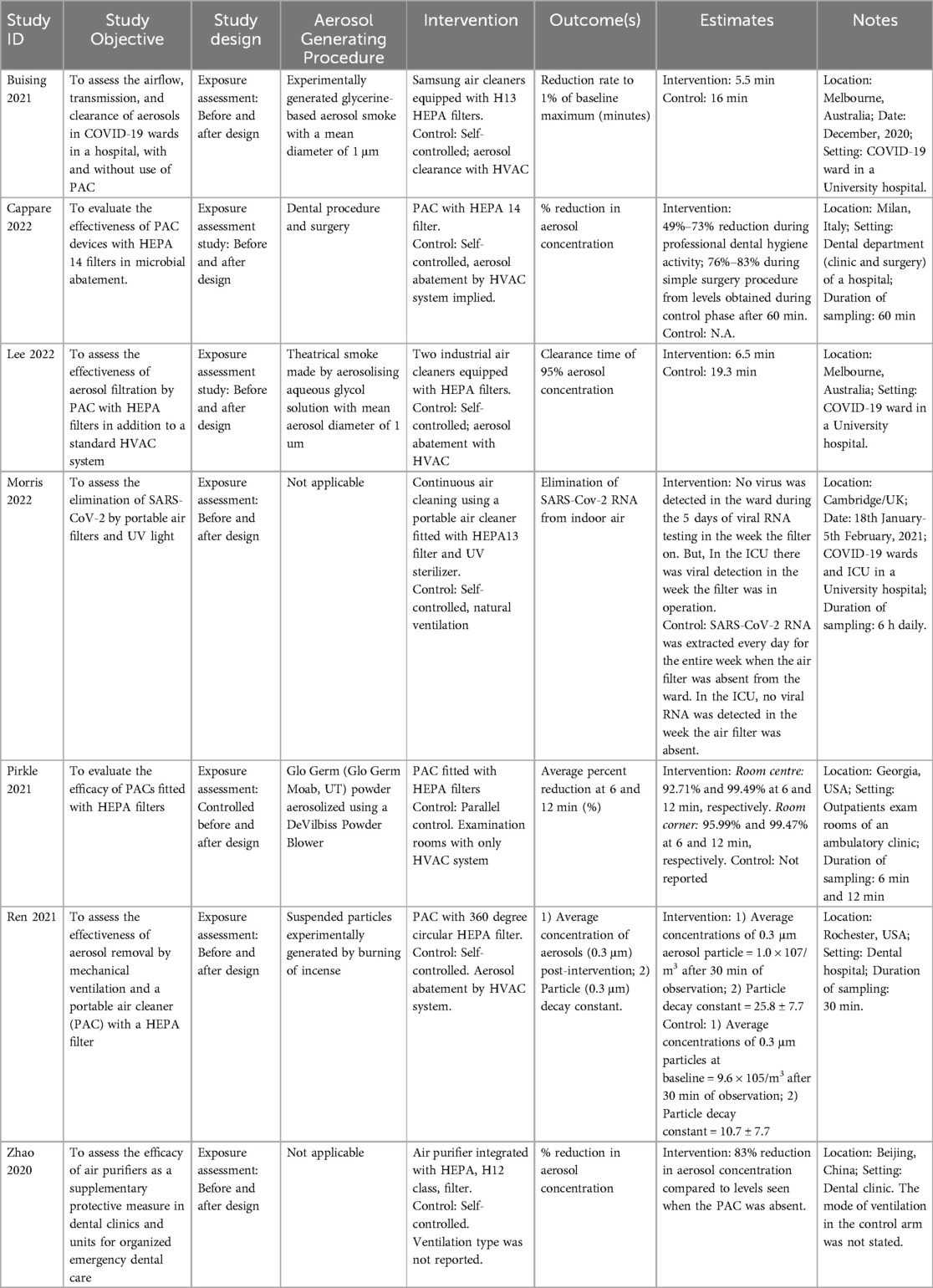
Three of studies were conducted in dental-care facilities, and three in designated COVID-19 hospital wards, including an intensive care unit (29). One study was conducted in an ambulatory clinic. Five of the studies used portable air cleaners (PAC) while two studies used fixed air cleaners. All air cleaners were fitted with HEPA filters, but one study used a HEPA-fitted PAC in combination with a ultra-violet (UV) sterilizer. Further details of the included studies are provided in Table 1.
Twelve studies were excluded in total. Three excluded studies were conducted outside the healthcare setting. Three others were authors' viewpoints and reports expressed in letters to the Editor, while three studies examined the efficacy of air cleaning against other microbial organisms (bacteria and bacteriophages). Two studies were computational fluid dynamic studies, one study was a methodological proposal, and another was a systematic review. A list of the excluded studies and justifications for the exclusion is presented in Supplementary S2.
Risk of bias in studies
An assessment of the risk of bias in the included studies is presented in Table 2.
Reporting bias assessment
One study (28), contrary to the stated methods, only reported results for the intervention arm but omitted results for the control arm. Another study (30) described the mode of ventilation in the intervention phase of the study, but omitted similar information for the control phase of the study.
Results of individual studies
We present the secondary outcomes of this systematic review in relation to comparisons made in the primary studies:
I. Air filter use vs. mechanical ventilation
II. Air filter + U.V. sterilization vs. natural ventilation
I Air filter use vs. mechanical ventilation
1. Rate of reduction of aerosol to 1% of baseline maximum concentration
This outcome was assessed in one study (31). The aerosol concentration dropped to 1% of the baseline maximum in 5.5 min in the intervention arm of the study while the control arm achieved similar abatement in 16 min.
2. Average percent reduction at 6 min and 12 min of sampling
In the intervention group, the mean aerosol decrease at 6 min was 92.71% in the centre of the rooms and 95.99% in the corner of the rooms. The mean decrease at the 12 min mark in the centre and corner of the rooms were 99.49% and 99.47%, respectively. Results from the control phase were unreported (28).
3. Percent reduction in aerosol concentration
The authors recorded about 49%–73% reduction of particles during professional dental hygiene activity and 76%–83% reduction during simple surgical procedures compared to the levels obtained without air cleaning (32).
4. Clearance time of 95% of aerosol concentration
In the intervention arm of the study, 95% aerosol clearance was achieved in 6.5 min, but in the control arm of the study, this was achieved in 19.3 min (33).
5. Aerosol abatement after 30 min of sampling
In the intervention group, the average concentrations of the 0.3 μm aerosol particle observed after 30 min was 1.0 × 107/m3, and 9.6 × 105/m3 for the control arm (34).
6. Particle decay constant
The study referenced above (34) also assessed the particle decay constant. In the intervention arm, this was 25.8 ± 7.7, against 10.7 ± 7.7 obtained in the control arm.
II Air filter + U.V. sterilization vs. natural ventilation
1. Elimination of SARS-Cov-2 RNA
Only one study (29) assessed this outcome. The SARS-CoV-2 RNA was extracted everyday in the first week of sampling, when the air filter was absent from the ward. The viral RNA extraction tests yielded no virus in the second week of sampling during which the PAC filter was operational. The viral RNA could not be extracted in the third week when the PAC was placed in the ward but wasn’t operational.
In the intensive care unit (ICU), air sampling results were contrary to what was obtained in the wards. The SARS-CoV-2 RNA could be detected during the intervention week but was indeterminate during the control week.
Zhao and colleagues (30) assessed the filtration efficiency of PAC expressed as percent-decrease in aerosol concentration from the control phase. There was an 83% reduction in aerosol concentration post-intervention compared to the control aerosol levels. This study did not specify the mode of ventilation in the control arm; therefore, it could not be placed in any of the comparison categories.
Certainty of evidence
The quality of evidence from these individual studies was low, firstly because of the indirectness of evidence which was a limitation in every study. Secondly, the majority of included studies appeared to base their results on just one reading from their experiment. The absence of a distribution of readings from these studies minimized our confidence in the representativeness of their findings. Thirdly, incomplete reporting of results was noticeable in several studies. Some studies completely omitted results for the control arm of the experiment. In reporting a diminished viral or bioaerosol count from the pre-intervention to post-intervention, completeness of reporting requires stating absolute pollutant levels for both arms of the study (Table 3).
Discussion
We found studies which examined different metrics representing partial, total, or time-bound clearance of airborne aerosols or viral RNA as a consequence of HEPA filtration. In general, conclusions from individual studies seem to suggest a faster or more effective clearance of aerosols by HEPA-filtration in comparison with mechanical or natural ventilation. Air cleaning by filtration produced a higher particle decay constant and higher percent clearance per unit of time. Viral RNA was found to be absent in a routine care ward when air filtration was implemented, but paradoxically, viral RNA was detected in the ICU despite the integration of HEPA-filtration with U.V. sterilisation.
Transmission by aerosols and indirectness of data
None of the included studies investigated human transmission as an endpoint of the intervention. Only one investigator looked at the impact of air cleaning on SARS-CoV-2 RNA airborne concentration. Airborne viruses are transmitted in aerosols and droplets which escape from the respiratory passages of infected persons. To wit: the aerosol concentration would be a proxy for viral levels, but the closeness of this approximation is uncertain. Subtypes of viable viruses may differ with regard to their infectivity (dose-response relationship) (35), while the current state of health and specific immune resistance of the exposed individual may modify deposition dynamics and infectivity despite airborne viral levels. Furthermore, not all viruses entrained in the expelled aerosol are viable. Furthermore, environmental factors like sunlight (36), temperature, and humidity (37) determine the stability and viability of viruses borne in aerosols.
The virus aerosols are a mixture of electrolytes, proteins, surfactants, organic material, surface-active compounds, and other components of the fluid lining of respiratory passages (7). During the transfer from the breathing zone of the shedding host and that of the healthy host, the situational temperature and relative humidity (RH) change from human physiologic values on exit (100% RH and 36°C) to that of the immediate room environment through which it transits. The room temperature and relative humidity modify the surface evaporation of the aerosols which in turn influence physico-chemical properties, phase-transition, and size distribution of the aerosols (38). The residence time, dispersal range, and stability of the virion are therefore subject to temperature and RH variations in different zones of the air volume in an enclosed space. Low temperature and RH are conducive to the survival and transmission of influenza viruses. At higher RH (40%–60%) the phospholipid–protein complexes of enveloped viruses are prone to denature in the air. Peak influenza virus decay is observed at RH 50% (range 40%–70%). At low RH (<30%), water evaporates more rapidly from aerosols leaving smaller involatile droplet nuclei which have higher content of proteins and salts, are environmentally more stable, capable of longer air residence times, and consequently have elevated transmission risk (38). The RH and temperature also modulate virus aerosol fate at the inhalation-deposition interface. Dry air provokes an inflammatory response which is accompanied by an increased mucous production and a depressed muco-ciliary clearance of deposits from the respiratory mucosal surfaces (39). Depositing aerosols are less likely to be removed. Moreover, inflammation creates breaches in the protective respiratory mucosa and impairs the local epithelial immune response. These conditions enable the settling of virus aerosols deeper down the respiratory tree and a greater likelihood of an established infective process (38).
The healthcare environment vs. other settings
Recommendations for health facility indoor ventilation are very specific and they seek to, among other things, limit air movements in between service areas and departments; attenuate and eliminate microbial, chemical, radioactive, and odoriferous contaminations; maintain the temperature and humidity demands which are conducive to the operations of different spaces (40). An important distinction of the ventilation systems of health facilities in comparison with office complexes, public buildings, schools, and aircrafts, for example, is the principle of zoning. Zoning achieves different ventilation system specifications in different department. Therefore, regular patients wards, isolation wards, intensive care units, operating theatres, recovery rooms, treatment rooms, nurses' stations, laboratories, sterilizing rooms, and administrative offices differ in environmental circulation components (40). In addition, health facilities are unique because they operate throughout the day every day of the year, so their air dilution and corresponding energy costs are enormous.
The effectiveness of a ventilation system in preventing virus transmission depends on the average amount of virus-free air that is provided to each occupant in a volume of space (41, 42). Effective air cleaning must incorporate the ventilation and preferred atmospherics of hospital spaces, but should also take into account the strength and persistence of virus production, and the number of sources (41). Studies included in this systematic review present a range of ventilation settings which were determined by the volume of the rooms, the room occupancy, the specific use to which a room was put, and the engineering and comfort-oriented requirements the healthcare setting (43, 44). Several studies specified the ventilation parameters of the study area and the adjustment of filtration devices to match the air-change rates of the inherent mode of room ventilation.
The HVAC modulation of airborne virus exposure in a room depends more on the occupancy density (which is the spatial volume of the room divided by the number of occupants), than it does on the outdoor air supplied per person, and reintroduced filtered air supplied per person. The occupancy density determines the time needed to attain the maximum virus concentration in uniformly mixed air while outdoor air supply and reintroduced filtered air determine the set point of virus maximum concentration. The implication is that spaces may have identical equilibrium concentration (when viral-laden aerosols settle into a uniform concentration in the entire volume of a well-mixed space) and exposure time, but higher infectivity is more likely to occur in those spaces with higher occupancy density because the highest inhalation dose is reached in shorter time (42). By implication, higher occupancy density reduces the fraction of virus-free outside air that is available for ventilation in demarcated floor area (45). The equilibrium concentration and the time to attain it also decreased in response to viral settling, and deactivation. At this concentration, the transmission rate of the virus is constant (6). Reviewed studies in this paper did not demonstrate or highlight how occupancy density affected their results.
The uniqueness of recommended ventilation in healthcare spaces justify focusing this review only on studies which were conducted in the health facility. It is worth emphasising that although the studies included in this review offer some evidence of effective aerosol abatement from air-filtration, this may not translate to similar abatement of viral transmission. The air cleaning technologies are designed to deplete background levels of aerosols. However, the immediate breathing zone of the patient would be rich in aerosols, thus increasing the exposure of the health worker, who often has to interact in close proximity with the patient, despite background air cleaning (20). Therefore, the potential viral abatement offered by air cleaning technologies should supplement other protective measures such as the use of personal protective equipment (PPE).
Absence of comparative analysis
Most of the included studies simply presented summary statistics which contrasted scenarios with and without air filtration. Actual comparative statistical analyses of aerosol concentrations between those scenarios were absent from most of these studies. This limitation makes it impractical to conclude that the observed differences in measured concentrations were not subject to extraneous influences. In addition, some methodological inadequacies did not permit such analysis as it seemed investigators used single experimental readings without replicating the experiment.
Side effects of air cleaners
The use of air-cleaning technologies can have undesired environmental consequences. Only two studies measured noise generated by the air filtering devices. Such side-effects may affect patients' recovery and overall well-being; especially, for noise-sensitive patients, but also for patients whose disease conditions may be worsened by the nature of the side-effect. For example, patients with overt or underlying respiratory conditions may experience worsening of symptoms from ozone, an established respiratory irritant. Ozone is known to be generated by U.V. light, electrostatic precipitators, and ion generators (20, 46).
Authors' conclusions
Implications for practice
In summary, the reviewed studies offer some evidence of accelerated aerosols removal by air filters. The implication of this finding for transmission have been supported by other studies which have shown the reduction in the transmission of other viruses and bacteria by HEPA filtration. However, a quantification of the expected reduction in the transmission of SAR-CoV-2 cannot be implied as the research evidence does not exist. Thus, the care of confirmed COVID-19 patients and potentially infected persons in the health facility cannot be separated from policies which prioritise the use of PPEs. The between-use wait-time of clinics, treatment rooms, and operating theatres would still be subject to existing standards as this systematic review does not provide sufficient evidence for a policy review.
Implications for research
Investigators in this discipline should agree on a limited set of metrics for quantifying the outcome of air-cleaning devices on the transmission potential of SARS-CoV-2 and other pathogens. There should be adherence to uniformity in the measurement and calculation of these metrics even when investigators explore different environmental and interventional scenarios. The consistency of findings between studies can be meaningfully assessed if methodologies and outcome metrics are comparable between studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EO: Writing – original draft, Writing – review & editing, Formal analysis, Investigation, Methodology, Project administration. MC: Investigation, Methodology, Writing – original draft. CE: Writing – original draft. MB: Investigation, Writing – original draft. VB: Investigation, Writing – original draft. AO-I: Conceptualization, Project administration, Writing – original draft. MM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This systematic review was funded under the “World Health Organisation Infection Prevention and Control in the context of COVID-19” Systematic Reviews project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvh.2025.1548272/full#supplementary-material
References
1. World Health Organisation. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. (2020). Available online at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (Accessed September 14, 2022).
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a pandemic. Acta Biomed. (2020) 91(1):157–60. doi: 10.23750/abm.v91i1.9397
4. Tang D, Comish P. The hallmarks of COVID-19 disease. PLoS Pathog. (2020) 16(5):e1008536. doi: 10.1371/journal.ppat.1008536
5. Christopherson DA, Yao WC, Lu M, Vijayakumar R, Sedaghat AR. High-efficiency particulate air filters in the era of COVID-19: function and efficacy. Otolaryngol Head Neck Surg. (2020) 163(6):1153–5. doi: 10.1177/0194599820941838
6. Bazant MZ, Bush JWM. A guideline to limit indoor airborne transmission of COVID-19. Proc Natl Acad Sci U S A. (2021) 118(17):e2018995118. doi: 10.1073/pnas.2018995118
7. Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. Airborne transmission of respiratory viruses. Science. (2021) 373(6558):eabd9149. doi: 10.1126/science.abd9149
8. van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382(16):1564–7. doi: 10.1056/NEJMc2004973
9. Gola M, Caggiano G, De Giglio O, Napoli C, Diella G, Carlucci M, et al. SARS-CoV-2 indoor contamination: considerations on anti-COVID-19 management of ventilation systems, and finishing materials in healthcare facilities. Ann Ig. (2021) 33(4):381–92. doi: 10.7416/ai.2020.2396
10. Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. (2020) 582(7813):557–60. doi: 10.1038/s41586-020-2271-3
11. Kogan V, Harto C, Hesse DJ, Hofacre KC. Final Report on Evaluation of In-Room Particulate Matter Air Filtration Devices. Washington, DC: Environmental Protection Agency (2008).
12. Morawska L, Tang JW, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. (2020) 142:105832. doi: 10.1016/j.envint.2020.105832
13. Centers for Disease Control and Prevention. Guidelines for envronmental infection control in health-care facilities. Appendix B: Air. (2003).
14. Atkinson J, Chartier Y, Pessoa-Silva CL, et al. Natural Ventilation for Infection Control in Health-care settings. Geneva, Switzerland: World Health Organisation (2009).
15. Elsaid AM, Mohamed HA, Abdelaziz GB, Ahmed MS. A critical review of heating, ventilation, and air conditioning (HVAC) systems within the context of a global SARS-cov-2 epidemic. Process Saf Environ Prot. (2021) 155:230–61. doi: 10.1016/j.psep.2021.09.021
16. Barnewall RE, Bischoff WE. Removal of SARS-CoV-2 bioaerosols using ultraviolet air filtration. Infect Control Hosp Epidemiol. (2021) 42(8):1014–5. doi: 10.1017/ice.2021.103
17. Nazarenko Y. Air filtration and SARS-CoV-2. Epidemiol Health. (2020) 42:e2020049. doi: 10.4178/epih.e2020049
18. Boretti A, Banik B, Castelletto S. Use of ultraviolet blood irradiation against viral infections. Clin Rev Allergy Immunol. (2021) 60(2):259–70. doi: 10.1007/s12016-020-08811-8
19. Cao J, Zhang Y, Chen Q, Yao M, Pei R, Wang Y, et al. Ozone gas inhibits SARS-Cov-2 transmission and provides possible control measures. Aerosol Sci Eng. (2021) 5(4):516–23. doi: 10.1007/s41810-021-00118-1
20. Environmental and Modelling Group. Potential application of air cleaning devices and personal decontamination to manage transmission of COVID-19. (2020).
21. McDevitt JJ, Rudnick SN, Radonovich LJ. Aerosol susceptibility of influenza virus to UV-C light. Appl Environ Microbiol. (2012) 78(6):1666–9. doi: 10.1128/AEM.06960-11
22. Elvis AM, Ekta JS. Ozone therapy: a clinical review. J Nat Sci Biol Med. (2011) 2(1):66–70. doi: 10.4103/0976-9668.82319
23. World Health Organisation. Year of health and care workers 2021 (2021). Available online at: https://www.who.int/campaigns/annual-theme/year-of-health-and-care-workers-2021 (Accessed February 10, 2023).
24. Okokon EO, Chibuzor M, Ezema C, Bernard M, Barde V, Oyo-Ita A, et al. The effectiveness of equivalent ventilation provided by air cleaning/purifier technologies against COVID-19 transmission in healthcare settings. PROSPERO. (2022) 2022:CRD42022356533.
25. World Health Organisation. Risk of bias assessment instrument for systematic reviews informing WHO global air quality guidelines. 2020.
26. Frampton G, Whaley P, Bennett M, Bilotta G, Dorne JCM, Eales J, et al. Principles and framework for assessing the risk of bias for studies included in comparative quantitative environmental systematic reviews. Environ Evid. (2022) 11(1):12. doi: 10.1186/s13750-022-00264-0
27. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group (2013). p. 2013. Available at: https://www.gradepro.org/terms/cite
28. Pirkle S, Bozarth S, Robinson N, Hester W, Wagner L, Broome S, et al. Evaluating and contextualizing the efficacy of portable HEPA filtration units in small exam rooms. Am J Infect Control. (2021) 49(12):1506–10. doi: 10.1016/j.ajic.2021.08.003
29. Morris AC, Sharrocks K, Bousfield R, Kermack L, Maes M, Higginson E, et al. The removal of airborne severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) and other microbial bioaerosols by air filtration on coronavirus disease 2019 (COVID-19) surge units. Clin Infect Dis Title. (2021) 75(1):e97–e101. doi: 10.1093/cid/ciab933
30. Zhao B, An N, Chen C. Using an air purifier as a supplementary protective measure in dental clinics during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. (2021) 42(4):493. doi: 10.1017/ice.2020.292
31. Buising KL, Schofield R, Irving L, Keywood M, Stevens A, Keogh N, et al. Use of portable air cleaners to reduce aerosol transmission on a hospital coronavirus disease 2019 (COVID-19) ward. Infect Control Hosp Epidemiol. (2022) 43(8):987–92. doi: 10.1017/ice.2021.284
32. Capparè P, D'Ambrosio R, De Cunto R, Darvizeh A, Nagni M, Gherlone E. The usage of an air purifier device with HEPA 14 filter during dental procedures in COVID-19 pandemic: a randomized clinical trial. Int J Environ Res Public Health. (2022) 19(9):5139. doi: 10.3390/ijerph19095139
33. Lee JH, Rounds M, McGain F, Schofield R, Skidmore G, Wadlow I, et al. Effectiveness of portable air filtration on reducing indoor aerosol transmission: preclinical observational trials. J Hosp Infect. (2022) 119:163–9. doi: 10.1016/j.jhin.2021.09.012
34. Ren Y-F, Huang Q, Marzouk T, Richard R, Pembroke K, Martone P, et al. Effects of mechanical ventilation and portable air cleaner on aerosol removal from dental treatment rooms. J Dent. (2021) 105:103576. doi: 10.1016/j.jdent.2020.103576
35. Nikitin N, Petrova E, Trifonova E, Karpova O. Influenza virus aerosols in the air and their infectiousness. Adv Virol. (2014) 2014:859090. doi: 10.1155/2014/859090
36. Schuit M, Gardner S, Wood S, Bower K, Williams G, Freeburger D, et al. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J Infect Dis. (2019) 221(3):372–8. doi: 10.1093/infdis/jiz582
37. Yang W, Marr Linsey C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol. (2012) 78(19):6781–8. doi: 10.1128/AEM.01658-12
38. Wolkoff P. Indoor air humidity revisited: impact on acute symptoms, work productivity, and risk of influenza and COVID-19 infection. Int J Hyg Environ Health. (2024) 256:114313. doi: 10.1016/j.ijheh.2023.114313
39. Wang S, Deng W, Wang H. Protecting healthcare workers against COVID-19: a fast-track ventilation system for hospitals [abstract]. Proceedings of the 11th Asian Conference on Emergency Medicine (ACEM) 2021. Hong Kong J Emerg Med. (2022) 29(11_suppl):2S–87S. doi: 10.1177/10249079221099636
40. ASHRAE. 2019 ASHRA Handbook: Heating Ventilating and Air-Conditioning Applications. S-I ed. Atlanta, GA: American Society of Heating Refrigerating and Air-Conditioning Engineers Inc. ASHRAE (2019).
41. Gupta JK, Lin CH, Chen Q. Risk assessment of an airborne disease inside the cabin of a passenger airplane. AE Int J Adv Curr Prac in Mobility. (2021) 3(3):1263–71. doi: 10.4271/2021-01-0036
42. Walkinshaw D. A brief introduction to passenger aircraft cabin air quality. ASHRAE J. (2020) 62:12–8.
43. Jung C-C, Wu P-C, Tseng C-H, Su H-J. Indoor air quality varies with ventilation types and working areas in hospitals. Build Environ. (2015) 85:190–5. doi: 10.1016/j.buildenv.2014.11.026
44. Yau YH, Chandrasegaran D, Badarudin A. The ventilation of multiple-bed hospital wards in the tropics: a review. Build Environ. (2011) 46(5):1125–32. doi: 10.1016/j.buildenv.2010.11.013
45. Walkinshaw DS, Horstman RH. COVID 19 and beyond: a procedure for HVAC systems to address infectious aerosol illness transmission. Front Built Environ. (2023) 9:999126. doi: 10.3389/fbuil.2023.999126
Keywords: air cleaning, aerosols, COVID-19, health facility, HEPA filter, indoor transmission
Citation: Okokon E, Chibuzor M, Ezema C, Bernard M, Barde V, Oyo-Ita A, Meremikwu M and WHO Colleagues (2025) The effectiveness of air-cleaning technologies against COVID-19 transmission in healthcare settings. Front. Environ. Health 4:1548272. doi: 10.3389/fenvh.2025.1548272
Received: 19 December 2024; Accepted: 7 April 2025;
Published: 1 May 2025.
Edited by:
Alessandro Giampieri, Durham University, United KingdomReviewed by:
Hasim Altan, Prince Mohammad bin Fahd University, Saudi ArabiaDouglas Walkinshaw, Indoor Air Technologies Inc, Canada
Copyright: © 2025 Okokon, Chibuzor, Ezema, Bernard, Barde, Oyo-Ita, Meremikwu and WHO Colleagues. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enembe Okokon, ZW5lbWJlb2tva29uQHVuaWNhbC5lZHUubmc=
 Enembe Okokon
Enembe Okokon Moriam Chibuzor3
Moriam Chibuzor3 Moses Bernard
Moses Bernard