- 1Phase I Clinical Trial Laboratory, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
- 2Department of Hematology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
- 3Department of Cardiology, The Second Affiliated Hospital of Tianjin Medical University, Tianjin, China
- 4Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Background: Essential thrombocythemia (ET) is one of the Philadelphia-negative (Ph−) classical myeloproliferative neoplasms (MPNs), characterized by increased megakaryocyte and platelet counts, as well as an increased risk of thrombo-hemorrhagic complications. The JAK2 V617F mutation is detected in 50%–60% of patients with ET and can drive ET occurrence through ligand-independent activation of the thrombopoietin (TPO) receptor, resulting in JAK/STAT pathway signaling. Phosphatase and tensin homolog (PTEN) has been found to be associated with many hematologic diseases, particularly in MPNs, and is involved in the JAK/STAT pathway. This study aimed to determine the role of PTEN in ET patients with the JAK2 V617F mutation.
Methods: In this study, we analyzed a series of 32 ET patients without treatment and 25 age- and sex-matched normal controls for the detection of PTEN expression and the STT motif phosphorylation of PTEN, as well as the expression patterns of STAT3 and STAT5 and their phosphorylated molecules (p-STAT3 and p-STAT5, respectively), in bone marrow mononuclear cells (BM-MNCs) by immunohistochemical (IHC) staining of bone marrow biopsies and Western blotting. Correlations between the PTEN expression and other clinical characteristics were also examined.
Results: The results showed a downregulated PTEN expression in the BM-MNCs of patients with ET, as well as an elevated p-STAT3 expression and a decreased p-STAT5 expression. In addition, analysis in combination with clinical data demonstrated a negative correlation between the expression of PTEN and the patients’ age and platelet counts. In contrast, a positive correlation was detected between the expression of PTEN and the level of p-STAT5.
Conclusions: The results of this study suggest that aberrant PTEN expression and JAK/STAT pathway signaling might be involved in the onset of ET. Our findings could shed new light on the pathogenesis of and the treatment for ET.
1 Introduction
Essential thrombocythemia (ET) is one of the Philadelphia-negative (Ph−) classical myeloproliferative neoplasms (MPNs), which additionally includes polycythemia vera (PV) and overt and primary myelofibrosis (PMF), according to the latest World Health Organization (WHO) classification (1). As a clonal hematopoietic stem cell (HSC) disease, ET is characterized by increased megakaryocytes in the bone marrow and platelet counts in peripheral blood, along with an increased risk of thrombo-hemorrhagic complications, leading to disability and even death (2). ET is also associated with elevated fibrotic progression rates and an increased risk of transformation into secondary acute myeloid leukemia (AML). Driver mutations in JAK2, CALR, and MPL are considered to be constitutive activation of the JAK/STAT signaling pathway, which are detected in approximately 90% of patients with ET and are crucial for diagnosis (3). Among the three types of mutations, the JAK2 V617F mutation is the most common, which is involved in all the different types of Ph− classical MPNs and detected in 50%–60% of patients with ET (4). While JAK2 V617F can drive the occurrence of ET through ligand-independent activation of the thrombopoietin (TPO) receptor, the megakaryocytic lineage expands and inflammatory cytokines are released, leading to various clinical features such as vasomotor symptoms, constitutional manifestations, splenomegaly, thrombosis, and hemorrhage (2, 5).
The evolutionally conserved JAK/STAT pathway acts as a critical intracellular mediator for cell surface receptor–ligand interactions and is a bridge for the activation of the downstream signaling cascade. Engagement of the cytokine receptor with ligand results in the phosphorylation of substrate molecules, including signal transducers and activators of transcription (STATs). As STAT family members, there are numerous studies on STAT3 and STAT5 in MPNs. A crucial role of STAT3 in ET has been supported by findings of higher levels of activated STAT3 (phosphorylated STAT3, p-STAT3) detected in an AML1/MDS1/EVI1 (AME) murine model that exhibited similar characteristics to ET compared with normal controls (6). An elevated level of p-STAT3 was also found in approximately half of bone marrow samples of patients with ET, regardless of the JAK2 mutation status. Conversely, the expression of p-STAT5 (phosphorylated STAT5) appeared to be downregulated (6, 7). This discrepancy in the expression of the STAT family members may be due to the roles they each play. STAT3 is an important regulator in the process of megakaryocytopoiesis (8). Therefore, the phosphorylation of this signal transducer may increase in patients with ET, while that of STAT5 does not.
Phosphatase and tensin homolog (PTEN) plays a negative regulatory role in phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling, which are downstream of RAS, termed as the PTEN/PI3K (or MAPK)/RAS pathway. PTEN has been found to be associated with various types of cancers, particularly some solid malignancies such as breast and lung cancers. In addition, deficiency in PTEN has been reported in many hematological diseases, including juvenile myelomonocytic leukemia (JMML), where it is deficient in 67% of patients (9). The phosphorylation of the Ser380, Thr382, and Thr383 residues (collectively named the STT motif) on the C-terminal tail of PTEN is connected to its stability; thus, the STT motif regulates its catalytic activity and stability, which may be associated with its antitumor activity (10). However, the role of PTEN and PTEN phosphorylation in Ph− classical MPNs remains largely unexplored. The relationship between PTEN and the STAT family has been described in many publications. The STAT3 pathway was observed to be positively regulated by mammalian target of rapamycin (mTOR) signaling, while PTEN serves as a negative regulator of both STAT3 and mTOR signaling in human breast cancer cell lines (11). Recent research on JAK2-mutant mouse models (12, 13) and MPN patient samples (14) has revealed that the JAK2 V617F mutation increases the fitness of HSCs and promotes megakaryocyte–erythroid differentiation. However, the function of PTEN and the STAT family in the pathogenesis of the classical JAK2 V617F mutation-positive ET has not been fully investigated. In this study, we aimed to identify the role of PTEN and the STAT family in the pathogenesis of the classical JAK2 V617F mutation-positive ET and to provide implications for the development of targeted therapies for this malignant disease.
As previous research works involving JAK2-mutant mouse models (12, 13) and MPN patient samples (14) have demonstrated that JAK2 V617F increases the fitness of HSCs and promotes megakaryocyte–erythroid differentiation, in this study, our aims were to identify the function of PTEN and the STAT family in the pathogenesis of the classical JAK2 V617F mutation-positive ET and to provide implications for the development of agents that target improved treatment of this malignant disease.
2 Methods
2.1 Patients
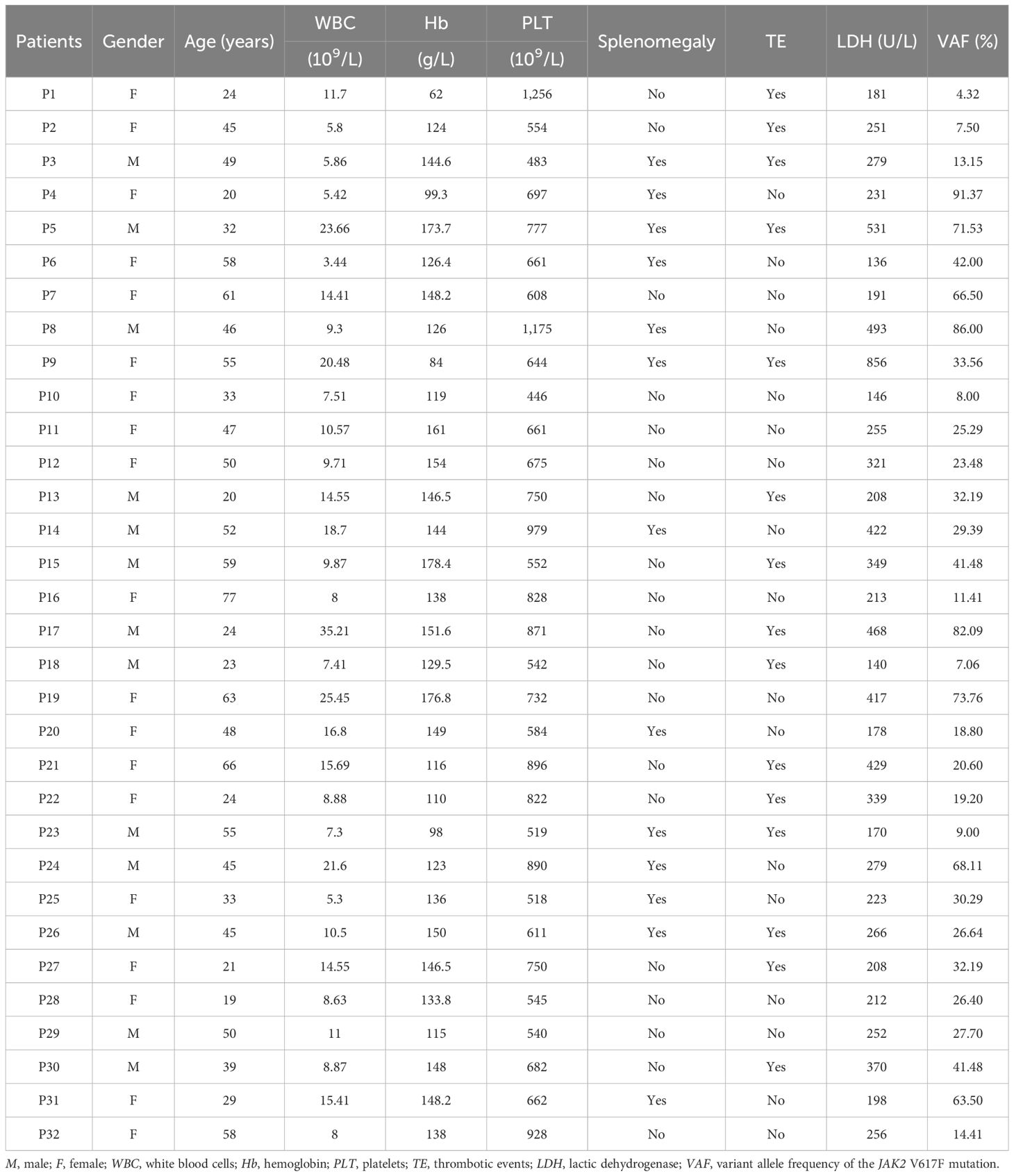
This research was approved by the hospital-based ethics committee (no. 2017004). Written informed consent was obtained from all participants or their legal guardians. The study was conducted in accordance with the Declaration of Helsinki. A total of 32 ET patients without treatment and 25 age- and sex-matched normal controls were enrolled in this study. The diagnosis of ET was based on the WHO 2016 diagnostic criteria (1). In all patients with ET, the presence of the JAK2 V617F mutation was documented using next-generation sequencing (NGS). The bone marrow aspirate and biopsy samples from patients with ET were collected at the time of diagnosis. The bone marrow samples of the control group were collected from normal donors for bone marrow transplantation. Information on the ET patients and normal controls enrolled in this study is provided in Table 1.
2.2 Bone marrow aspiration and biopsy
All study patients and normal controls underwent bone marrow aspiration and biopsy performed under sterile conditions using a 13-gauge, 200-mm bone marrow biopsy needle (Aisiai, Shanghai, China) on the anterior superior iliac spine. Approximately 10 ml of the bone marrow aspirate from each donor was collected in EDTA-coated tubes. The bone marrow biopsy materials (biopsy core of 1.5 cm) were transported to the pathology laboratories in 4% paraformaldehyde solution. The bone marrow pathological specimens were examined by the same histopathologist who is experienced in MPNs. All examinations were performed under an Olympus BX53 light microscope (Olympus, Tokyo, Japan).
2.3 Cell separation and Western blot
Bone marrow mononuclear cells (BM-MNCs) were isolated from EDTA anticoagulant bone marrow samples from the study subjects using Ficoll-Hypaque density gradient centrifugation at 1,800 rpm for 20 min at 20°C. All nucleated cells were then recovered, washed twice, and suspended in phosphate-buffered saline (PBS). Cell samples were incubated with 1 ml RIPA lysis buffer (Beyotime, Shanghai, China). After incubation on ice for 30 min, the cell lysates were centrifuged at 4°C and the supernatant proteins recovered and measured using a bicinchoninic acid (BCA) protein assay kit (Merck KGaA, Darmstadt, Germany), then dissolved in an SDS sample buffer (Beyotime), boiled for 5 min, and then separated on 10%–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). These proteins were then transferred into polyvinylidene difluoride (PVDF) membranes (Merck KGaA). After blocking with 5% nonfat milk, the PVDF membranes were incubated with primary antibodies in Tris-buffered saline (TBS) overnight at 4°C. The primary antibodies were anti-GAPDH, anti-PTEN, anti-STAT3, anti-p-STAT3, anti-STAT5, anti-p-STAT5 (1:1,000; Bioswamp, Wuhan, China), and anti-Phospho-PTEN (Ser380/Thr382/Thr383) (1:1,000; Absin, Shanghai, China). The following day, the PVDF membranes were incubated with goat anti-rabbit horseradish peroxidase (HRP)-conjugated antiserum (1:20,000; Bioswamp) for 1 h at room temperature. After soaking the membranes in an enhanced chemiluminescence reagent (Thermo Scientific, Waltham, MA, USA) for 5 min, the blots were visualized using an X-ray film.
2.4 Immunohistochemistry
Immunohistochemistry (IHC) staining was conducted to examine the expression of PTEN, STAT3, and p-STAT3 in the bone marrow cells of patients with ET. The aspirated material was centrifuged and the sediment transferred into 10% buffered formalin for overnight fixation. Tissue sections (5 μm) taken from each formalin-fixed, paraffin-embedded tissue block were deparaffinized, rehydrated, and rinsed in distilled water. Antigen retrieval was performed using a pressure cooker with 10 nM citrate buffer (pH 6.0) for 2 min. Endogenous peroxidase activity was then blocked by incubating the slides in 3% hydrogen peroxide in methanol for 10 min. The primary antibodies [anti-PTEN, anti-STAT3, anti-p-STAT3, and anti-p-STAT5 (Abcam, Cambridge, UK); anti-STAT5 (Bioss, Beijing, China); and anti-Phospho-PTEN (Ser380/Thr382/Thr383; Absin, Shanghai, China)] were incubated at room temperature for 2 h, and chromogen development was performed using the PV-9001 detection kit (ZSGB-BIO, Beijing, China). Sections were counterstained using hematoxylin, hydrated, cleaned, and then mounted under coverslips. The slides were visualized using an Olympus BX53 light microscope. The 3,30-diaminobenzidine (DAB)-positive areas were determined from the IHC images using the ImageJ program (v.1.51j8). Image-Pro Plus software was also used to analyze the optical density of protein expression.
2.5 Statistical analysis
Statistical analyses were performed with Stata 7.0 software (Stata, College Station, TX, USA). Data were given as the mean ± standard error of the mean (SEM). Statistical comparisons between groups were performed using one-way ANOVA followed by Scheff’s test or the Wilcoxon signed-rank test. The relationships between PTEN and the other clinical characteristics were evaluated using Pearson’s correlation test. A two-sided p-value <0.05 was considered statistically significant. Descriptive univariate analysis was also performed. Differences among groups were considered significant when p < 0.05.
3 Results
3.1 Demographic information of the recruited ET patients
As shown in Table 1, a total of 32 untreated patients with ET were recruited into this study. Of these patients, 16 (50%) were men, and the median age was 62.5 years (range, 14–83 years). In the overall cohort, the peripheral blood examination showed a median white blood cell (WBC) count of 10.19 × 109/L (range, 3.44–35.21 × 109/L), a median hemoglobin (Hb) volume of 138 g/L (range, 62–178.4 × 109/L), and a median platelet count of 668.5 × 109/L (range, 446–1,256 × 109/L). The indicators of disease burden, i.e., lactic dehydrogenase (LDH) and the variant allele frequency (VAF) of the JAK2 V617F mutation, were also evaluated. The median LDH value was 253.5 U/L (range, 136–856 U/L), while the median VAF for JAK2 V617F was 28.55% (range, 4%–91%). Splenomegaly and history of thrombotic events were found in 13 (40.63%) and 14 (43.75%) patients with ET, respectively.
3.2 The expression of PTEN decreased in ET patients compared with normal controls
In order to preliminarily understand the expression of PTEN in the bone marrow cells of patients with ET, the data from public databases were first analyzed. The public gene expression profile GSE103176 was downloaded from the Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo), a public functional genomic data repository. The GSE103176 data showed that the PTEN mRNA expression was significantly decreased in ET patients compared with normal controls (p < 0.001) (Figure 1A). The status of the JAK2 V617F mutation also appeared to affect the PTEN mRNA expression, as demonstrated in Figure 1B. Although the low incidence limited the sample sizes of the JAK2 V617F-negative ET in public databases, we were still able to observe that patients with ET who were negative for the JAK2 V617F mutation exhibited lower PTEN mRNA expression compared with those with a positive mutation status. Subsequently, we confirmed the database results for the detection of PTEN expression in the BM-MNCs and bone marrow biopsy tissue from 32 ET patients and 25 normal controls. In the Western blot analysis, densitometric analysis of the bands revealed that the ET patient samples exhibited lower PTEN levels compared with normal controls (Figures 2A, D). Furthermore, the IHC showed similar results (Figures 3A, B, K). The phosphorylation of the STT motif at the C-terminal of PTEN has been proven essential for its DNA repair activity and its contribution to cell survival in several research studies (15). To explore the phosphorylation level of the STT motif, Western blotting and IHC analysis were performed. The results showed no differences between the ET patients and normal controls (Figures 2A, D, 3C, D, N).
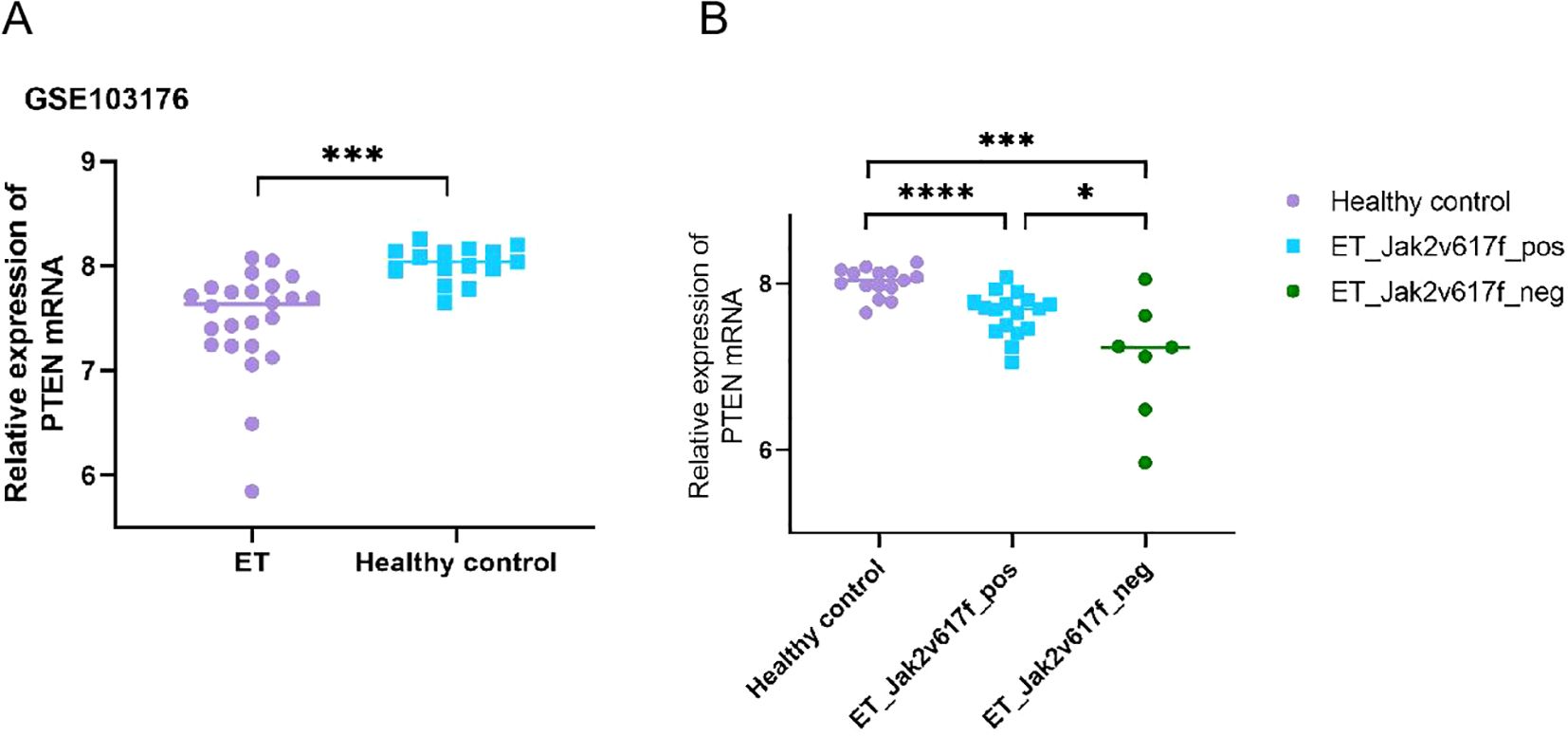
Figure 1. Data from the public dataset GSE103176 showing the phosphatase and tensin homolog (PTEN) mRNA expression in patients with essential thrombocythemia (ET) and in normal controls. (A) Data from the public dataset GSE103176 showed that the PTEN mRNA expression is significantly decreased in patients with ET. (B) JAK2 V617F mutation-negative ET patients had lower PTEN mRNA expression than those who were mutation-positive. *p < 0.05, ***p < 0.001, ****p < 0.0001.
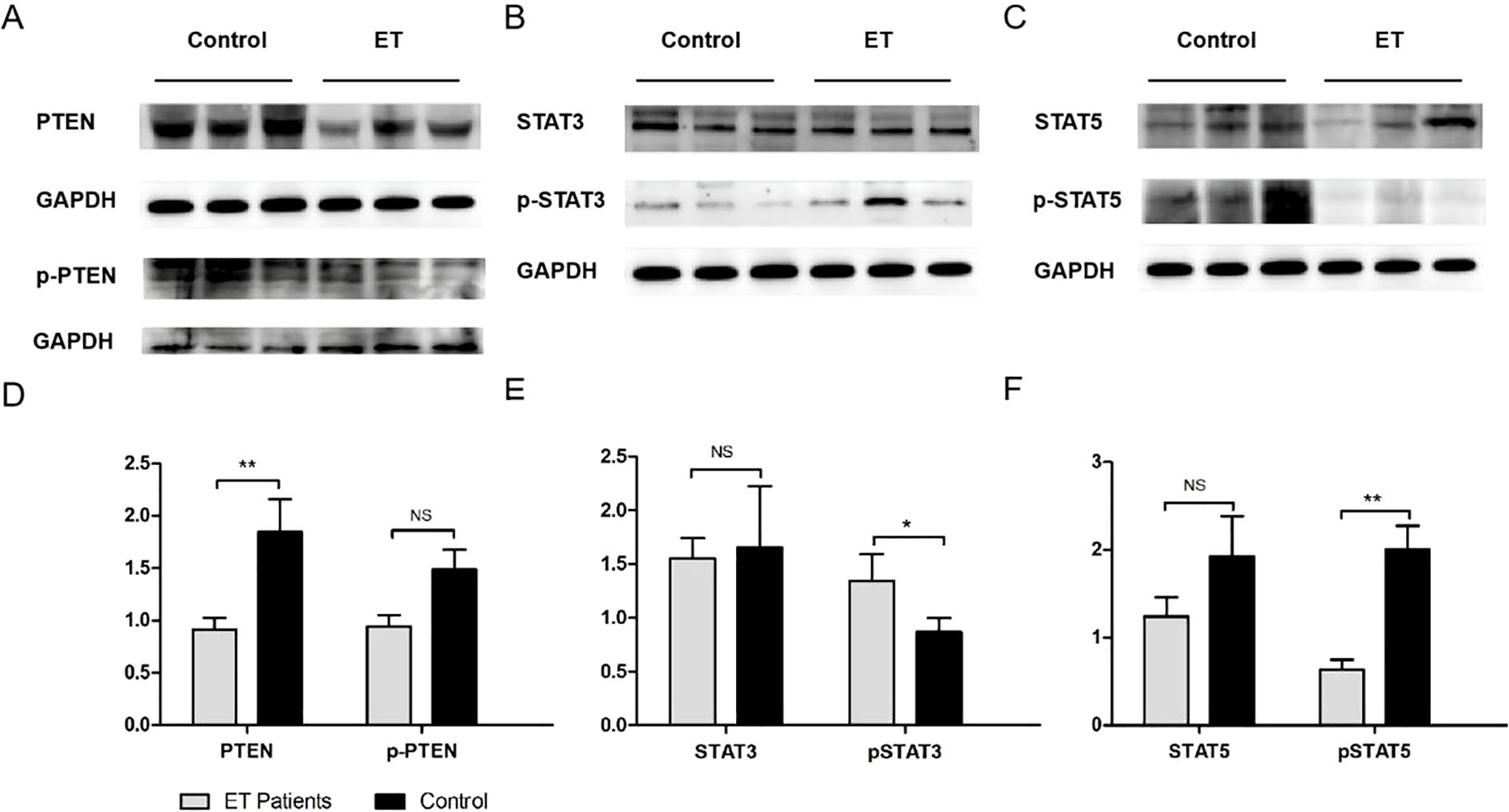
Figure 2. Western blot (WB) analysis of phosphatase and tensin homolog (PTEN), p-PTEN, STAT3, p-STAT3, STAT5, and p-STAT5 in the bone marrow mononuclear cells (BM-MNCs) of essential thrombocythemia (ET) patients and normal controls. (A–C) Three representative bands of the WB diagrams of PTEN, p-PTEN, STAT3, p-STAT3, STAT5, and p-STAT5 in ET patients and controls. (D–F) Quantitative results of the expression of PTEN and p-PTEN (PTEN/GAPDH and p-PTEN/GAPDH) (D); STAT3 and p-STAT3 (STAT3/GAPDH and p-STAT3/GAPDH) (E); and STAT5 and p-STAT5 (STAT5/GAPDH and p-STAT5/GAPDH) (F) in ET patients and normal controls. p-PTEN, phosphorylation of the STT (Ser380, Thr382, and Thr383 residues) motif on PTEN. *p < 0.05, **p < 0.01. NS, not significant.
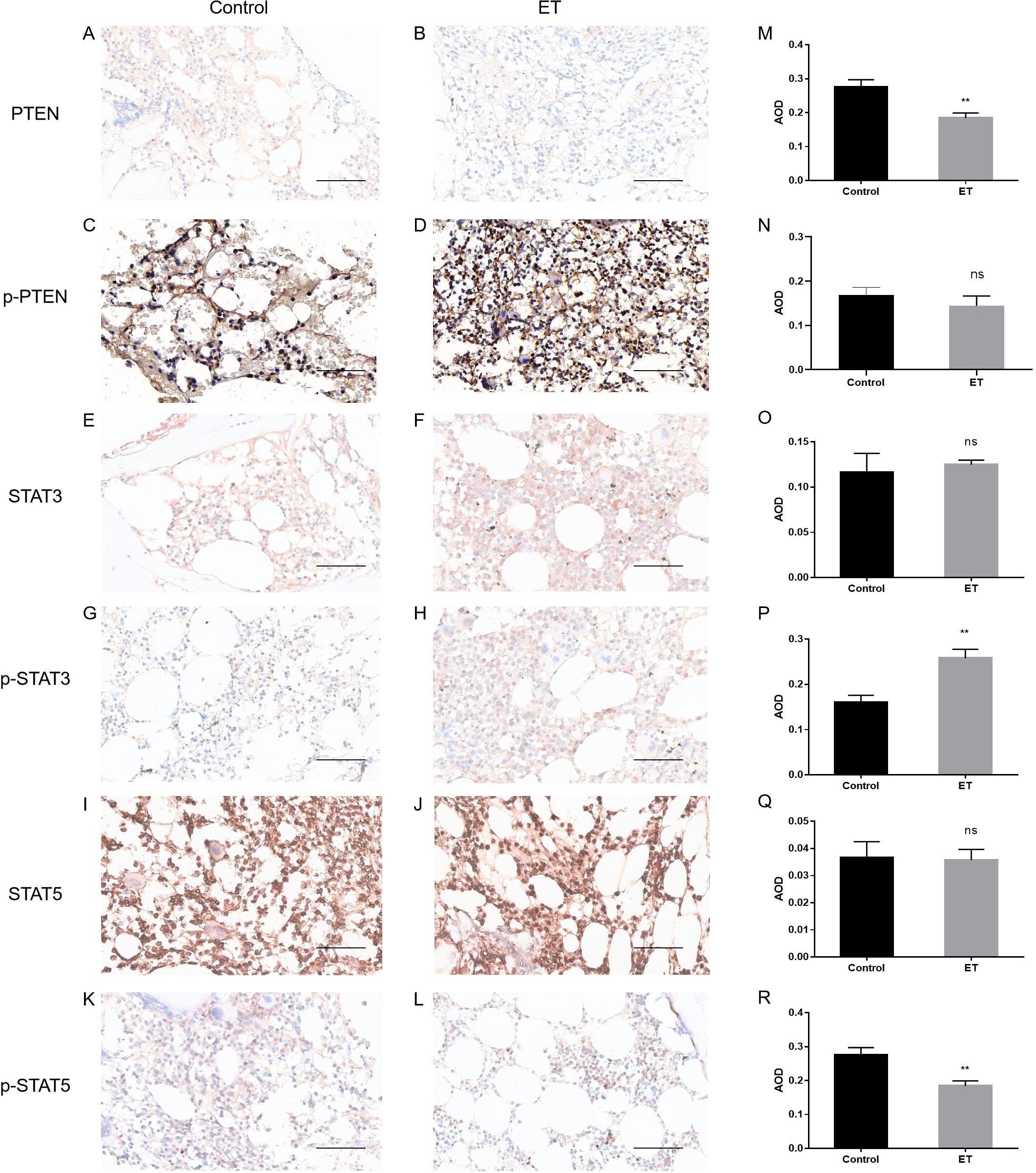
Figure 3. Immunohistochemistry (IHC) analysis of phosphatase and tensin homolog (PTEN), p-PTEN, STAT3, p-STAT3, STAT5, and p-STAT5 in the bone marrow mononuclear cells (BM-MNCs) of patients with essential thrombocythemia (ET). (A–L) Representative IHC diagrams of PTEN, p-PTEN, STAT3, p-STAT3, STAT5, and p-STAT5 in ET patients and normal controls. Scale bar, 50 μm (magnification, ×400). p-PTEN, phosphorylation of the STT (Ser380, Thr382, and Thr383 residues) motif on PTEN. (M–R) Average optical density per area (AOD; Integral optical density/Area) calculated with digital image analysis for the quantification of proteins. **p < 0.01, with t-test (n=3; each sample image was averaged from three fields of view). ns, not significant.
3.3 p-STAT3 is negatively correlated with PTEN in the BM-MNCs of ET patients
Based on literature reports, we hypothesized that PTEN may negatively regulate STAT3/p-STAT3, resulting in their excessive activation in the bone marrow cells of patients with ET. To verify this hypothesis, the protein expression levels of STAT3 and p-STAT3 were examined in the bone marrow cells from ET patients and normal controls. The results of the Western blot showed that the level of p-STAT3 was drastically elevated in the BM-MNCs of patients with ET compared with normal controls (Figures 2B, E), while the steady-state level of STAT3 remained unchanged. The results of IHC also supported the increased expression of p-STAT3 in patients with ET (Figures 3E, F, M). The reduced phosphorylation of STAT3 could be inversely correlated with the elevated level of PTEN found in ET bone marrow cells.
3.4 p-STAT5 is decreased in ET patients as confirmed by Western blot and IHC analysis
We also investigated the p-STAT5 expression of the BM-MNCs isolated from ET patients and normal controls. As shown in Figures 2C, F, compared with that in normal controls, the p-STAT5 level in patients with ET was significantly decreased, which was confirmed by densitometric analysis of the bands in the Western blot. Analysis of the IHC data also showed similar results (Figures 3I, J, O). Both the Western blot and IHC detection exhibited a slightly decreased STAT5 expression in patients with ET compared with normal controls; however, the differences did not reach significance.
3.5 Correlation of PTEN with ET clinical characteristics
To explore the association of the aberrant expression of PTEN and the JAK/STAT signaling pathway with the pathological characteristics of ET, the quantitative values of PTEN, p-PTEN, STAT3, STAT5, p-STAT3, and p-STAT5 expression were analyzed in ET patients with the clinical indicators of the same patients, including patient age, WBC count, Hb level, platelet count, LDH level, and the VAF of the JAK2 V617F mutation. The results showed that the expression level of PTEN was negatively correlated with the platelet count (p = 0.031) and the patient age (p = 0.01), but was positively correlated with the p-STAT5 level (p = 0.025), while no correlation with the VAF of the JAK2 V617F mutation was found (Figure 4). Moreover, there seemed to be a positive correlation between the WBC count and the LDH level (p = 0.015) and a negative correlation between the platelet count and the p-STAT5 level (p = 0.049). Univariate descriptive analysis was also performed. However, the PTEN expression and the molecule levels on the JAK/STAT signaling pathway were not found to have any impact on the splenomegaly and thrombotic events in patients with ET (data not shown). Subsequently, multivariate analysis was performed, which showed a negative correlation between PTEN and patient age and a positive correlation between PTEN and p-STAT5 (Supplementary Table S1).
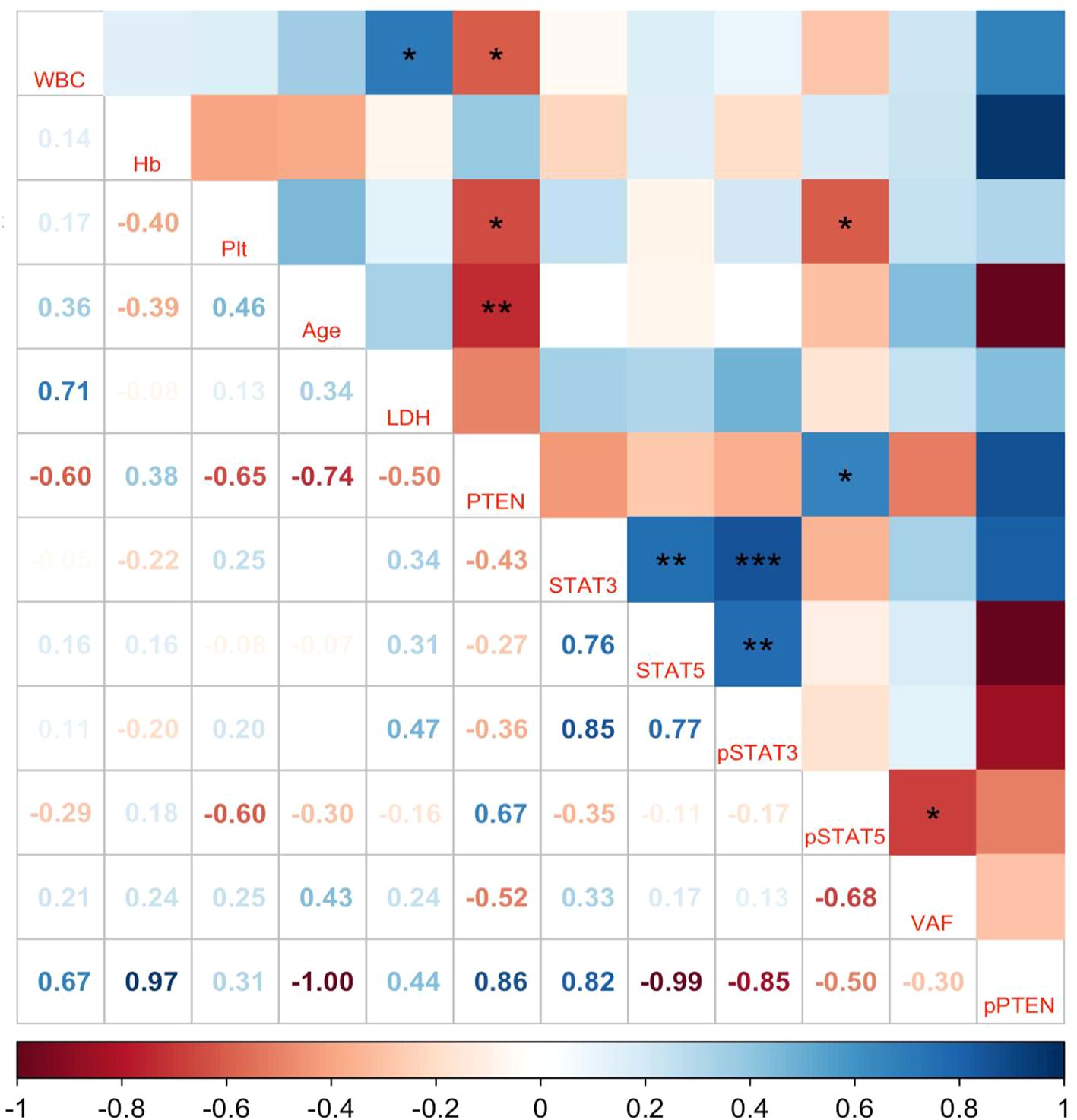
Figure 4. Pearson’s correlations for the relationships between the quantitative values of phosphatase and tensin homolog (PTEN), p-PTEN, STAT3, STAT5, p-STAT3, and p-STAT5 expression and the clinical indicators of patients with essential thrombocythemia (ET). WBC, white blood cells; Hb, hemoglobin; PLT, platelets; LDH, lactic dehydrogenase; VAF, variant allele frequency of the JAK2 V617F mutation; p-PTEN, phosphorylation of the STT (Ser380, Thr382, and Thr383 residues) motif on PTEN. The numbers represent Pearson’s coefficients. *p < 0.05, **p < 0.01, ***p < 0.001.
4 Discussion
In this study, a decreased PTEN level was detected in the BM-MNCs from untreated ET patients with the JAK2 V617F mutation for the first time. Previous research has reported PTEN as a negative regulator of STAT3 activity through the mediation of p-STAT3 dephosphorylation (16). Our findings are consistent with previous research, indicating elevated p-STAT3 levels in the BM-MNCs of ET patients with the JAK2 V617F mutation, whereas the p-STAT5 levels were observed to have decreased. Furthermore, analysis in combination with clinical data showed a negative correlation between PTEN expression and patient age and platelet count. Conversely, a positive correlation was found between the expression levels of PTEN and p-STAT5.
Previous studies on PTEN as a tumor suppressor gene mostly focused on solid tumors such as prostate cancer and colorectal cancer (17, 18), as well as hematological tumors such as myelodysplastic syndromes (MDS) and AML (19). Our study revealed, for the first time, that PTEN is poorly expressed in the BM-MNCs of patients with ET. Most research studies have reported a PTEN-regulated PI3K/AKT/mTOR signaling pathway (20, 21), and the loss of PTEN in pancreatic cancer-related fibroblasts resulted in increased STAT3 phosphorylation (22). In papillomavirus-infected cells, PTEN negatively regulated STAT3 phosphorylation activation, and mutant PTEN increased STAT3 phosphorylation compared with the wild-type PTEN group (16). The mTOR signaling pathway positively regulated STAT3 in MCF7, a model of cancer stem cell-like cells, while PTEN acted as a negative regulator of both STAT3 and mTOR signaling (11). In human cancer cell line U266, DU-145, and SCC4 cells, the sinoquinone-dependent expression of PTEN thereby inhibited the activation of STAT3 signaling, suppressed cell proliferation and invasion, and induced apoptosis through the activation of caspase-3 (23). In human glioblastoma cell lines, in both PTEN-expressing and PTEN-deficient cells, blockage of the JAK-2/STAT3 signaling pathway inhibited the migration and invasive potential of tumor cells, but the inhibition of STAT3 in PTEN-expressing cells might have a higher therapeutic potential (24). As the aberrant expression of PTEN and the subsequent dysregulation of the p-STAT3 and p-STAT5 levels exhibited, our results suggest that a low PTEN expression might be one of the key factors in the pathogenesis of ET. Platelet hyperreactivity and enhanced granule secretion have been reported as potential mechanisms for thrombosis in the setting of ET. Previous multiomic and functional analyses have identified PI3K/AKT/mTOR signaling as a principal contributor to platelet hyperreactivity in ET, while α-ketoglutarate (α-KG) supplementation has been proven to be an efficacious metabolic intervention by inhibiting PI3K/AKT/mTOR signaling, thereby modulating the metabolic responses and hyperreactivity in platelets from patients with ET (25). On the other hand, the activation of MEK/ERK has also been proven to contribute to the inefficacy/persistence of JAK2 inhibitor treatment in preclinical MPN studies (26). Thus far, the role of PTEN in the pathogenesis of ET has not been documented. Previous studies indicated that PTEN in imatinib-resistant chronic eosinophilic leukemia (CEL) EOL-1R cells dephosphorylated AKT, ERK, and STAT5, which led to the negative regulation of both PI3K/AKT and MEK/ERK signaling (27). It can be reasonably assumed that the downregulation of PTEN could lead to the activation of PI3K and MEK/ERK, which contributes to the occurrence of ET.
PTEN is mostly phosphorylated at the last 50 amino acid residues in the C-terminal, and the phosphorylation at the STT motif of PTEN is crucial for its DNA repair activities and aids in cell survival. However, there are hardly any studies exploring the phosphorylated PTEN in MPNs. This study also revealed for the first time the small differences in the phosphorylation levels of the STT motif of PTEN between ET patients and normal controls. These results might have been limited by the small sample size, and further studies are needed to be performed in order to explore whether the phosphorylation at other sites or other epigenetic regulation modes of PTEN are involved in the downregulation of its expression in patients with MPN.
As the predominant driver mutation of Ph− classical MPNs, the JAK2 V617F mutation requires the presence of one of three homodimeric type I myeloid cytokine receptors for pathological signaling—MPL, EPOR, or G-CSFR—while EPOR triggers the activation of signaling pathways including JAK2/STAT5, PI3K/AKT, and MAPK (28, 29). JAK2 V617F also activated persistent MPL-dependent STAT3 signaling that induced transcriptional activation at the promoter of programmed death ligand 1 (PD-L1), leading to the enhanced expression of PD-L1 at the surface of monocytes, megakaryocytes, and platelets (30). Studies have demonstrated STAT3 activation in the bone marrow samples of half of patients with ET, regardless of the presence of JAK2 mutation (6). While there was no positive correlation between the JAK2 V617F allele burden and the expression of p-STAT3 and p-STAT5 in patients with MPN, the phosphorylation of STAT5 and STAT3 in megakaryocyte nuclei was significantly higher in JAK2 V617F-positive MPN patients than that in negative samples. In addition, lower p-STAT5 levels were observed in the bone marrow biopsies from JAK2 V617F+ ET patients compared with JAK2 V617F+ early PMF patients (31).
Recent studies have highlighted the central pathogenic role of persistent JAK–STAT activation in MPN (32, 33). The activation of STAT3 signaling is a well-accepted mechanism in the pathogenesis of MPN, including ET. The JAK2 V617F, MPL W515K/L/A/R, and S505N mutations were believed to directly activate JAK2–STAT, resulting in a clonal myeloproliferation that is cytokine-independent or hypersensitive (34, 35). Although the central role of JAK–STAT activation in MPN has been highlighted (36, 37), this particular concept was confounded by the coexistence of an inflammatory state in MPN with aberrant cytokine expression and the fact that the activated JAK–STAT is a nonspecific phenomenon in cancer (38). Furthermore, “targeted therapy” with JAK inhibitors has so far failed to induce selective suppression of the disease clone in MPN (39). Regardless, the underlying mechanisms that enable single mutations to result in ET remain not fully understood. We found that the STAT3 phosphorylation level was negatively correlated with the PTEN expression levels and was not associated with the JAK2 V617F mutation. This suggests that PTEN might be another key regulator in the activation of the STAT3 pathway in ET bone marrow cells.
Although ET advances relatively slowly compared with PMF overall, some patients still show rapid disease progression, resulting in various complications such as thrombosis and transformation into AML in the short term. The rapid progression of the disease in these patients cannot be explained solely by the JAK2 V617F gene mutation, indicating abnormalities in other signaling pathways driving the disease progression. Here, both the univariate and multivariate analyses demonstrated that a lower PTEN expression was correlated with increased patient age and reduced p-STAT5 levels. However, research on CEL has indicated that forced PTEN expression might result in the dephosphorylation of STAT5 (27). This contradicted our findings of reduced p-STAT5 levels, which exhibited a positive correlation with PTEN. Possibly, it was the distinction in the pathogenesis between CEL and ET and will influence our new research direction. The limited sample size could have also potentially hindered further research into the PTEN and STAT signaling pathways. Nonetheless, based on our results, the combination of JAK2 inhibitors and PTEN agonists is likely to exert a synergistic function to provide new avenues for clinical ET treatment.
In conclusion, this study showed variations in the expression of PTEN and molecules such as STAT3, STAT5, p-STAT3, and p-STAT5 on the JAK/STAT signaling pathway in the BM-MNCs of ET patients without treatment. The downregulation of the PTEN level might play an essential role in the pathogenesis of ET, leading to elevated p-STAT3 and decreased p-STAT5 expression while having little impact on the expression of STAT3 and STAT5. Combined with clinical data, the expression of PTEN exhibited a negative correlation with patient age and platelet count and a positive correlation with p-STAT5, but no correlation with the JAK2 V617F mutation allele burden, which indicates that, although the JAK2 V617F mutation might initiate the onset of the disease, its allele burden is independent relative to PTEN expression. These results could therefore shed light on the new pathogenesis of and treatment strategies for ET.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhongnan Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. XL: Data curation, Formal Analysis, Project administration, Writing – original draft, Writing – review & editing. HS: Formal Analysis, Resources, Writing – original draft. HZ: Data curation, Formal Analysis, Software, Validation, Writing – original draft. YS: Investigation, Methodology, Resources, Writing – original draft. XZ: Conceptualization, Funding acquisition, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DZ: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by grants of National Natural Science Foundation of China (82000127, 81900116, 82470117) and the Health Commission of Hubei Province Scientific Research Project (WJ2021M163).
Acknowledgments
The authors thank all the patients and family members for their participation. We gratefully acknowledge Prof. Fuling Zhou for her scientific guidance and support in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2025.1479185/full#supplementary-material
Supplementary Figure 1 | The original image files for the western blots. Original and chemiluminescence photos of GADPH (A, B), PTEN (C, D), STAT3 (E, F), p-STAT3 (G, H), STAT5 (I, J), p-STAT5 (K, L). (M) shows the full scan of the entire original gel, highlighting the phosphorylated PTEN.
References
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
2. Tefferi A and Pardanani A. Essential thrombocythemia. N Engl J Med. (2019) 381:2135–44. doi: 10.1056/NEJMcp1816082
3. Loscocco GG, Guglielmelli P, and Vannucchi AM. Impact of mutational profile on the management of myeloproliferative neoplasms: A short review of the emerging data. Onco Targets Ther. (2020) 13:12367–82. doi: 10.2147/OTT.S287944
4. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. (2005) 365:1054–61. doi: 10.1016/S0140-6736(05)71142-9
5. Sangkhae V, Etheridge SL, Kaushansky K, and Hitchcock IS. The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood. (2014) 124:3956–63. doi: 10.1182/blood-2014-07-587238
6. Senyuk V, Rinaldi CR, Li D, Cattaneo F, Stojanovic A, Pane F, et al. Consistent up-regulation of Stat3 Independently of Jak2 mutations in a new murine model of essential thrombocythemia. Cancer Res. (2009) 69:262–71. doi: 10.1158/0008-5472.CAN-08-2534
7. Teofili L, Martini M, Cenci T, Petrucci G, Torti L, Storti S, et al. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood. (2007) 110:354–9. doi: 10.1182/blood-2007-01-069237
8. Kirito K, Osawa M, Morita H, Shimizu R, Yamamoto M, Oda A, et al. A functional role of Stat3 in in vivo megakaryopoiesis. Blood. (2002) 99:3220–7. doi: 10.1182/blood.V99.9.3220
9. Liu YL, Castleberry RP, and Emanuel PD. PTEN deficiency is a common defect in juvenile myelomonocytic leukemia. Leuk Res. (2009) 33:671–7. doi: 10.1016/j.leukres.2008.09.036
10. Vazquez F, Ramaswamy S, Nakamura N, and Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. (2000) 20:5010–8. doi: 10.1128/MCB.20.14.5010-5018.2000
11. Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. (2007) 104:16158–63. doi: 10.1073/pnas.0702596104
12. Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. (2010) 17:584–96. doi: 10.1016/j.ccr.2010.05.015
13. Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. (2008) 22:87–95. doi: 10.1038/sj.leu.2405043
14. Van Egeren D, Escabi J, Nguyen M, Liu S, Reilly CR, Patel S, et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell. (2021) 28:514–23 e9. doi: 10.1016/j.stem.2021.02.001
15. Misra S, Mukherjee A, and Karmakar P. Phosphorylation of PTEN at STT motif is associated with DNA damage response. Mutat Res. (2014) 770:112–9. doi: 10.1016/j.mrfmmm.2014.08.008
16. Sun S and Steinberg BM. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J Gen Virol. (2002) 83:1651–8. doi: 10.1099/0022-1317-83-7-1651
17. Mirzaei S, Paskeh MDA, Okina E, Gholami MH, Hushmandi K, Hashemi M, et al. Molecular Landscape of LncRNAs in Prostate Cancer: A focus on pathways and therapeutic targets for intervention. J Exp Clin Cancer Res. (2022) 41:214. doi: 10.1186/s13046-022-02406-1
18. Elrebehy MA, Al-Saeed S, Gamal S, El-Sayed A, Ahmed AA, Waheed O, et al. miRNAs as cornerstones in colorectal cancer pathogenesis and resistance to therapy: A spotlight on signaling pathways interplay - A review. Int J Biol Macromol. (2022) 214:583–600. doi: 10.1016/j.ijbiomac.2022.06.134
19. Vobugari N, Heuston C, and Lai C. Clonal cytopenias of undetermined significance: potential predictor of myeloid Malignancies? Clin Adv Hematol Oncol. (2022) 20:375–83.
20. Zhao X, Sun W, Guo B, and Cui L. Circular RNA BIRC6 depletion promotes osteogenic differentiation of periodontal ligament stem cells via the miR-543/PTEN/PI3K/AKT/mTOR signaling pathway in the inflammatory microenvironment. Stem Cell Res Ther. (2022) 13:417. doi: 10.1186/s13287-022-03093-7
21. Xu M, Liu Y, Mayinuer T, Lin Y, Wang Y, Gao J, et al. Mycoplasma bovis inhibits autophagy in bovine mammary epithelial cells via a PTEN/PI3K-Akt-mTOR-dependent pathway. Front Microbiol. (2022) 13:935547. doi: 10.3389/fmicb.2022.935547
22. Lefler JE, MarElia-Bennett CB, Thies KA, Hildreth BE 3rd, Sharma SM, Pitarresi JR, et al. STAT3 in tumor fibroblasts promotes an immunosuppressive microenvironment in pancreatic cancer. Life Sci Alliance. (2022) 5. doi: 10.26508/lsa.202201460
23. Heo JY, Kim HJ, Kim SM, Park KR, Park SY, Kim SW, et al. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. (2011) 308:71–80. doi: 10.1016/j.canlet.2011.04.015
24. Senft C, Priester M, Polacin M, Schroder K, Seifert V, Kogel D, et al. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol. (2011) 101:393–403. doi: 10.1007/s11060-010-0273-y
25. He F, Laranjeira AB, Kong T, Lin S, Ashworth KJ, Liu A, et al. Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms. J Clin Invest. (2024) 134. doi: 10.1172/JCI172256
26. Pandey G, Kuykendall AT, and Reuther GW. JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation. Blood Cancer J. (2022) 12:13. doi: 10.1038/s41408-022-00609-5
27. Nishioka C, Ikezoe T, Yang J, and Yokoyama A. Long-term exposure of leukemia cells to multi-targeted tyrosine kinase inhibitor induces activations of AKT, ERK and STAT5 signaling via epigenetic silencing of the PTEN gene. Leukemia. (2010) 24:1631–40. doi: 10.1038/leu.2010.145
28. Vainchenker W, Plo I, Marty C, Varghese LN, and Constantinescu SN. The role of the thrombopoietin receptor MPL in myeloproliferative neoplasms: recent findings and potential therapeutic applications. Expert Rev Hematol. (2019) 12:437–48. doi: 10.1080/17474086.2019.1617129
29. Tothova Z, Tomc J, Debeljak N, and Solar P. STAT5 as a key protein of erythropoietin signalization. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22137109
30. Prestipino A, Emhardt AJ, Aumann K, O’Sullivan D, Gorantla SP, Duquesne S, et al. Oncogenic JAK2(V617F) causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. (2018) 10. doi: 10.1126/scitranslmed.aam7729
31. Risum M, Madelung A, Bondo H, Bzorek M, Kristensen MH, Stamp IM, et al. The JAK2V617F allele burden and STAT3- and STAT5 phosphorylation in myeloproliferative neoplasms: early prefibrotic myelofibrosis compared with essential thrombocythemia, polycythemia vera and myelofibrosis. APMIS. (2011) 119:498–504. doi: 10.1111/j.1600-0463.2011.02754.x
32. James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. (2005) 434:1144–8. doi: 10.1038/nature03546
33. Vainchenker W and Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. (2017) 129:667–79. doi: 10.1182/blood-2016-10-695940
34. Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F, et al. The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. (2007) 110:1013–21. doi: 10.1182/blood-2006-10-054940
35. Tefferi A, Gangat N, Pardanani A, and Crispino JD. Myelofibrosis: genetic characteristics and the emerging therapeutic landscape. Cancer Res. (2022) 82:749–63. doi: 10.1158/0008-5472.CAN-21-2930
36. Constantinescu SN, Vainchenker W, Levy G, and Papadopoulos N. Functional consequences of mutations in myeloproliferative neoplasms. Hemasphere. (2021) 5:e578. doi: 10.1097/HS9.0000000000000578
37. Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. (2014) 123:e123–33. doi: 10.1182/blood-2014-02-554634
38. Constantinescu SN, Girardot M, and Pecquet C. Mining for JAK-STAT mutations in cancer. Trends Biochem Sci. (2008) 33:122–31. doi: 10.1016/j.tibs.2007.12.002
Keywords: essential thrombocythemia, myeloproliferative neoplasms, PTEN, STAT3, STAT5
Citation: Luo L, Liu X, Shen H, Zhang H, Shang Y, Zhang X and Zhang D (2025) Downregulated PTEN and aberrant JAK–STAT signal pathway in essential thrombocythemia patients. Front. Hematol. 4:1479185. doi: 10.3389/frhem.2025.1479185
Received: 11 August 2024; Accepted: 23 April 2025;
Published: 23 May 2025.
Edited by:
Savita Rangarajan, University of Southampton, United KingdomReviewed by:
Ram Babu Undi, University of Oklahoma Health Sciences Center, United StatesZeping Zhou, Second Affiliated Hospital of Kunming Medical University, China
Copyright © 2025 Luo, Liu, Shen, Zhang, Shang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian Zhang, emhhbmd4aWFuQHpuaG9zcGl0YWwuY24=; Donglei Zhang, emhhbmdkb25nbGVpMDhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lin Luo1†
Lin Luo1† Yufeng Shang
Yufeng Shang Donglei Zhang
Donglei Zhang