- 1Department of Haematology, University College London Hospital, London, United Kingdom
- 2Department of Haematology, Royal Free Hospital, London, United Kingdom
- 3UCL Respiratory, University College London, London, United Kingdom
- 4Biomarker and Drug Analysis Core Facility, University of Dundee, Dundee, United Kingdom
- 5Department of Dermatology, Royal Free Hospital, London, United Kingdom
Acquired cutis laxa (ACL) is a rare connective tissue disorder that is characterised by loose, pendulous skin and can be associated with systemic elastolysis and plasma cell dyscrasias (PCD). While myeloma therapy has been used for these patients, experience is limited, particularly when organ dysfunction limits a patient’s tolerance of chemotherapy. We present two cases of ACL with progressive pulmonary emphysema that received treatment for coexisting PCD, resulting in differing outcomes. For the first time, we also monitored desmosine as a direct measurement of elastolysis throughout treatment and suggest that this may be useful for guiding therapy in these rare and complex patients.
Introduction
Cutis laxa is a rare connective tissue disorder characterised by loose, pendulous skin and exists in inherited or acquired forms. Acquired cutis laxa (ACL) is associated with aetiologies such as drugs [penicillamine (1) and isoniazid (2)], infections (3), coeliac disease (4), and systemic lupus erythematosus (5). ACL has also been associated with B and T lymphomas and, especially, plasma cell dyscrasias (PCDs) (6). When ACL occurs with PCD and in the absence of myeloma-defining organ impairment, it is classified as one of the spectrum of monoclonal gammopathies of clinical significance (7, 8) with cutaneous manifestations (9–11).
While immunoglobulin deposition in the dermis can occasionally be seen (12), the aetiology of gammopathy-driven elastolysis in ACL and the determinants of its anatomical distribution remain unknown, which limits our ability to effectively diagnose and manage these patients. For example, a clonal driver for ACL is often only presumed in the presence of a coexisting PCD. And while tissue recovery is not possible, PC-directed therapy has been used to prevent the progression of immunoglobulin-driven ACL. However, the rarity of this condition and the heterogeneity of systemic involvement mean that there is little guidance as to whether or which PC-directed therapy is indicated. Posing further challenges in the management of these rare cases, organ impairment can complicate the delivery of cytotoxic therapy, and there is also no direct, objective, or replicable measure of connective tissue breakdown to guide management.
Desmosine is an amino acid found in elastin and increased concentrations in the plasma and urine have been correlated with pulmonary elastolysis caused by COPD (13). Here, we present two cases of ACL in patients presenting with progressive pulmonary emphysema who received treatment targeting a coexisting PCD, resulting in contrasting outcomes. We also describe, for the first time, longitudinal measurements of desmosine following chemotherapy for this condition.
Case descriptions
Patient 1: A 49-year-old woman presented in 2019 to a dermatologist reporting an initial circumferential rash that had appeared 3 years prior. The rash had slowly receded, leaving wrinkled skin over her torso, arms, and thighs. She had no significant medical or family history and had never smoked. An IgG Lambda paraprotein concentration of 3g/L was found by immunofixation, and there was no evidence of intradermal immunoglobulin deposition on skin biopsy.
Within months, the skin changes progressed to her face and extremities, and her dyspnoea had worsened. Basal-predominant emphysema was evident on computed tomography (CT) scans (Figures 1A–C), and pulmonary function tests (PFTs) were consistent with emphysema-predominant COPD [forced expiratory volume (FEV1): 71% of the predicted value, vital capacity (VC) 119%, residual volume (RV) 127%, diffusing capacity of the lungs for carbon monoxide (DLCO) 56%, and carbon monoxide transfer coefficient (KCO) 55%]. Alpha-1 antitrypsin levels were normal. Evidence of systemic elastolysis with skin changes indicated ACL.
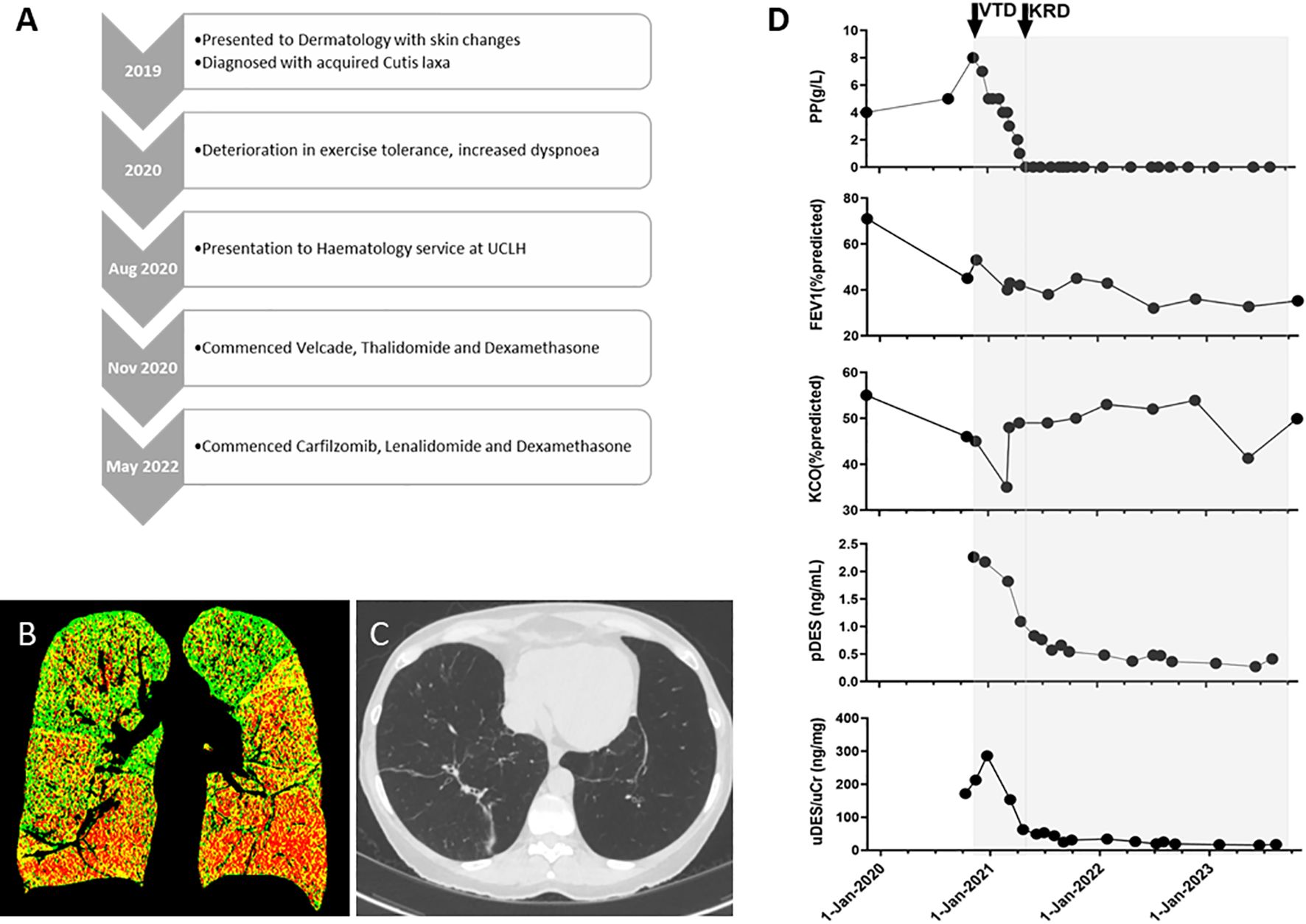
Figure 1. (A) Clinical timeline. (B) A representative coronal image of a paired and registered inspiratory-expiratory CT lung scan, analysed using parametric response mapping. Overall, this shows 29.6% emphysema with basal predominance (red voxels), 42.6% functional small-airway dysfunction (yellow voxels), and 27.8% normal lung (green voxels). (C) A representative transverse image from a later CT lung scan demonstrating significant bilateral basal airspace enlargement (and band atelectasis). (D) Graphs summarising paraprotein (PP), forced expiratory volume (FEV1), carbon monoxide transfer coefficient (KCO), and desmosine levels in plasma (pDES) and urine (uDES) correlated with PC-directed therapy (shaded grey). VTD, velcade, thalidomide, and dexamethasone chemotherapy; KRD, carfilzomib, lenalidomide, and dexamethasone chemotherapy.
Within a year, there was further respiratory decline, and this former runner could not manage a flight of stairs. Her PFTs deteriorated (FEV1 and KCO were 45% and 46% of the predicted values, respectively), her paraprotein level increased to 8g/L with normal free light chains, and there was no detectable Bence Jones proteinuria (BJP) or myeloma-defining events in the blood or by whole-body magnetic resonance imaging (WBMRI). A bone marrow (BM) biopsy revealed 20% lambda-restricted plasma cells (PCs), and normal cytogenetics by fluorescence in situ hybridisation (FISH). A diagnosis of smouldering myeloma was made, which was the presumed driver of the elastolysis.
In November 2020, velcade, thalidomide, and dexamethasone (VTD) were started with a view to a future stem cell harvest without GCSF (14) and a potential autologous stem cell transplant (ASCT). In January 2021, the patient developed COVID-19, which was managed with supplemental oxygen, remdesivir, and dexamethasone. Despite achieving a partial haematological response (PR) in March (PP 2g/dL), there was increased dyspnoea and deterioration in her PFTs (Figure 1D: FEV1 40% of the predicted value). The risk of an ASCT was now deemed unacceptably high; therefore, to improve cytoreduction, carfilzomib, lenalidomide, and dexamethasone (KRD) were started in May 2021.
The patient tolerated KRD well and has remained in a complete haematological response (CR) . Following #3, a repeat BM biopsy revealed minimal residual disease positivity (6 in 1x10-5 of total BM mononuclear cells).
The patient’s respiratory symptoms and lung function tests stabilised (Figure 1D), and she underwent a face and neck lift in March 2022 under local anaesthetic. In the summer of 2022, the patient experienced more exertional dyspnoea, and there was further deterioration in her FEV1 (32% of the predicted value), which has since remained largely stable. She continues to run a business and travel by air.
In keeping with marked systemic elastolysis, this patient’s plasma and urine desmosine levels were exceptionally elevated at baseline (urine concentrations>10x normal) and reduced to a plateau following therapy (Figure 1D).
Patient 2: A 54-year-old man presented with joint pains and skin changes and was diagnosed with ACL at the age of 47. An IgG kappa paraprotein concentration of 4g/L was detected and remained stable. He had been a smoker in his twenties and had no significant family history. At 50, he had been diagnosed with a hiatus hernia, along with COPD with emphysema. His respiratory symptoms had deteriorated in the months prior to presentation to the haematology department, with an exercise tolerance falling to 150m walking on flat ground and difficulty climbing stairs. He had frequent exacerbations of his COPD, prompting the use of multiple pulses of oral corticosteroids, and he had been prescribed ambulatory oxygen. At this stage, the patient became acquainted with Patient 1 through a cutis laxa Facebook group and requested a referral to the same haematology service for consideration of PC-directed therapy. CT scans demonstrated emphysema in a predominantly basal distribution. PFTs in April 2022 were consistent with emphysema-predominant COPD and gas trapping (FEV1: 33% of the predicted value, VC 57%, RV 239%, DLCO 46%, and KCO 75%). A skin biopsy of the affected skin demonstrated a marked reduction, and in some areas, the absence of elastic fibres throughout the dermis, consistent with a diagnosis of cutis laxa. There were no dermal immunoglobulin deposits by immunofluorescence. He had a paraprotein concentration of 5g/L, a Kappa light chain concentration of 258.3mg/L, a Lambda light chain concentration of 10.9mg/L, a low level of BJP (0.04g/L), normal biochemistry, and secondary polycythaemia with an HCT of 0.53. A WBMRI showed no focal myeloma bone lesions. A BM biopsy revealed 5% kappa-restricted PC. A diagnosis of monoclonal gammopathy of uncertain significance was made, which was thought to be a possible driver of the ACL.
Lenalidomide (15mg on D1–21 of a 28-D cycle) and dexamethasone (20mg weekly) were started in April 2022. The patient developed COVID-19 in September 2022 and was managed as an outpatient and with sotrovimab. He suffered frequent COPD exacerbations despite prophylactic azithromycin, leading to frequent interruptions to his chemotherapy regimen and precluding treatment intensification. The patient achieved a partial haematological response to chemotherapy (paraprotein and KLC response from 5 to 2g/L and 258 to 62mg/L, respectively).
The patient’s exercise tolerance between exacerbations was stable, although he was becoming more fatigued, and we observed no consistent reduction in desmosine (Figure 2). With the aim of improving his disease response, discussions regarding a change in chemotherapy had started when the patient was admitted with an exacerbation of COPD, and he died in May 2023.
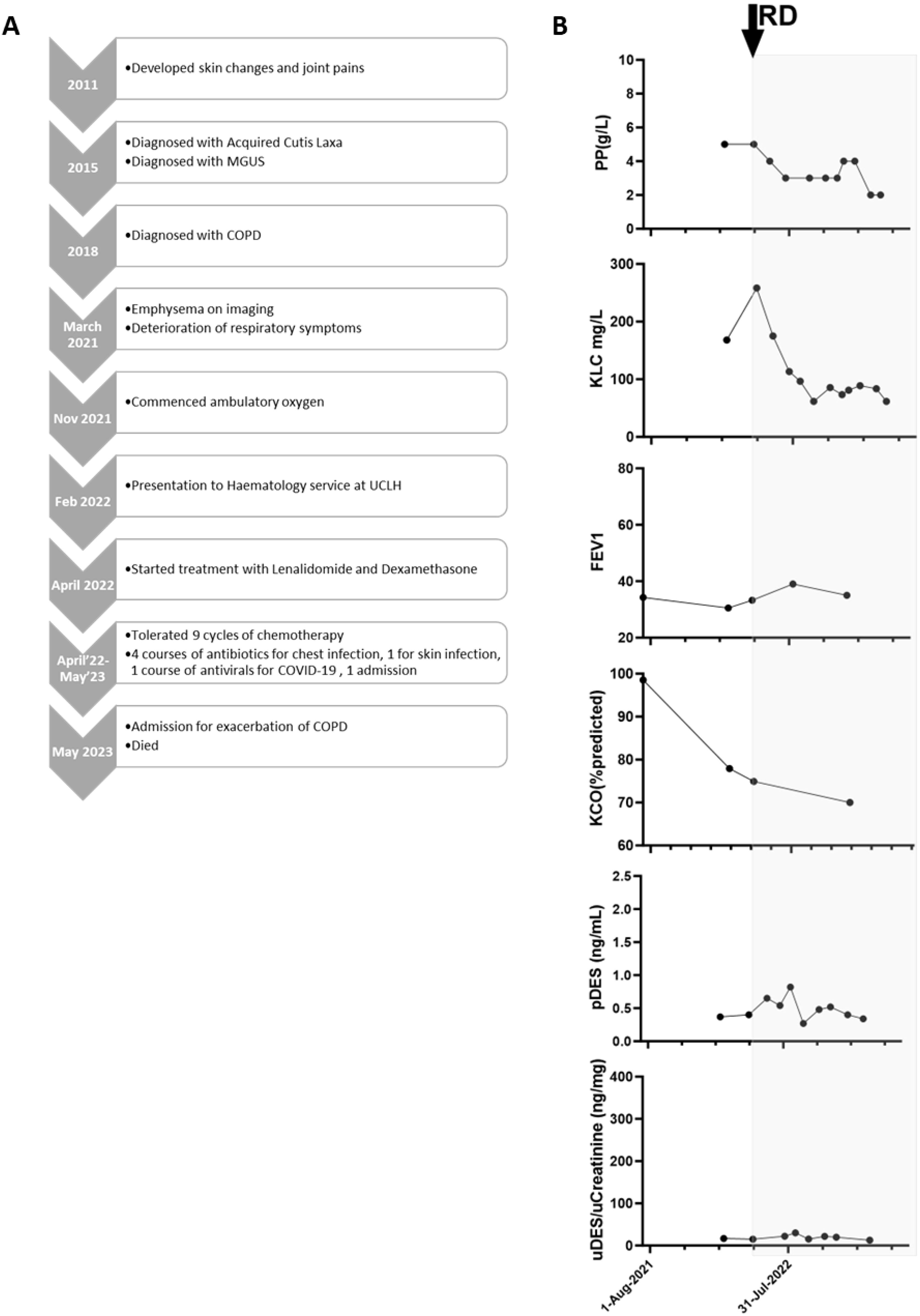
Figure 2. (A) Clinical timeline and (B) Summary of Patient 2. Graphs summarising paraprotein (PP), kappa light chains (KLC), forced expiratory volume (FEV1), carbon monoxide transfer coefficient (KCO) levels, along with desmosine levels in plasma (pDES) and urine (uDES), correlated with PC-directed therapy (shaded grey). RD, lenalidomide and dexamethasone chemotherapy.
Discussion
Both our cases of ACL had systemic elastolysis and pulmonary involvement, manifesting as emphysema, airflow obstruction, and gas trapping. In both cases, there was a coexisting PC clone without myeloma-related organ damage and, as is frequently the case, no direct evidence of PC-driven elastolysis (for example, through dermal deposition of immunoglobulins or their components (6)). However, there had been recent pulmonary deterioration, and both patients were well informed and understood that pulmonary damage was irreversible, but were keen for chemotherapy to stabilise the elastolysis.
Despite evidence of biochemical responses, pulmonary function stabilised and deteriorated in Patients 1 and 2, respectively. As a possible explanation for these contrasting outcomes, the baseline pulmonary defect was greater in Patient 2, which may have limited his potential benefit from chemotherapy. Furthermore, his frequent COPD exacerbations limited the delivery of chemotherapy and thus the depth of response. As has been observed in other monoclonal gammopathies of clinical significance (e.g., AL amyloidosis), organ dysfunction continues even with low tumour loads, thus therapy aims for early and deep (≥CR) responses (15, 16). Furthermore, there have been published cases of patients with ACL who have successfully undergone ASCT (9), but neither of our patients were deemed adequately fit for a bone marrow transplant. Our experience supports the importance of early treatment and deep responses over the type of therapy. Given the rarity of ACL, case-by-case and pragmatic considerations, including treatment availability and patient fitness, are needed to determine therapy.
In the treatment of both of these cases, desmosine measurements were not available in real-time, but were performed as a retrospective and direct measure of elastolysis. Treatment decisions were made clinically. The absolute values of plasma and urine desmosine can vary according to age and smoking history, but are typically <0.4ng/ml and 2–14ng/ml, respectively, as measured by mass spectrometry (17). Nonetheless, in Patient 1, there were exceptionally high levels at presentation, which then declined after attaining a CR and in parallel with stability in PFTs. This would indicate exaggerated elastolysis at presentation, which then reduced with PC-directed chemotherapy, likely preventing further pulmonary decline. Patient 2 had lower baseline levels, and there was no consistent reduction in desmosine with therapy, which reflected clinical decline.
Although the lack of clinical response in Patient 2 was likely due to incomplete suppression of the PC clone, it is also possible that this case of ACL was not PCD-driven. We also observed a dramatic difference in baseline desmosine levels between Patients 1 and 2, and we postulate that absolute baseline levels may correlate with the degree of elastolysis (i.e., possibly differentiating between high rates of ongoing elastolysis and low or “burnt out” disease). Further research is certainly needed to determine whether absolute desmosine levels correlate with the rate of connective tissue destruction in ACL and whether this could be further used to identify those patients most likely to benefit from PC-directed therapy.
Indeed, we suggest that desmosine may be useful in the management of ACL, particularly for monitoring response to PC-directed therapy. As neither patient had frank myeloma and was only treated to address progressive pulmonary elastolysis, desmosine assessment may be the only direct, quantitative measure of response to therapy. This is particularly important as any evolution of organ dysfunction caused by progressive connective tissue destruction is irreversible, and the sole reliance on symptoms, qualitative investigations (e.g., imaging), or highly variable readouts (e.g., PFTs) to determine response is far from ideal. In addition, in Patient 1, the decline in FEV1 and desmosine levels lagged behind myeloma marker levels, suggesting that myeloma markers should not serve as a surrogate for elastolysis and also further supporting the importance of attaining deep haematological responses.
Moreover, it is noteworthy that desmosine levels in Patient 1 plateaued at the higher end of concentrations measured in healthy patients, possibly indicating that there is ongoing, albeit dramatically reduced, levels of elastolysis. Indeed, more data are needed to determine whether desmosine levels after therapy correlate with outcomes and can be used to guide treatment. For example, in a therapeutic landscape firmly veering towards continuous therapy for multiple myeloma, desmosine may contribute to discussions about limiting the duration or intensity of therapy. Further, monitoring change in desmosine levels following treatment could reveal scenarios where there is a complete haematological response, but desmosine levels remain elevated (suggesting that PCD may be co-existent rather than causative), or there is a significant reduction in desmosine, suggesting the effective suppression of the causative PC clone.
In conclusion, we have presented two patients with ACL, systemic elastolysis, and associated PCDs, who had different clinical outcomes following chemotherapy. Supporting the importance of early specialist review, we have demonstrated that cytoreduction can successfully reduce further elastolysis, particularly when commenced before severe pulmonary deterioration, and therapeutic doses of chemotherapy are tolerated. In contrast to conventional myeloma treatment, we have emphasised the importance of complete vs. partial haematological responses over the type of therapy (chemotherapy vs. ASCT). We are also the first to report the use of desmosine in PC-driven ACL, suggesting that this may serve as a useful and direct marker to quantify the magnitude of elastolysis at presentation and track its decline following chemotherapy in patients with this rare disorder.
Patient 1’s perspective
Cutis laxa is rare, and acquired cutis laxa (the non-genetic version that I have) is even rarer. I saw several dermatologists before one diagnosed me. She also found a paraprotein in my blood and also noticed I was breathless, which then uncovered my lung damage.
I found other ACL patients around the world who were successfully treated with myeloma-type therapy. By this point, my lung function was declining at a rate that implied I had 1–2 years remaining, so I was willing to try anything, knowing that it could be risky and there were no guarantees. I believe the treatment was a success as the rapid decline of my lung damage looked to have stabilised or at least be declining at a slower rate.
My life is very different now, as my mobility is impaired and I have constant fatigue, but I still have a good life and am thankful for the treatment that saved my life. I hope this report will enable other doctors to spot the signs of ACL and consider chemotherapy if there is a paraprotein present.
Patient 2’s perspective
My husband was diagnosed with cutis laxa in 2015. There is so little known about the condition, and our quest for more information took us to meet a support group in France, where we met a Professor from Belgium, whom we subsequently visited to obtain more information and tests.
Sadly, his condition progressed, initially affecting his joints and skin but eventually spreading to his lungs. I will be forever grateful to the team at UCLH for taking an interest in his condition, and trialling chemotherapy to desperately try and halt the disease destroying his lungs. Unfortunately, he didn’t respond to the treatment, and he sadly passed away in 2023. He coped remarkably well with having such a rare condition, and I hope more research and knowledge of cutis laxa will be able to help other sufferers and prevent more lives from being lost.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the London Health Research Authority. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL: Data curation, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. EF: Writing – review & editing. RP: Writing – review & editing. KY: Writing – review & editing. AW: Writing – review & editing. JB: Writing – review & editing. JH: Data curation, Formal Analysis, Investigation, Writing – review & editing. SM: Investigation, Writing – review & editing. JH: Conceptualization, Methodology, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thank you to Bojidar Rangelov for his input on the processing of CT scan images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Poon E, Mason GH, and Oh C. Clinical and histological spectrum of elastotic changes induced by penicillamine. Australas J Dermatol. (2002) 43:147–50. doi: 10.1046/j.1440-0960.2002.00580.x
2. Koch SE and Williams ML. Acquired cutis laxa: case report and review of disorders of elastolysis. Pediatr Dermatol. (1985) 2:282–8. doi: 10.1111/j.1525-1470.1985.tb00466.x
3. Ozkan S, Fetil E, Günes AT, Bozkurt E, Sahin T, Erkizan V, et al. Cutis laxa acquisita: is there any association with Borrelia burgdorferi? Eur J Dermatol. (1999) 9:561–4.
4. García-Patos V, Pujol RM, Barnadas MA, Pérez M, Moreno A, Condomines J, et al. Generalized acquired cutis laxa associated with coeliac disease: evidence of immunoglobulin A deposits on the dermal elastic fibres. Br J Dermatol. (1996) 135:130–4. doi: 10.1111/j.1365-2133.1996.tb03625.x
5. Golisch KB, Gottesman SP, Ferrer P, and Culpepper KS. Cutis laxa acquisita after urticarial vasculitis in SLE patients. Am J Dermatopathol. (2018) 40:433–7. doi: 10.1097/DAD.0000000000001084
6. Jachiet M, Harel S, Saussine A, Battistella M, Rybojad M, Asli B, et al. Cutis laxa associated with monoclonal gammopathy: 14 new cases and review of the literature. J Am Acad Dermatol. (2018) 79:945–7. doi: 10.1016/j.jaad.2018.03.039
7. Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. (2018) 132:1478–85. doi: 10.1182/blood-2018-04-839480
8. Dispenzieri A. Monoclonal gammopathies of clinical significance. Hematology. (2020) 2020:380–8. doi: 10.1182/hematology.2020000122
9. Shalhout SZ, Nahas MR, Drews RE, and Miller DM. Generalized acquired cutis laxa associated with monoclonal gammopathy of dermatological significance. Case Rep Dermatol Med. (2020) 20207480607. doi: 10.1155/2020/7480607
10. Claveau J-S, Wetter DA, and Kumar S. Cutaneous manifestations of monoclonal gammopathy. Blood Cancer J. (2022) 12:58. doi: 10.1038/s41408-022-00661-1
11. Lipsker D. Monoclonal gammopathy of cutaneous significance: review of a relevant concept. J Eur Acad Dermatol Venereol. (2017) 31:45–52. doi: 10.1111/jdv.2017.31.issue-1
12. New HD and Callen JP. Generalized acquired cutis laxa associated with multiple myeloma with biphenotypic igG- λ and igA-κ Gammopathy following treatment of a nodal plasmacytoma. Arch Dermatol. (2011) 147:323–8. doi: 10.1001/archdermatol.2011.26
13. Huang JTJ, Chaudhuri R, Albarbarawi O, Barton A, Grierson C, Rauchhaus P, et al. Clinical validity of plasma and urinary desmosine as biomarkers for chronic obstructive pulmonary disease. Thorax. (2012) 67:502–8. doi: 10.1136/thoraxjnl-2011-200279
14. De Larrea CF, Rovira M, Mascaró Jr. JM, Torras A, Solé M, Lloreta J, et al. Generalized cutis laxa and fibrillar glomerulopathy resulting from IgG Deposition in IgG-lambda Monoclonal Gammopathy: pulmonary hemorrhage during stem cell mobilization and complete hematological response with bortezomib and dexamethasone therapy. Eur J Haematol. (2009) 82:154–8. doi: 10.1111/j.1600-0609.2008.01181.x
15. Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK, Leung N, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. (2007) 92:1415–8. doi: 10.3324/haematol.11413
16. Sidana S, Muchtar E, Sidiqi MH, Jevremovic D, Dispenzieri A, Gonsalves W, et al. Impact of minimal residual negativity using next generation flow cytometry on outcomes in light chain amyloidosis. Am J Hematol. (2020) 95:497–502. doi: 10.1002/ajh.25746
Keywords: monoclonal gammopathies, acquired cutis laxa, desmosine, chemotherapy, elastolysis
Citation: Lee L, Fosbury E, Popat R, Yong K, Wechalekar A, Brown JS, Huang J, Mcbride S and Hurst JR (2025) Case Report: Contrasting outcomes and use of desmosine to monitor two patients treated with plasma cell-directed chemotherapy for gammopathy-driven pulmonary elastolysis in acquired cutis laxa. Front. Hematol. 4:1502322. doi: 10.3389/frhem.2025.1502322
Received: 26 September 2024; Accepted: 22 May 2025;
Published: 23 June 2025.
Edited by:
Maria Giovanna Danieli, Università Politecnica delle Marche, ItalyReviewed by:
Bertrand Arnulf, Hôpital Saint-Louis, FranceKirsty Cuthill, King’s College Hospital NHS Foundation Trust, United Kingdom
Copyright © 2025 Lee, Fosbury, Popat, Yong, Wechalekar, Brown, Huang, Mcbride and Hurst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lydia Lee, bC5sZWVAdWNsLmFjLnVr
†These authors have contributed equally to this work and share last authorship
 Lydia Lee
Lydia Lee Emma Fosbury2
Emma Fosbury2 Jeffrey Huang
Jeffrey Huang Sandy Mcbride
Sandy Mcbride