- 1Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 2Department of Pathology, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, AR, United States
Background
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by a low platelet count, leading to purpura and bleeding episodes due to the presence of antiplatelet autoantibodies. In ITP, circulating platelets are sensitized by IgG autoantibodies, resulting in their rapid removal by antigen-presenting cells (macrophages or dendritic cells) in the spleen, and sometimes in the liver or other components of the monocyte-macrophage system. ITP can also occur in association with other autoimmune disorders, lymphoproliferative disorders, drugs, and various other bacterial and viral infections (1). COVID-19 has been recognized as a potential trigger for ITP, likely driven by autoimmune mechanisms like molecular mimicry, epitope spreading and polyclonal activation (2, 3). ITP is rarely associated with multiple myeloma (MM) (4), where thrombocytopenia is more commonly due to marrow infiltration by plasma cells or effects of chemotherapy. The standard therapy for ITP includes steroids and intravenous immune globulins (IVIG) for initial treatment followed by the monoclonal antibody rituximab and platelet stimulating agents, such as eltrombopag, for refractory cases.
B-cell maturation antigen (BCMA) targeting bispecific antibodies (bsAb), such as teclistamab, have recently revolutionized treatment for refractory MM with impressive response and survival rates (5). BCMA is widely present on MM cells but is also found on the vast majority of healthy plasma cells and late-stage B cells, making these healthy immune cells a target for BCMA directed therapy. The resulting immune suppression leads to increased susceptibility of infections, a well-known side effect of BCMA directed therapy. However, in this report we suggest that the depletion of healthy plasma cells and B cells by BCMA targeting bsAbs can be used therapeutically in MM patients who develop ITP.
Here, we present two cases of COVID-19 induced ITP in MM patients, both of whom were successfully treated for ITP and relapsed multiple myeloma with teclistamab.
Case presentation
Patient 1
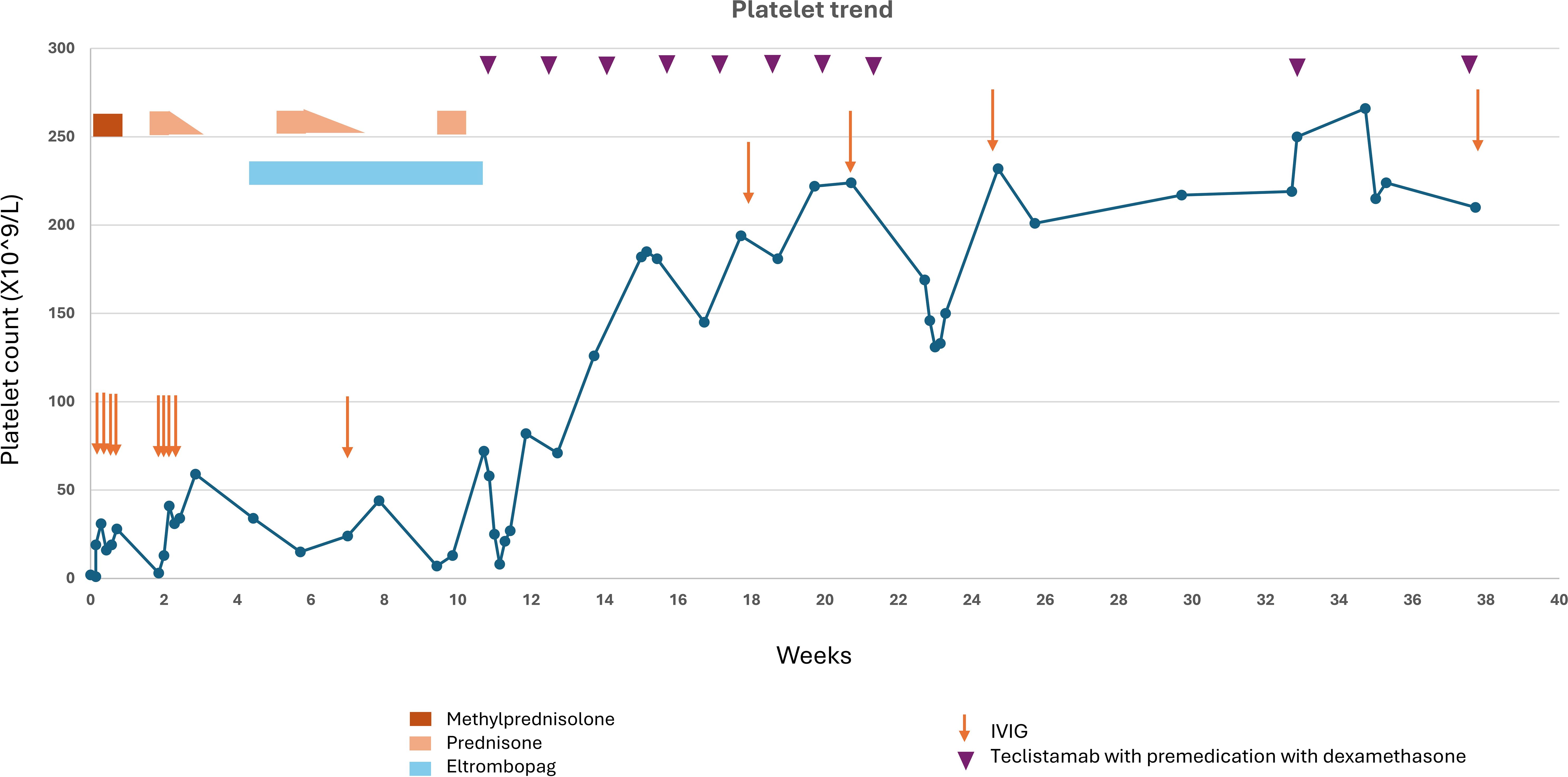
A 57-year-old female with relapsed refractory IgG lambda multiple myeloma, on therapy with cyclophosphamide 300 mg/m2 every other week, presented with mild confusion, mucocutaneous bleeding with epistaxis, gum bleeding, and left conjunctival hemorrhage. Laboratory findings revealed a white blood cell count of 7.09 X10^3/µL, hemoglobin 7.4 mg/dl (near baseline), MCV 114 fL, platelet 2 X10^9/L (dropped from 117 X10^9/L a month prior). She tested positive for COVID-19 and was treated with intravenous (iv) remdesivir and one unit of convalescent plasma. CT head was unremarkable. Further laboratory investigation indicated no evidence of hemolysis. Peripheral smear showed anisopoikilocytosis with normochromic anemia, mild left shifted granulocytes, lymphopenia, and thrombocytopenia with large platelets. Cyclophosphamide was held. Initial management included supportive platelet transfusions which did not improve her platelet count. Platelet antibody screen was negative. A bone marrow biopsy showed normal megakaryocyte morphology, negative for plasma cell myeloma and normal chromosome analysis at metaphase. Flow cytometry of bone marrow biopsy showed 0.05% minimal residual disease. FISH was negative for myelodysplastic syndrome. ITP was suspected and she was started on IVIG along with iv methylprednisolone 125 mg daily for 4 days. Following this treatment, her mental status improved, and her platelet counts rose to 28 X10^9/L at discharge in one week (Figure 1). Platelet count dropped to 3 X10^9/L at follow up two weeks later, and she received another cycle of IVIG for 4 days and was started on prednisone 60 mg daily, tapered over two weeks. Despite transient improvement with steroids, her platelet counts again declined after steroid tapering, prompting initiation of eltrombopag for persistent thrombocytopenia. PET CT showed mild increased FDG uptake in sacrum (SUVmax 3.7, previously 2.8). Concurrently, multiple myeloma markers began to rise with serum M protein reaching 0.3 g/dl with positive immunofixation and lambda light chains increasing to 6.29 mg/dl from normal values. To treat both her MM and to further obliterate the auto-Ab producing plasma and B cell compartments, we started therapy with teclistamab. Platelet counts improved with step up dosing of teclistamab and remained normal thereafter with weekly teclistamab 1.5 mg/kg. Monthly IVIG was pursued for infection prophylaxis, yet patient still developed an infection 2 months after teclistamab initiation with transient thrombocytopenia and teclistamab was held. Teclistamab was then resumed with monthly regimen. By four months, serum M protein and free light chains were undetectable. Her platelet counts remain within normal range 8 months into while on teclistamab therapy.
Patient 2
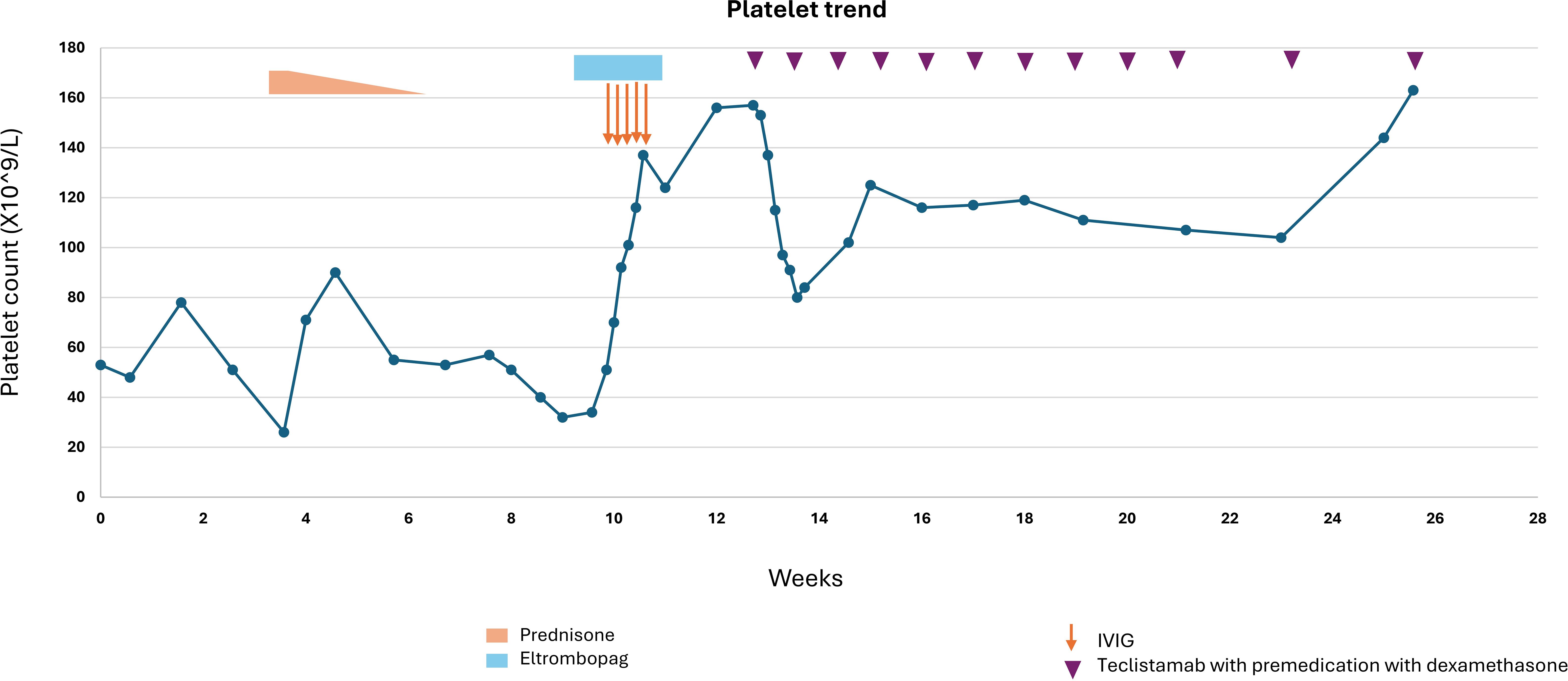
A 75-year-old male with history of coronary artery disease, hypertension, and IgG lambda plus free lambda multiple myeloma, in stringent complete remission, and off treatment for 4 years, developed COVID-19 which was treated with oral nirmatrelvir/ritonavir. After a month, routine lab work revealed a significant drop in platelet count from 201 X10^9/L to 24 X10^9/L. COVID-19 induced ITP was suspected, however platelet antibodies were negative, and his platelet counts were monitored initially without treatment. A bone marrow biopsy showed normal amount and morphology of megakaryocytes with no evidence of myelodysplastic syndrome or other etiologies that could cause thrombocytopenia. Due to persistent low platelet count, a diagnosis of ITP was made, and he was started on prednisone 1 mg/kg (90 mg daily), tapered over 3 weeks. Although platelet counts initially improved, they began to decline again as prednisone was tapered (Figure 2). A trial of IVIG, with iv hydrocortisone 100 mg daily for 5 days was then administered and eltrombopag was added to the regimen with improvement of his platelet counts. As the patient’s MM markers were starting to rise gradually, therapy with teclistamab was initiated to address both ITP and reactivation of MM. During step up dosing of teclistamab, the platelet counts initially declined, likely due to waning effects of IVIG and eltrombopag. However, with continued weekly teclistamab 1.5 mg/kg dose, platelet counts subsequently improved and stabilized. After two months, teclistamab was switched to biweekly regimen. The patient’s MM went into a complete remission after 4 months on teclistamab therapy with sustained normal platelet counts. Bone marrow biopsy with flow cytometry (6) showed eradication of all plasma cells, including malignant plasma cells and those healthy ones that likely serve as a reservoir for antibody production (Figure 3). The patient currently remains on monthly teclistamab regimen.
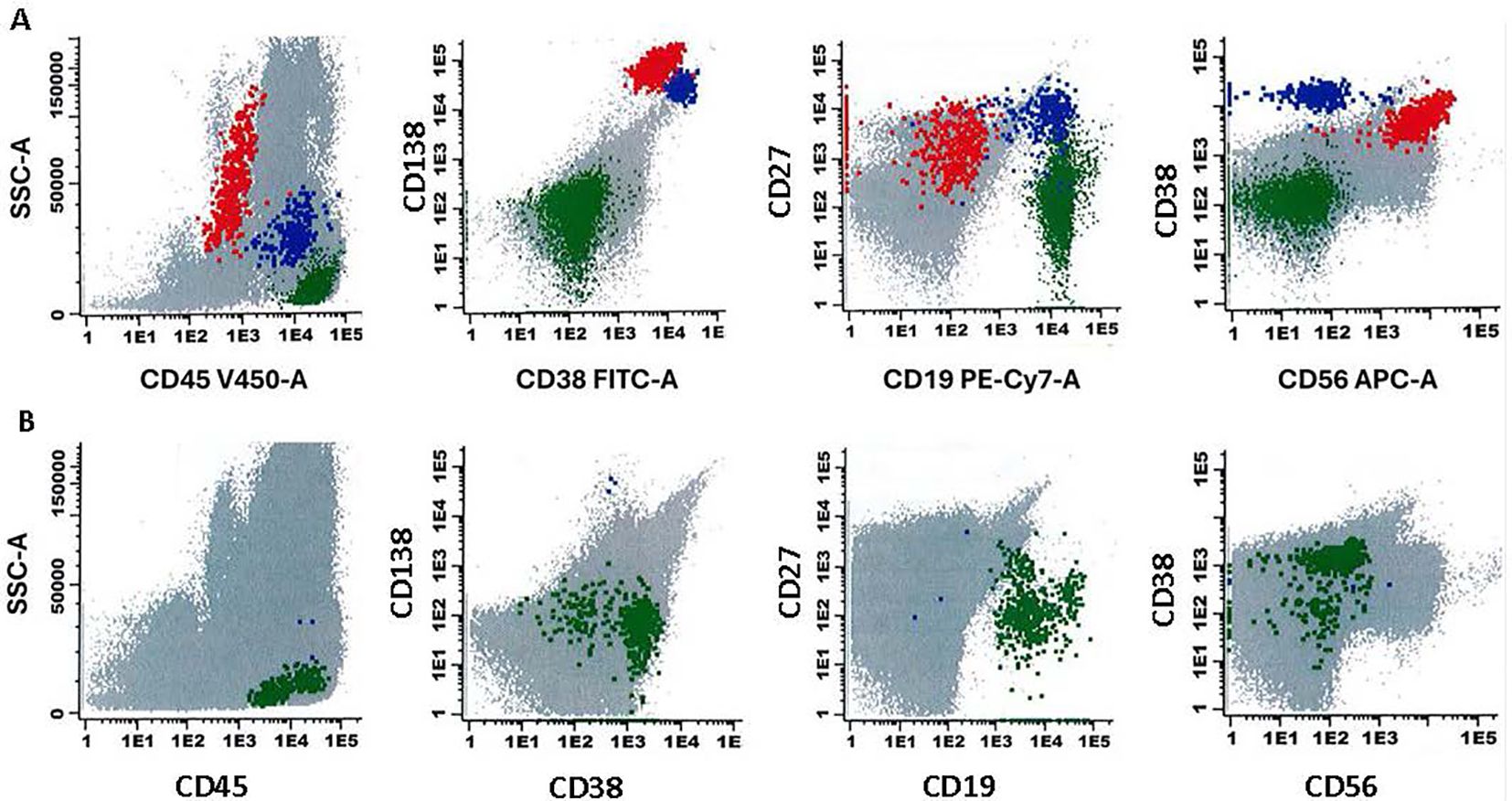
Figure 3. Flow cytometry analysis of a bone marrow aspirate in patient 2 with plasma cell neoplasm, with results given in 2-dimensional plots. The markers analyzed as previously published (6) are given on the x- and y-axes. Plasma cells with aberrant immunophenotype are depicted as red, normal plasma cells are depicted as blue, and normal B lymphoid cells are depicted as green. The upper (A) shows flow cytometry analysis results prior to treatment with Teclistamab. The lower (B) shows absence of both normal and abnormal plasma cells after treatment with Teclistamab.
Discussion
Teclistamab, a bispecific antibody, binds to CD3 receptor on T cell surface and BCMA on the myeloma cells, promoting T cell activation, cytokine release and subsequent lysis of the BCMA expressing myeloma cells (5, 7). This mechanism also impacts normal plasma cells and B cells, which share similar BCMA expression as that of malignant plasma cells, resulting in depleted humoral immune response, and a reduction in polyclonal immunoglobulin levels, including autoantibodies (7).
Both patients, diagnosed with ITP after Covid 19 infection and progressive multiple myeloma, were successfully treated with teclistamab leading to improved platelet counts and complete remission of myeloma. Platelet antibody tests were negative for both cases. Since the sensitivity of platelet antibody tests is low and is not demonstrable in close to 50% of patients (8), ITP was not excluded. Bone marrow biopsy showed no features of myelosuppression or other etiology, and infectious workup was notable only for COVID-19. Platelet counts did not improve despite multiple transfusions. Consequently, ITP was diagnosed by exclusion after relevant workup in both cases. Furthermore, while ITP secondary to COVID-19, can resolve within the first week with standard treatment (9), this was not observed in the presented patient cases. Both patients had prolonged thrombocytopenia for >10 weeks despite the repetitive application of steroids and IVIG which made the administration of anti-myeloma therapy very difficult and also increased the risk of bleeding.
In ITP, plasma cells and B cells are central to the production of pathogenic IgG autoantibodies that bind to platelets and megakaryocytes, leading to platelet opsonization and destruction in spleen and liver (10). Therefore, teclistamab, by targeting BCMA expressing myeloma and plasma cells could theoretically benefit both multiple myeloma and ITP by reducing the production of autoantibodies that contribute to ITP pathology. While teclistamab is not specifically approved for the treatment of ITP, its role in modulating immune responses may have implications for conditions characterized by autoimmune mechanisms, such as ITP. Case reports have also shown complete remission of systemic lupus erythematosus (SLE) with teclistamab, as well as improved disease activity in refractory autoimmune conditions, including systemic sclerosis, Sjogrens syndrome, MDA5-positive idiopathic myositis and seropositive rheumatoid arthritis (11, 12).
The first line treatment for ITP typically includes corticosteroids with or without IVIG. Second line therapies include immunosuppressive agents like rituximab, thrombopoietin receptor agonists, or splenectomy. Rituximab, a monoclonal antibody targeting CD20 on pan-B cells, is thought to eliminate B cells via apoptosis, antibody-dependent cytotoxicity and complement mediated lysis (13). Though rituximab has been used in management of chronic ITP, it has not been able to show favorable response in multiple myeloma due to downregulation and heterogenous expression of CD20 in plasma cells (14). Whereas long lived plasma cells cannot be completely removed by anti B cell therapies alone, plasma cell targeting therapies like bortezomib (15), daratumumab (16, 17) have been successfully attempted in treatment of ITP as reported in several case reports.
In addition to autoantibodies, other mechanisms, including T cell imbalances, impaired thrombopoiesis and defective megakaryopoeisis have been proposed in ITP pathogenesis. Rituximab, which depletes B cell population, and lowers platelet autoantibody titers, has an overall response rate of approximately 60%, with incomplete responses likely due to complex multimodal pathogenesis of ITP, including T cell mediated peripheral platelet destruction and megakaryocyte inhibition within the bone marrow (18). Although teclistamab effectively reduces plasma cells and autoantibodies, its role in addressing other immune factors involved in ITP is unclear.
While teclistamab effectively eliminates BCMA positive myeloma cells, it also depletes normal plasma cells, impairing humoral immunity and elevating infection risk. Teclistamab is also associated with cytokine release syndrome (CRS) and immune cell associated neurotoxicity syndrome (ICANS), however these were not observed in our patients. While our patients showed maintained platelet counts after introducing teclistamab, long term studies are necessary to determine the outcomes and safety of BCMA targeting bsAb in ITP, especially given the drugs recent introduction.
Conclusion
As BCMA is predominantly expressed on the myeloma cells, late-stage B cells and plasma cells responsible for producing autoantibodies implicated in ITP, BCMA-targeted therapies like teclistamab may provide a novel therapeutic approach for concurrently managing both refractory ITP and multiple myeloma. Further research is warranted to evaluate whether teclistamab might provide similar therapeutic benefits in other autoimmune conditions co-occurring with multiple myeloma. Additionally, long-term follow-up studies are essential to understand teclistamab’s impact on other immune pathways involved in ITP and to establish its long-term efficacy and safety.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AS: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis, Investigation. AK: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Investigation. SP: Data curation, Writing – review & editing. TP: Data curation, Writing – review & editing. HC: Data curation, Writing – review & editing. SN: Writing – review & editing, Data curation. RB: Writing – review & editing, Data curation. TS: Writing – review & editing, Data curation. ST: Writing – review & editing, Data curation, Supervision. MZ: Writing – review & editing, Data curation, Supervision. SH: Writing – review & editing, Data curation, Supervision. FV: Writing – review & editing, Data curation, Supervision. CS: Data curation, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author CS has received honoraria from Janssen, Pfizer and Arcellx.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. Front Immunol. (2018) 9:880. doi: 10.3389/fimmu.2018.00880
2. Nguyen H, Nguyen M, Olenik A. Immune thrombocytopenic purpura following COVID-19 infection: a case report and literature review. Cureus. (2023) 15:e39342. doi: 10.7759/cureus.39342
3. Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: A systematic review. S N Compr Clin Med. (2020) 2:2048–58. doi: 10.1007/s42399-020-00521-8
4. Sarfraz H, Anand K, Liu S, Shah S. Multiple myeloma with concurrent immune thrombocytopenic purpura. Ecancermedicalscience. (2020) 14:1012. doi: 10.3332/ecancer.2020.1012
5. Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. (2022) 387:495–505. doi: 10.1056/NEJMoa2203478
6. Rasche L, Alapat D, Kumar M, Gershner G, McDonald J, Wardell CP, et al. Combination of flow cytometry and functional imaging for monitoring or residual disease in myeloma. Leukemia. (2019) 33:1713–22. doi: 10.1038/s41375-018-0329-0
7. Frerichs KA, Verkleij CPM, Mateos MV, Martin TG, Rodriguez C, Nooka A, et al. Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: importance of immunoglobulin supplementation. Blood Adv. (2024) 8:194–206. doi: 10.1182/bloodadvances.2023011658
8. Vrbensky JR, Moore JE, Arnold DM, Smith JW, Kelton JG, Nazy I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: a systematic review and meta-analysis of a diagnostic test. J Thromb Haemost. (2019) 17:787–94. doi: 10.1111/jth.14419
9. Alharbi MG, Alanazi N, Yousef A, Alanazi N, Alotaibi B, Aljurf M, et al. COVID-19 associated with immune thrombocytopenia: a systematic review and meta-analysis. Expert Rev Hematol. (2022) 15:157–66. doi: 10.1080/17474086.2022.2029699
10. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. (2017) 6:16. doi: 10.3390/jcm6020016
11. Hagen M, Bucci L, Böltz S, Nöthling DM, Rothe T, Anoshkin K, et al. BCMA-targeted T-cell–engager therapy for autoimmune disease. New Engl J Med. (2024) 391:867–9. doi: 10.1056/NEJMc2408786
12. Alexander T, Krönke J, Cheng Q, Keller U, Krönke G. Teclistamab-induced remission in refractory systemic lupus erythematosus. New Engl J Med. (2024) 391:864–6. doi: 10.1056/NEJMc2407150
13. Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. (2005) 8:140–74. doi: 10.1159/000082102
14. Kapoor P, Greipp PT, Morice WG, Rajkumar SV, Witzig TE, Greipp PR. Anti-CD20 monoclonal antibody therapy in multiple myeloma. Br J Haematol. (2008) 141:135–48. doi: 10.1111/j.1365-2141.2008.07024.x
15. Beckman JD, Rollins-Raval MA, Raval JS, Park YA, Mazepa M, Ma A. Bortezomib for refractory immune-mediated thrombocytopenia purpura. Am J Ther. (2018) 25:e270–2. doi: 10.1097/MJT.0000000000000517
16. Migdady Y, Ediriwickrema A, Jackson PJ, Kadi W, Gupta R, Socola F, et al. Successful treatment of thrombocytopenia with daratumumab after allogeneic transplant: a case report and literature review. Blood Adv. (2020) 4:815–8. doi: 10.1182/bloodadvances.2019001215
17. Vernava I, Schmitt CA. Daratumumab as a novel treatment option in refractory ITP. Blood Cells Mol Dis. (2023) 99:102724. doi: 10.1016/j.bcmd.2023.102724
Keywords: immune thrombocytopenia, Teclistamab, BCMA, multiple myeloma, plasma cells
Citation: Shrestha A, Trikannad Ashwini Kumar AK, Pandey S, Patel T, Cheema HI, Naqvi SM, Bachu R, Shrivastava T, Thanendrarajan S, Zangari M, Al Hadidi S, van Rhee F and Schinke C (2025) Case Report: Teclistamab in the treatment of idiopathic immune thrombocytopenia in patients with multiple myeloma. Front. Hematol. 4:1547209. doi: 10.3389/frhem.2025.1547209
Received: 17 December 2024; Accepted: 28 March 2025;
Published: 24 April 2025.
Edited by:
Tomás José González-López, Burgos University Hospital, SpainReviewed by:
David Gomez-almaguer, Autonomous University of Nuevo León, MexicoSongsong Lu, Peking University People’s Hospital, China
Copyright © 2025 Shrestha, Trikannad Ashwini Kumar, Pandey, Patel, Cheema, Naqvi, Bachu, Shrivastava, Thanendrarajan, Zangari, Al Hadidi, van Rhee and Schinke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asis Shrestha, YXNocmVzdGhhQHVhbXMuZWR1
†These authors have contributed equally to this work
 Asis Shrestha
Asis Shrestha Anup Kumar Trikannad Ashwini Kumar1†
Anup Kumar Trikannad Ashwini Kumar1† Soumya Pandey
Soumya Pandey Maurizio Zangari
Maurizio Zangari Frits van Rhee
Frits van Rhee Carolina Schinke
Carolina Schinke