- 1Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 2Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
Acute promyelocytic leukemia (APL) during pregnancy is an exceptionally rare occurrence with high maternal and fetal mortality rates, requiring a highly tailored approach to therapy. We report a case of a patient diagnosed with APL during the third trimester of pregnancy who initially presented with spontaneous ecchymoses. Treatment was initiated with all-trans retinoic acid (ATRA) and a modified dose of idarubicin, leading to a rapid correction of the coagulopathy and a complete remission of the APL. The patient subsequently underwent induced labor, resulting in an uncomplicated vaginal delivery without significant hemorrhage and no evidence of embryopathy in the neonate. To date, no published reports of successful deliveries in patients with APL have been documented in Thailand. This case highlights how a multidisciplinary approach involving obstetricians, hematologists, and neonatologists ensured both maternal survival and optimal neonatal outcomes.
Introduction
Acute promyelocytic leukemia (APL) is characterized by the proliferation of malignant promyelocytes in the bone marrow (BM) that carry, most commonly, the t(15;17) chromosomal translocation and/or the PML/RARA fusion gene (1), which is diagnostic for this condition. APL comprises approximately 9% of acute myeloid leukemia (AML) in Thailand (2). The treatment of APL was completely revolutionized by the introduction of all-trans retinoic acid (ATRA), which improves the associated coagulopathy, contributing to a reduction in deaths (3–5). APL occurs infrequently during pregnancy and can lead to adverse outcomes, such as maternal and/or fetal death, without timely and appropriate management. However, data on treatment strategies are limited due to there being no standard guidelines for this special population (6). Therefore, it is essential to carefully weigh the benefits of immediate intervention against potential risks to the developing fetus, necessitating a multidisciplinary team approach. We report the first successful case of APL in pregnancy treated with ATRA and chemotherapy in Thailand. This case highlights the diagnosis and management of APL in a pregnant patient, detailing the clinical presentation, therapeutic strategies employed, and observed outcomes.
Case presentation
A previously healthy 33-year-old woman (Gravida 4 Para 3) presented to our hospital at 30+1 weeks of gestation. She had been referred by her general practitioner due to spontaneous bruising and fatigue for 1 week. An initial automated blood count showed a hemoglobin (Hb) level of 7.4 g/dL, white blood cell count (WBC) of 21,500/μL with 83% blasts, and a platelet count of 7,000/μL. Prothrombin time (PT) was 13.9 sec (normal range: 9.9–12.3 sec), with an activated partial thromboplastin time (aPTT) of 22.9 sec (normal range: 28.8–36.4 sec), and fibrinogen level of 115 mg/dl (normal range: 179.8–435.4 mg/dl). A peripheral blood smear showed leukocytosis with abnormal promyelocytes containing red to purple cytoplasmic granules and a decreased platelet count (Figure 1A) without a microangiopathic hemolytic blood picture of red blood cells. A bone marrow (BM) examination revealed marked hypercellular marrow with numerous abnormal hypergranular promyelocytes filled with stacks of Auer rods (Figures 1B–D), which were found to be CD34-/HLA-DR-/CD117+/CD33+ by flow cytometry (Figure 2). Routine karyotyping and molecular testing confirmed the diagnosis of APL with a karyotype of 46, XX, t(15;17)(q22;q21)[20] and reverse transcriptase polymerase chain reaction (RT-PCR) showed a translocation breakpoint within PML intron 6 (bcr1). According to her Sanz Score, she was classified as “high risk” APL. An obstetric ultrasound showed a male fetus without gross anomaly, normal fetal growth, normal doppler study, and no signs of intra- or retroplacental hemorrhage.
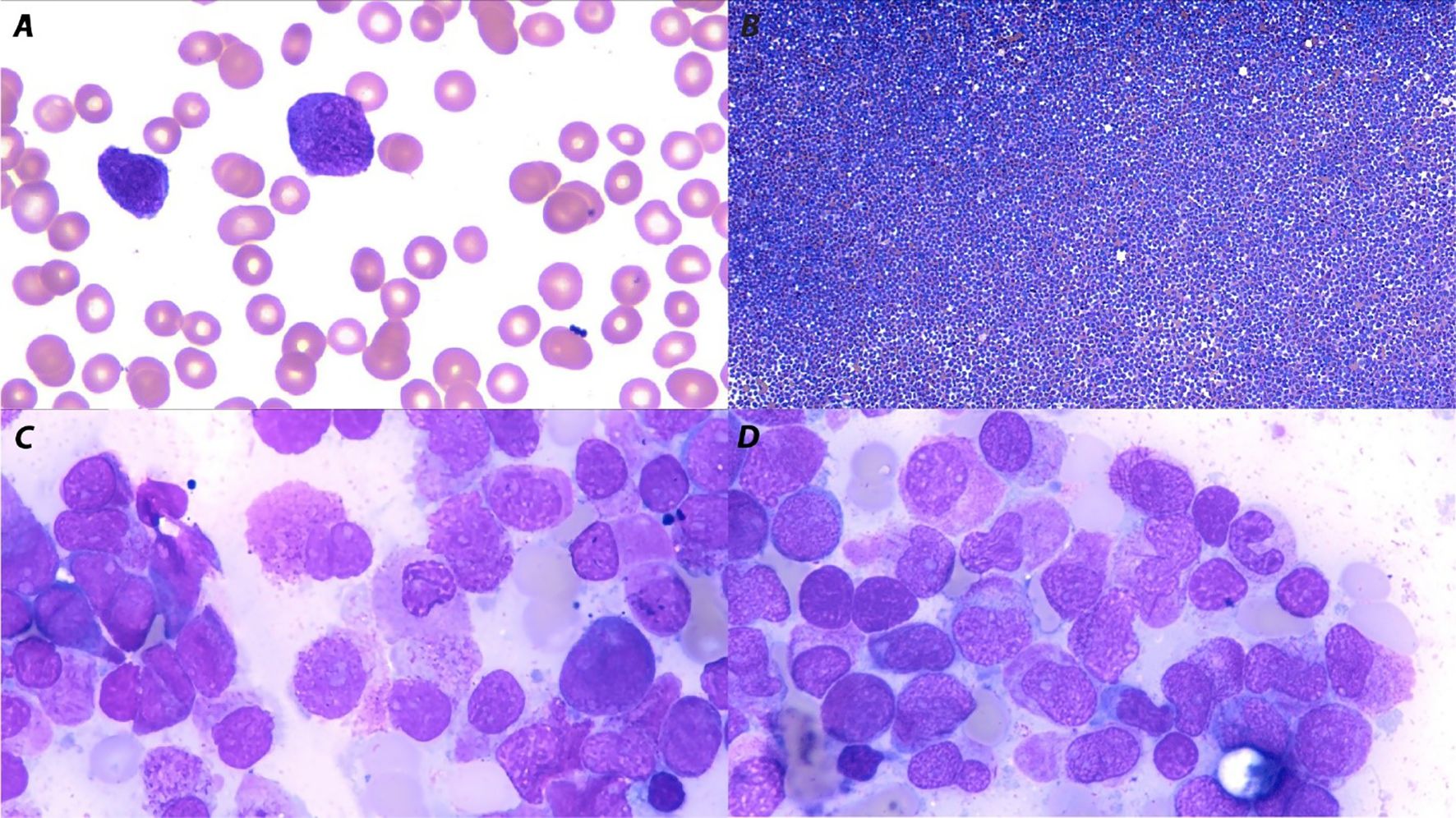
Figure 1. Peripheral blood smear and bone marrow aspiration morphology (Wright–Giemsa stain) in acute promyelocytic leukemia. (A) A peripheral blood smear showed abnormal promyelocytes containing red to purple cytoplasmic granules (magnification x100). (B) Bone marrow aspiration showed marked hypercellular bone marrow (magnification, x10) with (C, D) numerous abnormal hypergranular promyelocytes filled with stacks of Auer rods (magnification x100).
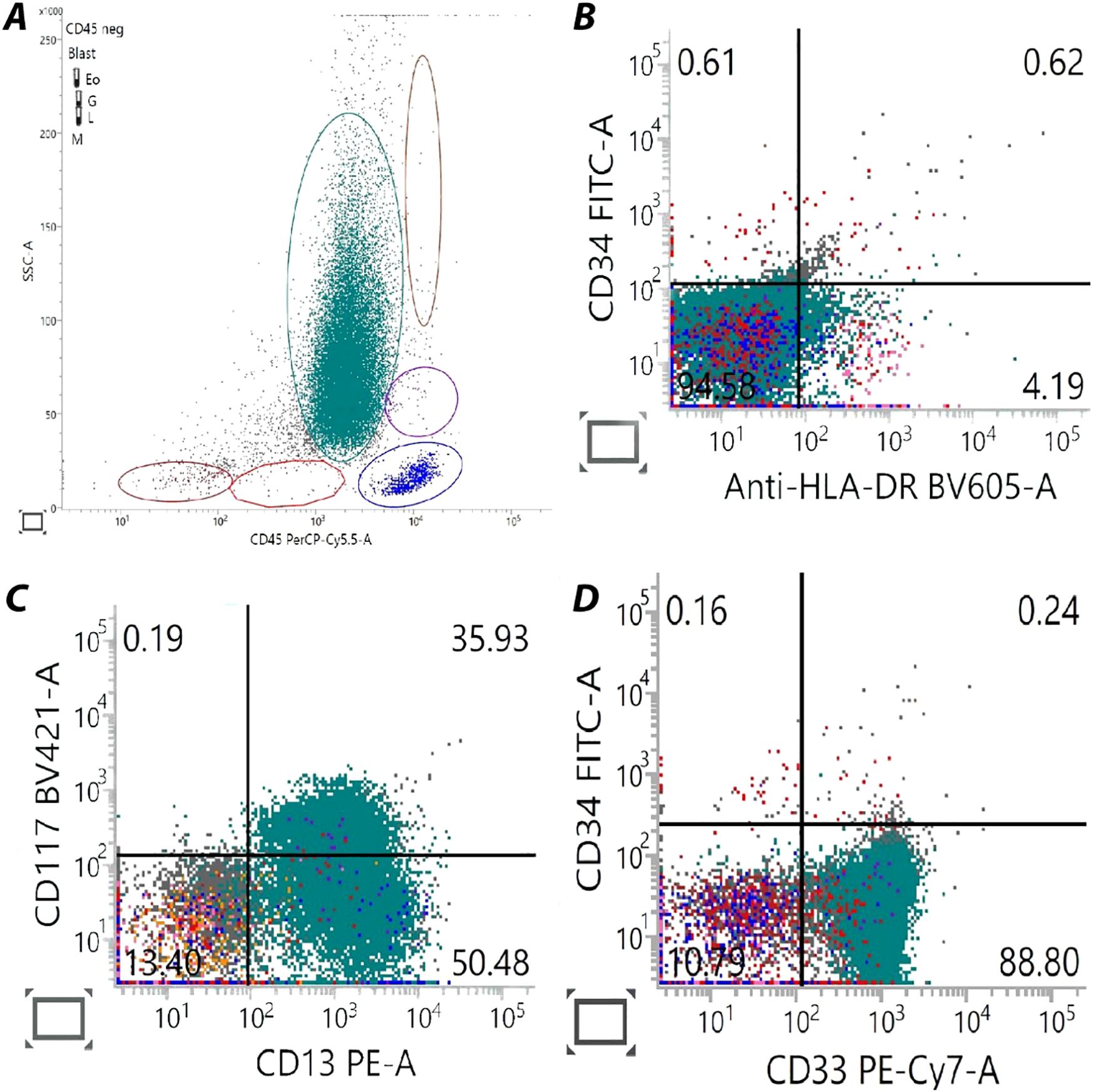
Figure 2. Flow cytometry analysis from the patient's bone marrow sample. These are features of the hypergranular variant of acute promyelocytic leukemia. (A) CD45 (x-axis)/SSC (y-axis) dot plot shows blasts circled in green with the noticeable of flame-shaped pattern (blast gate). (B) HLA-DR (x-axis)/CD34 (y-axis) dot plot indicated absence in expression for both markers. (C) CD13 (x-axis)/CD117 (y-axis) dot plot reveals CD13 positivity in blast gate and some of CD117 co-expression for 35.93%. (D) CD33 (x-axis)/CD34 (y-axis) dot plot represent CD33 expressed blasts in absence of CD34 population.
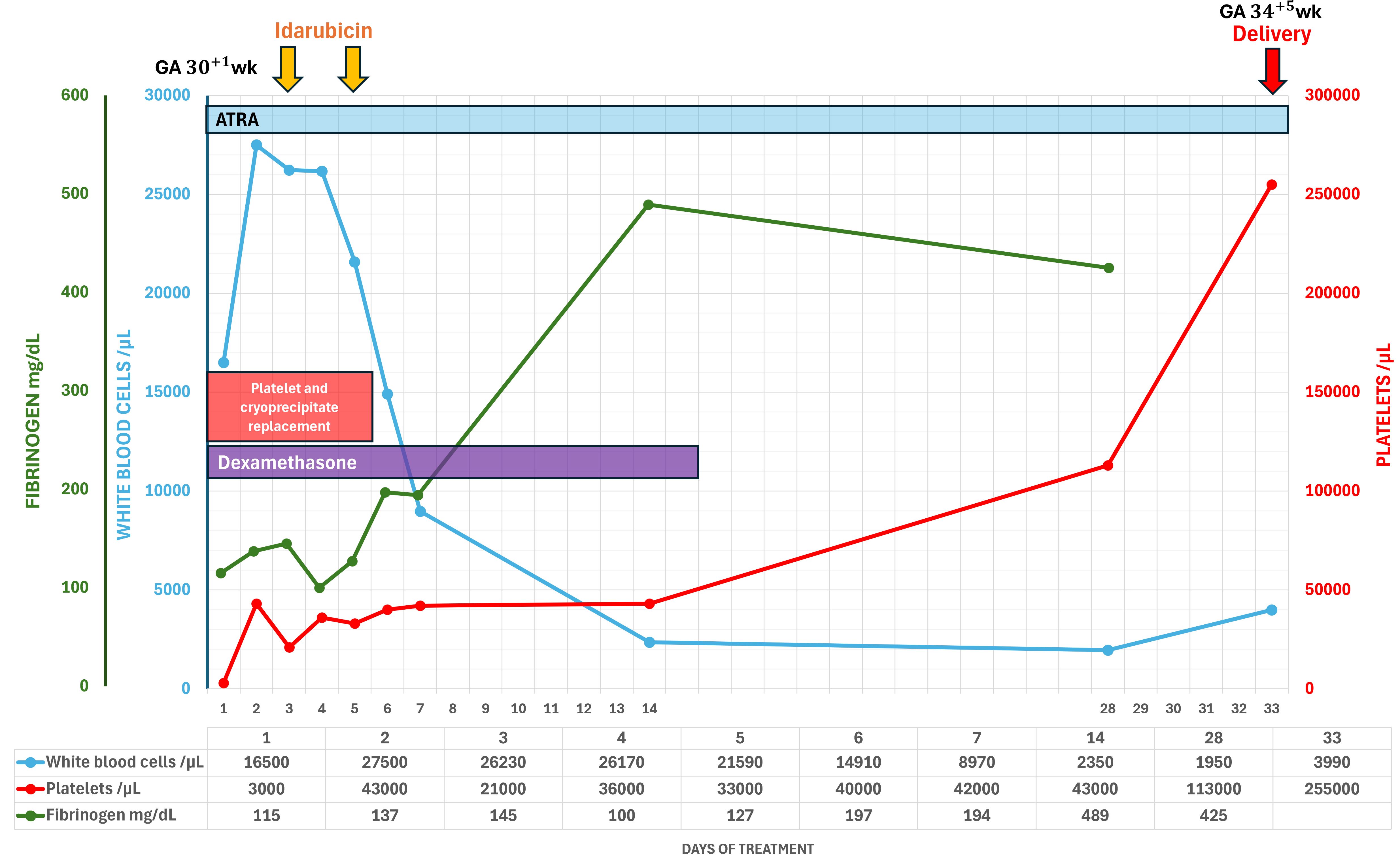
Although delivery at this gestational age is generally feasible, neither induction of labor nor cesarean delivery was considered to be appropriate because of the extremely high maternal bleeding risk caused by severe APL-associated coagulopathy. She was treated supportively with blood products to maintain her platelet and fibrinogen levels above 30,000/μL and 150 mg/dL, respectively. A multidisciplinary discussion was held between the patient, her husband, the hematologists, obstetricians, and neonatologists regarding treatment. She elected to continue with the pregnancy, understanding that this could pose risks for the fetus, such as intrauterine growth restriction, fetal cardiac function impairment, preterm delivery, and perinatal mortality. Induction was commenced according to the LPA2005 protocol and corticosteroid prophylaxis for differentiation syndrome (ATRA 45 mg/m2/day from day 1 until complete remission in divided doses; idarubicin 12 mg/m2/day on days 2, 4, 6, and 8; dexamethasone 10 mg IV every 12 hours from day 1 to day 15). Due to concern about potential risks to the fetus, we administered idarubicin in only two doses (omitting days 6 and 8) to control the white blood cell count. The obstetricians undertook twice-weekly ultrasound growth scans, with planned vaginal delivery at late preterm period (34–36 weeks of gestation), followed by consolidation and maintenance treatment.
Her admission was complicated by neutropenic fever, which was treated with piperacillin/tazobactam. Within the first few days of ATRA treatment, her coagulopathy deteriorated, however, her coagulation factors began to normalize afterwards. Hemorrhagic complications did not occur, and ecchymoses gradually disappeared. During the entire treatment period, specific signs of differentiation syndrome were not detected (Figure 3). Bone marrow assessment was done on day 30 of treatment (34+2 weeks of gestation), which revealed complete cytomorphologic remission. Fetal surveillance was unremarkable, including cardiotoxicity. Induction and vaginal delivery were successfully performed at 34+5 weeks gestation. The patient delivered an apparently healthy preterm male baby (2,430 grams, appropriate for gestational age, APGAR score 9-10-10). The newborn was immediately transferred to the neonatal intermediate care unit; no other complications developed postnatally. He was eventually discharged on his 17th day of life.
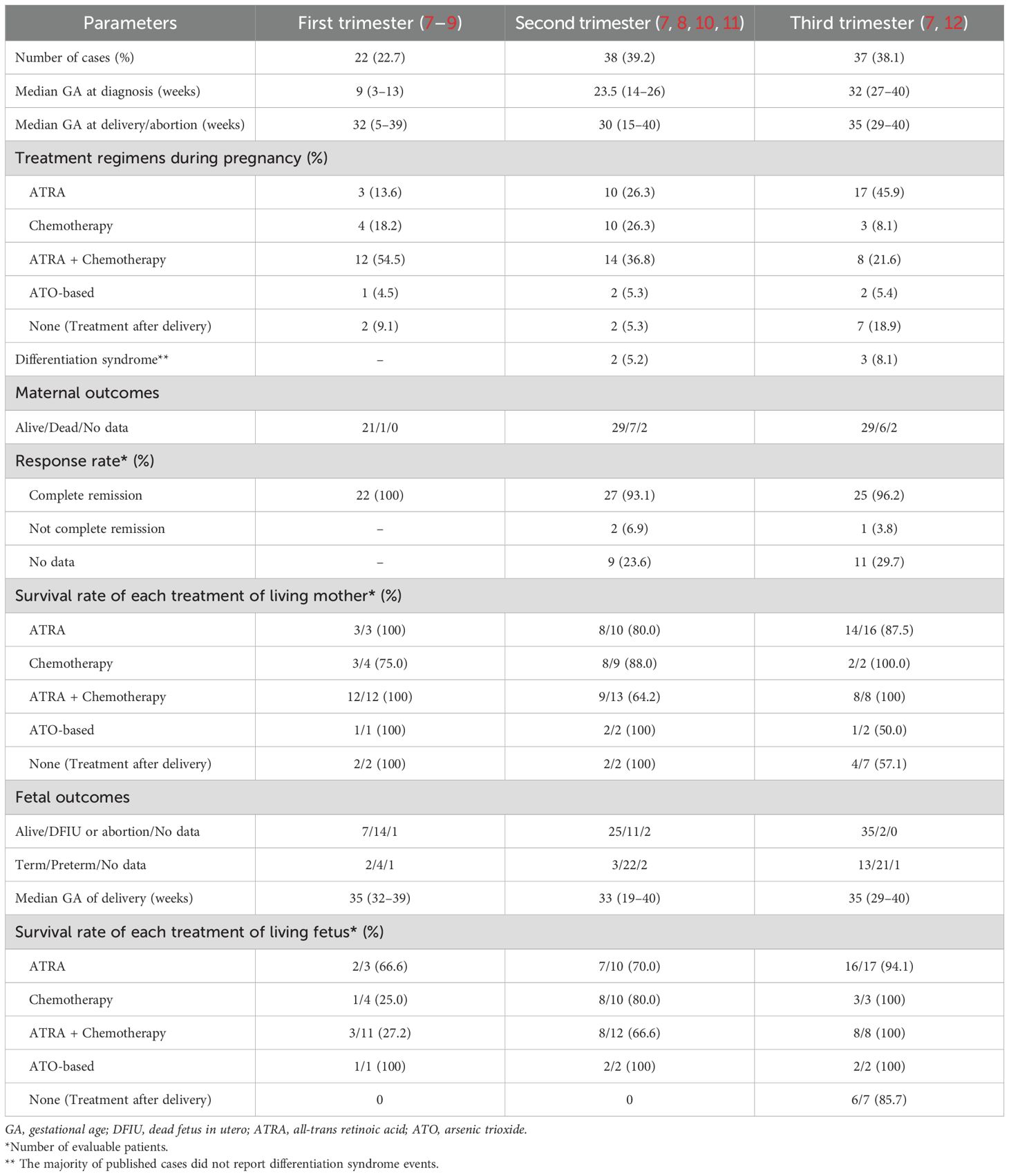
We reviewed cases of APL in pregnancy in PubMed from inception to December 2024 (not including our case). A PubMed search was conducted in December 2024 using the following search terms: {[(“acute promyelocytic leukemia” OR (“acute promyelocytic leukaemia”)] AND [(“pregnant”) OR (“pregnancy”)]}. A total of 70 articles, out of 140, met the eligibility criteria, containing 97 cases. The exclusions were due to the following criteria: APL case presentations were not specified (63 articles), non-English language (6 articles), and APL was diagnosed during the post-partum period (1 article). Only full articles or selected cases in the English language were included. The details are shown in Table 1.
Discussion
The incidence of APL during pregnancy is exceedingly rare, estimated to be approximately one per one million pregnancies (13, 14). Whether the pathogenesis of APL in pregnancy differs from that in non-gravid patients is unclear. However, some reports indicate that alterations in hormonal levels and immunological responses during pregnancy may play an important role in the development of malignancies (15, 16). APL is characterized by several distinctive clinical features, especially disseminated intravascular coagulation (DIC), and is associated with early hemorrhagic demise. Thus, rapid diagnosis and treatment are paramount to prevent early death. In Thailand, a resource-limited country, laboratory diagnostics such as flow cytometry, cytogenetics, and molecular testing are not available in primary care settings and in many secondary care hospitals. These tests are also time-consuming, and treatment is feasible only in specialized hematology centers, of which only a few exist in the country. As a result, the initial diagnosis is often based on a clinical evaluation and morphological examination of the peripheral blood smear. In cases where APL is suspected, prompt referral to a specialized hematology center is crucial to initiate appropriate management without delay.
APL is treated with ATRA and anthracycline-based chemotherapy, such as idarubicin or daunorubicin. Treatment outcomes are highly favorable, with an approximate 90% cure rate (17, 18). During pregnancy, this regimen is controversial due to potential teratogenic effects and fatal medical-related retinoic acid syndrome and requires careful consideration of both maternal and fetal health. During the first trimester, when organogenesis occurs, the use of these agents is generally contraindicated due to the high risk of teratogenicity. If acute leukemia is diagnosed during this period, the risk of delaying treatment must be weighed against the potential risks for severe fetal harm, particularly from ATRA. After the first trimester, the risks associated with idarubicin and ATRA are reduced, though concerns about fetal growth restriction and cardiotoxicity remain (19). Some cases of reversible fetal arrhythmias and other cardiac complications have been reported (20, 21). Therefore, fetal monitoring is necessary to detect and mitigate these risks.
Although the NCCN guidelines do not provide specific recommendations, the European LeukemiaNet guidelines for APL management during pregnancy advise against the use of arsenic trioxide throughout pregnancy. Women in the second and third trimesters may receive ATRA alone until delivery (due to the risk of abortion with chemotherapy) or ATRA in combination with chemotherapy. If chemotherapy is deferred to the postpartum period, therapy should be initiated without delay after delivery (6). Data from our review show that when a patient is diagnosed with APL in the third trimester, the survival rate for both the mother and fetus is better with treatment without delay. The survival outcomes for ATRA, chemotherapy, or ATRA with chemotherapy are greater than 85%. Surprisingly, there were no maternal or fetal deaths when treated with ATRA and chemotherapy. Therefore, we chose ATRA and modified the dose of idarubicin to reduce the potential for fetal cardiotoxicity while also administering dexamethasone prophylaxis for differentiation syndrome. Specifically, only two out of the four planned doses of idarubicin were given and consolidation therapy was continued after delivery. At the end of consolidation, the patient achieved a negative result in an RT-PCR for PML-RAR α and is continuing with maintenance treatment, demonstrating an individualized approach that considered both maternal and fetal health outcomes.
Conclusion
To the best of our knowledge, this is the first documented case of successful treatment of acute promyelocytic leukemia in pregnancy with ATRA and anthracycline-based chemotherapy resulting in favorable maternal and neonatal outcomes in Thailand. A multidisciplinary approach involving obstetricians, hematologists, and neonatologists ensured maternal survival and optimal neonatal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained for the publication of this case report.
Author contributions
SP: Writing – original draft, Writing – review & editing. BP: Writing – review & editing. NP: Writing – review & editing. SS: Supervision, Writing – review & editing. TR: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
2. Wanitpongpun C, Utchariyaprasit E, Owattanapanich W, Tantiworawit A, Rattarittamrong E, Niparuck P, et al. Types, clinical features, and survival outcomes of patients with acute myeloid leukemia in Thailand: A 3-year prospective multicenter study from the Thai acute leukemia study group (TALSG). Clin Lymphoma Myeloma Leukemia. (2021) 21:e635–e43. doi: 10.1016/j.clml.2021.03.004
3. Meng-er H, Yu-chen Y, Shu-rong C, Jin-ren C, Jia-Xiang L, Lin Z, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. (1988) 72:567–72. doi: 10.1182/blood.V72.2.567.567
4. Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results Multicenter Randomized Trial. Blood. (1993) 82:3241–9. doi: 10.1182/blood.V82.11.3241.3241
5. Tallman MS, Lefèbvre P, Baine RM, Shoji M, Cohen I, Green D, et al. Effects of all-trans retinoic acid or chemotherapy on the molecular regulation of systemic blood coagulation and fibrinolysis in patients with acute promyelocytic leukemia. J Thromb Haemostasis. (2004) 2:1341–50. doi: 10.1111/j.1538-7836.2004.00787.x
6. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood. (2019) 133:1630–43. doi: 10.1182/blood-2019-01-894980
7. Santolaria A, Perales A, Montesinos P, and Sanz MA. Acute promyelocytic leukemia during pregnancy: A systematic review of the literature. Cancers. (2020) 12:968. doi: 10.3390/cancers12040968
8. Valappil S, Kurkar M, and Howell R. Outcome of pregnancy in women treated with all-trans retinoic acid; a case report and review of literature. Hematology. (2007) 12:415–8. doi: 10.1080/10245330701448594
9. Ni W, Deng K, Chen Y, Li L, Liu J, Ju W, et al. Pregnant woman with acute promyelocytic leukemia delivers healthy twins and is cured successfully: A case report. Exp Ther Med. (2023) 25:206. doi: 10.3892/etm.2023.11905
10. Salter B, Radford JM, Berg T, and Leber B. The successful management of acute promyelocytic leukemia during pregnancy: a case report. Leuk Lymphoma. (2024) 65:526–9. doi: 10.1080/10428194.2023.2295786
11. Cochet C, Simonet M, Cattin J, Metz JP, Berceanu A, Deconinck E, et al. Arsenic trioxide treatment during pregnancy for acute promyelocytic leukemia in a 22-year-old woman. Case Rep Hematol. (2020) 2020:3686584. doi: 10.1155/2020/3686584
12. Li Y, Li PY, He JL, and Li QJ. Pregnancy complicated with acute promyelocytic leukemia: A case report and a literature review. Yangtze Med. (2020) 4:163–72. doi: 10.4236/ym.2020.43016
13. Aoki A, Yoneda N, Yoneda S, Miyazono T, Sugiyama T, and Saito S. Massive postpartum hemorrhage after chemotherapy in a patient with acute promyelocytic leukemia. J Obstet Gynaecol Res. (2011) 37:1759–63. doi: 10.1111/j.1447-0756.2011.01598.x
14. Catanzarite VA and Ferguson JE 2nd. Acute leukemia and pregnancy: a review of management and outcome, 1972-1982. Obstet Gynecol Surv. (1984) 39:663–78. doi: 10.1097/00006254-198411000-00001
15. Shakhar K, Valdimarsdottir HB, and Bovbjerg DH. Heightened risk of breast cancer following pregnancy: could lasting systemic immune alterations contribute? Cancer Epidemiology Biomarkers Prev. (2007) 16:1082–6. doi: 10.1158/1055-9965.EPI-07-0014
16. Verma V, Giri S, Manandhar S, Pathak R, and Bhatt VR. Acute promyelocytic leukemia during pregnancy: a systematic analysis of outcome. Leukemia Lymphoma. (2016) 57:616–22. doi: 10.3109/10428194.2015.1065977
17. Adès L, Sanz MA, Chevret S, Montesinos P, Chevallier P, Raffoux E, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): a comparison of French-Belgian-Swiss and PETHEMA results. Blood. (2008) 111:1078–84. doi: 10.1182/blood-2007-07-099978
18. Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. (2010) 116:3171–9. doi: 10.1182/blood-2010-03-276196
19. Milojkovic D and Apperley JF. How I treat leukemia during pregnancy. Blood. (2014) 123:974–84. doi: 10.1182/blood-2013-08-283580
20. Siu BL, Alonzo MR, Vargo TA, and Fenrich AL. Transient dilated cardiomyopathy in a newborn exposed to idarubicin and all-trans-retinoic acid (ATRA) early in the second trimester of pregnancy. Int J Gynecol Cancer. (2002) 12:399–402. doi: 10.1136/ijgc-00009577-200207000-00012
Keywords: acute promyelocytic leukemia, pregnancy, third trimester, multidisciplinary approaches, perinatal care
Citation: Puttirangsan S, Prasertkanchana B, Parapob N, Srichairatanakool S and Rattanathammethee T (2025) Successful management of acute promyelocytic leukemia in the third trimester of pregnancy: a case report and review of the literature. Front. Hematol. 4:1553336. doi: 10.3389/frhem.2025.1553336
Received: 30 December 2024; Accepted: 29 May 2025;
Published: 18 June 2025.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Anna Maria Testi, Sapienza University of Rome, ItalyAlessandro Perrella, Hospital of the Hills, Italy
Copyright © 2025 Puttirangsan, Prasertkanchana, Parapob, Srichairatanakool and Rattanathammethee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanawat Rattanathammethee, dGhhbmF3YXQuckBjbXUuYWMudGg=
 Sirichai Puttirangsan
Sirichai Puttirangsan B. Prasertkanchana1
B. Prasertkanchana1 Sirichai Srichairatanakool
Sirichai Srichairatanakool Thanawat Rattanathammethee
Thanawat Rattanathammethee