- 1Department of Hematology and Medical Oncology, Wheeling Hospital, West Virginia University Cancer Institute, Wheeling, WV, United States
- 2Division of Pharmaceutics and Pharmacology, College of Pharmacy, The Ohio State University, Columbus, OH, United States
- 3Division of Hematology, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, United States
Acute myeloid leukemia (AML) patients with FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) experience shorter disease-free survival and high relapse rates even with FLT3 inhibitor therapy. One of the main mechanisms for loss of response is the acquisition of molecular mutations that confer drug resistance to FLT3 inhibitors. TP-0903 is an oral multi-kinase inhibitor with activity against FLT3 and several other kinases known to mediate drug resistance. Three heavily treated relapsed/refractory AML patients with FLT3-ITD were treated with TP-0903 50 mg daily, on a phase 1b/2 clinical trial, for as long as disease response or clinical benefit was observed. The dose for one patient was reduced to 37 mg daily mid-cycle after developing grade 3 nausea that improved to grade 2 within 24 h of holding doses of TP-0903. All patients achieved stable disease; however, a significant reduction in bone marrow blast percentage after one to two cycles of treatment was observed in two patients, which correlated with a decrease in FLT3-ITD allelic ratio and variant allele frequency of co-occurring mutations (e.g., NPM1 and DNMT3A). TP-0903 was feasible with no unusual toxicity signals. Additional preclinical and clinical studies are needed in order to determine the role of TP-0903 in AML.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT04518345.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and is characterized by the acquisition of sequential genetic and molecular aberrations that culminate in the expansion of myeloid blasts in the blood and marrow leading to severely impaired hematopoiesis. Over the past two decades, the discovery of recurrent, and potentially actionable, mutational targets has led to the development of several agents that are specific for the treatment of certain subtypes of AML (1–4). Among the most common of these mutations are those in the FMS-like tyrosine kinase 3 (FLT3) gene, observed in approximately 30%–35% of AML patients and primarily included internal tandem duplications (ITDs) in 25% and tyrosine kinase domain (TKD) point mutations in 5% resulting in constitutive activation of FLT3 signaling (5–7). AML with FLT3-ITD has been well described as a particularly poor biological subtype as patients often present with proliferative disease and display a short disease-free interval following standard AML-directed chemotherapy (8–10). As such, molecularly targeted therapies directed at inhibiting FLT3 signaling have been an attractive treatment option.
Currently, there are three FLT3 inhibitors that are approved by the United States Food and Drug Administration. In 2017, the results from the landmark randomized phase 3 RATIFY study led to the approval of the first FLT3 inhibitor, midostaurin, in combination with cytarabine and daunorubicin (“7 + 3”) for newly diagnosed AML patients with FLT3 mutations, given the survival benefit that was observed in patients who received midostaurin compared to those who did not (2). However, the rate of CR/Cri was low in both cohorts in the upfront setting, and almost half of the patients achieving CR relapsed (11). In addition, as midostaurin is a multi-kinase inhibitor, toxicities due to off-target effects were an important limitation in terms of tolerability, prompting the development of more selective FLT3 inhibitors such as gilteritinib, quizartinib, and crenolanib (12–16). In 2018, gilteritinib became the second FLT3 inhibitor to receive approval for FLT3-mutated AML and the first to be approved as a single agent in the relapsed/refractory setting, based on the results of the phase 3 ADMIRAL study (12). Although gilteritinib displays an improved toxicity profile compared to midostaurin, it is not curative and is associated with a relatively short median overall survival of 9.3 months. Furthermore, the 2-year cumulative incidence of relapse after composite complete remission is reported to be 75.7% (17). Quizartinib, a more selective type II FLT3 inhibitor that has activity only against FLT3-ITD and not TKD mutations, was approved as frontline therapy in combination with 7 + 3 in 2023 based on the results of the QuANTUM-First study, which demonstrated a pronounced improvement in median overall survival of 31.9 months compared to 15.1 months in patients who received 7 + 3 alone (13). However, relapses remained common for quizartinib-treated patients, occurring in one-third of patients within 3 years (13).
One explanation for the lack of durable responses to FLT3 inhibitors is the development of resistance due to acquisition of new mutations at relapse. Molecular profiling of patients treated with midostaurin on the RATIFY trial showed that 46% of patients acquired new mutations in genes in the MAPK signaling pathway (e.g., MAP3K1, FAM53A, TMPRSS4, and RAS pathways) at relapse (11). In another study, molecular profiling of patients enrolled in the ADMIRAL trial demonstrated the acquisition of new RAS pathway mutations in 45% of patients and new FLT3 TKD F691L mutations in 13% of patients at relapse (18). TP-0903, a small molecule multi-kinase inhibitor, has features with the potential to overcome clinical resistance to FLT3 inhibitors. TP-0903 was initially studied in patients with advanced solid tumor malignancies (NCT: 02729298), where it was noted to be well tolerated and preliminary data demonstrated that it produced stable disease responses (19). Although developed as an AXL inhibitor, TP-0903 has activity against FLT3-ITD and TKD mutations and a variety of intracellular kinases related to MAPK, STAT, and AKT signaling (20). Recently, we demonstrated that TP-0903 had activity in preclinical models of drug-resistant AML, including those with RAS pathway and FLT3 F691L mutations (20). Here, we sought to evaluate the safety, tolerability, and preliminary clinical activity of this drug in a pilot phase 1 study of patients with relapsed or refractory AML and FLT3-ITD mutations.
Materials and methods
Patient population
Adults ≥18 years of age with relapsed/refractory histologically confirmed de-novo, therapy-related or secondary AML (excluding acute promyelocytic leukemia) and the presence of FLT3-ITD mutation were eligible. In addition, eligible patients were required to have an Eastern Cooperative Oncology Group performance status of ≤2 as well as a projected life expectancy of at least 6 months. Moreover, patients were required to have adequate organ function, defined as total bilirubin <2.0 mg/dL, liver transaminases less than 2.5 times the upper limit of normal, creatinine clearance of >50 mL/min, New York Heart Association congestive heart failure class II or better, and left ventricular ejection fraction of ≥40%.
The exclusion criteria included receipt of chemotherapy or radiation therapy within 2 weeks of protocol enrollment, although hydroxyurea was permitted during cycle 1 only to maintain a WBC count of <40,000 K/µL. Patients with active central nervous system involvement, uncontrolled infection, and advanced solid malignant tumors were also excluded. Pregnant patients, those with significant gastrointestinal disease, and patients who were unable to swallow pills were also not permitted to enroll. The complete eligibility criteria are provided in the Supplementary Material.
Study design
The protocol was approved by the Institutional Review Board of The Ohio State University (OSU) and was in accordance with the Declaration of Helsinki, the International Conference on Harmonization-Good Clinical Practice, and local laws. Informed consent was obtained from all patients. The primary objectives of the study were to determine the safety and tolerability of TP-0903. The secondary objectives were to characterize the toxicity profile of TP-0903 and to determine disease-free survival (DFS) and overall survival (OS) as well as the proportion of patients who were able to proceed with transplant. This protocol was registered at ClinicalTrials.gov (Identifier: NCT04518345).
Patients received TP-0903 monotherapy at a starting dose of 50 mg, by mouth, daily on days 1–21 of a 28-day cycle. A dose de-escalation trial design was utilized in which the dose of TP-0903 could be reduced to 37 mg depending on the toxicities experienced on the 50-mg dose. Patients were permitted to receive hydroxyurea during the first cycle of treatment only; however, patients whose WBC counts remained uncontrolled by the first day of cycle 2 were removed from the study. Participants could receive 28-day continuous cycles of treatment for as long as clinical benefit was observed. Patients achieving a clinical or hematologic response, defined as obtaining complete remission (CR), CR with hematologic recovery (CRh), or morphologic leukemia-free state (MLFS), were referred for allogeneic stem cell transplant if eligible. Patients who did not achieve clinical or hematologic benefit after four cycles of treatment were removed from the study. Transplant-ineligible responding patients were permitted to receive maintenance therapy with TP-0903 until loss of response or clinical benefit. Although the study was designed to accrue between 40 and 46 participants between the phase 1b and phase 2 components, the trial closed early due to the withdrawal of industry-sponsored funding.
Safety assessments
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to define hematologic and non-hematologic toxicities. Dose-limiting toxicity (DLT) was assessed during cycle 1 only and included any ≥ grade 4 non-hematologic toxicity, any non-Hy’s law ≥ grade 3 liver abnormality that did not resolve within 72 h, any ≥ grade 3 infection lasting more than 7 days in the absence of active AML, and any ≥ grade 3 bleeding with thrombocytopenia in the absence of AML. Any ≥ grade 3 non-hematologic toxicity not resulting directly from active leukemia constituted a DLT with the exception of alopecia, thrombosis in association with a central line, fatigue, anorexia, constipation, grade 3 or 4 electrolyte disturbances that resolved within 24 h with electrolyte correction and were not clinically significant, or grade 3 nausea or vomiting that did not require hospitalization or support with total parenteral nutrition and resolved to < grade 2 within 72 h. Hematologic DLTs were defined as any ≥ grade 4 neutropenia lasting more than 14 days past the end of the cycle (by day 42) in the absence of active AML.
Response assessment
Disease responses were determined based on the criteria defined by the 2017 European LeukemiaNet (21).
Mutation analysis
FLT3-ITD allelic ratio was determined by PCR amplification with the FAM-labeled forward primer 5′-GCAATTTAGGTATGAAAGCCAGC-3′ and the unlabeled reverse primer 5′-CTTTCAGCATTTTGACGGCAACC-3′ followed by capillary electrophoresis (2). Mutations co-occurring with FLT3-ITD before and during TP-0903 treatment were determined by targeted NGS. Libraries were generated using xGen™ DNA Lib Prep MC UNI kit and target capture was done using xGen AML Cancer Hybridization Panel in conjunction with a three-gene spike-in pool for ZRSR2, AXL, and ASXL2 (Integrated DNA Technologies, Coralville, IA, United States). DNA library preparations were performed according to the manufacturer’s instructions and sequenced on an Illumina NovaSeq (OSUCCC Genomics Shared Resource, Columbus, OH, United States). Sequenced reads were aligned to the GRCh38 genome build using the Burrows-Wheeler Aligner (BWA). Picard Tools was used to perform UMI-consensus calling on the aligned reads. The Genome Analysis Toolkit (GATK) was used to realign insertions and deletions in the aligned reads and to perform base quality score recalibration for those realigned regions. GATK’s MuTect2 was used to perform variant calling. After variant calling, variants were annotated using SnpEff and vcfanno along with the dbsnp, COSMIC, and gnomad variant databases. The Mucor3 algorithm was used as the baseline for integrative mutation assessment. Visual inspection of all variants was carried out using Integrative Genomics Viewer v.2.8 (Broad Institute, Cambridge, MA, United States). A variant allele fraction (VAF) cutoff of 0.01 was set for reporting mutations.
Pharmacodynamic studies
Ficoll-enriched bone marrow samples were processed, stained, and analyzed for AML immunophenotype markers (CD45 dim), FLT3, and pSTAT5 by flow cytometry as previously described (22). Sample analysis was performed in the OSUCCC Flow Cytometry Shared Resource and Immune Monitoring & Discovery Platform using the Cytek Aurora, and data were analyzed using FCS Express v.7 (De Novo Software, Pasadena, CA, United States).
Statistical analysis
As only three patients were enrolled in the trial, the statistical analysis was limited to descriptive statistics.
Results
Patient characteristics
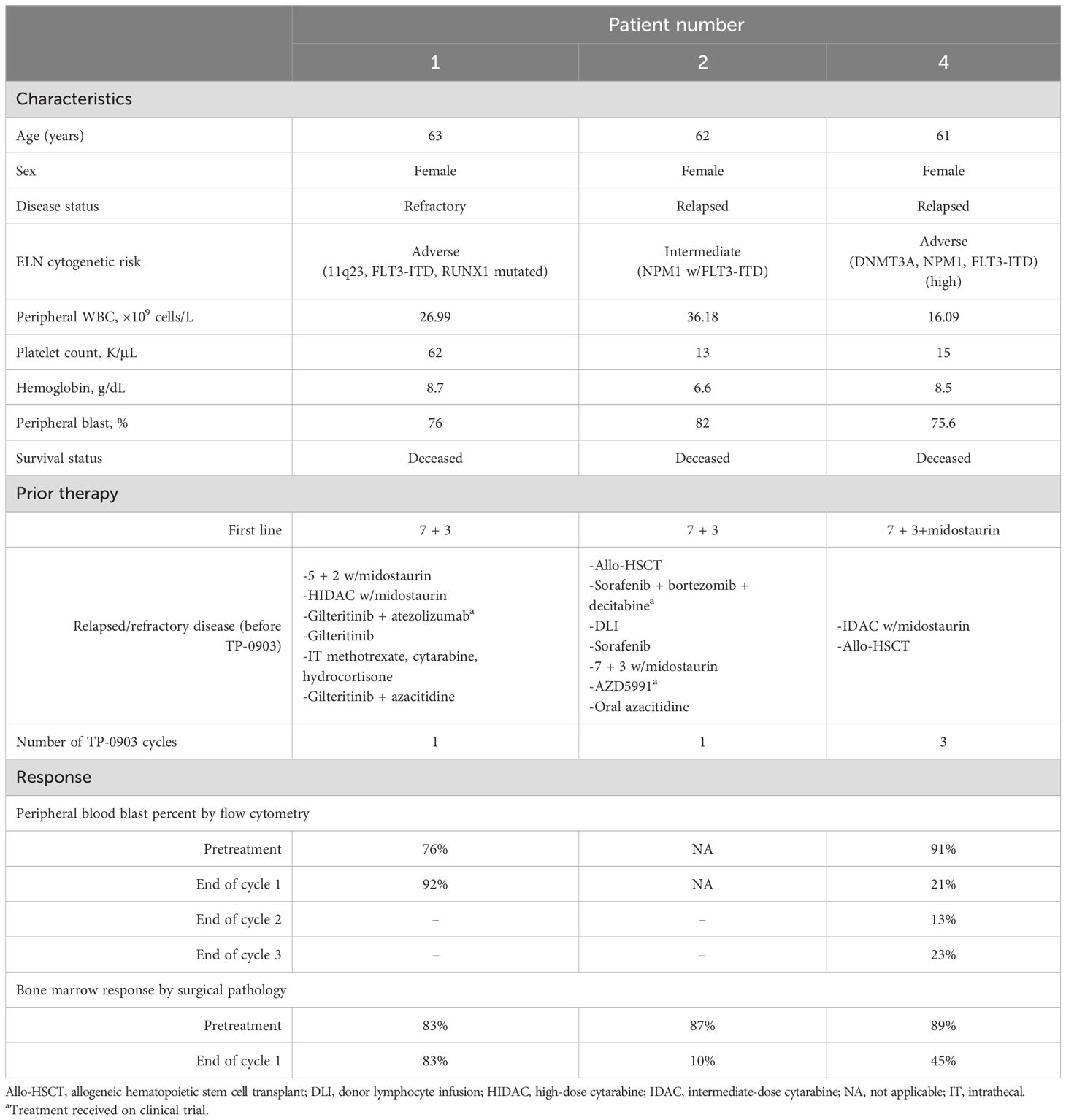
Between 5 November 2020 and 4 May 2022, this trial enrolled a total of three patients. Patient characteristics for each of the three patients are shown in Table 1. All participants were women between the ages of 61 and 63 years with ELN 2017 intermediate or adverse risk disease. Two patients had previously received an allogeneic hematopoietic stem cell transplant and two had received five or more prior lines of therapy at the time of enrollment. One patient had previously been treated with intrathecal chemotherapy for central nervous system involvement by AML. Only one patient (patient 4) was able to receive more than one cycle of treatment and eventually opted to be excluded from the study after receiving three cycles of TP-0903 in order to pursue a different therapy. Of the two patients who received only one cycle of therapy, one patient (patient 1) could not continue due to uncontrolled disease after cycle 1, necessitating ongoing use of hydroxyurea, and the second patient (patient 2) could not proceed due to a decline in her clinical condition and eventual death from AML.
Safety
We did not observe any DLTs in the three treated patients. Grade 1–2 non-hematologic toxicities observed in at least two of the three enrolled patients included nausea and vomiting, hypocalcemia, hypokalemia, renal insufficiency, and increased serum alkaline phosphatase. The most common ≥ grade 3 non-hematologic toxicities in at least one patient included tumor lysis syndrome, appendicitis, hyperbilirubinemia, sinus tachycardia, hypotension, pulmonary edema, fever, hypertension, and hypoxia. All three patients experienced ≥ grade 3 hematologic toxicities which consisted of anemia, thrombocytopenia, neutropenia, and lymphopenia. Patient 1 developed grade 3 nausea on cycle 1, day 13 despite being on maximal antiemetic therapy. The decision was made to hold TP-0903, and her nausea decreased to grade 2 within 24 h after treatment was withheld. TP-0903 was resumed at the 37-mg dose 11 days later, on cycle 1, day 24, because the patient was struggling with neutropenia-related infections, which were awaiting resolution before restarting treatment. On the 37-mg dose, which she continued for the remainder of the cycle, nausea remained grade 1–2. The toxicities of TP-0903 experienced by each patient are shown in Supplementary Table S1.
Response
Based on the ELN 2017 response criteria, all patients achieved stable disease. Two patients had a marked reduction in blood and marrow blasts following one cycle of therapy; both also displayed a significant decrease in the FLT3-ITD allelic ratio (Table 1).
Mutation analysis
In addition to harboring FLT3-ITD, other co-occurring mutations noted at the time of initial diagnosis are shown in Figure 1. In the two patients with a decline in blood or marrow blast counts, VAF in genes such as NPM1 and DNMT3A declined over one to two cycles of treatment. Mutation VAF remained unchanged in patient 1 who did not experience a decline in blast counts; a KRAS mutation with low VAF was observed in this patient who had received six prior regimens for relapsed disease, including three regimens that contained gilteritinib.
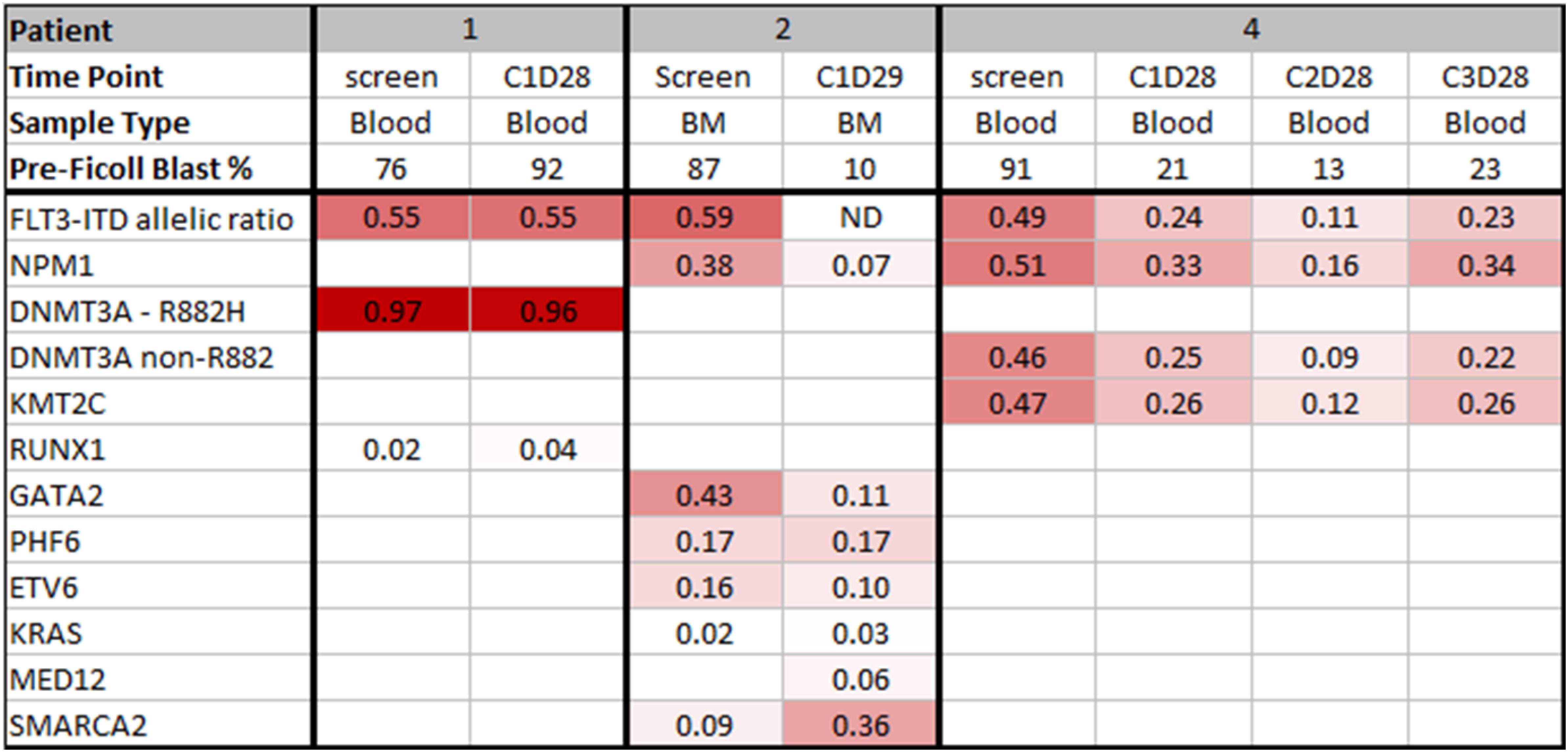
Figure 1. OncoPrint of mutations in TP-0903-treated patients pre- and post-treatment. Row 1 indicates the patient number. Rows 2 and 3 show the time of sample and sample type, respectively. Row 4 indicates the sample pre-Ficoll blast percentage. Row 5 designates the FLT3-ITD allelic ratio. Each remaining row represents genes mutated with a variant allele frequency (VAF) cutoff of 0.01. Shades of red indicate varying ranges of VAF (darker red color represents higher VAF). White color denotes wild-type genes. ND, not detected.
Pharmacodynamics
Pre- and post-treatment bone marrow samples during cycle 1 were available from patients 1 and 4 for pharmacodynamic analysis. Samples from days 1 (pretreatment), 5, and 28 were analyzed for inhibition of FLT3 and pSTAT5 expression via spectral flow cytometry. In patient 4 with a reduction in blast count, there was a decrease in the MFI for both FLT3 and pSTAT5, with a maximum decrease on day 28 (Figure 2B). For patient 2 who did not experience a reduction in blast count, there was an increase in MFI for both FLT3 and pSTAT5 by day 28 (Figure 2A).
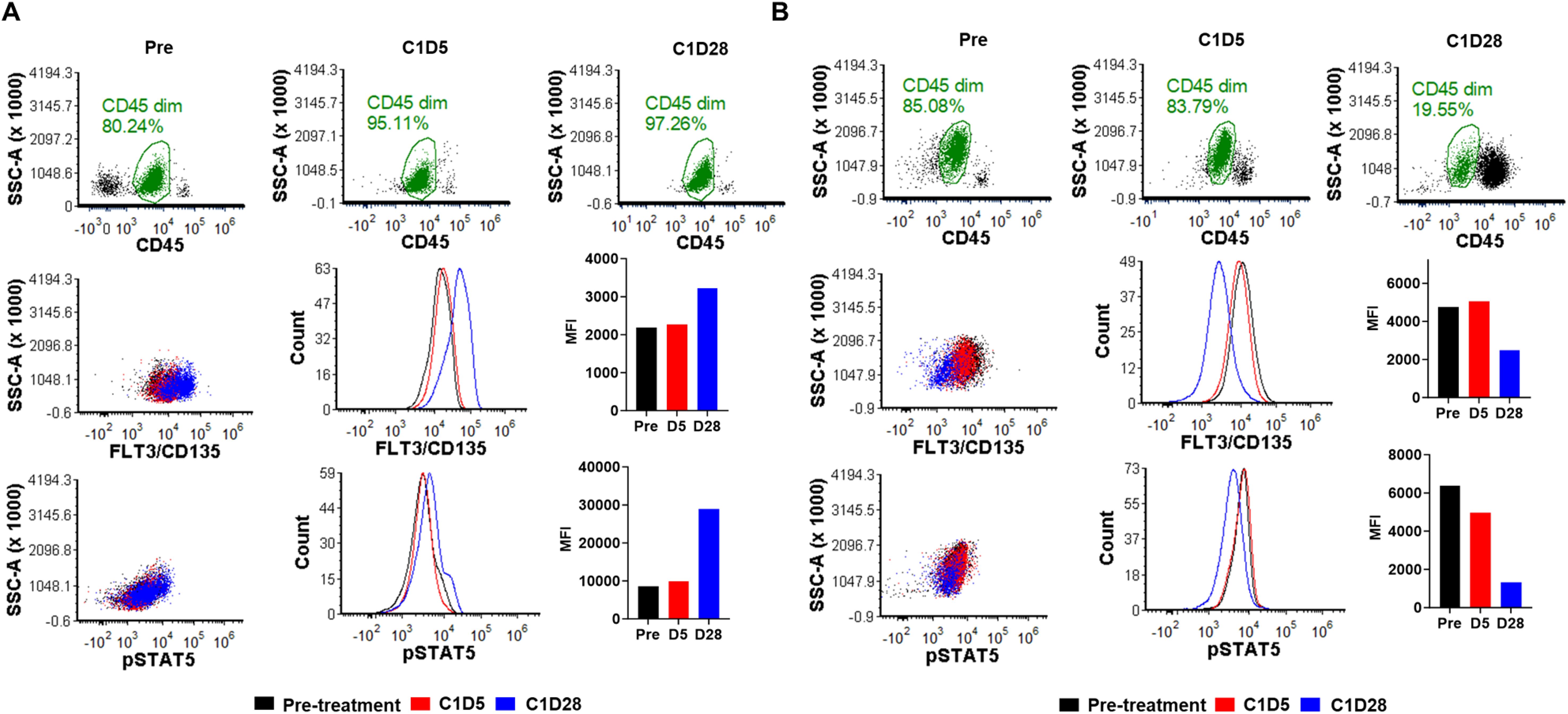
Figure 2. FLT3 and pSTAT5 expression in response to TP-0903 treatment. Using spectral flow cytometry, bone marrow samples from (A) patient 1 and (B) patient 4 were analyzed first by gating for AML blasts (CD45 dim; green) and then accessed for FLT3/CD135 and pSTAT5 expression. Pretreatment samples are indicated in black, C1D5 samples in red, and C1D28 samples in blue. MFI, mean fluorescent intensity.
Discussion
AML patients with FLT3-ITD mutations display aggressive disease biology, high relapse rates, and overall poor survival (23, 24). The development of FLT3 inhibitors and their integration into clinical practice has greatly impacted the care of such patients and has led to improved survival rates, though important limitations remain. Drug resistance and disease evolution characterized by the acquisition of resistance mutations are important mechanisms by which AML patients with FLT3-ITD commonly relapse on FLT3-directed therapy (17, 18, 20). As such, there is an opportunity to improve response rates and duration through the implementation of an FLT3 inhibitor that is able to abrogate potential resistance mutations. TP-0903, in preclinical models, has shown such activity rendering it an attractive agent to offer AML patients with FLT3-ITD. Herein, we report our findings on three heavily pretreated patients with AML and FLT3-ITD, which, to our knowledge, represents the first report of TP-0903 in AML.
Though we were only able to treat three patients with the single-agent TP-0903, we did not identify any toxicities that were unusual relative to other agents used in the relapsed/refractory AML space. Only one patient was able to receive more than one cycle of therapy. While no patients achieved CR or CRh, we observed a marked reduction in bone marrow blasts following one to two cycles of treatment in two patients (patients 2 and 4), concurrent with a decline in FLT3-ITD allelic ratio and co-occurring mutations such as NPM1 and DNMT3A. An on-target effect on inhibition of FLT3 and downstream pSTAT5 in bone marrow blasts was also observed.
Conclusions
TP-0903 was shown to be a feasible treatment option for AML patients with relapsed FLT3-ITD mutated AML. Since we were able to provide safety and disease-response data on only three patients, larger studies of TP-0903, both as a single agent and in combination with other therapies, are needed to better understand the utility of this agent. Although we did not observe any major clinical responses in three heavily pretreated patients, TP-0903 did show a marked effect in terms of reduction of FLT3-ITD allelic ratio and bone marrow blast percentage, which may point toward a potential efficacy signal, though future studies evaluating this finding are necessary.
Data availability statement
NGS data has been deposited in the NIH Sequence Read Archive (SRA) under BioProject ID PRJNA1250480 and can be accessed by using the following link: https://www.ncbi.nlm.nih.gov/bioproject/1250480.
Ethics statement
The studies involving humans were approved by the Ohio State University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BB: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. DB: Formal Analysis, Investigation, Project administration, Supervision, Writing – review & editing. KD-K: Data curation, Investigation, Project administration, Writing – review & editing. SO: Formal Analysis, Methodology, Project administration, Writing – original draft, Writing – review & editing. JY: Formal Analysis, Investigation, Methodology, Writing – review & editing. ZT: Formal Analysis, Investigation, Methodology, Writing – review & editing. AR: Conceptualization, Methodology, Writing – review & editing. JB: Formal Analysis, Methodology, Writing – review & editing. UB: Investigation, Writing – review & editing. SB: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. SPDO Pharma provided the funding support to conduct this research.
Acknowledgments
The authors wish to thank the patients and their family members, the inpatient acute leukemia nurses, staff, and pharmacists as well as the other physicians who assisted with the care of these patients.
Conflict of interest
BB received research support from Sumitomo Pharma America, Inc., Karyopharm Therapeutics, and Cell Therapeutics, Inc.; received advisory board honoraria from Servier Pharmaceuticals, Bristol Myers Squibb, Novartis Pharmaceuticals, Daiichi Sankyo US, Celgene Corp, and Kite Pharma; and is on the speaker’s bureau for Astra Zeneca US. AR served on an independent data monitoring committee for Telios Pharma, Inc. and is currently employed and holds stock with Eli Lilly and Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2025.1554764/full#supplementary-material
References
1. Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. (2010) 116:3622–6. doi: 10.1182/blood-2010-05-283648
2. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
3. Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. (2001) 98:1752–9. doi: 10.1182/blood.V98.6.1752
4. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. (2018) 378:2386–98. doi: 10.1056/NEJMoa1716984
5. Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. (2002) 99:4326–35. doi: 10.1182/blood.V99.12.4326
6. Reindl C, Bagrintseva K, Vempati S, Schnittger S, Ellwart JW, Wenig K, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. (2006) 107:3700–7. doi: 10.1182/blood-2005-06-2596
7. Pemmaraju N, Kantarjian H, Ravandi F, Cortes J. FLT3 inhibitors in the treatment of acute myeloid leukemia: the start of an era? Cancer. (2011) 117:3293–304. doi: 10.1002/cncr.v117.15
8. Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. (2001) 61:7233–9.
9. Breccia M, Frustaci AM, Cannella L, Stefanizzi C, Latagliata R, Cartoni C, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukaemia patients. Hematol Oncol. (2009) 27:148–53. doi: 10.1002/hon.v27:3
10. Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. (2005) 121:295–306. doi: 10.1016/j.cell.2005.02.013
11. Schmalbrock LK, Dolnik A, Cocciardi S, Sträng E, Theis F, Jahn N, et al. Clonal evolution of acute myeloid leukemia with FLT3-ITD mutation under treatment with midostaurin. Blood. (2021) 137:3093–104. doi: 10.1182/blood.2020007626
12. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
13. Erba HP, Montesinos P, Kim HJ, Patkowska E, Vrhovac R, Žák P, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1571–83. doi: 10.1016/S0140-6736(23)00464-6
14. Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. (2013) 31:3681–7. doi: 10.1200/JCO.2013.48.8783
15. Zimmerman EI, Turner DC, Buaboonnam J, Hu S, Orwick S, Roberts MS, et al. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood. (2013) 122:3607–15. doi: 10.1182/blood-2013-07-513044
16. Wang ES, Goldberg AD, Tallman M, Walter RB, Karanes C, Sandhu K, et al. Crenolanib and intensive chemotherapy in adults with newly diagnosed FLT3-mutated AML. J Clin Oncol. (2024) 42(15):Jco2301061. doi: 10.1200/JCO.23.01061
17. Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood. (2022) 139:3366–75. doi: 10.1182/blood.2021011583
18. Smith CC, Levis MJ, Perl AE, Hill JE, Rosales M, Bahceci E. Molecular profile of FLT3-mutated relapsed/refractory patients with AML in the phase 3 ADMIRAL study of gilteritinib. Blood Adv. (2022) 6:2144–55. doi: 10.1182/bloodadvances.2021006489
19. Sarantopoulos J, Fotopoulos G, Tsai FY-C, Beg MS, Adjei AA, Lou Y, et al. A phase Ia/b first-in-human, open-label, dose-escalation, safety, PK and PD study of TP-0903 in solid tumours. Ann Oncol. (2019) 30(Supplement 5). doi: 10.1093/annonc/mdz244.022
20. Jeon JY, Buelow DR, Garrison DA, Niu M, Eisenmann ED, Huang KM, et al. TP-0903 is active in models of drug-resistant acute myeloid leukemia. JCI Insight. (2020) 5(23):e140169. doi: 10.1172/jci.insight.140169
21. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
22. Buelow DR, Bhatnagar B, Orwick SJ, Jeon JY, Eisenmann ED, Stromatt JC, et al. BMX kinase mediates gilteritinib resistance in FLT3-mutated AML through microenvironmental factors. Blood Adv. (2022) 6:5049–60. doi: 10.1182/bloodadvances.2022007952
23. Ravandi F, Kantarjian H, Faderl S, Garcia-Manero G, O’Brien S, Koller C, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res. (2010) 34:752–6. doi: 10.1016/j.leukres.2009.10.001
24. Fröhling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. (2002) 100:4372–80. doi: 10.1182/blood-2002-05-1440
Keywords: acute myeloid leukemia, AML, relapsed/refractory, clinical trial, TP-0903
Citation: Bhatnagar B, Buelow DR, Dvorak-Kornaus KM, Orwick SJ, Yoon Jeon J, Talebi Z, Ruppert AS, Blachly JS, Borate U and Baker SD (2025) Preliminary clinical activity of TP-0903 in relapsed or refractory acute myeloid leukemia with FLT3-ITD mutations. Front. Hematol. 4:1554764. doi: 10.3389/frhem.2025.1554764
Received: 02 January 2025; Accepted: 21 March 2025;
Published: 22 April 2025.
Edited by:
Pellegrino Musto, University of Bari Aldo Moro, ItalyReviewed by:
Anna M. Eiring, The University of Texas at El Paso, United StatesFrancesco Tarantini, University of Bari Aldo Moro, Italy
Copyright © 2025 Bhatnagar, Buelow, Dvorak-Kornaus, Orwick, Yoon Jeon, Talebi, Ruppert, Blachly, Borate and Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhavana Bhatnagar, YmhhdmFuYS5iaGF0bmFnYXIxQGhzYy53dnUuZWR1; Sharyn D. Baker, YmFrZXIuMjQ4MEBvc3UuZWR1
 Bhavana Bhatnagar
Bhavana Bhatnagar Daelynn R. Buelow2
Daelynn R. Buelow2