- 1Department of Hematology-Oncology, College of Medicine, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 2Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AR, United States
- 3Department of Dermatology, University of Arkansas for Medical Sciences, Little Rock, AR, United States
Introduction: Sézary syndrome (SS) is an aggressive variant of cutaneous T-cell lymphoma (CTCL) that presents with generalized erythroderma, lymphadenopathy, peripheral blood involvement, and severe pruritus. Unfortunately, the disease can relapse or progress and generally has a poor prognosis. Mogamulizumab is a targeted monoclonal antibody that has been shown to be effective in relapsed disease, with particular benefit in the blood compartment, and has received the U.S. Food and Drug Administration (FDA) approval for CTCL. Data regarding the combination of mogamulizumab with other systemic treatments for CTCL are limited. Here, we present clinical outcomes in a real-world setting where patients with SS were treated using a mogamulizumab-based multi-agent approach.
Methods and results: We conducted a retrospective chart review of patients in a Cutaneous Lymphoma Clinic and identified 10 patients that met the International Society for Cutaneous Lymphomas (ISCL)/European Organization of Research and Treatment of Cancer (EORTC) diagnostic criteria for SS and were treated with mogamulizumab. These patients received at least four prior therapies, including systemics and topicals. All patients received mogamulizumab in addition to other systemic therapies. Responses were commonly observed in the blood after the first cycle. Global response, determined by assessment in four compartments including the skin, blood, viscera, and lymph nodes, identified four patients that achieved complete remission, three with partial response, two that maintained a stable disease, and one patient that experienced disease progression. The therapies most frequently used in combination were bexarotene and interferon. The median duration of response in our patient population was 23.5 months.
Conclusion: In this case series, a multi-agent approach combining mogamulizumab with traditional CTCL systemic agents was well tolerated and was effective in the treatment of relapsed or refractory SS and led to a period of extended clinical improvement.
1 Introduction
Sézary syndrome (SS) is an aggressive variant of cutaneous T-cell lymphoma (CTCL) with leukemic involvement. Patients with SS present with intense pruritus, generalized erythroderma, enlarged lymph nodes, and malignant cells in the peripheral blood (1, 2). A number of treatments that have activity in treating SS include retinoids, interferon, and photopheresis (3–5). As monotherapy, the response rate of these agents is limited (3, 6). Effective treatments for SS remain unsatisfactory, without a standard approach for relapsed or refractory (R/R) disease. The T cells in SS express surface cutaneous lymphocyte antigens (CLA) and CC chemokine receptor types 4 (CCR4) and 7 (CCR7), which play an important role in the chemotaxis of inflammation in the skin and cutaneous manifestations (7). In 2018, the U.S. Food and Drug Administration (FDA) approved mogamulizumab for the treatment of adult patients with R/R mycosis fungoides (MF) or SS after at least one previous systemic therapy. Mogamulizumab is a novel humanized defucosylated monoclonal antibody that targets CCR4 with enhanced antibody-dependent cellular cytotoxicity (5). The effectiveness of mogamulizumab has been substantiated in a phase 3 randomized controlled trial (MAVORIC), with the results showing an improved progression-free survival (PFS) of 7.7 months with mogamulizumab in comparison to 3.1 months with vorinostat. In addition, the response rate of patients treated with mogamulizumab was 28% compared with 5% in those who received vorinostat (8). Subset analysis of SS patients treated with mogamulizumab in the MAVORIC trial showed a response of 37%, which is particularly encouraging in this patient population. Currently, data regarding the efficacy of mogamulizumab in combination with other therapies are scarce. We hypothesize additional benefits in combination with standard therapies. Here, we describe our experience of using mogamulizumab in combination to treat 10 patients with R/R SS disease. The primary objective was to assess the objective response rate (ORR), with duration of response (DOR) and toxicity assessment as secondary objectives.
2 Patients and methods
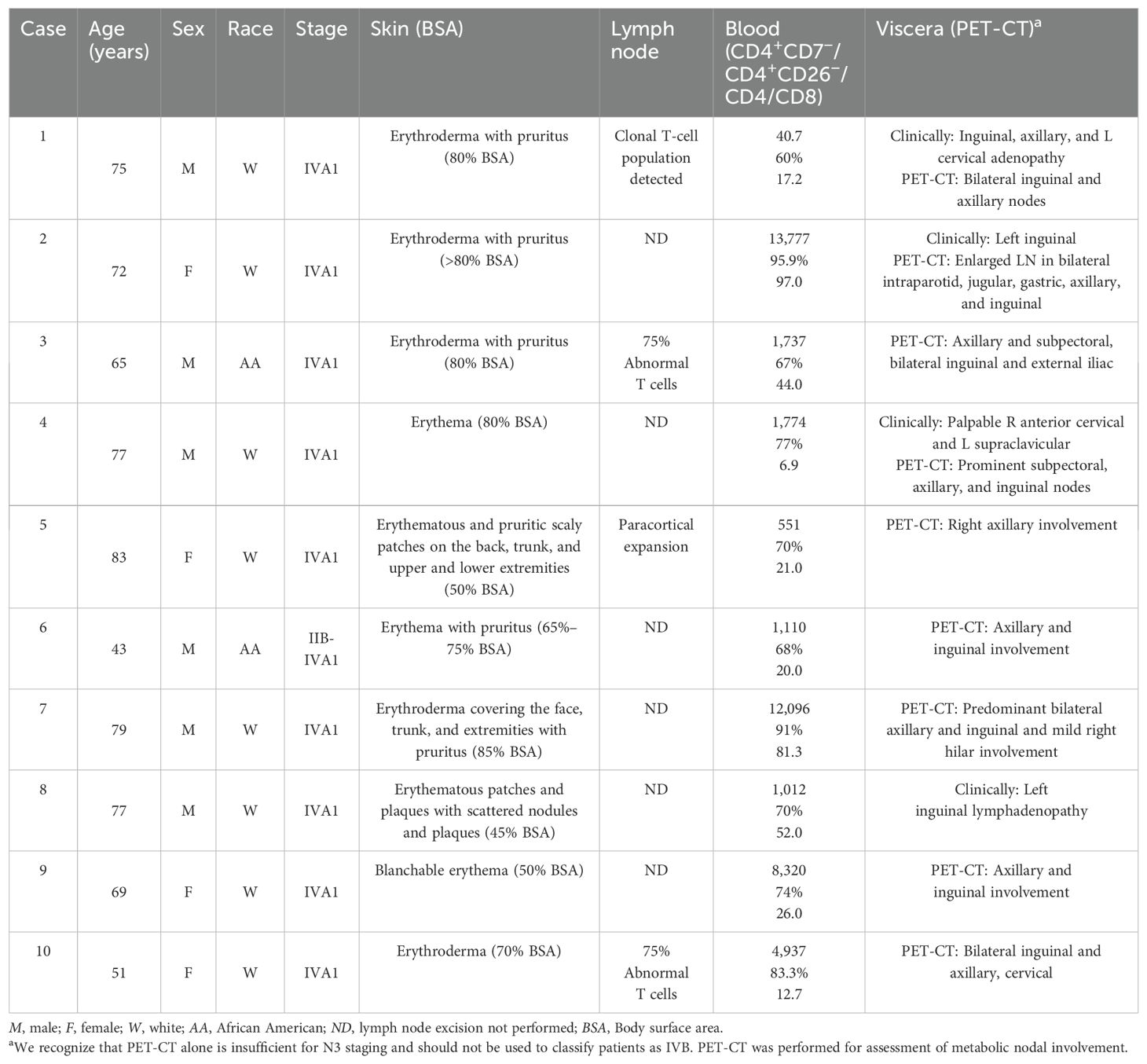
We performed a retrospective review of patients with SS treated in the Cutaneous Lymphoma Clinic at the University of Arkansas for Medical Sciences (UAMS) between January 2013 and January 2025 under a research protocol approved by the UAMS (IRB #276418). The UAMS database was queried for SS diagnosis, age, sex, therapy response, date of death, and laboratory studies, including hemoglobin, platelet count, white blood cell count, creatinine, bilirubin, and liver enzymes (Table 1). SS stage was graded based on the International Society for Cutaneous Lymphomas (ISCL)/European Organization of Research and Treatment of Cancer (EORTC) consensus recommendations (9). Initially, 15 patients with SS were identified. This number was reduced to 10 patients who matched the inclusion criteria below.
Patients with SS who failed at least one systemic treatment were included for analysis. The criteria assessed included the Sézary count based on flow cytometry for the CD4+/CD7− fraction and the CD4/CD8 ratio prior to mogamulizumab treatment, as well as clinical assessments including erythema, adenopathy, and pruritus. These data points were monitored post-treatment to assess the hematologic and clinical response. Mogamulizumab was administered intravenously (IV) using scheduled doses: 1.0 mg/kg weekly for the first 28-day cycle, then on days 1 and 15 of subsequent cycles. Patients were followed up on a monthly basis when initiating therapy; visits were spaced out as tolerated. Dose modifications were considered based on the occurrence of adverse events and on patient responses. No patients were lost to follow-up, and patients were followed until the end of the recruitment period or death.
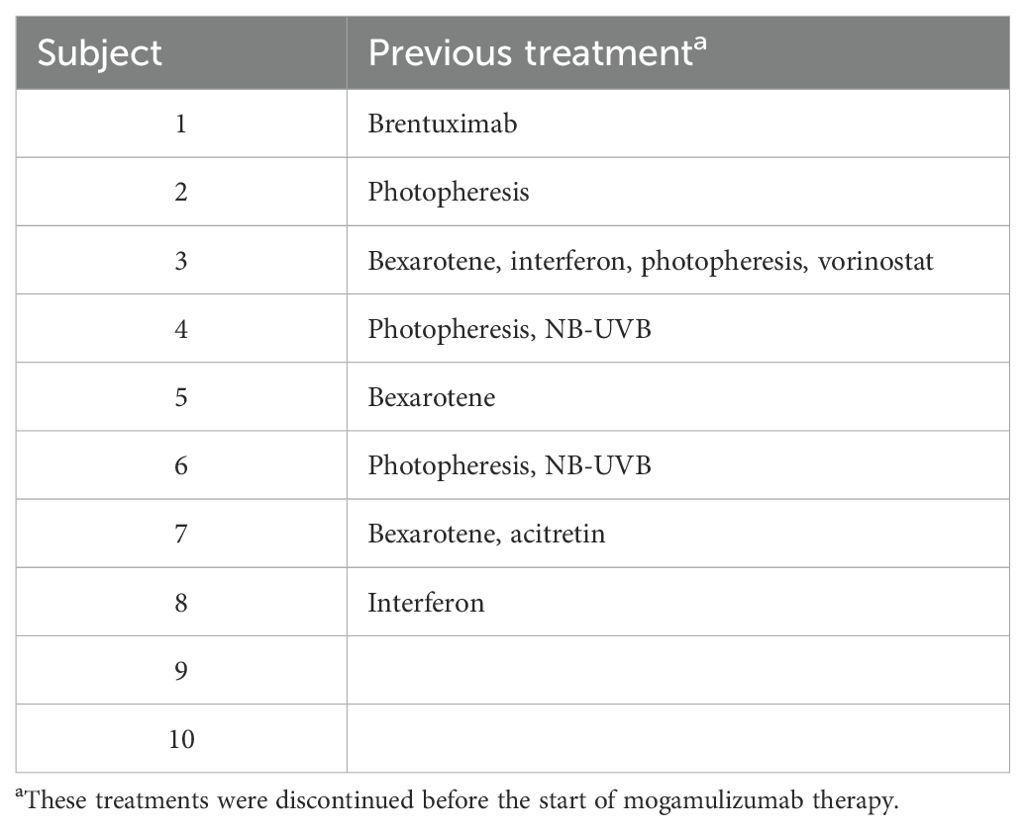
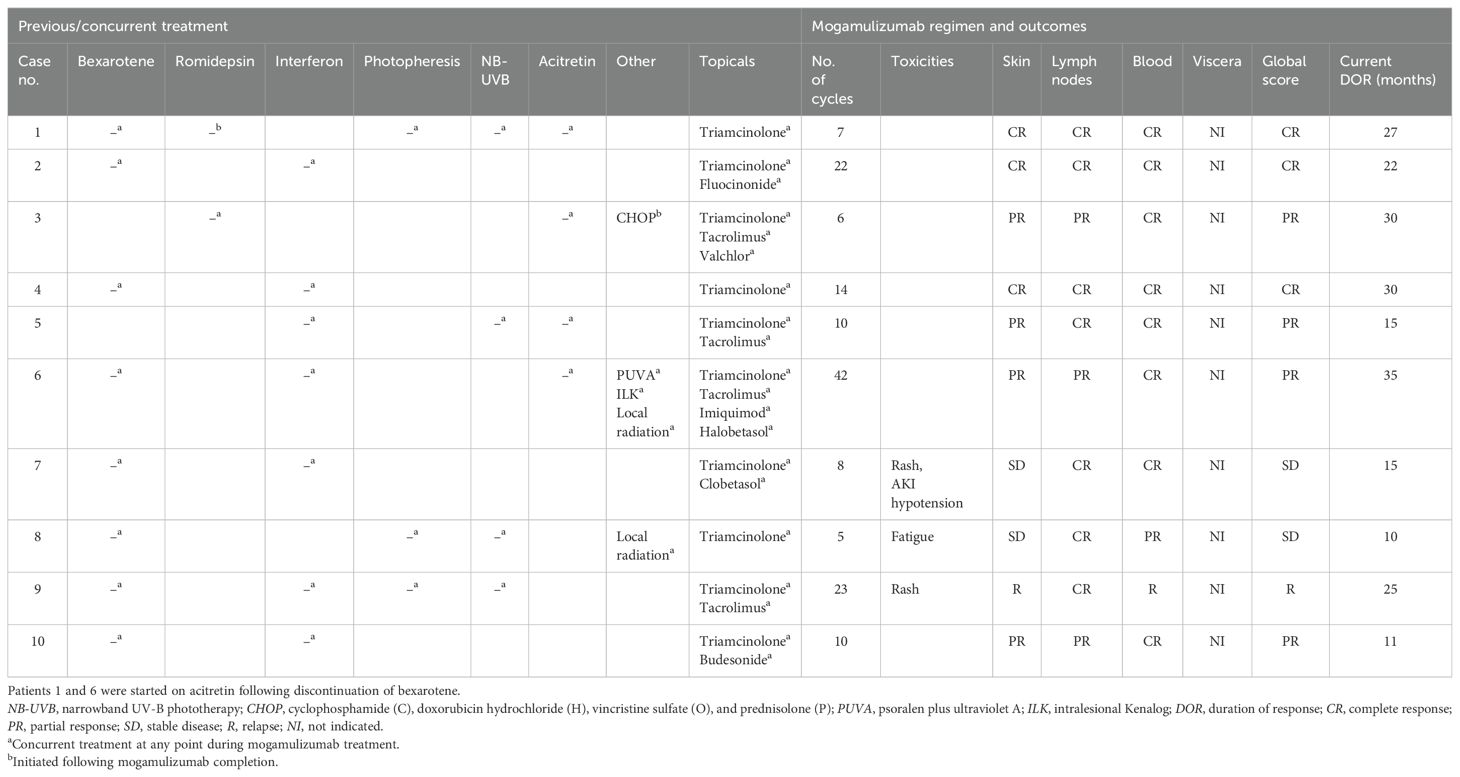
Clinical examinations for pruritus, erythema, and adenopathy were performed and assessed as complete response (CR), partial response (PR), stable disease (SD), or relapse (R). Laboratory assessments of the absolute Sézary count (CD4+/CD7−) and the CD4/CD8 ratio were trended to determine the hematologic response. The scoring parameters of the clinical exam findings and hematologic response were then combined into a global response score as outlined by the Clinical End Points and Response Criteria in Mycosis Fungoides and Sézary Syndrome paper published in 2011 by Olsen et al. (10). ORR was calculated as the proportion of patients with global responses of CR and PR. Table 2 summarizes the previous treatment of each patient. Table 3 provides information describing each patient’s global score determination and the treatments, treatment-emergent adverse events, and DOR. Response duration was followed from the initial response to disease progression or death as the endpoint. In addition, toxicities such as hematologic, hepatic, and renal function abnormalities were recorded.
3 Results
Patients with SS were identified from patients receiving systemic treatment at the UAMS Cutaneous Lymphoma Program. There were 10 patients with SS who received at least one cycle of mogamulizumab and who met the criteria for analysis. The median age of the patients treated with mogamulizumab was 71.1 years (range, 43–83 years), with a male/female ratio of 3:2 (Table 1). The average length of follow-up across all patients was 19 months, with a range of 4–37 months. The ORR was 70% (7/10). Subjects 1, 2, and 4 had a CR and subjects 3, 5, 6, and 10 had a PR, with subjects 7 and 8 having SD. Overall, the median DOR was 23.5 months. Subject 6 had skin involvement with tumors in addition to blood involvement and showed CR in the blood, while the skin showed PR. Subject 9 showed initial improvement in the blood and skin for a duration of 26 months, but subsequently relapsed in the blood, which was refractory to re-treatment with mogamulizumab. The overall and the individual laboratory responses after the first exposure to mogamulizumab are shown in Figure 1 (created in RStudio).
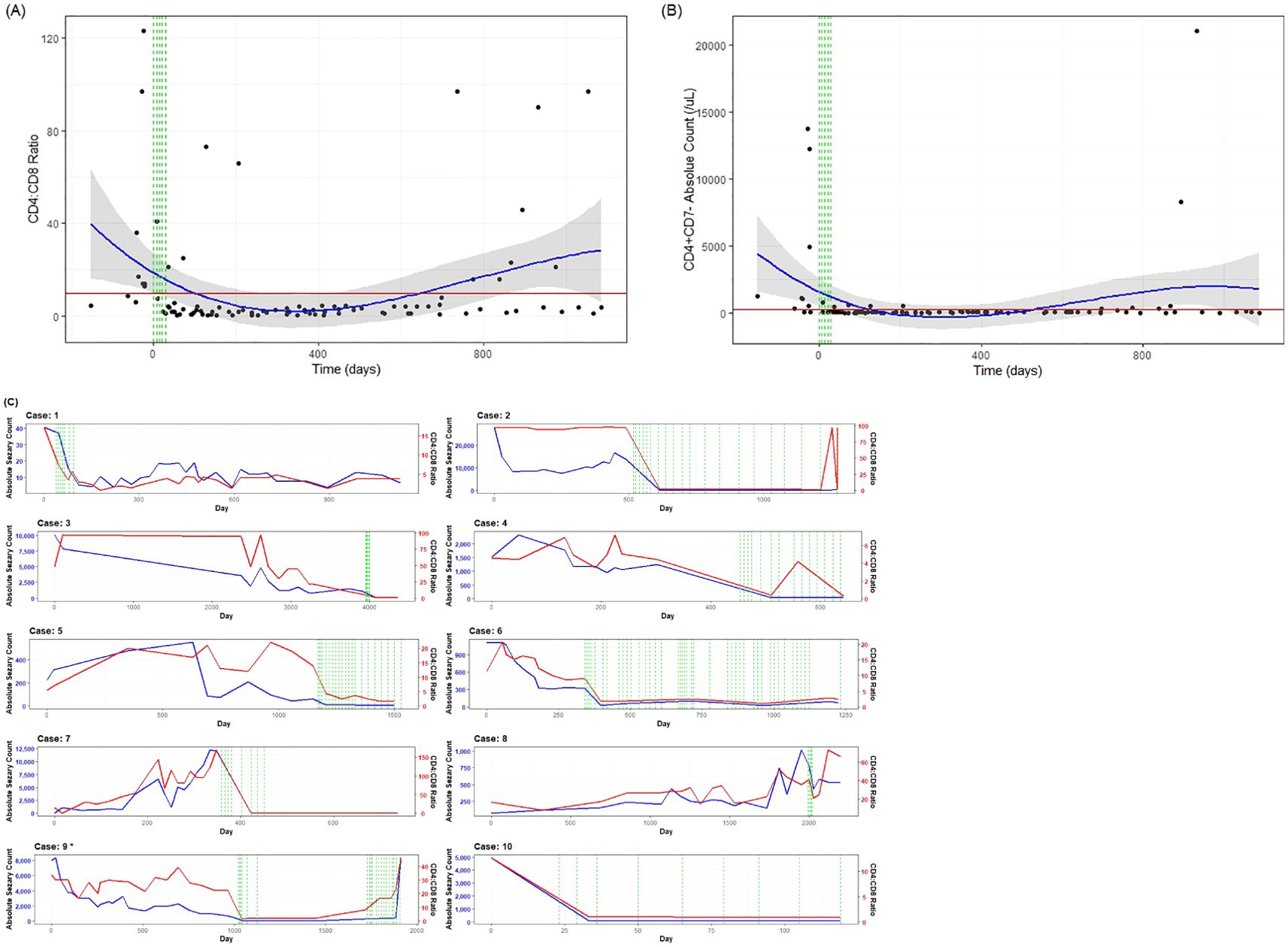
Figure 1. Laboratory values from flow cytometry for mogamulizumab-treated patients. The normal peripheral blood reference range for the absolute count of CD4+/CD7− cells is 21–189/μl and that for the CD4/CD8 ratio was 1.1:3.0. The green lines correspond to the first month of treatment during which the patients received mogamulizumab (days 0, 7, 14, 21, and 28). The red line represents the reference range threshold (Sézary count <250/μl and CD4/CD8 ratio <10:1). The blue line represents a polynomial regression model used to display changes over time. The gray area represents the 95% confidence interval (95%CI). (A) The mean CD4/CD8 ratio decreased from 37.9 at baseline to 4.9 after at least one cycle of mogamulizumab therapy. (B) The mean absolute Sézary count decreased from 3,509.9/μl at baseline to 69.1/μl after at least one cycle of mogamulizumab therapy. Data are derived from the observation of 10 patients. (C) Data displaying the individual response of each patient over time. The red lines correspond to the CD4/CD8 ratio, the blue lines indicate the Sézary count, and the green vertical dashed lines represent individual infusions of mogamulizumab. *The data points after relapse from patient 9 have not been included. This patient eventually reached an absolute count of 21,056/μl. She was re-treated with mogamulizumab and allogeneic stem cell transplant, with a complete response; however, she passed shortly after due to infection.
Mogamulizumab treatment was stopped in three patients (7, 8, and 9) after one, four, and eight cycles, respectively, due to adverse reactions, including an exfoliative, pruritic mogamulizumab-associated rash (MAR) and fatigue. However, these patients were continued on prior systemic treatments, with global response scores of SD, SD, and R, respectively. The rashes were effectively controlled with topical or systemic steroids. MAR was controlled in all subjects, and blood involvement remained stable post-treatment with mogamulizumab. Patients 1 and 6 were started on acitretin after discontinuing bexarotene due to insurance barriers and renal insufficiency, respectively. As shown in Figure 2, a complete blood response was most frequently observed within 4 weeks after the initiation of mogamulizumab therapy. A CR in the blood was determined as a normalization of the absolute Sézary count (CD4+/CD7−) to <250/mm3 and the CD4/CD8 ratio to <10. A PR in the blood was representative of a decrease in a patient’s absolute Sézary count by >50% from baseline. Relapse described patients that had a return in the absolute Sézary count or the CD4/CD8 ratio to >1,000/mm3 or >10:1, respectively.
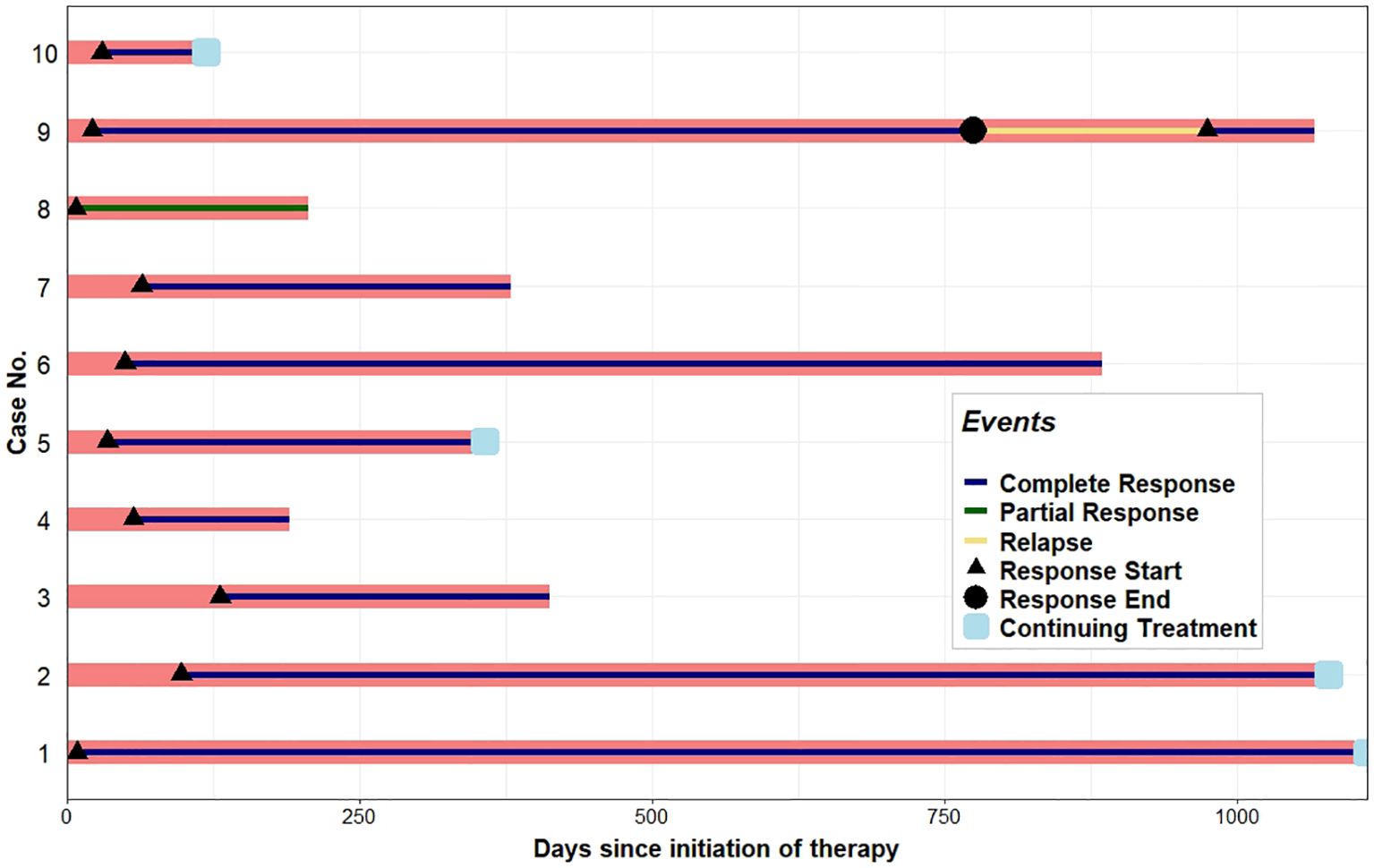
Figure 2. Swimmer plot displaying the hematologic response to mogamulizumab therapy. Each bar represents one subject in the study, numbered according to Table 3. A complete response denotes a normalization of the absolute Sézary count to <250/mm3 and the CD4/CD8 ratio to <10. A partial response in the blood was representative of a decrease in a patient’s absolute Sézary count by >50% from baseline. Relapse describes patients who had a return in the absolute Sézary count or the CD4/CD8 ratio to >1,000/mm3 or >10:1, respectively.
Concomitant treatments with mogamulizumab were well tolerated, without adverse events requiring cessation (Table 3). Retinoids were the most frequently used agents in combination with mogamulizumab, with bexarotene and acitretin continued in seven and in two subjects, respectively. Interferon was used in six subjects. Narrowband ultraviolet B (NB-UVB) phototherapy was administered concomitantly in five patients. One patient received a single course of mogamulizumab, with response in the blood and nodes. However, pruritus remained persistent, and he was transitioned to romidepsin and extracorporeal photopheresis (ECP), which maintained prolonged clinical remission.
4 Discussion
The introduction of mogamulizumab has offered an additional novel targeted therapy for the treatment of CTCL. The clinical trial data for mogamulizumab showed greater efficacy in patients with SS who exceeded the response in MF. Mogamulizumab offers a unique treatment for patients with SS. However, there remains a need for greater effectiveness and durable therapy in SS that is well tolerated. Here, we present a real-world experience in a series of SS patients who progressed/relapsed on at least one prior systemic therapy and who were then subsequently treated with mogamulizumab in combination with other systemic therapies, which included ECP, retinoids (bexarotene and acitretin), interferon alpha, and NB-UVB phototherapy. SS patients were maintained on their previous systemic drugs if no adverse effects were observed, which included bexarotene/acitretin, interferon, and phototherapy. In this patient cohort with progressive SS, all patients received at least one cycle of mogamulizumab added to their previous therapy. Overall, the DOR observed across these 10 patients exceeded a median duration of 23.5 months, which is greater than the DOR of 17.3 months seen in the MAVORIC trial (8). In our cohort, we observed 70% with long-term response in comparison to the response of 34% in the MAVORIC trial, with multiple patients clinically in remission without relapse at up to 35 months.
Importantly, we demonstrated that mogamulizumab therapy can be added in combination from insufficient efficacy on prior systemic treatments. The addition of mogamulizumab led to clinical and laboratory responses in our patients, with a limited subset experiencing adverse events in the skin after halting mogamulizumab. Six subjects tolerated long-term treatment, with excellent response. At the time of submission, five patients have maintained response to the current treatments with their disease under control. One patient relapsed with increased peripheral blood involvement after having an ongoing response for 26 months. This patient underwent allogeneic stem cell transplant and re-treatment with mogamulizumab, but succumbed to infectious complications. Even in patients with rash that required cessation, the short-term treatment with mogamulizumab was associated with clinical improvement, and these patients were able to be maintained with response on preexistent systemic drugs.
Other studies have reported mogamulizumab combinations in CTCL (11–18). A case series of four patients with CTCL who had previously failed bexarotene showed an increased stable response when treated with mogamulizumab plus bexarotene (11). This is a combination that was well tolerated in our patients, with nine being treated with retinoids, and with no additional adverse events detected.
In our patients, the combination with interferon alpha was found to be highly effective and was well tolerated. Five patients received low-dose interferon (1–3 million units three times per week), and most received concomitant bexarotene without adverse events.
One of our patients benefited from combination with ECP. This modality has been confirmed by Ninosu et al. in 11 erythrodermic CTCL patients treated with mogamulizumab in combination with ECP, showing an overall response of 73% in the skin and an overall response of 64% in the blood (19). In another study, Weiner et al. described the treatment of 16 CTCL patients (14 SS and 2 MF) with mogamulizumab in combination with ECP, bexarotene, and/or interferon, with a complete global response of 56% (20). Other reports of mogamulizumab in combination therapy include interferon (21), low-dose total skin electron beam therapy (13), gemcitabine (14), etoposide (15, 16), and others (22). Overall, the use of combination therapy has been well tolerated without limiting adverse effects and with potentially enhanced efficacy for the treatment of MF/SS to improve the global response rates compared with monotherapy alone, with a greater duration than monotherapy.
Treatment-emergent adverse events can negatively impact the quality of life and may affect compliance for long-term treatment, thereby limiting its efficacy and therapeutic potential. A rash was among the most prevalent treatment-emergent adverse events in the MAVORIC trial (23.9%), with 7% of patients discontinuing treatment due to its severity (8). The patients in this series reported symptoms of fatigue and lethargy, gastrointestinal side effects, appetite loss, and, most frequently, rash. In our case series, extensive dermatitis led to the discontinuation of mogamulizumab in two patients. For example, subject 9 developed an exfoliative and pruritic rash following the fourth infusion. A biopsy of the rash revealed a superficial dermal infiltration with enlarged lymphocytes and histiocytes, consistent with MAR. Nevertheless, within 4 months, the rash resolved, and clinical examination did not reveal any evidence of lymphadenopathy, indicating a continued response to treatment. A follow-up flow cytometry analysis showed a T-cell population with a CD4+ and CD7− phenotype comprising approximately 3.0% of all cells, with a normal total absolute count of CD4 T cells, a CD4/CD8 ratio of 1.75, and a recovery of CD26 expression. The patient continued to display CR in the blood compartment for 26 months post-mogamulizumab therapy, after which she relapsed. Following relapse, she was treated with salvage mogamulizumab infusions and allogeneic stem cell therapy, which led to a repeat CR in the blood; however, the patient eventually succumbed to infection. Another patient who developed extensive pruritic eruption was successfully treated with systemic corticosteroids and continued bexarotene with clearance of the blood compartment. This suggests that a rash in these patients did not affect the clinical outcome, which is consistent with studies (19, 20, 22, 23) suggesting that a mogamulizumab-induced rash may reflect an active immune response.
The responses in our subjects are consistent with the treatment-associated rash augmenting an immune phenomenon since our patients showed improvement in disease control. Subjects with rash on pathology showed histologic reaction patterns of spongiotic to lichenoid to granulomatous features, as well as a lack of a strong CD4 predominance. Furthermore, the pathology was not consistent with disease relapse or progression since T cells expressed CD7 (24, 25) and more strongly expressed CD26 (26). In the response in the peripheral blood, we observed by flow cytometry a consistent increase in the CD4+/CD26+ population in patients after treatment with mogamulizumab.
In this study, MAR occurred at a rate of 20%. Furthermore, the patients who developed a rash from mogamulizumab showed stable response even after its discontinuation; these subjects were maintained on other systemic treatments (such as bexarotene and interferon alpha). Mogamulizumab has been approved in other T-cell lymphomas, and rash has also been observed in patients and associated with positive outcomes. In the treatment of relapsed and aggressive adult T-cell leukemia/lymphoma (ATL), mogamulizumab monotherapy was found to enhance the PFS and overall survival (OS) in some patients, particularly in those who developed a drug-related skin rash (27). In another study, patients with acute-type ATL who developed a skin rash had a 1-year OS rate of 66.7%, whereas those without a skin rash had a 1-year OS rate of 34.3% (24). A study conducted in 2016 by Ureshino et al. provided evidence of the effectiveness of mogamulizumab in the treatment of two individuals diagnosed with ATL. During the treatment course, the patients experienced a severe skin rash and autoimmune brainstem encephalitis, adverse effects that coincided with the depletion of effector regulatory T cells (Tregs). Mogamulizumab inhibited the progression of ATL, until effector Tregs (eTreg) rebounded and the patients relapsed, suggesting that the inhibition of eTreg is associated with severe autoimmunity and should be monitored during mogamulizumab therapy (28).
The limitations in this study include the small number of patients and the need for greater long-term follow-up to accurately assess the DOR and/or additional treatment toxicities that may occur in patients who are continuing treatment. In addition, a larger systematic, multicenter analysis of patients with R/R SS receiving specific combination therapies with mogamulizumab may be necessary.
In conclusion, we present clinical data that provide valuable insights into the efficacy of mogamulizumab in combination with additional therapies (including bexarotene and interferon alpha concurrently). This case series suggests that a multi-agent approach using mogamulizumab in R/R SS is effective, well tolerated, and safe and leads to improved clinical outcomes. The most common side effect noted was a non-CTCL pruritic rash consistent with MAR, which, in our patients, appeared to correlate with disease control. Our data support the need for additional prospective studies to evaluate the optimal combination regimens using mogamulizumab in the relapsed/refractory setting for Sézary syndrome.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Arkansas for Medical Sciences IRB #276418. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JP: Formal Analysis, Software, Visualization, Writing – original draft, Writing – review & editing. DK: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. CG: Writing – review & editing. HW: Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer OA declared a past co-authorship with the author HW to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Larocca C, Kupper T. Mycosis fungoides and sézary syndrome: an update. Hematol Oncol Clin North Am. (2019) 33:103–20. doi: 10.1016/j.hoc.2018.09.001
2. Lee H. Mycosis fungoides and Sézary syndrome. Blood Res. (2023) 58:66–82. doi: 10.5045/br.2023.2023023
3. Rook AH, Zic J, Kim Y, Porcu P, Querfeld C, Wood G. Sézary syndrome: Immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol. (2013) 64:352. doi: 10.1016/j.jaad.2010.08.037
4. Stuver R, Geller S. Advances in the treatment of mycoses fungoides and Sézary syndrome: a narrative update in skin-directed therapies and immune-based treatments. Front Immunol. (2023) 14:1284045. doi: 10.3389/fimmu.2023.1284045
5. Alpdogan O, Kartan S, Johnson W, Sokol K, Porcu P. Systemic therapy of cutaneous T-cell lymphoma (CTCL). Chin Clin Oncol. (2019) 8:10. doi: 10.21037/cco.2019.01.02
6. Latzka J, Assaf C, Bagot M, Cozzio A, Dummer R, Guenova E. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2023. Eur J Cancer. (2023) 195:113343. doi: 10.1016/j.ejca.2023.113343
7. García-Díaz N, Piris MÁ, Ortiz-Romero PL, Vaqué JP. Mycosis fungoides and sézary syndrome: an integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers (Basel). (2021) 13:1931. doi: 10.3390/cancers13081931
8. Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. (2018) 19:1192–204. doi: 10.1016/S1470-2045(18)30379-6
9. Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. (2007) 110:1713–22. doi: 10.1182/blood-2007-03-055749
10. Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. International Society for Cutaneous Lymphomas; United States Cutaneous Lymphoma Consortium; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. (2011) 29:2598–607. doi: 10.1200/JCO.2010.32.0630
11. Afifi S, Mohamed S, Zhao J, Foss F. A drug safety evaluation of mogamulizumab for the treatment of cutaneous T-Cell lymphoma. Expert Opin Drug Saf. (2019) 18:769–76. doi: 10.1080/14740338.2019.1643837
12. Teoli M, Mandel VD, Franceschini C, Saraceni PL, Cicini MP, Ardigò M. Mogamulizumab and bexarotene are a promising association for the treatment of advanced cutaneous T-cell lymphomas: a case series. Eur Rev Med Pharmacol Sci. (2022) 26:8118–28. doi: 10.26355/eurrev_202211_30166
13. Fong S, Hong EK, Khodadoust MS, Li S, Hoppe RT, Kim YH, et al. Low-dose total skin electron beam therapy combined with mogamulizumab for refractory mycosis fungoides and sézary syndrome. Adv Radiat Oncol. (2020) 6:100629. doi: 10.1016/j.adro.2020.11.014
14. Caruso L, Castellino A, Dessì D, Flenghi L, Giordano A, Ibatici A. Italian real-life experience on the use of mogamulizumab in patients with cutaneous T-cell lymphomas. Cancer Manag Res. (2022) 14:3205–21. doi: 10.2147/CMAR.S377015
15. Amagai R, Kambayashi Y, Ohuchi K, Furudate S, Hashimoto A, Asano Y. Cutaneous T cell lymphoma treated with mogamulizumab monotherapy and mogamulizumab plus etoposide combined therapy: A real-world case series. Dermatol Ther. (2022) 35:e15858. doi: 10.1111/dth.15858
16. Ohuchi K, Fujimura T, Kambayashi Y, Amagai R, Lyu C, Tanita K. Successful treatment of mogamulizumab-resistant mycosis fungoides with mogamulizumab plus etoposide combined therapy: Investigation of the immunomodulatory effects of etoposide on the tumor microenvironment. Dermatol Ther. (2020) 33:e13487. doi: 10.1111/dth.13487
17. Zhao Y, Tang J, Chang C, Zheng Z, Takahashi T, Nomura T. HSR23-109: real-world combination use of mogamulizumab with other therapies among patients with mycosis fungoides and sézary syndrome. J Natl Compr Cancer Netw. (2023) 21. doi: 10.6004/jnccn.2022.7191
18. Dong N, Jaiswal S, Khadka S, Francisque C, Zhang Y, Glass L, et al. A retrospective study of extracorporeal photopheresis (ECP) in treatment of cutaneous T-cell lymphoma (CTCL) evaluating global, skin and blood responses. Blood. (2023) 142:3078. doi: 10.1182/blood-2023-191313
19. Ninosu N, Melchers S, Kappenstein M, Booken N, Hansen I, Blanchard M. Mogamulizumab combined with extracorporeal photopheresis as a novel therapy in erythrodermic cutaneous T-cell lymphoma. Cancers. (2023) 16:141. doi: 10.3390/cancers16010141
20. Weiner D, Rastogi S, Lewis D, Cohen L, Choi S, Vittorio C, et al. Mogamulizumab multimodality therapy with systemic retinoids, interferon, or extracorporeal photopheresis for advanced cutaneous T-cell lymphoma. Dermatol Ther. (2023), 7625926. doi: 10.1155/2023/7625926
21. Treitman R, Colton MD, Srivastava P, Tobin J, Marciano K, Rezac R, et al. A case series of patients with cutaneous t-cell lymphoma treated with combination mogamulizumab and other therapies after single agent mogamulizumab. Hematol Oncol. (2023) 41:772–4. doi: 10.1002/hon.3165_620
22. Bojanini L, Herrera M, Li S, Xu N, Ahamed AA, Khodadoust M. Long-term outcomes with mogamulizumab alone or in combination with other therapies for the treatment of cutaneous t-cell lymphoma. Hematol Oncol. (2023) 41:506–7. doi: 10.1002/hon.3164_374
23. Satake A, Konishi A, Azuma Y, Tsubokura Y, Yoshimura H, Hotta M. Clinical efficacy of mogamulizumab for relapsed/refractory aggressive adult T-cell leukemia/lymphoma: A retrospective analysis. Eur J Haematol. (2020) 105:704–11. doi: 10.1111/ejh.13474
24. Musiek ACM, Rieger KE, Bagot M, Choi JN, Fisher DC, Guitart J, et al. Dermatologic events associated with the anti-CCR4 antibody mogamulizumab: characterization and management. Dermatol Ther (Heidelb). (2022) 12:29–40. doi: 10.1007/s13555-021-00624-7
25. Wang JY, Hirotsu KE, Neal TM, Raghavan SS, Kwong BY, Khodadoust MS. Histopathologic characterization of mogamulizumab-associated rash. Am J Surg Pathol. (2020) 44:1666–76. doi: 10.1097/PAS.0000000000001587
26. Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. (2001) 115:885–92. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV
27. Ishida T, Utsunomiya A, Jo T, Yamamoto K, Kato K, Yoshida S. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. (2017) 108:2022–9. doi: 10.1111/cas.13343
Keywords: Sézary syndrome, cutaneous T-cell lymphoma (CTCL), mogamulizumab, mogamulizumab-associated rash, bexarotene, refractory Sézary syndrome
Citation: Pilkington J, Ly S, Kayishunge D, Gentille C and Wong HK (2025) Mogamulizumab in combination improves clinical outcomes in relapsed and refractory Sézary syndrome. Front. Hematol. 4:1557641. doi: 10.3389/frhem.2025.1557641
Received: 08 January 2025; Accepted: 07 April 2025;
Published: 14 May 2025.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Matilde Scaldaferri, AOU Città della Salute e della Scienza di Torino, ItalyOleg E. Akilov, University of Pittsburgh, United States
Marie Roelens, AP-HP, France
Copyright © 2025 Pilkington, Ly, Kayishunge, Gentille and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henry K. Wong, aGVucnkud29uZ0B2YS5nb3Y=; anRwaWxraW5ndG9uQHVhbXMuZWR1
 Jordan Pilkington1
Jordan Pilkington1 Henry K. Wong
Henry K. Wong